Introduction
As colorectal cancer (CRC) is one of the most
commonly diagnosed malignancies and the leading cause of
cancer-related mortality worldwide with >1.85 million cases and
850 000 deaths annually, it has become a major public health
concern. In recent years, the 5-year survival rate of patients with
CRC has markedly improved with the development of treatment
strategies, such as surgery, radiotherapy, chemotherapy and
immunotherapy (1,2); however, the effects of the current
treatment methods are limited for patients with advanced-stage or
metastatic CRC (3). Hence, the
identification of novel targets and the further elucidation of the
mechanisms underlying the progression of CRC is of utmost
urgency.
Tensin 4 (TNS4), also known as COOH-terminal
tensin-like, belongs to the tensin focal adhesion family (4). A number of studies have reported that
TNS4 is overexpressed in several types of cancer, including breast
cancer, pancreatic cancer, lung cancer and CRC (5–8).
Furthermore, TNS4 has been reported to be associated with several
biological processes, such as epithelial-mesenchymal transition
(EMT) (9–11), cell motility (6,12),
cell migration (13), metastasis
(8) and drug resistance (14,15).
For example, TNS4 overexpression promotes EMT, cell motility and
the colony formation of CRC cells through Src signaling (9). Liao et al (16) noted that TNS4 interacted with
β-catenin in colon cancer cells, which enhanced the colony
formation, anchorage-independent proliferation and invasiveness of
colon cancer cells.
Aerobic glycolysis, also known as the Warburg
effect, is a distinctive hallmark of cancer, which confers a
proliferation advantage on cancer cells by providing them with
energy and biosynthesis building blocks (17). Accumulating evidence has indicated
that targeting or modulating aerobic glycolysis may serve as an
antitumor therapeutic strategy (18). For example, a previous study
reported that the long non-coding (lnc)RNA AGPG promoted tumor cell
glycolysis and the proliferation of esophageal squamous cell
carcinoma cells by stabilizing PFKFB3 (19). Furthermore, Yu et al
(20) reported that OTU
deubiquitinase, ubiquitin aldehyde binding 2 suppressed CRC cell
proliferation and migration and promoted apoptosis and sensitivity
to chemotherapeutic drugs by regulating pyruvate kinase M (PKM)2
ubiquitination and glycolysis. However, the role of TNS4 in the
glycolysis and progression of CRC remains unclear.
The present study aimed to explore the roles of TNS4
in regulating aerobic glycolysis, migration and invasion of CRC
cells and investigate the underlying molecular mechanisms.
Materials and methods
Analysis of TNS4 expression in
patients with CRC
The expression of TNS4 in patients with colon
adenocarcinoma or rectum adenocarcinoma was obtained from the
University of Alabama at Birmingham Cancer data analysis Portal
(UALCAN; http://ualcan.path.uab.edu/) and Gene
Expression Profiling Interactive Analysis (GEPIA; http://gepia.cancer-pku.cn/) databases with data from
The Cancer Genome Atlas (TCGA).
Immunohistochemistry
A total of 92 pairs of paraffin blocks of CRC
tissues and corresponding normal adjacent tissues were obtained
from the First Affiliated Hospital of Soochow University (Suzhou,
China). The detailed clinicopathological information of the
patients is provided in Table SI.
Ethics approval was obtained from the Institutional Review Board of
the First Affiliated Hospital of Soochow University and written
informed consent was obtained from the patients prior to sample
collection. Immunohistochemistry was performed as previously
described (21). Briefly, tissues
were fixed with 4% paraformaldehyde (Beyotime Institute of
Biotechnology) at 25°C for 24 h. The specimens were embedded in
paraffin. 5-µm-thick sections from paraffin-embedded blocks were
deparaffinized and rehydrated. After antigen retrieval with 10 mM
sodium citrate buffer (pH 6.0, Beyotime Institute of
Biotechnology), the sections were incubated with 3% hydrogen
peroxide at room temperature for 10 min to block endogenous
peroxidase activity and non-specific protein interactions. The
sections were subsequently incubated with rabbit anti-human TNS4
antibodies (1:200; cat. no. 11580-1-AP; Proteintech Group, Inc.)
overnight at 4°C and then with biotinylated goat anti-rabbit
secondary antibody working solution (1:500; cat. no. SA1020; Boster
Biological Technology Co. Ltd.) at 37°C for 30 min. Subsequently,
the immunodetection was performed using the Dako EnVision detection
system (Agilent Technologies, Inc.). These slides were photographed
under a fluorescence microscope (Leica, Buffalo Grove, USA). The
semi-quantitative immunoreactive score system was adopted to obtain
the score of TNS4 immunostaining, as previously described (22).
Cells and cell culture
In total, two CRC cell lines (HCT116 and RKO) were
purchased from the American Type Culture Collection. Both the
HCT116 and RKO cells were cultured in DMEM (Biological Industries;
Sartorius AG) containing 10% fetal bovine serum (FBS, Biological
Industries; Sartorius AG), 100 U/ml penicillin and 100 mg/ml
streptomycin at 37°C in a humidified atmosphere of 5% CO2. The CRC
cells were treated with glycolysis activator DASA-58 (5 µM,
Selleck) and β-catenin activator SKL2001 (10 µM, Selleck) at 37°C
for 48 h.
Cell transfection and infection
In total, three commercial TNS4 small interfering
(si)RNAs (TNS4 siRNA-1, TNS4 siRNA-2 and TNS4 siRNA-3) were
purchased from Guangzhou RiboBio Co., Ltd. The three pairs of
synthesized siRNA sequences were as follows: siRNA-1 forward,
5′-CAAUCAUAGAAGAAGACCATT-3′ and reverse,
5′-UGGUCUUCUUCUAUGAUUGTT-3′; siRNA-2 forward,
5′-GGGCCAUCUCUCUGUGAUUTT-3′ and reverse,
5′-AAUCACAGAGAGAUGGCCCTT-3′; siRNA-3 forward,
5′-GCAAUGACCUCAUCCGACATT-3′ and reverse,
5′-UGUCGGAUGAGGUCAUUGCTT-3′; and siRNA negative control forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′. For cell transfection, the HCT116 or
RKO cells were transfected using Lipofectamine 2000®
(Invitrogen™; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions with TNS4 siRNA-1, TNS4 siRNA-2 or TNS4
siRNA-3 (100 pmol) at 37°C for 48 h. Scrambled siRNA negative
control (100 pmol) was used. After 48 h post-transfection, the
cells were harvested for RNA extraction and reverse transcription
(RT)-quantitative (q)PCR and Western blot. Besides, after 6 h of
transfection, the cells were used for Transwell analysis, glucose
consumption and lactate production assay.
TNS4 siRNA-3 had the highest inhibition efficiency
of TNS4 in HCT116 and RKO cells. Lentivirus pGLVU6/Puro vectors
carrying TNS4 shRNA containing the sequence of TNS4 siRNA-3 or
siRNA negative control sequence were manufactured using the 2nd
generation system by GenePharma Co., Ltd. The lentiviral TNS4 shRNA
plasmid (8 µg) was co-transfected with psPAX2 (2 µg, cat. no.
12260; Addgene, Inc.) and pMD2.G (6 µg, cat. no. 12259; Addgene,
Inc.) in HEK293T cells (American Type Culture Collection) in 10
cm-culture dish at 37° for 10 h. After replacing the transfection
medium using the culture medium, lentiviral particles in the
culture medium was collected every day for 3 days. For cell
transduction, the HCT116 and RKO cells in the logarithmic growth
period were infected with lentiviral particles (multiplicity of
infection=20) at 37°C for 12 h. The transfection efficiency was
observed under a fluorescence microscope at 24 h after transfection
After 72 h infection, puromycin (1 µg/ml, Beyotime Institute of
Biotechnology) was added to screen for stable TNS4 knockdown and
negative control cell lines. The screening period is about 8 to 12
days. The stable cells were cultured in complete medium with 0.5
µg/ml puromycin at 37°C.
RNA extraction and reverse
transcription (RT)-quantitative (q)PCR
Total RNA extraction from HCT116 and RKO cells was
performed using the Cell Total RNA Kit (cat. no. ES-RN001, Shanghai
Yishan Biotechnology Co., Ltd.). Subsequently, 1.0 µg total RNA was
used for cDNA synthesis using the RTIII AII-in-One Mix (cat. no.
MR05101, Monad Biotech Co., Ltd.) with the following conditions:
37°C for 2 min, 55°C for 15 min, and 85°C for 5 min. RT-qPCR was
performed using the CFX96 Touch Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc.) using the ChemoHS qPCR Mix (cat. no.
MQ00401, Monad Biotech Co., Ltd.) with SYBR Green. The cycling
conditions were as follows: one cycle at 95°C for 5 min, 40 cycles
of amplification at 95°C for 10 sec, and 60°C for 30 sec. RT-qPCR
was performed three times for each sample. β-actin was used for the
normalization of gene expression. All primers used for RT-qPCR are
listed in Table SII.
Western blot analysis
The HCT116 and RKO cells were lysed using RIPA
buffer containing protease inhibitors and phosphatase inhibitors
(cat. no. P0013D; Beyotime Institute of Biotechnology). The
Enhanced BCA Protein Assay kit (cat. no. P0010; Beyotime Institute
of Biotechnology) was used for protein determination. Subsequently,
10% SDS-PAGE; (cat. no. P0012AC; Beyotime Institute of
Biotechnology) was used to separate the total protein (30 µg),
which then was transferred onto PVDF membranes (Cytiva). After
blocking with 5% BSA (cat. no. FMS-WB021; Nanjing Fcmacs
Biotechnology Co., Ltd.) for 1.5 h at room temperature, the
membranes were incubated with rabbit anti-human TNS4 (1:1,000; cat.
no. 11580-1-AP; Proteintech Group, Inc.) or mouse anti-human/mouse
GAPDH (1:1,000; cat. no. 60004-1-Ig; Proteintech Group, Inc.)
antibodies at 4°C overnight. The membranes were then incubated with
the corresponding HRP-conjugated goat anti-rabbit (1:1,000; cat.
no. A0208; Beyotime Institute of Biotechnology) or anti-mouse
secondary antibodies (1:1,000; cat. no. A0216; Beyotime Institute
of Biotechnology) for 1 h at room temperature. Finally, the protein
bands were visualized using an ECL reagent (cat. no. 10100; NCM
Biotech) in a ChemiDoc™ MP Imaging System (Bio-Rad Laboratories,
Inc.). Additionally, ImageJ 2.0 software (National Institutes of
Health) was used to analyze the density of the protein bands.
Cell migration and invasion assay
To assess CRC cell migration and invasion, a
Transwell chamber was purchased from BD Biosciences (cat. no.
353097) and Matrigel from Corning, Inc. (cat. no. 356234). For the
cell migration assay, 3×104 HCT116 or RKO cells in serum-free
medium (400 µl) were seeded into the upper chamber (8-µm pore
size). Complete medium containing with 10% FBS was added to the
lower chamber. For the invasion assay, Matrigel (200 µg/ml) was
used to coat the upper chamber at room temperature for 2 h. For
migration or invasion, after cultured in the upper chamber for 24 h
or 48 h, the cells on the lower surface of the upper chamber were
fixed with 4% paraformaldehyde (Beyotime Institute of
Biotechnology) at room temperature for 30 min and stained with
crystal violet (Beyotime Institute of Biotechnology) at room
temperature for 15 min. Images were captured using a Nikon
Eclipse/NI-U fluorescence microscope and the number of
migrated/invaded cells was counted.
Glucose consumption and lactate
production assay
A Glucose Assay kit (cat. no. 361510; Shanghai Robio
Biotechnology Co., Ltd.) and a Lactate Assay kit (cat. no.
A019-2-1; Nanjing Jiancheng Taihao Biotechnology Co., Ltd.) were
used for the detection of glucose consumption and lactate
production according to the manufacturer's protocols,
respectively.
Statistical analysis
Data are presented as the mean ± standard deviation.
GraphPad Prism 6.0 software (Dotmatics) was used for statistical
analyses. The paired or unpaired Student's t-test, or one-way ANOVA
and Tukey's test's, were used to analyze the data. P<0.05 was
considered to indicate a statistically significant difference.
Results
TNS4 is highly expressed in clinical
CRC tissue and is associated with the TNM stage of patients with
CRC
To assess the role of TNS4 in CRC, its expression
was evaluated in the tissues of patients with CRC. The mRNA levels
of TNS4 were significantly upregulated in both colon and rectal
cancer tissues compared with normal tissues, according to data from
the UALCAN and GEPIA databases (Fig. 1A
and B). Furthermore, a CRC tumor cohort was used to assess the
protein expression of TNS4. Compared with the normal control, the
tumor tissues exhibited a significantly higher protein expression
of TNS4 (Fig. 1C). Moreover, TNS4
expression in patients with late-stage (III–IV) disease was
significantly higher than that in patients with early-stage (I–II)
disease (Fig. 1D). These data
suggest that TNS4 may function as an oncogene in CRC.
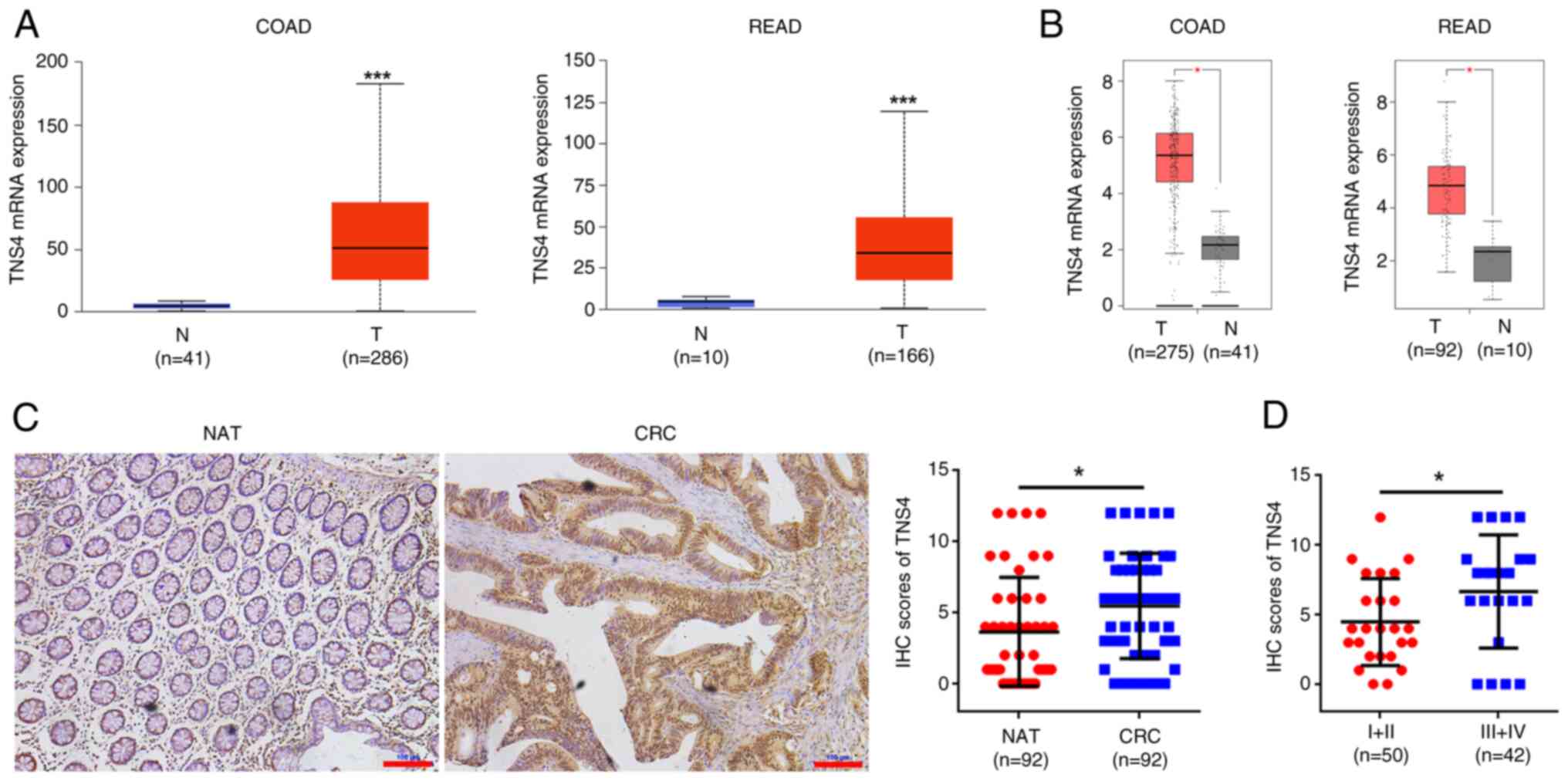 | Figure 1.TNS4 is overexpressed in CRC tissue
specimens and is associated with tumor-node-metastasis stage.
Relative TNS4 mRNA expression in COAD and READ tissue samples from
TCGA data based in the (A) University of Alabama at Birmingham
Cancer data analysis Portal and (B) Gene Expression Profiling
Interactive Analysis databases. T, tumor; N, normal. P<1×10-12;
P=1.11×10-16. (C) Representative images of IHC of TNS4 in CRC and
matched normal tissues from 92 patients with CRC. NAT, nonmalignant
adjacent tissues. Scale bar, 100 µm. (D) TNS4 protein expression
based on the staining index of CRC specimens at different clinical
stages. *P<0.05; ***P<0.001. TNS4, Tensin 4; CRC, colorectal
cancer; COAD, colon adenocarcinoma; READ, rectum adenocarcinoma;
TCGA, The Cancer Genome Atlas; IHC, immunohistochemistry. |
TNS4 is positively correlated with
glycolysis-related genes in patients with CRC
The GEPIA database was used to further evaluate the
association between TNS4 and glycolysis in CRC. It was demonstrated
that there were significant positive correlations between TNS4
expression and glycolysis-related genes, such as hexokinase 2
(HK2), lactate dehydrogenase (LDH)B, LDHM, pyruvate kinase M1/2
(PKM) and solute carrier (SLC)2A1, in the cancer tissues of
patients with CRC (Fig. 2). These
data indicate that TNS4 may function as a key regulator of
glycolysis in CRC.
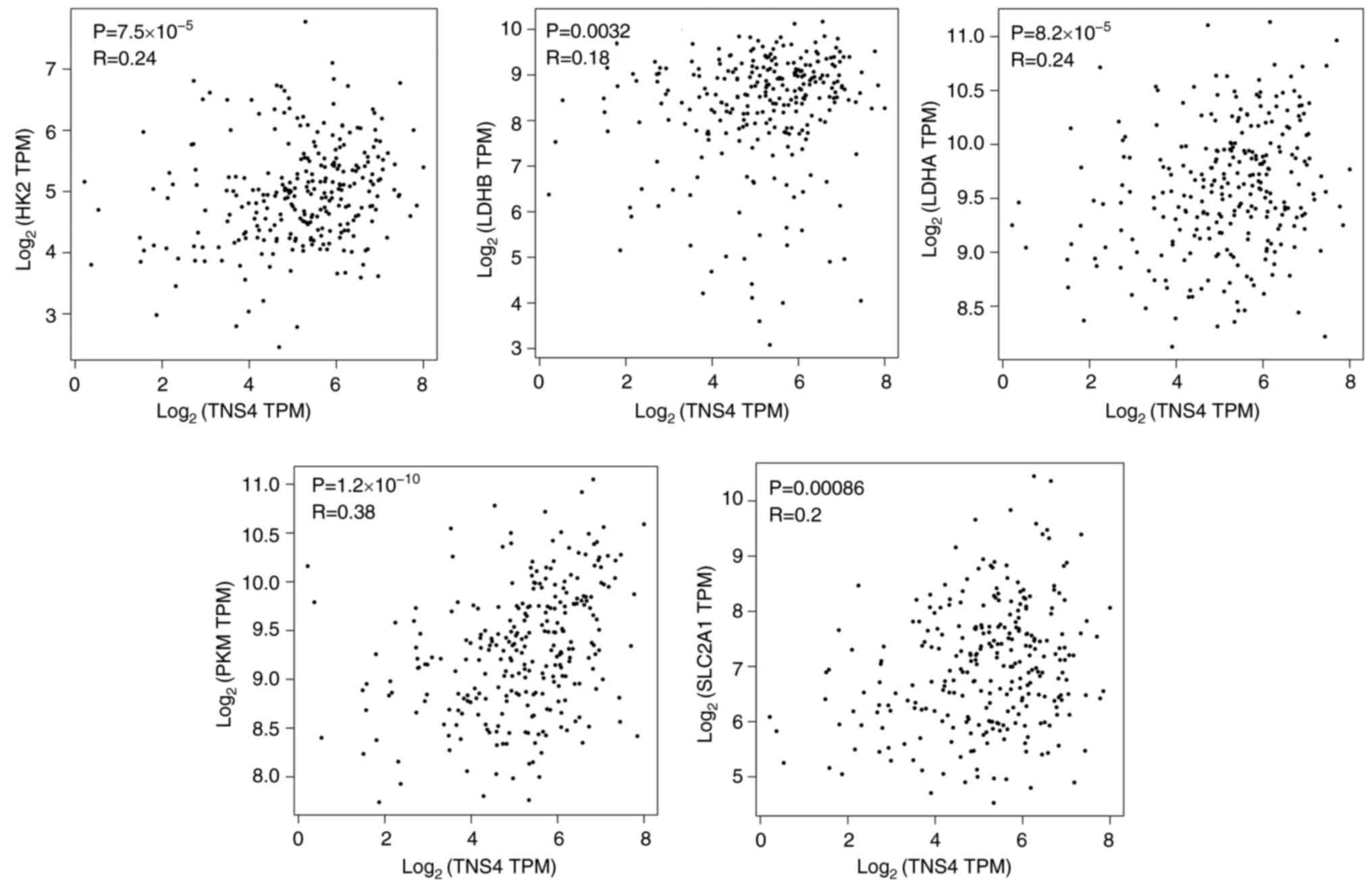 | Figure 2.TNS4 is positively correlated with
glycolysis-related genes in patients with CRC. The relationship
between TNS4 and HK2, LDHB, LDHM, PKM or SLC2A1 in CRC tissue
samples from The Cancer Genome Atlas data in the Gene Expression
Profiling Interactive Analysis database. TNS4, Tensin 4; CRC,
colorectal cancer; HK2, hexokinase 2; LDH, lactate dehydrogenase;
PKM, pyruvate kinase M1/2; SLC, solute carrier; TPM, transcripts
per million. |
TNS4 knockdown inhibits glucose
consumption and lactate production in CRC cells
To assess the potential role of TNS4 in glycolysis
in CRC cells, TNS4 was knocked down in the HCT116 and RKO cell
lines by transfecting them with three non-overlapping TNS4 siRNAs
(TNS4 siRNA-1, TNS4 siRNA-2 and TNS4 siRNA-3). Compared with NC,
transfection with TNS4 siRNA-1, TNS4 siRNA-2 and TNS4 siRNA-3
markedly decreased the protein expression of TNS4 in the HCT116 and
RKO cells (Fig. 3A). Moreover, TNS4
knockdown significantly decreased glucose consumption and lactate
production in the HCT116 and RKO cells compared with NC (Fig. 3B and C). Compared with NC, TNS4
silencing also led to a significant decrease in the mRNA expression
of multiple glycolysis-related genes, including glucose transporter
1, HK2, LDHA and pyruvate dehydrogenase kinase 1 in the CRC cells
(Fig. 3D).
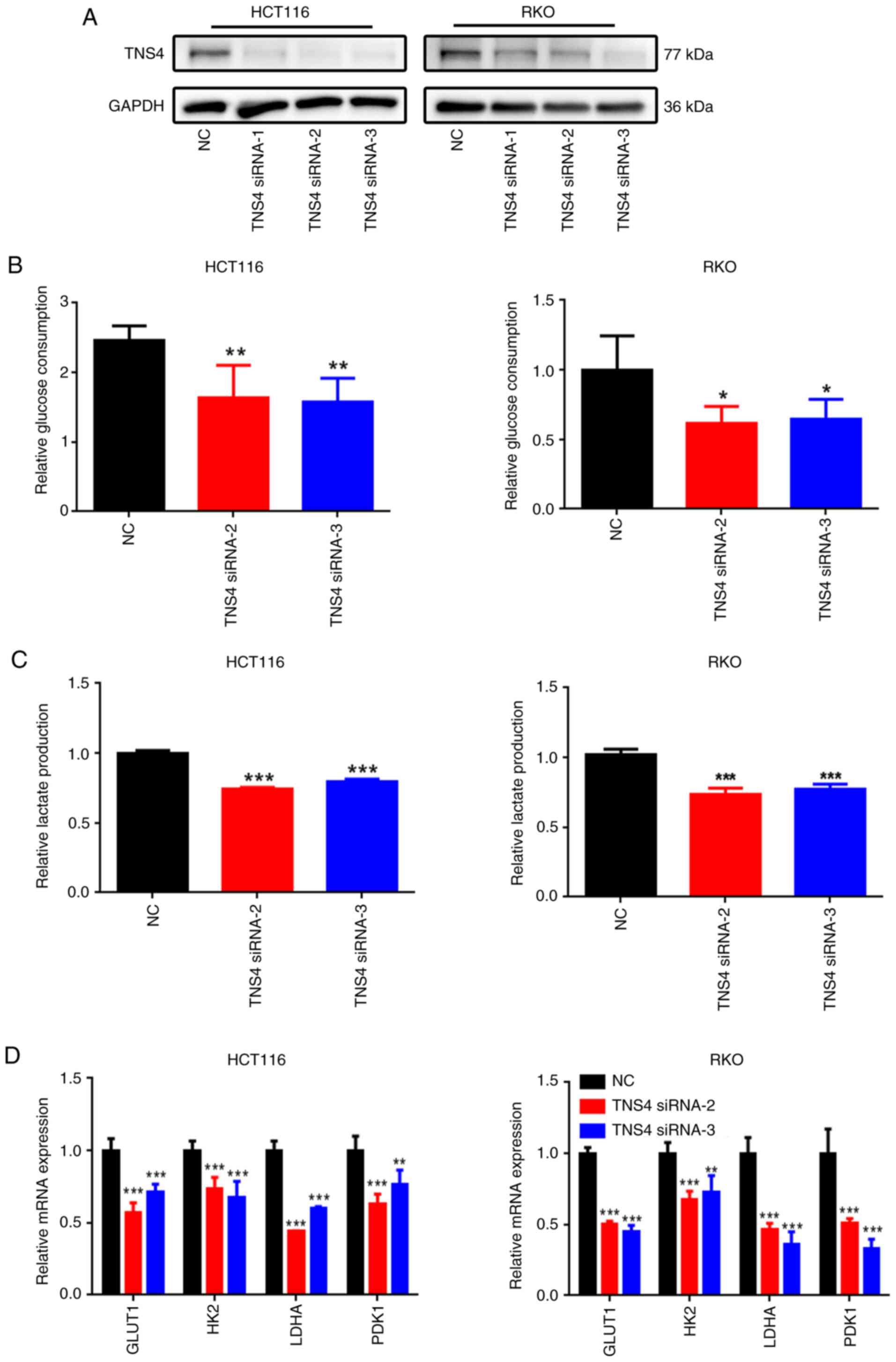 | Figure 3.TNS4 depletion inhibits glycolysis in
colorectal cancer cells. In both HCT116 and RKO cells transfected
with NC, TNS4 siRNA-1, TNS4 siRNA-2 or TNS4 siRNA-3: (A) TNS4
protein levels assessed using western blotting, with GAPDH served
as a loading control; (B) glucose consumption and (C) lactate
production; and (D) the expression of glycolysis-related genes
detected using reverse transcription-quantitative PCR. *P<0.05;
**P<0.01; ***P<0.001. TNS4, Tensin 4; NC, negative control;
siRNA, small interfering RNA; GLUT1, glucose transporter 1; HK2,
hexokinase 2; LDH, lactate dehydrogenase; PDK1, pyruvate
dehydrogenase kinase 1. |
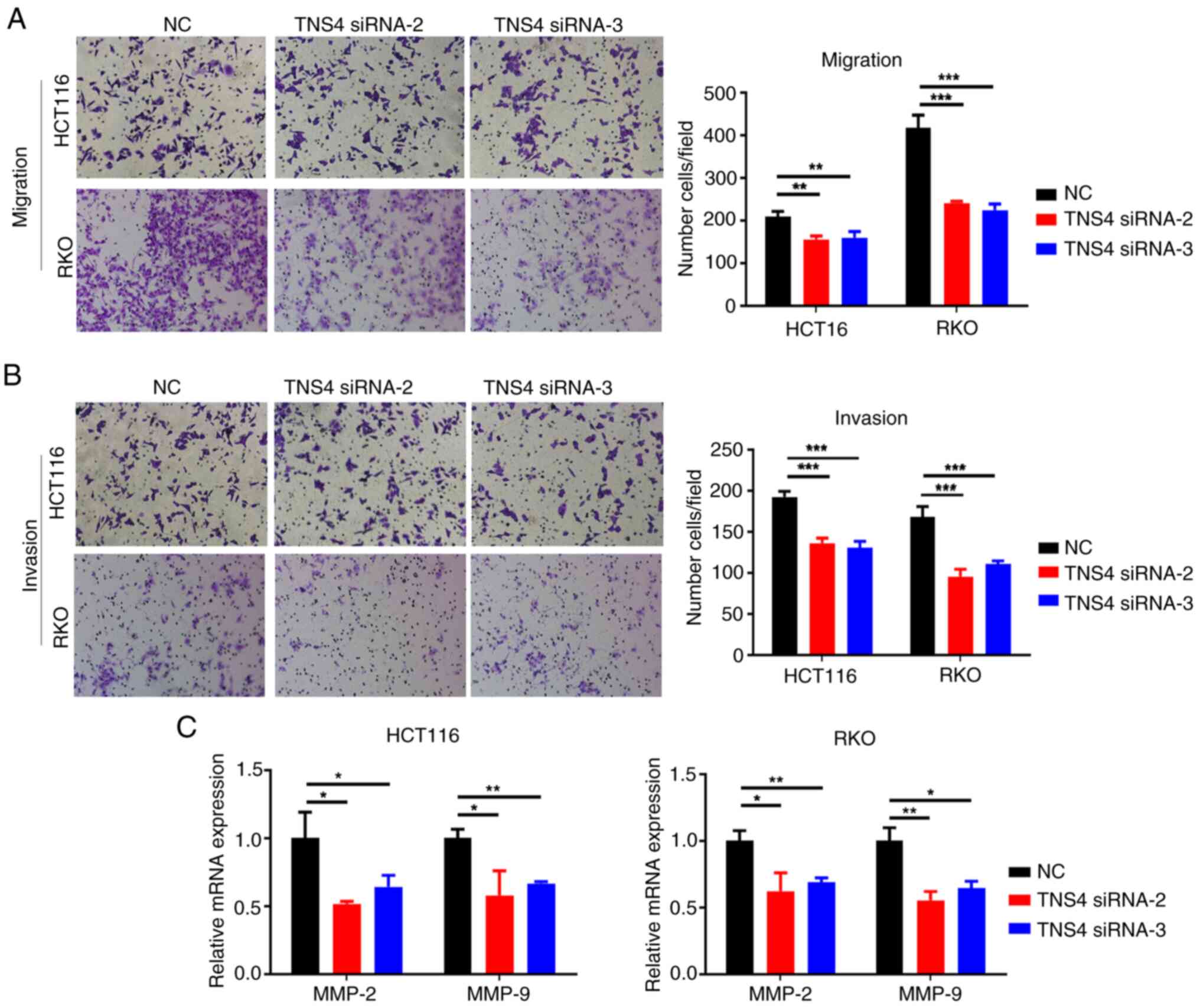
TNS4 silencing attenuates the
migration and invasion of CRC cells
The present study assessed whether TNS4 knockdown
regulated the migration and invasion of CRC cells. Compared with
the control group, the migration and invasion of the HCT116 and RKO
cells was significantly decreased in the TNS4 knockdown groups
(Fig. 4A and B). Furthermore,
transfection with TNS4 siRNA-2 and TNS4 siRNA-3 significantly
downregulated the mRNA expression of matrix metallopeptidase
(MMP)-2 and MMP-9 in the HCT116 and RKO cells (Fig. 4C). These data suggest that TNS4 may
serve a critical role in the migration and invasion of CRC
cells.
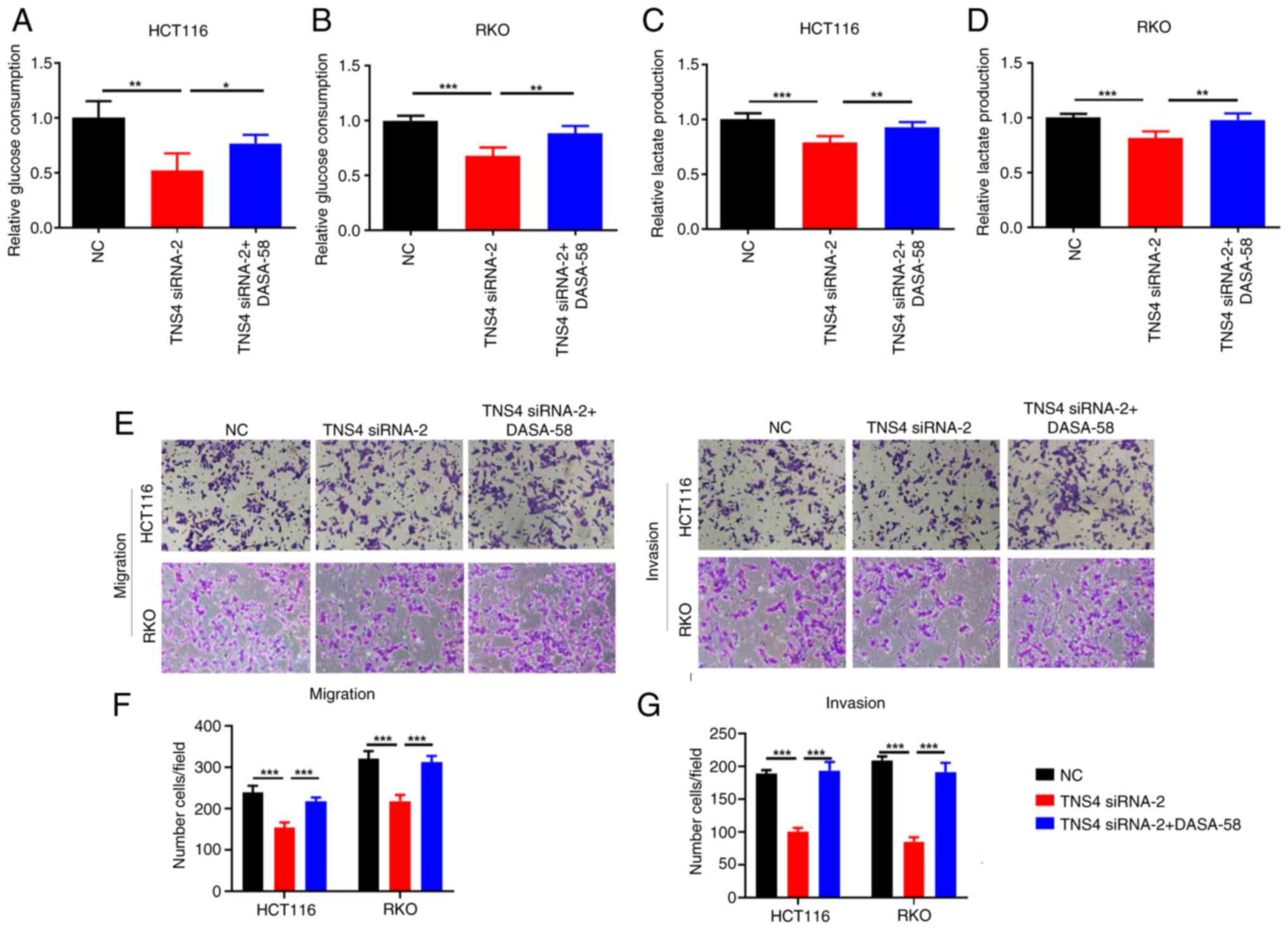
TNS4 silencing inhibits CRC cell
migration and invasion via glycolysis
Previous studies have demonstrated that the
activation of glycolysis serves a key role in the migration and
invasion of cancer cells (23,24).
As TNS4 knockdown significantly suppressed the glycolysis,
migration and invasion of CRC cells in the present study, the
ability of TNS4 to modulate CRC cell migration and invasion via
glycolysis was subsequently evaluated. The results demonstrated
that compared with NC, the glucose consumption and lactate
production were significantly reduced in HCT116 and RKO cells
following TNS4 knockdown, then with the addition of DASA-58, an
activator of glycolysis (25), they
significantly increased (Fig.
5A-D). Notably, treatment with DASA-58 significantly reversed
the effects of TNS4 silencing on the migration and invasion of
HCT116 and RKO cells (Fig.
5E-G).
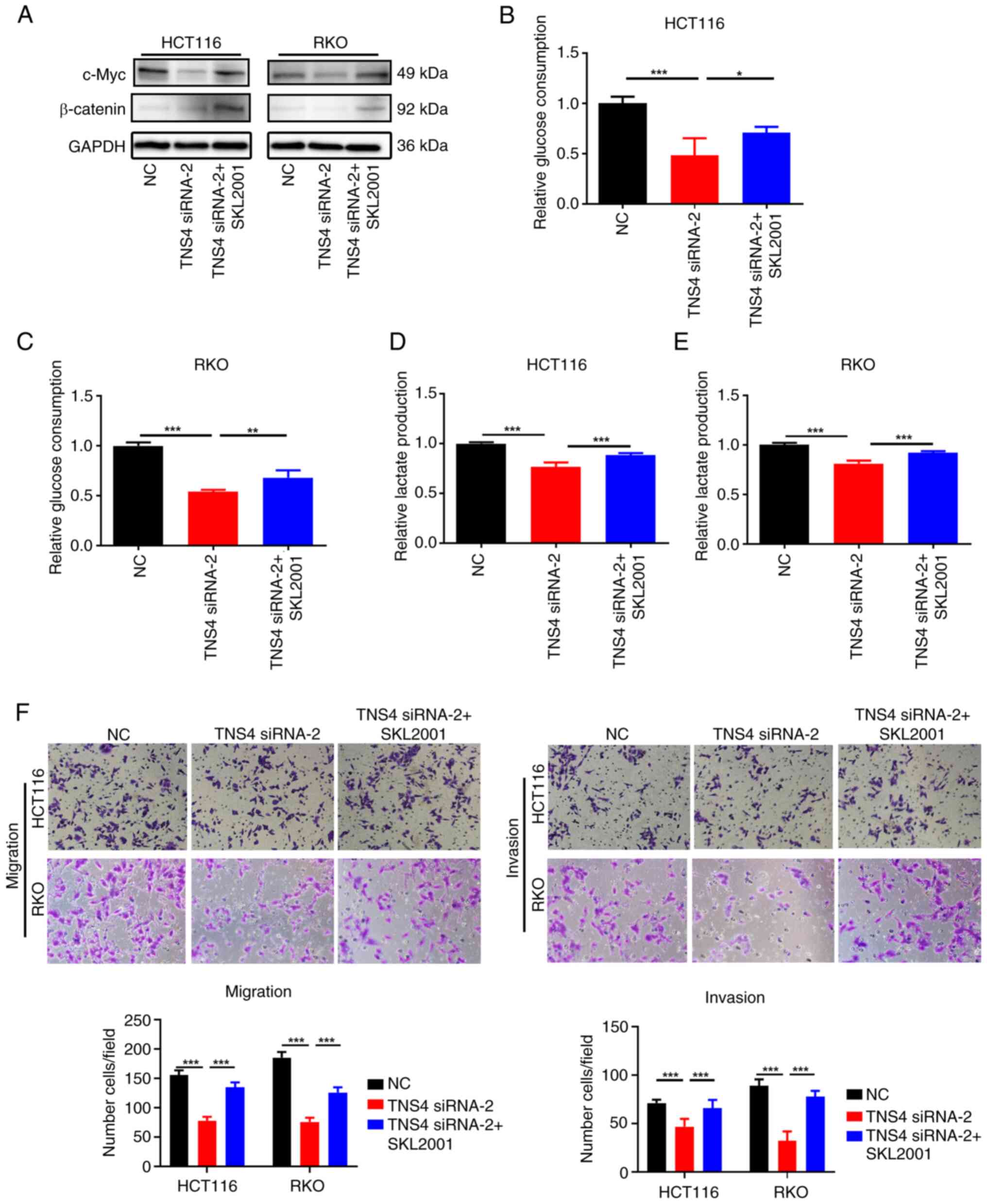
TNS4 knockdown inhibits glycolysis and
metastasis of CRC cells via the β-catenin/c-Myc pathway
To evaluate the mechanism of action of TNS4 in the
glycolysis and metastasis of CRC cells, a review of the literature
was performed. β-catenin/c-Myc signaling has been reported to be
involved in regulating both glycolysis and metastasis in cancers
(16,26–30).
In addition, TNS4 overexpression has been reported to activate
β-catenin signaling in colon cancer (16). Therefore, it we hypothesized that
TNS4 knockdown suppressed glycolysis, migration and invasion of CRC
cells via the β-catenin/c-Myc pathway. The results of western blot
analysis revealed that compared with NC, the knockdown of TNS4
markedly reduced the expression of β-catenin and c-Myc in the
HCT116 and RKO cells (Fig. 6A).
Furthermore, the addition of SKL2001, an activator of β-catenin
signaling (31), notably reversed
the inhibitory effects of TNS4 knockdown on β-catenin and c-Myc
expression in the HCT116 and RKO cells (Fig. 6A). In addition, it was demonstrated
that compared with NC, the glucose consumption and lactate
production were significantly reduced in HCT116 and RKO cells
following TNS4 knockdown, then with the addition of SKL2001, they
significantly increased (Fig.
6B-E). Additionally, treatment with SKL2001 significantly
increased the migration and invasion of the HCT116 and RKO cells
following transfection with TNS4 siRNA-2 (Fig. 6F). These results suggest that TNS4
may promote the glycolysis and metastasis of CRC cells via the
β-catenin/c-Myc pathway.
Discussion
There is increasing evidence to indicate that TNS4
is highly expressed in multiple cancer tissues (4). Albasri et al (8) analyzed the expression of TNS4 in a
series of 462 patients with CRC using immunohistochemistry and
reported that the expression of TNS4 was markedly increased, and
was associated with an advanced Dukes' stage, poor prognosis and
distant metastasis. In the present study, it was demonstrated that
TNS4 mRNA levels were significantly upregulated in CRC tissues
according to data from the UALCAN and GEPIA databases. Furthermore,
TNS4 protein expression was significantly increased in the tissue
samples of patients with CRC and was positively associated with the
TNM stage. These results suggest that TNS4 functions as an oncogene
and serves critical roles in the progression of CRC. In addition,
TNS4 is an important protein involved in maintaining normal
cellular functions, particularly those related to cell adhesion,
migration and signaling (4,32). However, as the current study focused
on the biological functions of TSN4 in CRC, the role of TNS4 in
normal cells by knocking down its expression was not assessed. It
is important to explore the roles of TNS4 in maintaining the normal
structure and function of the kidney and heart, as well as wound
regeneration (32).
Previous studies have demonstrated that TNS4 serves
a crucial role in the progression of cancer by regulating several
biological functions, such as EMT, cell motility, apoptosis and
tumorigenicity (4,9). Based on data from TCGA, the present
study revealed that the expression of TNS4 was significantly
associated with glycolysis-related genes (namely, HK2, LDHB, LDHM,
PKM and SLC2A1) in the CRC tissue samples, suggesting that TNS4 may
be involved in the regulation of aerobic glycolysis in CRC. Aerobic
glycolysis, as a key hallmark of cancer, has been demonstrated to
be associated with tumor growth, metastasis and drug resistance in
CRC (33,34). Shi et al (21) reported that B7 homolog 3, an
immunoregulatory protein, enhanced the chemoresistance of CRC cells
by promoting HK2-mediated aerobic glycolysis. Zhao et al
(35) reported that lncRNA MIR17HG
overexpression resulted in persistent glycolysis and the invasion
and liver metastasis of CRC cells. To the best of our knowledge,
the present study is the first to demonstrate that TNS4 knockdown
significantly inhibits glucose consumption, lactate production and
the mRNA expression of multiple glycolysis-related genes in CRC
cells. These results suggest that TNS4 is a key regulator of
aerobic glycolysis in CRC.
A number of studies have indicated that TNS4 is
associated with the metastasis of cancer cells (8,11,36,37). A
previous study reported that, in non-small cell lung cancer, TNS4
overexpression induced the EMT process and elevated the migratory
capacity of the cells (12). Asiri
et al (36) noted that TNS4,
as a direct mediator of TGF-β1 signaling, participated in
TGF-β1-induced EMT and cell motility in CRC. Moreover, the
TNS4-integrin-linked kinase interaction also modulated cell
motility in CRC (8). In the present
study, it was demonstrated that TNS4 knockdown significantly
decreased the migration and invasion of CRC cells. These results
suggest that TNS4 exerts promoting effects on the metastasis of CRC
cells. Furthermore, aerobic glycolysis is a crucial influencing
factor for cancer metastasis (38,39),
and in the present study, it was demonstrated that treatment with
DASA-58, an activator of glycolysis, significantly reversed the
effects of TNS4 silencing on the migration and invasion of CRC
cells. These results suggest that the TNS4-induced promotion of the
migration and invasion of CRC cells is glycolysis-dependent.
The activation of the β-catenin/c-Myc pathway has
been reported to be associated with cancer (29,30).
For example, CD36 has been reported to inhibit the glycolysis and
tumorigenesis of CRC through the glypican 4-β-catenin-c-Myc
signaling axis (40). Liang et
al (41) indicated that
butyrate controlled gastric cancer cell proliferation, migration,
invasion and aerobic glycolysis by downregulating
Wnt/β-catenin/c-MYC signaling. In human hepatocellular carcinoma,
Gankyrin has been reported to upregulate glycolysis and
glutaminolysis to promote tumorigenesis, metastasis and drug
resistance via upregulating c-Myc through the activation of
β-catenin signaling (26). In the
present study, it was revealed that TNS4 knockdown significantly
reduced the protein expression of β-catenin and c-Myc in CRC cells.
Consistent with these findings, it has been reported that TNS4 can
activate the β-catenin pathway to regulate tumorigenicity and tumor
angiogenesis (5,16,42).
Moreover, SKL2001, an activator of β-catenin signaling,
significantly reversed the effects of TNS4 knockdown on glucose
consumption, lactate production, and the migration and invasion of
CRC cells. Taken together, these results suggest that TNS4 promotes
the glycolysis and metastasis of CRC cells through the
β-catenin/c-Myc pathway.
In conclusion, the results of the present study
demonstrated that TNS4 was highly expressed in CRC tissues, and
that its high expression was positively associated with the TNM
stages of patients with CRC. Moreover, the silencing of TNS4
inhibited the glycolysis, migration and invasion of CRC cells via
the β-catenin/c-Myc pathway. This suggests that TNS4 may be an
efficient target for CRC diagnosis and therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YW and CX designed the experiments. YW and YL
performed the experiments and analyzed the data. YW wrote the
manuscript. CX and YL made suggestions during the writing. YW and
YL confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Board of the First Affiliated Hospital of Soochow University
(Suzhou, China; approval no. 2021-327). Written informed consent
was obtained from the patients prior to obtaining the samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Biller LH and Schrag D: Diagnosis and
treatment of metastatic colorectal cancer: A review. JAMA.
325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piawah S and Venook AP: Targeted therapy
for colorectal cancer metastases: A review of current methods of
molecularly targeted therapy and the use of tumor biomarkers in the
treatment of metastatic colorectal cancer. Cancer. 125:4139–4147.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holladay L, Luu J, Balendra V and Kmetz K:
Current and potential treatment of colorectal cancer metastasis to
bone. Cancer Treat Res Commun. 37:1007632023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lo SH: C-terminal tensin-like (CTEN): A
promising biomarker and target for cancer. Int J Biochem Cell Biol.
51:150–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu X, Zhou B, Cao M, Shao Q, Pan Y and
Zhao T: CTEN inhibits tumor angiogenesis and growth by targeting
VEGFA through down-regulation of β-catenin in breast cancer.
Technol Cancer Res Treat. 20:153303382110455062021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Ghamdi S, Cachat J, Albasri A, Ahmed M,
Jackson D, Zaitoun A, Guppy N, Otto WR, Alison MR, Kindle KB and
Ilyas M: C-terminal tensin-like gene functions as an oncogene and
promotes cell motility in pancreatic cancer. Pancreas. 42:135–140.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasaki H, Moriyama S, Mizuno K, Yukiue H,
Konishi A, Yano M, Kaji M, Fukai I, Kiriyama M, Yamakawa Y and
Fujii Y: Cten mRNA expression was correlated with tumor progression
in lung cancers. Lung Cancer. 40:151–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Albasri A, Al-Ghamdi S, Fadhil W,
Aleskandarany M, Liao YC, Jackson D, Lobo DN, Lo SH, Kumari R,
Durrant L, et al: Cten signals through integrin-linked kinase (ILK)
and may promote metastasis in colorectal cancer. Oncogene.
30:2997–3002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asiri A, Toss MS, Raposo TP, Akhlaq M,
Thorpe H, Alfahed A, Asiri A and Ilyas M: Cten promotes
epithelial-mesenchymal transition (EMT) in colorectal cancer
through stabilisation of Src. Pathol Int. 69:381–391. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thorpe H, Asiri A, Akhlaq M and Ilyas M:
Cten promotes epithelial-mesenchymal transition through the
post-transcriptional stabilization of Snail. Mol Carcinog.
56:2601–2609. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu X, Gao J, Zhang Y, Zhao T, Cai H and
Zhang T: CTEN induces epithelial-mesenchymal transition (EMT) and
metastasis in non small cell lung cancer cells. PLoS One.
13:e01988232018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Albasri A, Seth R, Jackson D, Benhasouna
A, Crook S, Nateri AS, Chapman R and Ilyas M: C-terminal
Tensin-like (CTEN) is an oncogene which alters cell motility
possibly through repression of E-cadherin in colorectal cancer. J
Pathol. 218:57–65. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang YX, Huang CY, Chiu HJ, Huang PH,
Chien HT, Jwo SH and Liao YC: Nuclear-localized CTEN is a novel
transcriptional regulator and promotes cancer cell migration
through its downstream target CDC27. J Physiol Biochem. 79:163–174.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu X, Zhang Y, Pan Y, Cao M, Zhou X and
Zhang T: Overexpression of CTEN is associated with gefitinib
resistance in non-small cell lung cancer. Oncol Lett.
21:402021.PubMed/NCBI
|
|
15
|
Li Y, Mizokami A, Izumi K, Narimoto K,
Shima T, Zhang J, Dai J, Keller ET and Namiki M: CTEN/tensin 4
expression induces sensitivity to paclitaxel in prostate cancer.
Prostate. 70:48–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liao YC, Chen NT, Shih YP, Dong Y and Lo
SH: Up-regulation of C-terminal tensin-like molecule promotes the
tumorigenicity of colon cancer through beta-catenin. Cancer Res.
69:4563–4566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schwartz L, Supuran CT and Alfarouk KO:
The Warburg effect and the hallmarks of cancer. Anticancer Agents
Med Chem. 17:164–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhong X, He X, Wang Y, Hu Z, Huang H, Zhao
S, Wei P and Li D: Warburg effect in colorectal cancer: The
emerging roles in tumor microenvironment and therapeutic
implications. J Hematol Oncol. 15:1602022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Liu ZX, Wu QN, Lu YX, Wong CW, Miao
L, Wang Y, Wang Z, Jin Y, He MM, et al: Long noncoding RNA AGPG
regulates PFKFB3-mediated tumor glycolytic reprogramming. Nat
Commun. 11:15072020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu S, Zang W, Qiu Y, Liao L and Zheng X:
Deubiquitinase OTUB2 exacerbates the progression of colorectal
cancer by promoting PKM2 activity and glycolysis. Oncogene.
41:46–56. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi T, Ma Y, Cao L, Zhan S, Xu Y, Fu F,
Liu C, Zhang G, Wang Z, Wang R, et al: B7-H3 promotes aerobic
glycolysis and chemoresistance in colorectal cancer cells by
regulating HK2. Cell Death Dis. 10:3082019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu M, Huang F, Zhang D, Ju J, Wu XB, Wang
Y, Wang Y, Wu Y, Nie M, Li Z, et al: Heterochromatin protein
HP1gamma promotes colorectal cancer progression and is regulated by
miR-30a. Cancer Res. 75:4593–4604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Z, Hu Z, Sui Q, Huang Y, Zhao M, Li
M, Liang J, Lu T, Zhan C, Lin Z, et al: LncRNA FAM83A-AS1
facilitates tumor proliferation and the migration via the
HIF-1α/glycolysis axis in lung adenocarcinoma. Int J Biol Sci.
18:522–535. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin S, Li Y, Wang D, Huang C, Marino D,
Bollt O, Wu C, Taylor MD, Li W, DeNicola GM, et al: Fascin promotes
lung cancer growth and metastasis by enhancing glycolysis and
PFKFB3 expression. Cancer Lett. 518:230–242. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rao J, Wang H, Ni M, Wang Z, Wang Z, Wei
S, Liu M, Wang P, Qiu J, Zhang L, et al: FSTL1 promotes liver
fibrosis by reprogramming macrophage function through modulating
the intracellular function of PKM2. Gut. 71:2539–2550. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu R, Li Y, Tian L, Shi H, Wang J, Liang
Y, Sun B, Wang S, Zhou M, Wu L, et al: Gankyrin drives metabolic
reprogramming to promote tumorigenesis, metastasis and drug
resistance through activating β-catenin/c-Myc signaling in human
hepatocellular carcinoma. Cancer Lett. 443:34–46. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai M, Song J, Wang L, Zhou K and Shu L:
HOXC13 promotes cervical cancer proliferation, invasion and Warburg
effect through β-catenin/c-Myc signaling pathway. J Bioenerg
Biomembr. 53:597–608. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Huang Y, Zhang J, Pei C, Hu J, Lyu
J and Shen Y: TIMMDC1 knockdown inhibits growth and metastasis of
gastric cancer cells through metabolic inhibition and
AKT/GSK3β/β-catenin signaling pathway. Int J Biol Sci.
14:1256–1267. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rennoll S and Yochum G: Regulation of MYC
gene expression by aberrant Wnt/β-catenin signaling in colorectal
cancer. World J Biol Chem. 6:290–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ashihara E, Takada T and Maekawa T:
Targeting the canonical Wnt/β-catenin pathway in hematological
malignancies. Cancer Sci. 106:665–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu YB, Li SY, Liu JY, Xue JJ, Xu JF, Chen
T, Cao TY, Zhou H, Wu TT, Dong CL, et al: Long non-coding RNA
NRSN2-AS1 promotes ovarian cancer progression through targeting
PTK2/β-catenin pathway. Cell Death Dis. 14:6962023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liao YC and Lo SH: Tensins-emerging
insights into their domain functions, biological roles and disease
relevance. J Cell Sci. 134:jcs2540292021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zafari N, Velayati M, Damavandi S, Pourali
G, Mobarhan MG, Nassiri M, Hassanian SM, Khazaei M, Ferns GA and
Avan A: Metabolic pathways regulating colorectal cancer: A
potential therapeutic approach. Curr Pharm Des. 28:2995–3009. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nenkov M, Ma Y, Gaßler N and Chen Y:
Metabolic reprogramming of colorectal cancer cells and the
microenvironment: Implication for therapy. Int J Mol Sci.
22:62622021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao S, Guan B, Mi Y, Shi D, Wei P, Gu Y,
Cai S, Xu Y, Li X, Yan D, et al: LncRNA MIR17HG promotes colorectal
cancer liver metastasis by mediating a glycolysis-associated
positive feedback circuit. Oncogene. 40:4709–4724. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Asiri A, Raposo TP, Alfahed A and Ilyas M:
TGFβ1-induced cell motility but not cell proliferation is mediated
through Cten in colorectal cancer. Int J Exp Pathol. 99:323–330.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fleming JC, Woo J, Moutasim K, Hanley CJ,
Frampton SJ, Wood O, Ward M, Woelk CH, Ottensmeier CH, Hafizi S, et
al: CTEN induces tumour cell invasion and survival and is
prognostic in radiotherapy-treated head and neck cancer. Cancers
(Basel). 12:29632020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang J, Ren B, Yang G, Wang H, Chen G, You
L, Zhang T and Zhao Y: The enhancement of glycolysis regulates
pancreatic cancer metastasis. Cell Mol Life Sci. 77:305–321. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng J, Li J, Wu L, Yu Q, Ji J, Wu J, Dai
W and Guo C: Emerging roles and the regulation of aerobic
glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res.
39:1262020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fang Y, Shen ZY, Zhan YZ, Feng XC, Chen
KL, Li YS, Deng HJ, Pan SM, Wu DH and Ding Y: CD36 inhibits
β-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4
to repress colorectal tumorigenesis. Nat Commun. 10:39812019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liang Y, Rao Z, Du D, Wang Y and Fang T:
Butyrate prevents the migration and invasion, and aerobic
glycolysis in gastric cancer via inhibiting Wnt/β-catenin/c-Myc
signaling. Drug Dev Res. 84:532–541. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Raposo TP, Alfahed A, Nateri AS and Ilyas
M: Tensin4 (TNS4) is upregulated by Wnt signalling in adenomas in
multiple intestinal neoplasia (Min) mice. Int J Exp Pathol.
101:80–86. 2020. View Article : Google Scholar : PubMed/NCBI
|