Introduction
Colorectal cancer (CRC) ranks as the third most
frequently diagnosed cancer and the fourth leading cause of
cancer-related mortality worldwide (1,2).
Surgical resection remains the preferred treatment strategy for
early-stage CRC, whilst systemic therapies, such as targeted
therapies and immunotherapy, have demonstrated promising outcomes
(3). Nonetheless, treatment options
for patients with advanced CRC recurrence and metastases remain
limited, particularly for those unable to withstand
chemotherapy.
Brucea javanica oil emulsion (BJOE) is
derived from a product that is extracted from the seeds of B.
javanica and emulsified by purified soybean lecithin (4). An increasing number of studies have
revealed the desirable antitumor effects of BJOE (5–7).
Studies have reported that BJOE may have a regulatory role in a
number of pathways, including MAPK and NF-κB signaling, the late
endosomal/lysosomal adaptor and MAPK and mTOR activator
3/mTOR/autophagy related protein 13 signaling axis and the PI3K/Akt
signaling pathway, as well as several other antitumor mechanisms,
resulting in a wide array of antitumor effects (8–10).
Moreover, BJOE is widely used in combination regimens to enhance
antitumor efficacy. For example: The combination of BJOE with
anti-programmed cell death protein-1 therapy has been reported to
markedly inhibit the growth of melanoma (11); BJOE in combination with radiation
increased the number of DNA double-strand breaks in esophageal
squamous cell carcinoma cells (12); and BJOE combined with chemotherapy
demonstrated an improved clinical efficacy compared with
chemotherapy alone in the treatment of malignant pleural effusion
(13).
Aidi injection (ADI) is a product derived from
natural medicinal sources that is composed of extracts from
Mylabris phalerata, Astragalus membranaceus, Panax ginseng
and Acanthopanax senticosus. The clinical antitumor efficacy
of Aidi injection is widely acknowledged in China, with the
treatment assessed for its positive impact on cancer survival
rates, tumor response, patient quality of life, and its ability to
mitigate the adverse effects of chemotherapy and radiotherapy,
especially in the treatment of lung cancer, liver cancer and CRC
(14). Furthermore, for patients
with advanced CRC, ADI has been reported to notable reduce the risk
of common chemotherapy side effects such as nausea, vomiting,
diarrhea, leukopenia and thrombocytopenia (15,16).
In the antitumor process, ADI promotes apoptosis and inhibits
angiogenesis and cell proliferation (17–19).
ADI also exhibits a protective effect on normal cells and tissues
(20).
The current report presents the case of a patient
with colon cancer with resectable lung metastasis that was treated
with BJOE + ADI following surgical resection of the metastatic
lesion.
Case report
A 69-year-old male patient presented to Fudan
University Shanghai Cancer Center (Shanghai, China) in March 2015
with increased bowel movements of 2–3 times daily, which was
accompanied by fresh blood and occasional abdominal pain. A
colonoscopy revealed a mass of 25–30 cm from the anal verge, which
occupied 75% of the circumference of the bowel lumen. Preoperative
pelvic computed tomography (CT) showed no significant abnormalities
(data not shown); however, abdominal magnetic resonance imaging
(MRI) identified a suspicious signal in segment S7 of the liver,
hepatic cysts and a slightly thickened gallbladder wall (data not
shown). In addition, chest CT demonstrated a pulmonary bulla in the
upper lobe and fibrotic lesions at the base of the right lung (data
not shown). Consequently, the patient underwent an open left
hemicolectomy in April 2015. The subsequent pathology results,
performed and interpreted by professional pathologists and
technicians at the Pathology Department of Fudan University
Shanghai Cancer Center, indicated that the tumor, located in the
transverse colon and classified as a polypoid type, measured
4.5×5.5×1.0 cm. The tumor was histologically categorized as a
mucinous adenocarcinoma based on the histological examination,
which adhered to the World Health Organization criteria (21). The histopathological examination was
conducted as follows: The tissue samples were fixed within 30 min
post-excision using a 10% neutral buffered formalin solution, with
the fixative volume being 10 times that of the tissue; the fixation
duration was 24 h at room temperature. Subsequently, 4-µm sections
were prepared from the paraffin-embedded tissues. Prior to
microscopy, the sections were stained using a standard hematoxylin
and eosin (H&E) staining protocol to visualize cellular
structures. The stained sections were observed under an Olympus
BX51 light microscope (Olympus Corporation). An examination of 13
lymph nodes revealed no signs of metastatic cancer, with the
specific counts being 0/1 for proximal tumor lymph nodes, 0/2 for
distal tumor lymph nodes, 0/5 for peritumoral lymph nodes and 0/5
for mesenteric lymph nodes. The colon tumor had invaded the
subserosal layer without evidence of vascular or neural invasion,
and the surgical margins were free of cancer cells (data not
shown). The microsatellite status was microsatellite stable (data
not shown). Immunohistochemistry of the tumor tissues, performed
and interpreted by professional technicians at the Pathology
Department of Fudan University Shanghai Cancer Center, demonstrated
p21 (+), p53 (+), CD44 (+), human epidermal growth factor receptor
2 (partially +), cyclooxygenase-2 (+), E-cadherin (+), Ki-67 (+;
70%), human mutL homolog-1 (+++), human mutS homolog (hMSH)6 (++),
postmeiotic segregation increased 2 (+++), EGFR (−), B-cell
lymphoma 2 (−), multi-drug resistance protein (−), topoisomerase II
(+), glutathione-S-transferase π (+) and hMSH2 (+++) (data
not shown). The 4-µm pathological sections were prepared as
aforementioned, and immunohistochemistry was performed as follows:
0.1% Triton X-100 was used for permeabilization at room temperature
for 10 min after cell fixation. The sections were then blocked with
5% BSA (cat. no. A7030; MilliporeSigma) at 4°C overnight and were
incubated with the following primary antibodies (Abcam): p21 (clone
EPR362; cat. no. ab109520; dilution 1:200), p53 (clone Y5; cat. no.
ab32049; dilution 1:200), CD44 (clone EPR1013Y; cat. no. ab51037;
dilution 1:500), human epidermal growth factor receptor 2 (clone
EP1045Y; cat. no. ab134182; dilution 1:500), cyclooxygenase-2
(clone EPR12012; cat. no. ab179800; dilution 1:500), E-cadherin
(clone EP700Y; cat. no. ab40772; dilution 1:500), Ki-67 (clone SP6;
cat. no. ab16667; dilution 1:300), human mutL homolog-1 (clone
EPR3894; cat. no. ab92312; dilution 1:200), hMSH6 (clone EPR3945;
cat. no. ab92471; dilution 1:200), postmeiotic segregation
increased 2 (clone EPR3947; cat. no. ab110638; dilution 1:200),
EGFR (clone EP38Y; cat. no. ab52894; dilution 1:500), B-cell
lymphoma 2 (clone 100:D5; cat. no. ab692; dilution 1:200),
multi-drug resistance protein (clone EPR10364-57; cat. no.
ab170904; dilution 1:250), topoisomerase II (clone EPR5377; cat.
no. ab109524; dilution 1:500), glutathione-S-transferase π
(clone EPR4236; cat. no. ab138491; dilution 1:200) and hMSH2 (clone
3A2B8C; cat. no. ab52266; dilution 1:200). All primary antibodies
were incubated at 4°C overnight. Subsequently, the sections were
incubated with secondary antibodies (1:1,000; Goat Anti-Rabbit IgG
H&L, cat. no. ab205718; Goat Anti-Mouse IgG H&L, cat. no.
ab6708; both from Abcam) at room temperature for 1 h. The DAB
chromogenic agent was purchased form Dako; Agilent Technologies,
Inc. (cat. no. K5007). The duration of DAB staining was 10 min at
room temperature. The sections were detected under an Olympus BX51
microscope. Following integration of the pathological findings and
immunohistochemical profile, the postoperative stage of the patient
was determined to be p-T3N0M0 (Tumor-Node-Metastasis Staging
System; American Joint Committee on Cancer, eighth edition)
(22), corresponding to stage IIa,
with proficient mismatch repair and no high-risk features
identified. Based on the intraoperative findings and postoperative
pathology results, the attending physicians from Fudan University
Shanghai Cancer Center recommended that the patient receive
adjuvant chemotherapy with 8 cycles of single-agent
Xeloda® (capecitabine) to reduce the risk of cancer
recurrence. Consequently, the patient received 8 cycles of
chemotherapy with Xeloda (3,500 mg, orally, days 1–14, every 3
weeks) from May 2015, and subsequently received regular
follow-ups.
In May 2016, during follow-up at Fudan University
Shanghai Cancer Center, several nodules were found in the upper
lobe of the left lung during the chest CT examination required for
periodic review (data not shown). The main symptoms of the patient
were fatigue, diarrhea, abnormal appetite and poor sleep quality,
and a physical assessment indicated that the abdomen was palpable
without any tenderness or rebound tenderness throughout. A
well-healed 15-cm surgical scar was also observed in the lower left
abdomen. In addition, there was no evidence of shifting dullness,
normal bowel sounds were present, and the abdominal reflexes
remained intact. The medical, family and psycho-social history of
the patient contained no relevant genetic information. At this
time, the patient presented to Yueyang Hospital of Integrated
Traditional Chinese and Western Medicine, Shanghai University of
Traditional Chinese Medicine (Shanghai, China) for further
diagnosis and treatment. Under the advice and guidance of a
Traditional Chinese Medicine physician, the patient took a Chinese
herbal decoction to relieve the aforementioned symptoms. A total of
3 months later, the lung nodules appeared to have progressed, with
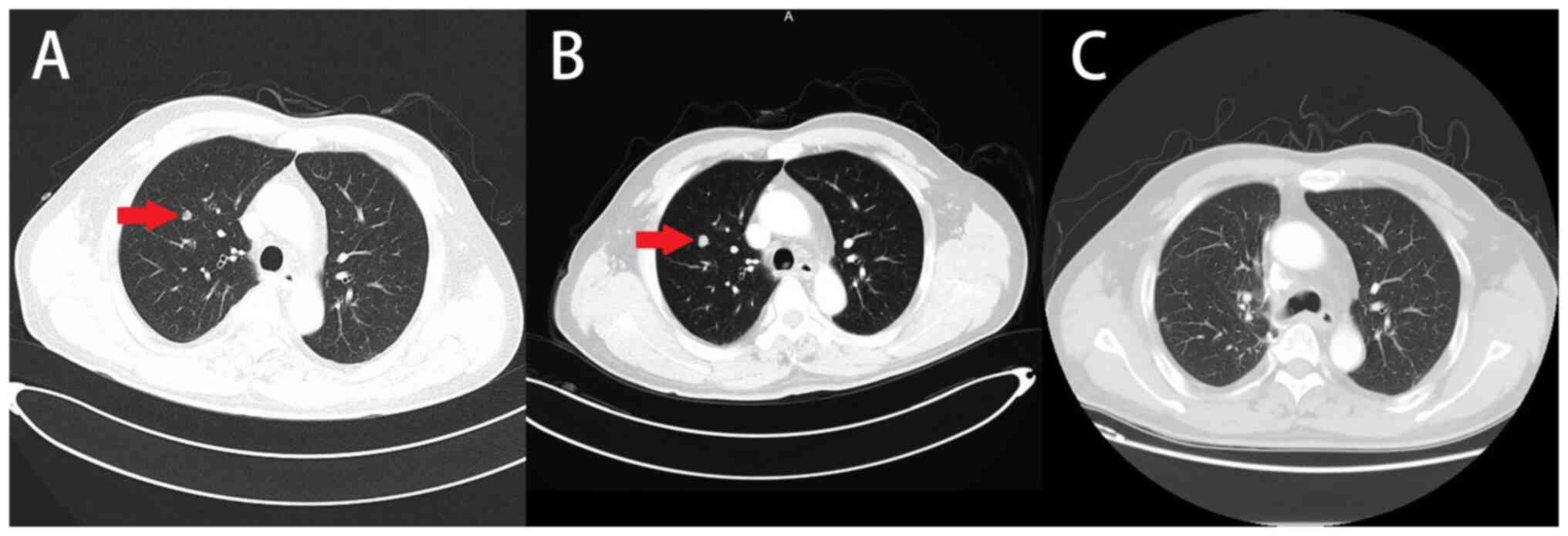
the largest nodule measuring 8-mm in diameter (Fig. 1A). At that time, the patient refused
to undergo further examinations, such as positron emission
tomography/CT, and opted for regular outpatient visits as follow-up
instead, as there was insufficient radiological evidence to suggest
pulmonary metastases. In January 2017, concerned about the
potential for further enlargement of the lung nodule, the patient
underwent a CT scan at Shanghai Pulmonary Hospital (Shanghai,
China), which revealed a nodule in the right upper lobe of the
lung, measuring 1 cm in diameter (Fig.
1B). The medical team, considering the patient's past medical
history, suspected lung metastasis. After completing a thorough
examination and ruling out contraindications for surgery, the
patient underwent single-port video-assisted thoracic surgery for
right upper lobectomy and lymphatic clearance. The postoperative
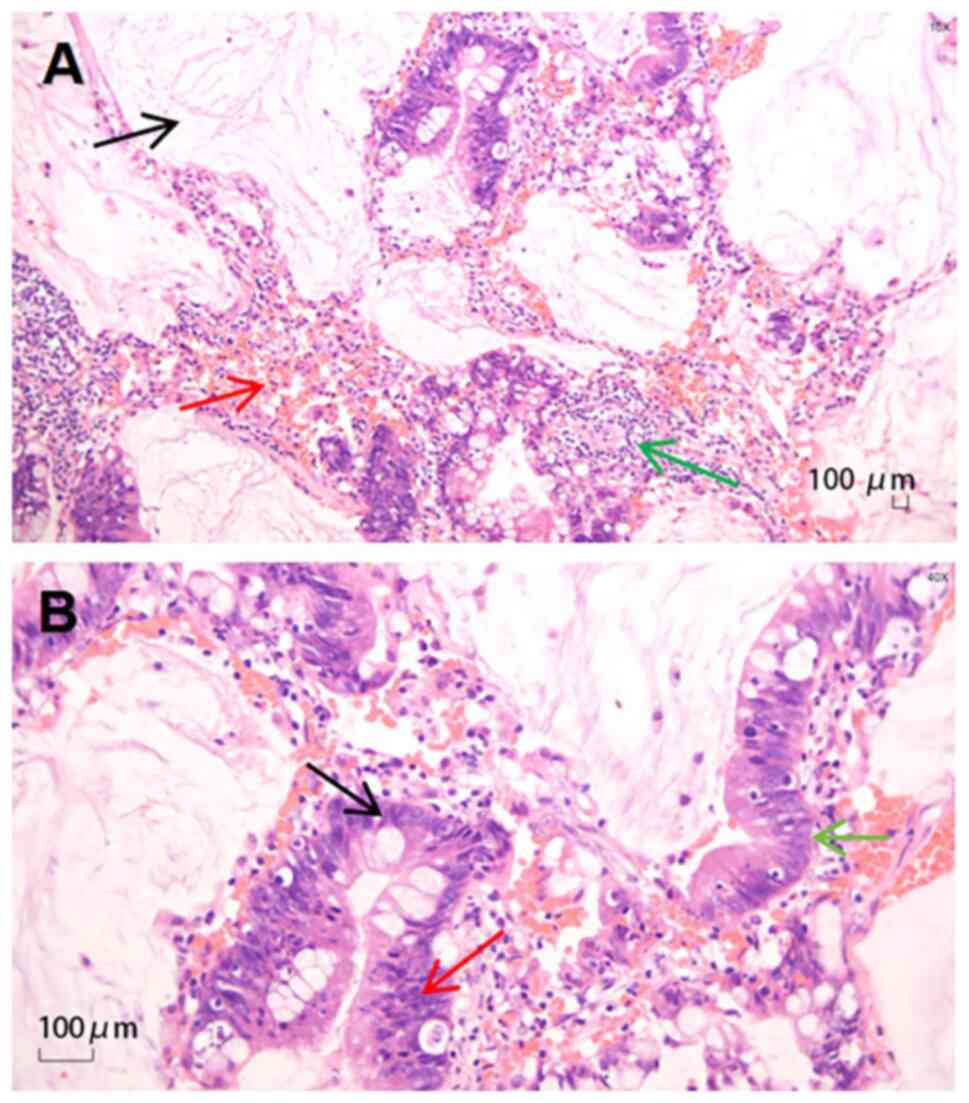
pathology, performed and interpreted by professional pathologists
and technicians at the Pathology Department of Shanghai Pulmonary
Hospital, indicated mucinous adenocarcinoma (Fig. 2). The histopathological examination
was conducted as follows: After fixation in 10% neutral formalin
solution for 24 h at room temperature, dehydration was performed
using absolute ethanol, followed by permeabilization with xylene at
room temperature for 10 min. The samples were then embedded in
regular paraffin, and 4-µm sections were cut and mounted on neutral
gum-coated slides. The sections were observed under an Olympus BX51
microscope. Immunohistochemical staining revealed cytokeratin
(CK)5/6 (−), CK7 (+), CK20 (+), thyroid transcription factor-1 (−),
p40 (−), CDK-2 (partially +) and Villin (+). The sections were
prepared as aforementioned for immunohistochemistry, and the
following antibodies (Abcam) were used: CK5 (clone EP1601Y; cat.
no. ab52635; dilution 1:200), CK6 (clone EPR1602Y; cat. no.
ab93279; dilution 1:200), CK7 (clone EPR17078; cat. no. ab181598;
dilution 1:500), CK20 (clone EPR1622Y; cat. no. ab76126; dilution
1:500), thyroid transcription factor-1 (clone EP1584Y; cat. no.
ab76013; dilution 1:500), p40 (clone EP2142Y; cat. no. ab76158;
dilution 1:200), CDX-2 (clone SP54; cat. no. ab101532; dilution
1:200) and Villin (clone SP145; cat. no. ab130751; dilution 1:200).
All primary antibodies were incubated at 4°C overnight. Secondary
antibody (goat anti-rabbit IgG H&L; cat. no. ab182016; dilution
1:1,000; goat anti-rabbit IgG H&L HPR; cat. no. Ab205718;
dilution 1:1,000) incubation, DAB staining and microscopy were
performed as aforementioned for immunohistochemistry. Positive
staining for CK7 and CK20 confirmed the presence of adenocarcinoma,
whilst partial positivity for CDK-2 suggested a potential
gastrointestinal origin (data not shown). Together with the
patient's history and enzyme markers, this supported the diagnosis
of pulmonary metastasis from colon cancer. At a follow-up in March
2017, it was confirmed that the nodule had been removed by chest CT
scan (Fig. 1C).
Subsequently, the patient received another round of
treatment: One cycle of XELOX [230 mg oxaliplatin, intravenous
drip, day 1 + 3,500 mg capecitabine, orally, days 1–14, every 3
weeks] in February 2017. However, the patient subsequently
developed severe abdominal pain and experienced an impaired grade I
alanine transaminase status, with an alanine transferase (ALT)
level of 292 U/l (normal range, 7–40 U/l) (23). Although the liver function returned
to normal (ALT, 37 U/l; tested in March 2017) and the abdominal
pain was relieved following treatment with an injection of 200 mg
magnesium isoglycyrate + 1.8 g reduced glutathione (intravenous
drip, days 1–10), the patient refused the original treatment plan
due to concerns regarding adverse reactions. Subsequently, the
regimen was adjusted to 550 mg irinotecan in March 2017
(intravenous drip, day 1, every 3 weeks). However, the patient
experienced a grade IV neutrophil count decrease
(0.1×109/l; normal range, 1.8–6.3×109/l)
following this regimen. Therefore, a 100 mg
tegafur-gimeracil-oteracil potassium capsule (orally, days 1–14,
every 3 weeks) for four cycles of chemotherapy (April-July 2017)
was administered. However, during cycles 3 and 4, the patient
developed myelosuppression again and showed a deteriorating trend
with a persistent decrease in platelet count to 36×109/l
(normal range, 125–350×109/l); thus, the chemotherapy
program was suspended. The patient was requested to attend regular
follow-ups.
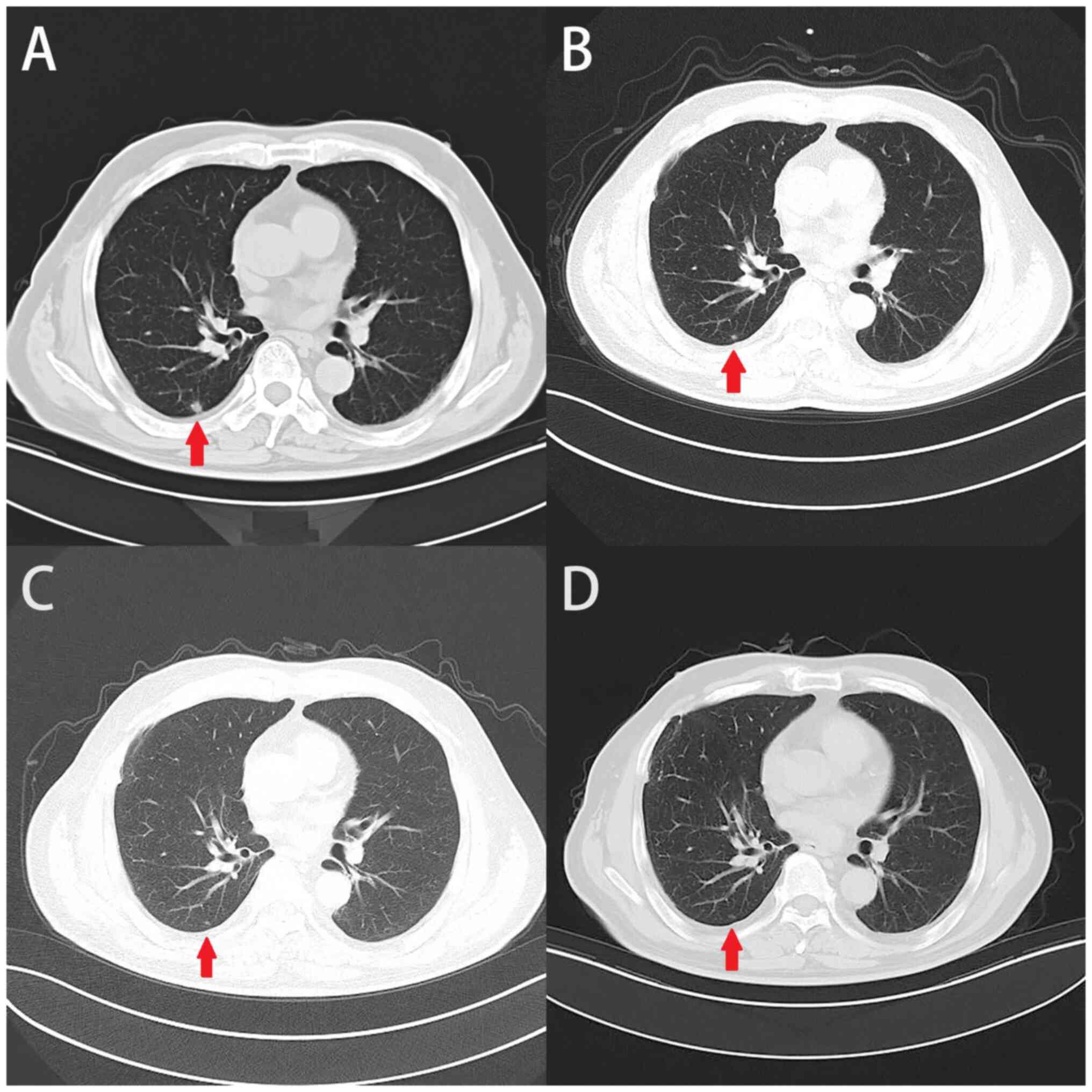
In September 2018, a new nodule in the dorsal
segment of the lower lobe of the right lung was observed by chest
CT scan (Fig. 3A). Due to the
distant metastases and intolerance to chemotherapy, the patient
appeared to be nearing termination of treatment. However, to
prolong the survival of the patient as much as possible, a 3-cycle
BJOE + ADI treatment (30 ml BJOE + 80 ml ADI, intravenous drip, on
days 1–8, every 4 weeks) was administered, which has been reported
to have an antitumor effect and few adverse reactions. This
combination is widely used in clinical practice and has
demonstrated promising application value (24). In the subsequent review period
(December 2018), an unexpected decrease in the nodule size was
observed (Fig. 3B).
The treatment regimen of the patient, including the
timing, medication, dosage and administration of therapies, is
presented in Table I. A chest CT
was performed every 3 months to monitor the changes in lung
lesions, an abdominal CT or MRI was performed every 3 months and a
colonoscopy was performed every 12 months to follow-up the
abdominal condition of the patient. During regular follow-up, no
tumor progression was observed (Fig. 3C
and D).
 | Table I.Treatment details. |
Table I.
Treatment details.
| Treatment date | Cycle | Treatment | Clinical outcome | Adverse event |
|---|
| 2015/5-2015/12 | 1st-8th | Xeloda (3,500 mg,
orally, days 1–14, q3w) (adjuvant chemotherapya) | - | - |
| 2017/2 | 1st | Oxaliplatin (230 mg,
ivgtt, d1) + capecitabine (3,500 mg, po, d1-14, q3w) | - | ALT increased, grade
IIIb |
| 2017/3 | 1st | Irinotecan (550 mg,
ivgtt, d1, q3w) | - | Neutrophil count
decreased, grade IV |
| 2017/4 | 1st | S-1 (100 mg, po,
d1-14, q3w) | - | - |
| 2017/5 | 2nd | S-1 (100 mg, po,
d1-14, q3w) | - | - |
| 2017/6 | 3rd | S-1 (100 mg, po,
d1-14, q3w) | - | Platelet count
decreased, grade II |
| 2017/6 | 4th | S-1 (100 mg, po,
d1-14, q3w) | - | Platelet count
decreased, grade II |
| 2017/7 | 5th | S-1 (100 mg, po,
d1-14, q3w) | 2017/8 CT: SD | Platelet count
decreased, grade III |
| 2018/9 | 1st | 30 ml BJOE + 80 ml
ADI (ivgtt, d1-d8, q4w) | 2018/9 CT: PD
(baseline) | - |
| 2018/10 | 2nd | 30 ml BJOE + 80 ml
ADI (ivgtt, d1-d8, q4w) | - | - |
| 2018/11 | 3rd | 30 ml BJOE + 80 ml
ADI (ivgtt, d1-d8, q4w) | 2018/12 CT: PR | - |
CT in the present case was performed using the
following parameters: A slice thickness of 5 mm, a reconstruction
interval of 1.25 mm and exposure parameters set with a tube voltage
of 120 kVp, employing automatic current modulation for broad-beam
exposure. Consistency was maintained across all follow-ups by using
the same CT machine and medical team for scan collection. The liver
and kidney function of the patient was assessed before and after
the BJOE + ADI intervention, as well as other clinical indicators
such as routine blood (leukocyte, neutrophil, platelet and
hemoglobin) and tumor markers (Table
II). However, the results indicated that there were no
significant changes in the liver and kidney function, and no
notable adverse reactions were observed. The treatment history is
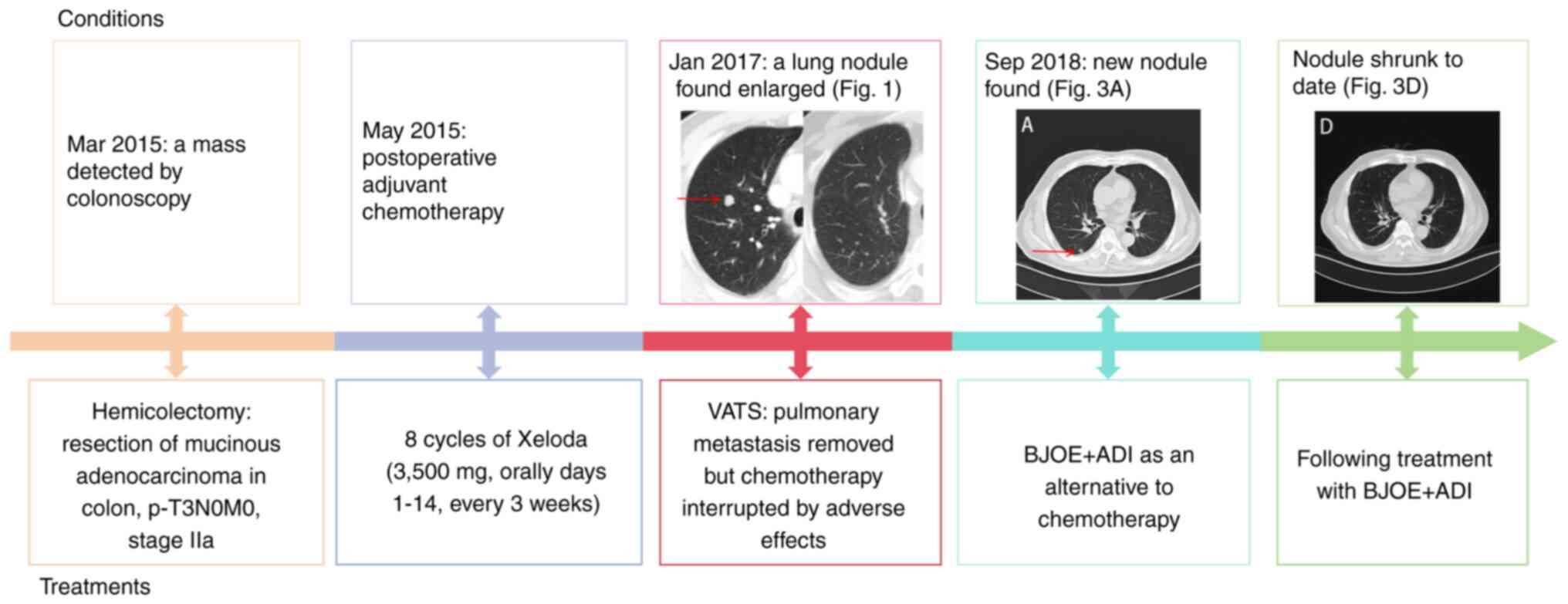
shown in Fig. 4, and it is revealed
that, from the first injection of BJOE + ADI in 2018 until the
present day, the patient has >5 years of progression-free
survival (PFS), which is uncommon for a patient with distant
metastases and intolerance to chemotherapy, as the overall 5-year
survival rate for stage IV CRC is ~15.1% (25).
 | Table II.Clinical indicators of the patient
during the treatment of BJOE + ADI. |
Table II.
Clinical indicators of the patient
during the treatment of BJOE + ADI.
| Indicator | Normal range | 2018/9 | 2018/11 | 2018/12 | 2019/1 | 2019/2 | 2019/3 | 2019/7 | 2019/11 |
|---|
| CEA, ng/ml | ≤5 | 6.30 | 5.20 | 4.90 | 2.20 | 2.60 | 2.70 | 2.50 | 2.70 |
| CA 19-9, U/ml | ≤30 | 29.4 | 26.3 | 22.5 | 20.6 | 30.8 | 25.2 | 29.5 | 30.7 |
| WBCs,
×109/l | 3.5–9.5 | 5.8 | 4.9 | 4.5 | 7.1 | 5.1 | 5.2 | 8.1 | 4.5 |
| PLTs,
×109/l | 125-350 | 121 | 111 | 123 | 119 | 108 | 112 | 135 | 125 |
| ALT, U/l | 9-52 | 17 | 22 | 25 | 19 | 25 | 13 | 12 | 22 |
| Cr, µmol/l | 46-92 | 75 | 88 | 82 | 85 | 78 | 82 | 83 | 84 |
Discussion
The advanced stage of CRC often poses significant
challenges for achieving effective control of metastasis and
progression, which causes a mortality rate that is hard to
mitigate. Furthermore, when distant metastases develop, patients
are typically faced with a poor prognosis (26). Compounding the issue, the patient
described in the present study was unable to tolerate chemotherapy.
During the development of the treatment plan, the healthcare team
underwent significant deliberation and encountered moments of
uncertainty; however, through persistent communication and careful
consideration of alternative options, the medical team was able to
offer the patient a combination of two traditional Chinese
medicinal products. The subsequent response of the patient to this
treatment was encouraging, highlighting the potential of these
therapies in prolonging the survival of patients with advanced CRC.
Nevertheless, it should be acknowledged that chemotherapy remains
the mainstay of CRC treatment, particularly for advanced and
metastatic cases. However, certain patients may not tolerate
chemotherapy well, or may develop resistance or recurrence
following chemotherapy (27). In
the present case, BJOE + ADI was used as an alternative to
chemotherapy as the patient could not tolerate chemotherapy and
developed a new pulmonary nodule following resection surgery.
Whilst it is not suggested that this combination treatment could
replace chemotherapy for all patients with CRC, it may provide a
possible option for those who cannot benefit from chemotherapy.
To contextualize the findings of the present study,
the existing literature was reviewed for similar cases. Several
reports have documented a prolonged PFS in patients with advanced
cancer using complementary and alternative therapies (28). A meta-analysis of 52 studies
reported that ADI, when used as an adjunctive therapy in
combination with modern medicine, may have a complementary
beneficial role in improving survival time, tumor response and
quality of life, and reducing the side effects of
chemoradiotherapy. The most commonly studied types of cancer were
lung cancer, liver cancer and CRC (14). By summarizing these studies, the
understanding of the potential benefits and limitations of such
treatment can be improved. Additionally, a comparative analysis
with other cases would provide valuable insights into the efficacy
and safety profiles of these herbal products.
The antitumor mechanisms of BJOE and ADI remain
speculative; however, based on the available evidence, we
hypothesize the following: Both BJOE and ADI may have enhanced the
immune response of the patient. BJOE contains active compounds that
stimulate natural killer cells and promote cytokine production. In
addition, ADI has been associated with increased T-cell activation
and improved tumor surveillance (29). It was also been reported that the
combination of bruceitol and cisplatin may enhance the antitumor
effect of cisplatin and induce apoptosis of CT-26 colon cancer
cells (30). Furthermore, a
previous study screened the optimal compound formulation of ADI
(covalent-organic framework: Composed of cantharidin,
calyptoflavone-7-O-β-D-glucoside, ginsenoside Rc and ginsenoside
Rd; molar ratio, 1:12:12:8). It was reported that this formulation
exhibited a marked synergistic effect, leading to the inhibition of
cancer cell viability, increased cell death and induction of
apoptosis in nude mice models with liver cancer and CRC.
Additionally, the formulation reduced mitochondrial membrane
potential levels, promoted cytochrome c leakage and reduced tumor
volume and weight (31).
In clinical practice, BJOE or ADI are often used as
an alternative to chemotherapeutic agents in certain patients who
cannot tolerate chemotherapy. These drugs exhibit a potent
inhibitory effect on tumors, leading to prolonged stable disease in
patients (14,28). Notably, the patient described in the
present study attained an extended period of PFS following the use
of this drug combination. This was an unexpected outcome that
serves as a reminder of the importance of re-evaluating the
antitumor potential of Chinese medicine, particularly when
considering its safety profile. Nonetheless, the present case
report does have limitations, such as the lack of molecular
biomarkers; however, the present case report provides valuable
evidence for the potential benefits of administering BJOE and ADI
in patients with CRC with lung metastases. Further studies that
investigate the intricate antitumor mechanisms underlying this drug
combination are required, to potentially offer new therapeutic
options and renewed hope for patients with advanced cancer who
cannot tolerate chemotherapy.
Acknowledgements
Not applicable.
Funding
The present work was supported by the High Level of Peak Plateau
(Shanghai Municipal Education Commerce, grant no. KY110.01.400),
the Shanghai Frontiers Science Center of Disease and Syndrome
Biology of Inflammatory Cancer Transformation (grant no.
2021KJ03-12), the Shanghai Sailing Program (grant no. 21YF1448200),
the National Natural Science Foundation of China (grant no.
82305333) and the Shanghai 2022 ‘Science and Technology Innovation
Action Plan’ medical innovation research project (grant no.
22Y31920103).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JS and HZ took the lead in writing the original
draft of the manuscript. JS, HZ and CS contributed to data curation
and charted the course of the disease by dedicating their efforts
to data collection and the case study. JS, LJ and YG participated
in the discussion and analysis of the case, contributing to
charting the course of the disease. JY and LX were responsible for
the conceptualization of the research and the development of the
methodology. JY and LX confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present case report was approved by The Ethics
Committee of Yueyang Hospital (Shanghai, China; approval no.
2023-150).
Patient consent for publication
The patient provided written informed consent to
publish the present case report, including the publication of
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y,
Chen H and Dai M: Incidence, mortality, survival, risk factor and
screening of colorectal cancer: A comparison among China, Europe,
and northern America. Cancer Lett. 522:255–268. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma R, Abbasi-Kangevari M, Abd-Rabu R,
Abidi H, Abu-Gharbieh E, Acuna JM, Adhikar S, Advan SM, Sohail
Afzal M, Meybodi MA, et al: Global, regional, and national burden
of colorectal cancer and its risk factors, 1990–2019: A systematic
analysis for the Global Burden of Disease Study 2019. Lancet
Gastroenterol Hepatol. 7:627–647. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruff SM, Brown ZJ and Pawlik TM: A review
of targeted therapy and immune checkpoint inhibitors for metastatic
colorectal cancer. Surg Oncol. 51:1019932023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fuhong D, Xiang G, Haiying L, Jiangye W,
Xueming G and Wenxiao C: Evaluation of efficacy and safety for
Brucea javanica oil emulsion in the control of the malignant
pleural effusions via thoracic perfusion. BMC Cancer. 18:4112018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Wang H, Cao L, Wu J, Lu T, Li S
and Li J: Efficacy and safety of Brucea Javanica oil
emulsion injection in the treatment of gastric cancer: A systematic
review and meta-analysis. Front Nutr. 8:7841642021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen J, Chen S, Yang X, Wang S and Wu W:
Efficacy and safety of Brucea javanica oil emulsion
injection as adjuvant therapy for cancer: An overview of systematic
reviews and meta-analyses. Phytomedicine. 102:1541412022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou H, Wang Q, Jiao L, Bi L, Sang S, Han
Y, Gan S, Liu R, AG and Gong Y: Intrapleural injection of Brucea
javanica oil emulsion provided a long-term benefits in patient
with malignant pleural effusion from pleural mesothelioma: A case
report. Explore (NY). 20:126–129. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye L, Zhao JF, Wang YM, Chen WH, Qian S,
Zhou ZG and Xu M: Brucea javanica oil emulsion suppresses
tumor growth in human cervical cancer cells through inhibition of
the E6 oncogene and induction of apoptosis. Transl Cancer Res.
9:918–929. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Chen B, Xiao M, Wang X and Peng Y:
Brucea javanica oil emulsion promotes autophagy in ovarian
cancer cells through the miR-8485/LAMTOR3/mTOR/ATG13 signaling
axis. Front Pharmacol. 13:9351552022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Yin SL, Wang LH, Jia LN, Su GY,
Liu XQ, Zhou F, Breslin P, Meng R, Li QY, et al: Seed oil of
Brucea javanica induces apoptosis through the PI3K/Akt
signaling pathway in acute lymphocytic leukemia Jurkat cells. Chin
J Nat Med. 19:608–620. 2021.PubMed/NCBI
|
|
11
|
Meng J, Yu Z, Chen H, Yu X, Jiang M, Zeng
XA and You J: Brucea javanica oil emulsion significantly
improved the effect of anti-programmed cell death protein-1
immunotherapy. Phytomedicine. 107:1544462022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan P, Yang BX and Ge XL: Brucea
javanica seed oil enhances the radiosensitivity of esophageal
cancer by inhibiting hypoxia-inducible factor 1α, in vitro and in
vivo. Oncol Lett. 15:3870–3875. 2018.PubMed/NCBI
|
|
13
|
Shi R, Wu Z, Wang H, Zhang J, Zhang F,
Stalin A, Chen M, Huang J, Zhai Y, Zhang Q, et al: Investigation on
the efficiency of Brucea javanica oil emulsion injection
with chemotherapy for treating malignant pleural effusion: A
meta-analysis of randomized controlled trials. Front Pharmacol.
13:9982182022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang M, Shen C, Zhu SJ, Zhang Y, Jiang HL,
Bao YD, Yang GY and Liu JP: Chinese patent medicine Aidi injection
for cancer care: An overview of systematic reviews and
meta-analyses. J Ethnopharmacol. 282:1146562022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang T, Nan H, Zhang C, Wang Y, Zhang X,
Li Y and Mulati: Aidi injection combined with FOLFOX4 chemotherapy
regimen in the treatment of advanced colorectal carcinoma. J Can
Res Ther. 10:522014. View Article : Google Scholar
|
|
16
|
Xie G, Cui Z, Peng K, Zhou X, Xia Q and Xu
D: Aidi injection, a traditional Chinese medicine injection, could
be used as an adjuvant drug to improve quality of life of cancer
patients receiving chemotherapy: A propensity score matching
analysis. Integr Cancer Ther. 18:1534735418810792019. View Article : Google Scholar
|
|
17
|
Lu Y, Zhang S, Zhu X, Wang K, He Y, Liu C,
Sun J, Pan J, Zheng L, Liu W, et al: Aidi injection enhances the
anti-tumor impact of doxorubicin in H22 tumor-containing mice. J
Ethnopharmacol. 303:1159682023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lan HY, An P, Liu QP, Chen YY, Yu YY, Luan
X, Tang JY and Zhang H: Aidi injection induces apoptosis of
hepatocellular carcinoma cells through the mitochondrial pathway. J
Ethnopharmacol. 274:1140732021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang W, Peng W, Li Y, Pan T, Feng F, Xu J
and Zhou X: Network pharmacology and molecular docking analysis on
molecular targets and mechanisms of aidi injection treating of
nonsmall cell lung cancer. Evid Based Complement Alternat Med.
2022:83502182022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu Y, Liu W, Lv T, Wang Y, Liu T, Chen Y,
Jin Y, Huang J, Zheng L, Huang Y, et al: Aidi injection reduces
doxorubicin-induced cardiotoxicity by inhibiting carbonyl reductase
1 expression. Pharm Biol. 60:1616–1624. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
WHO Classification of Tumours, . Digestive
System Tumours. 1. 5th edition. International Agency for Research
on Cancer; Lyon: 2019
|
|
22
|
American Joint Committee on Cancer, . AJCC
Cancer Staging Manual. 8th edition. Springer; New York, NY:
2017
|
|
23
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 4.03. 2010.
|
|
24
|
Gao W and Zhang K: Network meta-analysis
of 8 types of traditional Chinese medicine injection combined with
chemotherapy in colorectal cancer treatment. J Cancer Res Clin
Oncol. 149:9823–9838. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hernandez Dominguez O, Yilmaz S and Steele
SR: Stage IV colorectal cancer management and treatment. J Clin
Med. 12:20722023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA A Cancer J
Clin. 73:233–254. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu AZ, Graves KD, Jensen RE, Marshall JL,
Formoso M and Potosky AL: Patient preference and decision-making
for initiating metastatic colorectal cancer medical treatment. J
Cancer Res Clin Oncol. 142:699–706. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jazieh AR, Abuelgasim KA, Ardah HI,
Alkaiyat M and Da'ar OB: The trends of complementary alternative
medicine use among cancer patients. BMC Complement Med Ther.
21:1672021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen HM, Lai ZQ, Liao HJ, Xie JH, Xian YF,
Chen YL, Ip SP, Lin ZX and Su ZR: Synergistic antitumor effect of
brusatol combined with cisplatin on colorectal cancer cells. Int J
Mol Med. 41:1447–1454. 2018.PubMed/NCBI
|
|
30
|
Lee SM, Choi HC and Hyun MK: An overview
of systematic reviews: Complementary therapies for cancer patients.
Integr Cancer Ther. 18:15347354198900292019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
An P, Lu D, Zhang L, Lan H, Yang H, Ge G,
Liu W, Shen W, Ding X, Tang D, et al: Synergistic antitumor effects
of compound-composed optimal formula from Aidi injection on
hepatocellular carcinoma and colorectal cancer. Phytomedicine.
103:1542312022. View Article : Google Scholar : PubMed/NCBI
|