Introduction
Giant cell tumor of bone (GCTB) is a locally
aggressive intermediate bone tumor occurring in long bones such as
the femur, tibia, radius, humerus, and spine. GCTB predominantly
arises in the second to fourth decades of life and is slightly more
common in women (1,2). The treatment mainstays are surgical
treatment including curettage or en bloc resection (1,2).
Complete surgical excision is preferred for optimal oncological
outcomes. However, surgical treatment is often challenging, with
severe impairment in cases of GCTB in the spine and pelvis due to
the proximity of critical structures, including major nerves and
vessels, and the high incidence of postoperative complications
(3,4). Furthermore, GCTB recurs
postoperatively in 10–50% of patients (1,2). While
radiotherapy is used to treat unresectable or advanced GCTB, the
risk of malignant transformation after radiotherapy is relatively
high (5,6). Although selective serial embolization
may be useful for treating GCTB in the spine and pelvis, the
recurrence rate is 22–75% (7,8).
GCTB comprises reactive non-neoplastic
multinucleated giant cells (osteoclasts) expressing the receptor
activator of nuclear factor-κB (RANK) and neoplastic mononuclear
stromal cells expressing RANK ligand (RANKL) (9). RANK-RANKL interactions promote
osteoclast proliferation and survival, thus facilitating bone
resorption. Denosumab, a monoclonal antibody inhibitor of RANKL,
recently showed effectiveness in GCTB treatment (10,11).
Denosumab suppresses the osteoclast activity promoted by
mononuclear tumor cells, induces new bone formation in the
destroyed bone, and improves bone stability. The marked and
extensive re-ossification of osteolytic lesions indicates the
tumor-suppressive effect of denosumab (12). Multicenter phase 2 studies
demonstrated that denosumab was an effective and promising
treatment option for advanced or unresectable GCTB (13–16).
However, the severe adverse events (AEs) of denosumab treatment
include hypocalcemia, osteonecrosis of the jaw (ONJ), atypical
femoral fracture (AFF), and malignant transformation of GCTB
(9–11,17),
likely because 4-weekly denosumab treatment is required for
difficult-to-treat GCTB. Furthermore, denosumab is not generally
used in women who want to become pregnant, although GCTB is likely
to occur at childbearing age. Denosumab cessation to address these
issues resulted in high rates of local recurrence (15,18,19).
Chawla et al reported disease or recurrence progression
after denosumab cessation in 26% of patients with surgically
unsalvageable GCTB within a median of 39 months (15). Palmerini et al observed that
40% of patients with unresectable GCTB developed tumor progression
at a median of 8 months after denosumab cessation (18).
Denosumab was widely used in managing bone
metastases before GCTB treatment. Recent studies demonstrated the
efficacy and safety of extended-interval denosumab treatment
(de-escalation) for bone metastases (20,21).
Clemons et al showed similar clinical results, including
symptomatic skeletal events related to bone metastases, for a
12-weekly treatment of bone-modifying agents, including denosumab,
compared to a 4-weekly treatment (20). However, the benefits of denosumab
de-escalation for unresectable GCTB have not been well discussed,
with only two related case reports regarding denosumab
de-escalation for GCTB (22,23).
The denosumab dosing interval was extended after achieving good
therapeutic effects with standard 4-weekly treatment, mainly to
avoid AEs. The present study investigated the efficacy and safety
of denosumab de-escalation in a series of cases with unresectable
GCTB to address i) how long the denosumab dosing interval can be
extended, ii) whether denosumab de-escalation can sustain the
therapeutic change in GCTB on imaging, and iii) if denosumab
de-escalation can prevent AEs.
Patients and methods
Study population
The medical records of nine patients (2 men and 7
women) with GCTB that were either unresectable or resectable but
not candidates for resection, who received de-escalated denosumab
treatment at Okayama University Hospital (Okayama, Japan) between
April 2014 and December 2023 were retrospectively reviewed.
Patients who had undergone radiotherapy or surgery with neoadjuvant
denosumab treatment were excluded (Table I). The median age at initial
denosumab treatment was 44 (range, 25–77) years. The tumors were
located in the sacrum (5 patients), femur (2 patients), thoracic
spine (1 patient), and lumbar spine (1 patient). Four patients were
newly diagnosed with primary tumors, while five had locally
recurrent tumors, two of whom were previously treated with repeated
embolization for sacral GCTB, one with intralesional resection
(curettage) followed by repeated embolization for sacral GCTB, and
two with intralesional resection and bone grafting for femoral
GCTB. Two patients with resectable recurrent femoral GCTB received
definitive denosumab treatment as both refused prosthetic
replacement. One patient (case 7) who originally participated in a
multicenter phase 2 clinical trial on denosumab and received
denosumab therapy in another hospital was treated in our hospital
after the therapy for GCTB was covered by the National Health
Insurance in Japan.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Case | Sex | Age, years | Location | Campanacci
grading | Prior
treatment |
|---|
| 1 | Female | 77 | L5 | 2 |
|
| 2 | Female | 44 | T10 | 2 |
|
| 3 | Female | 25 | S1 | 3 |
|
| 4 | Female | 64 | S3 | 3 | Embolization |
| 5 | Female | 30 | S2 | 3 | Embolization and
surgery |
| 6 | Male | 27 | S1 | 3 | Embolization |
| 7 | Female | 44 | S2 | 3 | Embolization |
| 8 | Male | 59 | Femur | 2 | Surgery |
| 9 | Female | 26 | Femur | 2 | Surgery |
Treatment
All patients received 120 mg of denosumab on days 1,
8, 15, and 29 and every 4 weeks thereafter. The treatment interval
was gradually extended to every 8, 12, and 24 weeks after careful
discussion with the patient based on the clinical symptoms and
radiological findings. The patients were administered calcium and
vitamin D supplements daily. The denosumab treatment was defined
based on the treatment interval: standard treatment (every 4 weeks)
and de-escalated treatment (every 8, 12, and 24 weeks). We
investigated the duration and dose of denosumab treatment in each
period, as well as the AEs related to denosumab treatment.
Imaging assessment
Computed tomography (CT) (Discovery CT750 HD, GE)
images, obtained at 120 kV and a 5-mm slice thickness, were viewed
in a routine bone window setting (window level 200 HU, window width
2,000 HU) in the axial, sagittal, and coronal planes. The magnetic
resonance imaging (MRI) (MEGNETOM Prisma, Siemens) findings
included T1- and T2-weighted images obtained in the axial,
sagittal, and coronal planes. All patients underwent plain
radiography, CT, and MRI before denosumab treatment, every 1–5
months in patients administered standard denosumab treatment, and
every 4–12 months in patients administered de-escalated treatment.
The radiological responses of the tumor to denosumab treatment were
assessed using the MD Anderson (MDA) criteria (24–26)
(Table II). Re-ossification of
osteolytic lesions on CT images indicated successful repair with
denosumab treatment (10). The time
to response (TTR) was defined as the duration from the first
denosumab treatment to the identification of sclerotic changes
inside the tumor based on the MDA criteria. Five tumors were
associated with extraskeletal masses, which were evaluated as grade
3 according to the Campanacci classification, while four tumors
were classified as grade 2 (27).
The sizes of the extraskeletal masses were measured after
completing the course of denosumab treatment. The spinal
instability neoplastic score (SINS) was evaluated in seven patients
with spinal GCTB (Table III). The
total score was divided into three categories: stable (0–6 points),
potentially unstable (7–12 points), and unstable (13–18
points).
 | Table II.MD Anderson criteria. |
Table II.
MD Anderson criteria.
| Response
category | Plain X-ray or
computed tomography | Magnetic resonance
imaging |
|---|
| Complete | Complete sclerotic
fill-in of lytic lesions | Disappearance of
tumor signal |
| response | Normalization of
bone density |
|
| Partial
response | Development of a
sclerotic rim or partial sclerotic change or sclerosis of lytic
lesions | Regression of
measurable lesion |
|
| Regression of
measurable lesion |
|
| Stable disease | No change in
measurable lesion | No change in
measurable lesion |
| Progressive
disease | Increase in the
size of measurable lesions | Increase in the
size of measurable lesions |
 | Table III.Spinal instability neoplastic
scores. |
Table III.
Spinal instability neoplastic
scores.
| Component | Score |
|---|
| Location |
|
|
Junctional (occiput-C2, C7-T2,
T11-L1, L5-S1) | 3 |
| Mobile
spine (C3-C6, L2-L4) | 2 |
|
Semirigid (T3-T10) | 1 |
| Rigid
(S2-S5) | 0 |
| Paina |
|
|
Yes | 3 |
|
Occasional pain but not
mechanical | 1 |
|
Pain-free lesion | 0 |
| Bone lesion |
|
|
Lytic | 2 |
| Mixed
(lytic/blastic) | 1 |
|
Blastic | 0 |
| Radiographic spinal
alignment |
|
|
Subluxation/translation
present | 4 |
| De novo
deformity (kyphosis/scoliosis) | 2 |
| Normal
alignment | 0 |
| Vertebral body
collapse |
|
| >50%
collapse | 3 |
| <50%
collapse | 2 |
| No
collapse with >50% body involved | 1 |
| None of
the above | 0 |
| Posterolateral
involvement of spinal elementsb |
|
|
Bilateral | 3 |
|
Unilateral | 1 |
| None of
the above | 0 |
Clinical and physical
examinations
Local pain was assessed using the numerical rating
scale (NRS), with scores ranging from 0 (no pain) to 10 (worst
pain). Patients with GCTB of the spine were also examined.
Functional impairment was assessed using the American Spinal Injury
Association (ASIA) impairment scale for patients with spinal GCTB
(28).
Statistical analysis
The median sizes of the extraskeletal masses before
and after standard treatment, the median SINS for spinal GCTB
before and after standard treatment, and at the latest follow-up,
and median NRS before and after standard treatment were analyzed
using Wilcoxon signed-rank tests. All analyses were conducted using
IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Tokyo,
Japan). For all analyses, P<0.05 was considered significant.
Results
Denosumab de-escalation and clinical
course
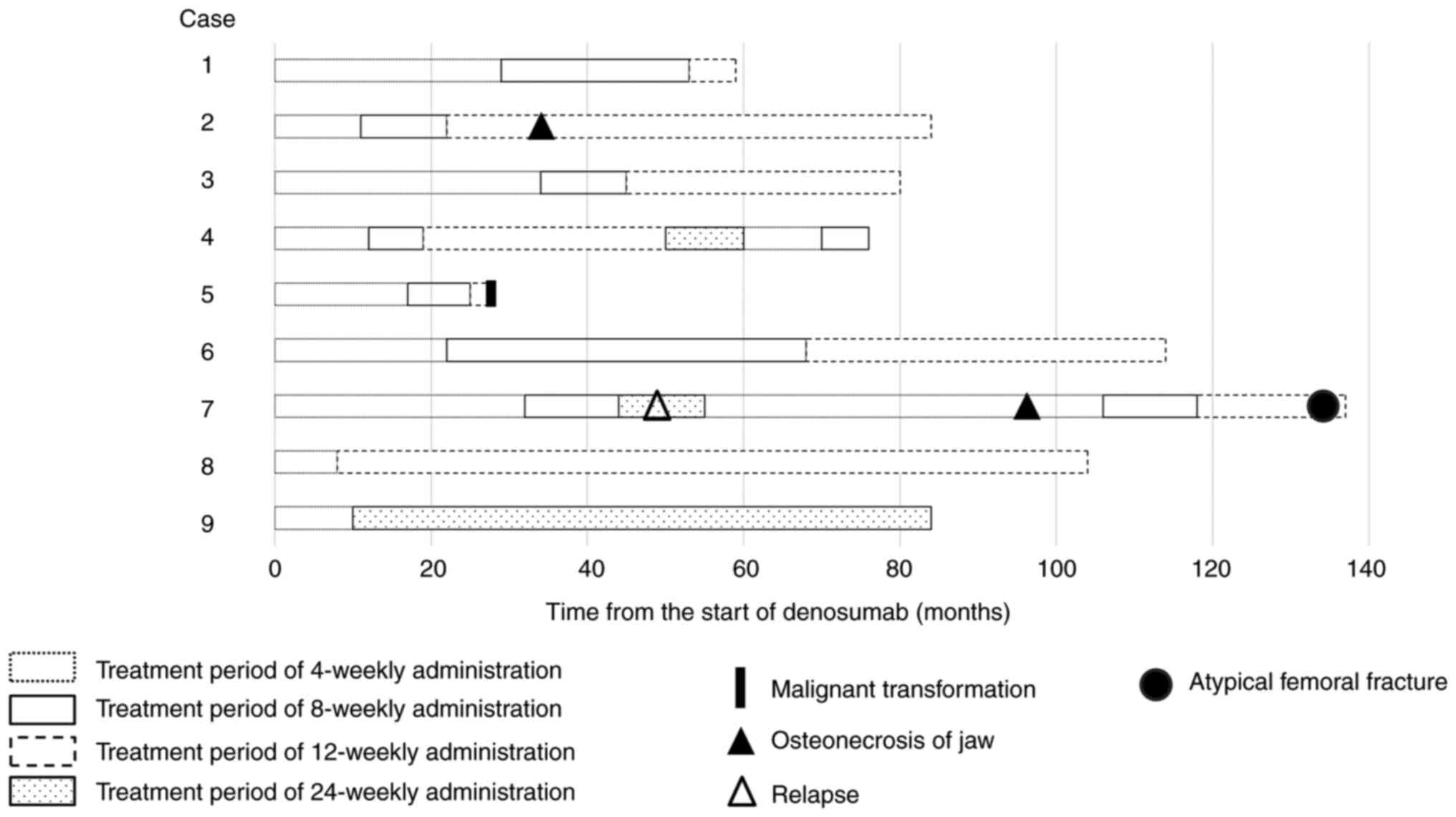
The median overall duration of denosumab treatment
was 84 (range, 28–137) months (Fig.
1). The interval of denosumab treatment was extended after a
median of 12 (range, 12–34) months, with a median treatment time
for de-escalation of 46 (range, 11–96) months (Table IV). The median number of denosumab
injections was 40 (range, 20–110), with medians of 17 (range, 8–37)
injections for standard treatment and 15 (range, 5–41) injections
for de-escalated treatment. Eight of the nine patients had received
de-escalated treatment at the latest follow-up.
 | Table IV.Denosumab treatment. |
Table IV.
Denosumab treatment.
|
|
| Number of denosumab
doses | Duration of
denosumab treatment (months) |
|---|
|
|
|
|
|
|---|
| Case | Denosumab
treatment | 4-weekly | 8-weekly | 12-weekly | 24-weekly | 4-weekly | 8-weekly | 12-weekly | 24-weekly |
|---|
| 1 | 8-weekly → | 17 | 12 | 2 |
| 29 | 24 | 6 |
|
|
| 12-weekly |
|
|
|
|
|
|
|
|
| 2 | 8-weekly → | 14 | 7 | 18 |
| 11 | 11 | 62 |
|
|
| 12-weekly |
|
|
|
|
|
|
|
|
| 3 | 8-weekly → | 37 | 4 | 11 |
| 34 | 11 | 35 |
|
|
| 12-weekly |
|
|
|
|
|
|
|
|
| 4 | 8-weekly → | 16 | 4 | 10 | 2 | 12 | 7 | 31 | 10 |
|
| 12-weekly → |
|
|
|
|
|
|
|
|
|
| 24-weekly |
|
|
|
|
|
|
|
|
|
| (progression) | 10 | 2 |
|
| 10 | 6 |
|
|
|
| → 4-weekly → |
|
|
|
|
|
|
|
|
|
| 8-weekly |
|
|
|
|
|
|
|
|
| 5 | 8-weekly → | 20 | 4 | 1 |
| 17 | 8 | 3 |
|
|
| 12-weekly |
|
|
|
|
|
|
|
|
|
| (malignant
transformation) |
|
|
|
|
|
|
|
|
| 6 | 8-weekly → | 27 | 24 | 17 |
| 22 | 46 | 46 |
|
|
| 12-weekly |
|
|
|
|
|
|
|
|
| 7 | 8-weekly → | 37 | 5 |
| 2 | 32 | 12 |
| 11 |
|
| 24-weekly |
|
|
|
|
|
|
|
|
|
| (progression) | 52 | 8 | 6 |
| 51 | 12 | 19 |
|
|
| → 4-weekly → |
|
|
|
|
|
|
|
|
|
| 8-weekly → |
|
|
|
|
|
|
|
|
|
| 12-weekly |
|
|
|
|
|
|
|
|
| 8 | 12-weekly | 8 |
| 32 |
| 8 |
| 96 |
|
| 9 | 24-weekly | 8 |
|
| 12 | 10 |
|
| 74 |
All seven patients with spinal GCTB had stable
disease following 8-weekly treatment for a median of 11 (range,
7–46) months. Six patients proceeded to 12-weekly treatment, with
five showed stable disease following 12-weekly treatment for a
median of 35 (range, 6–62) months. One patient (case 4) proceeded
to 24-weekly treatment and experienced tumor regrowth 10 months
after de-escalation to 24-weekly treatment. The patient was
re-treated with standard denosumab therapy. Imaging showed tumor
reduction with sclerotic changes; hence, the patient received
8-weekly treatment of denosumab and demonstrated stable disease.
One patient (case 5) developed malignant transformation 2.3 years
after the first denosumab treatment and 3 months after the first
12-weekly treatment. One patient (case 7) treated with de-escalated
therapy from 8-weekly to 24-weekly treatment experienced tumor
regrowth 6 months after de-escalation to 24-weekly treatment. The
patient was re-treated with standard denosumab therapy. Imaging
showed tumor reduction with sclerotic changes; hence, the patient
received 8-weekly treatment, then 12-weekly treatment of denosumab
and demonstrated stable disease. One (case 8) of the two patients
with femoral GCTB proceeded to 12-weekly treatment of denosumab;
the other patient (case 9) proceeded to 24-weekly treatment of
denosumab and both patients had stable disease at the latest
follow-up. All patients were alive at the latest follow-up visit.
None of the patients, except for one with malignant transformation,
developed pulmonary metastasis.
Imaging assessments
Plain radiography and computed tomography (CT)
revealed sclerotic changes inside and around the tumor in eight
patients after receiving standard denosumab treatment. The median
TTR was 3.5 (range, 1.1–5.3) months (Table V), and the median time to identify
the maximum sclerotic change was 11 (range, 6–17) months after the
first denosumab treatment. Sclerotic changes were continuously
identified on CT without progressive osteolytic change after
8-weekly and 12-weekly treatment in all nine patients. One patient
achieved a complete response (CR), while eight achieved partial
response (PR) following a standard denosumab treatment according to
the MDA criteria and remained unchanged during de-escalated
treatment (Table V). One patient
(case 9) showed stable radiographic changes after 24-weekly
treatment of denosumab, while two patients (case 4 and 7) developed
a recurrent tumor with progressive osteolytic change after
24-weekly treatment of denosumab, which demonstrated sclerotic
changes after resuming standard treatment.
 | Table V.Radiological responses. |
Table V.
Radiological responses.
|
| Extraskeletal mass,
cm (% of the original site) | MDA criteria |
| SINS |
|---|
|
|
|
|
|
|
|---|
| Case | Before
denosumab | Standard
period | De-escalation
period | Standard
period | De-escalation
period | TTR, months | Primary | Standard
period | De-escalation
period |
|---|
| 1 |
|
|
| PR | PR | 4 | 10 | 9 | 9 |
| 2 |
|
|
| PR | PR | 2 | 7 | 4 | 4 |
| 3 | 8.3 | 7 (84%) | 7 (84%) | PR | PR | 3 | 8 | 3 | 3 |
| 4 | 5.6 | 5.5 (98%) | 5.5 (98%) | PR | PR | 3 | 6 | 2 | 2 |
| 5 | 2.6 | 0.2 (8%) | 0.2 (8%) | PR | PR | 4.6 | 5 | 1 | 1 |
| 6 | 14.2 | 8.9 (62%) | 8.9 (62%) | PR | PR | 1.1 | 10 | 6 | 6 |
| 7 | 15 | 11.8 (79%) | 11.8 (79%) | PR | PR | 5.3 | 9 | 5 | 2 |
| 8 |
|
|
| PR | PR | 4 |
|
|
|
| 9 |
|
|
| CR | CR | 4.3 |
|
|
|
Extraskeletal masses were identified in five
patients, with a median size of 8.3 (range, 2.6–15) cm before
denosumab treatment (Table V). The
sizes of the masses decreased significantly to a median of 7.0
(range, 0.2–11.8) cm after standard treatment in all five patients
compared to the sizes before treatment (P=0.043), corresponding to
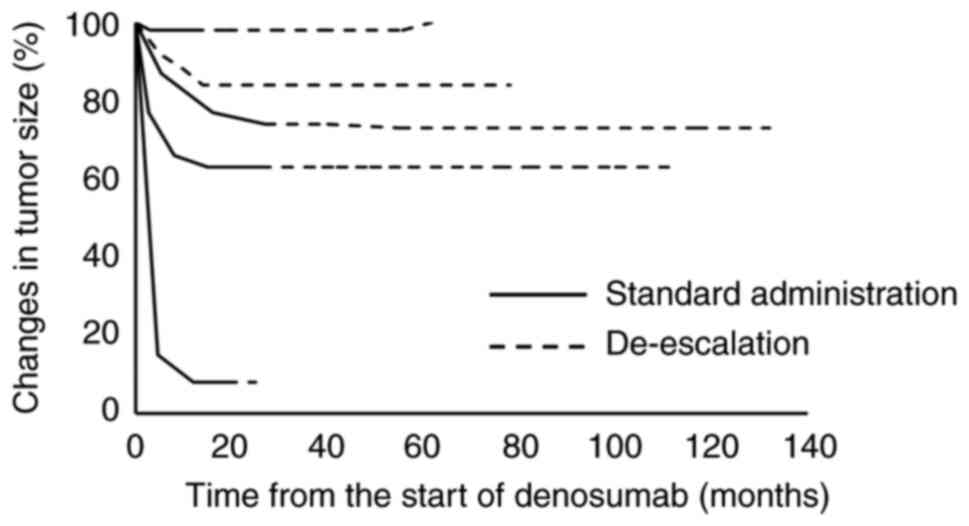
a mean of 79% (range, 8–98%) of the original sizes. The median time
to identify initial tumor reduction was 4.6 (range, 1.1–5.3) months
after the first denosumab treatment (Fig. 2), while the median time to identify
maximum tumor reduction was 14 (range, 3–17) months. Three patients
showed stable disease following de-escalated treatment, while two
patients (case 4 and 7) experienced tumor regrowth.
Imaging of spinal GCTB before denosumab treatment
demonstrated <50% collapse in two patients, no collapse with
>50% body involvement in three patients, and no collapse with
≤50% body involvement in two patients according to SINS. At the
latest follow-up, six patients did not experience further spinal
collapse, while one patient with T10 disease (case 2) progressed
from <50 to >50% collapse. The median SINS for spinal GCTB
before denosumab treatment was 8 (range, 5–10) points; two patients
had stable disease, while five patients had potentially unstable
disease based on their SINS. The SINS improved to a median of 4
(range, 1–10) points after standard treatment (P=0.024): six
patients had stable disease, while one patient had potentially
unstable disease based on their SINS. One patient showed further
improvement in SINS after de-escalated treatment. The SINS further
improved to a median of 3 (range, 1–10) points at the latest
follow-up (P=0.026), which was not significantly different from
that after the standard treatment (P=0.317). The tumors in six
patients were classified as stable, while that in one patient was
classified as potentially unstable after receiving de-escalated
treatment.
Clinical symptoms
Eight of the nine patients experienced local pain
before denosumab treatment, with a median NRS score of 4 (range,
0–10) (Table VI). Seven patients
became pain-free (NRS=0) after standard denosumab treatment, with a
median NRS score of 0 (range, 0–2) (P=0.001). Pain disappeared at a
median of 97 (range, 7–181) days after the first denosumab
treatment and after a median of five (range, 2–8) injections of
standard treatment. One patient (case 1) experienced pain (NRS=2)
at the latest follow-up. Tumor recurrence (case 4 and 7) was
identified after treatment de-escalation to 24-weekly treatment due
to progressive severe pain (NRS=10), which subsequently disappeared
(NRS=0) after resuming standard treatment.
 | Table VI.Pain assessment. |
Table VI.
Pain assessment.
|
| NRS |
|---|
|
|
|
|---|
| Case | Before denosumab
treatment | Standard
administration period | De-escalation
period |
|---|
| 1 | 7 | 2 | 2 |
| 2 | 1 | 0 | 0 |
| 3 | 9 | 0 | 0 |
| 4 | 10 | 0 | 0 |
| 5 | 4 | 0 | 0 |
| 6 | 4 | 0 | 0 |
| 7 | 10 | 0 | 0 |
| 8 | 0 | 0 | 0 |
| 9 | 2 | 0 | 0 |
All seven patients with spinal GCTB experienced
numbness or hypoesthesia in the lower legs, and two patients had
bladder and bowel dysfunction. Two patients were classified as
having grade D, while five were classified as having grade E
impairments before denosumab treatment, based on the ASIA scale.
Sensory disturbance improved in all seven patients after receiving
standard treatment, although three had slight numbness during
standard and de-escalated treatment. One of the two patients with
bladder and bowel dysfunction before treatment completely
recovered; the other experienced persistent mild constitution
symptoms after denosumab treatment. All seven patients were
classified as having grade E impairments based on the ASIA scale
after receiving de-escalated treatment.
AEs
Two patients experienced ONJ after the treatment was
de-escalated to every 12 weeks. Both patients received ONJ
treatment without denosumab discontinuation. One patient (case 2)
developed pain, mucosal swelling, and bone exposure of the jaw 3.3
years after the first denosumab treatment and 2.3 years after the
initial de-escalation. A dental panoramic radiograph showed an
osteolytic lesion of the jaw, which the dentist diagnosed as ONJ.
The symptoms improved with oral antibacterial medication. However,
the patient underwent sequestrectomy after experiencing purulent
discharge from the exposed bone for 1 year. Although the pain
subsequently improved, the patient still had a small amount of
purulent discharge. The other patient (case 7) also showed pain,
mucosal swelling, and bone exposure in the jaw at 7.8 years after
the first denosumab treatment and 5.2 years after the initial
de-escalation. CT showed an osteolytic lesion of the jaw, which the
dentist diagnosed as ONJ. The symptoms improved with periodic
cleaning of the affected area and oral antibacterial medication.
However, mucosal pain and swelling recurred 1 year and 3 months
later. CT showed progression of the osteolytic lesion. The patient
underwent sequestrectomy, after which the symptoms improved. AFF
occurred in one patient (case 7) during 12-weekly treatment of
denosumab. At that time, the patient had received denosumab
treatment for 11 years. Internal fixation with intramedullary nail
was performed and bone union could obtain without delay. Four
patients had asymptomatic hypocalcemia (median: 8.6; range, 8.2–8.7
mg/dl) that did not require additional treatment. Hypocalcemia was
identified transiently at a median of 1 (range, 1–2) weeks after
the initial denosumab treatment and was not detected thereafter.
One patient (case 5) with grade 3 sacral GCTB according to the
Campanacci classification developed malignant transformation after
12-weekly treatment of denosumab. The patient had undergone primary
surgical resection elsewhere and developed local recurrence 4
months postoperatively. After referring to this hospital, multiple
courses of selective embolization were performed for treatment of
the recurrent tumor, which remained stable for >6 years. MRI
showed enlargement of a soft tissue mass adjacent to the sacrum 6.5
years after the initial embolization. The pathological diagnosis by
CT-guided biopsy was recurrent GCTB without malignant change. The
recurrent tumor reduced in size following denosumab treatment and
remained stable for 2 years, after which regular MRI revealed tumor
regrowth. Denosumab therapy was discontinued, and the recurrent
tumor was finally resected. The surgical specimen was
pathologically diagnosed as a malignant transformation of GCTB.
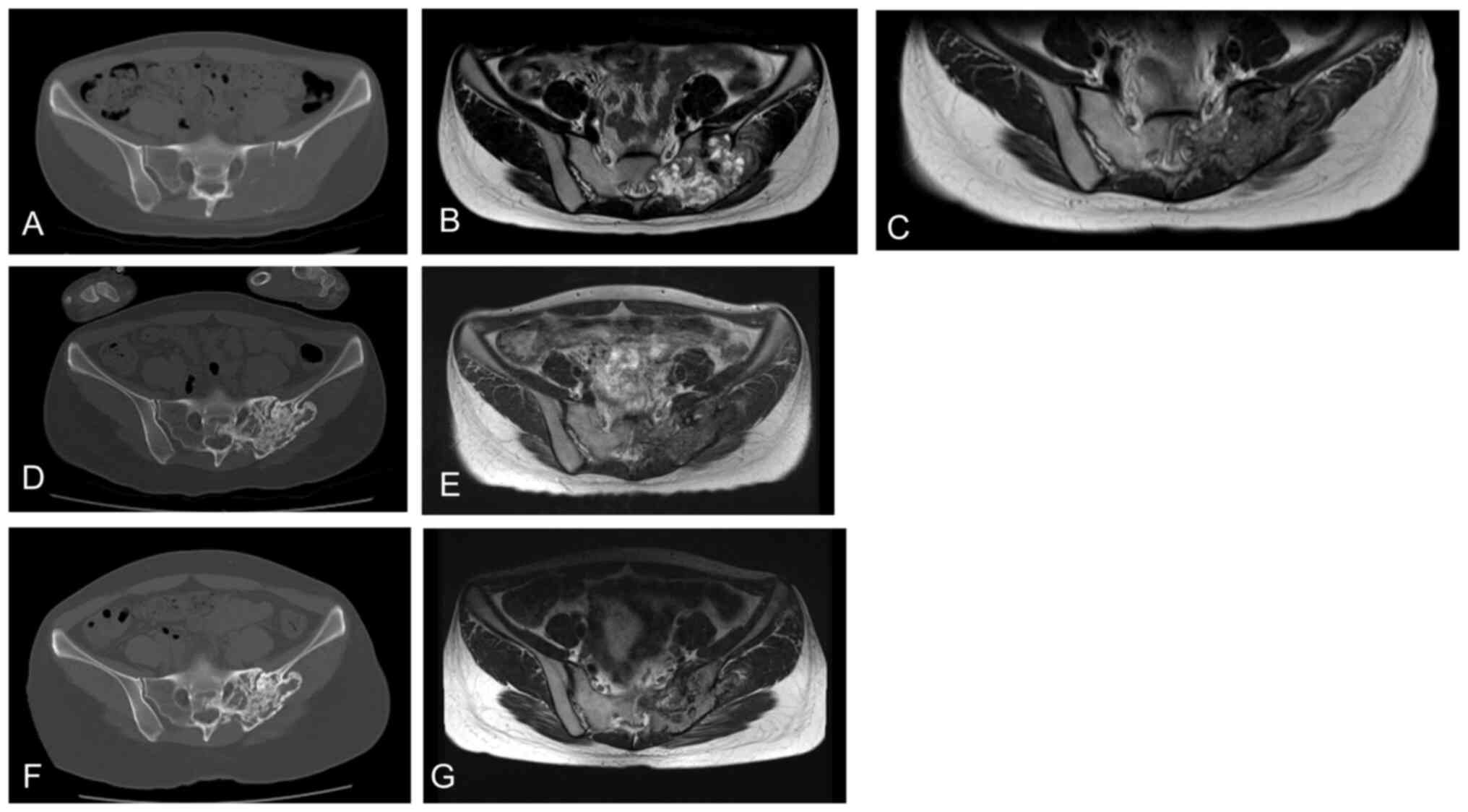
Case 3
A 25-year-old woman with sacral GCTB presented with
severe pain (NRS=9) and numbness in her left buttocks and lower
limbs. CT imaging revealed a huge mass in the sacrum with cortical
destruction and extensive soft tissue involvement (Fig. 3A). MRI revealed a large tumor in the
sacrum with high intensity on T2-weighted images (Fig. 3B). The patient underwent standard
denosumab therapy. Her pain decreased (NRS=2) after 1 week and
disappeared after 2 weeks. Five months later, MRI revealed
remarkable tumor shrinkage (Fig.
3C). The patient showed stable disease over the next 2 years,
with remarkable sclerosis of the lytic lesions (Fig. 3D). After 8-weekly treatment of
denosumab for 6 months, the patient achieved stable disease
(Fig. 3E). She then received
12-weekly denosumab therapy. At the last follow-up, she continued
to show stable disease (Fig. 3F,
G).
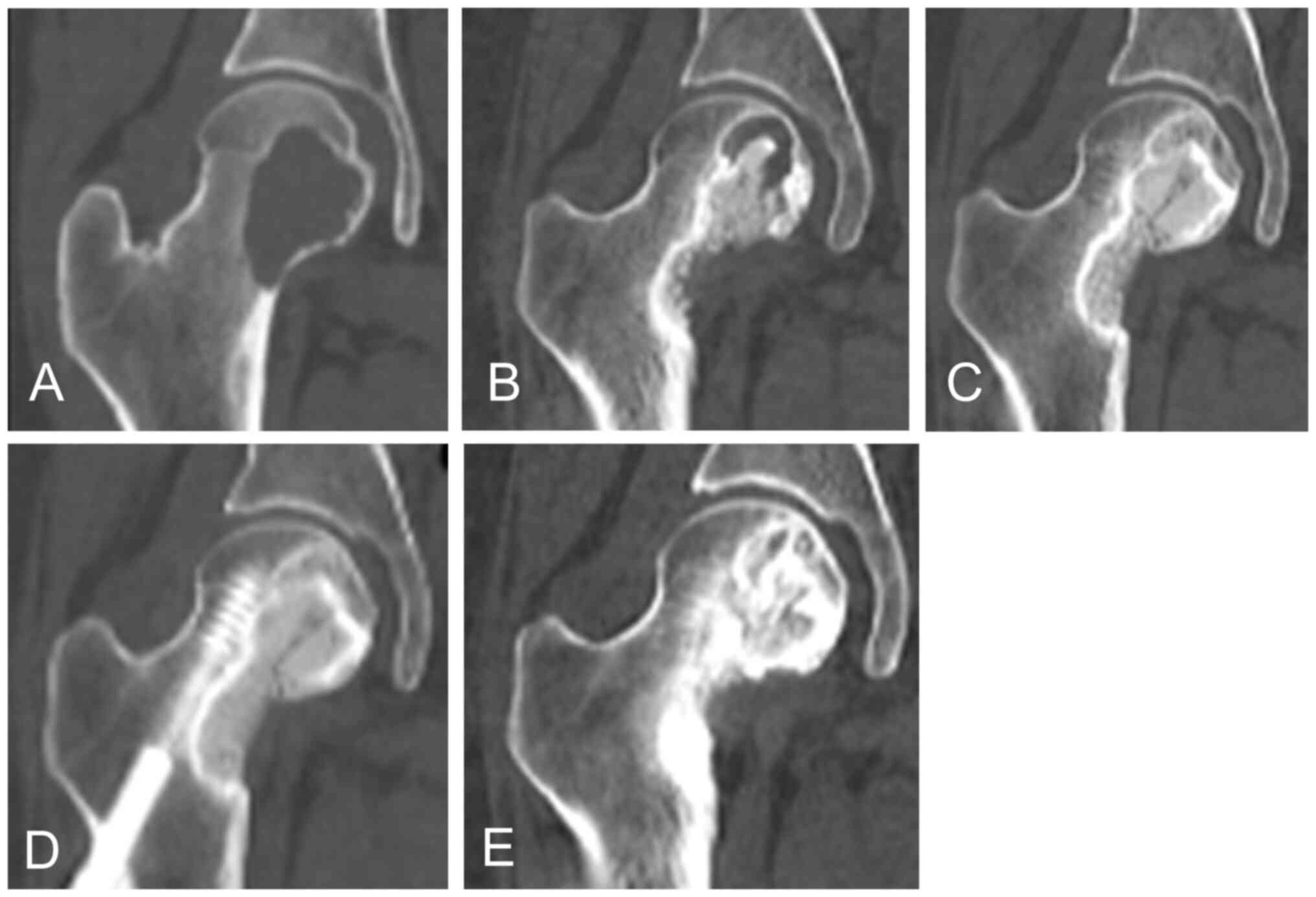
Case 9
A 26-year-old woman had GCTB of the femoral head. CT
images (Fig. 4A) showed a lytic
lesion. She underwent two rounds of curettage and bone grafting
with internal fixation. CT revealed a lytic lesion in the femoral
neck (Fig. 4B). Hence, denosumab
was initiated. After 4 months, a change in sclerosis was observed
(Fig. 4C). After 10 months, the
lytic lesion was completely filled with newly formed bone (Fig. 4D). Denosumab was administered every
24 weeks. At the last follow-up, the patient showed stable disease
(Fig. 4E).
Discussion
The interval of denosumab treatment to unresectable
GCTB was extended to minimize the risk of AEs associated with
long-term denosumab treatment. A phase 2 clinical study was planned
in Europe to evaluate the risks and benefits of a reduced dose
density of denosumab for unresectable GCTB (29). However, this study was discontinued
before its completion owing to poor accrual. Only two related case
reports have described denosumab de-escalation for GCTB (22,23).
Tanikawa et al demonstrated that extending the dosing
interval to 6 months was useful and appropriate measure (22). Hence, whether denosumab
de-escalation is beneficial for unresectable GCTB remains unclear.
The present study confirmed that gradual de-escalation to 8- and
12-weekly treatment achieved stable disease with therapeutic
changes inside the tumor, including sclerotic changes and
extraskeletal mass reduction, which were obtained via standard
4-weekly treatment. However, two of the three patients who received
24-weekly treatments developed tumor recurrence during 24-weekly
treatment. Thus, 24-weekly treatment should be performed carefully
for local recurrence.
In the present study, all patients demonstrated
sclerotic change within a median of 3.5 months after the first
denosumab treatment, which was consistent with previous reports.
Many studies have reported re-ossification inside and around the
tumor after denosumab treatment on plain radiographs and CT images
(10–12). Sclerotic changes with newly formed
bone identified on CT images suggest successful therapeutic effects
due to the reparative process of denosumab treatment (9,10). New
bone formation is important, especially in patients with spinal
structural instability caused by tumor invasion since most spinal
GCTBs are considered unresectable (9,10).
Nakazawa et al reported dramatic tumor regression and
sclerosis 6 months after 4-weekly denosumab treatment in a patient
with GCTB arising from the C5 vertebral body, which led to complete
regression on CT 24 months after denosumab treatment (30). In the present study, the good
therapeutic changes in imaging with standard treatment remained
unchanged after de-escalated 8-weekly and 12-weekly treatments.
After 24-weekly treatment, one lesion showed stable disease and two
patients developed local recurrence. However, re-de-escalation was
effective for these recurrent lesions, and long-term control had
been obtained by 8-weekly and 12-weekly treatment. These results
suggest that de-escalated 12-weekly treatment, but not 24-weekly
treatment can maintain good therapeutic responses, as shown on
imaging.
All five patients with an extraskeletal mass showed
tumor reduction at a median of 17 months after the first denosumab
treatment. Chawla et al reported that 72% of patients
achieved partial or complete responses, as assessed based on RECIST
version 1.1, within a median of 95 weeks after 4-weekly denosumab
treatments for surgically unresectable GCTB including 63 patients
with spinal GCTB (14). In the
present study, one and eight patients achieved CR and PR,
respectively, according to the MDA criteria, during standard
treatment. The MDA criteria are used to assess for sclerotic
changes in the tumor and changes in tumor size following denosumab
treatment. RECIST1.1 might not be suitable for assessing the
therapeutic effect on GCTB without extraskeletal masses, as it only
measures tumor size, which often remains unchanged within the bone
after denosumab treatment (14,15,31).
The MDA criteria are more useful for the assessment of GCTB on
imaging after denosumab treatment (32), although these criteria were
initially developed to evaluate bone metastases. The median
duration to identify maximum sclerotic change was 11 months after
denosumab treatment, with a median duration of this maximum change
of 14 months, suggesting that the maximum effect of denosumab on
GCTB can be achieved approximately 1 year after the first
treatment.
Denosumab can improve the clinical symptoms such as
pain due to GCTB in patients with unsalvageable GCTB (8–14).
Chawla et al reported the clinical benefit of denosumab
treatment in 67 of 169 (40%) patients with unresectable GCTB, with
pain reduction the most frequent benefit (28%) (15). Bukata et al assessed only
patients with GCTB of the spine and observed the clinical benefit
of denosumab treatment in 87 of 103 (85%) patients with
unresectable GCTB of the spine; with pain reduction reported in 77
of 103 patients (75%) (33). In the
present study, all eight patients who experienced local pain at
diagnosis reported pain reduction after denosumab treatment, which
was sustained after 8-weekly and 12-weekly treatments.
The reported AEs related to denosumab treatment in
patients with GCTB include ONJ (9–13%), hypocalcemia (5%), AFF
(1–4%), and malignant transformation (1–4%) (15,18,34).
ONJ was reported in two patients who received a 12-weekly denosumab
treatment: one patient developed ONJ 3.3 years after the first
denosumab treatment and 2.3 years after the first de-escalated
treatment; the other patient developed ONJ 7.8 years after the
first denosumab treatment and 5.2 years after the initial
de-escalated treatment. Previous studies demonstrated that ONJ
occurs in a dose-dependent manner. Raimondi et al reported
that 4 of 29 (13.8%) patients experienced ONJ during denosumab
treatment at 125, 119, 85, and 41 months after denosumab
administration, respectively (35).
Bukata et al reported a median time to ONJ onset of 41
months after standard denosumab treatment (33). No previous paper reported the
occurrence of ONJ after de-escalated denosumab treatment. Palmerini
et al reported a 5-year ONJ-free survival rate of 92% after
standard denosumab treatment (18).
However, whether denosumab de-escalation can prevent ONJ remains
unclear. Hence, careful attention should be paid to the occurrence
of ONJ in patients receiving denosumab treatment for >3 years,
even if treatment is de-escalated. Further clinical trials are
needed to determine whether denosumab de-escalation prevents ONJ.
Chawla et al reported four cases (1%) of AFF in a phase II
study showing the clinical benefits of denosumab treatment in 532
patients with GCTB (15). They all
occurred after 48 months of denosumab treatment. In the present
study, one patient developed AFF after the start of denosumab
treatment for 11 years. Then, careful observation is required and
X-rays should be undertaken to investigate AFFs when clinical signs
such as thigh pain arise in patients with long-term denosumab
treatment.
This study has several limitations. First, the
sample size was small, as only nine patients were analyzed. GCTB is
relatively rare, accounting for approximately 3–5% of all primary
bone tumors; moreover, unresectable GCTB such as spinal and pelvic
GCTB occurs in only 2–15% of all GCTB. As mentioned above, the
international clinical study on reduced dose density of denosumab
for unresectable GCTB was discontinued before its completion owing
to poor accrual. Thus, it was relatively difficult to study enough
patients with unresectable GCTB. Second, this study had no
comparison group and did not perform randomization. The patients
treated with denosumab de-escalation were only described and the
result of de-escalation could not be compared to that of standard
treatment because all patients in this hospital with unresectable
GCTB received de-escalated denosumab treatment. Owing to the small
number of patients with unresectable GCTB, it is difficult to
provide a control group for comparison. Third, the patients in this
study were not managed according to a set protocol. Most patients
underwent gradual denosumab de-escalation (4-, 8-, 12-, and
24-weekly treatments). However, some patients skipped the 8- or
12-weekly treatments and directly received a 24-weekly de-escalated
treatment after standard treatment. In addition, there remains no
single clinical indication to determine the duration of
de-escalated treatment. De-escalation was initiated after
discussion among doctors, patients, and/or their families based on
the patient's clinical symptoms and imaging findings. Multicenter
trials are needed to compare groups of patients and confirm the
appropriate dosing interval for denosumab treatment, although one
multicenter study was discontinued before its completion due to
poor accrual. Lastly, structured chart review methodology was not
used in this study, which can influence the outcome.
In conclusion, 12-weekly de-escalated denosumab
treatment showed clinical benefits as a maintenance treatment in
patients with unresectable GCTB, in addition to sustained stable
tumor control and improved clinical symptoms with standard
treatment. A 24-weekly treatment can also be administered, with
careful attention to detecting local recurrence. Meanwhile, whether
denosumab de-escalation prevented ONJ, AFF, and malignant
transformation remained unknown. Patients receiving long-term
denosumab treatment should be carefully examined for ONJ and AFF,
even during the period of de-escalated treatment. Further
investigation and more case studies are warranted to identify the
clinical significance of denosumab de-escalation in severe AEs.
Acknowledgements
Not applicable.
Funding
This work was supported by JSPS KAKENHI (grant nos. 22K09401,
23K08678 and 23K08698).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
EN and TK wrote the main manuscript text. EN, TF, TK
and TO designed the study. EN, HK and TI treated the patients and
collected the data. EN, HK, and TI collected and analyzed data. EN
and TI confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This retrospective chart review study involving
human participants was performed in accordance with the ethical
standards of the institutional and national research committee and
with the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards. The Human Investigation Committee
(IRB) of Okayama University Hospital approved this study (approval
no. K 2103-040). The opt-out strategy was used for patient
consent.
Patient consent for publication
As this study is a retrospective study, patient
consent was obtained using an opt-out consent method.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van der Heijden L, Dijkstra S, van de
Sande M and Gelderblom H: Current concepts in the treatment of
giant cell tumour of bone. Curr Opin Oncol. 32:332–338. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Basu Mallick A and Chawla SP: Giant cell
tumor of bone: An update. Curr Oncol Rep. 22:512021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boriani S, Bandiera S, Casadei R, Boriani
L, Donthineni R, Gasbarrini A, Pignotti E, Biagini R and Schwab JH:
Giant cell tumor of the mobile spine: A review of 49 cases. Spine
(Phila Pa 1976). 37:E37–E45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jamshidi K, Bagherifard A, Mirzaei A and
Bahrabadi M: giant cell tumor of the sacrum: series of 19 patients
and review of the literature. Arch Bone Jt Surg. 5:443–450.
2017.PubMed/NCBI
|
|
5
|
Tsukamoto S, Mavrogenis AF, Kido A and
Errani C: Current concepts in the treatment of giant cell tumors of
bone. Cancers (Basel). 13:36472021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palmerini E, Picci P, Reichardt P and
Downey G: Malignancy in giant cell tumor of bone: A review of the
literature. Technol Cancer Res Treat.
1:15330338198400002019.PubMed/NCBI
|
|
7
|
Hosalkar HS, Jones KJ, King JJ and Lackman
RD: Serial arterial embolization for large sacral giant-cell
tumors: Mid- to long-term results. Spine (Phila Pa 1976).
32:1107–1115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He SH, Xu W, Sun ZW, Liu WB, Liu YJ, Wei
HF and Xiao JR: Selective arterial embolization for the treatment
of sacral and pelvic giant cell tumor: A systematic review. Orthop
Surg. 9:139–144. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Gao J, Gao Y, Lin N, Zheng M and Ye
Z: Denosumab in giant cell tumor of bone: Current status and
pitfalls. Front Oncol. 10:5806052020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palmerini E, Staals EL, Jones LB, Donati
DM, Longhi A and Randall RL: Role of (Neo) adjuvant denosumab for
giant cell tumor of bone. Curr Treat Options Oncol. 21:682020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta A, Durocher-Allen L, Popovic S,
Tozer R, Yao X and Ghert M: The role of denosumab for surgical
outcomes in patients with giant cell tumour of bone: A systematic
review. Curr Oncol. 22:1302–1313. 2021. View Article : Google Scholar
|
|
12
|
van Langevelde K and McCarthy CL:
Radiological findings of denosumab treatment for giant cell tumours
of bone. Skeletal Radiol. 49:1345–1358. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomas D, Henshaw R, Skubitz K, Chawla S,
Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, et al:
Denosumab in patients with giant-cell tumour of bone: An
open-label, phase 2 study. Lancet Oncol. 11:275–280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chawla S, Henshaw R, Seeger L, Choy E,
Blay JY, Ferrari S, Kroep J, Grimer R, Reichardt P, Rutkowski P, et
al: Safety and efficacy of denosumab for adults and skeletally
mature adolescents with giant cell tumour of bone: Interim analysis
of an open-label, parallel-group, phase 2 study. Lancet Oncol.
14:901–908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chawla S, Blay JY, Rutkowski P, Le Cesne
A, Reichardt P, Gelderblom H, Grimer RJ, Choy E, Skubitz K, Seeger
L, et al: Denosumab in patients with giant-cell tumour of bone: A
multicentre, open-label, phase 2 study. Lancet Oncol. 20:1719–1729.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rutkowski P, Ferrari S, Grimer RJ, Stalley
PD, Dijkstra SP, Pienkowski A, Vaz G, Wunder JS, Seeger LL, Feng A,
et al: Surgical downstaging in an open-label phase II trial of
denosumab in patients with giant cell tumor of bone. Ann Surg
Oncol. 22:2860–2868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alaqaili SI, Abduljabbar AM, Altaho AJ,
Khan AA and Alherabi J: A: Malignant sarcomatous transformation of
benign giant cell tumor of bone after treatment with denosumab
therapy: A literature review of reported cases. Cureus.
28:e37922018.PubMed/NCBI
|
|
18
|
Palmerini E, Chawla NS, Ferrari S, Sudan
M, Picci P, Marchesi E, Leopardi MP, Syed I, Sankhala KK,
Parthasarathy P, et al: Denosumab in advanced/unresectable
giant-cell tumour of bone (GCTB): For how long? Eur J Cancer.
76:118–124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matcuk GR Jr, Patel DB, Schein AJ, White
EA and Menendez LR: Giant cell tumor: Rapid recurrence after
cessation of long-term denosumab therapy. Skeletal Radiol.
44:1027–1031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clemons M, Liu M, Stober C, Pond G, Jemaan
Alzahrani M, Ong M, Ernst S, Booth C, Mates M, Abraham Joy A, et
al: Two-year results of a randomised trial comparing 4- versus
12-weekly bone-targeted agent use in patients with bone metastases
from breast or castration-resistant prostate cancer. J Bone Oncol.
30:1003882021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu C, Wang L, Liu L, Zhuang J, Tang S,
Zhang T, Zhou C, Feng F, Liu R, Zhang J, et al: Efficacy and safety
of de-escalation bone-modifying agents for cancer patients with
bone metastases: A systematic review and meta-analysis. Cancer
Manag. Res. 10:3809–3823. 2018.
|
|
22
|
Tanikawa M, Yamada H, Sakata T and Mase M:
Dosing interval adjustment of denosumab for the treatment of giant
cell tumor of the sphenoid bone: A case report. Surg Neurol Int.
11:3702020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park A, Cipriano CA, Hill K, Kyriakos M
and McDonald DJ: Malignant transformation of a giant cell tumor of
bone treated with denosumab: A case report. JBJS Case Connect.
6:e782016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamaoka T, Costelloe CM, Madewell JE, Liu
P, Berry DA, Islam R, Theriault RL, Hortobagyi GN and Ueno NT:
Tumour response interpretation with new tumour response criteria vs
the World Health Organisation criteria in patients with bone-only
metastatic breast cancer. Br J Cancer. 102:651–657. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Costelloe CM, Chuang HH, Madewell JE and
Ueno NT: cancer response criteria and bone metastases: RECIST 1.1,
MDA and PERCIST. J Cancer. 1:80–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayashi N, Costelloe CM, Hamaoka T, Wei C,
Niikura N, Theriault RL, Hortobagyi GN, Madewell JE and Ueno NT: A
prospective study of bone tumor response assessment in metastatic
breast cancer. Clin Breast Cancer. 13:24–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Campanacci M, Baldini N, Boriani S and
Sudanese A: Giant-cell tumor of bone. J Bone Joint Surg Am.
69:106–114. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Priebe MM and Waring WP: The interobserver
reliability of the revised American Spinal Injury Association
standards for neurological classification of spinal injury
patients. Am J Phys Med Rehabil. 70:268–270. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
European Organisation for Research and
Treatment of Cancer, . Reduced Dose-density of Denosumab for
Unresectable GCTB (REDUCE). ClinicalTrials.gov Identifier:
NCT03620149. National Library of Medicine; Bethesda, MD: 2021,
https://www.clinicaltrials.gov/ct2/show/NCT03620149January
6–2021
|
|
30
|
Nakazawa T, Inoue G, Imura T, Miyagi M,
Saito W, Namba T, Shirasawa E, Uchida K, Takahira N and Takaso M:
Remarkable regression of a giant cell tumor of the cervical spine
treated conservatively with denosumab: A case report. Int J Surg
Case Rep. 24:22–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sambri A, Medellin MR, Errani C,
Campanacci L, Fujiwara T, Donati D, Parry M and Grimer R: Denosumab
in giant cell tumour of bone in the pelvis and sacrum: Long-term
therapy or bone resection? J Orthop Sci. 25:513–519. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bukata SV, Blay JY, Rutkowski P, Skubitz
K, Henshaw R, Seeger L, Dai T, Jandial D and Chawla S: Denosumab
treatment for giant cell tumor of the spine including the sacrum.
Spine(Phila Pa 1976). 46:277–284. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Broehm CJ, Garbrecht EL, Wood J and
Bocklage T: Two cases of sarcoma arising in giant cell tumor of
bone treated with denosumab. Case Rep. Med.
2015:7671982015.PubMed/NCBI
|
|
35
|
Raimondi A, Simeone N, Guzzo M, Maniezzo
M, Collini P, Morosi C, Greco FG, Frezza AM, Casali PG and
Stacchiotti S: Rechallenge of denosumab in jaw osteonecrosis of
patients with unresectable giant cell tumour of bone: A case series
analysis and literature review. ESMO Open. 5:e0006632020.
View Article : Google Scholar : PubMed/NCBI
|