Introduction
Lung cancer consistently demonstrates a high
incidence and mortality rate worldwide, with figures standing at
12.4 and 18.7% respectively (1).
Historically, the standard first-line treatment involved
platinum-based doublet chemotherapy, which offers limited benefits
to patients (2); however, the
advent of programmed cell death protein 1 (PD-1)/programmed
death-ligand 1 (PD-L1) inhibitors has substantially enhanced
treatment outcomes for advanced non-small cell lung cancer,
revolutionizing contemporary therapeutic strategies (3,4).
Tumor lysis syndrome (TLS) is a rare but severe
complication predominantly seen in malignant lymphomas and acute
lymphocytic leukemia. It is marked by profound metabolic
disturbances and associated clinical symptoms, triggered by the
release of cytoplasmic and nuclear components into the bloodstream
due to tumor cell lysis. Manifestations include hyperkalemia,
hyperuricemia, hyperphosphatemia, hypocalcemia, acute uric acid
nephropathy and acute renal failure. TLS is infrequently observed
in solid tumors; however, cases have been documented in small-cell
lung cancer, neuroblastoma and testicular tumors (5). High tumor burden and responsiveness to
treatment constitute significant risk factors for the development
of TLS. Current therapeutic strategies primarily encompass
intravenous hydration, stabilization of electrolyte balance, uric
acid reduction, and preservation of renal function. Despite these
interventions, the prognosis remains unfavorable, with a high
mortality rate spanning from 29 to 79% (6,7). To
date, no cases of TLS have been reported in patients with lung
squamous cell carcinoma (SCC) treated with PD-1/PD-L1 inhibitors
combined with chemotherapy, to the best of our knowledge.
The present study reports a novel case of TLS in a
patient with advanced lung SCC following first-line treatment with
a PD-1 inhibitor and chemotherapy. The present case underscores the
critical need for increased vigilance, enhanced early detection,
and prompt intervention to manage the rare occurrence of TLS in
this patient population.
Case report
A 61-year-old male patient presented to the Second
People's Hospital of Guiyang, (Guiyang City, Guizhou Province, in
July 2023, exhibiting a persistent cough accompanied by hemoptysis
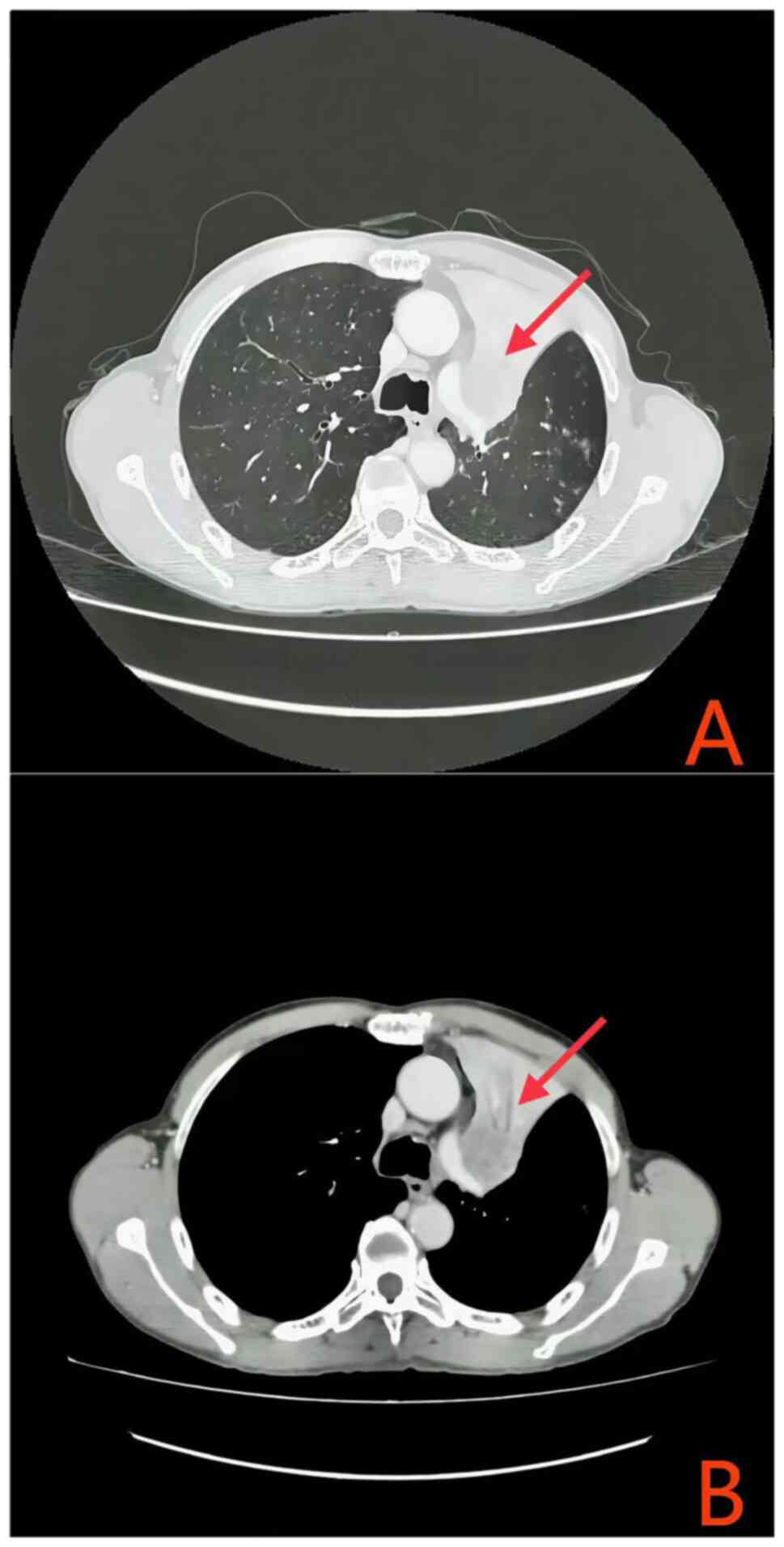
for a duration exceeding two months. Enhanced chest computed
tomography (CT) revealed a suspected left upper lobe lung cancer
with obstructive pneumonia (Fig.
1A). Further CT imaging delineated atelectasis in the left
upper lobe, the primary lung lesion measuring only 36 mm in
diameter, multiple pulmonary nodules indicative of metastatic
disease, and enlarged mediastinal lymph nodes (Fig. 1B); however, cranial magnetic
resonance imaging, enhanced upper abdominal CT and whole-body bone
imaging showed no significant abnormalities. Bronchoscopic
pathological biopsy was performed and the pathological analysis of
the tissue specimens demonstrated the presence of squamous cell
carcinoma of the lung (For routine tissue staining, we utilize
Hematoxylin and Eosin (HE) staining. Specifically, tissues are
fixed in 10% formalin at room temperature for 6 h, and the slices
are cut to a thickness of 3 µm. The staining protocol proceeds as
follows: three xylene baths, each lasting 3 min; two baths of
absolute ethanol, each for 3 min; a single bath of 95% alcohol for
2 min; two hematoxylin baths, each for 5 min; a brief 3-sec
exposure to hydrochloric acid-alcohol; a 1-min treatment with
lithium carbonate; eosin staining for 30 sec; and rinsing through a
series of alcohol baths with varying concentrations (75% for 10
sec, 85% for 10 sec, 90% for 10 sec, and 95% for 1 min), followed
by two baths of absolute ethanol, each lasting 2 min. Microscopic
examination is performed using a LEICA2000 microscope. For
immunostaining, paraffin-embedded tissues are fixed in 10% formalin
at room temperature for 6 h. No resin treatment is employed, and
the slice thickness remains at 3 um. No permeabilization reagents
are used. Antigen retrieval and exposure for paraffin-embedded
tissues are primarily achieved through high pressure and
high-temperature treatment with EDTA repair solution, facilitating
superior binding of antibodies to their respective antigens. A 3%
H2O2 blocking reagent is applied for 15 min
at room temperature, and no serum blocking is utilized. The primary
antibodies and their respective dilutions, catalog numbers, and
suppliers are: CK 1:300, CgA 1:200, Vimentin 1:400, EMA 1:200,
Ki-67 1:200, P53 1:200, CK5/6 1:250, P40 1:200, P63 1:200, TTF-1
1:200, Napsin-A 1:150, Desmin 1:100, S-100 1:200, CD56 1:150, Syn
1:200. All antibodies are provided by Wuxi OriGene Biotechnology
Co., Ltd. and incubated at 37°C for 1 h. The secondary antibodies
are prepared as working solutions, with the catalog number
specified as HRP (horseradish peroxidase). The supplier is Venta
Medical Group, and the incubation conditions. The secondary
antibody is diluted to a working solution, with the catalog number
specified as HRP (horseradish peroxidase). The supplier is Venta
Medical Group, and the incubation conditions are 37°C for 30 min.
DAB is used, with the detection reagent named as DAB Chromogenic
Solution. The type of microscope used is a LEICA2000 microscope),
devoid of any indicia of small cell components (Fig. 2). This observation unequivocally
precluded the possibility of small cell carcinoma in the
differential diagnostic considerations. The laboratory evaluation
pertaining to liver and kidney functionality, electrolytes and
lymphocyte subsets are outlined in Tables I and II. Upon performing a thorough assessment
of the driver genes of the patient, no clinically significant
genetic mutations were identified. PD-L1 tumor proportion score of
the patient was noted to be <1%. The patient was diagnosed with
SCC of the left upper lung, obstructive pneumonia and atelectasis,
with invasion into the left main bronchus, mediastinum and major
blood vessels. Lymph node metastases were confirmed in regions
1R/L, 2R, 3A, 4R/A, 5, 6, 7 and 10R/L, with multiple pulmonary
metastases. The disease was staged as cT4N3M1a IVA according to the
International Association for the Study of Lung Cancer staging
system 9th Edition (8), with PD-L1
expression <1%.
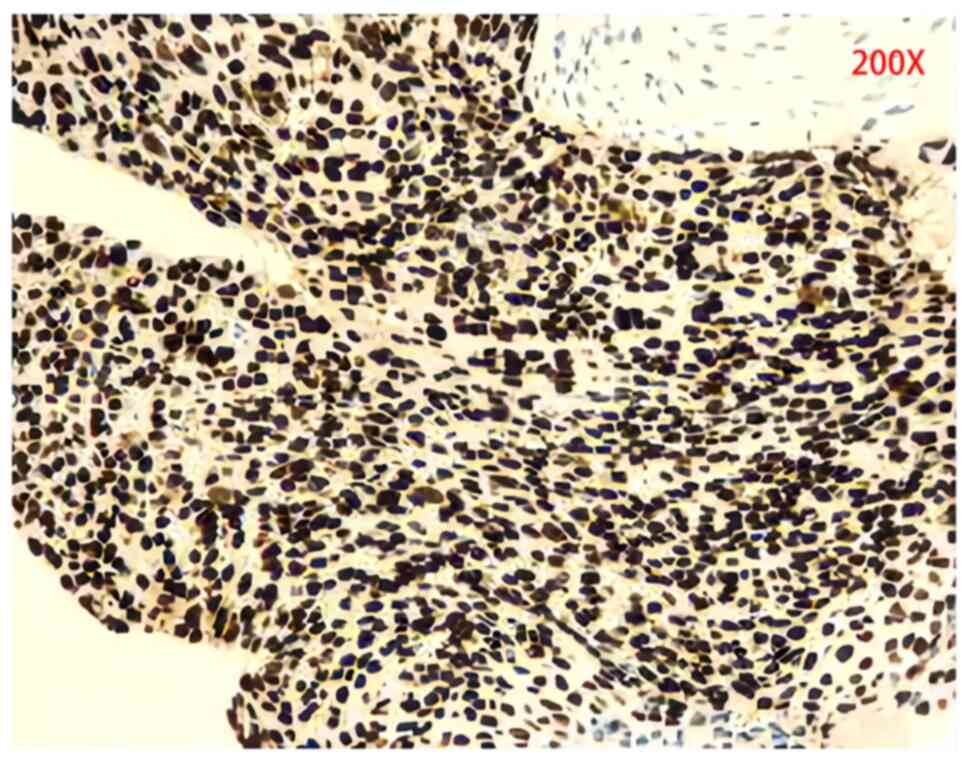 | Figure 2.Biopsy histological images of squamous
cell carcinoma. Immunohistochemical staining revealed Cytokeratin
(+), vimentin (focal +), Epithelial Membrane Antigen (−), Ki-67
Antigen (+40%), Tumor Protein P53 (mutant type), Cytokeratin 5/6
(+), P40 (+), P63 (+), Thyroid Transcription Factor-1 (−), napsin-A
(−), desmin (−), S-100 (−), CD56 (−), Synaptophysin (−) and CgA
(−). |
 | Table I.Laboratory tests. |
Table I.
Laboratory tests.
| Item | Laboratory reference
values | 2023-07 | 2023-08 | 2023-08 | 2023-08 | 2023-10 | 2023-10 |
|---|
| Potassium | 3.50–5.30 mmol/l | 3.80 | 5.41 | 4.70 | 5.16 | 3.80 | 3.90 |
| Phosphorus | 0.85–1.51 mmol/l | 1.31 | 1.76 | 1.79 | 1.38 | 1.07 | 1.04 |
| Uric acid | 208.00–428.00
µmol/l | 402.00 | 547.04 | 412.00 | 300.39 | 300.00 | 229.00 |
| Calcium | 2.11–2.52 mol/l | 1.96 | 2.16 | 2.06 | 2.15 | 1.86 | 2.00 |
| Creatinine | 57.00–111.00
µmol/l | 95.00 | 422.02 | 337.00 | 197.58 | 152.00 | 107.00 |
 | Table II.Peripheral blood lymphocyte
subsets. |
Table II.
Peripheral blood lymphocyte
subsets.
| Lymphocyte
subset | Proportion, % |
|---|
| T cells
(CD45+CD3+) | 63.6 |
| Cytotoxic/suppressor
T cells (CD3+CD8+) | 39.2 |
| Inducible/helper T
cells (CD3+CD4+) | 53.0 |
| Positive T cells
(CD3+CD4+CD8+) | 0.6 |
| Double-negative T
cells (CD3+CD4−CD8−) | 7.1 |
| Double-NK cells
(CD3−CD16+CD56+) | 29.3 |
| CIK cells
(CD3+CD16+CD56+) | 5.2 |
| B cells
(CD3−CD19+) | 6.9 |
| Cytotoxic T cells
(CD3+CD18+CD28+) | 56.4 |
| Suppressor T cells
(CD3+CD18+CD28−) | 44.0 |
| Regulatory T cells
(CD25+CD127low−T4) | 10.0 |
In July 2023, the patient began treatment with a
combination of albumin-bound paclitaxel, cisplatin and a PD-1
inhibitor. Tislelizumab 200 mg was administered via intravenous
drip on Day 1. Paclitaxel (albumin-bound) 395 mg was administered
via intravenous drip on Day 2. Cisplatin 110 mg was administered
via intravenous drip from Day 2 to Day 3, divided into two
administrations. The treatment cycle is 21 days, and this is the
first cycle. Subsequently, in August 2023, a comprehensive
re-evaluation of the hepatic and renal function of the patient,
along with electrolyte profiling, revealed hyperkalemia,
hyperphosphatemia, hyperuricemia, hypocalcemia and signs of acute
renal impairment (Table I);
however, the patient remained asymptomatic for arrhythmia, tetany,
muscular spasms, hypotension, nausea, vomiting, abdominal
discomfort or oliguria. Based on consensus guidelines from the TLS
expert panel (9), a diagnosis of
laboratory-confirmed TLS was considered. Treatment included rectal
administration of Shenkang Shuan (4 g (4× daily), oral Niuduqing
granules 5 g for kidney protection (3× daily) Until renal function
returns to normal, oral cyclosilicate sodium powder to reduce
potassium levels (3× times daily, adjusted to 5 g orally 3× daily
to maintain stability once the blood potassium levels normalized),
and febuxostat 20 mg (once daily) to decrease uric acid levels.
Fluid replacement and dynamic monitoring of liver, kidney and heart
function were initiated, along with symptomatic treatment
[Administer intravenous infusion of 0.9% sodium chloride injection
at a dosage of 3 l/m2 daily, and maintain urine output at a rate
greater than 100 ml/(m2•h)]. After fluid replacement, urine
alkalinization, electrolyte correction and kidney protection, the
parameters of the patient improved (Table I). In accordance with the monitoring
recommendations outlined in the National Comprehensive Cancer
Network® guidelines during initial therapy (10), due to the manifestation of TLS
symptoms in the patient, a timely assessment of any changes in the
condition of the patient was imperative. Therefore, following a
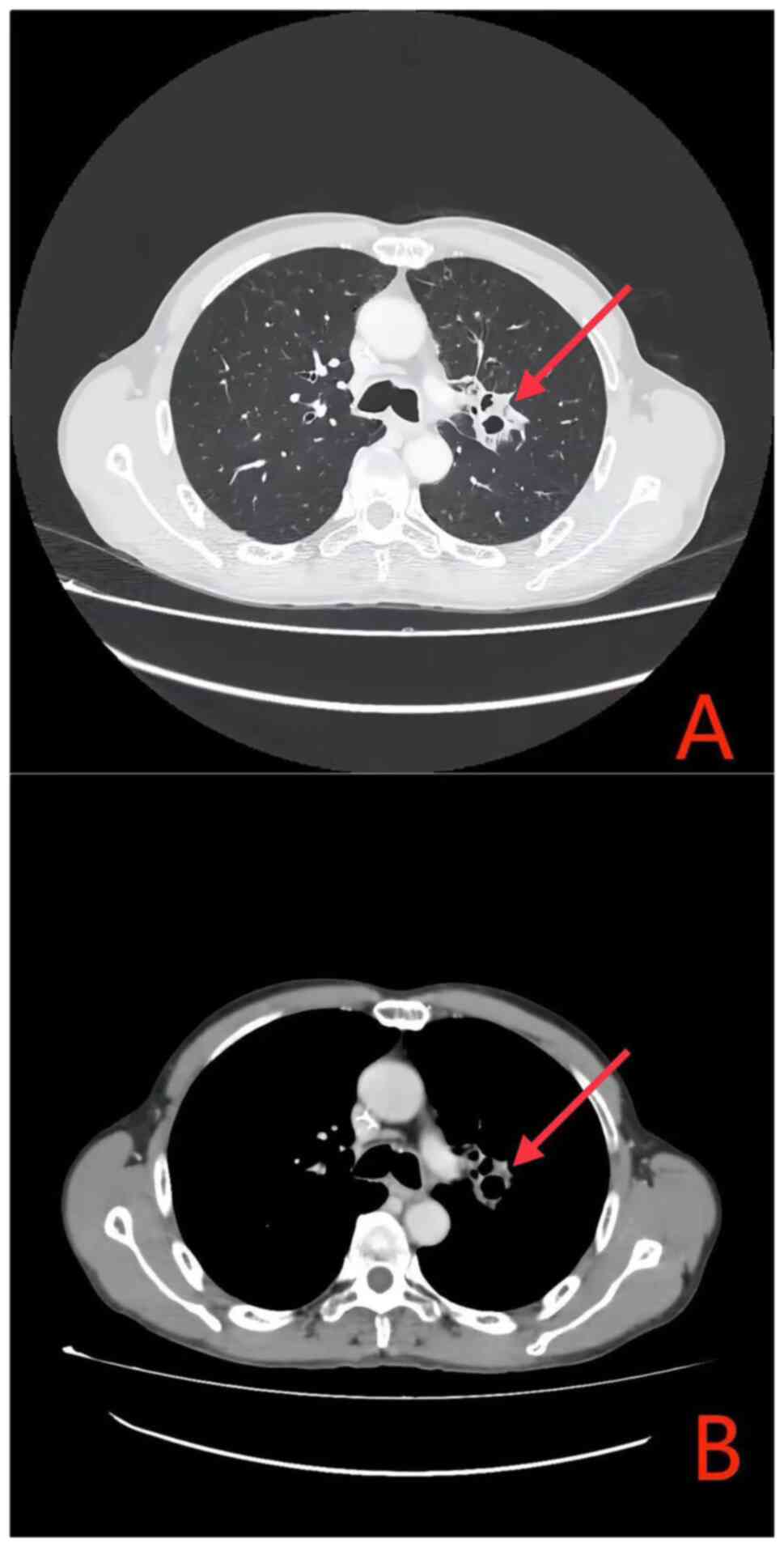
single cycle of chemotherapy combined with PD-1 immunotherapy,
radiological reassessment performed at 4 weeks post-treatment
demonstrated a marked decrease in the size of both the primary lung
lesion and the metastatic lymph nodes when compared with the
baseline prior to treatment (Fig.
3). The treatment efficacy was classified as a partial response
(PR) based on the Response Evaluation Criteria in Solid Tumours
(RECIST) 1.1 criteria (11)
(Figs. 2 and 3). From the second cycle onwards, the
chemotherapy drug was adjusted to albumin-bound paclitaxel +
carboplatin (Tislelizumab 200 mg was administered via intravenous
drip on Day 1. Paclitaxel (albumin-bound) 395 mg was administered
via intravenous drip on Day 2. Carboplatin 440 mg was administered
via intravenous drip on Day 2. The treatment cycle is 21 days).
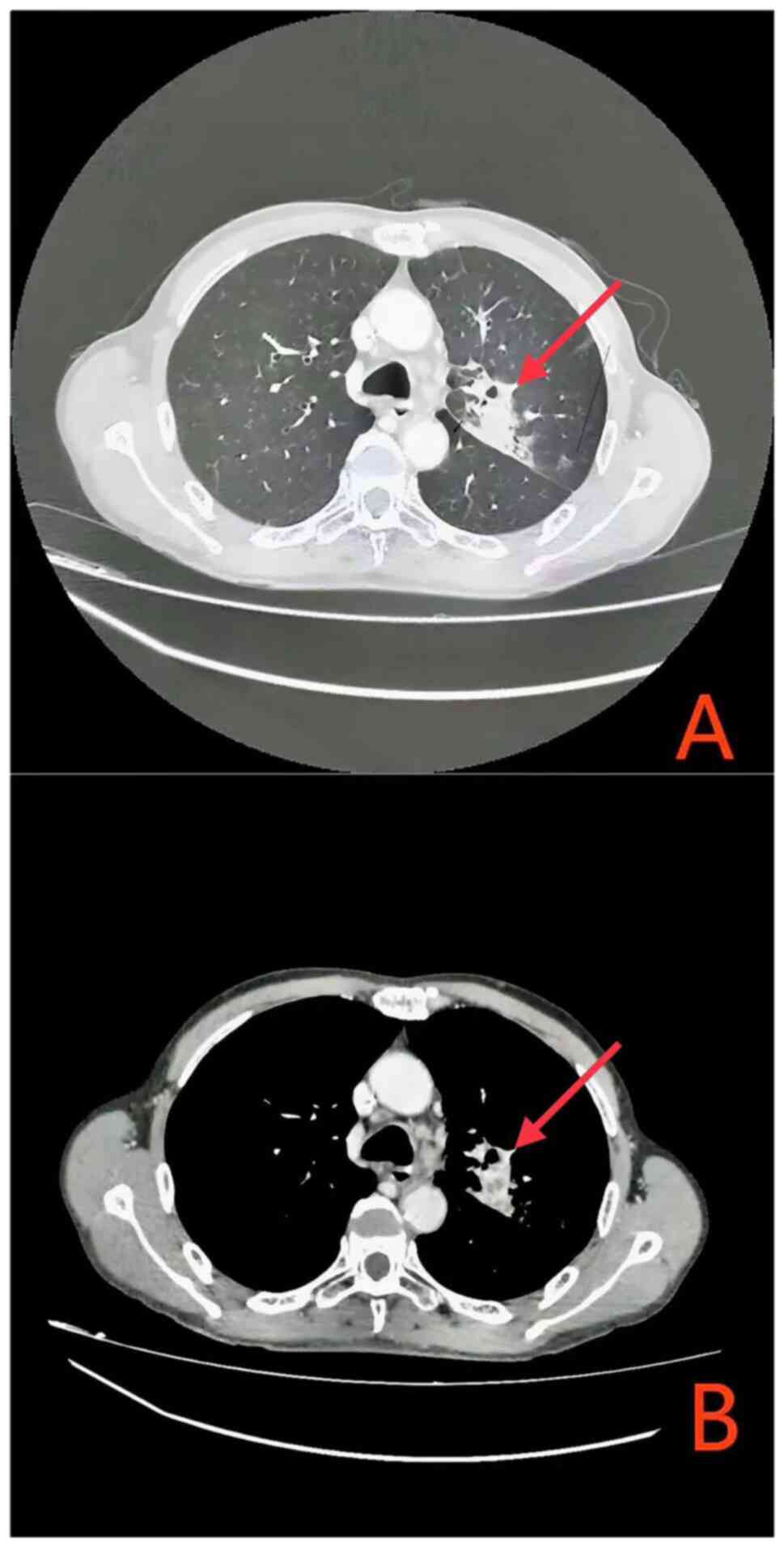
Upon completion of the systematic treatment, the efficacy
evaluation according to RECIST 1.1 criteria was classified as a PR
(Fig. 4). As of April 2024, the
patient's condition remains stable.
Discussion
Non-small cell lung cancer accounts for ~80% of lung
cancer cases, with lung squamous cell carcinoma (SCC) identified as
a subtype linked to a poor prognosis. Recent advancements in immune
checkpoint blockade therapy have notably improved the prognosis for
patients with advanced lung SCC, PD-1 inhibitors plus chemotherapy
showed a clinically meaningful improvement in OS (HR, 0.71;95% CI,
0.58 to 0.88) and PFS (HR, 0.57; 95% CI, 0.47 to 0.69) vs. placebo
plus chemotherapy in the protocol-specified (11,12).
PD-1 inhibitors, which are IgG4 antibodies, target the PD-1
receptors on T cells, disrupting the immune evasion mechanism of
the tumor and preserving the tumoricidal activity of these cells
(13).
TLS is an oncologic emergency that, whilst rare in
solid tumors, has been observed in drug-sensitive malignancies such
as small-cell lung cancer, neuroblastoma and testicular tumors
(5). TLS results from extensive
necrosis of tumor cells, leading to intracellular changes,
increased membrane permeability and the release of necrotic
contents into the bloodstream. This causes metabolic disturbances
such as hyperkalemia, hyperuricemia, hyperphosphatemia,
hypocalcemia and acute renal failure. A significant tumor burden is
considered a high-risk factor for TLS development. In the present
case, despite the primary lung lesion measuring only 36 mm in
diameter, the patient had multiple metastatic lymph nodes across
several areas (1R/L, 2R, 3A, 4R/L, 5, 6, 7 and 10R/L), contributing
to a substantial overall tumor burden. Notably, the PD-L1 tumor
proportion score of the patient was noted to be <1%.
Immunohistochemical analysis of PD-L1 expression is
the first clinically validated biomarker predictive of response to
immune therapy, with higher expression levels generally associated
with improved outcomes (14).
Nevertheless, patients with low PD-L1 expression can still benefit
from PD-1/PD-L1 inhibitors (15),
highlighting the need for additional biomarkers to guide
immunotherapy in distinct patient subgroups. Studies have assessed
the relationship between peripheral blood lymphocytes and
immunotherapy outcomes (16–18).
In the present patient, cytotoxic T cells
(CD3+CD8+CD28+) accounted for
56.4% of the peripheral lymphocyte subset, notably above the normal
reference values. After one cycle of administration of a PD-1
inhibitor combined with chemotherapy, the patient achieved a PR.
Evidence suggests that high levels of circulating CD8+ T
cells are associated with improved clinical outcomes in patients
receiving PD-1 inhibitors (18–20),
indicating that monitoring lymphocyte subsets could be a valuable
strategy for identifying high-risk patients. Furthermore,
management of TLS involves active hydration to optimize renal
perfusion and glomerular filtration rate, reduce deposition of uric
acid and calcium phosphate in the renal tubules, and enhance uric
acid solubility and excretion. In cases of refractory electrolyte
disturbances, fluid overload or rapid renal function decline,
dialysis should be initiated promptly.
In conclusion, the widespread use of PD-1 inhibitors
necessitates vigilant monitoring for TLS in solid tumors. Prompt
intervention, dynamic post-treatment monitoring and early TLS
detection are crucial for improving patient compliance, ensuring
treatment continuity and optimizing outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LC contributed to the conceptualization, design,
analysis, and writing of the original and subsequent drafts. WC
contributed to data collection, advised on patient treatment,
interpretation of data, and reviewed and edited the manuscript. LC
and WC confirm the authenticity of all the raw data. Both authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient gave written informed consent for the
publication of patient data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morgensztern D, Waqar S, Subramanian J,
Gao F and Govindan R: Improving survival for stage IV non-small
cell lung cancer: A surveillance, epidemiology, and end results
survey from 1990 to 2005. J Thorac Oncol. 4:1524–1529. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gadgeel S, Rodríguez-Abreu D, Speranza G,
Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell
SF, et al: Updated Analysis From KEYNOTE-189: Pembrolizumab or
placebo plus pemetrexed and platinum for previously untreated
metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol.
38:1505–1517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Novello S, Kowalski DM, Luft A, Gümüş M,
Vicente D, Mazières J, Rodríguez-Cid J, Tafreshi A, Cheng Y, Lee
KH, et al: Pembrolizumab Plus Chemotherapy in Squamous
Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III
KEYNOTE-407 Study. J Clin Oncol. 41:1999–2006. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Howard SC, Jones DP and Pui CH: The tumor
lysis syndrome. N Engl J Med. 364:1844–1854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klemencic S and Perkins J: Diagnosis and
management of oncologic emergencies. West J Emerg Med. 20:316–322.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papapanou M, Athanasopoulos AE, Georgiadi
E, Maragkos SA, Liontos M, Ziogas DC, Damaskos D and Schizas D:
Spontaneous tumor lysis syndrome in patients with solid tumors: A
scoping review of the literature. Med Oncol. 40:2332023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asamura H, Nishimura KK, Giroux DJ,
Chansky K, Hoering A, Rusch V and Rami-Porta R; Members of the
IASLC Staging and Prognostic Factors Committee and of the Advisory
Boards, and Participating Institutions, : IASLC lung cancer staging
project: The New Database to Inform Revisions in the Ninth Edition
of the TNM classification of lung cancer. J Thorac Oncol.
18:564–575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perissinotti AJ, Bishop MR, Bubalo J,
Geyer MB, Goodrich A, Howard SC, Kula J, Mandayam S, Cairo MS and
Pui CH: Expert consensus guidelines for the prophylaxis and
management of tumor lysis syndrome in the United States: Results of
a modified Delphi panel. Cancer Treat Rev. 120:1026032023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M,
et al: NCCN Guidelines® Insights: Non-Small Cell Lung
Cancer, Version 2.2023. J Natl Compr Canc Netw. 21:340–350. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong H, Sun S, Chen J, Wang Z, Zhao Y,
Zhang G, Chen G, Zhou M, Zhou J, Du Y, et al: First-line penpulimab
combined with paclitaxel and carboplatin for metastatic squamous
non-small-cell lung cancer in China (AK105-302): A multicentre,
randomised, double-blind, placebo-controlled phase 3 clinical
trial. Lancet Respir Med. 12:355–365. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SH, Lee HT, Lim H, Kim Y, Park UB and
Heo YS: Crystal structure of PD-1 in complex with an antibody-drug
tislelizumab used in tumor immune checkpoint therapy. Biochem
Biophys Res Commun. 527:226–2312. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Updated Analysis of KEYNOTE-024: Pembrolizumab versus
platinum-based chemotherapy for advanced non-small-cell lung cancer
With PD-L1 tumor proportion score of 50% or Greater. J Clin Oncol.
37:537–546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Man J, Millican J, Mulvey A, Gebski V and
Hui R: Response rate and survival at key timepoints with PD-1
blockade vs chemotherapy in PD-L1 Subgroups: Meta-Analysis of
Metastatic NSCLC Trials. JNCI Cancer Spectr. 5:pkab0122021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gridelli C, Ardizzoni A, Barberis M,
Cappuzzo F, Casaluce F, Danesi R, Troncone G and De Marinis F:
Predictive biomarkers of immunotherapy for non-small cell lung
cancer: Results from an Experts Panel Meeting of the Italian
Association of Thoracic Oncology. Transl Lung Cancer Res.
6:373–386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gascón M, Isla D, Cruellas M, Gálvez EM,
Lastra R, Ocáriz M, Paño JR, Ramírez A, Sesma A, Torres-Ramón I, et
al: Intratumoral versus circulating lymphoid cells as predictive
biomarkers in lung cancer patients treated with immune checkpoint
inhibitors: Is the Easiest Path the Best One? Cells. 9:15252020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim KH, Kim CG and Shin EC: Peripheral
blood immune cell-based biomarkers in anti-PD-1/PD-L1 therapy.
Immune Netw. 20:e82020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geng R, Tang H, You T, Xu X, Li S, Li Z,
Liu Y, Qiu W, Zhou N, Li N, et al: Peripheral CD8+CD28+ T
lymphocytes predict the efficacy and safety of PD-1/PD-L1
inhibitors in cancer patients. Front Immunol. 14:11258762023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong JY, Cho HJ, Sa JK, Liu X, Ha SY, Lee
T, Kim H, Kang W, Sinn DH, Gwak GY, et al: Hepatocellular carcinoma
patients with high circulating cytotoxic T cells and intra-tumoral
immune signature benefit from pembrolizumab: Results from a
single-arm phase 2 trial. Genome Med. 14:12022. View Article : Google Scholar : PubMed/NCBI
|