Introduction
Gastric cancer, a widespread malignancy on a global
scale, imposes a significant burden in terms of both incidence and
mortality in numerous countries (1). The prognosis of patients with gastric
cancer is influenced by a myriad of factors, including tumor
staging, histological type and the overall health status of the
individuals (2–4). Due to its high malignancy,
investigating the factors influencing the clinical outcomes of
gastric cancer remains of significant importance (5,6).
Pyloric stenosis, a relatively common complication,
is known to significantly impact the nutritional status of the
patients (7,8). It not only disrupts dietary intake and
reduces the quality of life but also has a substantial influence on
the treatment and prognosis of gastric cancer (9–11).
Since pyloric stenosis can lead to decreased treatment tolerance
and rapid disease progression, nutritional status has always been a
focal point of concern (12–14).
While previous studies have primarily examined the nutritional
status of pyloric stenosis patients, few have delved into their
systemic inflammatory status (15,16).
Inflammatory status can impact the disease progression of patients
with gastric cancer through various pathways, and a substantial
body of previous research has also identified the close
relationship and interaction between inflammation and nutritional
status (17–19). Therefore, it is still necessary to
explore the inflammatory status of patients with gastric cancer
with pyloric stenosis and its impact on prognosis.
Hence, the primary objective of the present study
was to probe the intricate relationship between systemic
inflammatory status and prognosis in patients with gastric cancer
who were concurrently afflicted by pyloric stenosis and underwent
radical resection, employing a retrospective approach. To mitigate
potential biases, propensity score-matching analysis was employed,
which enabled the authors to more accurately assess this
association. The present study is the first, to the best of the
authors' knowledge, to examine the inflammatory status of patients
with early pyloric stenosis using multiple classic inflammatory
indices and to analyze their impact on patient prognosis.
Additionally, the predictive abilities of different inflammatory
indices were compared to identify the one with the highest
prognostic value.
Patients and methods
Patients
The present retrospective study enrolled 242
patients with gastric cancer at the Affiliated Hospital of
Southwest Medical University (Luzhou, China) between July 2016 and
December 2020. Inclusion criteria were as follows: i) Patients were
confirmed to have early pyloric stenosis. The diagnosis of early
pyloric stenosis in patients was established through a
comprehensive assessment, including: a) Clinical symptoms:
Presentation of upper abdominal pain, bloating, weight loss, acid
reflux and fatigue; b) gastroscopic examination: Direct
visualization of the stenosis in the pyloric region via
gastroscopy, with a biopsy conducted to rule out or confirm
malignancy; c) imaging studies: Utilization of upper
gastrointestinal barium meal X-ray, CT scan, or MRI to ascertain
the location and extent of the stenosis; d) clinical
manifestations: Evaluation of the nutritional status of the patient
to assess the impact of the stenosis on digestion and absorption.
ii) Patients with tumor-node-metastasis (TNM) stage II or III who
had received radical resection. iii) Patients who had received
complete treatment and follow-up, with comprehensive clinical and
medical record data. Exclusion criteria were as follows: i)
Patients suffering from chronic inflammatory conditions. A chronic
inflammatory condition refers to a prolonged and persistent
inflammatory response in the body, which is closely associated with
the development, progression and exacerbation of diseases. This
state can be induced by various factors, including persistent
infections, autoimmune reactions, long-term exposure to harmful
substances, chronic stress, or the presence of chronic diseases. It
is typically characterized by elevated white blood cell counts,
increased levels of inflammatory markers in the blood (such as
C-reactive protein, tumor necrosis factor-α and interleukin-6), and
infiltration of inflammatory cells in the affected tissues. This
condition can lead to tissue damage, fibrosis, impaired organ
function, and the onset and progression of chronic diseases. ii)
Patients who received preoperative chemotherapy, radiation therapy,
or immunotherapy. iii) Patients who were lost to follow-up or had
incomplete clinical and pathological information. Since all
patients were in TNM stage II and III, they all underwent standard
curative resection surgery, and received adjuvant chemotherapy with
either the standard SOX (oxaliplatin + S1) or XELOX (oxaliplatin +
capecitabine) regimen based on pathological staging 3 weeks
postoperatively. This study received approval and support from the
Ethics Committee of The Affiliated Hospital of Southwest Medical
University (approval no. KY2023224; Luzhou, China).
Data collection and follow-up
Information regarding the general health status and
disease progression of patients through the medical record system
was screened and collected. To investigate the inflammatory and
nutritional status of the patients, pertinent blood parameters,
which included total protein, albumin, globulin, prealbumin,
neutrophil (NEU), lymphocyte (LYM), monocyte (MON) and platelet
counts were concurrently gathered. For preoperative blood
collection, 5 ml of fasting venous blood from the elbow was drawn.
In total, 2 ml of the blood was transferred into an
ethylenediaminetetraacetic acid anticoagulant tube and mixed well,
then automatically analyzed for routine hematological parameters
using a BC-6000 Automated Hematology Analyzer (Shenzhen Mindray
Bio-Medical Electronics Co., Ltd.). Additionally, 3 ml of the blood
was transferred into a dry tube and left to stand at room
temperature to obtain the upper layer fluid, which was then
centrifuged (Sorvall ST8 Benchtop Room Temperature Centrifuge,
20–25 degrees Celsius; Thermo Fisher Scientific, Inc.) at a
relative centrifugal force of 3,260 × g for 5 min to separate the
serum. The serum biochemical parameters were automatically measured
using a cobas® c 311 analyzer (Roche Diagnostics). To
investigate systemic inflammation status, neutrophil-to-lymphocyte
ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic
immune-inflammation index (SII) and systemic inflammation response
index (SIRI) based on the blood parameters of patients were
calculated (Table I). The ranges
for NLR, PLR, SII and SIRI were 0.77–38.82, 66.27–741.18,
178.89–1,891.76 and 0.24–19.41, respectively. Overall survival (OS)
was obtained through routine telephone follow-up and was defined as
the period from the first day of surgery to either the date of
death or the last follow-up.
 | Table I.Calculation formulas. |
Table I.
Calculation formulas.
| Parameters | Calculation
formula |
|---|
|
Neutrophil-to-lymphocyte ratio | Neutrophil
(109/l)/lymphocyte (109/l) |
|
Platelet-to-lymphocyte ratio | Platelet
(109/l)/lymphocyte (109/l) |
| Systemic
immune-inflammation index | Platelet (109/l) ×
neutrophil (109/l)/lymphocyte (109/l) |
| Systemic
inflammation response index | Monocyte (109/l) ×
neutrophil (109/l)/lymphocyte (109/l) |
Statistical analysis
Categorical variables were described using n (%) and
differences were assessed through chi-square test and Fisher's
exact test. Continuous variables were presented as mean [standard
deviations (SD)] and differences were analyzed using an unpaired
Student's t-test. Kaplan-Meier survival curves and log-rank tests
were employed to evaluate survival disparities. Cox regression
analysis and Lasso regression analysis were utilized to identify
independent prognostic factors in the present study and address
issues related to multicollinearity. The proportional hazards
assumption test was performed using the Cox model and Schoenfeld
residual plots. Additionally, to mitigate potential selection bias,
propensity score matching (PSM) was employed. Finally, a risk
prognosis model and nomogram were constructed to further validate
the prognostic value of the inflammatory markers. Two-sided
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were conducted using SPSS 25
(IBM, Corp.) and R 4.1.3 (The R Foundation for Statistical
Computing; http://www.r-project.org/).
Results
Patient characteristics
The present study included a total of 242
participants, consisting of 193 male and 49 female patients. The
average age of the study cohort was 63.18 (SD, 9.91) years. The age
range was 40–81 years. Due to the concurrent presence of pyloric
stenosis, most patients exhibited distinct clinical symptoms. Among
these patients, 110 (45.5%) experienced stomachache, 128 (52.9%)
reported abdominal distention, 105 (43.4%) had weight loss, 94
(38.8%) suffered from sour regurgitation and 61 (25.2%) presented
with fatigue. Furthermore, the patients also demonstrated rapid
tumor progression, with over half of them having a tumor size of
≥50 mm (55.4%) and being in TNM stage III (66.1%), as shown in
Table II.
 | Table II.Characteristics of patients with
gastric cancer. |
Table II.
Characteristics of patients with
gastric cancer.
| Parameters | Number of patients
with pyloric stenosis (total n=242) |
|---|
| Mean age, years
(SD) | 63.18 (9.91) |
| Mean BMI, kg/m2
(SD) | 20.84 (3.36) |
| Mean total protein,
g/l (SD) | 61.45 (7.70) |
| Mean albumin
levels, g/l (SD) | 35.65 (4.49) |
| Mean globulin
levels, g/l (SD) | 26.02 (5.30) |
| Mean prealbumin
levels, g/l (SD) | 177.59 (53.75) |
| Median neutrophil
levels, 109/l (IR) | 3.68 (2.94,
4.88) |
| Median lymphocyte
levels, 109/l (IR) | 1.34 (1.00,
1.90) |
| Median monocyte
levels, 109/l (IR) | 0.41 (0.34,
0.52) |
| Median platelet
levels, 109/l (IR) | 244.00 (200.00,
288.00) |
| Median
neutrophil-to-lymphocyte ratio, (IR) | 2.74 (2.02,
3.94) |
| Median
platelet-to-lymphocyte ratio, (IR) | 185.47 (128.24,
246.67) |
| Median systemic
immune-inflammation index, (IR) | 671.22 (447.55,
1015.01) |
| Median systemic
inflammation response index, (IR) | 1.15 (0.84,
1.76) |
| Sex, n (%) |
|
|
Male | 193 (78.9) |
|
Female | 49 (20.2) |
| Stomach ache, n
(%) |
|
|
Yes | 110 (45.5) |
| No | 132 (54.5) |
| Abdominal
distension, n (%) |
|
|
Yes | 128 (52.9) |
| No | 114 (47.1) |
| Weight loss, n
(%) |
|
|
Yes | 105 (43.4) |
| No | 137 (56.6) |
| Fatigue, n (%) |
|
|
Yes | 61 (25.2) |
| No | 181 (74.8) |
| Acid reflux, n
(%) |
|
|
Yes | 94 (38.8) |
| No | 148 (61.2) |
| Primary tumor site,
n (%) |
|
| Upper
1/3 | 8 (3.3) |
| Middle
1/3 | 26 (10.7) |
| Lower
1/3 | 204 (84.3) |
|
Whole | 4 (1.7) |
| Borrmann type, n
(%) |
|
| I | 44 (18.2) |
| II | 170 (70.2) |
|
III | 22 (9.1) |
| IV | 6 (2.5) |
| Peripheral lymph
node, n (%) |
|
|
Positive | 192 (79.3) |
|
Negative | 50 (20.7) |
| Tumor size, n
(%) |
|
| <50
mm | 108 (44.6) |
| ≥50
mm | 134 (55.4) |
|
Tumor-node-metastasis stage, n (%) |
|
| II | 82 (33.9) |
|
III | 160 (66.1) |
| Human epidermal
growth factor receptor 2, n (%) |
|
|
Positive | 114 (47.1) |
|
Negative | 128 (52.9) |
Inflammatory markers
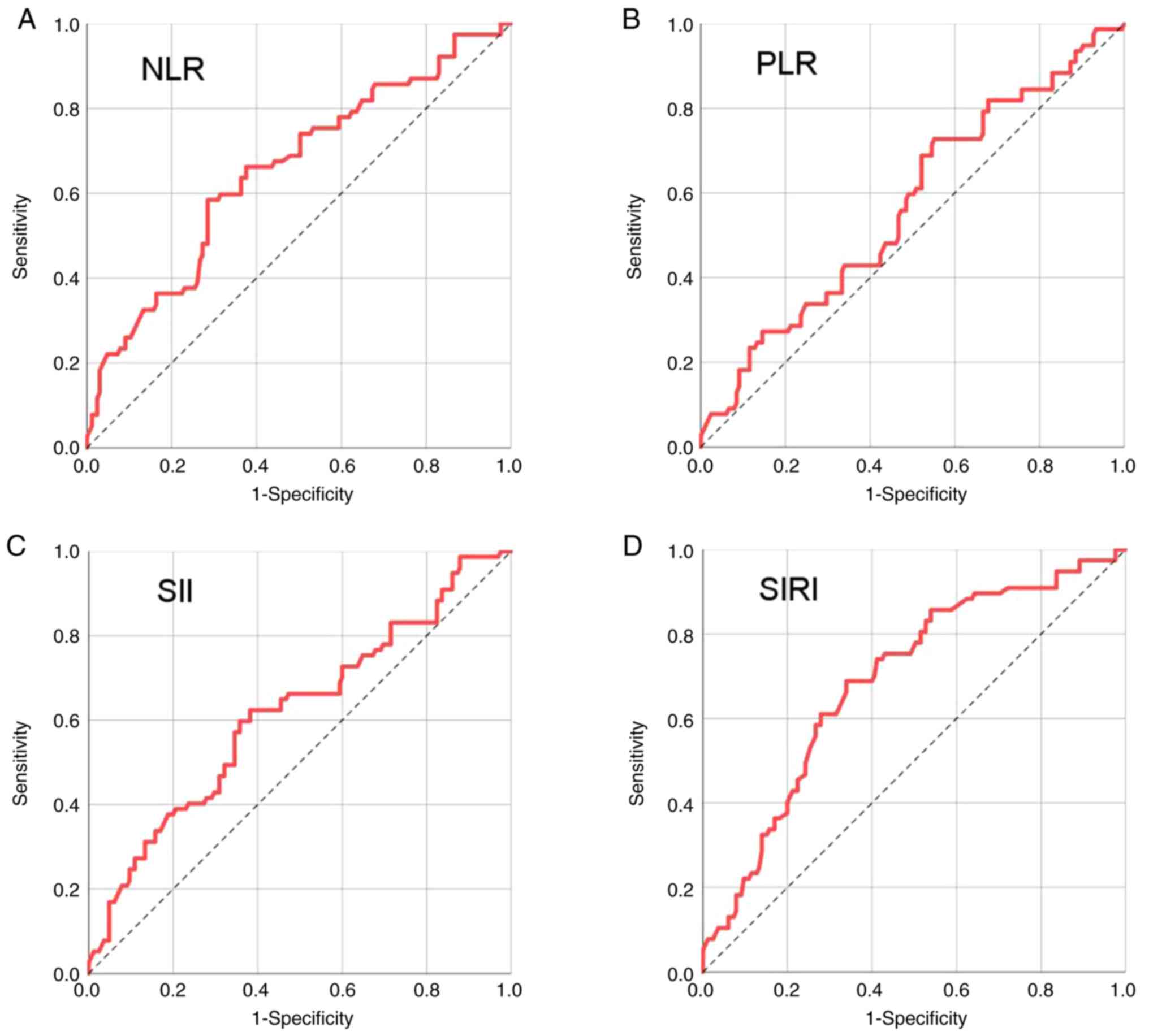
Receiver operating characteristic (ROC) curves were
generated using patient survival status to compare the predictive
capabilities of various inflammatory markers and identify their
optimal cutoff values (Fig. 1). The
optimal cut-off values, determined using the maximum Youden index,
were 3.150 for NLR (150 vs. 92 patients), 150.200 for PLR (95 vs.
147 patients), 718.025 for SII (131 vs. 111 patients) and 1.215 for
SIRI (133 vs. 109 patients). Their corresponding AUC values were
0.655, 0.569, 0.616 and 0.690, respectively. Notably, SIRI
exhibited the highest AUC, demonstrating its strong predictive
efficacy (Table III).
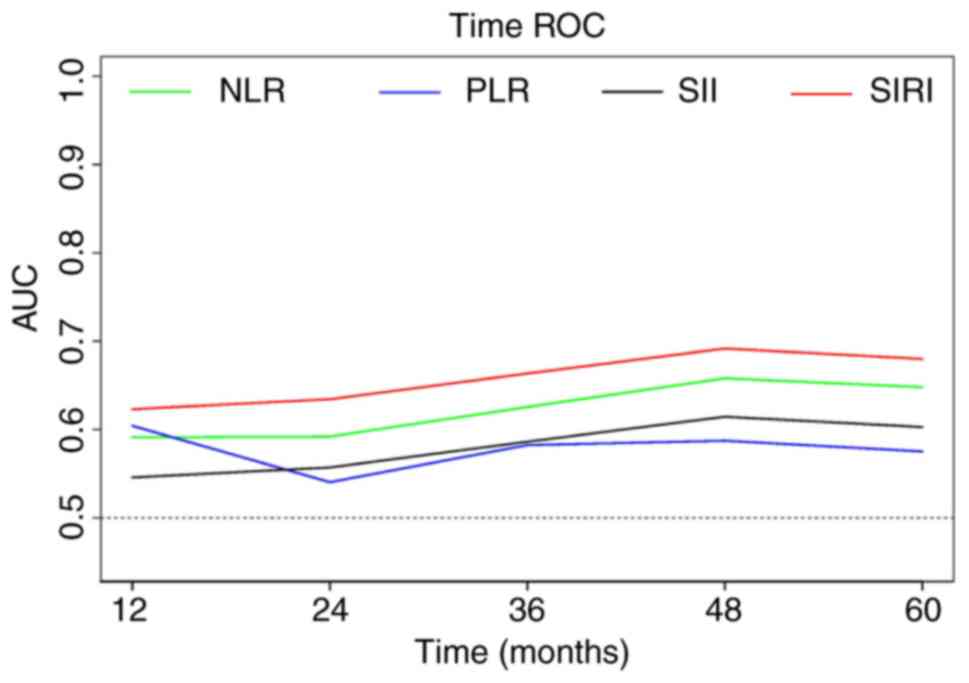
Furthermore, to further compare their predictive abilities,
time-dependent ROC curves were created for the inflammatory markers
(Fig. 2). The results revealed that
SIRI consistently exhibited the highest AUC at all time points,
providing additional confirmation of its excellent predictive
performance.
 | Table III.AUC and cutoff values of inflammatory
markers. |
Table III.
AUC and cutoff values of inflammatory
markers.
| Parameters | AUC | 95% CI | Youden index | Cut-off |
|---|
|
Neutrophil-to-lymphocyte ratio | 0.655 | 0.580–0.730 | 0.300 | 3.150 |
|
Platelet-to-lymphocyte ratio | 0.569 | 0.492–0.647 | 0.176 | 150.200 |
| Systemic
immune-inflammation index | 0.616 | 0.539–0.694 | 0.242 | 718.025 |
| Systemic
inflammation response index | 0.690 | 0.619–0.760 | 0.349 | 1.215 |
Survival analysis of inflammatory
markers
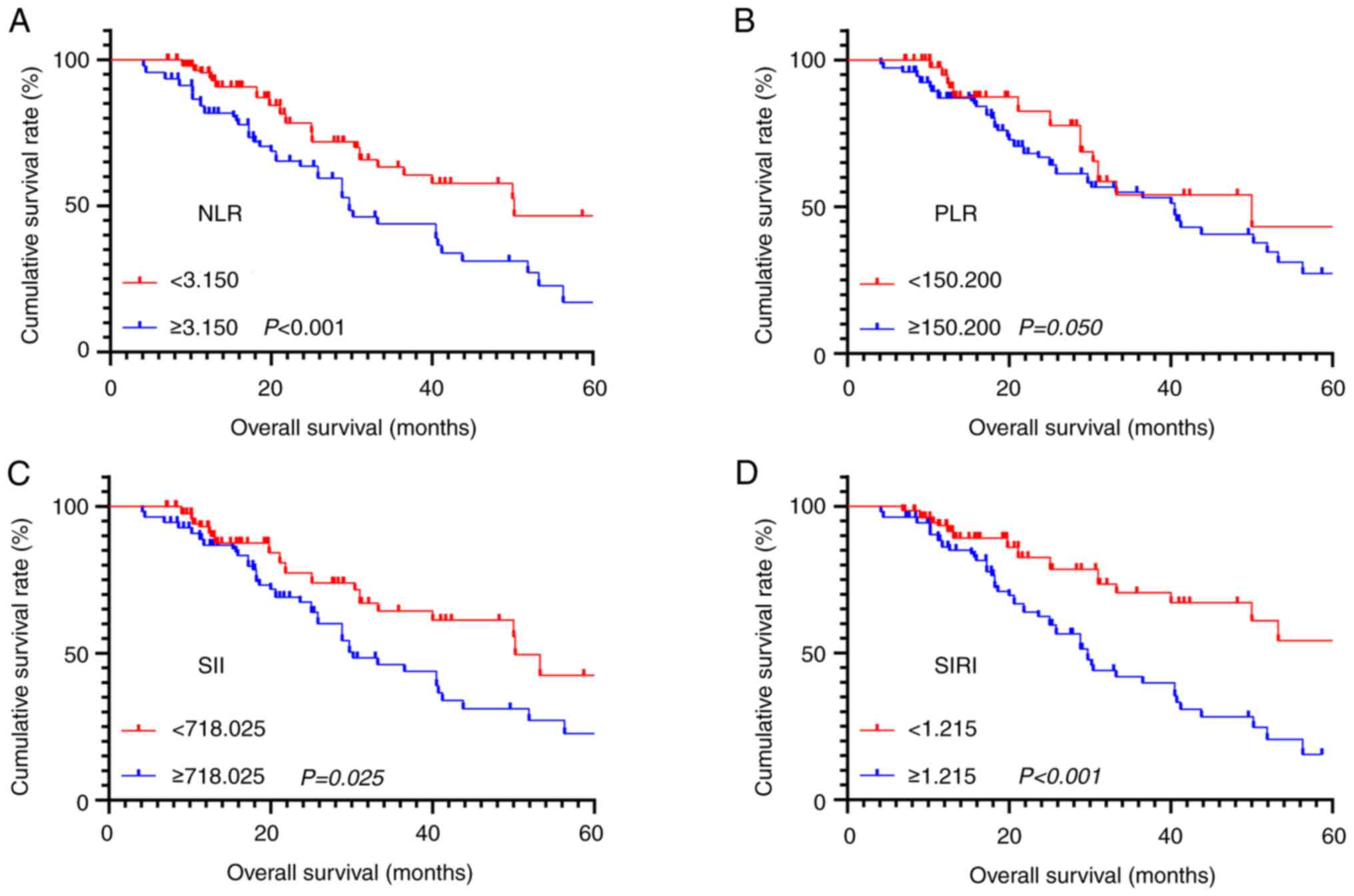
Survival analysis was performed on all inflammatory
markers and survival curves were plotted. The results indicated
that higher NLR (χ2=10.522, P<0.001), SII (χ2=6.733, P=0.025)
and SIRI (χ2=15.490, P<0.001) were all associated with shorter
OS, while there was no significant survival difference among
patients with different PLR (χ2=2.561, P=0.050) (Fig. 3A-D). In addition, to further explore
their prognostic value, univariate and multivariate survival
analyses were conducted (Table
IV). It was found that neutrophil (P=0.002), monocyte
(P=0.023), NLR (P=0.002), SII (P=0.011), SIRI (P<0.001),
positive peripheral lymph node (P=0.001) and TNM stage (P<0.001)
were associated with the OS of the patients. Given the high
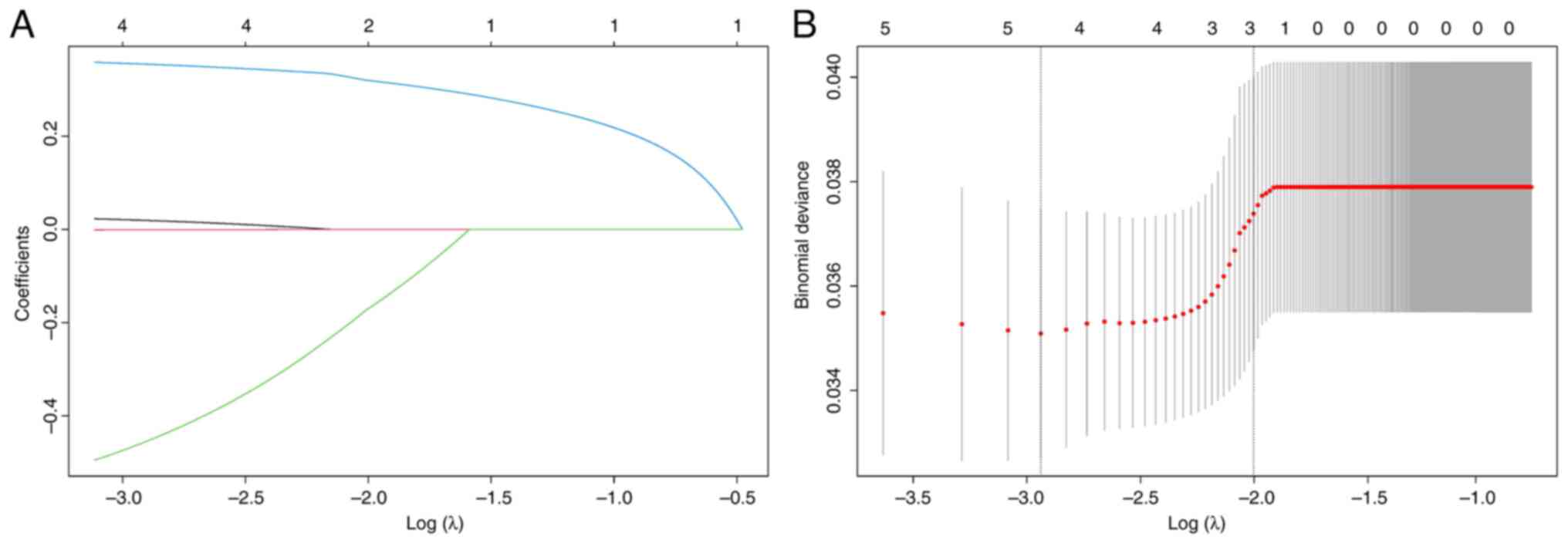
correlations among inflammation-related blood parameters and
inflammatory markers, Lasso regression analysis was performed on
these variables before conducting multivariate analysis to mitigate
multicollinearity. After 285 rounds of cross-validation, the
optimal λ value was identified as 0.009. Based on the optimal λ
value, monocyte and SII were found to be collinear and were
excluded from the multivariate analysis (Fig. 4A and B). Finally, SIRI (HR=1.851,
P=0.046) and TNM stage (HR=2.906, P=0.033) were identified as
independent prognostic factors in this study (Table IV).
 | Table IV.The univariate and multivariate
survival analysis. |
Table IV.
The univariate and multivariate
survival analysis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameters | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age, years | 1.022
(0.998–1.047) | 0.075 |
|
|
| BMI, kg/m2 | 0.964
(0.898–1.034) | 0.306 |
|
|
| Total protein,
g/l | 1.034
(0.999–1.069) | 0.057 |
|
|
| Albumin, g/l | 1.025
(0.969–1.085) | 0.383 |
|
|
| Globulin, g/l | 1.035
(0.991–1.082) | 0.123 |
|
|
| Prealbumin,
g/l | 0.997
(0.993–1.002) | 0.292 |
|
|
| Neutrophil,
g/l | 1.190
(1.064–1.331) | 0.002 | 1.068
(0.933–1.223) | 0.341 |
| Lymphocyte,
g/l | 0.793
(0.516–1.218) | 0.290 |
|
|
| Monocyte, g/l | 3.343
(1.184–9.441) | 0.023 |
|
|
| Platelet, g/l | 0.999
(0.993–1.001) | 0.325 |
|
|
|
Neutrophil-to-lymphocyte ratio (<3.150
vs. ≥3.150) | 2.080
(1.321–3.277) | 0.002 | 1.217
(0.667–2.219) | 0.523 |
|
Platelet-to-lymphocyte ratio (<150.200
vs. ≥150.200) | 1.502
(0.908–2.486) | 0.113 |
|
|
| Systemic
immune-inflammation index | 1.822
(1.148–2.891) | 0.011 |
|
|
| (<718.025 vs.
≥718.025) |
|
|
|
|
| Systemic
inflammation response index | 2.542
(1.568–4.122) | <0.001 | 1.851
(1.010–3.394) | 0.046 |
| (<1.215 vs.
≥1.215) |
|
|
|
|
| Sex (male vs.
female) | 0.597
(0.318–1.120) | 0.108 |
|
|
| Stomach ache (yes
vs. no) | 1.201
(0.763–1.889) | 0.429 |
|
|
| Abdominal
distention (yes vs. no) | 0.900
(0.569–1.422) | 0.651 |
|
|
| Weight loss (yes
vs. no) | 1.375
(0.869–2.176) | 0.174 |
|
|
| Fatigue (yes vs.
no) | 0.987
(0.613–1.588) | 0.957 |
|
|
| Acid reflux (yes
vs. no) | 1.406
(0.886–2.230) | 0.148 |
|
|
| Primary tumor site
(low 1/3 vs. others) | 1.204
(0.763–1.898) | 0.425 |
|
|
| Borrmann type (I+II
vs. III+IV). | 0.991
(0.646–1.520) | 0.967 |
|
|
| Peripheral lymph
node (positive vs. negative) | 3.867
(1.770–8.446) | 0.001 | 1.493
(0.475–4.696) | 0.493 |
| Tumor size (<50
mm vs. >50 mm) | 1.004
(0.641–1.573) | 0.985 |
|
|
|
Tumor-note-metastasis stage (II vs.
III) | 4.082
(2.098–7.941) | <0.001 | 2.906
(1.087–7.768) | 0.033 |
| Human epidermal
growth factor receptor 2 | 1.337
(0.851–2.099) | 0.208 |
|
|
Propensity score matching analysis for
SIRI
In the present study, SIRI not only exhibited the
highest AUC but also proved to be an independent prognostic
indicator. To minimize interference factors as much as possible,
PSM analysis was conducted on SIRI. Before PSM, there were 133
patients with low SIRI and 109 patients with high SIRI. The
chi-square test and Fisher's exact test showed that SIRI was
associated with ALB, sex, fatigue, primary tumor site and HER2
expression (all P<0.05). After incorporating all interference
factors and setting the matching tolerance to 0.02, a total of 174
patients were successfully matched. After PSM, there were 87
patients in both the low and high SIRI groups, and SIRI was not
associated with any clinical or pathological parameter (all
P>0.05) (Table V).
 | Table V.Patient characteristics related to
SIRI before and after PSM. |
Table V.
Patient characteristics related to
SIRI before and after PSM.
|
| Before PSM |
| After PSM |
|
|---|
|
|
|
|
|
|
|---|
| Parameters | Low SIRI (total
n=133) | High SIRI (total
n=109) | P-value | Low SIRI (total
n=87) | High SIRI (total
n=87) | P-value |
|---|
| Mean age, years
(SD) | 63.39 (10.30) | 62.93 (9.47) | 0.718 | 63.23 (9.81) | 62.45 (9.60) | 0.596 |
| Mean BMI, kg/m2
(SD) | 21.04 (2.95) | 20.59 (3.80) | 0.309 | 20.94 (2.93) | 20.26 (3.72) | 0.379 |
| Mean total protein,
g/l (SD) | 61.82 (7.87) | 60.11 (7.43) | 0.087 | 61.96 (7.90) | 61.79 (7.30) | 0.862 |
| Mean albumin, g/l
(SD) | 36.66 (4.10) | 34.42 (4.64) | <0.001 | 35.75 (4.23) | 35.46 (4.47) | 0.911 |
| Mean globulin, g/l
(SD) | 25.44 (4.78) | 26.73 (5.81) | 0.060 | 25.47 (4.96) | 26.55 (5.97) | 0.195 |
| Mean prealbumin,
g/l (SD) | 183.03 (51.39) | 170.96 (56.01) | 0.082 | 187.00 (52.96) | 175.38 (56.90) | 0.165 |
| Sex (%) |
|
| 0.009 |
|
| 0.839 |
|
Male | 98 (73.7) | 95 (87.2) |
| 72 (82.8) | 73 (83.9) |
|
|
Female | 35 (26.3) | 14 (12.8) |
| 15 (17.2) | 14 (16.1) |
|
| Stomach ache
(%) |
|
| 0.524 |
|
| 0.442 |
|
Yes | 58 (43.6) | 52 (47.7) |
| 34 (39.1) | 39 (44.8) |
|
| No | 75 (56.4) | 57 (52.3) |
| 53 (60.9) | 48 (55.2) |
|
| Abdominal
distention (%) |
|
| 0.866 |
|
| 0.649 |
|
Yes | 71 (53.4) | 57 (52.3) |
| 46 (52.9) | 43 (49.4) |
|
| No | 62 (46.6) | 52 (47.7) |
| 41 (47.1) | 44 (50.6) |
|
| Weight loss
(%) |
|
| 0.854 |
|
| 0.649 |
|
Yes | 57 (42.9) | 48 (44.0) |
| 43 (49.4) | 40 (46.0) |
|
| No | 76 (57.1) | 61 (56.0) |
| 44 (50.6) | 47 (54.0) |
|
| Fatigue (%) |
|
| 0.001 |
|
| 0.159 |
|
Yes | 22 (16.5) | 39 (35.8) |
| 16 (18.4) | 22 (25.3) |
|
| No | 111 (83.5) | 70 (64.2) |
| 71 (81.6) | 65 (74.7) |
|
| Acid reflux
(%) |
|
| 0.861 |
|
| 0.878 |
|
Yes | 51 (38.3) | 43 (39.4) |
| 37 (42.5) | 38 (43.7) |
|
| No | 82 (61.7) | 66 (60.6) |
| 50 (57.5) | 49 (56.3) |
|
| Primary tumor site
(%) |
|
| 0.009 |
|
| 0.065 |
| Upper
1/3 | 2 (1.5) | 6 (5.5) |
| 2 (2.3) | 6 (6.9) |
|
| Middle
1/3 | 20 (15.0) | 6 (5.5) |
| 13 (14.9) | 5 (5.7) |
|
| Low
1/3 | 111 (83.5) | 93 (85.3) |
| 72 (82.8) | 72 (82.8) |
|
|
Whole | 0 (0.0) | 4 (3.7) |
| 0 (0.0) | 4 (4.6) |
|
| Borrmann type
(%) |
|
| 0.913 |
|
| 0.447 |
| I | 22 (16.5) | 22 (20.2) |
| 14 (16.1) | 17 (19.5) |
|
| II | 95 (71.4) | 75 (68.8) |
| 66 (75.9) | 60 (69.0) |
|
|
III | 12 (9.0) | 10 (9.2) |
| 5 (5.7) | 9 (10.3) |
|
| IV | 4 (3.0) | 2 (1.8) |
| 2 (2.3) | 1 (1.1) |
|
| Peripheral lymph
node (%) |
|
| 0.637 |
|
| 0.999 |
|
Positive | 107 (80.5) | 85 (78.0) |
| 71 (81.6) | 71 (81.6) |
|
|
Negative | 26 (19.5) | 24 (22.0) |
| 16 (18.4) | 16 (18.4) |
|
| Tumor size (%) |
|
| 0.142 |
|
| 0.167 |
| <50
mm | 65 (48.9) | 43 (39.4) |
| 45 (51.7) | 33 (37.9) |
|
| ≥50
mm | 68 (51.1) | 66 (60.6) |
| 42 (48.3) | 54 (62.1) |
|
|
Tumor-node-metastasis stage (%) |
|
| 0.799 |
|
| 0.873 |
| II | 46 (34.6) | 36 (33.0) |
| 30 (34.5) | 29 (33.3) |
|
|
III | 87 (65.4) | 73 (67.0) |
| 57 (65.5) | 58 (66.7) |
|
| Human epidermal
growth factor receptor 2 (%) |
|
| 0.049 |
|
| 0.095 |
|
Positive | 69 (51.9) | 45 (41.3) |
| 47 (54.0) | 36 (41.4) |
|
|
Negative | 64 (48.1) | 64 (58.7) |
| 40 (46.0) | 51 (58.6) |
|
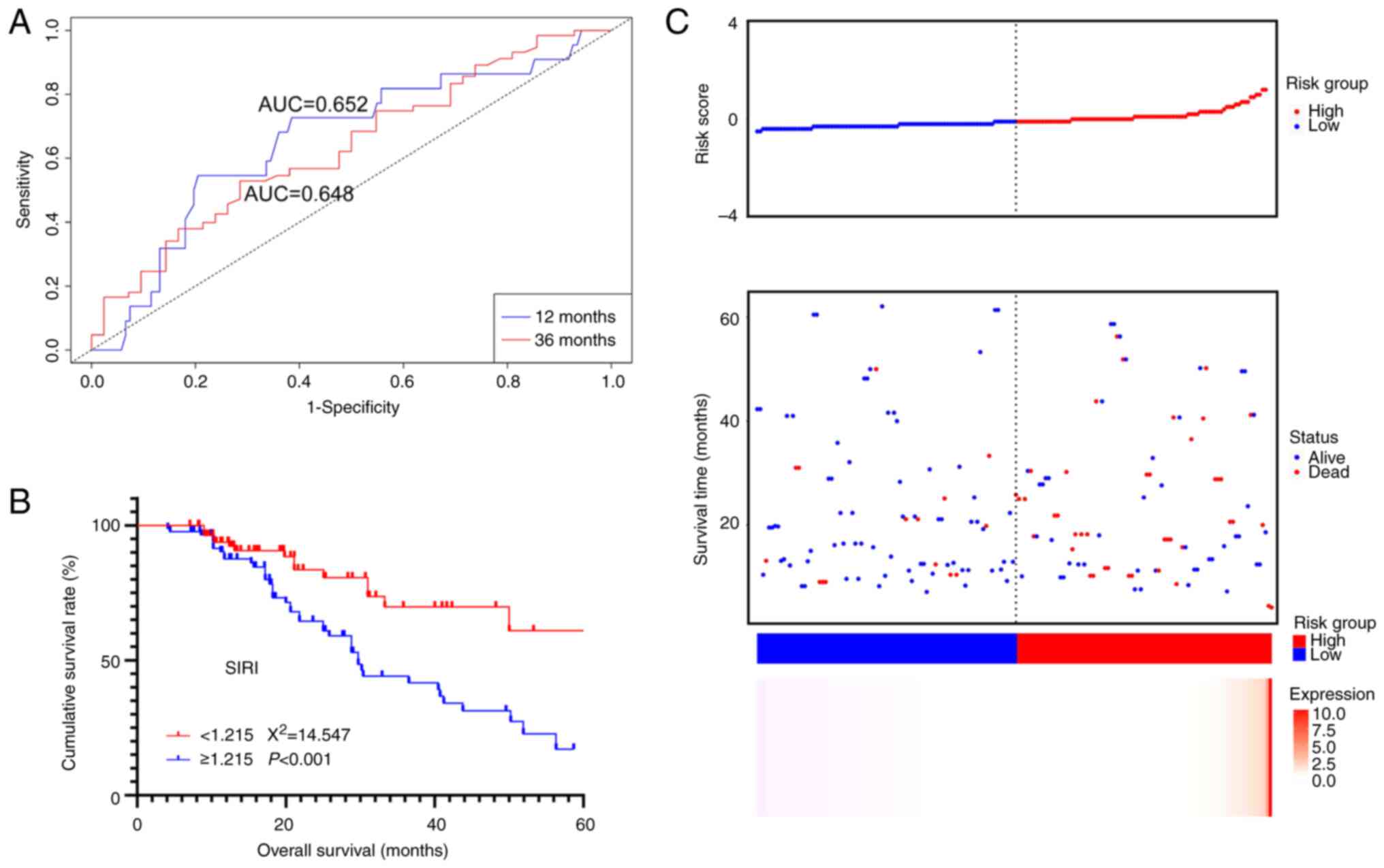
The ROC curve and survival curve for SIRI were
plotted based on the new dataset. The 1 and 3-year AUC for SIRI
were 0.648 and 0.652, respectively, which remained relatively high
(Fig. 5A). Additionally, survival
analysis indicated that SIRI was still significantly associated
with the clinical outcome of the patients, with higher SIRI values
associated with lower OS (χ2=14.547, P<0.001, Fig. 5B).
In addition, a prognostic risk model based on SIRI
using the β coefficient from the Cox analysis was established. The
risk score was calculated as SIRI value ×0.392. The risk factor
correlation plot demonstrated a significant association between
higher risk scores and lower survival rates (Fig. 5C). This further validated the
predictive capacity of SIRI for patient prognosis.
Nomogram for SIRI
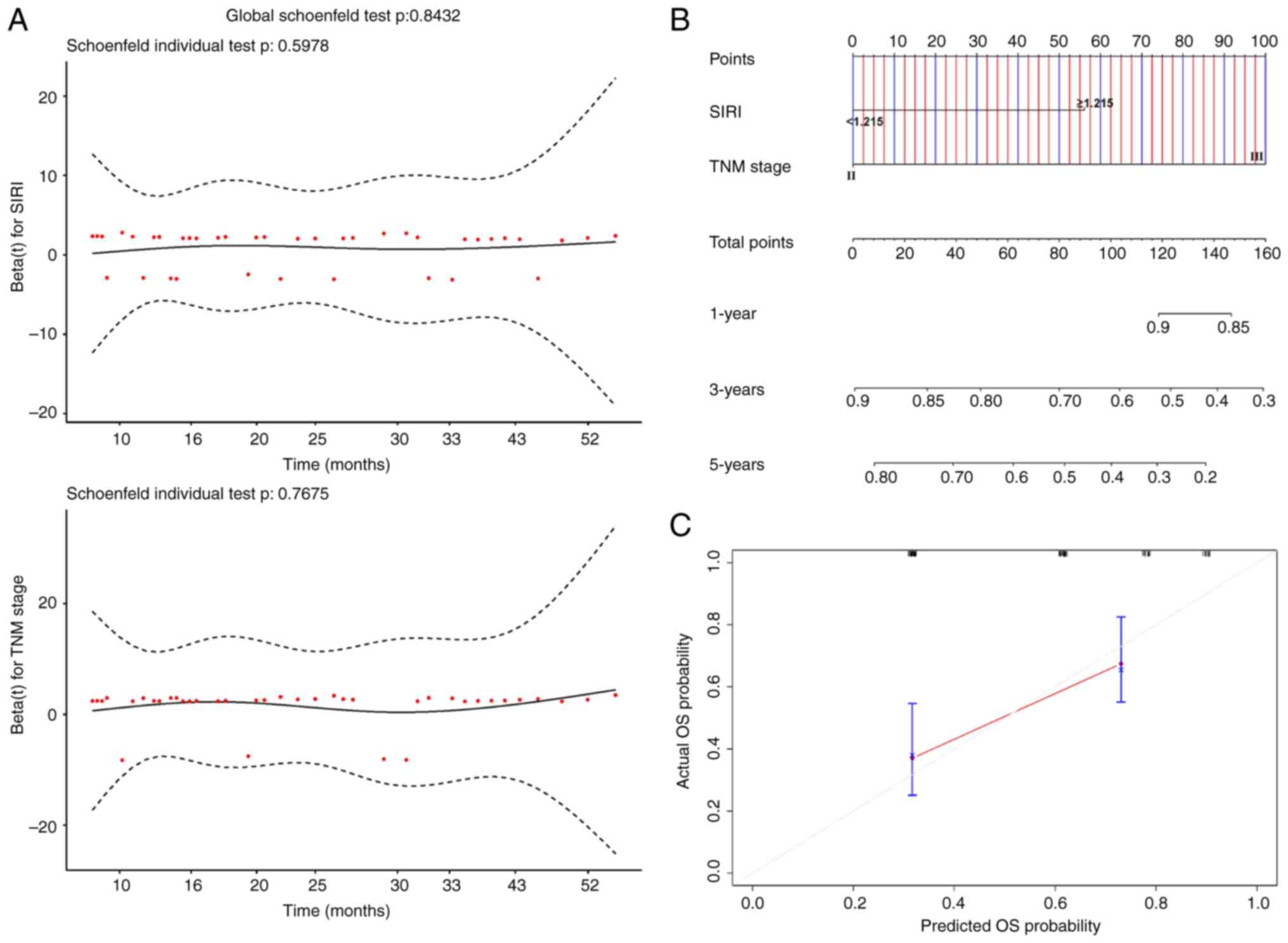
A nomogram was constructed to predict patient
survival probabilities based on TNM stage and SIRI. Schoenfeld
residual plots for TNM stage (P=0.5978) and SIRI (P=0.7675)
indicated that neither of them violated the proportional hazards
assumption (Fig. 6A). The C-index
of the nomogram was 0.671 (Fig.
6B). Furthermore, calibration curves based on bootstrapping
also demonstrated a high level of consistency between predicted
probabilities and actual probabilities (Fig. 6C). These findings collectively
highlighted the high accuracy of the SIRI nomogram.
Discussion
Nutritional status has been demonstrated to be
related to the prognosis of various cancers (20–23). A
study conducted by Sun et al (24) on the Prognostic Nutritional Index
(PNI) in gastric cancer confirmed the association between poor
nutritional status and poor survival outcomes. In 2022, they
collected data from 146 patients who received postoperative
immunotherapy or chemotherapy and found a correlation between low
PNI and poor OS. A meta-analysis conducted by Zhang et al
(25) in 2023 also reached the same
conclusion. Early pyloric stenosis often leads to a poor
nutritional status, which significantly impacts the prognosis of
patients with gastric cancer. Notably, two retrospective studies
conducted between 2021 and 2023 investigated the clinical
characteristics and survival outcomes of patients with early
pyloric stenosis. Jiao et al (26) and Li et al (27) collected data from 73 and 221
patients, respectively, and found that individuals with early
pyloric stenosis not only exhibited poorer nutritional status and
tumor burden but also experienced significantly worse survival
outcomes compared with patients without early pyloric stenosis. The
impact of systemic inflammation on gastric cancer had been a topic
of interest for numerous researchers. Hashimoto et al
(28) explored the impact of
hematologic inflammatory markers on the prognosis of soft tissue
sarcomas (STSs). The study included a total of 22 patients with STS
treated at their institution and analyzed the correlation between
pretreatment blood markers and tumor characteristics. The findings
suggested that C-reactive protein levels, white blood cell and
neutrophil counts, and NLR may be poor prognostic factors for
highly aggressive STSs. Zurlo et al (29) conducted a retrospective analysis of
the application of NLR, tumor infiltrating lymphocytes (CD4+/CD8+)
and programmed death-ligand 1 expression in patients with gastric
cancer who underwent neoadjuvant treatment. Through their analysis
of data collected from 65 patients in 2022, they found significant
associations between these factors and patient prognosis,
indicating that the pre-treatment systemic inflammatory and immune
status could influence clinical outcomes. In addition, Wu et
al (30) and Qiu et al
(31) conducted two meta-analyses
that confirmed the significant impact of SII on the prognosis of
patients with gastric cancer. These findings indirectly validated
the importance of exploring the inflammatory status in patients
with pyloric stenosis.
In the present study, the inflammatory status was
determined by calculating the NLR, PLR, SII and SIRI of the
patient. In the survival analysis of all patients, all inflammatory
markers except PLR were significantly associated with OS. In
addition, after excluding multicollinearity through Lasso
regression analysis, SIRI was also found to be an independent
prognostic factor. In addition, SIRI had the highest AUC,
indicating its high prognostic value. In further analysis of SIRI,
the SIRI after PSM still demonstrated a high AUC and was
significantly correlated with OS. The risk prognosis model and
nomogram established based on SIRI also revealed high accuracy.
This further confirms the significant correlation between
inflammatory status and the prognosis of patients with early
pyloric stenosis.
The inflammatory status of the patients was
reflected through various classic inflammatory indices, including
NLR, PLR, SII and SIRI. In the preliminary analysis, it was found
that NLR, SII and SIRI were all associated with patient prognosis.
Moreover, it was also discovered that SIRI had the highest
prognostic value among the inflammatory indices. Therefore, the
predictive ability of SIRI for prognosis in detail through PSM was
further analyzed and ultimately confirmed its strong predictive
power. The specific mechanisms through which inflammatory status
affects the clinical outcomes of patients with gastric cancer with
early pyloric stenosis remain unclear. Systemic inflammatory status
may lead to immune function suppression in patients with gastric
cancer (32). This not only weakens
the ability of the immune system to inhibit and destroy tumors but
also renders patients more susceptible to complications such as
infections, thereby accelerating disease progression and reducing
treatment effectiveness (33–35).
Additionally, some inflammatory mediators can stimulate tumor cell
proliferation and promote new blood vessel formation, thus directly
contributing to tumor progression (36,37).
SIRI consists of NEU, MON and LYM, all of which have been found to
be significantly associated with the prognosis of patients with
gastric cancer in previous studies (38–41).
NEU and MON were demonstrated to play pivotal roles in
immune-inflammatory responses, often correlating with the degree of
inflammation (42). In certain
situations, elevated counts of NEU and MON indicated an overactive
immune system or an inflammatory state, which could signal a high
tumor burden and potentially exacerbate tumor progression (43,44).
Conversely, LYM was revealed as a major component of the antitumor
immune response, playing a critical role in resistance against both
tumors and infections (45,46). Low counts of LYM indicated immune
suppression, which could have led to tumor evasion and
dissemination (47). Inflammation
status and pyloric stenosis exhibit a close interplay (48). On the one hand, pyloric stenosis
directly reduces the energy intake of the patients, leading to
malnutrition (49,50). On the other hand, certain
inflammatory mediators not only affect the energy intake and
absorption of the patients but also induce protein breakdown,
decrease muscle mass, result in weight loss, thereby further
exacerbating the malnutrition status of the patients (51,52).
These factors could all potentially explain why the inflammatory
status, particularly SIRI, was closely associated with the clinical
outcomes of the patients.
However, the present study was a retrospective
study, and despite efforts to minimize selection bias through PSM
analysis, there may have been unaccounted-for confounding factors
that could have impacted the research results. In addition, the
data of this study was only retrieved from one hospital, which
might have regional and population specificity, limiting the
applicability of the research results. Finally, the optimal cut-off
value for inflammatory indicators was calculated through the ROC
curve, and there was still no recognized standard. The conclusions
of this study require further validation in larger and broader
studies. Additionally, while past research on inflammatory indices
in other cancers has yielded numerous positive results, the
pathogenesis of different cancers varies, as do the factors
inducing malnutrition and their impact on tumors. Therefore, the
applicability of the findings of the present study to other cancers
still requires confirmation through further research involving
larger sample sizes and multiple types of cancer.
In conclusion, inflammatory status was significantly
associated with the prognosis of patients with gastric cancer with
early pyloric stenosis who underwent radical resection. The NLR,
SII and SIRI could all predict patient outcomes. Moreover, SIRI
exhibited the highest prognostic value among the inflammatory
indices and has been identified as an independent prognostic factor
in the present study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
The design and writing of the original manuscript
draft was conducted by LH. Data acquisition was performed by JL. XL
and XW conducted the analysis and interpretation of data. QY
contributed to the allocation of resources, conception, design and
project administration. All authors read and approved the final
version of the manuscript. LH and QY confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Southwest Medical
University (approval no. KY2023224; Luzhou, China). The Ethics
Committee of the Affiliated Hospital of Southwest Medical
University has waived the need for patient informed consent due to
the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
López MJ, Carbajal J, Alfaro AL, Saravia
LG, Zanabria D, Araujo JM, Quispe L, Zevallos A, Buleje JL, Cho CE,
et al: Characteristics of gastric cancer around the world. Crit Rev
Oncol Hematol. 181:1038412023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2021 (6th edition).
Gastric Cancer. 26:1–25. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guan WL, He Y and Xu RH: Gastric cancer
treatment: Recent progress and future perspectives. J Hematol
Oncol. 16:572023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Röcken C: Predictive biomarkers in gastric
cancer. J Cancer Res Clin Oncol. 149:467–481. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan Z: Recent advances in the surgical
treatment of advanced gastric cancer: A review. Med Sci Monit.
25:3537–3541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka Y, Fujii S, Kusaka T and Kokuryu H:
Gastric mucosal carcinoma with pyloric stenosis. Internal Med.
60:807–808. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwai N, Okuda T and Kagawa K:
Gastrointestinal: Natural progression of early gastric cancer
causing pyloric stenosis. J Gastroenterol Hepatol. 35:92020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimura T, Kataoka H, Sasaki M, Yamada T,
Hayashi K, Togawa S, Okumura F, Kubota E, Ohara H and Joh T:
Feasibility of self-expandable metallic stent plus chemotherapy for
metastatic gastric cancer with pyloric stenosis. J Gastroenterol
Hepatol. 24:1358–1364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abe S, Kondo H, Sumiyoshi T, Mizushima T,
Sugawara M, Shimizu Y and Okushiba S: Treatment strategy for early
gastric cancer with the risk of pyloric stenosis after endoscopic
resection. Endoscopy. 41:1101–1103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JU, Park MS, Yun SH, Yang MA, Han SH,
Lee YJ, Jung GM, Kim JW, Cho YK and Cho JW: Risk factors and
management for pyloric stenosis occurred after endoscopic
submucosal dissection adjacent to pylorus. Medicine (Baltimore).
95:e56332016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Acker SN, Kulungowski AM, Hodges M,
Crombleholme TM, Somme S and Partrick DA: Pyloric
stenosis-postoperative care on a nonsurgical ward. J Surg Res.
199:149–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trindade AJ, Sejpal DV and Benias PC:
Palliation of malignant pyloric stenosis using a lumen-apposing
metal stent. Clin Gastroenterol Hepatol. 17:A182019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saeed SM, Bilal S, Siddique MZ, Saqib M,
Shahid S, Ghumman AN and Yusuf MA: Pyloric stent insertion in
malignant gastric outlet obstruction: Moving beyond palliation.
Ther Adv Gastrointest Endosc. 14:263177452110470122021.PubMed/NCBI
|
|
15
|
Jomrich G, Paireder M, Kristo I, Baierl A,
Ilhan-Mutlu A, Preusser M, Asari R and Schoppmann SF: High systemic
immune-inflammation index is an adverse prognostic factor for
patients with gastroesophageal adenocarcinoma. Ann Surg.
273:532–541. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi H, Wang H, Pan J, Liu Z and Li Z:
Comparing prognostic value of preoperative platelet indexes in
patients with resectable gastric cancer. Sci Rep. 12:64802022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu YY, Ruan GT, Ge YZ, Li QQ, Zhang Q,
Zhang X, Tang M, Song MM, Zhang XW, Li XR, et al: Systemic
inflammation with sarcopenia predicts survival in patients with
gastric cancer. J Cancer Res Clin. 149:1249–1259. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He K, Si L, Pan X, Sun L, Wang Y, Lu J and
Wang X: Preoperative systemic immune-inflammation index (SII) as a
superior predictor of long-term survival outcome in patients with
stage I–II gastric cancer after radical surgery. Front Oncol.
12:8296892022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding P, Guo H, Sun C, Yang P, Kim NH, Tian
Y, Liu Y, Liu P, Li Y and Zhao Q: Combined systemic
immune-inflammatory index (SII) and prognostic nutritional index
(PNI) predicts chemotherapy response and prognosis in locally
advanced gastric cancer patients receiving neoadjuvant chemotherapy
with PD-1 antibody sintilimab and XELOX: A prospective study. BMC
Gastroenterol. 22:1212022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li P, Huang CM, Zheng CH, Russo A,
Kasbekar P, Brennan MF, Coit DG and Strong VE: Comparison of
gastric cancer survival after R0 resection in the US and China. J
Surg Oncol. 118:975–982. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng Q, Tan S, Jiang Y, Han J, Xi Q,
Zhuang Q and Wu G: Post-discharge oral nutritional supplements with
dietary advice in patients at nutritional risk after surgery for
gastric cancer: A randomized clinical trial. Clin Nutr. 40:40–46.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miao X, Ding L, Hu J, Zhu H, Zhao K, Lu J,
Jiang X, Xu Q and Zhu S: A web-based calculator combining geriatric
nutritional risk index (GNRI) and tilburg frailty indicator (TFI)
predicts postoperative complications among young elderly patients
with gastric cancer. Geriatr Gerontol Int. 23:205–212. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruan GT, Zhang Q, Zhang X, Tang M, Song
MM, Zhang XW, Li XR, Zhang KP, Ge YZ, Yang M, et al: Geriatric
nutrition risk index: Prognostic factor related to inflammation in
elderly patients with cancer cachexia. J Cachexia Sarcopenia
Muscle. 12:1969–1982. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun H, Chen L, Huang R, Pan H, Zuo Y, Zhao
R, Xue Y and Song H: Prognostic nutritional index for predicting
the clinical outcomes of patients with gastric cancer who received
immune checkpoint inhibitors. Front Nutr. 9:10381182022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Ma W, Qiu Z, Kuang T, Wang K, Hu
B and Wang W: Prognostic nutritional index as a prognostic
biomarker for gastrointestinal cancer patients treated with immune
checkpoint inhibitors. Front Immunol. 14:12199292023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiao X, Wang Y, Qu X, Qu J and Wang X:
Effects of preoperative pyloric stenosis on outcomes and
nutritional status in 73 patients following curative gastrectomy
for gastric cancer: A retrospective study from a single center. Med
Sci Monit. 27:e9309742021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li G, He L and Sun H: Nutritional risk
index predicts the prognosis of gastric cancer patients with
pyloric stenosis who received preoperative parenteral nutrition.
Oncol Lett. 26:4012023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hashimoto K, Nishimura S, Shinyashiki Y,
Ito T and Akagi M: Characterizing inflammatory markers in highly
aggressive soft tissue sarcomas. Medicine (Baltimore).
101:e306882022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zurlo IV, Schino M, Strippoli A, Calegari
MA, Cocomazzi A, Cassano A, Pozzo C, Di Salvatore M, Ricci R,
Barone C, et al: Predictive value of NLR, TILs
(CD4+/CD8+) and PD-L1 expression for
prognosis and response to preoperative chemotherapy in gastric
cancer. Cancer Immunol Immunother. 71:45–55. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu J, Wu XD and Gao Y and Gao Y:
Correlation between preoperative systemic immune-inflammatory
indexes and the prognosis of gastric cancer patients. Eur Rev Med
Pharmacol Sci. 27:5706–5720. 2023.PubMed/NCBI
|
|
31
|
Qiu Y, Zhang Z and Chen Y: Prognostic
value of pretreatment systemic immune-inflammation index in gastric
cancer: A meta-analysis. Front Oncol. 11:5371402021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Venet F and Monneret G: Advances in the
understanding and treatment of sepsis-induced immunosuppression.
Nat Rev Nephrol. 14:121–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N,
Yi P, Tang L, Pan Q, Rao S, et al: The cancer metabolic
reprogramming and immune response. Mol Cancer. 20:282021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen DS and Mellman I: Elements of cancer
immunity and the cancer-immune set point. Nature. 541:321–330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Weverwijk A and de Visser KE:
Mechanisms driving the immunoregulatory function of cancer cells.
Nat Rev Cancer. 23:193–215. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Andrejeva G and Rathmell JC: Similarities
and distinctions of cancer and immune metabolism in inflammation
and tumors. Cell Metab. 26:49–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vinay DS, Ryan EP, Pawelec G, Talib WH,
Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, et
al: Immune evasion in cancer: Mechanistic basis and therapeutic
strategies. Semin Cancer Biol. 35 (Suppl 35):S185–S198. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fang T, Wang Y, Yin X, Zhai Z, Zhang Y,
Yang Y, You Q, Li Z, Ma Y, Li C, et al: Diagnostic sensitivity of
NLR and PLR in early diagnosis of gastric cancer. J Immunol Res.
2020:91460422020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cupp MA, Cariolou M, Tzoulaki I, Aune D,
Evangelou E and Berlanga-Taylor AJ: Neutrophil to lymphocyte ratio
and cancer prognosis: An umbrella review of systematic reviews and
meta-analyses of observational studies. BMC Med. 18:3602020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hirahara T, Arigami T, Yanagita S,
Matsushita D, Uchikado Y, Kita Y, Mori S, Sasaki K, Omoto I,
Kurahara H, et al: Combined neutrophil-lymphocyte ratio and
platelet-lymphocyte ratio predicts chemotherapy response and
prognosis in patients with advanced gastric cancer. BMC Cancer.
19:6722019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ye Z, Yu P, Cao Y, Chai T, Huang S, Cheng
X and Du Y: Prediction of Peritoneal cancer index and prognosis in
peritoneal metastasis of gastric cancer using NLR-PLR-DDI score: A
retrospective study. Cancer Manag Res. 14:177–187. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ikegame A, Kondo A, Kitaguchi K, Sasa K
and Miyoshi M: Presepsin production in monocyte/macrophage-mediated
phagocytosis of neutrophil extracellular traps. Sci Rep.
12:59782022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fendl B, Berghoff AS, Preusser M and Maier
B: Macrophage and monocyte subsets as new therapeutic targets in
cancer immunotherapy. ESMO Open. 8:1007762023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Que H, Fu Q, Lan T, Tian X and Wei X:
Tumor-associated neutrophils and neutrophil-targeted cancer
therapies. Biochim Biophys Acta Rev Cancer. 1877:1887622022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
MacPherson S, Kilgour M and Lum JJ:
Understanding lymphocyte metabolism for use in cancer
immunotherapy. FEBS J. 285:2567–2578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pan JH, Zhou H, Cooper L, Huang JL, Zhu
SB, Zhao XX, Ding H, Pan YL and Rong L: LAYN is a prognostic
biomarker and correlated with immune infiltrates in gastric and
colon cancers. Front Immunol. 10:62019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Q, Li S, Qiao S, Zheng Z, Duan X and
Zhu X: Changes in T lymphocyte subsets in different tumors before
and after radiotherapy: A meta-analysis. Front Immunol.
12:6486522021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Soldati L, Di Renzo L, Jirillo E, Ascierto
PA, Marincola FM and De Lorenzo A: The influence of diet on
anti-cancer immune responsiveness. J Transl Med. 16:752018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Matsunaga T, Miyata H, Sugimura K, Motoori
M, Asukai K, Yanagimoto Y, Takahashi Y, Tomokuni A, Yamamoto K,
Akita H, et al: Prognostic significance of sarcopenia and systemic
inflammatory response in patients with esophageal cancer.
Anticancer Res. 39:449–458. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu Z, Jin K, Guo M, Long J, Liu L, Liu C,
Xu J, Ni Q, Luo G and Yu X: Prognostic value of the CRP/Alb ratio,
a novel inflammation-based score in pancreatic cancer. Ann Surg
Oncol. 24:561–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu JX, Li A, Zhou LY, Liu XF, Wei ZH,
Wang XZ and Ying HQ: Significance of combined preoperative serum
Alb and dNLR for diagnosis of pancreatic cancer. Future Oncol.
14:229–239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stumpf F, Keller B, Gressies C and Schuetz
P: Inflammation and nutrition: Friend or foe? Nutrients.
15:11592023. View Article : Google Scholar : PubMed/NCBI
|