Introduction
Advanced gastric cardiac cancer (AGCC) is a
challenging malignancy that typically presents with dysphagia and
primarily affects the proximal stomach, often extending to the
gastroesophageal junction. Previous studies showed that 23.3% of
early gastric cancer was gastric cardiac carcinoma in China
(1), which is a higher rate
compared with the 7.0% recorded in Japan (2) and the 11.9% recorded in a Western
cohort (3). Patients with AGCC face
the risk of malnutrition and decreased quality of life (QoL)
(4–8). In one study, the 5-year survival rate
was <10% at the advanced stage (9). Dysphagia further complicates
treatment, as it hampers the delivery of oral medications, reducing
the effectiveness of systemic chemotherapy (SYS). As a result,
malignant dysphagia is a common and difficult clinical
manifestation in cases of AGCC, necessitating effective symptom
management for enhanced patient comfort (4–6,10).
According to literature reports, 60–90% of patients with cancer at
the gastroesophageal junction experience significant swallowing
difficulties (11–14). SYS is the mainstay treatment for
AGCC, aiming to improve symptom remission rates and prolong patient
survival; however, the efficacy of SYS alone is limited,
highlighting the need for alternative approaches (15).
Endoscopic stent implantation is a conventional
treatment option for alleviating dysphagia caused by AGCC; however,
stent placement carries the risk of complications such as stent
obstruction, migration and perforation (7,16–19).
Other interventions, such as percutaneous gastrostomy and
naso-jejunal feeding, have demonstrated a certain amount of
symptomatic relief, but are associated with higher risks of
complications, including infections, bleeding and dislodgement
(20,21). Therefore, gastric transcatheter
chemoembolization (GTC), a minimally invasive technique, has
emerged as a promising therapeutic option for AGCC with dysphagia.
This technique involves the intra-arterial infusion of
chemotherapeutic drugs directly into the tumor-feeding artery,
followed by the injection of embolic agents to induce ischemic
necrosis of the tumor. GTC allows for high local drug
concentrations, effectively reducing tumor vascularity and size,
thereby improving dysphagia control in patients with AGCC (4–6,10,22–24).
Despite its potential benefits, the use of GTC in
combination with SYS for AGCC with dysphagia remains controversial,
and comparative studies with SYS alone are lacking. Therefore, the
objective of the present retrospective study is to evaluate the
efficacy of combining GTC with SYS compared with SYS alone in
patients with AGCC and dysphagia.
Materials and methods
Study design and participants
A retrospective review was performed of consecutive
patients with AGCC who presented with dysphagia and underwent
either SYS alone or a combination of GTC and SYS between January
2018 and December 2022. The patients and their families made the
decision to undergo GTC after consultation with the
multidisciplinary team of experts from the gastric cancer group of
the Affiliated Cancer Hospital of Shandong First Medical University
of China (Jinan, China). This decision was influenced by the
presence of dysphagia symptoms, irrespective of tumor size. AGCC
diagnosis was confirmed through endoscopy and biopsy, and staging
was performed using the 8th edition of the American Joint Committee
on Cancer (AJCC) criteria (25). To
be included in the present study, patients had to meet the
following criteria: i) Histologically-confirmed AGCC; ii) advanced
stage of the disease (AJCC stage III or IV); iii) dysphagia as a
presenting symptom [at least Ogilvie Dysphagia Scale (26) grade 1]; iv) age ≥18 years; v)
Eastern Cooperative Oncology Group (ECOG) performance status of
0–2; vi) treatment with either GTC combined with SYS or SYS alone;
and vii) sufficient medical records available for review. The
exclusion criteria included the following: i) Previous treatment
for AGCC; ii) co-existing malignancies; and iii) other significant
comorbidities.
Procedures
Systemic chemotherapy
The SYS group received a combination of two
first-line chemotherapy regimens: tegafur-gimeracil-oteracil (S-1)
plus oxaliplatin (SOX) (27)r
capecitabine and oxaliplatin (XELOX) (28). In the SOX regimen, patients took
oral S-1 at a dosage of 40–60 mg twice a day on days 1–14, every 3
weeks, and received intravenous oxaliplatin at a dosage of 130
mg/m2 on day 1, every 3 weeks. For the XELOX regimen,
patients took oral capecitabine at a dosage of 1,000
mg/m2 twice a day on days 1–14, every 3 weeks, and
received intravenous oxaliplatin at a dosage of 130
mg/m2 over 2 h on day 1, every 3 weeks.
Second-line chemotherapy regimens included
irinotecan (29) and paclitaxel
monotherapy (30). In the
irinotecan monotherapy regimen, patients received intravenous
irinotecan at a dosage of 150 mg/m2 on days 1 and 15,
every 4 weeks. For the paclitaxel monotherapy regimen, patients
received intravenous paclitaxel at a dosage of 60 mg/m2
on days 1 and 8, every 3 weeks.
The decision to switch from first-line to
second-line chemotherapy was based on several factors, including
disease progression, treatment response and tolerance. The specific
timing and criteria for switching were determined by the treating
oncologist.
GTC
GTC was performed by an experienced interventional
radiologist under local anesthesia and conscious sedation. The
patient was positioned supine, and access to the right femoral
artery was obtained using the Seldinger technique. A 5-French (5-F)
sheath was inserted, and a 5-F diagnostic catheter was advanced
into the abdominal aorta under fluoroscopic guidance. Arteriography
of the abdominal trunk was then performed to identify the supply
arteries of the tumor. If necessary, additional arterial
angiography, including the right gastric artery, short gastric
arteries, posterior gastric artery, gastro-epiploic artery and left
inferior phrenic artery, was performed to gain a comprehensive
understanding of the blood supply arteries of the tumor. If the
tumor received blood supply from sources other than the left
gastric artery, 300–500-µm Embosphere microspheres (Merit Medical
Systems, Inc.) were used to embolize certain non-primary blood
supply arteries. Subsequently, a 0.022-inch microcatheter
(PROGREAT®; Terumo Medical Corporation) was inserted
into the left gastric artery, and fluorouracil (5-FU) (at a dose of
1,000 mg/m2) was slowly injected via the microcatheter
over a period of >10 min. Following the chemotherapy infusion,
Embosphere microspheres (300–500 µm) were injected to embolize the
left gastric artery. The procedure was determined to be successful
when angiography demonstrated that there was no residual arterial
flow to the tumor. The imaging data for a 55-year-old patient
diagnosed with AGCC who underwent GTC are presented in Fig. S1A-M.
After the intervention, pressure was applied to the
femoral artery for 15 min, and patients were closely monitored for
24 h post-procedure to detect any signs of abdominal pain, fever or
other complications. Routine blood tests, including liver and
kidney function tests, were performed before and after the
procedure to identify any changes.
Each cycle was scheduled at intervals of 4 weeks,
and patients received GTC treatment once within each cycle. The
number of cycles administered to each patient varied based on their
response to treatment and the discretion of the treating
physician.
Assessment of dysphagia severity,
nutritional status, QoL and adverse events (AEs)
The primary outcome measure of the present study was
symptom remission, which included improvement in dysphagia
severity, QoL and nutritional status. These measures were evaluated
at baseline, 4 weeks, and 8 weeks after the first cycle of SYS, and
were regularly assessed via telephone or at an outpatient clinic
visit.
Dysphagia was evaluated using the Ogilvie Dysphagia
Scale, which grades dysphagia into 5 levels: 0, no dysphagia; 1,
normal diet with certain food restrictions (such as raw apple and
steak); 2, semi-solid diet; 3, fluids only; and 4, complete
dysphagia, even for liquids.
Nutritional status was assessed by experienced
dietitians using the scored Patient-Generated-Subjective Global
Assessment (PG-SGA) scale (31).
The PG-SGA scale consists of two parts: Patient self-assessment and
assessment by medical staff. It evaluates weight, intake, symptoms
affecting intake, functional capacity, metabolic demands and
physical assessment. Patients assessed the first four aspects
themselves, whilst medical staff assessed the last two. Each item
was given a score of 0–4 based on its impact on nutritional status,
and the total scores were then summed. Nutritional status was
categorized as well-nourished (0–1 points), suspected malnutrition
(2–3 points), moderate malnutrition (4–8 points), or severe
malnutrition (≥9 points) based on the total score.
QoL was assessed using the Functional Assessment of
Cancer Therapy-General (FACT-G)7 scale (32), which is a condensed version of the
FACT-G scale (33). It includes
three items from the physical wellbeing subscale (fatigue, pain and
nausea), one item from the emotional wellbeing subscale (concern
about the condition worsening), and three items from the functional
wellbeing subscale (enjoyment of life, contentment with QoL and
sleep). Each item is graded on a 5-point scale, ranging from ‘not
at all’ (0) to ‘very much’ (4). The
functional wellbeing subscale is directly scored from 0–4 points,
whilst the physical wellbeing subscale and emotional wellbeing are
scored in reverse. The scores from FACT-G7 are summed on a scale of
0–28, with higher scores indicating a better QoL.
AEs were recorded and graded according to the Common
Terminology Criteria for Adverse Events version 5.0 (34). Specific AEs related to GTC such as
fever, abdominal pain and gastrointestinal ulcers were also
assessed.
Treatment response
All patients were regularly followed up after
receiving treatment. The response to treatment was assessed using
computed tomography (CT) or magnetic resonance imaging at 1, 2 and
6 months after the first cycle of GTC. Evaluation of treatment
response was performed in accordance with the Response Evaluation
Criteria in Solid Tumors version 1.1 (35). Overall survival (OS) was defined as
the duration from the initiation of treatment to either death from
any cause or the last follow-up. Progression-free survival (PFS)
was defined as the period from the start of treatment to disease
progression or death from any cause. Objective response rate (ORR)
was defined as the % of patients who achieved either a complete
response (CR) or partial response (PR) to the treatment. CR was
defined as the disappearance of all target lesions, whilst PR was
defined as a ≥30% decrease in the sum of the diameters of target
lesions. Disease control rate (DCR) was defined as the % of
patients who achieved CR, PR or stable disease (SD). SD was defined
as neither PR nor PD.
Statistical analysis
All statistical analyses were performed using
MatchIt package version 4.5.5 (36)
and the R software version 4.2.2 (R Core Team; http://www.R-project.org/). Baseline characteristics
are presented as the median (interquartile range) for continuous
variables and the n (%) for categorical variables. The maximum
diameter of the tumor at the cardia, Ogilvie score, PG-SGA score
and FACT-G7 score were compared between the two groups at 4 and 8
weeks after the first cycle of SYS. For comparison between groups
of categorical data, Fisher's exact test or the χ2 test
was used. The Wilcoxon rank sum test was used for the analysis of
continuous variables. Univariate and multivariate logistic
regression analysis was performed to determine the independent
factors associated with presence of dysphagia after 8 weeks of the
first cycle of SYS. Kaplan-Meier curves and the log-rank test were
used to compare the OS and PFS between the groups. Subgroup
analyses were performed to evaluate the impact of baseline
characteristics on OS, and the interaction between the treatment
groups and subgroups was tested using Cox proportional hazards
regression analysis. P<0.05 was considered to indicate a
statistically significant difference.
Propensity score matching (PSM) was used to minimize
the influence of confounding factors and improve the validity of
the findings, making the results more applicable to real-world
clinical practice. PSM were calculated using logistic regression,
accounting for the aforementioned demographic and clinical
characteristics. Furthermore, 1:1 greedy nearest neighbor matching
was performed with a caliper of 0.3. The method functionally relied
on the MatchIt R package. The distance between the unit of one
group and another was calculated, and each unit was assigned a
control unit as a match. The matching was ‘greedy’ as no action was
taken to optimize an overall criterion; each match was selected
without accounting for the other matches that may have subsequently
occurred. Fig. S2 presents the
balance of baseline covariates before and after PSM for both the
GTC + SYS and SYS alone groups.
Results
Study population
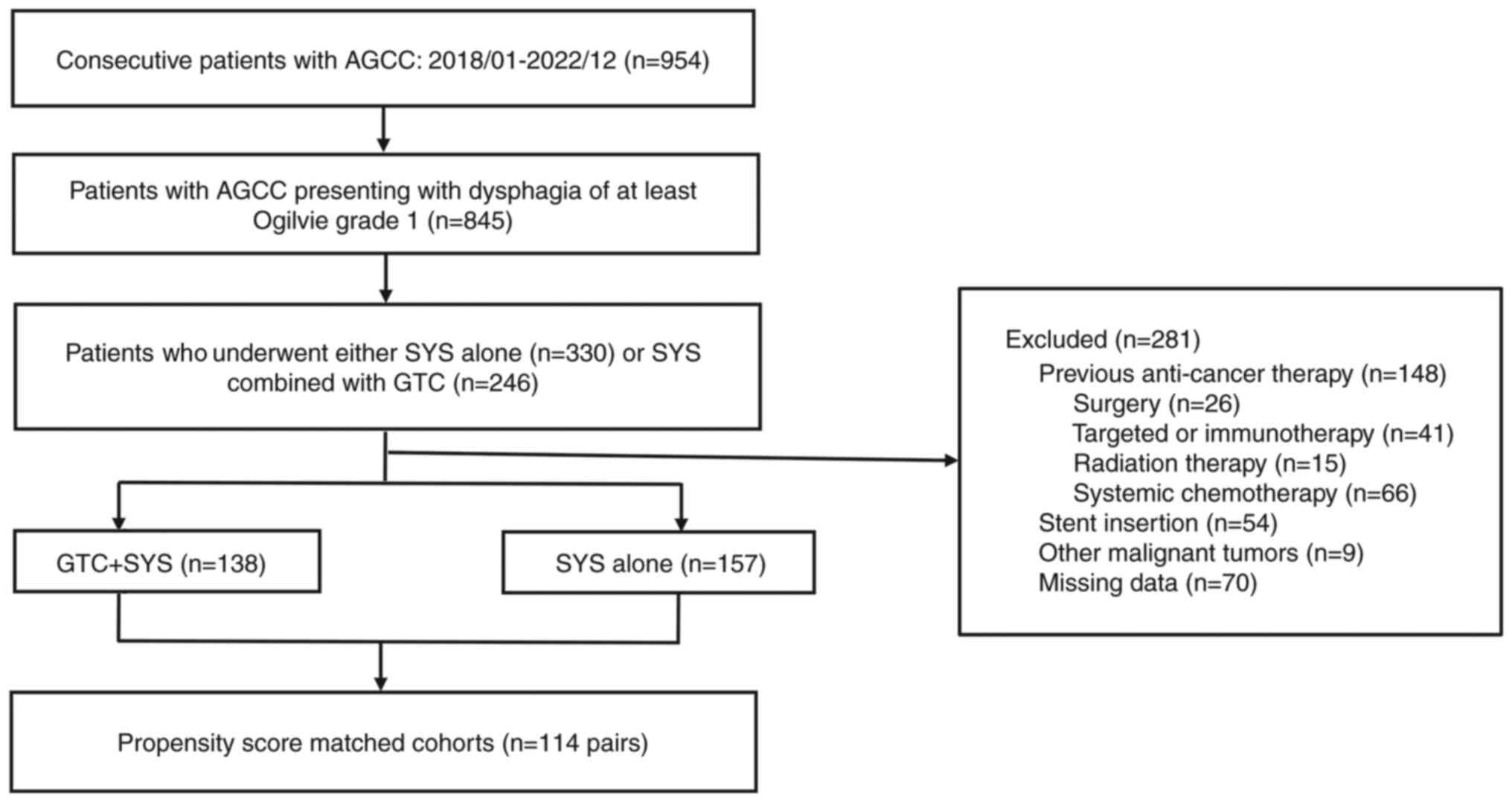
Initially, 954 patients were screened, and a total
of 295 patients with AGCC presenting with dysphagia, who matched
the inclusion criteria, were included in the present retrospective
study. Of these, 138 patients received GTC combined with SYS,
whilst 157 patients received SYS alone. PSM yielded 114
well-balanced pairs in terms of baseline demographics and clinical
characteristics. A notable reduction in SMDs after matching can be
observed, indicating that the PSM effectively improved the balance
of covariates between the two treatment groups. This improvement in
balance allowed for a more reliable comparison of treatment
effectiveness, as the potential influence of confounding factors
was minimized. The screening process is presented in Fig. 1.
Table I presents the
baseline characteristics of the study population before and after
PSM. Prior to PSM, there were significant differences in ECOG, WBC,
Ogilvie score, PG-SGA score, and the first-line chemotherapy
regimen between the two groups. Following PSM, the baseline
characteristics were well balanced between the two groups and there
were no significant differences. The majority of participants were
male, accounting for 73% in the GTC + SYS group and 70% in the SYS
group. The age distribution showed a median of 62 years in both
groups. Biomarker analysis revealed no significant differences
between the two groups. The main tumor characteristics also did not
significantly differ. The median follow-up time for the GTC + SYS
group was 12 months (range, 6–24 months), whilst it was 10 months
(range, 5–22 months) for the SYS alone group. There was no
statistically significant difference in the follow-up time between
the two groups (P=0.234). In the GTC + SYS group, the median number
of GTC procedures performed was 2, with a range of 1–4
procedures.
 | Table I.Baseline characteristics of patients
before and after propensity score matching. |
Table I.
Baseline characteristics of patients
before and after propensity score matching.
|
| Unmatched | Matched |
|---|
|
|
|
|
|---|
| Characteristic | SYS (n=157) | GTC + SYS
(n=138) | P-value | SYS (n=114) | GTC + SYS
(n=114) | P-value |
|---|
| Sex |
|
| 0.320 |
|
| 0.660 |
|
Male | 103 (66) | 98 (71) |
| 80 (70) | 83 (73) |
|
|
Female | 54 (34) | 40 (29) |
| 34 (30) | 31 (27) |
|
| Age, years | 62 (57–65) | 63 (55–68) | 0.193 | 62 (59–66) | 62 (55–68) | 0.638 |
| ECOG |
|
| 0.014a |
|
| 0.618 |
| 0 | 15 (10) | 12 (9) |
| 12 (11) | 11 (10) |
|
| 2 | 36 (23) | 53 (38) |
| 35 (31) | 42 (37) |
|
| 1 | 106 (68) | 73 (53) |
| 67 (59) | 61 (54) |
|
| WBCs, n
(×109/l) | 5.69
(4.31–7.16) | 6.23
(4.65–8.19) | 0.026a | 5.70
(4.22–7.14) | 6.07
(4.58–8.19) | 0.099 |
| HGB, g/l | 129 (114–141) | 129 (112–138) | 0.687 | 129 (114–141) | 130 (112–139) | 0.926 |
| PLTs, n
(×109/l) | 229 (164–290) | 216 (162–278) | 0.432 | 211 (155–289) | 216 (165–278) | 0.674 |
| AST, U/l | 23 (16–31) | 25 (17–39) | 0.405 | 25 (17–32) | 24 (17–37) | 0.759 |
| Albumin, g/l | 40.6
(38.3–43.7) | 39.7
(36.0–42.6) | 0.263 | 40.6
(37.7–43.7) | 40.1
(36.0–43.8) | 0.586 |
| Cr, µmol/l | 59 (37–78) | 61 (52–72) | 0.069 | 60 (37–78) | 61 (52–72) | 0.195 |
| CEA, ng/ml | 4 (2–9) | 4 (2–16) | 0.283 | 4 (2–11) | 4 (2–16) | 0.658 |
| CA 19-9, U/ml | 30 (12–166) | 36 (12–288) | 0.398 | 28 (12–152) | 36 (12–216) | 0.528 |
| Maximum diameter of
the tumor at the cardia, cm | 3.40
(2.70–4.10) | 3.50
(2.90–4.30) | 0.268 | 3.40
(2.80–4.10) | 3.50
(2.90–4.18) | 0.930 |
| Grade of
differentiation |
|
| 0.634 |
|
| >0.999 |
|
Poor | 111 (71) | 101 (73) |
| 80 (70) | 80 (70) |
|
|
Well | 10 (6) | 11 (8) |
| 9 (8) | 9 (8) |
|
|
Moderate | 36 (23) | 26 (19) |
| 25 (22) | 25 (22) |
|
| TNM stage |
|
| 0.811 |
|
| 0.791 |
|
III | 82 (52) | 74 (54) |
| 62 (54) | 60 (53) |
|
| IV | 75 (48) | 64 (46) |
| 52 (46) | 54 (47) |
|
| Ogilvie score |
|
| 0.024a |
|
| 0.684 |
| 1 | 6 (4) | 11 (8) |
| 6 (5) | 8 (7) |
|
| 2 | 39 (25) | 49 (36) |
| 35 (31) | 39 (34) |
|
| 3 | 112 (71) | 78 (57) |
| 73 (64) | 67 (59) |
|
| PG-SGA Score | 9 (7–10) | 9 (8–11) | 0.024a | 9 (8–11) | 9 (8–10) | 0.570 |
| FACT-G7 Score | 9 (7–11) | 9 (7–10) | 0.537 | 9 (7–11) | 9 (7–10) | 0.548 |
| Trastuzumab |
|
| 0.052 |
|
| 0.467 |
|
Yes | 60 (38) | 38 (28) |
| 31 (27) | 36 (32) |
|
| No | 97 (62) | 100 (72) |
| 83 (73) | 78 (68) |
|
| Immunotherapy |
|
| 0.094 |
|
| 0.546 |
| No | 60 (38) | 38 (28) |
| 31 (27) | 36 (32) |
|
|
Tislelizumab | 60 (38) | 55 (40) |
| 49 (43) | 41 (36) |
|
|
Sintilimab | 37 (24) | 45 (33) |
| 34 (30) | 37 (32) |
|
| First line
chemotherapy regimen |
|
| 0.033a |
|
| 0.891 |
|
SOX | 82 (52) | 89 (64) |
| 72 (63) | 73 (64) |
|
|
XELOX | 75 (48) | 49 (36) |
| 42 (37) | 41 (36) |
|
| Second line
chemotherapy regimen |
|
| 0.872 |
|
| 0.891 |
|
Irinotecan | 97 (62) | 84 (61) |
| 71 (62) | 72 (63) |
|
|
Paclitaxel | 60 (38) | 54 (39) |
| 43 (38) | 42 (37) |
|
| Number of first
line therapy cycles | 5 (4–6) | 5 (4–6) | 0.859 | 5 (4–6) | 5 (4–6) | 0.585 |
| Number of second
line therapy cycles | 6 (4–7) | 6 (4–7) | 0.732 | 6 (4–7) | 6 (4–7) | 0.881 |
Comparison of the clinical symptoms
and clinical symptom relief between the two groups
Comparison of the severity of dysphagia between
the groups
At baseline, there were no significant differences
in the severity of dysphagia between the two treatment groups
(P>0.05). After 4 weeks of the initial cycle of SYS, the
severity of dysphagia in the SYS group was significantly higher
compared with the GTC + SYS group (P<0.001). Following the
initial treatment period, it was observed that ~25% of patients in
the GTC + SYS group no longer experienced symptoms of dysphagia,
whilst all patients in the SYS group continued to report
difficulties with swallowing. This trend persisted at 8 weeks after
the first cycle of SYS, with the SYS group also experiencing
significantly more severe dysphagia than the GTC + SYS group
(P<0.001; Table SI).
Specifically, at both 4 and 8 weeks, the GTC + SYS group
demonstrated significantly lower median Ogilvie scores than the SYS
only group (Table II).
Additionally, at 8 weeks after the initial treatment, the
percentage of patients with dysphagia in the SYS group was
significantly higher compared with that in the GTC + SYS group (84
vs. 28%; P<0.001). Regarding clinical symptom relief, the GTC +
SYS group had a significantly higher rate of improvement in
dysphagia compared with the SYS group (P<0.05). Specifically,
110/114 patients in the GTC + SYS group reported improvement in
dysphagia symptoms, whilst only 86/114 patients in the SYS group
reported similar improvements at 8 weeks after the first cycle of
SYS (96 vs. 75%; P<0.001) (data not shown).
 | Table II.Summary of symptom scores and maximum
diameter of the tumor at the cardia of patients before and after
treatment. |
Table II.
Summary of symptom scores and maximum
diameter of the tumor at the cardia of patients before and after
treatment.
|
| Treatment |
|
|---|
|
|
|
|
|---|
| Characteristic | GTC + SYS
(n=114) | SYS (n=114) | P-value |
|---|
| Ogilvie score |
|
|
|
|
Baseline | 3.00
(2.00–3.00) | 3.00
(2.00–3.00) | 0.393 |
| 4 weeks
after the first cycle of SYS | 1.00
(1.00–1.00) | 2.00
(2.00–2.00) |
<0.001a |
| 8 weeks
after the first cycle of SYS | 0.00
(0.00–1.00) | 2.00
(1.25–2.00) |
<0.001a |
| PG-SGA score |
|
|
|
|
Baseline | 9.00
(8.00–10.00) | 9.00
(8.00–11.00) | 0.570 |
| 4 weeks
after the first cycle of SYS | 2.00
(1.00–2.00) | 6.00
(4.25–8.75) |
<0.001a |
| 8 weeks
after the first cycle of SYS | 1.00
(0.00–1.00) | 3.00
(3.00–6.00) |
<0.001a |
| FACT-G7 score |
|
|
|
|
Baseline | 9.0 (7.0–10.0) | 9.0 (7.0–11.0) | 0.548 |
| 4 weeks
after the first cycle of SYS | 13.0
(11.0–14.0) | 10.50
(9.0–12.0) |
<0.001a |
| 8 weeks
after the first cycle of SYS | 17.5
(16.0–19.0) | 12.0
(11.0–14.0) |
<0.001a |
| Maximum diameter of
the tumor at the cardia, cm |
|
|
|
|
Baseline | 3.50
(2.90–4.18) | 3.40
(2.80–4.10) | 0.954 |
| 4 weeks
after the first cycle of SYS | 1.60
(1.10–2.38) | 2.70
(1.93–3.30) |
<0.001a |
| 8 weeks
after the first cycle of SYS | 0.90
(0.60–1.20) | 2.20
(1.40–2.80) |
<0.001a |
| Reduction in
maximum diameter of the tumor at the cardia, cm |
|
|
|
| 4 weeks
after the first cycle of SYS | 1.7 (1.6–2.0) | 0.8 (0.6–1.1) |
<0.001a |
| 8 weeks
after the first cycle of SYS | 2.7 (1.9–3.2) | 1.3 (1.2–1.5) |
<0.001a |
Comparison of nutritional status and
QoL between groups
There were significant differences for nutritional
status and QoL between the groups at 4 and 8 weeks following the
initial cycle of SYS. Specifically, both at 4 and 8 weeks post the
first cycle of SYS, the GTC + SYS group demonstrated significantly
lower median PG-SGA scores and significantly higher median FACT-G7
scores compared with the SYS group (P<0.001; Table II). At 4 weeks post the initial SYS
cycle, there was a significantly higher proportion of
well-nourished individuals in the GTC + SYS group than in the SYS
group, whilst the SYS group had a significantly higher proportion
of severe and moderate malnutrition cases than the GTC + SYS group
(P<0.001; Table SI). By the end
of 4 weeks of treatment, ~41% of patients in the GTC + SYS group no
longer displayed signs of malnutrition, whereas all patients in the
SYS group still exhibited varying degrees of malnutrition (Table SI). This pattern persisted at 8
weeks post the first SYS cycle, with a significantly higher
proportion of well-nourished individuals in the GTC + SYS group
than the SYS group, and the SYS group displaying significantly more
cases of suspected, moderate and severe malnutrition than the GTC +
SYS group (P<0.001). After 8 weeks following the initial SYS
cycle, the improvement in malnutrition status was compared between
the two groups. In the GTC + SYS group, 99 patients (87%) showed
notable improvements in their malnutrition status, whilst in the
SYS alone group, only 8 patients (7%) showed a marked improvement.
The difference in improvement rates between the two groups was
found to be statistically significant (P<0.001; Table SI).
Comparison of the maximum diameter of
the tumor at the cardia between groups
A similar trend was observed for the maximum
diameter of the tumor at the cardia. Significant reductions in
tumor size were seen in the GTC + SYS group compared with the SYS
group at 4 and 8 weeks after the first cycle of SYS. Specifically,
after 4 weeks of the first cycle of SYS, the median reduction in
the maximum tumor diameter in the GTC + SYS group was 1.7 cm,
compared with 0.8 cm in the SYS alone group (P<0.001).
Similarly, after 8 weeks of the first cycle of SYS, the median
reduction in the maximum tumor diameter in the GTC + SYS group was
2.7 cm, compared with 1.3 cm in the SYS alone group (P<0.001).
After 4 weeks of the first cycle of SYS, the median maximum tumor
diameter at the gastric cardia in the GTC + SYS group was
significantly smaller than in the SYS alone group (P<0.001).
This difference was even more pronounced at 8 weeks after the first
cycle of SYS, with the median maximum tumor diameter being
significantly smaller in the GTC + SYS group compared with the SYS
alone group (P<0.001; Table
II).
Independent predictors of the presence
of dysphagia at 8 weeks after the first cycle of SYS
Univariate logistic regression analysis of factors
associated with presence of dysphagia at 8 weeks after the first
cycle of SYS demonstrated that treatment with GTC + SYS was the
only significant independent factor for reducing the occurrence of
dysphagia [odds ratio (OR)=0.07; 95% CI, 0.04–0.14; P<0.001).
Multivariable logistic regression then confirmed that treatment
with GTC + SYS remained the only significant factor for reducing
the occurrence of dysphagia at 8 weeks after the first cycle of SYS
(OR=0.07; 95% CI, 0.03–0.13; P<0.001; Table III). The model coefficients for
dysphagia and the model fit measures are shown in Tables SII and SIII, respectively, and the Omnibus
likelihood ratio tests are shown in Table SIV.
 | Table III.Univariate and multivariate analysis
of predictive factors for presence of dysphagia symptoms after 8
weeks of the first cycle of SYS. |
Table III.
Univariate and multivariate analysis
of predictive factors for presence of dysphagia symptoms after 8
weeks of the first cycle of SYS.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Characteristic | n | Event n | OR (95% CI) | P-value | n | Event n | OR (95% CI) | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
Male | 163 | 93 | - |
| 163 | 93 | - |
|
|
Female | 65 | 35 | 0.88
(0.49–1.57) | 0.659 | 65 | 35 | 0.65
(0.31–1.35) | 0.250 |
| Age |
|
|
|
|
|
|
|
|
| ≤60
years | 90 | 46 | - |
| 90 | 46 | - |
|
| >60
years | 138 | 82 | 1.40
(0.82–2.40) | 0.217 | 138 | 82 | 1.10
(0.55–2.18) | 0.781 |
| ECOG |
|
|
|
|
|
|
|
|
| 0 | 23 | 11 | - |
| 23 | 11 | - |
|
| 1 +
2 | 205 | 117 | 1.45
(0.61–3.49) | 0.399 | 205 | 117 | 1.84
(0.59–5.85) | 0.291 |
| CEA, ng/ml |
|
|
|
|
|
|
|
|
| ≤5 | 125 | 71 | - |
| 125 | 71 | - |
|
|
>5 | 103 | 57 | 0.94
(0.56–1.60) | 0.825 | 103 | 57 | 0.94
(0.47–1.86) | 0.851 |
| CA 19-9, U/ml |
|
|
|
|
|
|
|
|
|
>40 | 106 | 60 | - |
| 106 | 60 | - |
|
|
≤40 | 122 | 68 | 0.97
(0.57–1.63) | 0.895 | 122 | 68 | 0.84
(0.41–1.72) | 0.638 |
| Grade of
differentiation |
|
|
|
|
|
|
|
|
|
Poor | 160 | 89 | - |
| 160 | 89 | - |
|
|
Well | 18 | 9 | 0.80
(0.30–2.15) | 0.650 | 18 | 9 | 0.78
(0.22–2.79) | 0.697 |
|
Moderate | 50 | 30 | 1.20
(0.63–2.31) | 0.586 | 50 | 30 | 1.36
(0.58–3.31) | 0.487 |
| Trastuzumab |
|
|
|
|
|
|
|
|
|
Yes | 67 | 36 | - |
| 67 | 36 | - |
|
| No | 161 | 92 | 1.15
(0.65–2.04) | 0.636 | 161 | 92 | 1.20
(0.57–2.53) | 0.639 |
| First line
chemotherapy regimen |
|
|
|
|
|
|
|
|
|
SOX | 145 | 80 | - |
| 145 | 80 | - |
|
|
XELOX | 83 | 48 | 1.11
(0.65–1.93) | 0.697 | 83 | 48 | 1.11
(0.52–2.37) | 0.784 |
| Number of first
line therapy cycles |
|
|
|
|
|
|
|
|
| ≤5 | 152 | 89 | - |
| 152 | 89 | - |
|
|
>5 | 76 | 39 | 0.75
(0.43–1.30) | 0.300 | 76 | 39 | 0.86
(0.42–1.75) | 0.671 |
| Second line
chemotherapy regimen |
|
|
|
|
|
|
|
|
|
Irinotecan | 143 | 75 | - |
| 143 | 75 | - |
|
|
Paclitaxel | 85 | 53 | 1.50
(0.87–2.61) | 0.146 | 85 | 53 | 1.76
(0.87–3.66) | 0.121 |
| Number of second
line therapy cycles |
|
|
|
|
|
|
|
|
| ≤5 | 104 | 59 | - |
| 104 | 59 | - |
|
|
>5 | 124 | 69 | 0.96
(0.56–1.62) | 0.869 | 124 | 69 | 0.96
(0.49–1.88) | 0.911 |
| Treatment |
|
|
|
|
|
|
|
|
|
SYS | 114 | 96 | - |
| 114 | 96 | - |
|
| GTC +
SYS | 114 | 32 | 0.07
(0.04–0.14) |
<0.001a | 114 | 32 | 0.07
(0.03–0.13) |
<0.001a |
Comparison of treatment prognosis
between groups
Short-term treatment efficacy
There were no cases of complete remission in either
treatment group. The rates of CR and PR, SD and PD were not
significantly different between the two groups. Additionally, the
ORR (45% vs. 45%) and DCR) (82% vs. 79%) showed no statistically
significant difference between the two groups (P>0.05) (data not
shown).
Long-term treatment efficacy
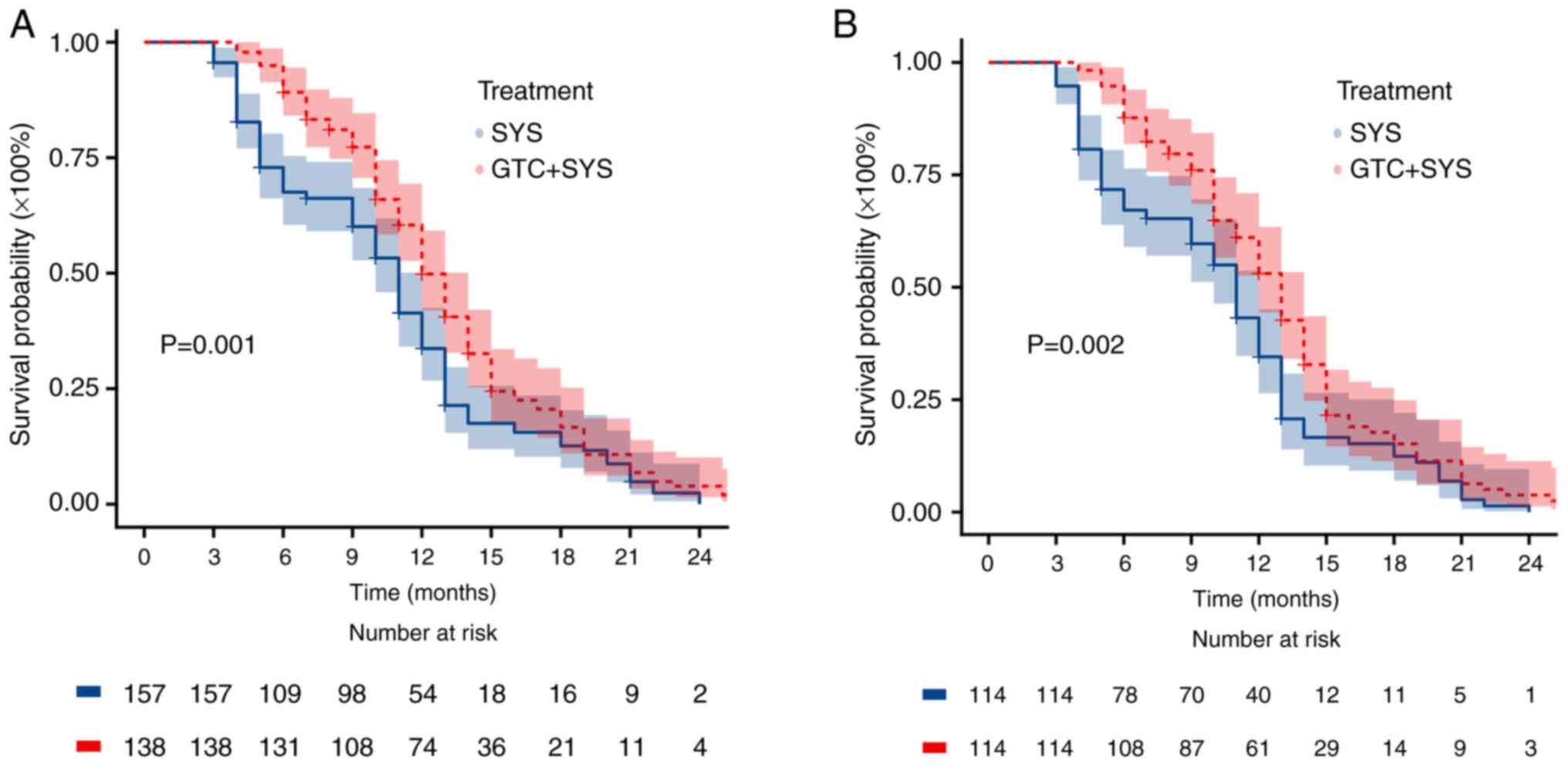
The Kaplan-Meier survival curves for OS and PFS
before and after PSM are presented in Figs. 2 and 3, respectively. The median OS was
significantly higher in the GTC + SYS group than in the SYS group
before (Fig. 2A) and after
(Fig. 2B) PSM. Before PSM, the
median OS was 13.0 months (95% CI, 12.0–14.0) in patients who
received GTC + SYS vs. 11.0 months (95% CI, 10.0–12.0) in patients
who received SYS alone (log-rank P=0.001). After PSM, the median OS
was 13.0 months (95% CI, 12.0–14.0) in patients who received GTC +
SYS vs. 11.0 months (95% CI, 10.0–12.0) in patients who received
SYS alone (log-rank P=0.002) (data not shown). However, there was
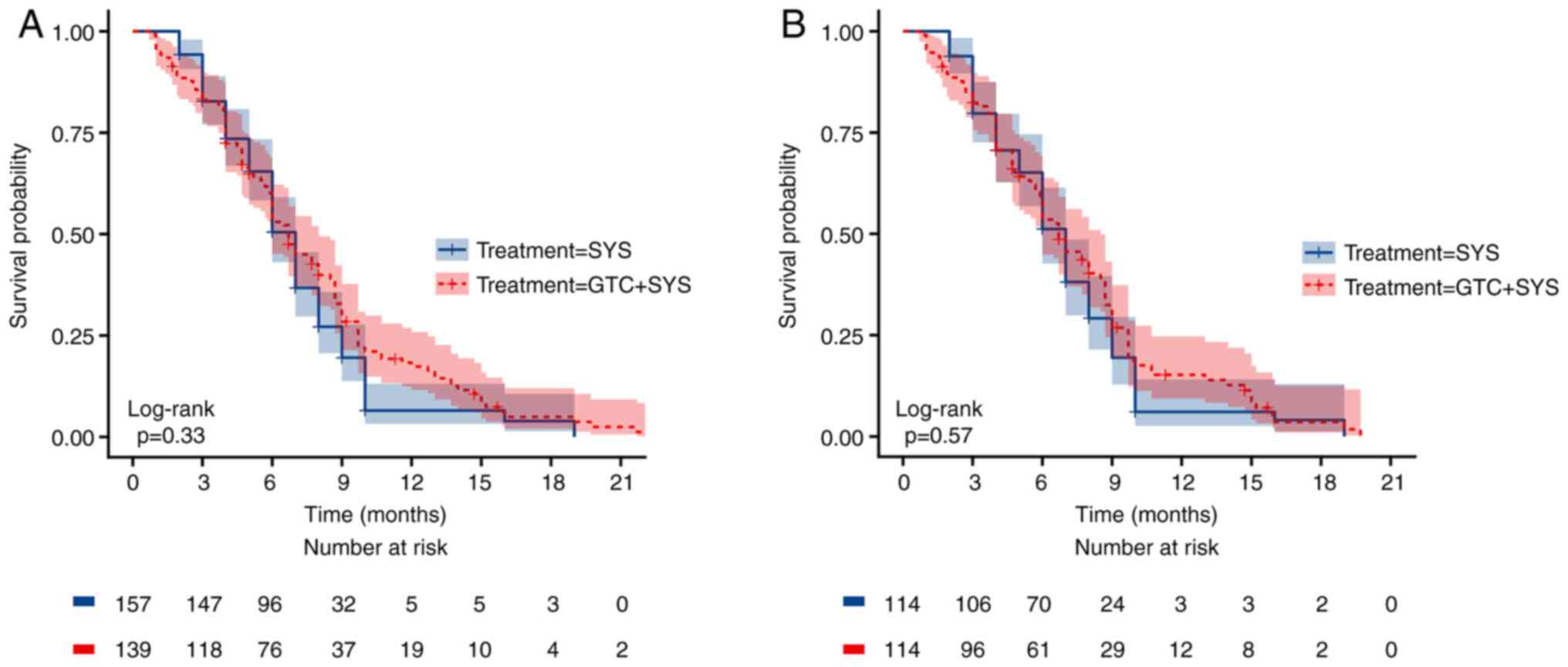
no significant difference in PFS between the two groups before
(Fig. 3A) and after (Fig. 3B) PSM (Table IV). Before PSM, the median PFS was
6.7 months (95% CI, 6.0–8.0) in patients who received GTC + SYS vs.
7.0 months (95% CI, 6.0–7.0) in patients who received SYS alone
(log-rank P=0.330). After PSM, the median PFS was 6.7 months (95%
CI, 6.0–8.5) in patients who received GTC + SYS vs. 7.0 months (95%
CI, 6.0–7.0) in patients who received SYS alone (log-rank P=0.570).
Furthermore, 12-month OS was 53.5% in the GTC + SYS group and 35.1%
in the SYS alone group. 12-month OS results were generally
consistent across patient subgroups and did not differ
significantly (Fig. 4).
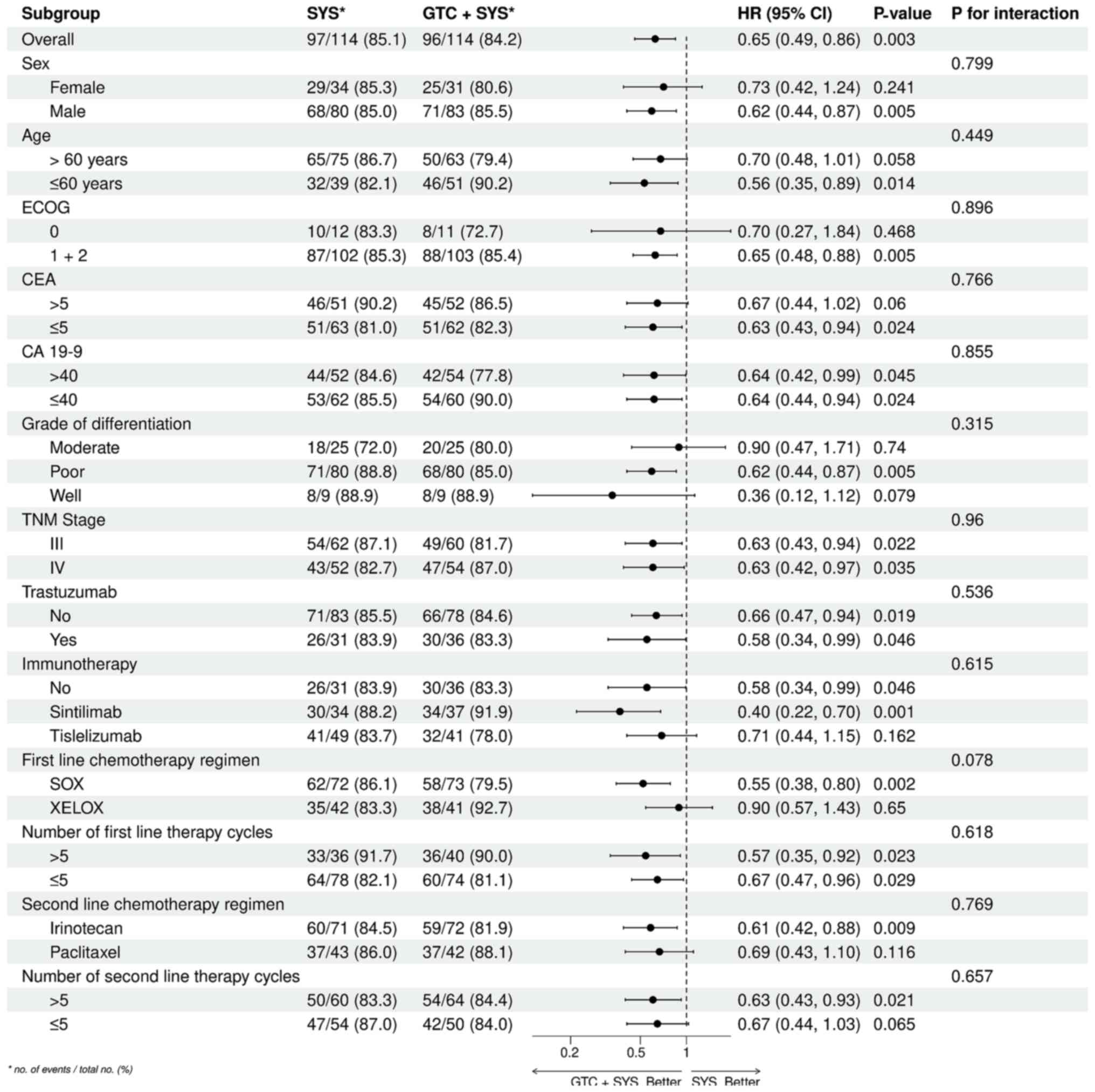 | Figure 4.ORs and 95% CIs for OS by subgroup.
The forest plot displays the ORs and their corresponding 95% CIs
for the comparison of GTC + SYS with SYS alone. Th end-time point
of OS calculation was at 12 months. HR, odds ratio; CI, confidence
interval; OS, overall survival; GTC, gastric transcatheter
chemoembolization; SYS, systemic chemotherapy; ECOG, Eastern
Cooperative Oncology; CEA, carcinoembryonic antigen; CA 19-9,
carbohydrate antigen 19-9; TNM, tumor-node-metastasis; SOX,
tegafur-gimeracil-oteracil + oxaliplatin; XELOX, capecitabine +
oxaliplatin. |
 | Table IV.Treatment efficacy of response and
survival outcomes. |
Table IV.
Treatment efficacy of response and
survival outcomes.
|
| Treatment |
|
|---|
|
|
|
|
|---|
| Characteristic | GTC + SYS
(n=114) | SYS (n=114) | P-value |
|---|
| OS, months (95%
CI) | 13.0
(12.0–14.0) | 11.0
(10.0–12.0) | 0.002a |
| 6
months OS, % (95% CI) | 87.7
(81.9–91.4) | 67.1
(59.0–76.4) | <0.001 |
| 12
months OS, % (95% CI) | 53.5
(44.4–63.4) | 35.1
(26.4–45.1) | 0.010 |
| PFS, months (95%
CI) | 6.7 (6.0–8.5) | 7.0 (6.0–7.0) | 0.570 |
| 6
months PFS, % (95% CI) | 53.49
(44.86–63.78) | 51.18
(42.62–61.44) | 0.887 |
| 12
months PFS, % (95% CI) | 15.18
(9.33–24.70) | 6.07
(2.63–14.04) | 0.063 |
| PR, n (%) | 51 (45) | 51 (45) | >0.999 |
| SD, n (%) | 42 (37) | 39 (34) | 0.768 |
| PD, n (%) | 21 (18) | 24 (21) | 0.722 |
| ORR, n (%) | 51 (45) | 51 (45) | >0.999 |
| DCR, n (%) | 93 (82) | 90 (79) | 0.739 |
The logistic regression analysis revealed that the
number of GTC treatment sessions was not significantly associated
with the 12-month survival rate of patients (Table SV) or the presence of dysphagia at
8 weeks after initial treatment (Table
SVI; P>0.05).
Comparison of AEs between groups
In the GTC + SYS group, the most common severe AEs
(grades 3/4) were leukopenia (7.3%), neutropenia (7.3%), and nausea
and vomiting (7.3%). In the SYS group, the most common severe AEs
were neutropenia (9.8%) and thrombocytopenia (8.2%). No patients in
either group discontinued treatment or died due to AEs. The
incidence of AEs was similar between the two groups, with no
significant difference in the incidence of grade 3/4 events
(Table SVII). Out of the 114
patients in the GTC + SYS group, 14 patients experienced
superficial mucosal ulceration in the gastric body along the lesser
curvature proximate to the fundus. This was confirmed by upper
abdominal pain and subsequent gastric endoscopy. These patients
were managed with symptomatic supportive treatment and showed
improvement without any serious complications such as perforation
(data not shown).
Discussion
In the present retrospective study, the
effectiveness of GTC combined with SYS compared with SYS alone in
the treatment of AGCC presenting with dysphagia was assessed. The
findings highlight that GTC may reduce the local tumor burden in
the cardia more rapidly and efficiently than SYS alone, leading to
improved dysphagia symptom remission, enhanced nutritional status,
and an improved QoL for patients. Additionally, GTC + SYS was
associated with a longer OS. Overall, the results of the present
study suggest that GTC + SYS, as a therapeutic intervention, holds
promise in effectively managing dysphagia symptoms and improving
outcomes in patients with AGCC. However, it is important to note
that data on endoscopic evaluation of esophageal-gastric stenosis
and gastric cancer size were not included in the present study. The
main reason for excluding endoscopic evaluation data was the
retrospective nature of this study. The endoscopic report for
patients in the present study described the size of the tumor along
the lower end of the esophagus and the longitudinal axis of the
cardia but did not specify the thickness of the tumor protruding
into the lumen. The thickness of the tumor protruding into the
gastric cavity directly influences the degree of stenosis.
Therefore, the maximum diameter of the cardiac tumor on the CT
image of the patient before initial treatment was considered as a
quantitative and accurate index, without including the content of
the endoscopic report as a matching factor. Additionally, in
certain patients with severe stenosis, the endoscope could not pass
through the stenosis segment, limiting the information available
about the mass and the stenosis segment. This limitation is one
reason why the content of the endoscopic report was not used as a
matching factor.
Several studies have reported the use of GTC in the
treatment of gastric cancer, highlighting its potential benefits in
terms of symptom remission and survival outcomes (1,3,6,16).
Peng et al (6) evaluated the
safety and efficacy of GTC in 42 patients with advanced gastric
cancer with obstruction and reported that GTC could be an
alternative treatment for advanced gastric cancer with obstruction.
Similarly, Li et al (10)
reported that GTC could shrink tumors and improve QoL in elderly
patients with advanced gastric cancer. Wang et al (5) performed a retrospective analysis of
patients with advanced gastric cardiac cancer who underwent GTC and
reported a marked improvement in dysphagia symptom remission rates,
with 100% of patients experiencing complete or partial relief.
However, the study design lacked a control group receiving only
systematic chemotherapy, which is the unique advantage of the
present study.
One of the crucial decisions in the present study
was the selection of 5-FU as the primary treatment for GTC. This
choice is supported by existing literature, which has demonstrated
the effectiveness of 5-FU in treating advanced gastric cancer
(27,28). These agents have shown efficacy in
both first-line and adjuvant settings, and their use in GTC can
complement SYS by directly targeting the tumor. Additionally, 5-FU
has shown favorable tolerability and safety profiles, making it
suitable for combination therapy regimens. Furthermore, the
selection of 5-FU for GTC is supported by its ability to exert
local cytotoxic effects on the tumor whilst minimizing systemic
toxicity (37,38). By delivering the chemotherapy
directly to the tumor via the gastric arteries, GTC allows for a
higher concentration of the drug at the tumor site, potentially
enhancing its antitumor effects. The present study demonstrated
that patients in the GTC + SYS group experienced a more rapid and
significant reduction in cardia tumor diameter at both the 4- and
8-week follow-up compared with those in the SYS alone group. This
suggests that the addition of GTC to SYS resulted in a more
effective shrinking of localized tumors in the cardia, leading to a
more pronounced alleviation of dysphagia symptoms.
Embospheres are biocompatible and biodegradable, and
they have been demonstrated to effectively occlude the blood
vessels supplying the tumor without causing significant adverse
events. Several studies have investigated the safety of using
embospheres for embolization in GTC (39,40).
An important factor that has been reported to influence the
occurrence of postoperative complications in GTC is the size of the
embospheres. Smaller diameter microspheres have been associated
with a higher risk of complications such as ischemic necrosis,
gastric ulcers and gastric perforation. Conversely, larger
microspheres have been reported to have a higher embolization
efficacy but may also increase the risk of non-target embolization
(41). Consistent with these
studies, embolization using 300–500 µm-sized embospheres was well
tolerated in patients with AGCC in the present study, without any
major complications such as gastric perforation, hepatic infarction
or embolic events.
Limitations of the present study need to be
acknowledged. Firstly, the study was retrospective and performed at
a single center, which may limit the generalizability of the
findings. To address this, future studies should consider employing
a prospective design with randomization in multiple centers.
Secondly, the study lacked long-term follow-up data on the
dysphagia, nutritional status and QoL of the patients, which could
provide more comprehensive insights into the efficacy and safety of
GTC combined with SYS in the treatment of AGCC. Further research
with longer-term follow-up is required to validate the efficacy of
GTC in improving the nutritional status and QoL of patients.
Another limitation of the present study is the lack of data on
endoscopic evaluation of esophageal gastric stenosis and gastric
cancer size. This information could have provided valuable insights
into the severity of dysphagia and the extent of disease in
patients. Future studies should consider incorporating these
parameters to enhance the robustness of the findings.
In conclusion, the findings of the present study
suggest that combining GTC with SYS may be a more effective
treatment approach for AGCC presenting with dysphagia than SYS
alone. The higher dysphagia symptom remission and improved
nutritional status and QoL in the combined treatment group indicate
that this approach may help improve OS for these patients. However,
further studies are needed to confirm these findings and determine
the optimal treatment approach for this challenging condition.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science Foundation
of Shandong Province (grant no. ZR2023QH127).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZL, PS and RX contributed to the study conception
and design, as well as material preparation, data collection and
analysis. The first draft of the manuscript was written by ZL and
all authors commented on previous versions of the manuscript. All
authors have read and approved the final manuscript. ZL and PS
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shandong Cancer Hospital and Institute (Jinan, China;
approval no. SDTHEC 2023004007; approved 4/14/2023). The need for
informed consent was waived due to the retrospective nature of the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
The ORCID of ZL is 0000-0002-8886-4764. The ORCID of
PS is 0000-0001-5141-6039.
Glossary
Abbreviations
Abbreviations:
|
GTC
|
gastric transcatheter
chemoembolization
|
|
SYS
|
systemic chemotherapy
|
|
AGCC
|
advanced gastric cardiac cancer
|
|
QoL
|
quality of life
|
|
PSM
|
propensity score matching
|
|
FACT-G7
|
Functional Assessment of Cancer
Therapy-General 7
|
|
PG-SGA
|
Patient-Generated Subjective Global
Assessment
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
AE
|
adverse effect
|
References
|
1
|
Chen L, Wang YH, Cheng YQ, Du MZ, Shi J,
Fan XS, Zhou XL, Zhang YF, Guo LC, Xu GF, et al: Risk factors of
lymph node metastasis in 1620 early gastric carcinoma radical
resections in Jiangsu Province in China: A multicenter
clinicopathological study. J Dig Dis. 18:556–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoteya S, Matsui A, Iizuka T, Kikuchi D,
Yamada A, Yamashita S, Furuhata T, Domon K, Nakamura M, Mitani T,
et al: Comparison of the clinicopathological characteristics and
results of endoscopic submucosal dissection for esophagogastric
junction and non-junctional cancers. Digestion. 87:29–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tate DJ, Klein A, Sidhu M, Desomer L,
Awadie H, Lee EYT, Mahajan H, McLeod D and Bourke MJ: Endoscopic
submucosal dissection for suspected early gastric cancer: Absolute
versus expanded criteria in a large Western cohort (with video).
Gastrointest Endosc. 90:467–479.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang J, Li J, Deng Q, Chen Z, He K, Chen Y
and Fu Z: Effect of neoadjuvant chemotherapy combined with arterial
chemoembolization on short-term clinical outcome of locally
advanced gastric cancer. BMC Cancer. 23:2462023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Yin M, Ma Y, Li X, Jin S, Liu T,
Zhu M, Liu C and Wu G: Transcatheter arterial chemoembolization
with lipiodol for advanced gastric fundus and cardia cancer. Eur J
Cancer Prev. 32:305–306. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng D, Zhang B, Yuan C, Tong Y and Zhang
W: Gastric transcatheter chemoembolization can resolve advanced
gastric cancer presenting with obstruction. Front Surg.
9:10040642022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sunde B, Ericson J, Kumagai K, Lundell L,
Tsai JA, Lindblad M, Rouvelas I, Friesland S, Wang N and Nilsson M:
Relief of dysphagia during neoadjuvant treatment for cancer of the
esophagus or gastroesophageal junction. Dis Esophagus. 29:442–447.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cools-Lartigue J, Jones D, Spicer J,
Zourikian T, Rousseau M, Eckert E, Alcindor T, Vanhuyse M, Asselah
J and Ferri LE: Management of dysphagia in esophageal
adenocarcinoma patients undergoing neoadjuvant chemotherapy: Can
invasive tube feeding be avoided? Ann Surg Oncol. 22:1858–1865.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arnold M, Moore SP, Hassler S,
Ellison-Loschmann L, Forman D and Bray F: The burden of stomach
cancer in indigenous populations: A systematic review and global
assessment. Gut. 63:64–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li N, Wang G, Duan G, Li Z, Zheng Y, Wang
Z and Li G: Clinical observation of transcatheter arterial
chemoembolization in super-aged patients with advanced gastric
cancer. Support Care Cancer. 30:1441–1450. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siddiqui AA, Sarkar A, Beltz S, Lewis J,
Loren D, Kowalski T, Fang J, Hilden K and Adler DG: Placement of
fully covered self-expandable metal stents in patients with locally
advanced esophageal cancer before neoadjuvant therapy. Gastrointest
Endosc. 76:44–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Fiore F, Lecleire S, Pop D, Rigal O,
Hamidou H, Paillot B, Ducrotté P, Lerebours E and Michel P:
Baseline nutritional status is predictive of response to treatment
and survival in patients treated by definitive chemoradiotherapy
for a locally advanced esophageal cancer. Am J Gastroenterol.
102:2557–2563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lagergren J and Lagergren P: Recent
developments in esophageal adenocarcinoma. CA Cancer J Clin.
63:232–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagaraja V, Cox MR and Eslick GD: Safety
and efficacy of esophageal stents preceding or during neoadjuvant
chemotherapy for esophageal cancer: A systematic review and
meta-analysis. J Gastrointest Oncol. 5:119–126. 2014.PubMed/NCBI
|
|
15
|
Tey J, Soon YY, Koh WY, Leong CN, Choo BA,
Ho F, Vellayappan B, Lim K and Tham IW: Palliative radiotherapy for
gastric cancer: A systematic review and meta-analysis. Oncotarget.
8:25797–25805. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kato H, Tsutsumi K and Okada H: Recent
advancements in stent therapy in patients with malignant
gastroduodenal outlet obstruction. Ann Transl Med. 5:1862017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hori Y, Naitoh I, Hayashi K, Ban T,
Natsume M, Okumura F, Nakazawa T, Takada H, Hirano A, Jinno N, et
al: Predictors of stent dysfunction after self-expandable metal
stent placement for malignant gastric outlet obstruction: tumor
ingrowth in uncovered stents and migration of covered stents. Surg
Endosc. 31:4165–4173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mariette C, Gronnier C, Duhamel A, Mabrut
JY, Bail JP, Carrere N, Lefevre JH, Meunier B, Collet D, Piessen G,
et al: Self-expanding covered metallic stent as a bridge to surgery
in esophageal cancer: Impact on oncologic outcomes. J Am Coll Surg.
220:287–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee H, Min BH, Lee JH, Shin CM, Kim Y,
Chung H and Lee SH: Covered metallic stents with an anti-migration
design vs. uncovered stents for the palliation of malignant gastric
outlet obstruction: A multicenter, randomized trial. Am J
Gastroenterol. 110:1440–1449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sinapi I, Navez B, Hamoir M, Schmitz S,
Machiels JP, Deprez PH and Van den Eynde M: Seeding of the
percutaneous endoscopic gastrostomy site from head and neck
carcinoma: Case report and review of the literature. Head Neck.
35:E209–E212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mariette C, De Botton ML and Piessen G:
Surgery in esophageal and gastric cancer patients: What is the role
for nutrition support in your daily practice? Ann Surg Oncol.
19:2128–2134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Shi H, Che Y, Sun W, Niu X and Lu
W: Efficacy of endostatin combined with continuous transcatheter
arterial infusion and chemoembolization on gastric cancer with
liver metastasis and analysis of prognosis. J BUON. 25:1469–1475.
2020.PubMed/NCBI
|
|
23
|
Wu P and Wang J: Efficacy of
interventional therapy and effect on inflammatory factors in
patients with gastric cancer after chemotherapy. Oncol Lett.
18:1733–1744. 2019.PubMed/NCBI
|
|
24
|
Wang J, Shi H, Yang G, Han G, Zhao M, Duan
X, Mi L, Han X, Li N, Shi J, et al: Combined intra-arterial and
intravenous chemotherapy for unresectable, advanced gastric cancer
has an improved curative effect compared with intravenous
chemotherapy only. Oncol Lett. 15:5662–5670. 2018.PubMed/NCBI
|
|
25
|
Huang SF, Chien TH, Fang WL, Wang F, Tsai
CY, Hsu JT, Yeh CN, Chen TC, Wu RC, Chiu CT and Yeh TS: The 8th
edition American Joint Committee on gastric cancer pathological
staging classification performs well in a population with high
proportion of locally advanced disease. Eur J Surg Oncol.
44:1634–1639. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ogilvie AL, Dronfield MW, Ferguson R and
Atkinson M: Palliative intubation of oesophagogastric neoplasms at
fibreoptic endoscopy. Gut. 23:1060–1067. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu RH, Wang ZQ, Shen L, Wang W, Lu JW, Dai
G, Xu JM, Zhang YQ, Chen XB, Deng YH, et al: S-1 plus oxaliplatin
versus S-1 plus cisplatin as first-line treatment for advanced
diffuse-type or mixed-type gastric/gastroesophageal junction
adenocarcinoma: A randomized, phase 3 trial. J Clinical Oncol.
37:40172019. View Article : Google Scholar
|
|
28
|
Luo HY, Xu RH, Wang F, Qiu MZ, Li YH, Li
FH, Zhou ZW and Chen XQ: Phase II trial of XELOX as first-line
treatment for patients with advanced gastric cancer. Chemotherapy.
56:94–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hawkes E, Okines AF, Papamichael D, Rao S,
Ashley S, Charalambous H, Koukouma A, Chau I and Cunningham D:
Docetaxel and irinotecan as second-line therapy for advanced
oesophagogastric cancer. Eur J Cancer. 47:1146–1151. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hironaka S, Ueda S, Yasui H, Nishina T,
Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki
T, et al: Randomized, open-label, phase III study comparing
irinotecan with paclitaxel in patients with advanced gastric cancer
without severe peritoneal metastasis after failure of prior
combination chemotherapy using fluoropyrimidine plus platinum: WJOG
4007 trial. J Clin Oncol. 31:4438–4444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gabrielson DK, Scaffidi D, Leung E,
Stoyanoff L, Robinson J, Nisenbaum R, Brezden-Masley C and Darling
PB: Use of an abridged scored Patient-Generated Subjective Global
Assessment (abPG-SGA) as a nutritional screening tool for cancer
patients in an outpatient setting. Nutr Cancer. 65:234–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yanez B, Pearman T, Lis CG, Beaumont JL
and Cella D: The FACT-G7: A rapid version of the functional
assessment of cancer therapy-general (FACT-G) for monitoring
symptoms and concerns in oncology practice and research. Ann Oncol.
24:1073–1078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chiu L, Chiu N, Chow E, Cella D, Beaumont
JL, Lam H, Popovic M, Bedard G, Poon M, Wong E, et al: Comparison
of three shortened questionnaires for assessment of quality of life
in advanced cancer. J Palliat Med. 17:918–923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-Version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nishino M, Jagannathan JP, Ramaiya NH and
Van den Abbeele AD: Revised RECIST guideline version 1.1: What
oncologists want to know and what radiologists need to know. AJR Am
J Roentgenol. 195:281–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao QY, Luo JC, Su Y, Zhang YJ, Tu GW and
Luo Z: Propensity score matching with R: conventional methods and
new features. Ann Transl Med. 9:8122021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li M, Zhang J, Wang D, Zhong B, Tucker S,
Lu C, Cheng J, Cao C, Xu J, Xu J and Pan H: A phase II study of
intra-arterial chemotherapy of 5-fluorouracil, cisplatin, and
mitomycin C for advanced nonresectable gastric cancer. Anticancer
Drugs. 20:941–945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang CW, Zou SC, Shi D and Zhao DJ:
Clinical significance of preoperative regional intra-arterial
infusion chemotherapy for advanced gastric cancer. World J
Gastroenterol. 10:3070–3072. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu Y, Weiss CR, Paudel K, Shin EJ,
Kedziorek D, Arepally A, Anders RA and Kraitchman DL: Bariatric
arterial embolization: Effect of microsphere size on the
suppression of fundal ghrelin expression and weight change in a
swine model. Radiology. 289:83–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bai ZB, Qin YL, Deng G, Zhao GF, Zhong BY
and Teng GJ: Bariatric embolization of the left gastric arteries
for the treatment of obesity: 9-Month data in 5 patients. Obes
Surg. 28:907–915. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weiss CR, Akinwande O, Paudel K, Cheskin
LJ, Holly B, Hong K, Fischman AM, Patel RS, Shin EJ, Steele KE, et
al: Clinical safety of bariatric arterial embolization: Preliminary
results of the BEAT obesity trial. Radiology. 283:598–608. 2017.
View Article : Google Scholar : PubMed/NCBI
|