Introduction
In Europe, prostate cancer ranks first among the
most frequently diagnosed cancers in men (1). It is more common in men aged >65
years (2), however studies have
documented an age-migratory pattern towards an increase in prostate
cancer cases in younger age groups (50–59 years) (3). As in other developed countries,
prostate cancer incidence has been rising in Portugal since 1998
(4). This cancer was the most
common among Portuguese men with 7,529 new diagnoses in 2022 (19.9%
of all new cases of malignancy in men) and an age-standardized
incidence rate of 62.6 cases/100,000 men (vs. 59.9/100,000 men in
Europe) (1). Prostate cancer
mortality has declined in most high-income countries since the
mid-1990s, including in Portugal (4), likely reflecting advances in treatment
and earlier detection with the increased use of prostate-specific
antigen (PSA) testing (5–7). In Portugal, there were 2,083 prostate
cancer-related deaths in 2022 and the age-standardised mortality
rate was 11.1 deaths/100,000 men (vs. 11.2 deaths/100,000 men in
Europe (1). Localised disease
accounts for >80% of prostate cancer diagnoses and of these 15%
are at high risk of cancer recurrence (8,9).
Although the aetiology of prostate cancer is not
well understood, risk factors such as age, family history, certain
genetic mutations, such as BRCA gene mutation (10), and ethnicity may contribute to the
development of prostate cancer (11,12).
These may influence both genetic and epigenetic factors (13). Of all the men who receive a
diagnosis of prostate cancer, 30–50% may not have a
life-threatening condition (14).
Therefore, choice of treatment may be complex since it must
consider tumour staging, risk stratification, comorbidities, life
expectancy, potential side effects of the different treatments and
the preference of the patient (14).
Localized prostate cancer has often an indolent
course but disease progression and metastases can develop in the
long-term (15), thus reinforcing
the importance of early diagnosis and adequate clinical management.
Nevertheless, the clinical approach to patients with early-stage
prostate cancer remains controversial (16). Active surveillance is considered
appropriate for selected patients, namely for those with a
clinically low-risk and for some with an (favourable)
intermediate-risk. Remaining patients (intermediate- and high-risk)
may undergo radical prostatectomy, radiotherapy including external
beam radiotherapy (EBRT) or brachytherapy, or even experimental
focal therapies, including high-intensity focused ultrasound,
cryotherapy or laser ablation therapy (17).
The natural course of prostate cancer, the time of
diagnosis and the available treatment options make the
characterisation of patients at the time of diagnosis extremely
relevant. Portuguese Oncology Institute of Porto (IPO Porto; Porto,
Portugal) is a Portuguese comprehensive cancer centre with
integrated oncology and palliative care that serves a large
population region. It includes multidisciplinary clinical units,
termed the Pathology Clinics, which ensure, in the same physical
space, that all the needs of healthcare patients are met according
to their diagnosis. Each case is evaluated by different medical
specialists, and decision-making occurs through knowledge and
expertise, whilst taking into account the preferences, values and
priorities of the patients (18).
It is of utmost importance to understand current
multidisciplinary clinical practice in the management of these
patients and the clinical outcomes resulting from this practice in
a real-world setting outside of clinical trials. In this context,
the Prostate Early Cancer (PEarlC) study was conducted primarily to
investigate real-world characteristics and treatment patterns of a
retrospective cohort of patients with early-stage prostate cancer
followed up at IPO Porto and to evaluate the response to treatment
by clinical outcomes commonly used in real-world practice. As a
secondary objective, the present study compared clinical outcomes
between different disease risk subgroups.
Materials and methods
Study design
The present study was a retrospective,
non-interventional, single-centre cohort study of patients with
early-stage prostate cancer diagnosed between January 2015 and
December 2017 (index date). For each patient, all available data
between the index date and end of clinical activity, end of
follow-up (December 2020) or death (whichever occurred first) were
retrospectively collected. The data set for the statistical
analysis was prepared between July and December 2021, and the final
study report was released in May 2022.
This study was approved by The Board of
Administration (Contract number 1604247; 21 May 2021) and Ethics
Committee of IPO Porto (approval no. CES126/021; 6 May 2021; Porto,
Portugal) and by the Data Protector Officer of IPO Porto (DPO
Opinion 47/2021; 16 April 2021) before data collection. Patient
informed consent was exempt due to the retrospective observational
nature of the study and data anonymization. The reporting of the
present study conforms to the Strengthening the Reporting of
Observational Studies in Epidemiology statement (19).
Patient selection
The study inclusion criteria was as follows: i) Male
patients aged ≥18 years; ii) topographic location (ICD-O-3) C61
(malignant neoplasm of prostate); iii) histologically confirmed
prostate adenocarcinoma (ICD-O-3) 8140–8480 (20); iv) diagnosed between 1 January 2015
and 31 December 2017 at IPO Porto; v) disease stage I–III (21); vi) tumour behaviour 3 (malignant);
vii) and start of treatment or active surveillance at IPO
Porto.
Exclusion criteria included: i) Distant metastasis
(clinical stage M1); ii) continuation of prostate cancer treatment
in another hospital following admission to IPO Porto; iii) no
follow-up in IPO outpatient clinics (urology, medical oncology,
radiation oncology and multidisciplinary tumour board); iv) other
malignancies in the 5 years before or during the study (except
basal/squamous cell carcinoma); v) and participation in clinical
trials.
The cohort was stratified into non-high-risk
localised prostate cancer (LPC), high-risk LPC and locally advanced
prostate cancer (LAPC), based on criteria defined by the guidelines
of The European Association of Urology (Table I) (22).
 | Table I.EAU risk groups for biochemical
recurrence of LPC and LAPC (22). |
Table I.
EAU risk groups for biochemical
recurrence of LPC and LAPC (22).
| Low risk |
Intermediate-risk | High-risk |
|---|
| PSA <10 ng/ml
and GS <7 | PSA 10–20 ng/ml or
GS 7 | PSA >20 ng/ml or
GS >7 | Any PSA Any GS
(any |
| (ISUP grade 1) and
cT1-2a | (ISUP grade 2/3) or
cT2b | (ISUP grade 4/5) or
cT2c | ISUP grade) cT3-4
or cN+ |
| Localized |
|
| Locally
advanced |
Data
Demographic and disease characteristics, prostate
cancer treatment and healthcare resource utilization data were
abstracted from electronic medical and administrative records from
IPO Porto. Retrieved data were subject to rigorous quality-control
procedures. Confidentiality and anonymization of data for analysis
were ensured. Data sources were linked by the IPO Porto internal
unique patient identifier.
Definition of clinical outcomes
The following clinical outcomes were calculated
based on extracted data: i) PSA progression-free survival
(evaluated in patients treated with curative intent). If submitted
to surgery, defined as time from radical prostatectomy to first PSA
>0.2 ng/ml over baseline. If treated with radiation therapy
(EBRT or brachytherapy) (23),
defined as time from the initial treatment to first PSA level
according to the Phoenix Criteria (23). If treated with systemic therapy
(24), defined as time from
systemic treatment initiation to first PSA level according to the
Phoenix Criteria; ii) metastasis-free survival (evaluated in
patients treated with curative or palliative intent) defined as
time from treatment start to diagnosis of first metastasis; iii)
disease-free survival (evaluated in patients in curative treatment)
defined as time from treatment start to first relapse; iv)
progression-free survival (evaluated in patients in palliative
treatment) defined as time from start of palliative treatment to
progression or death in the hospital, whichever occurred first.
Progression was evaluated considering PSA level progression,
clinical progression or radiographic progression (bone scintigraphy
or CT-scan); v) overall survival (OS; evaluated in all patients)
defined as time from date of diagnosis to death by any cause; vi)
PSA response rate (evaluated in patients in palliative treatment
after PSA progression) defined as the percentage of patients with a
decline of at least 50% in the PSA level. Patients with death due
to disease progression or without any evaluation during follow-up
were excluded; and vii) no evidence of residual tumour rate
(evaluated in patients who underwent radical prostatectomy) defined
as the percentage of patients who had an anatomopathological result
of no residual tumour (R0) or microscopic residual tumour (R1).
Statistical analysis
Data was summarized using descriptive statistics for
the overall sample and stratified by study subgroups. The
characteristics of the patients at diagnosis were compared between
LPC and LAPC subgroups using non-parametric statistical tests for
independent samples [Mann-Whitney test for age, Chi-square test for
age groups and Fisher's exact test for disease stage and Eastern
Cooperative Oncology Group (ECOG)]. The Kaplan-Meier method was
used to analyse time-to-event variables and to estimate the median,
corresponding 95% confidence intervals (CI), and 1- and 5-year
rates. If the event of interest was not observed, time was censored
at the end of the follow-up (except progression-free survival,
where time was censored at the end of the follow-up or death
outside the hospital, whichever occurred first). Survival curves
were compared between subgroups using the log-rank test. Cox
proportional-hazards regression analysis was used to compute hazard
ratios (HR) and 95% CI. Missing data was not replaced. A two-sided
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using R software
version 4.1.0 (R Foundation for Statistical Computing) (25).
Results
Number of patients
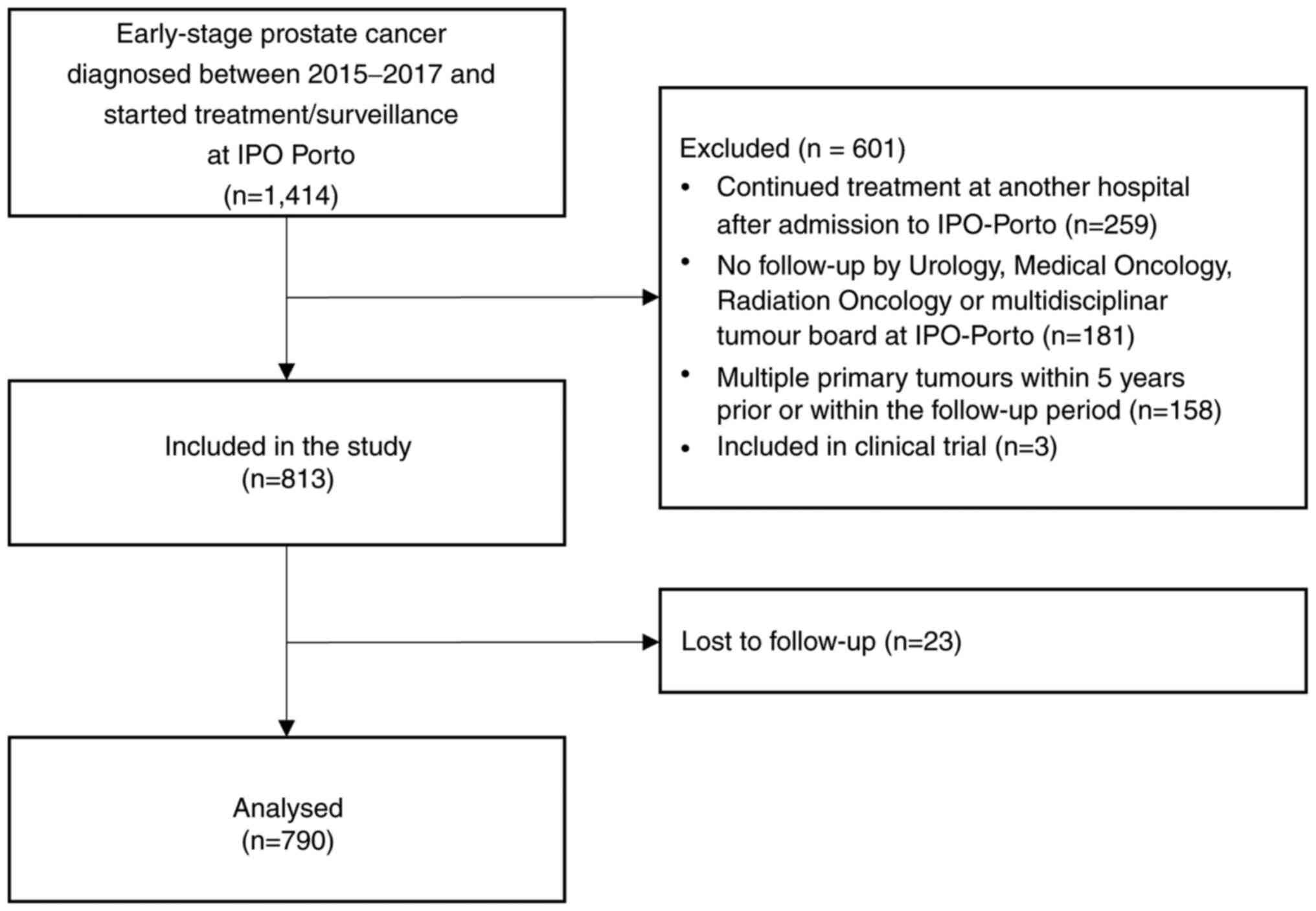
In the present retrospective cohort study, a total
of 1,414 patients were assessed for eligibility and 601 were
excluded, including 259 who continued treatment in another hospital
following admission to IPO Porto, 181 who had no follow-up visits
in IPO outpatient clinics, 158 who had multiple primary tumours
(within 5 years before or within the follow-up period) and 3 who
were enrolled in clinical trials. Of the 813 eligible patients, 23
were lost to follow-up during the study period and excluded from
statistical analysis (n=790; Fig.
1).
Demographic and clinical
characteristics
Table II describes
the characteristics at diagnosis and vital status of patients at
the end of the follow-up. Overall, the median age was 68.0 years.
The majority of patients had LPC (85.7%), of whom ~1/3 (36.0%) were
high-risk. Only 1.1% had a ECOG performance status ≥2. The majority
of the patients had been diagnosed with stage II (52.9%) or stage
III (30.1%). The median follow-up was 46.7 months and was similar
between subgroups. There were ~94.8% patients alive at the end of
the follow-up. There were significant statistical differences in
age (P=0.001) and disease stage (P<0.001) of LPC vs. LAPC
patients but not in ECOG (P=0.627).
 | Table II.Demographic and clinical
characteristics at diagnosis and status at end of follow-up. |
Table II.
Demographic and clinical
characteristics at diagnosis and status at end of follow-up.
|
| LPC, n=677 |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Non-high-risk,
n=433 | High-risk,
n=244 | LAPC, n=113 | Overall, n=790 | P-value, LPC vs.
LAPC |
|---|
| Median age (range),
years | 66.0
(44.0–83.0) | 71.0
(46.0–89.0) | 70.0
(42.0–85.0) | 68.0
(42.0–89.0) | 0.001 |
| Age group, years
(%) |
|
|
|
|
|
|
<60 | 87 (20.1) | 23 (9.4) | 5 (4.4) | 115 (14.6) | 0.001 |
|
60–69 | 211 (48.7) | 87 (35.7) | 48 (42.5) | 346 (43.8) |
|
|
>=70 | 135 (31.2) | 134 (54.9) | 60 (53.1) | 329 (41.6) |
|
| Stage, n (%) |
|
|
|
|
|
| I | 125 (28.9) | 6 (2.5) | 0 (0) | 131 (16.6) |
<0.001a |
|
II | 207 (47.8) | 211 (86.5) | 0 (0) | 418 (52.9) |
|
|
III | 99 (22.9) | 26 (10.7) | 113 (100) | 238 (30.1) |
|
|
Unknown | 2 (0.5) | 1 (0.4) | 0 (0) | 3 (0.4) |
|
| ECOG, n (%) |
|
|
|
|
|
|
0–1 | 419 (96.8) | 230 (94.3) | 110 (97.3) | 759 (96.1) | 0.627a |
|
2–3 | 3 (0.7) | 4 (1.6) | 2 (1.8) | 9 (1.1) |
|
|
Unknown | 11 (2.5) | 10 (4.1) | 1 (0.9) | 22 (2.8) |
|
| Status at end of
follow-up, n (%) |
|
|
|
| - |
|
Dead | 11 (2.5) | 21 (8.6) | 9 (8.0) | 41 (5.2) |
|
|
With evidence of
disease | 8 (1.8) | 16 (6.6) | 4 (3.5) | 28 (3.5) |
|
|
Without evidence
of disease | 3 (0.70) | 5 (2.00) | 5 (4.40) | 13 (1.60) |
|
| Alive | 422 (97.5) | 223 (91.4) | 104 (92.0) | 749 (94.8) |
|
|
With evidence of
disease | 77 (17.80) | 39 (16.00) | 10 (8.80) | 126 (15.90) |
|
|
Without evidence
of disease | 345 (79.70) | 184 (75.40) | 94 (83.20) | 623 (78.90) |
|
First treatment characterization
The majority of the patients received treatment with
curative intent (EBRT, brachytherapy or radical prostatectomy;
Table III). Only 4.3% received
palliative treatment (androgen deprivation therapy) and 10.3%
(n=81) had no treatment until the end of the follow-up period (in
active surveillance or watch-and-wait; Table III). The majority of the untreated
patients had a short life expectancy, comorbidities or a poor
performance status (data not shown). The percentage of patients in
palliative treatment was higher in high-risk (10.2%) than in
non-high-risk (1.2%) LPC and LAPC (3.5%) groups. The most common
first-line treatment was radical prostatectomy (38.8%) in patients
with non-high-risk LPC and radiotherapy plus hormone therapy (HT)
both in patients with high-risk LPC (50.6%) and patients with LAPC
(89.1%).
 | Table III.Characterization of the first
treatment. |
Table III.
Characterization of the first
treatment.
|
| LPC, n=677 |
|
|
|---|
|
|
|
|
|
|---|
| Treatment
characteristic | Non-high-risk,
n=433 | High-risk,
n=244 | LAPC, n=113 | Overall, n=790 |
|---|
| First treatment
intention, n (%) |
|
|
|
|
|
Curative | 361 (83.4) | 208 (85.2) | 106 (93.8) | 675 (85.4) |
|
Palliative | 5 (1.2) | 25 (10.2) | 4 (3.5) | 34 (4.3) |
| No
treatment | 67 (15.5) | 11 (4.5) | 3 (2.7) | 81 (10.3) |
| First treatment, n
(%) |
|
|
|
|
|
ADTb | 5 (1.4) | 25 (10.7) | 4 (3.6) | 34 (4.8) |
|
Brachytherapy | 114 (31.1) | 16 (6.9) | 1 (0.9) | 131 (18.5) |
| Radical
prostatectomy | 142 (38.8) | 38 (16.3) | 5 (4.5) | 185 (26.1) |
|
Radiotherapy | 59 (16.1) | 36 (15.5) | 2 (1.8) | 97 (13.7) |
|
Radiotherapy + HT | 46 (12.6) | 118 (50.6) | 98 (89.1) | 262 (37.0) |
| First management
approacha, n (%) |
|
|
|
|
|
Bicalutamide | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.1) |
|
Goserelin | 4 (1.1) | 18 (7.7) | 4 (3.6) | 26 (3.7) |
|
Orchidectomy | 1 (0.3) | 6 (2.6) | 0 (0.0) | 7 (1.0) |
|
Brachytherapy | 114 (31.1) | 16 (6.9) | 1 (0.9) | 131 (18.5) |
| Radical
prostatectomy | 87 (23.8) | 21 (9.0) | 4 (3.6) | 112 (15.8) |
| Radical
prostatectomy + radiotherapy | 53 (14.5) | 17 (7.3) | 1 (0.9) | 71 (10.0) |
| Radical
prostatectomy + radiotherapy + HT | 2 (0.5) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
|
Radiotherapy | 59 (15.8) | 36 (15.4) | 2 (1.8) | 97 (13.7) |
|
Radiotherapy + HT | 46 (12.6) | 107 (45.9) | 83 (75.5) | 236 (33.3) |
|
Radiotherapy + brachytherapy +
HT | 0 (0.0) | 11 (4.7) | 15 (13.6) | 26 (3.7) |
Clinical outcomes
After 5 years, the OS rate was 92.9% (95% CI,
90.2–95.7%; all patients), 82.9% (95% CI, 77.3–100.0%) of the
patients treated with curative intent were free of PSA progression,
87.5% (95% CI, 82.6–92.7%) of patients with curative/palliative
treatment were metastasis-free, 83.7% (95% CI, 78.0–89.8%) of
patients treated with curative intent were disease-free. In
time-to-event outcomes, the median was only reached in
progression-free survival (29.9 months; 95% CI, 26.5–41.0 months)
with no patients free of progression after 5 years (Table IV).
 | Table IV.Kaplan-Meier estimates of
time-to-event outcomes. |
Table IV.
Kaplan-Meier estimates of
time-to-event outcomes.
|
| LPC |
|
|
|---|
|
|
|
|
|
|---|
| Time-to-event
outcome | Non-high-risk | High-risk | LAPC | Overall |
|---|
| PSA
progression-free survivala |
|
|
|
|
| n | 361 | 208 | 106 | 675 |
| Median
(95% CI), months | n.a. | n.a. | n.a. | n.a. |
| 1-year
rate (95% CI), % | 98.9 | 97.1 | 100.0 | 98.5
(97.6–99.4) |
| 5-year
rate (95% CI), % | 87.4 | 78.7 | 79.9 | 82.9
(77.3–100.0) |
| Metastasis-free
survivalb |
|
|
|
|
| n | 366 | 233 | 110 | 709 |
| Median
(95% CI), months | n.a. | n.a. | n.a. | n.a. |
| 1-year
rate (95% CI), % | 98.9 | 98.3 | 100.0 | 98.9
(98.1–99.7) |
| 5-year
rate (95% CI), % | 91.8 | 78.5 | 91.7 | 87.5
(82.6–92.7) |
| Disease-free
survivala |
|
|
|
|
| n | 361 | 208 | 106 | 675 |
| Median
(95% CI), months | n.a. | n.a. | n.a. | n.a. |
| 1-year
rate (95% CI), % | 99.2 | 98.1 | 100.0 | 98.9%
(98.2–99.7) |
| 5-year
rate (95% CI), % | 88.4 | 79.9 | 79.1 | 83.7
(78.0–89.8) |
| Progression-free
survivalc |
|
|
|
|
| n | 22 | 39 | 10 | 71 |
| Median
(95% CI), months | 32.9
(27.1–46.0) | 29.5
(24.4–44.5) | 29.1
(26.3–41.8) | 29.9
(26.5–41.0) |
| 1-year
rate (95% CI), % | 90.5 | 86.5 | 100.0 | 89.7
(92.7–97.2) |
| 5-year
rate (95% CI), % | 0.0 | 0.0 | 0.0 | 0.0 |
| Overall
survivald |
|
|
|
|
| n | 433 | 244 | 113 | 790 |
| Median
(95% CI), months | n.a. | n.a. | n.a. | n.a. |
| 1-year
rate (95% CI), % | 100.0 | 99.2 | 100.0 | 99.8
(99.4–100) |
| 5-year
rate (95% CI), % | 96.8 | 88.8 | 87.9 | 92.9
(90.2–95.7) |
Kaplan-Meier curves were not statistically different
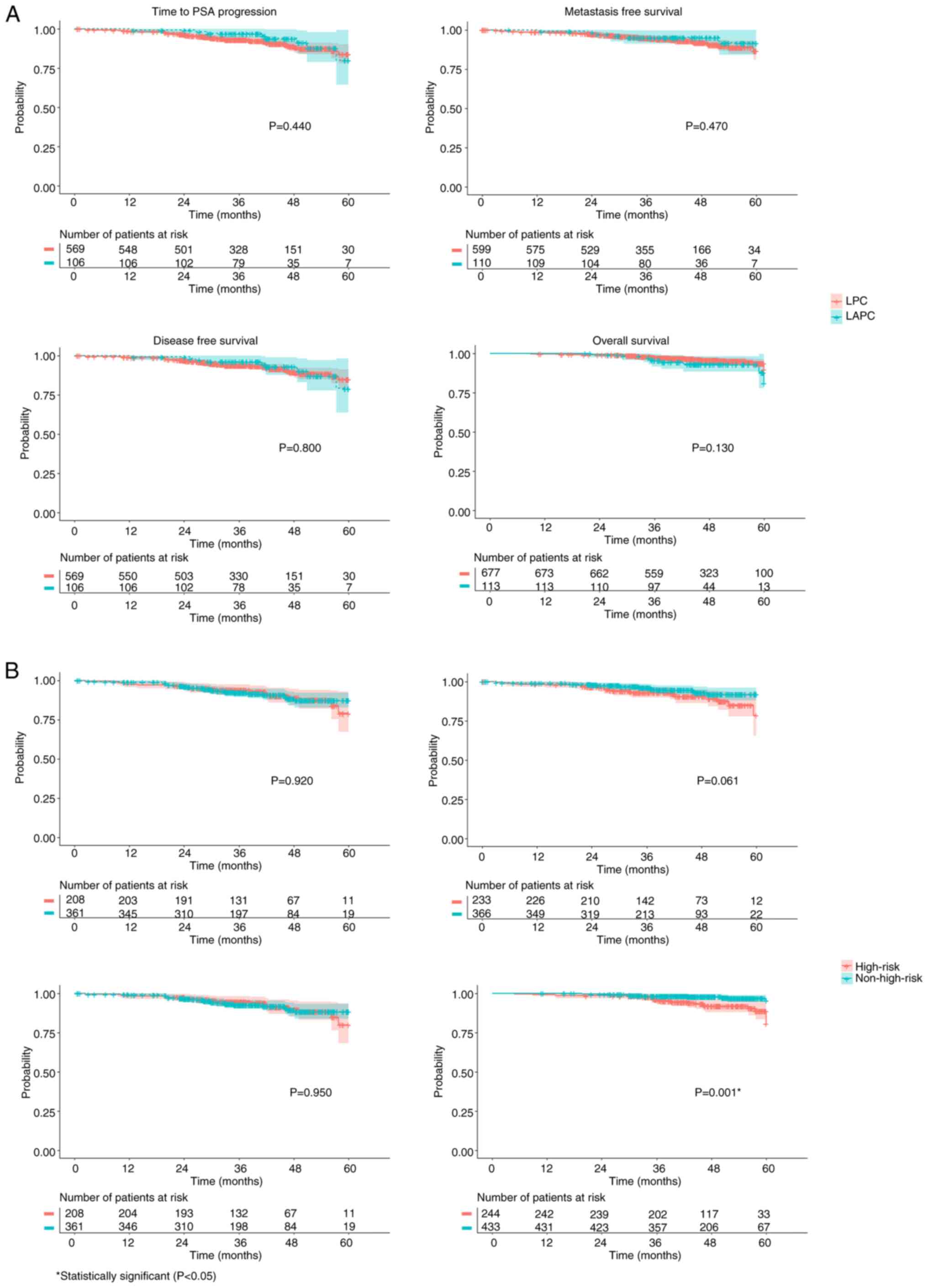
between patients with LPC vs. LACP patients (Fig. 2A). Within patients with LPC,
high-risk patients showed a worse OS in comparison with
non-high-risk patients (5-year OS rate, 88.8% vs. 96.8%; HR=3.34,
95% CI, 1.64–7.05, P=0.001; Fig.
2B). The 5-year metastasis-free survival rate was worse in
high-risk (78.5%) vs. non-high-risk patients (91.8%), although the
difference was not statistically significant (P=0.061; Fig. 2B).
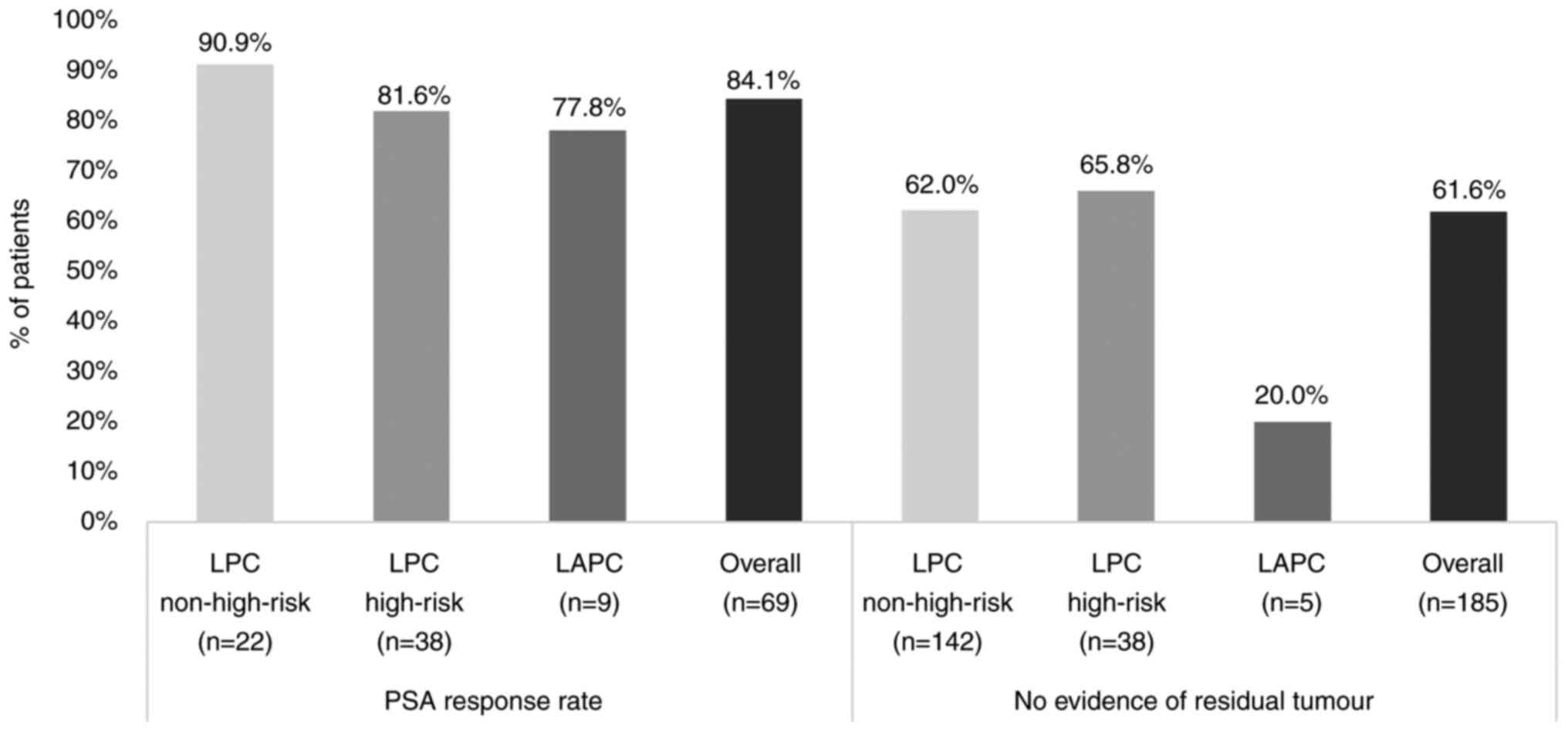
A total of ~84.1% (58/69 patients; CI 95%,
73.3–91.8%) of the patients treated with palliative treatment
following PSA progression had a PSA response (Fig. 3). In patients who underwent radical
prostatectomy, 61.6% (114/185 patients; 95% CI, 54.2–68.7%) showed
no evidence of residual tumour (Fig.
3).
Discussion
It is known that the number of patients in treatment
for LPC has been increasing in Portugal (26) likely due to the widespread use of
PSA (contributing to an increase in incidence and early cancer
detection), improved access to healthcare (mainly hospital
specialist consultations) and population aging (26). However, there is still a lack of
knowledge on the characteristics and clinical outcomes of these
patients with early prostate cancer, namely those with high-risk
LPC and LAPC. Real-world data is important to characterise patients
treated in clinical practice and measure outcomes outside of the
clinical trial setting where highly selected patients are
frequently enrolled (27). It can
also be of importance to provide insights about health care
patterns, including treatments (27). Real-world data is increasingly being
used to propose and support decision-making in the clinical
management of patients (27).
To the best of our knowledge, the present
retrospective study provided the first characterisation of a large
cohort of patients with early-stage prostate cancer treated in
real-world conditions at a tertiary cancer institute in Portugal,
together with an exhaustive set of clinical outcomes. The present
study covered the clinical practice at IPO Porto over 6 years
(2015–2020). Clinical practice at IPO Porto for early-stage
prostate cancer has not suffered major changes since then.
Therefore, it is expected that the results presented here are still
valid for the present setting. Although this is a single-centre
study, IPO Porto is one of the most relevant Portuguese centres
treating oncologic diseases, including prostate cancer, and is
responsible for the management of ~850 new patients with prostate
cancer per year, equating to ~11.3% of all new cases in Portugal
(1). The present study conducted at
this centre allowed the inclusion of a large sample and the
calculation of precise estimates for the overall sample with a low
margin of error.
In the present study, the exclusion criteria were
mainly defined to exclude patients with incomplete follow-up data,
as they would not be suitable to estimate 5-year clinical outcomes.
A total of 259 patients were excluded from the study due to
continuation of treatment outside IPO Porto, which is common in
reference oncology centres; namely, when patients looked for a
second opinion on the treatment protocol suggested by another
hospital, or wished to have access to treatment techniques, such as
robotic or laparoscopic surgery, that were not available at IPO at
the time of the study. However, these excluded patients are not
expected to have more severe disease than the included patients,
and therefore their exclusion likely did not limit the internal
validity of the results for all patients treated at IPO Porto.
Furthermore, a characterisation of the subjects lost to follow-up
was not performed, but their exclusion did not introduce any bias
in the results considering their low number (n=23), as compared
with the total sample size (n=790). Thus, the present sample
appears to have been highly representative of all early-stage
prostate cancer cases attended at IPO Porto.
It was not expected that the characteristics of the
patients treated at IPO Porto differed significantly from those of
patients treated at other reference Portuguese oncology centres.
Given the small number of patients in palliative care, non-treated
or only in HT, the sample of the present study mainly reflected
patients being followed up using a multidisciplinary approach and
treated with curative intentions including brachytherapy, radical
prostatectomy or radiotherapy combined with HT. Missing data was
minimal (only missing for ECOG status) due to exclusion criteria
and data quality control. The quality of the data retrospectively
collected in the present study was ensured by the standard
operating procedures for data management and statistical analysis
implemented at IPO Porto.
In the present study, 14.3% of all PC diagnoses were
high-risk, which is aligned with what has been described in the
literature (15%) (8,9). Within the LPC subgroup, there was a
higher percentage of patients submitted to prostatectomy in the
non-high-risk patients (38.8%) compared with high-risk patients
(16.3%). There is likely a judicious use of surgery as a single
recommendation for high-risk patients, mainly because most of the
time there is a need for multimodal treatments, and this is of the
utmost importance to the multidisciplinary board of IPO Porto.
There is still a discussion about the use of radical prostatectomy
as a first-line treatment for LAPC (28). At IPO Porto, only 4.5% (n=5) of
patients with LAPC were submitted to this type of surgery. At IPO
Porto, the clinical protocol is to treat patients with LAPC with
radiotherapy combined with hormonotherapy, a practice aligned with
the European Association of Urology recommendations (22).
Only four studies with Portuguese patients with
LPC/LAPC published in indexed journals were identified for the
purpose of the present study. A previous study (29), including 300 Portuguese patients
with LPC and a first prostate biopsy at another Portuguese oncology
reference centre (Portuguese Oncology Institute of Coimbra,
Coimbra, Portugal) between January 2014 and December 2018, reported
17.3% (vs. 30.1% at IPO Porto) patients who underwent radical
prostatectomy, 39.3% (vs. 43.2%) who underwent external
radiotherapy, 29.3% (vs. 21.7%) who underwent brachytherapy and
14.1% (vs. 5.0%) who were treated with other options (active
surveillance, cryotherapy and hormonal therapy). Since there is no
information on the risk of these patients, the differences in
treatment practices between IPO Coimbra and IPO Porto could not be
justified.
A registry-based study (30) carried out in 43 Portuguese centres
and published in 2010 included 1,767 patients with early-stage
prostate cancer, of whom 69.8% had LPC (vs. 85.7% at IPO Porto) and
30.2% had LAPC. In patients with LPC, the most common treatments
were radical prostatectomy (43.9%), radical radiotherapy (25.3%)
and pelvic adjuvant radiotherapy (9.3%). In LAPC, 67.8% were first
treated with HT. However, the study was published >10 years ago,
and the authors recognized limitations on the sampling methods and
external validity.
In addition, a Portuguese single-centre
retrospective study (31) was
identified that reported 6-year real-world outcomes in
non-high-risk LPC [defined according to European Organisation for
Research and Treatment of Cancer (32)] between 2003 and 2013; however, in
this previous study, the patients were only treated with
brachytherapy, and therefore it cannot be used for comparing
outcomes or treatment patterns with the present study.
Botelho et al (26) performed a nationwide study which
described treatment patterns over time in localized prostate cancer
in the Portuguese National Health System hospitals between 2000 and
2020. Still, the results were mainly presented at treatment-level
rather than patient-level, focused mostly on hospital case volume
(cumulative number of treatments per year) and data for
radiotherapy were not available after 2012. Therefore, the lack of
recent real-world Portuguese studies in patients with LPC and/or
LAPC limited our understanding and the comparison of the current
clinical practice and outcomes in other hospitals vs. IPO
Porto.
Furthermore, the comparison with published studies
from other countries is not straightforward due to differences in
the included patient population (namely the stage of the disease),
treatments, outcome definitions and study period. Goy et al
(33) compared clinical outcomes of
radical prostatectomy vs. EBRT vs. brachytherapy for patients with
intermediate-risk prostate cancer only (a subgroup included in the
non-high-risk LPC of PEarlC study) treated between 2004 and 2007
and followed-up for 10 years. The 5-year survival rate seen in the
PEarlC study for the non-high-risk group (96.8%) is within the
range estimates by treatment in the former study (90.6–98.1%) as
well as the PSA progression-free survival rate (87.4% in PEarlC
study vs. 73–90.7% in Goy et al).
In the PEarlC study, the majority of patients with
early-stage prostate cancer appear to have a favourable 5-year
prognosis, with positive real-world outcomes, namely
metastasis-free survival, disease-free survival, progression-free
survival and OS. No statistical difference was observed between the
results of patients with LPC vs. LAPC patients. Notably, patients
with high-risk LPC exhibited worse outcomes compared with
non-high-risk but only the difference in OS was statistically
significant. Indeed, the short follow-up to measure clinical
outcomes of patients at an early stage of the disease may have been
a study limitation, due to the availability and quality of
electronic data and urgency to accelerate evidence generation,
which is at present scarce. In addition, when analysing some
outcomes (progression-free survival, PSA response rate, no evidence
of residual tumour), the number of available patients was small,
particularly in the LAPC subgroup. This may have also contributed
to the lack of statistical significance. In fact, the study was not
powered to compare results between subgroups of patients. The
comparison of clinical outcomes between different treatments were
not aimed in the present study, since the choice of the treatment
depends on patient and disease characteristics, including severity
which is itself a prognostic variable. Finally, the healthcare
resource use and corresponding costs estimated for these patients
were previously presented (34) and
beyond the scope of the current analysis.
To conclude, the clinical management of patients
with early-stage prostate cancer is multifactorial, considering
patient-related factors, such as age, comorbidities, previous
treatments or conditions, and cancer-related factors, such as
tumour grading, staging or risk group, with some patients showing
an indolent course of the disease and being more suited to
surveillance, and others with indications to start active local
treatment with curative intent, such as surgery or radiotherapy.
The best approach should be decided in a multidisciplinary team
setting. At IPO Porto and following recommendations from several
medical societies (especially from the European Urology Oncology),
the treatment modality is always decided in a multidisciplinary
meeting, and patients included in this study were treated with a
wide range of therapies. In LPC or LAPC, systemic therapies have a
potentiating role in curative treatment in selected patient
subgroups, or as subsequent treatment lines following the failure
of local treatment. The range of prognostic factors and therapeutic
options, often with overlapping efficacy rates but different
toxicity profiles, highlights the importance of taking the
therapeutic decision in a truly multidisciplinary setting, of which
IPO Porto was a pioneer. The PEarlC study highlighted that the
current strategy has high success rates in disease control and
survival, and there is a need for long follow-ups, due to the often
indolent course of this disease. Overall, the prognosis for most
patients is rather positive, as there is a possibility to adjust
the type of therapy to the clinical characteristics of the patient.
There is a confined subgroup of patients (high-risk), who in most
cases are not eligible for curative therapy, and for whom future
pharmacological innovation may bring improvements, namely in OS or
metastasis-free survival.
In the future, treatments closely tailored to
disease risk, the advancement of the technical development of local
therapies, such as surgery and radiotherapy, with improved results
in clinical outcomes and less side effects, together with the
development of new medicines and an improved disease control with a
first-line treatment or following treatment failure are expected.
Furthermore, it will be relevant to periodically update the results
of the present study for a close monitoring of the ongoing
practice, its clinical benefits and expand evidence on respective
outcomes. The present large real-world study is an important
contribution to reducing the evidence gap for early stages of
prostate cancer, ultimately improving public health impact of this
disease in the future.
Acknowledgements
The authors would like to thank Dr Catarina Silva
(Institute for Evidence-Based Health, Lisbon, Portugal) for medical
writing support.
Funding
This study was funded by Johnson & Johnson Innovative
Medicine.
Availability of data and materials
The data generated in the present study are not
publicly available due to data access and ownership compliance
regulations but may be requested from the corresponding author.
Authors' contributions
IB, SGM, RC, MR, EM, JLC, AR, AO, ACF, SS, PR and
MJB provided substantial contributions to the conception and design
of the study. SGM, RC and PR were involved in the acquisition,
analysis and interpretation of the study data. All authors
substantially contributed to critically reviewing the manuscript
for important intellectual content. IB and PR confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript. All authors agreed to be
accountable for the work in ensuring that questions related to the
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
IPO Porto (approval no. CES126/021; Porto, Portugal) and by the
Data Protector Officer at IPO Porto. Patient's informed consent was
exempt from the Ethics Committee of IPO Porto.
Patient consent for publication
Not applicable.
Competing interests
IB, SGM, RC, MR, EM, JLC, AR, AO, PR and MJB are
employees of IPO Porto, which established a financial contract with
Johnson & Johnson Innovative Medicine for the data extraction,
statistical analysis and data interpretation. ACF and SS are
employees of Johnson & Johnson Innovative Medicine.
References
|
1
|
Ferlay J, Ervik M, Lam F, Laversanne M,
Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I and Bray F:
Global cancer observatory: Cancer today, Lyon, France:
International agency for research on cancer. Available from:.
https://gco.iarc.who.int/todayMarch
13–2024
|
|
2
|
Daniyal M, Siddiqui ZA, Akram M, Asif HM,
Sultana S and Khan A: Epidemiology, etiology, diagnosis and
treatment of prostate cancer. Asian Pac J Cancer Prev.
15:9575–9578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Situmorang GR, Umbas R, Mochtar CA and
Santoso RB: Prostate cancer in younger and older patients: Do we
treat them differently? Asian Pac J Cancer Prev. 13:4577–4580.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pina F, Castro C, Ferro A, Bento MJ and
Lunet N: Prostate cancer incidence and mortality in Portugal:
Trends, projections and regional differences. Eur J Cancer Prev.
26:404–410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Printz C: Early-stage prostate cancer, PSA
screening rates decline. Cancer. 122:8252016. View Article : Google Scholar
|
|
7
|
Sridharan S, Dal Pra A, Catton C, Bristow
RG and Warde P: Locally advanced prostate cancer: Current
controversies and optimisation opportunities. Clin Oncol.
25:499–505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wani M and Madaan S: What is new in the
management of high-risk localized prostate cancer? J Clin Med.
12:4552023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khauli R, Ferrigno R, Guimarães G,
Bulbulan M, Uson Junior PLS, Salvajoli B, Palhares DMF, Racy D, Gil
E, de Arruda FF, et al: Treatment of localized and locally
advanced, high-risk prostate cancer: A report from the first
prostate cancer consensus conference for developing countries. JCO
Glob Oncol. 7:530–537. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nyberg T, Frost D, Barrowdale D, Evans DG,
Bancroft E, Adlard J, Ahmed M, Barwell J, Brady AF, Brewer C, et
al: Prostate cancer risks for male BRCA1 and BRCA2 mutation
carriers: A prospective cohort study. Eur Urol. 77:24–35. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kgatle MM, Kalla AA, Islam MM, Sathekge M
and Moorad R: Prostate cancer: Epigenetic alterations, risk
factors, and therapy. Prostate Cancer. 2016:56538622016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gathirua-Mwangi WG and Zhang J: Dietary
factors and risk of advanced prostate cancer. Eur J Cancer Prev.
23:96–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adjakly M, Ngollo M, Dagdemir A, Judes G,
Pajon A, Karsli-Ceppioglu S, Penault-Llorca F, Boiteux JP, Bignon
YJ, Guy L and Bernard-Gallon D: Prostate cancer: The main risk and
protective factors-Epigenetic modifications. Ann Endocrinol
(Paris). 76:25–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Budäus L, Huland H and Graefen M:
Controversies in the management of localized prostate cancer:
Radical prostatectomy still the standard of care. Crit Rev Oncol
Hematol. 84 (Suppl 1):e24–e29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Popiolek M, Rider JR, Andrén O, Andersson
SO, Holmberg L, Adami HO and Johansson JE: Natural history of
early, localized prostate cancer: A final report from three decades
of follow-up. Eur Urol. 63:428–435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marhold M, Kramer G, Krainer M and Le
Magnen C: The prostate cancer landscape in Europe: Current
challenges, future opportunities. Cancer Lett. 526:304–310. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Evans AJ: Treatment effects in prostate
cancer. Mod Pathol. 31((S1)): S110–S121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
ESMO, . Available at. https://www.esmo.org/for-patients/esmo-designated-centres-of-integrated-oncology-palliative-care/esmo-accredited-designated-centres/instituto-portugues-de-oncologia-do-porto-francisco-gentil-epeJanuary
12–2023
|
|
19
|
von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC and Vandenbroucke JP: STROBE: Initiative: The
strengthening the reporting of observational studies in
epidemiology (STROBE) statement: Guidelines for reporting
observational studies. Int J Surg. 12:1495–1499. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
ICD-O-3 Coding Materials Archive.
https://seer.cancer.gov/archive/icd-o-3/January
12–2023
|
|
21
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mottet N, Bellmunt J, Briers E, et al:
EAU-ESTRO-ESUR-SIOG guidelines on prostate cancer. Available at.
https://uroweb.org/guidelines/prostate-cancerJanuary
12–2023
|
|
23
|
Roach M III, Hanks G, Thames H Jr,
Schellhammer P, Shipley WU, Sokol GH and Sandler H: Defining
biochemical failure following radiotherapy with or without hormonal
therapy in men with clinically localized prostate cancer:
Recommendations of the RTOG-ASTRO phoenix consensus conference. Int
J Radiat Oncol Biol Phys. 65:965–974. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
The Prostate Cancer Clinical Trials
Working Group 3, . Available at. https://pcctc.org/news/pcwg3/January 12–2023
|
|
25
|
R Core Team (2021), . R: A language and
environment for statistical computing. R foundation for statistical
computing Vienna, Austria: Available at. https://www.R-project.org/April 18–2024
|
|
26
|
Botelho F, Lopes R, Pina F, Silva C,
Pacheco-Figueiredo L and Lunet N: Prostate cancer treatment in
Portugal: A nationwide analysis. Sci Rep. 13:193622023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
George DJ, Sartor O, Miller K, Saad F,
Tombal B, Kalinovský J, Jiao X, Tangirala K, Sternberg CN and
Higano CS: Treatment patterns and outcomes in patients with
metastatic castration-resistant prostate cancer in a real-world
clinical practice setting in the United States. Clin Genitourin
Cancer. 18:284–294. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li PI, Chen SJ, Chen YH, Chen WC and Huang
CP: Comparative outcomes of robotic radical prostatectomy in
patients with locally advanced prostate cancer. Medicina (Kaunas).
58:18202022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pereira-Lourenço M, Vieira E, Brito D,
Peralta JP, Godinho R, Conceiçao P, Reis M, Rabaça C and Sismeiro
A: Influence of sociodemographic factors on treatment's choice for
localizd prostate cancer in Portugal. Arch Ital Urol Androl.
92:45–49. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nunes P, Pimentel FL, Pina F and Rolo F:
National registry of prostate cancer, in Portugal-ReNaCaP. Acta
Urologica. 3:39–45. 2010.
|
|
31
|
Galego P, Silva FC and Pinheiro LC:
Analysis of monotherapy prostate brachytherapy in patients with
prostate cancer. Initial PSA and Gleason are important for
recurrence? Int Braz J Urol. 41:353–359. 2015.PubMed/NCBI
|
|
32
|
Ash D, Flynn A, Battermann J, Reijke T,
Lavagnini P and Blank L; ESTRA/EAU Urological Brachytherapy Group
and EORTC Radiotherapy Group, : ESTRO/EAU/EORTC recommendations on
permanent seed implantation for localized prostate cancer.
Radiother Oncol. 57:315–321. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goy BW and Burchette R: Ten-year treatment
complication outcomes of radical prostatectomy vs external beam
radiation vs brachytherapy for 1503 patients with intermediate risk
prostate cancer. Brachytherapy. 20:1083–1089. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Braga I, Monteiro S, Calisto R, Rangel M,
Medeiros E, Cunha JS, Rosinha A, Oliveira A, Bento MJ, Lopes R, et
al: Healthcare resource utilization (HCRU) in treatment of patients
with localized/locally advanced prostate cancer (LPC/LAPC) in a
Portuguese comprehensive cancer center (PCCC). Value in Health.
25:S2802022. View Article : Google Scholar
|