Introduction
Breast cancer is the most commonly diagnosed cancer
in women and the leading cause of cancer death, followed by lung
and colorectal cancer (1). Decades
of research have shown that breast cancer is a complex and
heterogeneous disease. Clinical outcomes of breast cancer have
substantially improved over the years with significant advances in
treatment options. The latest developments in adjuvant therapies,
particularly immunotherapy and antibody-drug conjugates, have
demonstrated improved survival rates in patients with breast
cancer. However, their therapeutic effects are known to be
transient, and development of drug resistance in some patients
limits its further use. Breast cancer remains a major contributor
to mortality and morbidity (2).
Therefore, it is necessary to continue efforts to identify novel
adjuvant treatment strategies to optimize survival and quality of
life for patients with breast cancer. Traditional Chinese herbs
(TCHs) have demonstrated unique potential as they have shown
efficacy in breast cancer treatment while boasting a significantly
more favorable side effect profile compared with standard
radiotherapy and chemotherapy. The mechanisms of action for TCHs is
not yet fully elucidated but has been associated with the
inhibition of cancer growth, the reduction of metastasis and
invasion, the promotion of cancer cell apoptosis and the
enhancement of the immune response (3). The Pingxiao capsule (PXC) contains a
well-known TCH formula that has been widely used by Chinese
patients as an alternative medicine adjunct in the treatment of
cancer (4). The typical PXC formula
consists of a mixture of Strychnos nux-vomica L., Curcuma
wenyujin Y. H., Agrimonia pilosa Ledeb.,
Toxicodendron vernicifluum, Trogopterus dung, alumen,
potassium nitrate (saltpeter) and Citrus aurantium L.
(5). Of these ingredients,
Curcuma wenyujin, which contains curcumin, has been
identified to have anti-tumor and anti-inflammatory effects
(3).
Early breast cancer (EBC) is cancer is found only in
the breast or nearby lymph nodes and has not spread to other parts
of the body. The present retrospective cohort study was conducted
in patients with operable EBC who were treated at a university
affiliated academic hospital in order to investigate the clinical
efficacy of PXC as a novel adjuvant agent and its potential to
alleviate the side effects of systemic chemotherapy.
Patients and methods
Data source
A chart review was retrospectively performed on 371
patients who underwent surgery for EBC in the Department of Breast
Surgery of the First Hospital of China Medical University (CMU;
Shenyang, China) between August 2011 and March 2016.
Patient selection
Inclusion criteria used were as follows: i) Patients
with an established diagnosis of EBC, defined as histological
diagnosis of operable stage I to stage III invasive ductal
carcinoma with achievement of an R0 resection; ii)
patients aged between 18 and 75 years; and iii) patients who
underwent standard surgical treatment and adjuvant chemotherapy.
All patients included in the study received at least one of the
following: Chemotherapy, radiotherapy, endocrine therapy and/or
anti-human epidermal growth factor receptor 2 (HER2) therapy,
according to the NCCN guidelines (6,7). This
included those with N0 cancer, who received chemotherapy due to
high-risk factors such as an estrogen receptor (ER) expression
level of <50% and/or a Ki67 level ≥30%. PXC used by the study
population was manufactured by Xi'an Charoen Pokphand
Pharmaceutical, Co., Ltd., (batch no. 1712140), which is the sole
manufacturer and distributor of PXC. Patients were divided into the
PXC group (patients who received PXC treatment) and the non-PXC
group (patients who did not receive PXC) per chart review.
Treatment
The present study adjusted for confounders using the
principle of restriction and matching. Subjects in the two cohorts
(PXC vs. non-PXC) were matched in terms of age, tumor stage,
axillary metastasis, hormonal receptor status, HER2 status and
molecular subtypes to eliminate potential confounding factors. In
addition, only those who had received PXC treatment within 1 year
of diagnosis and had received standard dosing of PXC (4–8 capsules,
three times a day for >1 month, for a maximum of 6 months; the
dosage of each capsule is 230 milligrams) were included. Patients
must also have completed any indicated standard treatment in
addition to surgery (i.e. chemotherapy, radiotherapy, endocrine
therapy accordingly) before PXC treatment, and those who did not
comply with standard treatments were excluded from the study.
Disease-free survival (DFS) was defined as the time from the date
of surgery to either tumor recurrence or death due to any cause,
and overall survival (OS) was defined as the interval between the
date of surgery and the date of patient death or the last follow-up
visit.
The study protocol was reviewed by the CMU Ethics
Committee Institutional Review Board (approval no. 2020 NO.203).
Approval was granted prior to the commencement of the study and the
requirement for written consent was waived.
Adverse events (AEs) after
chemotherapy
All subjects underwent chemotherapy. A total of 105
patients were treated with a docetaxel + anthracyclines +
cyclophosphamide (TEC or EC-T) regimen, 12 patients were treated
with an anthracyclines + cyclophosphamide (EC) regimen, 88 patients
were treated with a docetaxel + cyclophosphamide (TC) regimen, 20
patients were treated with a docetaxel + platinum (DP) regimen, 121
patients were treated with an anthracyclines + cyclophosphamide +
docetaxel + herceptin (EC-TH) regimen, 14 patients were treated
with a 5-fluorouracil + anthracyclines + cyclophosphamide (CEF)
regimen and 11 patients were treated with a CEF-T regimen. For all
patients, treatments were initiated within 6 weeks of surgery
according to NCCN. In addition, for patients with ER+ expression
≥10%, regardless of PR expression, endocrine therapy should be
completed for 5 years. Data for AEs was obtained through chart
review of routine documentation. All patients with breast cancer
were routinely followed up in the Outpatient Clinic immediately
before starting chemotherapy and all patients underwent routine
laboratory tests. Patients were also followed up at an interval of
14 days after starting chemotherapy and then at the end of the
first treatment cycle, 21 days after starting chemotherapy, during
which they were admitted for observation. AEs were documented for
every cycle of treatment by phone or during follow-up visits using
the Common Terminology Criteria for Adverse Events version 4.0
(8). Treatment was either suspended
or terminated for patients with AEs that were of grade 2 or higher
during chemotherapy, and the period for which the agent was
administered at the prescribed dose (not including the period when
the dose was reduced) was recorded. Incidence of AEs, and the
completion rate of the first cycle of chemotherapy at the
prescribed dose were tabulated.
Statistical analysis
The primary objective of the present study was to
assess the efficacy and survival benefit of PXC-based adjuvant
therapy, and if this was significant, to identify prognostic
factors for its use. Participant demographics and characteristic
such as age, tumor stage, axillary metastasis status, hormonal
receptor status, HER2 status and molecular subtypes were reviewed
and tabulated. Descriptive statistics were reported as proportions
and medians. Frequencies of tumor characteristics and response
rates were compared using the χ2 test or Fisher's exact
test. DFS and OS were demonstrated using the Kaplan-Meier survival
curve. The log-rank test was used to compare two or more survival
curves, and Cox's proportional hazards regression models were used
to analyze the independent predictors for recurrence. Survival
duration was calculated starting from the time of surgery. P≤0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed with SPSS 12.0 for Windows
(SPSS Inc.).
Results
Patient characteristics
A total of 371 patients (age range, 27–65 years;
median age, 50) met the inclusion criteria and were included in
this study. Patients were followed up from 22 to 137 months, with
the median follow-up time being 101 months. During the follow-up
period, 122 patients (32.9%) experienced recurrence and 73 patients
(19.7%) died. There were 154 patients (41.5%) in the PXC treatment
group and 217 (58.5%) in the non-PXC group. Of those in the PXC
group, 62 (16.7% of total) patients received treatment for 1 month,
and 92 (24.8% of total) patients received treatment for ≥3 months,
with an overall range of treatment duration of 1–6 months.
Subjects in the two cohorts (PXC vs. non-PXC) were
similar in terms of age (P=0.172), tumor stage (P=0.075), axillary
metastasis (P=0.259), hormonal receptor status (P=0.206), HER2
status (P=0.520) and molecular subtype (P=0.509), as demonstrated
in Table I. Most patients had tumor
ER+ expression ≥10% (79% in PXC group, 73% in non-PXC
group) and >70% of patients in each group had PR+
tumors. Of the patients in the PXC group with ER+
tumors, 100% patients received endocrine therapy when
ER+ expression ≥10%, and of the patients in the non-PXC
group with ER+ tumors, 96% received endocrine therapy.
Most HER2+ patients (98.0% of patients in the PXC group
and 96.1% of patients in the non-PXC group) received anti-HER2
therapy. All cases of TNBC received 6–8 cycles of chemotherapy with
TEC, EC-T or DP regimens (data not shown).
 | Table I.Demographics and baseline
characteristics. |
Table I.
Demographics and baseline
characteristics.
| Parameters | PXC, n (%) | Non-PXC, n (%) |
χ2/Fisher's exact test
value | P-value |
|---|
| Sex |
|
|
|
|
| Female | 154 (100) | 217 (100) |
|
|
| Age, years (%) |
|
|
|
|
| ≤50 | 87 (56) | 107 (49) | 1.864 | 0.172 |
|
>50 | 67 (44) | 110 (51) |
|
|
| Tumor stage |
|
| 6.795 | 0.075 |
| T1 | 35 (23) | 41 (19) |
|
|
| T2 | 103 (67) | 154 (71) |
|
|
| T3 | 9 (6) | 20 (9) |
|
|
| T4 | 7 (5) | 2 (1) |
|
|
| Axillary
metastasis |
|
| 3.994 | 0.259 |
| N0 | 88 (57) | 119 (55) |
|
|
| N1 | 40 (26) | 44 (20) |
|
|
| N2 | 21 (14) | 43 (20) |
|
|
| N3 | 5 (3) | 11 (5) |
|
|
| Hormonal
receptor |
|
| 1.603 | 0.206 |
|
Positive | 121 (79) | 158 (73) |
|
|
|
Negative | 33 (21) | 59 (27) |
|
|
| HER2 |
|
| 0.414 | 0.520 |
|
Positive | 49 (32) | 76 (35) |
|
|
|
Negative | 105 (68) | 141 (65) |
|
|
| Molecular
subtype |
|
| 2.316 | 0.509 |
| Luminal
A | 28 (18) | 36 (17) |
|
|
| Luminal
B | 93 (60) | 122 (56) |
|
|
|
HER2-positive | 13 (8) | 18 (8) |
|
|
|
Triple-negative | 20 (13) | 41 (19) |
|
|
Efficacy analysis
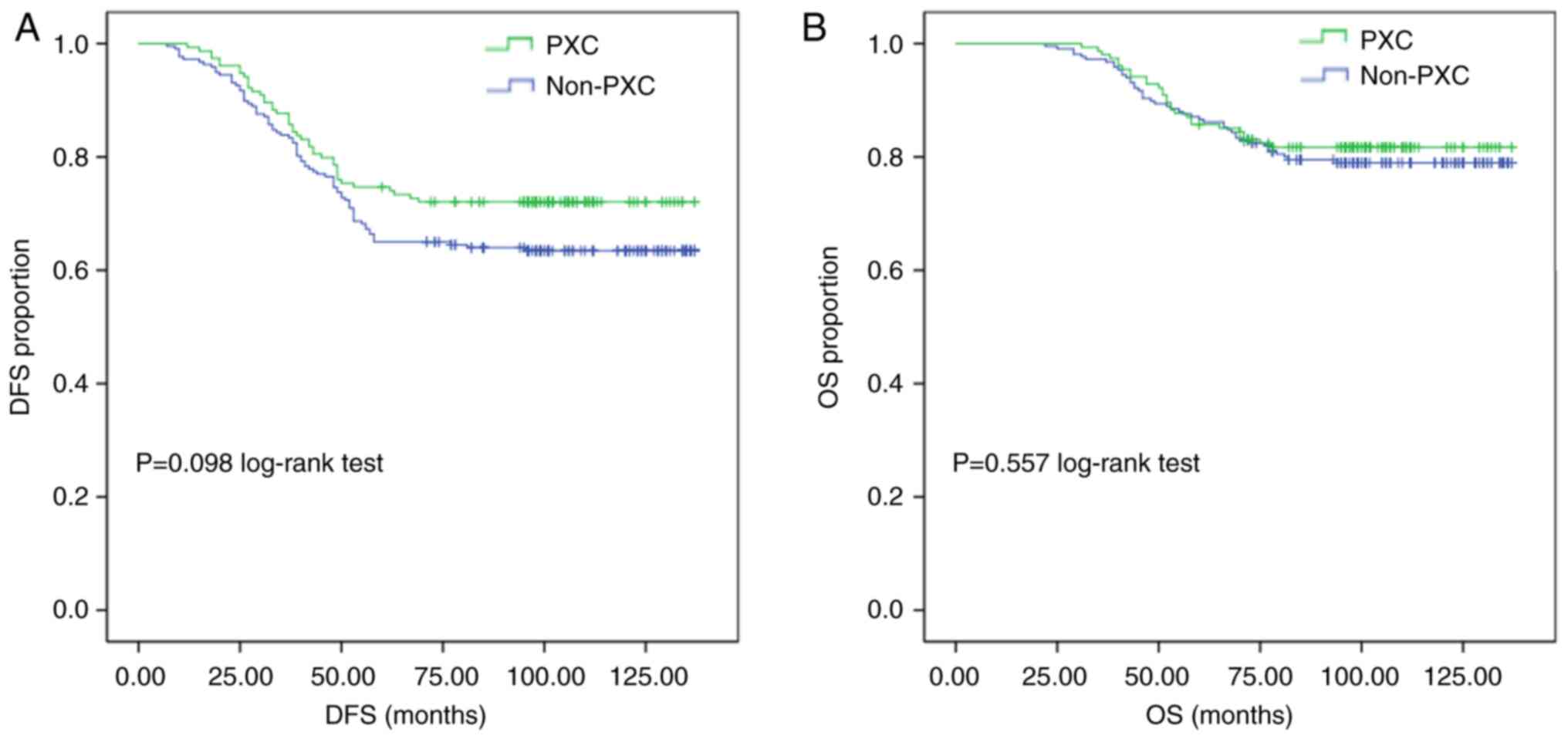
At the conclusion of the study period, the overall
DFS rate was 72.1% in the PXC group compared with 63.6% in the
non-PXC group. However, Kaplan-Meier analysis showed no significant
difference between the DFS rates of the PXC and non-PXC groups
(P=0.098; Fig. 1A). In addition, OS
was not significantly different between the PXC and non-PXC groups
(P=0.557; Fig. 1B).
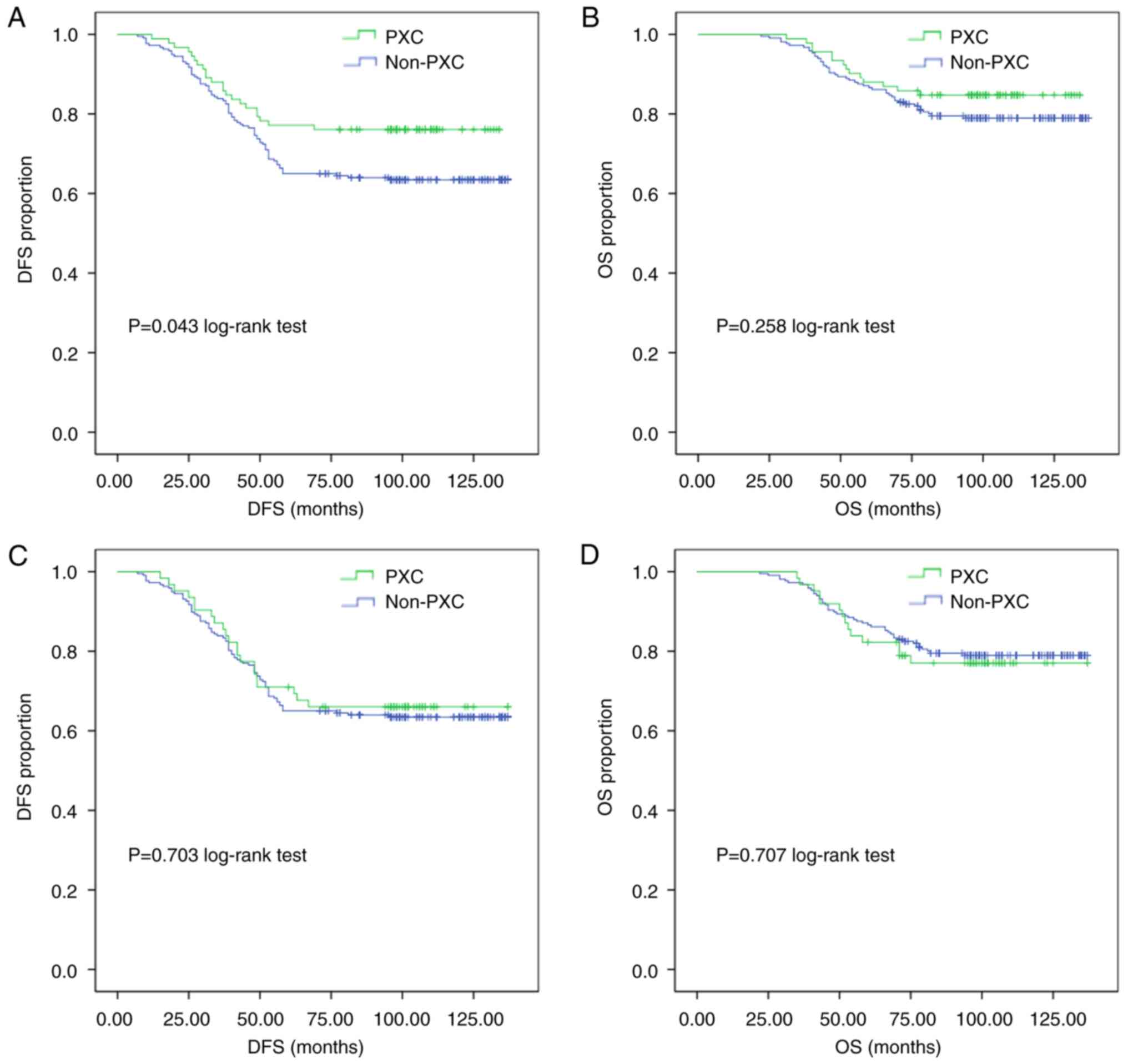
Subgroup analyses were performed to assess the
effects of the administration duration of PXC, with subjects
divided into groups who have received 1–3 months of PXC therapy and
those with ≥3 months of PXC therapy, both of which were compared
with the non-PXC group (Fig. 2).
The results showed that the DFS rates were significantly higher in
the PXC group with at least 3 months of treatment compared with
those in the non-PXC group (76.1 vs. 63.6%, respectively; P=0.043
Fig. 2A). However, such a
difference was not observed for OS (P=0.258 Fig. 2B). There was no significant
difference in DFS (P=0.703) or OS (P=0.707) between those who used
PXC for 1–3 months and those who did not use PXC (Fig. 2C and D).
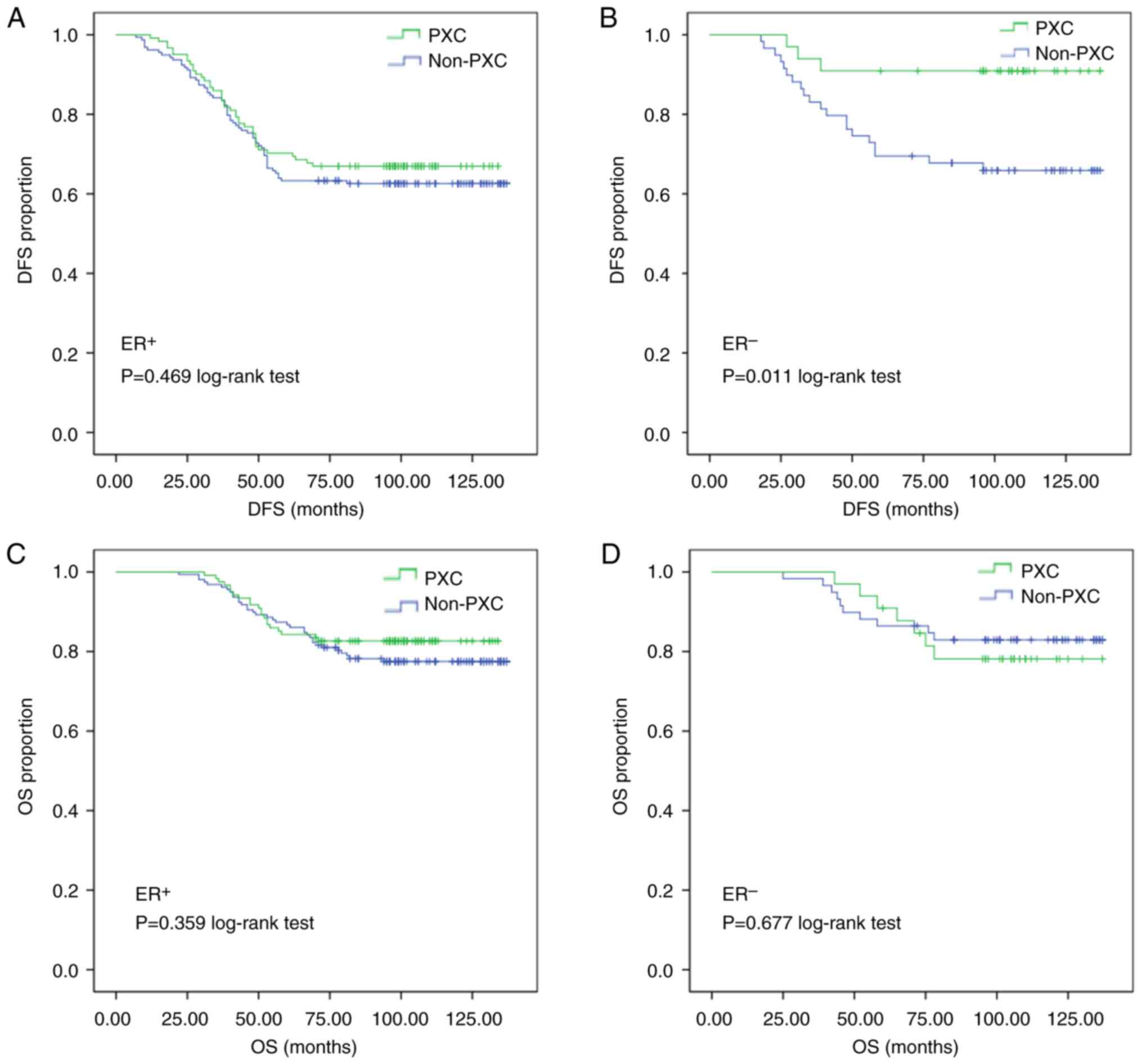
Subgroup analysis was conducted between those who
took PXC for ≥3 months and those who did not. In patients with
hormone ER-negative tumors, the DFS rate in the PXC group (90.9%)
was significantly higher than that in the non-PXC group (66.1%;
P=0.011, Fig. 3B) regardless of
node status; but there was no significant difference in DFS with
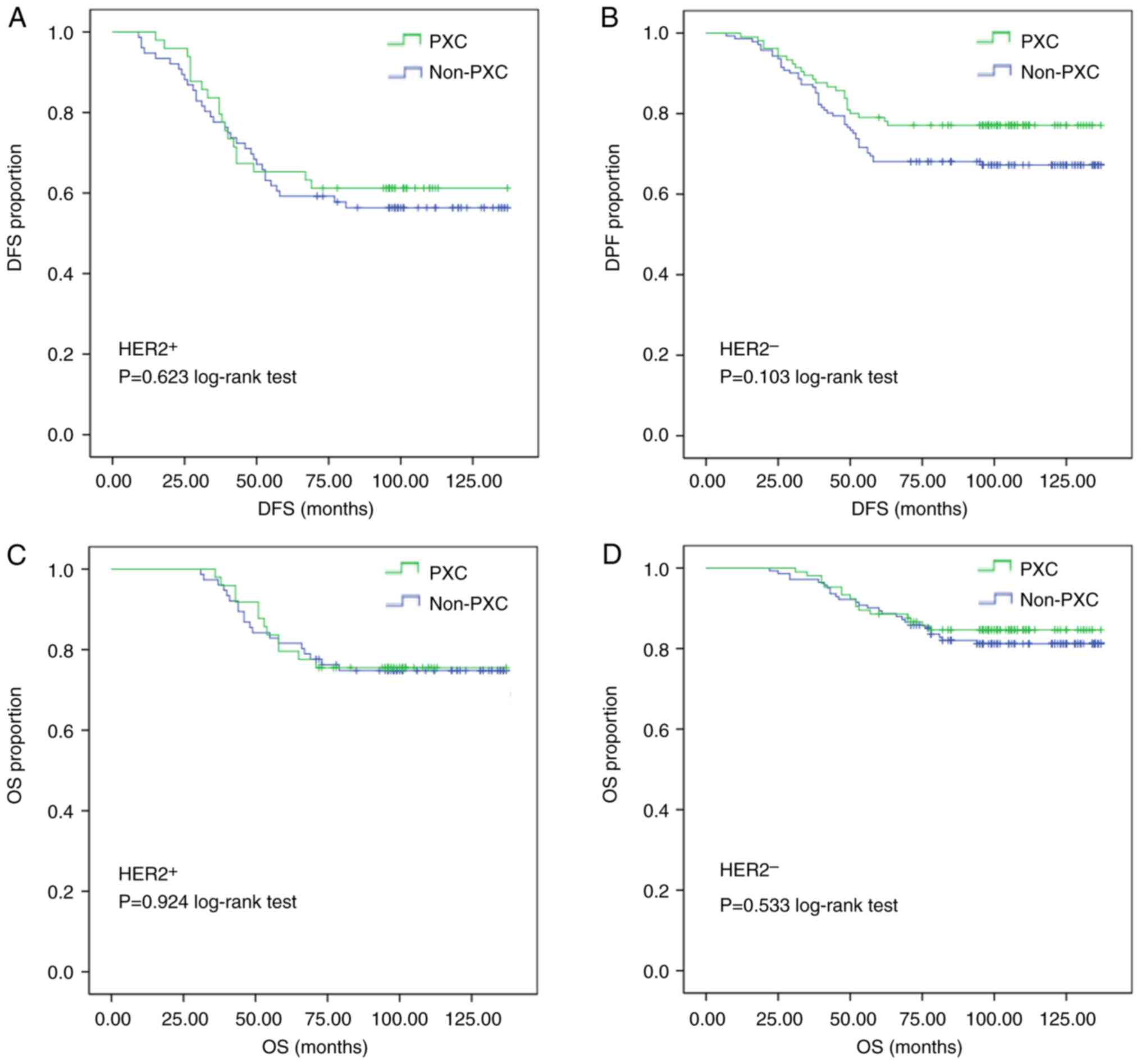
PXC use in those with ER+ tumors (P=0.469, Fig. 3A). As per HER2 status, there was no
statistically significant difference in both DFS and OS between the
PXC group and the non-PXC group (Fig.
4). In summary, DFS was significantly improved in patients with
hormone receptor-negative tumors and in those with ≥3 months of
treatment with PXC.
Prognostic factor analysis
Univariate and multivariate analyses for the risk of
recurrence are summarized in Table
II. Among the six variables in both univariate and multivariate
analyses, three variables were identified to have prognostic
significance: Tumor stage (P<0.001 and P=0.001, respectively),
axillary metastasis (P<0.001 and P<0.001, respectively), and
HER2 status (P=0.009 and P=0.018, respectively), all of which were
associated with a higher risk of recurrence. By contrast, PXC use
was associated with significantly lower risk of recurrence upon
univariate analysis (P=0.046).
 | Table II.Analysis of potential risk factors
for recurrence. |
Table II.
Analysis of potential risk factors
for recurrence.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 1.197
(0.840–1.708) | 0.320 | 1.081
(0.752–1.553) | 0.674 |
| Tumor stage | 1.890
(1.458–2.449) | <0.001 | 1.644
(1.219–2.217) | 0.001 |
| Axillary
metastasis | 2.302
(1.929–2.746) | <0.001 | 2.155
(1.797–2.583) | <0.001 |
| Hormonal
receptor | 1.159
(0.760–1.768) | 0.492 | 0.976
(0.637–1.459) | 0.910 |
| HER2 | 1.613
(1.127–2.310) | 0.009 | 1.548
(1.078–2.224) | 0.018 |
| PXC | 0.853
(0.731–0.997) | 0.046 | 0.918
(0.785–1.073) | 0.284 |
The results of univariate and multivariate analysis
for DFS are summarized in Table
III. Among the six variables in the univariate analysis, tumor
stage (P=0.002) and axillary metastasis (P<0.001) were
identified to have prognostic significance. Multivariate analysis
using Cox's proportional hazard model identified axillary
metastasis (P<0.001) to be an independent prognostic factor for
DFS.
 | Table III.Analysis of possible risk factors for
survival. |
Table III.
Analysis of possible risk factors for
survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 1.197
(0.840–1.708) | 0.320 | 1.049
(0.656–1.677) | 0.842 |
| Tumor stage | 1.711
(1.225–2.389) | 0.002 | 1.302
(0.870–1.950) | 0.200 |
| Axillary
metastasis | 3.180
(2.522–4.010) | 0.000 | 3.045
(2.398–3.867) | 0.000 |
| Hormonal
receptor | 1.114
(0.647–1.916) | 0.697 | 0.861
(0.495–1.497) | 0.596 |
| HER2 | 1.541
(0.968–2.451) | 0.068 | 1.365
(0.852–2.187) | 0.195 |
| PXC | 0.898
(0.739–1.091) | 0.278 | 0.918
(0.785–1.073) | 0.284 |
AEs in patients receiving
chemotherapy
The following incidences of AEs (only those rated
grade 2 or higher were included) were observed in the non-PXC group
and the PXC group with ≥3 months of treatment: 6.0 and 3.3% for
anemia (P=0.409); 41.5 and 23.9% for neutropenia (P=0.003), 4.1 and
0.0% for nausea (P=0.062); 6.0 and 8.7% for fatigue (P=0.388), 5.1
and 5.4% for oral mucositis (P>0.999); 3.7 and 1.1% for diarrhea
(P=0.289); and 6.0 and 3.3% for anorexia (P=0.409), as detailed in
Table IV. The incidences of
neutropenia (P=0.003) of grade 2 or higher were significantly lower
in the PXC group than those in the control group. Compared with
that in the non-PXC group, the incidence of nausea (≥ grade 2) in
the PXC group was markedly reduced (4.1 vs. 0%), although it did
not reach statistical significance (P=0.062; Fisher's exact test).
Notably, all patients had no obvious side effects caused by PXC.
These results suggest that PXC treatment may play a role in
preventing neutropenia and alleviating nausea in patients with EBC
receiving chemotherapy.
 | Table IV.Chemotherapy-related adverse events
(PXC ≥3 months). |
Table IV.
Chemotherapy-related adverse events
(PXC ≥3 months).
|
| PXC ≥3 months | Non-PXC |
|
|---|
|
|
|
|
|
|---|
| Graded groups | n | % | n | % | P-value |
|---|
| Anemia |
|
|
|
| 0.409 |
|
0-1 | 89 | 96.7 | 204 | 94.0 |
|
|
2-4 | 3 | 3.3 | 13 | 6.0 |
|
| Neutropenia |
|
|
|
| 0.003 |
|
0-1 | 70 | 76.1 | 127 | 58.5 |
|
|
2-4 | 22 | 23.9 | 90 | 41.5 |
|
| Nausea |
|
|
|
| 0.062 |
|
0-1 | 92 | 100.0 | 208 | 95.9 |
|
| 2 | 0 | 0 | 9 | 4.1 |
|
| Fatigue |
|
|
|
| 0.388 |
|
0-1 | 84 | 91.3 | 204 | 94.0 |
|
| 2 | 8 | 8.7 | 13 | 6.0 |
|
| Oral mucositis |
|
|
|
| >0.999 |
|
0-1 | 87 | 94.6 | 206 | 94.9 |
|
|
2-3 | 5 | 5.4 | 11 | 5.1 |
|
| Diarrhea |
|
|
|
| 0.289 |
|
0-1 | 91 | 98.9 | 209 | 96.3 |
|
|
2-3 | 1 | 1.1 | 8 | 3.7 |
|
| Anorexia |
|
|
|
| 0.409 |
|
0-1 | 89 | 96.7 | 204 | 94.0 |
|
|
2-3 | 3 | 3.3 | 13 | 6.0 |
|
Discussion
Conventional and targeted therapies for breast
cancer currently include a combination of cytotoxic chemotherapy
drugs and pathway-selective small molecule inhibitors as adjuncts.
However, these treatment options are typically associated with
systemic toxicity, intrinsic or acquired therapeutic resistance,
and the emergence of drug-resistant cancer stem cell populations.
Traditional Chinese medicine is an alternative school of medicine
that utilizes time-tested formulas of medicinal herbs, acupuncture
and mind-body practices to promote holistic healing and its use
dates back >3,000 years (9). In
China, TCH use has been a part of conventional cancer treatment for
>50 years (10). Yet, there
remains a paucity of high-quality clinical trials on TCH regimens.
This gap in knowledge can be attributable to the fact that TCH
formulas are often complex, relying on a concoction of medicinal
herbs that exert varying but synergistic effects. The benefit is
its ability to deliver a multi-target, multi-media and multi-link
system of therapeutics, although its active ingredients, toxicity
and adverse reactions, are often difficult to elucidate. In recent
years, several popular traditional Chinese medicine regimens have
gained increasing recognition as safe adjuncts to cancer treatment,
with little detectable systemic toxicity and adverse effects
(11). Medicinal plants, such as
Tripterygium wilfordii, have been reported to contain
components that have the capability of directly initiating
apoptosis, and T. wilfordii has been reported to induce
apoptosis of cancer cells in hepatocellular carcinoma and lung
cancer A549 cells (12). Similarly,
PXC might be one popular formulation of TCH that also has
components with antitumor effects documented in the literature
(13).
The present study retrospectively analyzed patients
with EBC to investigate the benefits of PXC as an adjunct to
standard of care chemotherapy, endocrine therapy and anti-HER2
therapy. Among all the study subjects, the median DFS time was 101
months, and the completion of adjuvant PXC therapy for ≥3 months
was associated with an increase in DFS rate of 12.5%. In addition,
an increase in DFS rate of 24.8% was observed in ER-negative
patients associated with PXC use. These findings are in support of
the existing body of evidence that Chinese herbal regimens may play
an active role in targeting multiple functional signaling pathways
in hyper-proliferative breast cancer (14,15),
and are associated with the inhibition of cancer growth, the
reduction of metastasis and invasion, the promotion of cancer cell
apoptosis and the enhancement of immunity (16). Notably, curcumin, one of the
bioactive components in PXC, has been reported to inhibit the
proliferation and migration of cervical cancer cells, and cause the
apoptosis of metastatic cells without affecting the normal
epithelial cells (17). Curcumin is
also able to reverse TGF-induced epithelial-mesenchymal
transformation (EMT) in hepatocellular carcinoma by down regulating
the expression of Snail (3).
Distinct EMT transition states present different functions, with
the hybrid EMT state presenting the highest metastatic potential
(18).
Neutropenia and nausea are commonly reported
toxicities associated with chemotherapy (19,20).
Chemotherapy-induced neutropenia and nausea/vomiting are often
ranked by patients as one of the most distressing and feared
consequences of chemotherapy (21,22).
In addition to its antitumor effects, TCH also has the advantages
of a more favorable side-effect profile and lower systemic
toxicity. Use of traditional Chinese herbal formulas has been
reported to reduce the side effects of radiotherapy and
chemotherapy, such as insomnia, depression and fatigue, which
significantly improves the quality of life of patients with cancer
(3). The present results are
consistent with these findings, showing that TCH capsule combined
with chemotherapy significantly improving the survival time and
alleviating adverse events with similar TCH formula use to PXC
(23,24).
Limitations of the present study include a
relatively small sample size and recruitment restricted to a single
institution. Although, to the best of our knowledge, the present
study is first and largest study to date to specifically evaluate
the role of PXC as an adjunct therapy in patients with EBC, the
patient sample did not reveal significant benefits of PXC use in
terms of OS, which is possibly attributable to the limited duration
of PXC treatment and an insufficient length of follow-up. Despite
these factors, the study remains of high generalizability among the
Asian population of Chinese descent, given that the population in
China is highly homogenous in ethnicity, with >90% being Han
Chinese. As with other retrospective cohort studies, the present
study is also inevitably subject to potential selection bias.
Side effects from PXC use itself are rare, but a
non-specific mild drug rash, dizziness and diarrhea have been
reported in the literature (5).
Given its favorable side-effect profile and low level of systemic
toxicity, adjuvant therapy with a PXC course of 5–10 years (as in
current standard endocrine therapy) should be considered for more
robust evaluation of its therapeutic effects and survival benefits.
Side-effect profile assessment with long-term use would also be
beneficial. Therefore, large-scale prospective cohort studies or
randomized clinical trials with a longer duration of treatment and
follow-up (5 to 10 years) would be crucial to further delineate the
efficacy of PXC as an adjunctive therapy for EBC along with its
long-term side-effect profile.
In conclusion, to the best of our knowledge, the
present retrospective cohort study is the first to demonstrate
significant improvement in DFS in patients who used PXC as an
adjunct to their standard breast cancer treatment. This benefit was
most notable in patients with hormone receptor-negative early
breast cancer and when duration of use for PXC was ≥3 months. A
well-designed, largescale prospective study with longer term PXC
treatment duration may be beneficial to further elucidate the
therapeutic effect of PXC as a potent adjuvant agent.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Natural Science
Foundation of Liaoning Province (grant no. 20180551215), the Key
Research and Development Plan Guidance Project of Liaoning Province
(grant no. 2019JH8/10300020) and the Key Project of China Health
Promotion Foundation (grant no. CHPF-RXO180301).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SW and XZ conceived and designed the experiments.
SW, LZ, HJ and XZ analyzed data. CZ and XZ interpreted data and
drafted the manuscript. All authors read and approved the final
manuscript. SW, CZ, LZ, HJ, and XZ confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
The study protocol was reviewed by the China Medical
University Ethics Committee Institutional Review Board (Shenyang,
China; approval no. 2020 NO. 203). Approval was granted prior to
commencement of the study and the requirement for written consent
was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun X, Liu K, Lu S, He W and Du Z:
Targeted therapy and immunotherapy for heterogeneous breast cancer.
Cancers (Basel). 14:54562022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu W, Yang B, Yang L, Kaur J, Jessop C,
Fadhil R, Good D, Ni G, Liu X, Mosaiab T, et al: Therapeutic
effects of ten commonly used chinese herbs and their bioactive
compounds on cancers. Evid Based Complement Alternat Med.
2019:60578372019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang D, Fu Z, Xing Y, Tan Y, Han L, Yu H
and Wang T: Rapid identification of chemical composition and
metabolites of Pingxiao capsule in vivo using molecular networking
and untargeted data-dependent tandem mass spectrometry. Biomed
Chromatogr. 34:e48822020. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao Q, Zhai H, Huang H, Lin J and He W: A
comparative study of the efficacy of tamoxifen and Chinese patented
medicine (Pingxiao capsules) in gynecomastia: A retrospective
cohort study. Andrologia. 54:e146402022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gradishar W and Salerno KE: NCCN
guidelines update: Breast cancer. J Natl Compr Canc Netw. 14 (5
Suppl):S641–S644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Theriault RL, Carlson RW, Allred C,
Anderson BO, Burstein HJ, Edge SB, Farrar WB, Forero A, Giordano
SH, Goldstein LJ, et al: Breast cancer, version 3.2013: Featured
updates to the NCCN guidelines. J Natl Compr Canc Netw. 11:753–761.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen AP, Setser A, Anadkat MJ, Cotliar J,
Olsen EA, Garden BC and Lacouture ME: Grading dermatologic adverse
events of cancer treatments: The common terminology criteria for
adverse events version 4.0. J Am Acad Dermatol. 67:1025–1039. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou P: Traditional Chinese medicine. Comb
Chem High Throughput Screen. 13:8362010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Mao JJ, Wang XS and Lin H:
Evaluation of traditional Chinese medicine herbs in oncology
clinical trials. Cancer J. 25:367–371. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Telang NT: Natural products as drug
candidates for breast cancer (review). Oncol Lett. 26:3492023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Hao J and Chen D: Role of
oxidative stress in anti-cancer activity of Tripterygium
wilfordii. Tradit Med Res. 6:502021. View Article : Google Scholar
|
|
13
|
Barreto C and Jandus A: Role of natural
products in combating cancer. Cancer Insight. 1:35–52. 2022.
|
|
14
|
Arzi L, Mollaei H and Hoshyar R:
Countering triple negative breast cancer via impeding Wnt/β-catenin
signaling, a phytotherapeutic approach. Plants (Basel).
11:21912022.PubMed/NCBI
|
|
15
|
Yang Z, Zhang Q, Yu L, Zhu J, Cao Y and
Gao X: The signaling pathways and targets of traditional Chinese
medicine and natural medicine in triple-negative breast cancer. J
Ethnopharmacol. 264:1132492021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zulfiker AH, Hashimi SM, Good DA, Grice ID
and Wei MQ: Cane toad skin extract-induced upregulation and
increased interaction of serotonin 2A and D2 receptors via Gq/11
signaling pathway in CLU213 cells. J Cell Biochem. 118:979–993.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He G, Mu T, Yuan Y, Yang W, Zhang Y, Chen
Q, Bian M, Pan Y, Xiang Q, Chen Z and Sun A: Effects of notch
signaling pathway in cervical cancer by curcumin mediated
photodynamic therapy and its possible mechanisms in vitro and in
vivo. J Cancer. 10:4114–4122. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abe M, Iihara H and Aogi K: Fosnetupitant
for the prevention of chemotherapy-induced nausea and vomiting: A
short review and clinical perspective. Adv Ther. 40:1913–1925.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gascón P, Awada A, Karihtala P, Lorenzen S
and Minichsdorfer C: Optimal use of granulocyte colony-stimulating
factor prophylaxis to improve survival in cancer patients receiving
treatment: An expert view. Wien Klin Wochenschr. Nov 27–2023.(Epub
ahead of print). PubMed/NCBI
|
|
21
|
Kuchuk I, Bouganim N, Beusterien K,
Grinspan J, Vandermeer L, Gertler S, Dent SF, Song X, Segal R,
Mazzarello S, et al: Preference weights for chemotherapy side
effects from the perspective of women with breast cancer. Breast
Cancer Res Treat. 142:101–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun CC, Bodurka DC, Weaver CB, Rasu R,
Wolf JK, Bevers MW, Smith JA, Wharton JT and Rubenstein EB:
Rankings and symptom assessments of side effects from chemotherapy:
Insights from experienced patients with ovarian cancer. Support
Care Cancer. 13:219–227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ge A, Yang K, Deng X, Zhao D, Ge J and Liu
L: The efficacy and safety of Xihuang Pill/capsule in adjuvant
treatment of breast cancer: A systematic review and meta-analysis
of 26 randomized controlled trials. J Ethnopharmacol.
295:1153572022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng W, Xu Y, Feng F, Gu C, Wang Z, Han D,
Zhou X and He H: Meta-analysis of therapy of cinobufacini capsule
adjunct with first-line platinum-based chemotherapy for the
treatment of advanced NSCLC. Evid Based Complement Alternat Med.
2021:55964152021. View Article : Google Scholar : PubMed/NCBI
|