Introduction
The human zinc finger and BTB domain containing 20
(ZBTB20) gene (also termed DPZF, HOF and Zfp288) is located in
chr3q13.31 and encodes a protein composed of 741 amino acids.
ZBTB20 is a member of the poxvirus and zinc-finger (POZ) domain and
Kruppel-like family of transcription repressors (1,2), which
are characterized by a distinct and unique N-terminal BTB/POZ
domain and C-terminal DNA-binding zinc finger domains. ZBTB20
participates in various physiological and pathophysiological
processes, including glucose metabolism (3), growth regulation (2) and nervous system development (4–6).
The role of ZBTB20 in tumorigenesis is complex.
Several studies have identified ZBTB20 as a tumor promoter. The
expression of ZBTB20 is increased in hepatocellular carcinoma (HCC)
(7,8), gastric carcinoma (9) and non-small cell lung cancer (10) and is correlated with poor prognosis.
Increased expression of ZBTB20 in HCC was positively correlated
with tumor vein invasion, recurrence and metastasis (7). In HCC (8) and non-small cell lung cancer (10), ZBTB20 promotes cell proliferation by
inhibiting forkhead box O1 expression. ZBTB20 knockdown
significantly reduces cell proliferation, migration and invasion
through induction of IκBα expression and inhibits the NF-κB
signaling pathway in gastric cancer cells, whereas overexpression
exerts the opposite effects (9). A
report has also indicated that ZBTB20 may act as a tumor suppressor
gene. ZBTB20 mRNA levels were significantly reduced in primary
prostate cancer samples compared with benign tissues, and the
recurrence-free survival of patients with tumors expressing low
levels of ZBTB20 was markedly reduced (11). ZBTB20 expression is positively
associated with the expression of phosphatase and tensin homolog
(PTEN) and has a role in human PTEN-regulated tumor suppressor
networks (11,12).
Glioma is a type of neuroepithelial tumor and the
most common primary intracranial tumor (13). These tumors are graded from I to IV
based on morphology and malignant behavior, according to the 2021
World Health Organization (WHO) classification (14). Glioblastoma (GBM), which is
classified as a grade IV tumor, has the highest incidence (~46.1%)
among all primary malignant brain tumors in the US (15). Liu et al (16) reported that miR-758-5p suppresses
glioblastoma proliferation, migration and invasion by targeting
ZBTB20. However, the clinical expression profile and role of ZBTB20
in GBM remain to be further explored. The objective of the present
study was to investigate the clinical role of ZBTB20 in GBM and
identify the possible molecular mechanisms underlying this role.
For this purpose, the expression levels of ZBTB20 in glioma
specimens were analyzed using publicly available data and the
function of ZBTB20 was investigated through in vitro gain-
and loss-of-function experiments.
Materials and methods
Cell culture
The human GBM cell lines U251 (cat no. TCHu58) was
purchased from the National Collection of Authenticated Cell
Cultures in August 2018 and May 2019, respectively. The cell line
was authenticated by short tandem repeat analysis and subjected to
mycoplasma detection at the National Collection of Authenticated
Cell Cultures. 293T cells were provided by the Stem Cell Research
Center of Zhengzhou University (Zhengzhou, China) and tested for
mycoplasma contamination using a Mycoplasma Detection Kit (Beijing
Solarbio Science & Technology Co., Ltd.) at the same
institution. The cells were cultured in Dulbecco's Modified Eagle's
Medium (DMEM; Hyclone) supplemented with 10% fetal bovine serum
(FBS; Biological Industries) and 1% (v/v) penicillin-streptomycin
(GIBCO; Thermo Fisher Scientific, Inc.). Cultures were maintained
in a 37°C incubator with 5% CO2 and passaged with
Trypsin-EDTA (0.25%) (GIBCO; Thermo Fisher Scientific, Inc.).
Lentiviral infection
The GeneCopoeia HIV-Based Lentiviral Expression
System, a modified version of the third-generation
self-inactivating (SIN) lentiviral vector system, was used to
produce lentivirus. ZBTB20 (NM_001164342.2) knock-in and knockdown
experiments in U251 cells were performed using a lentiviral system
(pReceiver-Lv105 and psi-LVRU6GP; GeneCopoeia, Inc.), with ZBTB20
complementary DNA (cDNA) or short hairpin RNAs (shRNAs) targeting
5′-GCACTGGACTTCAGGATAAGT-3′ (shZBTB20-a),
5′-GCATGTGTCTGACGGATAAGT-3′ (shZBTB20-b) and
5′-GCCAAACACTTCTAGAGAAAT-3′ (shZBTB20-c). Lentivirus packaging was
performed using the Lenti-Pac™ HIV Expression Packaging Kit
(GeneCopoeia, Inc.). A total of 2 days before transfection,
1.3–1.5×106 293T lentiviral packaging cells were seeded
in a 10-cm dish in 10 ml DMEM supplemented with 10%
heat-inactivated FBS. In a sterile polypropylene tube, 2.5 µg
lentiviral open reading frame/shRNA expression plasmid and 5.0 µl
(0.5 µg/µl) Lenti-Pac HIV packaging mix (ratio: 1:1) were diluted
in 200 µl Opti-MEM® I (Invitrogen; Thermo Fisher
Scientific, Inc.). In a separate tube, 15 µl EndoFectin Lenti was
diluted in 200 µl Opti-MEM I. The diluted EndoFectin Lenti reagent
was added drop-wise to the DNA solution while the DNA-containing
tube was gently vortexed. The mixture was then incubated for 10–25
min at room temperature. The DNA-EndoFectin Lenti complex was added
directly to each dish and the cells were incubated in a 37°C
incubator with 5% CO2 overnight. The overnight culture
medium was then replaced with fresh DMEM supplemented with 2–5%
heat-inactivated FBS. TiterBoost reagent (1:500) was added to the
culture medium and incubation was continued in a 37°C incubator
with 5% CO2. The pseudovirus-containing culture medium
was collected in sterile capped tubes 48 h post transfection and
the tubes were centrifuged at 500 × g for 10 min at room
temperature. The supernatant was then filtered through 0.45-µm
polyethersulfone low protein-binding filters. The lentivirus titer
was estimated by transduction of U251 cells and the fraction of
eGFP fluorescent cells was counted using a fluorescent
microscope.
The cells were transduced with lentivirus (MOI 10)
in the presence of 5 µg/ml polybrene (MilliporeSigma). After 8–10 h
of transduction, the culture medium was replaced. After 72 h of
transduction, the cells were selected using 2 µg/ml puromycin
(Beijing Solarbio Science & Technology Co., Ltd.) for 2–3
weeks, after which the cells were maintained in culture medium
containing 2 µg/ml puromycin. The expression of ZBTB20 in cells was
detected by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blotting. The cells transfected with
lentivirus containing empty pReceiver-Lv105 (NC group) were used as
the control for the ZBTB20 overexpression experiments (ZBTB20
group), and cells transfected with lentivirus containing
psi-LVRU6GP with a scramble sequence (5′-GCTTCGCGCCGTAGTCTTA-3′;
GeneCopoeia, Inc.; shNC group) were used as the control for the
ZBTB20 knockdown experiments (shZBTB20 group).
RT-qPCR
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
was added to the U251 cell culture for the extraction of total RNA.
First-strand cDNA was prepared using the RT Reagent Kit (Takara
Bio, Inc.), according to the manufacturer's instructions. Next,
qPCR was performed using diluted cDNA and the One Step SYBR
PrimeScript PLUS RT-PCR Kit (Takara Bio, Inc.) with an ABI 7500
fluorescence quantitative PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The primers used in the present study are
shown in Table SI. The PCR cycling
conditions included an initial holding period at 95°C for 5 min,
followed by a two-step PCR program consisting of 40 cycles of 95°C
for 5 sec and 60°C for 10 sec. The expression levels were
normalized to those of the housekeeping gene,
glyceraldehyde-3-phosphate dehydrogenase and analyzed using the
2−ΔΔCq method (17).
Western blot analysis
Cell lysis buffer for Western and IP (Beyotime
Institute of Biotechnology) was added to the cultured U251 cells.
Cell lysates were collected and centrifuged at 10,000 × g for 5 min
at 4°C. The supernatant was taken and the protein concentrations
were determined using a Bicinchoninic Acid Protein Assay kit (CoWin
Biosciences). The protein was subjected to SDS-PAGE (Shanghai
Sangong Pharmaceutical Co., Ltd.) using a PowerPAC HC High-Current
Power Supply electrophoresis machine (Bio-Rad Laboratories, Inc.);
6% SDS-PAGE was used for the detection of ten-eleven translocation
1 (TET1), 12% SDS-PAGE was used for the detection of cleaved
caspase-3 (CASP3) and 10% SDS-PAGE was used for all other proteins.
When detecting ZBTB20 expression in the ZBTB20 overexpression
group, 15 µg sample per well was loaded, whereas 30 µg per well was
used for all other experiments. After sample loading was completed,
a constant voltage of 100V was used to separate proteins. The
separated proteins were then transferred to methanol pretreated
polyvinylidene fluoride membranes (MilliporeSigma) at 4°C. For
TET1, a constant current of 270 mA for 4 h was used and for cleaved
CASP3, a constant current of 200 mA for 50 min was used. For all
other proteins a constant current of 200 mA for 60 min was used.
After membrane transfer, the membrane was incubated in Western
Blocking Buffer (Beyotime Institute of Biotechnology) at room
temperature for 1 h. The membranes were then washed with
Tris-buffered saline with 0.05% Tween-20 three times, 5 min each
time. Next, the membranes were separately incubated overnight at
4°C with the following antibodies: Polyclonal rabbit anti-human
ZBTB20 (dilution, 1:2,000; cat. no. ab127702; Abcam), polyclonal
rabbit anti-TET1 (1:1,000; cat. no. A21766; ABclonal Biotech Co.,
Ltd.), polyclonal rabbit anti-human FAS (1:1,000; cat. no. A2639;
ABclonal Biotech Co., Ltd.), polyclonal rabbit anti-human B-cell
lymphoma-2 (BCL2; 1:2,000; cat. no. BA0412; Wuhan Boster Biological
Technology, Ltd.), polyclonal rabbit anti-human BCL2 associated X
(BAX; 1:2,000, cat. no. BA0315-2; Wuhan Boster Biological
Technology, Ltd.), monoclonal rabbit anti-human CASP3 (1:1,000,
cat. no. AF1213; Beyotime Institute of Biotechnology), monoclonal
rabbit anti-human cleaved CASP3 (1:1,000; cat. no. AF1150; Beyotime
Institute of Biotechnology), monoclonal rabbit anti-human
phosphorylated-extracellular signal regulated kinase (p-ERK;
1:1,000; cat. no. AP0485; ABclonal Biotech Co., Ltd.), polyclonal
rabbit anti-human ERK (1:1,000; cat. no. A16686; ABclonal Biotech
Co., Ltd.), monoclonal rabbit anti-human p-cyclic
AMP-responsive-element-binding protein (CREB; 1:500; cat. no.
ab32096; Abcam), monoclonal mouse anti-human CREB (1:500; cat. no.
ab178322; Abcam) and monoclonal rabbit anti-human β-actin
(1:10,000; cat. no. AC026; ABclonal Biotech Co., Ltd.). The
membranes were then incubated with goat anti-rabbit IgG H&L
(HRP) (1:20,000; cat. no. ab205718; Abcam) or goat anti-mouse IgG
H&L (HRP) (1:10,000; cat. no. ab205719; Abcam) for 30 min at
37°C. Finally, the bands were visualized using enhanced
chemiluminescence (Beyotime Institute of Biotechnology) and the
Bio-Rad Gel Doc XR + IMAGELAB system.
Cell proliferation assay
The U251 cells were digested with Trypsin-EDTA
(0.25%) (GIBCO; Thermo Fisher Scientific, Inc.) and seeded into a
96-well plate. At 0, 24, 48 and 72 h after seeding, Cell Counting
Kit-8 (Beyotime Institute of Biotechnology) solution was added to
the wells [1:10 (v/v)]. After incubation for 1 h at 37°C, the
optical density at a wavelength of 450 nm was determined through
spectrophotometry (Life Science Analyzer: BioMate 3
Spectrophotometer; Thermo Fisher Scientific Inc.).
Cell cycle analysis
The U251 cells were seeded and cultured for 48 h
before use. Then the cells were trypsinized and collected by
centrifugation at 800 × g for 5 min at room temperature. The cells
were washed with cold PBS, collected by centrifugation at 800 × g
for 5 min at 4°C and fixed in 70% ethanol at −20°C overnight. After
centrifugation at 1,800 × g for 5 min at 4°C, the cells were
collected and washed with cold PBS. The cells were again collected
by centrifugation at 1,800 × g for 5 min at 4°C and the supernatant
was discarded. Next, 200 µl of propidium iodide/RNase staining
buffer (cat. no. 550825; BD Pharmingen; BD Biosciences) was added
to each tube, mixed and incubated at room temperature for 15 min,
avoiding light. A total of three parallel samples were set up for
each group. The samples were run on a customized BD LSR II Flow
Cytometer (BD Biosciences), and the data were analyzed using FlowJo
software (v10.6.2; FlowJo LLC).
Bobcat339 drug treatment
The U251 cells were seeded into a 6-well plate and
cultured to ~70% confluency. Subsequently, 30 µM Bobcat339 (Beijing
Solarbio Science & Technology Co., Ltd.) was added to the
culture medium. After a 24-h incubation in a 37°C incubator at
37°C, the cells were harvested and proteins were extracted for
western blotting as aforementioned.
Bioinformatics
TCGA cohorts of mRNA expression and survival data in
low-grade glioma (LGG) (18) and
GBM (19) were downloaded from
University of California Santa Cruz (UCSC) Xena (https://tcga.xenahubs.net; dataset ID:
TCGA.GBMLGG.sampleMap/HiSeqV2_PANCAN). TCGA brain LGG and GBM gene
expression had been determined by RNAseq, mean-normalized (per
gene) across all TCGA cohorts. Values in this dataset are generated
at UCSC by combining ‘gene expression RNAseq’ values of all TCGA
cohorts and values are then mean-centered per gene, and then, the
extracted converted data only belong to this cohort. The mRNA
expression and survival data of patients with glioma (DataSet ID:
mRNA-array_301) were obtained from the Chinese Glioma Genome Atlas
(CGGA; http://www.cgga.org.cn/) (20). The patients with incomplete
information were excluded from analysis. Data regarding the
association between the expression level of ZBTB20 and other genes
were downloaded from the cBioPortal (http://cbioportal.org).
Statistical analysis
Statistical analysis was conducted using SPSS
version 19.0 software (IBM Corp.). The correlation between ZBTB20
expression and the clinicopathological parameters of glioma was
evaluated through Pearson correlation analysis. Univariate analysis
of ZBTB20 expression and survival data was performed using the
Kaplan-Meier and log-rank tests. For the in vitro
experiments, independent sample t-tests were used for the
comparisons between two groups. One-way analysis of variance
followed by the Bonferroni test was conducted for the comparison of
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Relationship between ZBTB20 expression
and the clinicopathological characteristics of GBM
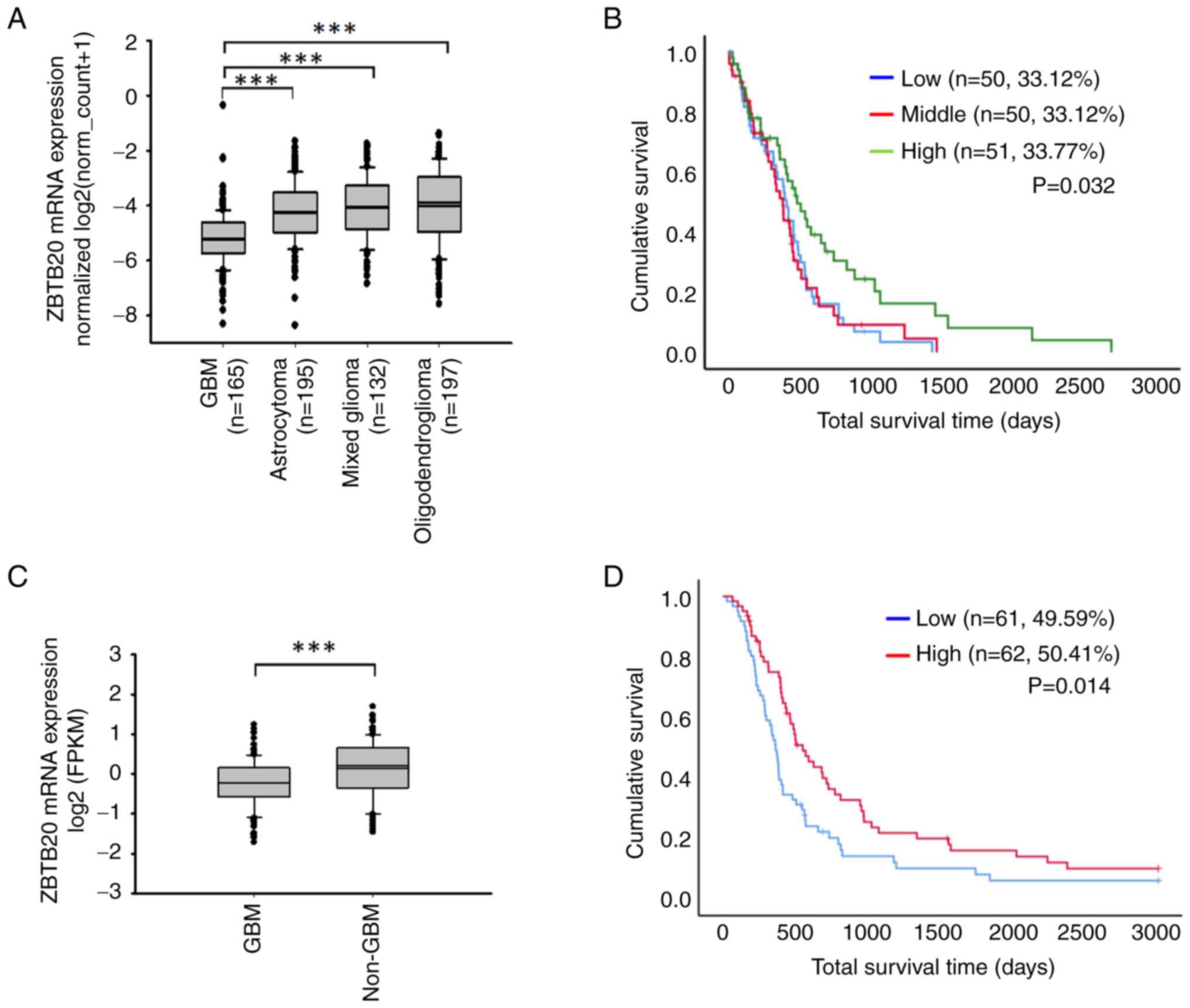
The analysis based on TCGA data demonstrated that
the ZBTB20 mRNA expression level was higher in LGG tissues
(including astrocytoma, mixed glioma and oligodendroglioma tissues)
vs. GBM (WHO grade IV) tissues (Fig.
1A). The results obtained from the analysis of CGGA and TCGA
data were consistent. The expression of ZBTB20 in GBM (WHO grade
IV) was significantly lower than that observed in non-GBM (WHO
grade II and III) (Table I and
Fig. 1C). After sorting patients
with primary glioma into the high, middle and low ZBTB20 expression
groups, analysis of TCGA data demonstrated that the survival time
of the top 33.77% expression group was significantly higher than
that of the middle 33.12% and the bottom 33.12% expression groups
(Fig. 1B). Furthermore, analysis of
the CGGA data demonstrated that the overall survival time of
patients in the top 50% expression group was significantly higher
than that of the bottom 50% expression group (Fig. 1D).
 | Table I.Number of samples in the different
WHO grade subgroups. |
Table I.
Number of samples in the different
WHO grade subgroups.
| Tumor type | WHO | n |
|---|
| GBM | IV | 124 |
| non-GBM | II, III | 172 |
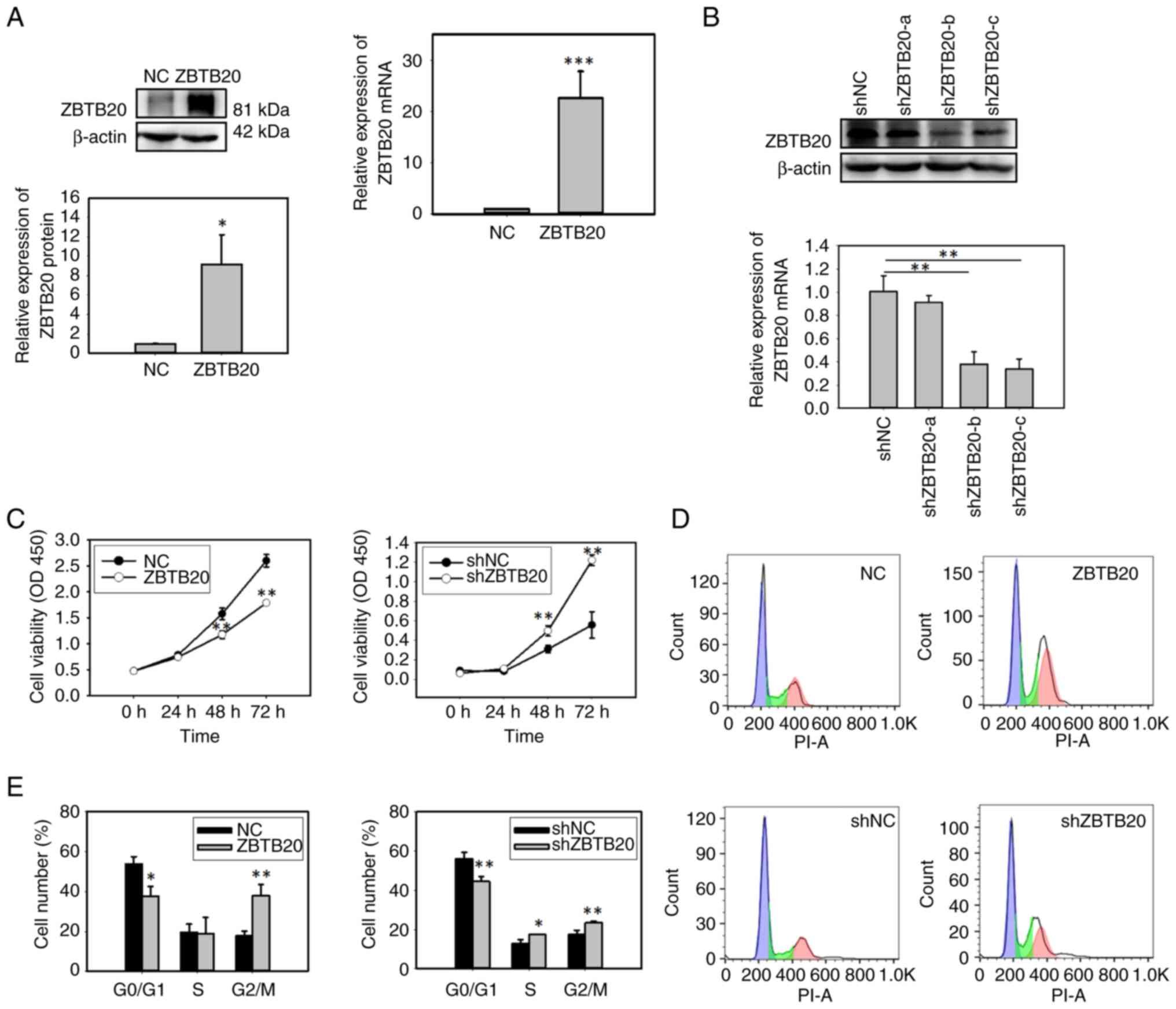
ZBTB20 inhibits the proliferation of
GBM cells
Gain- and loss-of-function experiments in U251 GBM
cells verified that ZBTB20 expression was increased in
ZBTB20-overexpressing cells (ZBTB20 group) and decreased in
ZBTB20-knockdown cells (shZBTB20 group) (Fig. 2A and B). ZBTB20 overexpression
significantly repressed cell proliferation, whereas ZBTB20
knockdown exerted the opposite effect (Fig. 2C). Cell cycle analysis showed that
ZBTB20 overexpression increased the percentage of cells in the G2/M
phase, but not that of cells in S phase (Fig. 2D and E). These findings suggested
that ZBTB20 overexpression may inhibit cell proliferation through
cell cycle arrest at the G2/M phase. In addition, ZBTB20 knockdown
increased the percentages of cells in both the S phase and G2/M
phase (Fig. 2D and E).
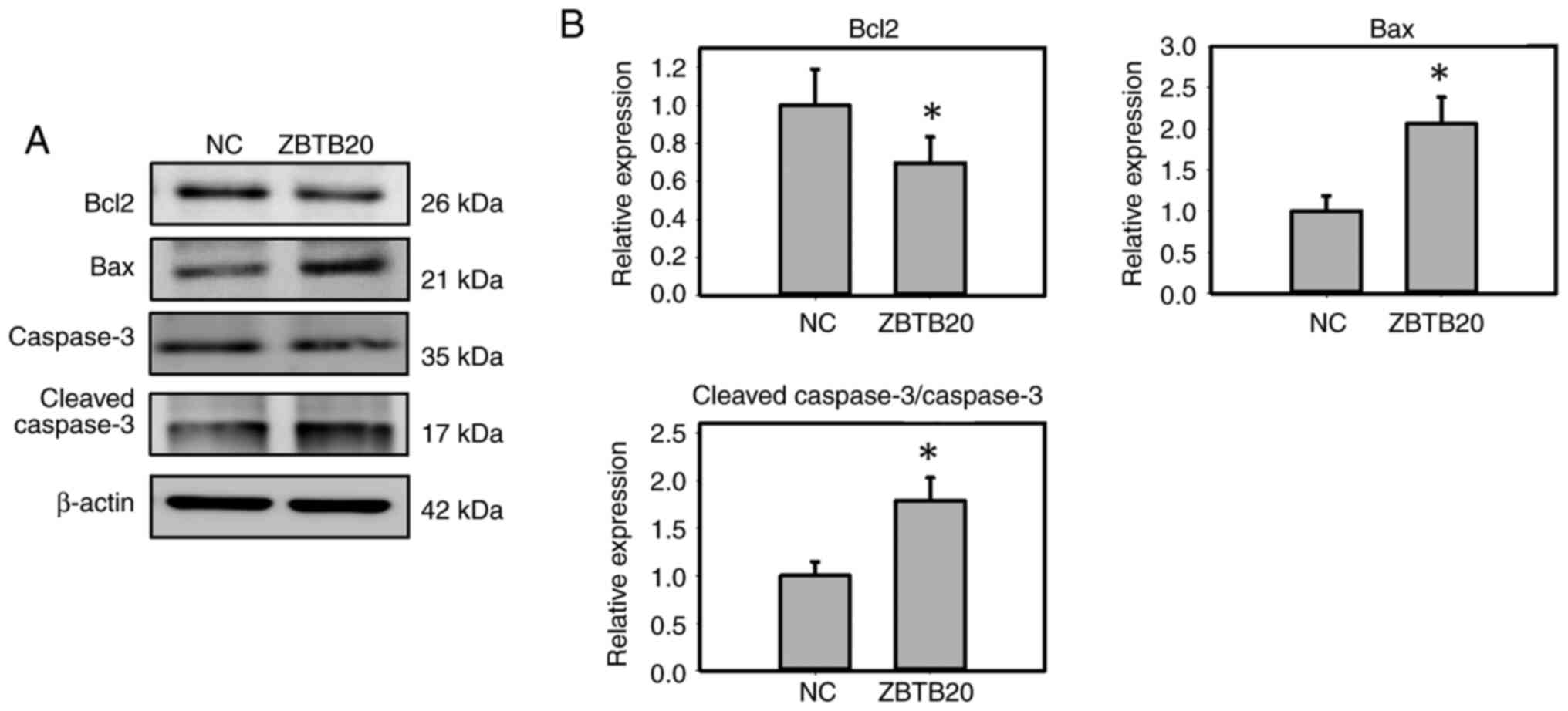
ZBTB20 promotes apoptosis in GBM
cells
To analyze the role of ZBTB20 in apoptosis, the
expression levels of BAX, BCL2 and CASP3 in ZBTB20-overexpressing
GBM cells were detected, and it was found that ZBTB20
overexpression increased the expression of BAX and cleaved CASP3,
whereas it decreased the level of BCL2 (Fig. 3A and B).
ZBTB20 upregulates TET1/FAS/CASP3 in
GBM cells
TET1 is a member of the TET family, which are tumor
suppressor genes in glioma (21–24).
Therefore, the expression levels of ZBTB20 and TET1 in glioma
tissues were analyzed, and a positive correlation between the two
proteins was found in both GBM and LGG tissues (Fig. 4A and B). In vitro, ZBTB20
overexpression increased TET1 expression in GBM cells at both the
mRNA and protein levels (Fig.
4C-E). It was further demonstrated that ZBTB20 overexpression
decreased the expression of p-ERK and its downstream signal, p-CREB
(Fig. 4F and G), indicating that
ZBTB20 may decrease the activity of the ERK signaling pathway.
Previous studies have shown that TET1 is transcriptionally
suppressed via the ERK signaling pathway in non-malignant and lung
cancer cell lines (25,26). Therefore, we hypothesize that ZBTB20
may increase TET1 expression through inactivation of the ERK
signaling pathway in GBM cells.
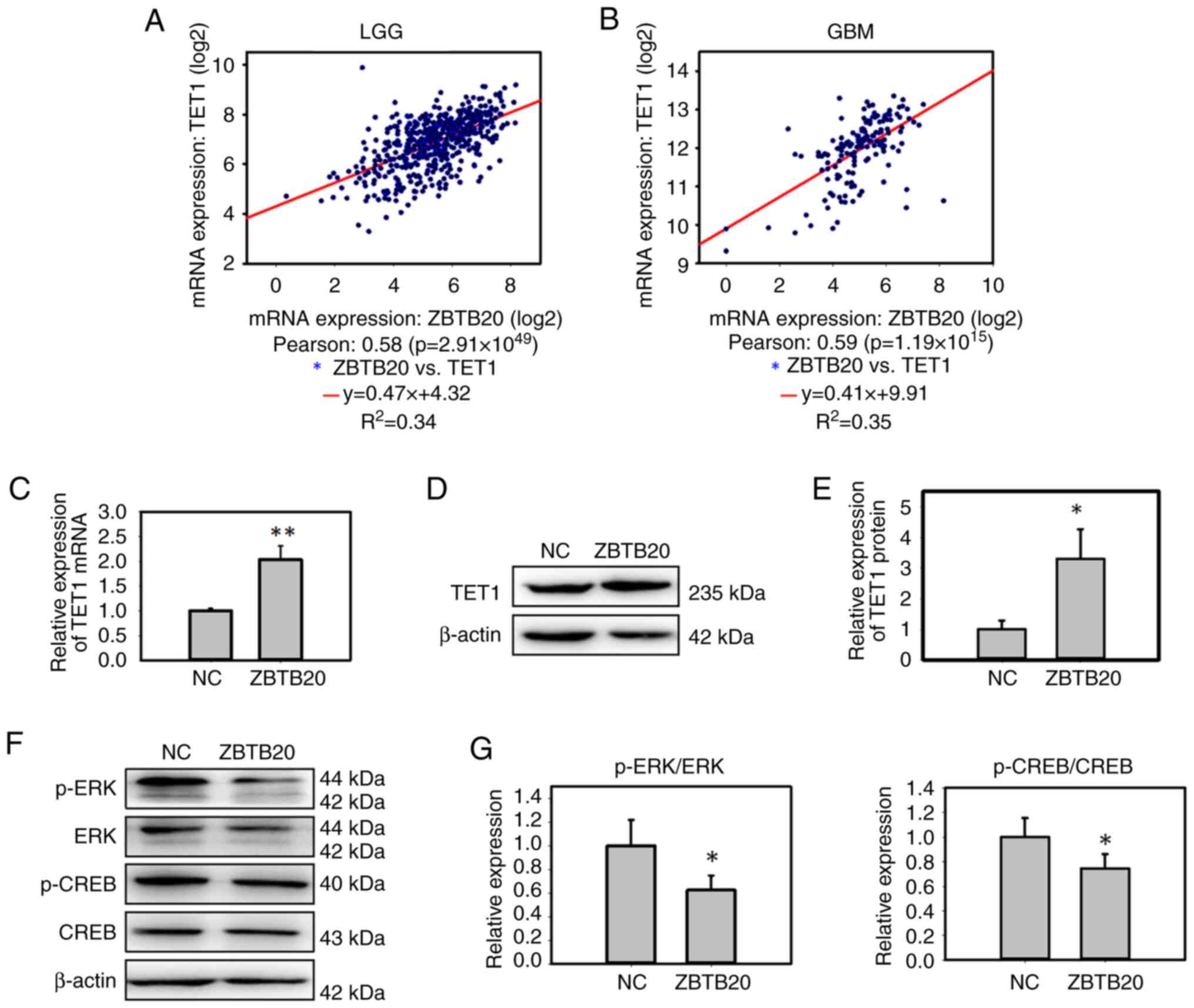 | Figure 4.ZBTB20 increases TET1 expression and
decreases the activity of the ERK signaling pathway in GBM cells.
Analysis of the correlation between ZBTB20 and TET1 transcriptional
expression in (A) LGG and (B) GBM tissues. The results were
downloaded from the cBioPortal. Expression of TET1 in
ZBTB20-overexpressing U251 cells was determined using (C) reverse
transcription-quantitative polymerase chain reaction and (D)
western blotting. (E) Histogram of the western blotting analysis of
ZBTB20-overexpressing U251 cells. (F) Expression of key genes in
the ERK signaling pathway were detected by western blotting, (G)
which were semi-quantified and presented as histograms. All
experiments were independent experiments and were repeated three
times. Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01. ERK, extracellular signal regulated
kinase; CREB, cyclic AMP-responsive-element-binding protein; GBM,
glioblastoma; LGG, lower grade glioma; NC, negative control; p-,
phosphorylated; TET1, ten-eleven translocation 1; ZBTB20, zinc
finger and BTB domain containing 20. |
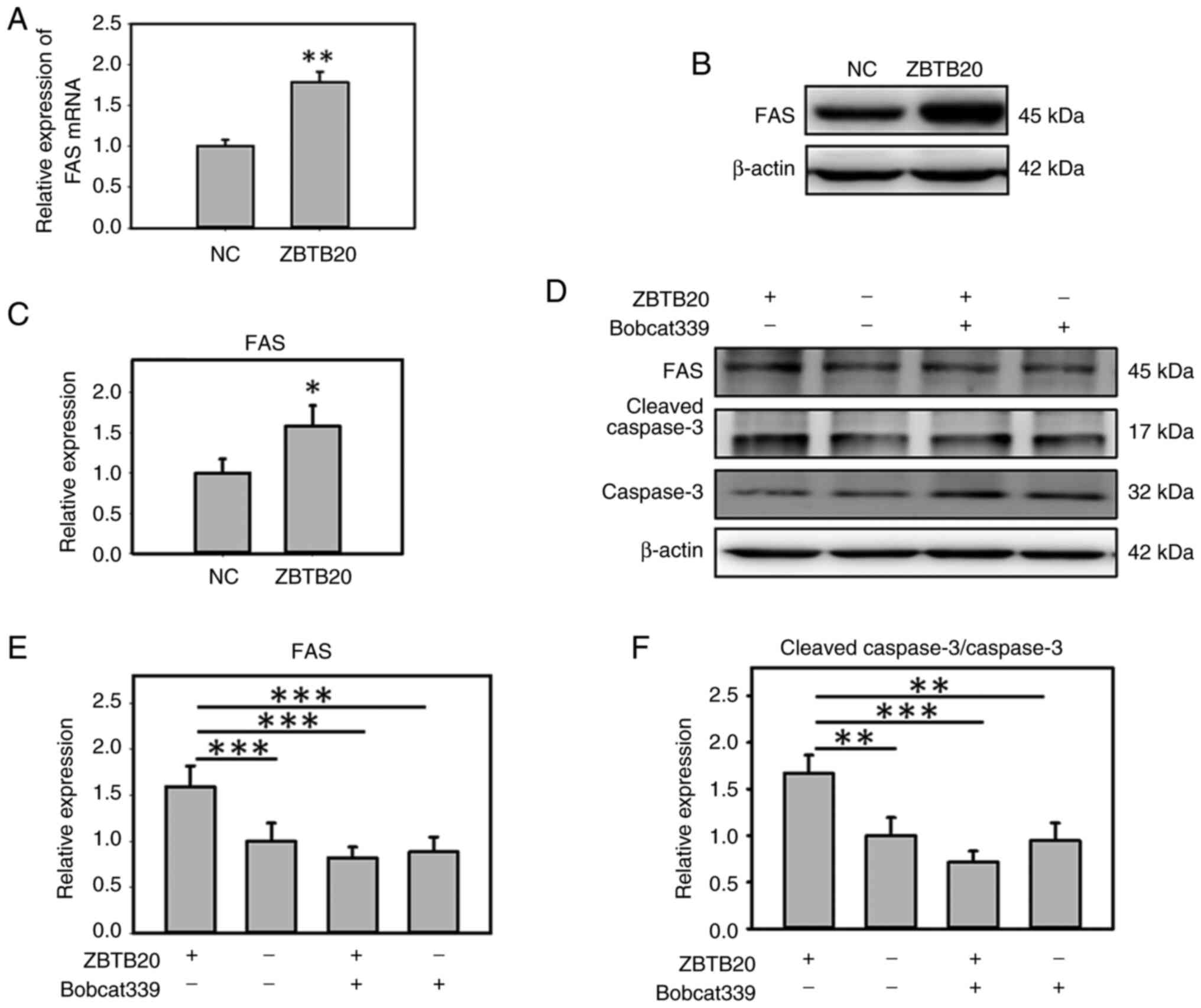
A previous study has reported that TET1 can maintain
the 5-hydroxymethylcytosine (5-hmC)-modified state of the FAS
promoter and hence promote FAS expression (25). The activation of FAS can induce
apoptotic signaling and activate CASP8/10/3/6/7, thus initiating
the apoptotic process (27,28). In the present study, it was
demonstrated that ZBTB20 overexpression increased the expression of
FAS both at the mRNA and protein levels in GBM cells (Fig. 5A-C). To study the possible effect of
TET1 on FAS expression, the TET1 inhibitor, Bobcat339, was used to
treat ZBTB20-overexpressing GBM cells. It was demonstrated that the
TET1 inhibitor decreased the activation of FAS/CASP3 and the
apoptosis process induced by ZBTB20 overexpression (Fig. 5D-F), indicating that ZBTB20
activated the FAS/CASP3 pathway through TET1.
Discussion
At present, the role of ZBTB20 in glioma is largely
unclear. In the present study, it was found that ZBTB20 expression
in GBM (the most malignant type of glioma) was lower than that
measured in LGG. Furthermore, lower ZBTB20 expression was
correlated with a poorer prognosis in patients with GBM. The
clinical characteristics of ZBTB20 therefore indicated that ZBTB20
may be a tumor suppressor gene in GBM. The anticancer effect of the
ZBTB20 gene was further demonstrated in the present study through
gain- and loss-of-function experiments. Overexpression and
knockdown of ZBTB20 promoted and suppressed cell proliferation,
respectively. These results were inconsistent with those previously
reported in a study by Liu et al (16), in which it was demonstrated that
ZBTB20 knockdown inhibited the proliferation of glioma cells. This
divergence might be due to the use of different experimental
methods. For example, Liu et al (16) used transient transfection through
the delivery of chemosynthetic ZBTB20 siRNA into glioma cells and
drew conclusions based on the short-term effect of ZBTB20
knockdown. By contrast, in the present study, cell models with
stable expression of ZBTB20 or shRNA against ZBTB20 were used,
which more closely resemble the in vivo condition for tumor
formation (a long-term process).
It should be noted that the ZBTB20 gene may play
different roles in different tumor types and the mechanisms
involved may be different. Based on previous reports, how ZBTB20
inhibits tumor growth in GBM cannot be explained. However, in the
present study, an interesting finding in the correlation analysis
of gene expression in glioma samples was observed, that is, ZBTB20
was correlated with another tumor suppressor, TET1. TET1 is a
member of the TET protein family, which contains three members
(TET1, TET2 and TET3) (29). These
proteins possess a similar C-terminal catalytic domain, which can
catalyze the transformation of 5-methylcytosine into 5-hmC
(30). Evidence has indicated that
TET1 acts as a tumor suppressor in different glioma types,
including GBM. The levels of 5-hmC, the product of TET1 action,
were markedly depleted in astrocytomas compared with normal brain
(21) and have been associated with
poor survival in patients with anaplastic glioma (22) and GBM (23). TET1 expression in GBM cells inhibits
tumor formation (24), while
downregulation of TET1 promotes glioma cell proliferation and
invasion by targeting the Wnt/β-catenin pathway (31). In the present study, to demonstrate
a possible regulatory relationship between ZBTB20 and TET1, the
expression levels of TET1 and the ERK pathway [which has been shown
to transcriptionally inhibit TET1 expression (25,26)]
in ZBTB20-overexpressing cells were examined. The results
demonstrated that ZBTB20 may promote TET1 expression through
inactivation of ERK pathway. It is also worth noting that
inhibition of ERK signaling may be involved in the ZBTB20-mediated
cell proliferation and apoptosis as a previous study has
demonstrated the role of ERK signaling in cell proliferation,
differentiation, migration, senescence and apoptosis (32). Further evidence also indicates that
ERK signaling is hyperactive in malignant glioma and therefore
targeting ERK signaling may be a novel therapeutic strategy for
targeting malignant gliomas (33).
In addition, the activating pathway of ERK/CREB is associated with
the malignant behaviors of GBM cells (34). ERK inhibition in GBM is associated
with autophagy activation and tumorigenesis suppression (35). The ERK/CREB signaling pathway also
regulates the tumor characteristics of other carcinomas such as
acute myoloid leukemia (36) and
gastric cancer (37). Based on the
aforementioned reports, it may be hypothesized that ZBTB20 inhibits
the proliferation and promote the apoptosis of GBM cells by
inhibiting the ERK/CREB signaling pathway. However, in the present
study, further experiments exploring how ZBTB20 changed the ERK
pathway were not performed.
The results of the present study also implied that
the TET1/FAS/CASP3 pathway was activated in GBM cells by ZBTB20.
FAS, also termed the tumor necrosis factor receptor superfamily 6
gene, encodes a type I transmembrane glycoprotein containing 319
amino acids (38). A previous study
has reported that the FAS promoter is maintained in a
5-hmC-modified state by TET1 and hence, it cannot be silenced by
methylation (25). The activation
of FAS can induce and activate CASP3/6/7/8/9/10, thus initiating
the apoptotic process (28,39–42).
Limitations remain in the present study. Although it
was demonstrated that the ZBTB20 gene inhibited glioma growth
through the TET1/FAS/CASP3 signaling pathway, the mechanism through
which ZBTB20 promotes TET1 expression remains unclear. In addition,
ZBTB20 is regarded as a transcriptional repressor. However, the
genes that ZBTB20 specifically transcriptionally regulates in this
process were not identified in the present study. Another
limitation of the present study is that there were few
observational indicators in the ZBTB20 knockdown group experiments,
such as a lack of detection of apoptosis indicators.
In summary, the results of the present study
demonstrated that low expression of ZBTB20 was associated with a
poorer prognosis in patients with GBM. It was also demonstrated
that ZBTB20 inhibited cell proliferation and promoted apoptosis by
activating the TET1/FAS/CASP3 pathway in GBM cells. Therefore,
ZBTB20 may be a tumor suppressor gene and may serve as a target for
the diagnosis and treatment of glioma.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This project was supported by the Science and Technology
Department of Henan Province (grant no. 201403003).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PD designed the experimental process and contributed
to bioinformatics analyses and cell experiments. YX contributed to
the conception of the study and assisted with performing the
analysis with constructive discussions. YZ contributed to cellular
experiments. BL contributed to cell cycle detection by flow
cytometry. HC and SC contributed to molecular biology testing. All
authors read and approved the final version of the manuscript. PD
and YX confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CGGA
|
Chinese Glioma Genome Atlas
|
|
TCGA
|
The Cancer Genome Atlas
|
|
TET1
|
ten-eleven translocation 1
|
|
ZBTB20
|
zinc finger and BTB domain containing
20
|
|
GBM
|
glioblastoma
|
References
|
1
|
Xie Z, Zhang H, Tsai W, Zhang Y, Du Y,
Zhong J, Szpirer C, Zhu M, Cao X, Barton MC, et al: Zinc finger
protein ZBTB20 is a key repressor of alpha-fetoprotein gene
transcription in liver. Proc Natl Acad Sci USA. 105:10859–10864.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang W, Mi J, Li N, Sui L, Wan T, Zhang
J, Chen T and Cao X: Identification and characterization of DPZF, a
novel human BTB/POZ zinc finger protein sharing homology to BCL-6.
Biochem Biophys Res Commun. 282:1067–1073. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sutherland AP, Zhang H, Zhang Y, Michaud
M, Xie Z, Patti ME, Grusby MJ and Zhang WJ: Zinc finger protein
Zbtb20 is essential for postnatal survival and glucose homeostasis.
Mol Cell Biol. 29:2804–2815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitchelmore C, Kjaerulff KM, Pedersen HC,
Nielsen JV, Rasmussen TE, Fisker MF, Finsen B, Pedersen KM and
Jensen NA: Characterization of two novel nuclear BTB/POZ domain
zinc finger isoforms. Association with differentiation of
hippocampal neurons, cerebellar granule cells, and macroglia. J
Biol Chem. 277:7598–7609. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nielsen JV, Blom JB, Noraberg J and Jensen
NA: Zbtb20-induced CA1 pyramidal neuron development and area
enlargement in the cerebral midline cortex of mice. Cereb Cortex.
20:1904–1914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nielsen JV, Nielsen FH, Ismail R, Noraberg
J and Jensen NA: Hippocampus-like corticoneurogenesis induced by
two isoforms of the BTB-zinc finger gene Zbtb20 in mice.
Development. 134:1133–1140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q, Tan YX, Ren YB, Dong LW, Xie ZF,
Tang L, Cao D, Zhang WP, Hu HP and Wang HY: Zinc finger protein
ZBTB20 expression is increased in hepatocellular carcinoma and
associated with poor prognosis. BMC Cancer. 11:2712011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kan H, Huang Y, Li X, Liu D, Chen J and
Shu M: Zinc finger protein ZBTB20 is an independent prognostic
marker and promotes tumor growth of human hepatocellular carcinoma
by repressing FoxO1. Oncotarget. 7:14336–14349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Zhou X, Zhang M, Cheng L, Zhang Y
and Wang X: ZBTB20 promotes cell migration and invasion of gastric
cancer by inhibiting IkappaBalpha to induce NF-κB activation. Artif
Cells Nanomed Biotechnol. 47:3862–3872. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao JG, Ren KM and Tang J: Zinc finger
protein ZBTB20 promotes cell proliferation in non-small cell lung
cancer through repression of FoxO1. FEBS Lett. 588:4536–4542. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de la Rosa J, Weber J, Friedrich MJ, Li Y,
Rad L, Ponstingl H, Liang Q, de Quirós SB, Noorani I, Metzakopian
E, et al: A single-copy sleeping beauty transposon mutagenesis
screen identifies new PTEN-cooperating tumor suppressor genes. Nat
Genet. 49:730–741. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
de la Rosa J, Weber J, Rad R, Bradley A
and Cadinanos J: Disentangling PTEN-cooperating tumor suppressor
gene networks in cancer. Mol Cell Oncol. 4:e13255502017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lapointe S, Perry A and Butowski NA:
Primary brain tumours in adults. Lancet. 392:432–446. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ostrom QT, Gittleman H, Fulop J, Liu M,
Blanda R, Kromer C, Wolinsky Y, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 17
(Suppl 4):iv1–iv62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Jiang J, Hui X, Wang W, Fang D and
Ding L: Mir-758-5p suppresses glioblastoma proliferation, migration
and invasion by targeting ZBTB20. Cell Physiol Biochem.
48:2074–2083. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cancer Genome Atlas Research Network, .
Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA,
Rheinbay E, Miller CR, Vitucci M, et al: Comprehensive, integrative
genomic analysis of diffuse lower-grade gliomas. N Engl J Med.
372:2481–2498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brennan CW, Verhaak RG, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: The somatic genomic landscape of glioblastoma.
Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao Z, Zhang KN, Wang Q, Li G, Zeng F,
Zhang Y, Wu F, Chai R, Wang Z, Zhang C, et al: Chinese glioma
genome atlas (CGGA): A comprehensive resource with functional
genomic data from Chinese glioma patients. Genomics Proteomics
Bioinformatics. 19:1–12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Orr BA, Haffner MC, Nelson WG,
Yegnasubramanian S and Eberhart CG: Decreased
5-hydroxymethylcytosine is associated with neural progenitor
phenotype in normal brain and shorter survival in malignant glioma.
PLoS One. 7:e410362012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang F, Liu Y, Zhang Z, Li J, Wan Y,
Zhang L, Wang Y, Li X, Xu Y, Fu X, et al: 5-hydroxymethylcytosine
loss is associated with poor prognosis for patients with WHO grade
II diffuse astrocytomas. Sci Rep. 6:208822016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson KC, Houseman EA, King JE, von
Herrmann KM, Fadul CE and Christensen BC: 5-Hydroxymethylcytosine
localizes to enhancer elements and is associated with survival in
glioblastoma patients. Nat Commun. 7:131772016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Forloni M, Gupta R, Nagarajan A, Sun LS,
Dong Y, Pirazzoli V, Toki M, Wurtz A, Melnick MA, Kobayashi S, et
al: Oncogenic EGFR represses the TET1 DNA demethylase to induce
silencing of tumor suppressors in cancer cells. Cell Rep.
16:457–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu BK and Brenner C: Suppression of
TET1-dependent DNA demethylation is essential for KRAS-mediated
transformation. Cell Rep. 9:1827–1840. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thakur S and Brenner C: KRAS-driven
miR-29b expression is required for tumor suppressor gene silencing.
Oncotarget. 8:74755–74766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scaffidi C, Schmitz I, Zha J, Korsmeyer
SJ, Krammer PH and Peter ME: Differential modulation of apoptosis
sensitivity in CD95 type I and type II cells. J Biol Chem.
274:22532–22538. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Neumann L, Pforr C, Beaudouin J, Pappa A,
Fricker N, Krammer PH, Lavrik IN and Eils R: Dynamics within the
CD95 death-inducing signaling complex decide life and death of
cells. Mol Syst Biol. 6:3522010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ito S, D'Alessio AC, Taranova OV, Hong K,
Sowers LC and Zhang Y: Role of Tet proteins in 5mC to 5hmC
conversion, ES-cell self-renewal and inner cell mass specification.
Nature. 466:1129–1133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q,
Ding J, Jia Y, Chen Z, Li L, et al: Tet-mediated formation of
5-carboxylcytosine and its excision by TDG in mammalian DNA.
Science. 333:1303–1307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji J, You Q, Zhang J, Wang Y, Cheng J,
Huang X and Zhang Y: Downregulation of TET1 promotes glioma cell
proliferation and invasion by targeting Wnt/β-catenin pathway. Anal
Cell Pathol (Amst). 2021:89807112021.PubMed/NCBI
|
|
32
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lo HW: Targeting Ras-RAF-ERK and its
interactive pathways as a novel therapy for malignant gliomas. Curr
Cancer Drug Targets. 10:840–848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang F, Xu M, Wang S, Song L, Yu D, Li Y,
Cao R, Xiong Z, Chen Z, Zhang Q, et al: Gain-Of-function
E76K-mutant SHP2 promotes cell proliferation, metastasis, and tumor
growth in glioblastoma through activation of the ERK/CREB pathway.
Onco Targets Ther. 12:9435–9447. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang K, Luan L, Li X, Sun X and Yin J: ERK
inhibition in glioblastoma is associated with autophagy activation
and tumorigenesis suppression. J Neurooncol. 156:123–137. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gong R, Li H, Liu Y, Wang Y, Ge L, Shi L,
Wu G, Lyu J, Gu H and He L: Gab2 promotes acute myeloid leukemia
growth and migration through the SHP2-Erk-CREB signaling pathway. J
Leukoc Biol. 112:669–677. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen J, Li M and Min L: HSPB8 promotes
cancer cell growth by activating the ERK-CREB pathway and is
indicative of a poor prognosis in gastric cancer patients. Oncol
Rep. 39:2978–2986. 2018.PubMed/NCBI
|
|
38
|
Itoh N, Yonehara S, Ishii A, Yonehara M,
Mizushima S, Sameshima M, Hase A, Seto Y and Nagata S: The
polypeptide encoded by the cDNA for human cell surface antigen Fas
can mediate apoptosis. Cell. 66:233–243. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rokhlin OW, Glover RA and Cohen MB:
Fas-mediated apoptosis in human prostatic carcinoma cell lines
occurs via activation of caspase-8 and caspase-7. Cancer Res.
58:5870–5875. 1998.PubMed/NCBI
|
|
40
|
Pirnia F, Schneider E, Betticher DC and
Borner MM: Mitomycin C induces apoptosis and caspase-8 and −9
processing through a caspase-3 and Fas-independent pathway. Cell
Death Differ. 9:905–914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Chun HJ, Wong W, Spencer DM and
Lenardo MJ: Caspase-10 is an initiator caspase in death receptor
signaling. Proc Natl Acad Sci USA. 98:13884–13888. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suita H, Shinomiya T and Nagahara Y:
Caspase-6 induces 7A6 antigen localization to mitochondria during
FAS-induced apoptosis of Jurkat cells. Anticancer Res.
37:1697–1704. 2017. View Article : Google Scholar : PubMed/NCBI
|