Introduction
Hepatocellular carcinoma (HCC) is a principal
histologic type of liver cancer, which primarily arises in
cirrhotic livers when repeated inflammation and fibrinogenesis
leads to dysplasia and malignant transformation of the liver
(1). China has the highest number
of HCC cases worldwide, attributable to a high incidence rate of
18.3 cases per 100,000 people (2).
The recognized risk factors for HCC include HBV/HCV infection,
alcohol, nonalcoholic fatty liver disease (NAFLD), Aflatoxin
B1 and several metabolic syndromes (2). Traditional treatments for HCC include
surgery, local ablation and transarterial chemoembolization (TACE).
In recent years, a variety of target drugs and immune checkpoint
inhibitors (ICIs) have been used in the treatment of HCC. However,
the prognosis of patients with HCC remains poor (3).
There has been a growing emphasis on the
investigation of the tumor immune microenvironment of HCC, given
the use of ICIs for HCC therapy. The HCC tumor microenvironment
consists of a multifaceted and dynamic system that is formed of
cancer cells, a complex cytokine milieu, extracellular matrix
components, immune cells, physical and chemical characteristics
(4). In terms of immune cells,
tumor-infiltrating CD8+ T cells are considered to be one
of the principal T cell subsets responsible for mediating effective
antitumor responses (5). The
function of tumor-infiltrating CD8+ T cells have been
associated with improved survival outcomes in patients with lung
cancer, breast cancer and malignant pleural mesothelioma (6–8).
However, T cell exhaustion frequently occurs in the tumor
microenvironment due to the prolonged activation of the immune
response (9). The exhausted T cells
show upregulated inhibitory receptors, decreased effector cytokine
production and cytolytic activity, leading to cancer immune evasion
(9). CD39 is an enzyme expressed on
the cell surface that can attenuate the functionality of effector T
cells by hydrolyzing extracellular ATP and impeding effector
responses in lymphocytes (10).
Previous studies have indicated that the expression level of CD39
in tumor-infiltrating CD8+ T cells may be associated
with the state of T cell exhaustion, with T cells exhibiting high
CD39 expression potentially manifesting as ‘bystander’ T cells
(11,12). In contrast to this, programmed cell
death ligand 1 (PD-L1), a transmembrane cell surface protein of
tumor cells that interacts with programmed cell death protein 1
(PD-1), regulates T cell proliferation, cytokine production and
cellular exhaustion by binding to the PD-1 molecule on immune
cells, ultimately leading to tumor immune evasion (13–16).
Several studies have attempted to elucidate the correlation between
PD-L1 expression levels in HCC and patient prognosis. However,
these investigations have yielded inconsistent conclusions
(17–22). Therefore, it could be suggested that
the combined influence of PD-L1 expression levels in tumors and the
functionality of tumor-infiltrating CD8+ T cells may be
a significant factor affecting the prognosis of patients with
HCC.
The present study utilized multiplex
immunofluorescence assays to analyze CD39 expression levels in
tumor-infiltrating CD8+ T cells and PD-L1 expression
levels in HCC tumor cells. A comprehensive examination of the
combined influence of immune cell exhaustion and
PD-1/PD-L1-mediated tumor immune evasion was conducted to further
elucidate their potential as a prognostic factors and therapeutic
targets for HCC.
Materials and methods
Patients
The present study evaluated HCC tumor samples
collected from 91 patients with HCC treated at Hebei Medical
University 4th Hospital (Shijiazhuang, China) between January 2012
to December 2015. The age range of the patients was 34–73 years,
with a median age of 57 years. All tissue specimens were obtained
from patients who were diagnosed with HCC by a pathologist and
subsequently underwent hepatectomy. All patients provided written
informed consent for the collection of tissue specimens. The study
was approved by the Clinical Research Ethics Committee of Hebei
Medical University 4th Hospital (approval no. 2018MEC149;
Shijiazhuang, P.R. China). All participants were regularly followed
up for 5 years by outpatient clinics and telephone interviews at
least every 3 months. Imaging examinations were performed every 3
months within 2 years after surgery, and every 6 months after 2
years. Overall survival (OS) and recurrence-free survival (RFS)
were assessed to analyze the survival status of patients with
HCC.
Inclusion and exclusion criteria
The inclusion criteria of patient enrollment were
patients with a diagnosis of primary HCC by histopathological
examination and who had received no systematic therapy, including
chemotherapy, targeted therapy or immunotherapy, prior to radical
resection of the tumor. The exclusion criteria were patients who
had other types of cancer and had a past history of systematic
therapy before surgery.
Multiplex immunofluorescence
assay
Samples from patients were immersed in 10% neutral
buffered formalin for fixation for 3 days at room temperature.
Slides of the tissue microarray were cut into 4-µm slides from
paraffin embedded samples. Slides were heated in an oven at 63°C
for 1 h, dewaxed in xylene and a series of graded ethanol (100, 95,
85 and 75%), boiled in citrate buffer (100°C for 3 min), and cooled
down to room temperature for 15–20 min. To block non-specific
peroxidase reactions, 3% hydrogen peroxide was used for 20 min at
room temperature. After cooling and washing with PBS (3 times, 5
min each), the slides were incubated with the following primary
antibodies at 4°C overnight: Anti-PD-L1 (cat. no. ab205921; Abcam),
anti-CD39 (1:2,000; cat. no. ab223842; Abcam), anti-CD8 (cat. no.
ab4055; Abcam) and anti-pan-cytokeratin (CK; cat. no. PA125; Suzhou
Baidao Medical Technology Co., Ltd.). After rewarming to room
temperature, slides were washed by PBS (3 times, 5 min each).
HRP-conjugated goat anti-rabbit secondary antibodies (cat. no.
GK500705, Dako; Agilent Technologies, Inc.) were subsequently added
and incubated for 15 min at room temperature for visualization.
Staining with Opal dye solution (1:100; cat. no. NEL820001KT, Akoya
Biosciences, Inc.) was performed according to the manufacturer's
instructions. The samples were counterstained with DAPI (cat. no.
28718-90-3; MilliporeSigma) for 10 min at room temperature, and
imaged using an automatic quantitative pathology imaging system
(Vectra Polaris; PerkinElmer, Inc.).
Statistical analysis
The data were analyzed using R (version 4.2.2;
Rstudio, Inc.) and GraphPad Prism (version 10; Dotmatics) software.
The survival receiver operating characteristic (ROC) method was
used to determine the optimal cutoff values for CD39 expression.
Kaplan-Meier analysis with the log-rank test was utilized to
analyze differences in the survival rates between different groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline patient characteristics
The present study analyzed 91 clinical samples from
patients with HCC (Table I). Among
the patients, there were 79 male patients (86.8%) and 12 female
patients (13.2%). Within the patient cohort, 87 patients had
hepatitis B (95.6%), while 89 patients had cirrhosis (97.8%).
According to the Barcelona Clinic Liver Cancer staging system
(23), 3 patients (3.3%) had stage
0 disease, 58 patients (63.7%) had stage A disease and 30 patients
(33%) had stage B or C disease. According to the China Liver Cancer
staging system (24), 61 patients
(67.1%) had stage Ia or Ib disease, 13 patients (14.3%) had stage
IIa or IIb disease and 17 patients (18.6%) had stage IIIa or IIIb
disease. Within the 5-year follow-up period, 69 patients (75.8%)
experienced tumor recurrence, while 22 patients (24.2%) did not
experience tumor recurrence.
 | Table I.Baseline characteristics of patients
with hepatocellular carcinoma. |
Table I.
Baseline characteristics of patients
with hepatocellular carcinoma.
| Patient
characteristic | Patients, n | Percentage of total
patients, % |
|---|
| Sex |
|
|
|
Male | 79 | 86.8 |
|
Female | 12 | 13.2 |
| Hepatitis |
|
|
|
None | 4 | 4.4 |
|
Hepatitis B | 87 | 95.6 |
|
Hepatitis C | 0 | 0.0 |
| Cirrhosis |
|
|
|
Yes | 89 | 97.8 |
| No | 2 | 2.2 |
| Tumor number |
|
|
|
Single | 81 | 89.0 |
|
Multiple | 10 | 11.0 |
| Vascular
invasion |
|
|
|
Yes | 16 | 17.6 |
| No | 75 | 82.4 |
| Barcelona
Clinic |
|
|
| Liver Cancer
stage |
|
|
| 0 | 3 | 3.3 |
| A | 58 | 63.7 |
| B | 13 | 14.3 |
| C | 17 | 18.7 |
| China Liver Cancer
stage |
|
|
| Ia | 29 | 31.9 |
| Ib | 32 | 35.2 |
|
IIa | 8 | 8.8 |
|
IIb | 5 | 5.5 |
|
IIIa | 16 | 17.5 |
|
IIIb | 1 | 1.1 |
| α-fetoprotein |
|
|
| ≤7.86
(normal) | 26 | 28.6 |
|
>7.86 (high) | 65 | 71.4 |
| Recrudescence |
|
|
|
Yes | 69 | 75.8 |
| No | 22 | 24.2 |
Association of PD-L1 and CD39
expression with survival of patients with HCC
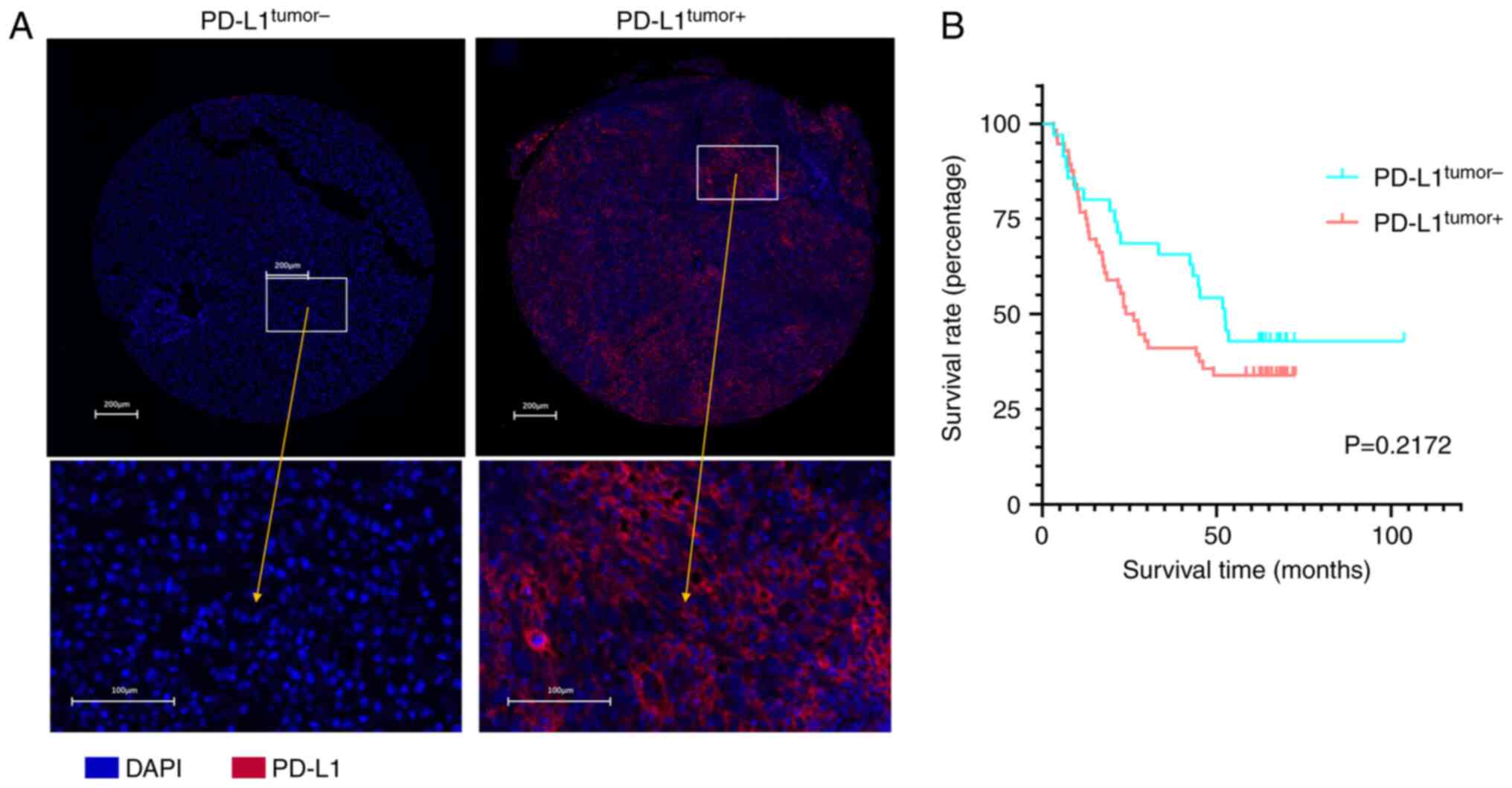
In order to investigate the relationship between
PD-L1 expression levels in HCC tumor cells and patient prognosis,
patients were divided into either PD-L1tumor+ or
PD-L1tumor− groups based on expression levels of PD-L1
calculated using multiplex immunofluorescent assay results
(Fig. 1A; Table II). PD-L1 expression levels were
selectively quantified within CK+ areas to evaluate
PD-L1 expression levels specifically in tumor cells and to exclude
stromal cells (Fig. S1A).
PD-L1+ cells ≥1% were defined as PD-L1tumor+
representing high expression of PD-L1 and PD-L1+ cells
<1% were defined as PD-L1tumor− reflecting low
expression of PD-L1. OS and RFS were compared between the
PD-L1tumor+ and PD-L1tumor− groups.
Kaplan-Meier survival analysis results demonstrated no significant
difference between a median OS of 25.1 months for
PD-L1tumor+ group (n=56) and 52.4 months for
PD-L1tumor− group (n=35; P=0.2172; Fig. 1B). The median RFS was 15.76 months
for PD-L1tumor+ group and 32.02 months for
PD-L1tumor− group. No significant difference in RFS was
observed between the PD-L1tumor+ and
PD-L1tumor− groups (P=0.5877; Fig. S1B).
 | Table II.Patient grouping information. |
Table II.
Patient grouping information.
| Group | Patients, n | Percentage, % |
|---|
|
PD-L1tumor |
|
|
| High
expression (+) | 56 | 61.5 |
| Low
expression (−) | 35 | 38.5 |
| CD39T
cell |
|
|
| High
expression (+) | 32 | 35.2 |
| Low
expression (−) | 59 | 64.8 |
| CD39T
cell/PD-L1tumor |
|
|
|
Co-upregulated | 23 | 25.3 |
|
Non-co-upregulated | 68 | 74.7 |
|
Co-low-expression | 26 | 28.6 |
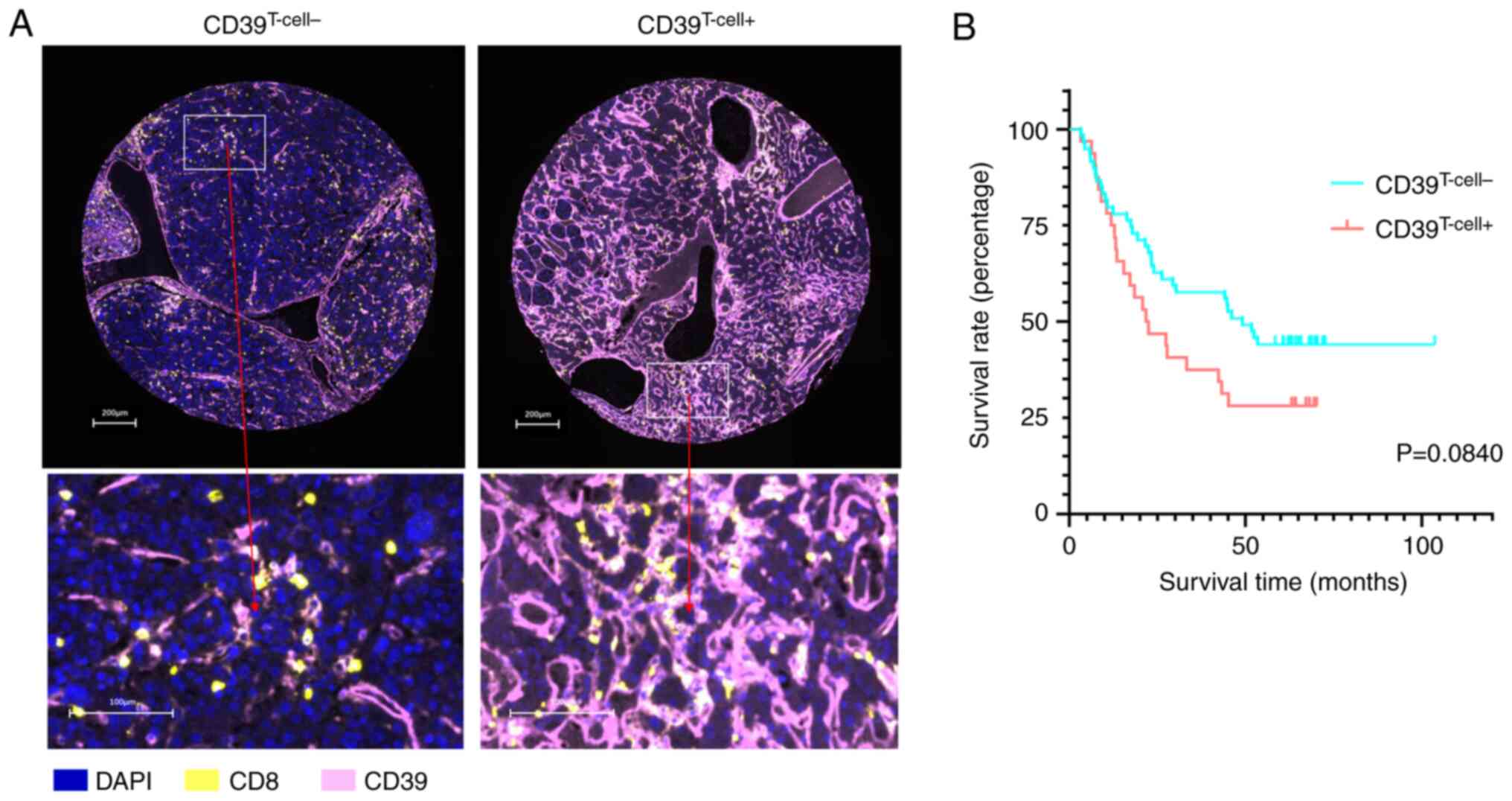
To further investigate the impact of
tumor-infiltrating CD8+ T cell exhaustion on the
prognosis of patients with HCC, patients were divided into
CD39T cell+ or CD39T cell− groups reflecting
high and low expression of CD39 respectively (Fig. 2A; Table
II). Using the survival ROC method, the cut-off value for the
CD39T cell+ group was CD39+ T
cells/CD39− T cells ≥1.42, and CD39+ T
cells/CD39− T cells <1.42 for the CD39T
cell− group (Fig. S2).
Similar to aforementioned PD-L1 expression levels, CD39 expression
levels on CD8+ T cells were selectively quantified
within CK+ areas (Fig.
S3A). Survival analysis demonstrated a median OS of 22.1 months
for CD39T cell+ group (n=32) and 49.1 months for
CD39T cell− group (n=59). Results demonstrated no
statistically significant difference in OS between the CD39T
cell+ and CD39T cell− groups (P=0.0840; Fig. 2B). The median RFS was 11.69 months
for CD39T cell+ group and 31.54 months for CD39T
cell− group and no significant difference in RFS was observed
between the two groups (P=0.0584; Fig.
S3B).
Based on the findings of the present study, it could
be suggested that tumor cell PD-L1 expression levels, as well as
CD39 expression levels on CD8+ T cells individually,
were insufficient to influence the overall prognosis of patients
with HCC.
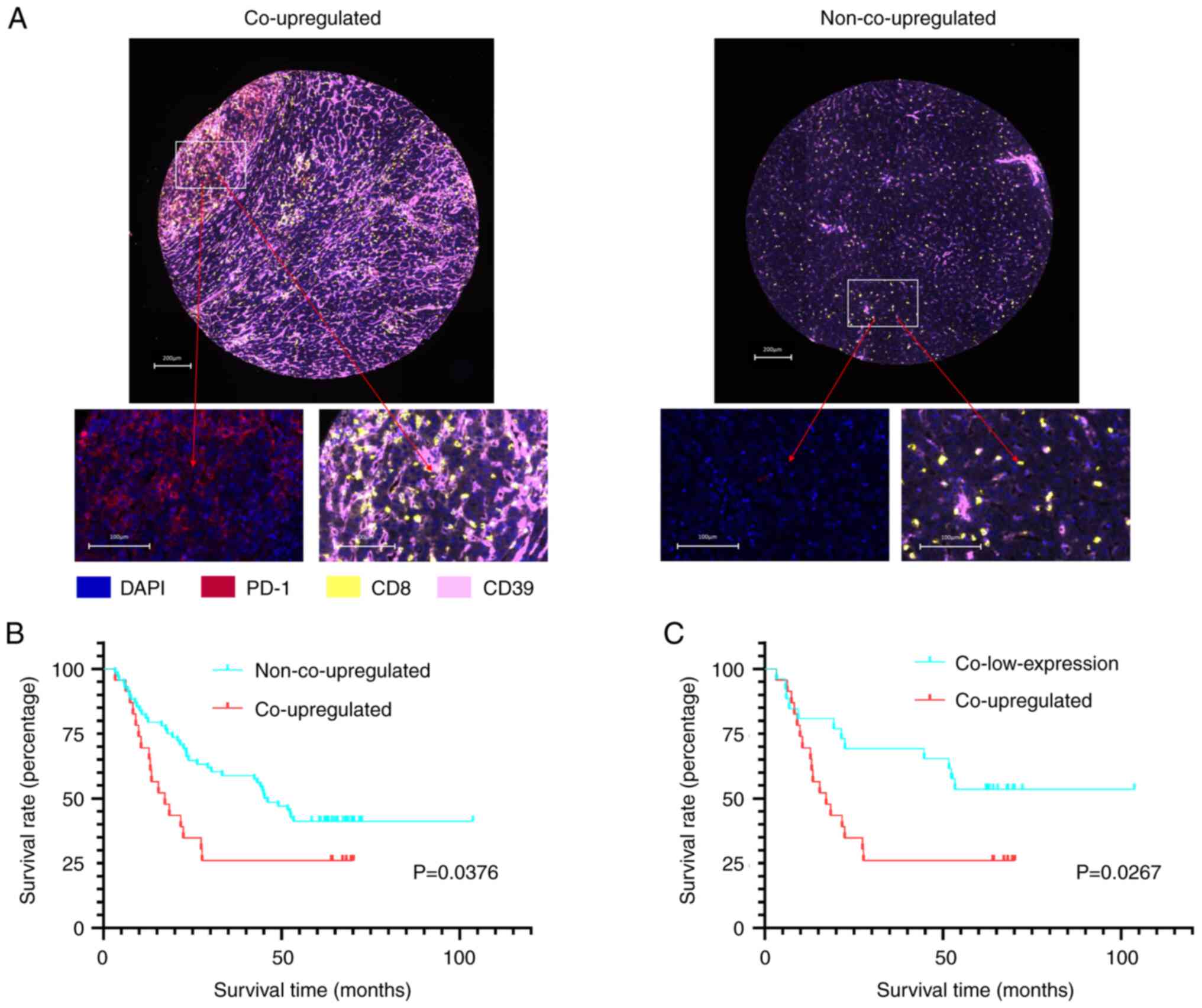
Co-upregulation of
PD-L1tumor and CD39T cell as a prognostic
factor for patients with HCC
In the context of HCC progression, it could be
suggested that the co-upregulation of PD-L1 in tumor cells and CD39
in tumor-infiltrating CD8+ T cells may synergistically
impact the prognosis of patients with HCC. Accordingly, patients
were divided into two groups: PD-L1tumor/CD39T
cell co-upregulated group (PD-L1+ cells ≥1% and
CD39+ T cells/CD39− T cells ≥1.42) and the
PD-L1tumor/CD39T cell non-co-upregulated
group (PD-L1+ cells <1% or CD39+ T
cells/CD39− T cells <1.42) (Fig. 3A; Table
II). Survival analysis between these two cohorts demonstrated a
median OS of 17.2 months for the co-upregulated group (n=23) and
45.6 months for the non-co-upregulated group (n=68). Kaplan-Meier
analysis demonstrated that the OS significantly reduced among
patients in the co-upregulated group compared with the
non-co-upregulated group (P=0.0376; Fig. 3B). The median RFS was 9.26 months
for co-upregulated patients and 31.42 months for non-co-upregulated
group. The RFS between the co-upregulated and non-co-upregulated
groups was statistically significant (P=0.0269; Fig. S4A).
The median OS of the co-upregulated group was 17.2
months and 57.8 months in the PD-L1tumor/CD39T
cell co-low-expression group (PD-L1+ cells <1%
and CD39+ T cells/CD39− T cells <1.42;
n=26). Survival analysis demonstrated that the OS of the
co-upregulated group was significantly different compared with the
PD-L1tumor/CD39T cell co-low-expression group
(P=0.0267; Fig. 3C; Table II). The median RFS of the
co-upregulated group was 9.26 months and 37.37 months in the
co-low-expression group. The median RFS of the co-upregulated group
compared with the co-low-expression group was not significantly
different (P=0.0864; Fig.
S4B).
Additionally, within the PD-L1tumor+
group, patients with upregulated CD39 expression (n=33) had a
markedly shorter OS (17.2 months) compared with patients with low
CD39 expression (n=23; 44.1 months), but there was no significant
difference in OS between the two subgroups (P=0.1017; Fig. S4C).
Collectively, the findings of the present study
indicated that the co-upregulation of PD-L1tumor and
CD39T cell expression significantly correlated with poor
prognosis in patients with HCC and therefore could potentially
serve as an independent prognostic factor for patients with
HCC.
Discussion
In previous years, ICIs, such as PD-1 inhibitors,
has become an important approach for the systemic treatment of
advanced HCC (25). However, the
efficacy of systemic therapy for patients with HCC, whether as
monotherapy with ICIs or in combination with antiangiogenic agents,
remains limited (26). The efficacy
of immunotherapy depends on the interaction between immune cells
and tumor cells within the tumor immune microenvironment (27). Therefore, it is important to assess
the impact of both tumor cells and immune cell states within the
tumor microenvironment of HCC on patient prognosis.
The present study employed a multiplex
immunofluorescence assay to simultaneously assess PD-L1 expression
in tumor cells and CD39 expression in tumor-infiltrating
CD8+ T cells. The results indicated that the individual
upregulation of PD-L1 in tumor cells or CD39 in tumor-infiltrating
CD8+ T cells had no significant impact on the prognosis
of patients with HCC. However, patients exhibiting co-upregulation
of these two indicators had significantly decreased OS and RFS. To
the best of our knowledge, the present study represented the first
report of the synergistic impact of tumor cell and
tumor-infiltrating CD8+ T cell states within the immune
microenvironment of HCC on patient prognosis.
PD-L1 is an immunosuppressive molecule expressed in
tumor cells. Inhibiting the activity of immune cells regulates the
immune system (28). Increased
expression of PD-L1 in tumor cells has been associated with poor
overall survival times in certain types of cancers, including
breast cancer, colorectal cancer and non-small cell lung cancer
(29–31). However, the impact of PD-L1
expression levels on the prognosis of cancer patients is
inconsistent in the currently available literature. Meta-analysis
in a previous study of HCC indicated that PD-L1 upregulation
predicted reduced disease-free survival and progression-free
survival, however did not impact OS (22). A further two studies reported that
the upregulation of C-type lectin domain family 1 member B or CMTM6
with PD-L1 in tumor cells was associated with poor prognosis of
patients with HCC (20,21). Conversely, other studies have
suggested that PD-L1 expression on tumor cells does not
significantly correlate with the prognosis of patients with HCC or
that PD-L1 expression was associated with an improved prognosis of
patients with HCC (18,19). These studies did not analyze the
synergistic impact of the PD-L1 expression on tumor cells and the
function of tumor-infiltrating immune cells. The results in the
present study indicated that high PD-L1 expression level in tumor
cells was not associated with the prognosis of patients with HCC.
However, patients with high PD-L1 expression levels in tumor cells,
in conjunction with high CD39 expression levels in
tumor-infiltrating CD8+ T cells exhibited a
significantly worse prognosis, in terms of OS and RFS.
Tumor-infiltrating CD8+ T cells serve a
pivotal role in mediating the antitumor response, but their
efficacy can be compromised by T cell exhaustion (5,9).
Previous studies have demonstrated that in settings of chronic
antigen exposure, such as chronic infections and cancer, T cells
progressively lose their effector functions, which is referred to
as T cell exhaustion (32). High
expression levels of CD39 is considered a hallmark of
CD8+ T cell exhaustion (11,12).
In the present study, it was suggested that the immune escape
induced by the upregulation of PD-L1 in tumor cells may be coupled
with the functional exhaustion of tumor-infiltrating
CD8+ T cells to result in reduced OS and RFS in patients
with HCC. The research findings reported in the present study
indicated that the expression levels of PD-L1 in tumor cells and
the functional status of immune cells synergistically impact the
prognosis of patients with HCC.
However, there were several limitations of the
present study. Firstly, as sample size was limited (n=91), it was
not possible to include all factors including tumor size, tumor
number and vascular invasion into the multivariate survival
analysis. Furthermore, the sample sizes of certain subgroups
(PD-L1tumor/CD39T cell co-upregulated group
and PD-L1tumor/CD39T cell co-low-expression
group) were small, which limited the conclusions that could be
drawn from the statistical analysis. Furthermore, it was not
possible to explore the effect of ICIs as treatment in the patients
with HCC included in the present study, as ICIs were not widely
used in the treatment of HCC in China during sample collection
(January 2012-December 2015). The treatments received by patients
were limited to surgical treatment, ablation therapy,
interventional therapy and traditional targeted therapy such as
tyrosine kinase inhibitors. In order to explore the impact of tumor
cells PD-L1 expression and immune cell exhaustion on ICI treatment,
future work should focus on the collection of clinical samples from
patients with HCC who have received ICI treatment for further
investigation.
In conclusion, the findings of the present study
demonstrated a significant impact of the interplay between tumor
cells and tumor-infiltrating immune cells on the prognosis of
patients with HCC. The upregulation of PD-L1 expression in tumor
cells, coupled with functional exhaustion of tumor-infiltrating
CD8+ T cells reflected by upregulation of CD39
expression, may potentially be associated with poor prognosis in
patients with HCC. These findings suggested that the
co-upregulation of PD-L1 expression in tumor cells and CD39
expression in tumor-infiltrating CD8+ T cells could
possibly serve as a clinical indicator for prognostic assessment of
patients with HCC and has the potential to guide immunotherapeutic
approaches in the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Hebei Province Medical Research
Project Plan (grant no. 20200092).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XK designed the study, analyzed the data and drafted
the manuscript. SZ, SL and JL performed data acquisition, data
analysis and edited the manuscript. XK and SL confirm the
authenticity of all the raw data. SW designed the study and edited
the manuscript. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Fourth
Hospital of Hebei Medical University Research Ethics Committee
(approval no. 2018MEC149; Shijiazhuang, China). All procedures
performed were in accordance with the ethical standards of the
Institutional and/or National Research Committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. Written informed consent was obtained from all patients
for the retention of diagnostic samples for future experimental use
at the time of collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Block TM, Mehta AS, Fimmel CJ and Jordan
R: Molecular viral oncology of hepatocellular carcinoma. Oncogene.
33:5093–5107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGlynn KA, Petrick JL and El-Serag HB:
Epidemiology of hepatocellular carcinoma. Hepatology. 73 (Suppl
1):S4–S13. 2021. View Article : Google Scholar
|
|
3
|
Han Z, Yang F, Zhang Y, Wang J, Ni Q, Zhu
H, Zhou X, Gao H and Lu J: Prognostic efficacy and prognostic
factors of TACE plus TKI with ICIs for the treatment of
unresectable hepatocellular carcinoma: A retrospective study. Front
Oncol. 12:10299512022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chew V, Lai L, Pan L, Lim CJ, Li J, Ong R,
Chua C, Leong JY, Lim KH, Toh HC, et al: Delineation of an
immunosuppressive gradient in hepatocellular carcinoma using
high-dimensional proteomic and transcriptomic analyses. Proc Natl
Acad Sci USA. 114:E5900–E5909. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu P and Fu YX: Tumor-infiltrating T
lymphocytes: Friends or foes? Lab Invest. 86:231–245. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Djenidi F, Adam J, Goubar A, Durgeau A,
Meurice G, de Montpréville V, Validire P, Besse B and Mami-Chouaib
F: CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific
tissue-resident memory T cells and a prognostic factor for survival
in lung cancer patients. J Immunol. 194:3475–3486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anraku M, Cunningham KS, Yun Z, Tsao MS,
Zhang L, Keshavjee S, Johnston MR and de Perrot M: Impact of
tumor-infiltrating T cells on survival in patients with malignant
pleural mesothelioma. J Thorac Cardiovasc Surg. 135:823–829. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mahmoud SM, Paish EC, Powe DG, Macmillan
RD, Grainge MJ, Lee AH, Ellis IO and Green AR: Tumor-infiltrating
CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin
Oncol. 29:1949–1955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Y, Li Y and Zhu B: T-cell exhaustion
in the tumor microenvironment. Cell Death Dis. 6:e17922015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antonioli L, Pacher P, Vizi ES and Haskó
G: CD39 and CD73 in immunity and inflammation. Trends Mol Med.
19:355–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Canale FP, Ramello MC, Núñez N, Araujo
Furlan CL, Bossio SN, Gorosito Serrán M, Tosello Boari J, Del
Castillo A, Ledesma M, Sedlik C, et al: CD39 Expression defines
cell exhaustion in tumor-infiltrating CD8+ T cells.
Cancer Res. 78:115–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simoni Y, Becht E, Fehlings M, Loh CY, Koo
SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, et al:
Bystander CD8+ T cells are abundant and phenotypically
distinct in human tumour infiltrates. Nature. 557:575–579. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Latchman YE, Liang SC, Wu Y, Chernova T,
Sobel RA, Klemm M, Kuchroo VK, Freeman GJ and Sharpe AH:
PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting
cells, and host tissues negatively regulates T cells. Proc Natl
Acad Sci USA. 101:10691–10696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang Y, Chen M, Nie H and Yuan Y: PD-1
and PD-L1 in cancer immunotherapy: Clinical implications and future
considerations. Hum Vaccin Immunother. 15:1111–1122. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dolina JS, Van Braeckel-Budimir N, Thomas
GD and Salek-Ardakani S: CD8+ T cell exhaustion in
cancer. Front Immunol. 12:7152342021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung HI, Jeong D, Ji S, Ahn TS, Bae SH,
Chin S, Chung JC, Kim HC, Lee MS and Baek MJ: Overexpression of
PD-L1 and PD-L2 is associated with poor prognosis in patients with
hepatocellular carcinoma. Cancer Res Treat. 49:246–254. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo M, Yuan F, Qi F, Sun J, Rao Q, Zhao Z,
Huang P, Fang T, Yang B and Xia J: Expression and clinical
significance of LAG-3, FGL1, PD-L1 and CD8+T cells in
hepatocellular carcinoma using multiplex quantitative analysis. J
Transl Med. 18:3062020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang CY, Wang Y, Luo GY, Han F, Li YQ,
Zhou ZG and Xu GL: Relationship between PD-L1 expression and CD8+
T-cell immune responses in hepatocellular carcinoma. J Immunother.
40:323–333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yugawa K, Itoh S, Yoshizumi T, Iseda N,
Tomiyama T, Morinaga A, Toshima T, Harada N, Kohashi K, Oda Y and
Mori M: CMTM6 stabilizes PD-L1 expression and is a new prognostic
impact factor in hepatocellular carcinoma. Hepatol Commun.
5:334–348. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu K, Wang ZM, Li JN, Zhang S, Xiao ZF and
Tao YM: CLEC1B expression and PD-L1 expression predict clinical
outcome in hepatocellular carcinoma with tumor hemorrhage. Transl
Oncol. 11:552–558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Q, Liu F and Liu L: Prognostic
significance of PD-L1 in solid tumor: An updated meta-analysis.
Medicine (Baltimore). 96:e63692017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou J, Sun H, Wang Z, Cong W, Zeng M,
Zhou W, Bie P, Liu L, Wen T, Kuang M, et al: Guidelines for the
diagnosis and treatment of primary liver cancer (2022 edition).
Liver Cancer. 12:405–444. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang A, Yang XR, Chung WY, Dennison AR
and Zhou J: Targeted therapy for hepatocellular carcinoma. Signal
Transduct Target Ther. 5:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xing R, Gao J, Cui Q and Wang Q:
Strategies to improve the antitumor effect of immunotherapy for
hepatocellular carcinoma. Front Immunol. 12:7832362021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ribas A and Wolchok JD: Cancer
immunotherapy using checkpoint blockade. Science. 359:1350–1355.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
current researches in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
29
|
Wang S, Yuan B, Wang Y, Li M, Liu X, Cao
J, Li C and Hu J: Clinicopathological and prognostic significance
of PD-L1 expression in colorectal cancer: A meta-analysis. Int J
Colorectal Dis. 36:117–130. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brody R, Zhang Y, Ballas M, Siddiqui MK,
Gupta P, Barker C, Midha A and Walker J: PD-L1 expression in
advanced NSCLC: Insights into risk stratification and treatment
selection from a systematic literature review. Lung Cancer.
112:200–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang
Y and Zhang Q: Expression of PD-L1 and prognosis in breast cancer:
A meta-analysis. Oncotarget. 8:31347–31354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wherry EJ and Kurachi M: Molecular and
cellular insights into T cell exhaustion. Nat Rev Immunol.
15:486–499. 2015. View Article : Google Scholar : PubMed/NCBI
|