Introduction
It is predicted that there will be 58,450 new
patients with head and neck squamous cell carcinoma (HNSCC)
diagnoses in the United States in 2024 and more than 12,000 deaths
(1). A comparable incidence was
noted in Korea, where it constituted ~2.1% and ranked as the 10th
most prevalent cancer among male patients (2). The most common cause of HNSCC is
smoking, with human papillomavirus (HPV) contributing to
oropharyngeal cancer (OPC) and Epstein-Barr virus linked to
nasopharyngeal cancer among other associated causative factors.
Notably, HPV-positive OPC is more prevalent among younger
individuals, particularly in relatively high-income countries
(3). Recently, there has been a
growing trend in the prevalence of HPV-positive OPC among older
individuals. A previous study demonstrated an increase in the
median age at diagnosis from 53 to 58 years between 1998 and 2013,
aligning with the observed trend in Korea (4–6).
Furthermore, the incidence of HPV-positive OPC is increasing in
both younger and older patients, with a noticeable shift in the
burden towards older patients. However, it is noteworthy that most
cases occur in patients aged <60 years.
HPV-positive OPC exhibits distinctive
histopathological characteristics that impact clinical outcomes.
The expression of viral E6 and E7 oncoproteins by HPV deactivates
the tumor suppressor protein p53 and the retinoblastoma protein
(pRb), respectively (7). Typically,
HPV-positive OPC exhibits high expression of p16 protein and a
lower prevalence of genetic mutations, such as those in the
TP53 (p53) gene, compared with HPV-negative OPC. These
features are associated with chemo- or radiosensitivity (8). Moreover, HPV-positive OPC displays a
unique immune microenvironment characterized by CD8+
T-cell activation and elevated programmed death-ligand 1 (PD-L1)
expression, setting it apart from HPV-negative OPC. These cells can
trigger an immune response; consequently, immunotherapy is used to
treat HPV-positive OPC. The favorable clinical and biological
characteristics of the HPV-positive OPC led to its downstaging in
the 8th edition of the American Joint Committee on Cancer (AJCC)
staging system (9). Moreover,
distinct treatment strategies have been proposed in the National
Comprehensive Cancer Network (NCCN) guidelines for HPV-positive OPC
compared with HPV-negative OPC (10).
Given this context, a number of clinical studies
have actively investigated de-intensified treatment approaches to
mitigate the toxicity in patients with HPV-positive OPC (11,12).
De-intensified treatments include measures such as reducing the
chemotherapy dose, omitting induction chemotherapy (IC), lowering
the radiation dose, and opting for less invasive surgery.
Clinical studies on de-intensified treatment have
been attempted; however, there is insufficient evidence for
selecting suitable patients. As a result, this approach is not
widely used in clinical practice and not endorsed by the NCCN
guidelines (13). Previously, it
has been reported that patients with OPC with high p53 expression
had inferior survival outcomes than those with low p53 expression
among patients with HPV-positive OPC (14). These results suggested that even in
patients with HPV-positive OPC, treatment results may differ due to
a combination of variable etiologies, and it is necessary to
identify high-risk factors that can predict survival and develop
treatment strategies. These disparities between the theoretically
favorable prognosis and practical evidence of HPV-positive OPC may
derive from tumor heterogeneity in addition to staging, biological
features and patient-specific characteristics.
Hence, in the present retrospective study, the
authors aimed to identify clinical high-risk factors for
HPV-positive OPC and conducted a survival analysis in patients with
OPC treated with IC followed by chemoradiotherapy (CRT). The
outcomes of the present study can provide insights into
chemoresistance.
Patients and methods
Study population
Patients who underwent treatment for OPC at Chonnam
National University Hwasun Hospital between June 2004 and October
2020 were retrospectively reviewed. Inclusion criteria were as
follows: i) Patients with pathologically confirmed, HNSCC (stage
II–III or stage IV M0); ii) underwent at least two cycles of IC
followed by CRT; and iii) older than 19 years at the time of
diagnosis. Patients with an initial diagnosis of stage IV M1
disease or nasopharyngeal cancer were excluded. Briefly, 100 (95%)
were male and the median age at diagnosis was 60 years (range,
40–67). To be eligible for the study, their medical records needed
to include information on staging using computed tomography (CT) or
magnetic resonance imaging (MRI) along with
18F-fluorodeoxyglucose positron emission tomography
(18F-FDG-PET) scans as necessary. Baseline patient
demographics collected encompassed sex, age, Eastern Cooperative
Oncology Group (ECOG) performance status score, body mass index
(BMI) (15), smoking history,
pathologic differentiation, chemotherapy regimen and cumulative
radiation dose. To diagnose HPV-positive OPC, p16 and HPV status
were confirmed through medical records. In cases where HPV was not
tested, additional PCR using My HPV Chip Kit™ (AG Bio Diagnostics
Co. Ltd.) was performed with archived tissue from Biobank of
Chonnam National University Hwasun Hospital, a member of the Korean
Biobank Network. The HPV DNA chip contained 12 types of high-risk
HPV (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 54, 56, 58) and 8
types of low-risk HPV (HPV 6, 11, 34, 40, 42, 43, 44, 54) strains.
The PCR products from all samples were subjected to electrophoresis
on 2.0% agarose gels, and size of HPV DNA products was 100 base
pairs. After 10 µl of the amplified HPV product was denatured at
95°C for 5 min, it was mixed with a hybridization solution and then
applied to the DNA chip. Hybridization HPV DNA was visualized using
a DNA chip scanner (GenePix 4000B; Molecular Devices, LLC). HPV
amplicons were hybridized with the corresponding type of specific
oligonucleotide probe and visualized on HPV DNA chip slides as
double positive spots. Among patients diagnosed before 2017,
eligible individuals were re-staged according to the AJCC 8th
edition staging system based on HPV status. The protocol of the
present study was approved (approval no. CNUHH-2023-232) by the
Institutional Review Board of Chonnam National University Hwasun
Hospital (Hwasun, Republic of Korea).
Treatment and tumor assessment
The patients received IC every 3 weeks, with a dose
of 70 mg/m2 docetaxel and 75 mg/m2 cisplatin
administered as a 4-h intravenous infusion on day 1. Additionally,
1,000 mg/m2 5-fluorouracil was administered as a 24-h
continuous infusion for 4 days. After IC, definitive treatment,
such as CRT or surgery, was conducted based on the consensus of the
multidisciplinary head and neck cancer team. CRT commenced within 4
weeks of IC completion, involving concurrent administration of
cisplatin at a dose of 100 mg/m2 every 3 weeks (on days
1, 22 and 43) or at a dose of 40 mg/m2 weekly for 7 days
(days 1, 8, 15, 22, 29, 36 and 43). Radiotherapy (RT) was
administered using three-dimensional conformal radiation therapy
(3D-CRT) or intensity-modulated RT (IMRT). Response was evaluated
by the Response Evaluation Criteria in Solid Tumors 1.1 and
involved assessing complete response (CR), partial response, stable
disease and progressive disease after IC and 8 weeks post CRT
completion (16). Alongside imaging
studies, all patients underwent a physical examination by an
otolaryngologist to validate the response evaluation. Patients who
achieved a CR upon physical examination and CT or MRI scan,
underwent 18F-FDG-PET scans for confirmation 1 month
after CR confirmation. Most patients were evaluated for response by
CT, but in cases of radiation-induced swelling on CT, it was
difficult to assess the response, thus in these cases, MRI was
performed. After completing treatment, patients underwent monthly
follow-ups with physical examinations. CT or MRI scans were
conducted every 4 months for the initial 2 years, followed by
biannual scans until disease progression.
Statistical analysis
Overall survival (OS) was characterized as the time
from diagnosis to death. Progression-free survival (PFS) was
defined as the time from diagnosis to death or the initially
documented recurrence, further categorized as locoregional
recurrence (tumor at the primary site or regional nodes) or distant
metastasis. Patient characteristics were compared using the
Chi-square test, Fisher's exact test and unpaired Student's t-test.
Continuous variables are expressed as median, and categorical
variables are presented in terms of frequency and percentage
values. Survival analysis utilized Kaplan-Meier survival curves,
and the log-rank test was used for comparison. Univariate and
multivariate survival analyses were conducted using Cox
proportional hazards. The level of significance adopted in all
analysis was 5% with a 95% confidence interval and P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using R, version 4.2.2 (R
Foundation for Statistical Computing; http://www.r-project.org/).
Results
Patient characteristics
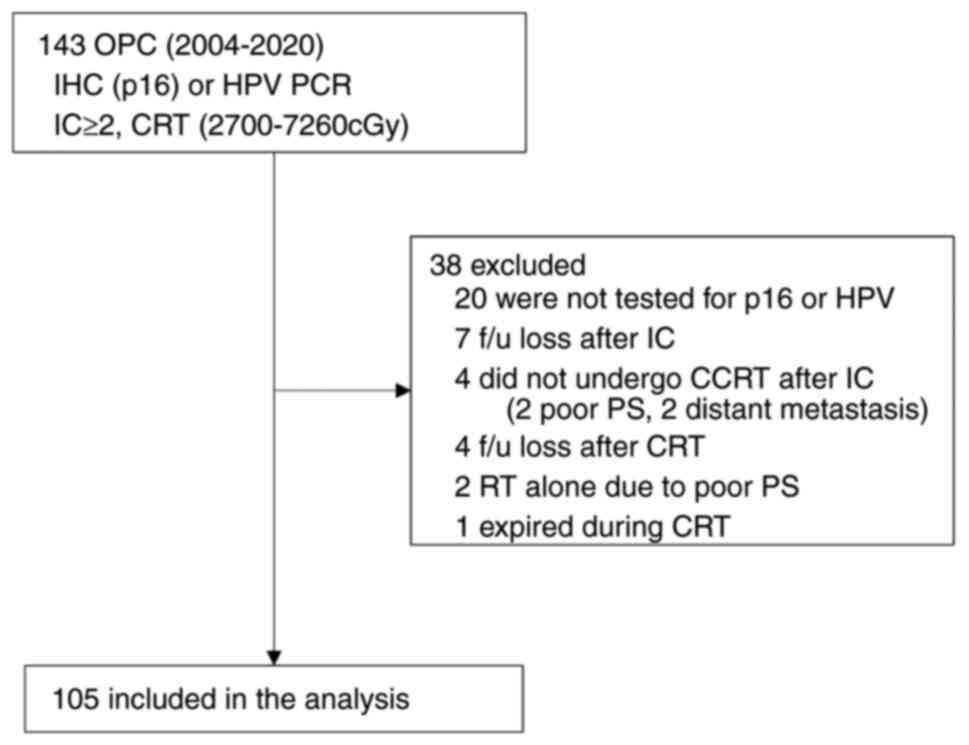
The present study included 105 patients, comprising
40 HPV-negative and 65 HPV-positive individuals (Fig. 1). Baseline patient characteristics
are presented in Table I. The
median follow-up period for surviving patients was 60 months, and
the median age at diagnosis was 60 years (range, 40–76 years). The
study population primarily comprised males (95.2%), with 74.3%
having a smoking history. In comparison to patients with
HPV-negative OPC, those with HPV-positive OPC were younger
(P=0.046), showed a lower percentage of N2 stage (P=0.024), had a
less frequent smoking history (P=0.049), and a higher incidence of
poorly differentiated tumors (P=0.001). Upon restaging according to
the AJCC 8th edition staging system, 51 out of 65 patients (78%)
with HPV-positive OPC were down-staged from their initial
diagnosis, indicating a higher proportion of patients in the early
stages of disease (P<0.01) compared with HPV-negative OPC.
Specifically, 3 individuals were down-staged from stage III to
stage II, 10 were down-staged from stage IV to stage III and 38
were downgraded from stage IV to stage II.
 | Table I.Patient characteristics and response
rate. |
Table I.
Patient characteristics and response
rate.
| Characteristics | All (N=105) | HPV-negative
(N=40) | HPV-positive
(N=65) | P-value |
|---|
| Male | 100 (95) | 40 (100) | 60 (92) | 0.154 |
| Age, years |
|
|
|
|
|
Median | 60 | 62 | 57 |
|
|
<60 | 55 (52) | 16 (40) | 39 (60) | a0.046 |
|
≥60 | 50 (48) | 24 (60) | 26 (40) |
|
| WHO PS |
|
|
|
|
| 0 | 29 (28) | 9 (22) | 20 (31) | 0.380 |
| 1 | 76 (72) | 31 (78) | 45 (69) |
|
| BMI,
kg/m2 |
|
|
|
|
|
Normal | 45 (43) | 17 (43) | 28 (43) | 0.136 |
|
Overweight | 45 (43) | 14 (35) | 31 (48) |
|
|
Low | 15 (14) | 9 (23) | 6 (9) |
|
| Smoking status,
pk-yrs |
|
|
| a0.049 |
| No | 27 (26) | 6 (15) | 21 (32) |
|
|
Yes | 78 (74) | 34 (85) | 44 (68) |
|
|
<20 | 12 (15) | 4 (12) | 8 (18) | 0.536 |
|
≥20 | 66 (85) | 30 (88) | 36 (82) |
|
| Differentiated |
|
|
| a0.001 |
| WD | 29 (28) | 19 (48) | 10 (15) |
|
| MD | 40 (38) | 15 (38) | 25 (39) |
|
| PD | 22 (21) | 5 (13) | 17 (26) |
|
|
Unknown | 14 (13) | 1 (3) | 13 (20) |
|
| Stage |
|
|
| a<0.01 |
| II | 53 (51) | 0 (0) | 53 (82) |
|
|
III | 18 (17) | 6 (15) | 12 (18) |
|
| IV | 34 (32) | 34 (85) | 0 (0) |
|
| Tumor status |
|
|
| 0.361 |
| T1 | 8 (8) | 4 (10) | 4 (6) |
|
| T2 | 40 (38) | 18 (45) | 22 (34) |
|
| T3 | 37 (35) | 10 (25) | 27 (42) |
|
| T4 | 20 (19) | 8 (20) | 12 (18) |
|
| Node status |
|
|
| a0.024 |
| N0 | 5 (5) | 0 (0) | 5 (8) |
|
| N1 | 37 (35) | 10 (25) | 27 (41) |
|
| N2 | 63 (60) | 30 (75) | 33 (51) |
|
| Total radiation
dose, cGy |
|
|
| 0.819 |
|
<6,600 | 27 (26) | 11 (27) | 16 (35) |
|
|
≥6,600 | 78 (74) | 29 (73) | 49 (75) |
|
| Response of IC |
|
|
|
|
|
ORR | 92.4 | 92.5 | 90.3 | >0.999 |
| CR | 29 (28) | 11 (28) | 18 (28) | >0.999 |
| PR | 68 (65) | 26 (65) | 42 (63) |
|
| SD | 8 (8) | 3 (8) | 5 (8) |
|
| Response after
CRT |
|
|
|
|
|
ORR | 94.3 | 92.5 | 95.4 | 0.672 |
| CR | 87 (83) | 32 (80) | 55 (85) | 0.088 |
| PR | 12 (11) | 5 (13) | 7 (11) |
|
| SD | 3 (3) | 3 (8) | 0 (0) |
|
| PD | 3 (3) | 0 (0) | 3 (5) |
|
Treatment delivery and outcomes
Of the total participants, 102 (97.1%) underwent
three cycles of IC. At the same time, 3 patients received
additional cycles beyond the initial three to elicit further
responses due to a minor response after the initial treatment.
There was no significant difference in the relative dose intensity
between patients with HPV-negative and HPV-positive OPC
(HPV-negative vs. HPV-positive; docetaxel, 92.9 vs. 91.6%;
cisplatin, 92.2 vs. 89.5; and 5-FU, 92.1 vs. 91.3%). Most patients
(N=99, 94.3%) who completed the planned IC cycle advanced to CRT
and 18 patients (17%) underwent surgery following CRT. A total of 6
patients discontinued the scheduled cycle of CRT; 2 experienced
acute kidney injury and 4 declined planned CRT due to grade 3 oral
mucositis, chemotherapy-induced nausea and vomiting, and
odynophagia. Among them, 5 out of 6 patients who discontinued
planned CRT had HPV-positive OPC. The median cumulative radiation
dose was 66 Gray (Gy) (range, 27.0–72.6 Gy) and the median
cumulative dose of cisplatin was 200 mg/m2 (range,
60–300 mg/m2). There were no significant differences
observed between HPV-negative OPC and HPV-positive OPC, nor between
older (≥60 years) and younger patients (<60 years) with a median
age of 60 years. Additionally, all but three patients received
radiation with a cumulative dose exceeding 50 Gy. RT modalities
comprised 3D-CRT in 52 patients (50%) and IMRT in 53 (50%).
CR to IC was observed in 29 patients (27.6%), with
68 (64.8%) showing PR. Following CRT, CR was achieved in 87
patients (82.8%), and 12 (11.4%) exhibited PR. The overall response
rates after IC followed by CRT, detailed in Table I, revealed no significant difference
between HPV-negative and HPV-positive OPC (92.5 vs. 95.4%).
Survival analysis and patterns of
recurrence
Throughout the follow-up period, recurrence was
observed in 34 patients (32.4%), with 67.6% experiencing
locoregional recurrence and 32.4% developing distant metastases
(lung, liver, or bone). No significant differences were noted in
the patterns of locoregional recurrence and distant metastasis
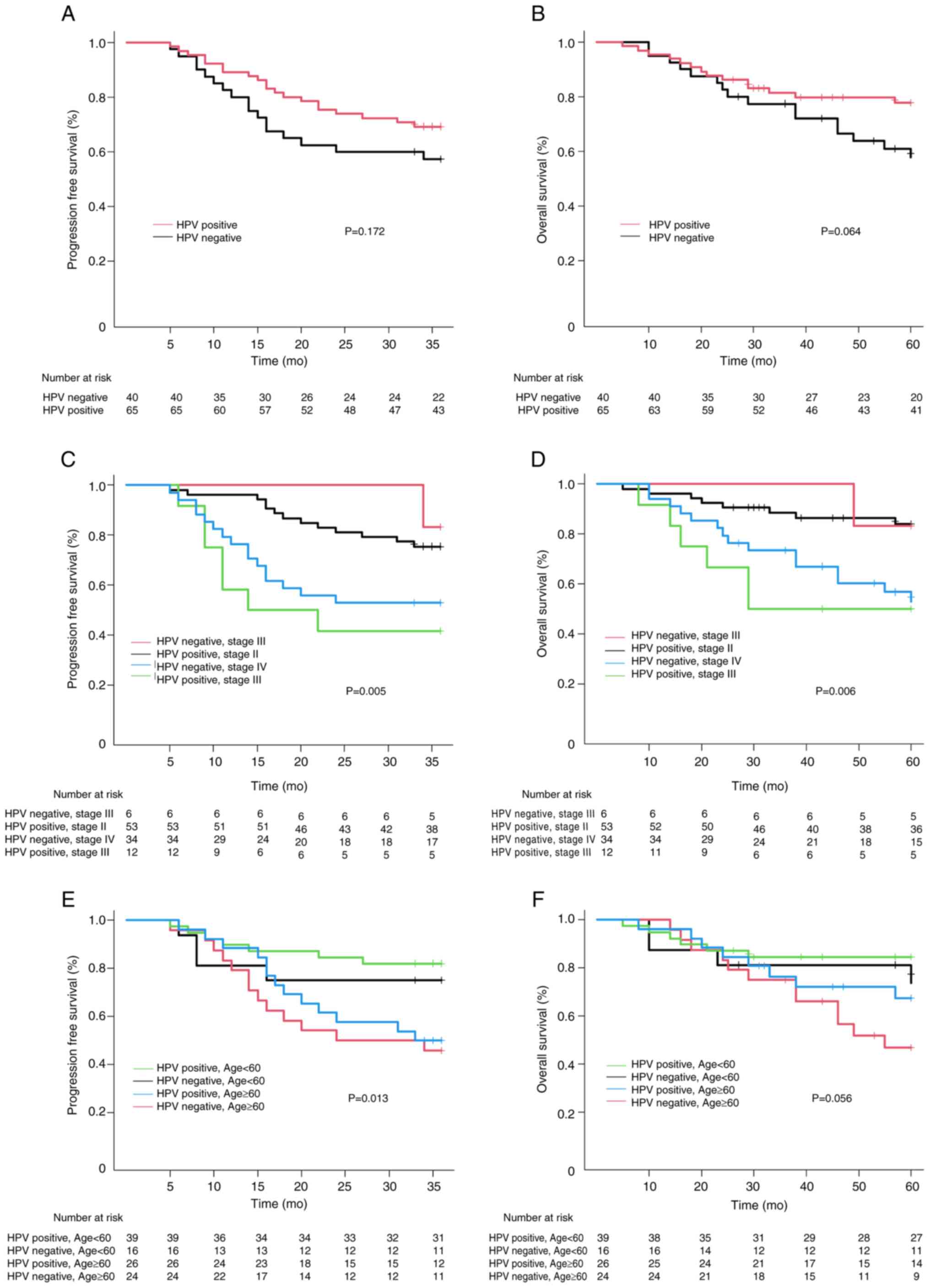
between HPV-positive and HPV-negative OPC. The 3-year PFS for
HPV-negative OPC was 57.4% [95% confidence interval (CI):
42.11–72.69] and 69.2% (95% CI: 58.03–80.37) in HPV-positive OPC.
Additionally, 5-year OS was 60.9% in HPV-negative OPC (95% CI:
45.42–76.38) and 77.8% in HPV-positive OPC (95% CI: 67.41–88.19)
(data not shown). While the 3-year PFS and 5-year OS exhibited a
more favorable trend in HPV-positive OPC compared with HPV-negative
OPC, neither the 3-year PFS (P=0.172) nor the 5-year OS (P=0.064)
exhibited a statistically significant association with HPV status
(Fig. 2A and B). To assess
treatment outcomes based on HPV status and stage, patients were
grouped into four categories: HPV-positive/stage II,
HPV-negative/stage III, HPV-positive/stage II and
HPV-negative/stage IV. A decline in survival outcomes with a higher
stage for HPV-positive OPC and HPV-negative OPC in the 3-year PFS
(P=0.005) and the 5-year OS (P=0.006) was observed (Fig. 2C and D). Notably, the survival
outcomes of HPV-positive/stage III and HPV-negative/stage IV were
comparable in the 3-year PFS and 5-year OS. Furthermore, the
outcomes in HPV-positive/stage II and HPV-negative/stage III were
similar, with no statistically significant differences among group.
However, when the groups A of HPV-negative/stage III and
HPV-positive/stage II and B of HPV-negative/stage IV and
HPV-positive/stage III were compared, both PFS and OS were
significantly worse in group A, which had a relatively poor
prognosis (P=0.001) (data not shown).
Risk factors associated with survival
outcomes
To identify distinct clinical prognostic factors in
HPV-positive OPC, factors related to survival outcomes based on HPV
status were explored. It was found that individuals ≥60 years of
age and non-CR after CRT were linked to a higher risk for PFS in
all patients compared with patients <60 years of age and CR
after CRT (Table II). Regarding
OS, non-CR at the primary site following IC, and non-CR after CRT
were identified as significant adverse factors, while being
overweight emerged as a favorable factor (Table III). Although age had no
statistical significance for OS in the multivariate analysis, this
data revealed a close relationship between survival outcomes and
age. Hence, the survival outcomes were examined based on the median
age of 60 years (<60 vs. ≥60 years) to ascertain the association
depending on HPV status. It was demonstrated that PFS (P=0.013) and
OS (P=0.056) were poorer in patients aged ≥60 years than in those
<60 years (Fig. 2E and F).
 | Table II.Uni- and multivariate analyses of
survival outcomes in all patients, HPV-negative OPC and
HPV-positive OPC for progression-free survival. |
Table II.
Uni- and multivariate analyses of
survival outcomes in all patients, HPV-negative OPC and
HPV-positive OPC for progression-free survival.
|
| All patients | HPV-negative
OPC | HPV-positive
OPC |
|---|
|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|
|
|---|
| Characteri
stics | HR | P-value | 95% CI | HR | P-value | 95% CI | HR | P-value | 95% CI | HR | P-value | 95% CI | HR | P-value | 95% CI | HR | P-value | 95% CI |
|---|
| Male | 1.77 | 0.572 | 0.24–12.9 |
|
|
|
|
|
|
|
|
| 1.51 | 0.690 | 0.20–11.2 |
|
|
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| <60 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ≥60 | a2.96 | a0.003 | a1.46–6.00 | a2.70 | a0.006 | a1.33–5.50 | 2.35 | 0.136 | 0.76–7.21 |
|
|
| a3.18 | a0.014 | a1.27–8.00 | a3.38 | a0.011 | a1.32–8.63 |
| WHO PS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 1 | 1.31 | 0.441 | 0.66–2.61 |
|
|
| 0.64 | 0.407 | 0.23–1.83 |
|
|
| 0.79 | 0.627 | 0.32–1.99 |
|
|
|
| BMI,
kg/m2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Normal | 1 |
|
|
|
|
| 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
Overweight | 0.53 | 0.089 | 0.26–1.10 |
|
|
| 0.40 | 0.402 | 0.12–1.31 |
|
|
| 0.66 | 0.386 | 0.26–1.68 |
|
|
|
|
Low | 0.96 | 0.924 | 0.38–2.40 |
|
|
| 0.81 | 0.806 | 0.25–2.62 |
|
|
| 0.93 | 0.921 | 0.20–4.23 |
|
|
|
| Smoking,
pk-yrs |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | 1 |
|
|
|
|
| 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
<20 | 0.81 | 0.762 | 0.22–3.07 |
|
|
| 0.68 | 0.749 | 0.06–7.46 |
|
|
| 0.85 | 0.844 | 1.17–4.22 |
|
|
|
|
≥20 | 1.43 | 0.375 | 0.65–3.16 |
|
|
| 1.40 | 0.656 | 0.62–6.17 |
|
|
| 1.22 | 0.686 | 0.46–3.26 |
|
|
|
| Tumor status |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
T0-T3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| T4 | 2.02 | 0.058 | 0.98–4.18 |
|
|
| 0.84 | 0.858 | 0.26–3.11 |
|
|
| a3.59 | a0.007 | a1.43–9.08 | a3.16 | a0.019 | a1.21–8.27 |
| Node status |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N0-1b, N1-N2bc |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N2b, N2cc | a2.10 | a0.045 | a1.02–4.34 |
|
|
| 2.63 | 0.057 | 0.97–7.14 | a3.02 | a0.035 | a1.08–8.43 | 1.54 | 0.345 | 0.63–3.77 |
|
|
|
| Total radiation,
cGy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<6,600 | 1.01 | 0.985 | 0.49–2.08 |
|
|
| 1.03 | 0.956 | 0.36–2.93 |
|
|
| 1.01 | 0.992 | 0.37–2.77 |
|
|
|
|
≥6,600 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| After IC,
non-CR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Overall
response | 1.90 | 0.127 | 0.83–4.32 |
|
|
| 2.10 | 0.245 | 0.60–7.30 |
|
|
| 1.77 | 0.306 | 0.59–5.31 |
|
|
|
|
Primary | a1.95 | a0.043 | a1.02–3.73 |
|
|
| 2.09 | 0.137 | 0.79–5.49 |
|
|
| 2.03 | 0.114 | 0.84–4.89 |
|
|
|
|
LNs | 2.00 | 0.098 | 0.88–4.56 |
|
|
| 2.43 | 0.164 | 0.70–8.45 |
|
|
| 1.77 | 0.306 | 0.59–5.31 |
|
|
|
| After CRT |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| CR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-CR | a4.71 | a<0.01 | a2.38–9.33 | a4.29 | a<0.01 | a2.16–8.51 | a4.12 | a0.006 | a1.50–11.3 | a4.68 | a0.004 | a1.64–13.4 | a5.15 | a0.001 | a2.03–13.0 | a3.88 | a0.005 | a1.50–10.0 |
 | Table III.Uni- and multivariate analysis of
survival outcomes in all patients, HPV-negative OPC and
HPV-positive OPC for overall survival. |
Table III.
Uni- and multivariate analysis of
survival outcomes in all patients, HPV-negative OPC and
HPV-positive OPC for overall survival.
|
| All patients | HPV-negative
OPC | HPV-positive
OPC |
|---|
|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|
|
|---|
|
Characteristics | HR | P-value | 95% CI | HR | P-value | 95% CI | HR | P-value | 95% CI | HR | P-value | 95% CI | HR | P-value | 95% CI | HR | P-value | 95% CI |
|---|
| Male | 1.44 | 0.720 | 0.19–10.6 |
|
|
|
|
|
|
|
|
| 0.99 | 0.992 | 0.13–7.57 |
|
|
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<60 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
≥60 | a2.34 | a0.028 | a1.09–5.01 | 2.06 | 0.068 | 0.95–4.49 | 2.27 | 0.159 | 0.73–7.07 |
|
|
| 2.03 | 0.190 | 0.70–5.86 |
|
|
|
| WHO PS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 1 | 0.58 | 0.146 | 0.27–1.21 |
|
|
| 0.481 | 0.177 | 0.17–1.39 |
|
|
| 0.54 | 0.257 | 0.19–1.56 |
|
|
|
| BMI,
kg/m2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Normal | 1 |
|
| 1 |
|
| 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
Overweight | a0.29 | a0.011 | a0.12–0.76 | a0.28 | a0.009 | a0.11–0.73 | 0.39 | 0.159 | 0.10–1.45 |
|
|
| a0.26 | a0.044 | a0.07–0.96 |
|
|
|
|
Low | 1.26 | 0.613 | 0.52–3.03 | 1.51 | 0.370 | 0.62–3.69 | 1.17 | 0.776 | 0.38–3.61 |
|
|
| 1.07 | 0.936 | 0.23–4.94 |
|
|
|
| Smoking,
pk-yrs |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | 1 |
|
|
|
|
| 1 |
|
|
|
|
| 1 |
|
|
|
|
|
|
<20 | 1.75 | 0.403 | 0.47–6.53 |
|
|
| 1.62 | 0.632 | 0.23–11.5 |
|
|
| 1.67 | 0.573 | 0.28–10.0 |
|
|
|
|
≥20 | 1.71 | 0.283 | 0.64–4.52 |
|
|
| 1.19 | 0.818 | 0.27–5.34 |
|
|
| 1.77 | 0.390 | 0.48–6.56 |
|
|
|
| Tumor status |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
T0-T3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| T4 | 1.93 | 0.112 | 0.86–4.33 |
|
|
| 0.65 | 0.570 | 0.15–2.87 |
|
|
| a4.19 | a0.008 | a1.45–12.1 | a3.15 | a0.042 | a1.04–9.53 |
| Node status |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N0-1b, N1-N2bc |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N2b, N2cc | a2.93 | a0.019 | a1.19–7.16 |
|
|
| 2.18 | 0.150 | 0.75–6.30 |
|
|
| 1.79 | 0.297 | 0.59–5.34 |
|
|
|
| Total radiation,
cGy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<6,600 | 0.98 | 0.969 | 0.44–2.21 |
|
|
| 1.23 | 0.725 | 0.39–3.80 |
|
|
| 0.89 | 0.847 | 0.28–2.85 |
|
|
|
|
≥6,600 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| After IC,
non-CR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Overall
response | a3.03 | a0.039 | a1.06–8.69 |
|
|
| 1.85 | 0.338 | 0.53–6.49 |
|
|
| 6.01 | 0.084 | 0.79–46.0 |
|
|
|
|
Primary | a3.43 | a0.001 | a1.66–7.10 | a2.76 | a0.015 | a1.22–6.23 | a3.41 | a0.015 | a1.27–9.13 |
|
|
| a3.86 | a0.016 | a1.29–11.6 | a5.64 | a0.006 | a1.66–19.2 |
|
LNs | 2.44 | 0.069 | 0.93–6.38 |
|
|
| 1.45 | 0.517 | 0.47–4.51 |
|
|
| 6.01 | 0.084 | 0.79–46.0 |
|
|
|
|
After CRT |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-CR | a7.44 | a<0.01 | a3.58–15.5 | a3.93 | a0.001 | a1.17–8.82 | a7.83 | a<0.01 | a2.86–21.6 | a6.56 | a<0.01 | a2.31–18.6 | a6.43 | a0.001 | a2.20–18.8 |
|
|
|
In addition to the aforementioned data, Tables II and III revealed significant clinical factors
in HPV-negative and HPV-positive OPC. Non-CR after CRT emerged as
an adverse risk factor for survival in HPV-negative OPC. In
contrast to HPV-negative OPC, age ≥60 years, T4 stage and non-CR
after CRT were identified as significant risk factors for PFS,
while T4 stage and non-CR at the primary site after IC were
associated with significantly worse survival outcomes for OS in
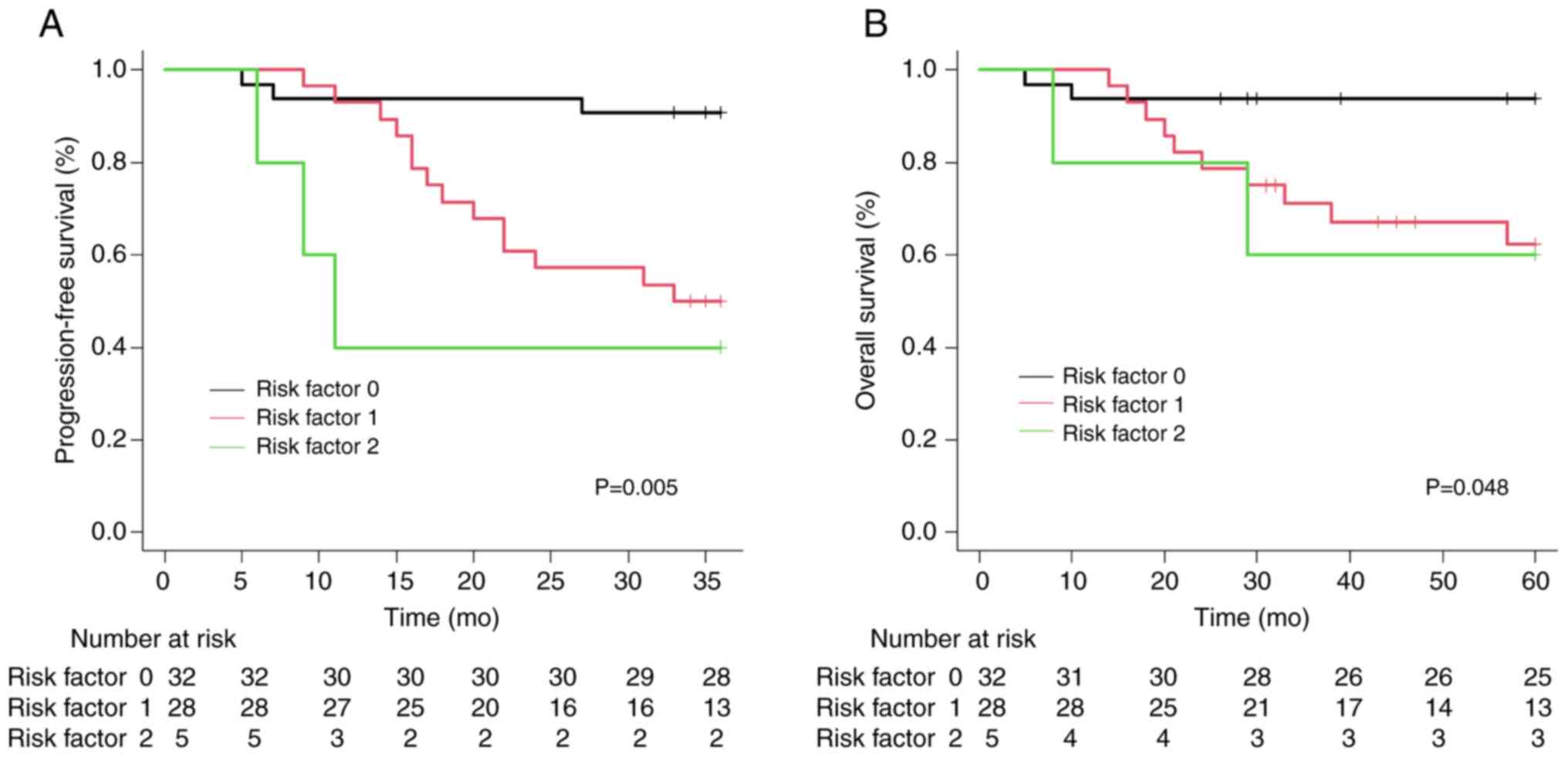
patients with HPV-positive OPC. Utilizing these results, patients
with HPV-positive OPC were categorized into three groups based on
the number of clinical risk factors at diagnosis (age and T4
stage). As depicted in Fig. 3, the
PFS and OS demonstrated significant stratification across each
group as the number of clinical risk factors was increased.
Discussion
Theoretically, HPV-positive OPC is expected to
exhibit more favorable survival outcomes than HPV-negative OPC due
to its better responsiveness to chemotherapy (7,17).
Contrary to previous studies (7,17), the
data of the present study revealed no significant survival
difference between the two groups. This could be because the
tumorigenesis of HPV-positive OPC involves multiple confounding
factors, such as smoking, alcohol consumption and environmental
factors, alongside viral pathogens in head and neck cancer
(18,19). Consequently, using a single factor
such as HPV status to predict treatment response in all cases is
challenging. Furthermore, interpersonal or intrapersonal
heterogeneity can lead to varied treatment outcomes depending on
host factors, even if it involves a single pathogen. To identify
the clinical factors in patients with HPV-positive OPC that
exhibited a poor prognosis comparable with those in patients with
HPV-negative OPC, each group was analyzed. Therefore, it is crucial
to consider known pathogens and additional clinical indicators when
evaluating prognostic factors.
As aforementioned, the routine use of IC is not
recommended in current standard guidelines because OPC typically
responds well to chemotherapy and radiation (20). Nevertheless, IC has advantages of
reducing the rate of distant metastasis, selecting chemosensitive
patients to minimize subsequent therapies, and facilitating organ
preservation. In previous randomized clinical trials with subgroup
analyses of patients with and without OPC, no survival benefit was
observed in the OPC group (21–23).
Nevertheless, these trials had several limitations, such as the
absence of HPV assessment, diverse tumor stages or IC regimens and
lack of standard CRT. Despite these limitations, IC remains an
attractive treatment option due to its potential to reduce bulky
masses, thereby increasing the possibility of de-intensifying
radiotherapy or enhancing operability, ultimately improving the
likelihood of achieving CR (22).
Furthermore, it is established that the response to
IC is a reliable predictor of survival outcomes in head and neck
cancer, a trend consistent with that of OPC (24–27).
Consequently, attempts to de-escalate CRT based on the IC response
have been made in most clinical studies involving HPV-positive OPC,
which tends to be more sensitive to radiation. This approach aims
to mitigate adverse effects associated with radiation.
The ECOG E1308 study demonstrated that reduced-dose
IMRT benefited patients who achieved clinical CR at the primary
site after IC with cisplatin, paclitaxel and cetuximab. However, in
a subgroup analysis, T4 stage, N2c [metastasis in bilateral or
contralateral lymph node(s), non-larger than 6-cm in greatest
dimension and extranodal extension (ENE) negative], or a smoking
history exceeding 10 years demonstrated a statistically significant
decrease in PFS (11). In the
OPTIMA study, patients with HPV-positive OPC were classified into
low-risk [T1-3, N0-2b (N0, no regional lymph node; N1 metastasis in
a single ipsilateral lymph node, ≤3 cm in greatest dimension and
ENE negative; N2a, metastasis in a single ipsilateral node >3 cm
but not >6 cm in greatest dimension and ENE negative; N2b,
metastases in multiple ipsilateral nodes, non-larger than 6 cm in
greatest dimension and ENE negative), smoking history ≤10 years]
and high-risk {T4 or N2c-N3 [N2c, metastasis in bilateral or
contralateral lymph nodes, non-larger than 6 cm in greatest
dimension and ENE negative; N3, metastasis in a lymph node larger
than 6 cm in greatest dimension and ENE negative; or metastasis in
any node(s) and clinically overt ENE positive], smoking history
>10 years} categories. De-escalation treatment was subsequently
administered with 50 Gy RT alone, 45 Gy CRT, or 75 Gy CRT based on
the response to IC (12).
De-escalated RT or CRT for HPV-positive OPC based on risk
stratification and response to IC showed no significant difference
in the 2-year PFS or OS, and toxicity could be minimized by
reducing the RT dose. This study highlighted that risk-stratified
patients with <50% response following IC exhibited comparable
outcomes, with 28 and 27% for low- and high-risk patients,
respectively.
While Ang et al (7) illustrated risk stratification based on
the N stages of the AJCC 5th edition, the results of the present
study indicated that the T stage remained a crucial prognostic
factor for survival in HPV-positive OPC even after IC. This
difference could be attributed to alterations in the AJCC staging
system. In the earlier AJCC 5–7th staging systems, there was no
distinction in the TNM stage system or treatment strategy for
HPV-positive OPC. However, in the AJCC 8th staging system, the N
stage of HPV-positive OPC has been modified and defined as multiple
ipsilateral, equivalent to N1. By contrast, in the AJCC 7th staging
system, this stage was identical to N2 in multiple ipsilateral
regardless of HPV status. As a result, most patients in the present
study were downstaged to stage II or III according to the AJCC 8th
staging system. This prompts the question of whether the current
staging system for HPV-positive OPC is sufficient to reflect the
prognosis as depicted in Fig. 2C and
D. Yoo et al (28)
similarly highlighted an advanced T stage in the univariate
analysis, indicating that patients may not be suitable for
de-escalation treatment. T4 stage emerged as a risk factor for poor
prognosis in other clinical trials that had previously attempted
de-escalation treatment (11).
Combining previous findings with the results of the present study,
it has become evident that the T4 stage is a distinct high-risk
factor in HPV-positive OPC. The N stage in HPV-positive OPC in the
AJCC 8th staging system requires modification to enhance its
applicability in clinical practice.
The present study revealed no differences in
treatment outcomes, including overall response rate and survival
between HPV-positive and HPV-negative OPCs. This is likely due to
the improved treatment outcomes compared with those in previously
published results for HPV-negative OPC. In a trial in the United
States by Ang et al (7), the
3-year PFS and 3-year OS of HPV-negative OPC were 43.4 and 57.1%,
respectively, in a retrospective analysis of the OPC patients who
were treated with CRT. Compared with this report, a higher survival
rate with a 3-year PFS of 57.4% and a 5-year OS of 60.9% was
observed. Furthermore, after CRT, there was a high CR rate of 80%
for the HPV-negative OPC in the present study. Therefore, the
enhanced survival outcome in patients with HPV-negative OPC who
received the IC followed by CRT may mitigate the survival gap
between HPV-negative and HPV-positive OPCs. This result could
explain why the two groups exhibited no significant difference in
the survival rate.
To identify other clinical factors, several studies
have explored smoking, a significant etiological factor for head
and neck cancer, as a prognostic factor. However, the findings were
inconsistent (29–31). A recent study has indicated that
age-related molecular alterations and genomic, immunological and
tumor differences may contribute to tumor responses in older adults
(32). The present study similarly
found no relationship between survival and smoking. This could be
attributed to inaccuracies in medical records or individual
variations in susceptibility to smoking. de la Iglesia et al
(33) demonstrated that smoking
status was not linked to tumor heterogeneity based on gene mutation
burden. Instead, active smoking manifests an immunosuppressive
effect by inhibiting the infiltration of cytotoxic T cells into the
tumor. Hence, there is a need to identify a biomarker that can
reflect the molecular and immunological changes induced by current
smoking rather than relying solely on smoking history.
Extensive research has been conducted on genes
influencing the prognosis and novel treatments for HPV-positive OPC
(5). Khwaja et al
demonstrated that elevated E6 expression was linked to a high risk
of distant metastasis and poor survival outcomes (34). A recent study revealed that patients
with HPV-positive OPC exhibiting high levels of FGF11, recognized
for its oncogenic functions, had poor survival outcomes (35). TP53, identified in
HPV-positive OPC in heavy smokers, has been linked to poor
prognosis. However, it can function as a target for viral enzymes
E6/E7, resulting in the degradation of TP53 (36,37).
Hence, next-generation sequencing (NGS) enables the analysis of
biomarkers related to tumor response to chemotherapy and
facilitates the exploration of age-related biomarkers, aiding in
the identification of patients at high risk for HPV-positive OPC
and complementing traditional markers such as HPV or p16.
The present study has several limitations in terms
of adopting a clinical treatment strategy based on the results.
First, the present study relied on retrospective data collected
from a single institution. Therefore, further validation with data
from multiple centers is necessary. However, the eligible patients
in the present study received a consistent IC regimen and CRT
method, minimizing treatment-related bias. Second, biomarkers such
as p53 expression or NGS were not assessed. Finally, recently
proposed as standard treatment, immune checkpoint inhibitors were
not utilized during this period. Therefore, future studies are
warranted to conduct these evaluations.
In conclusion, a clinical T4 stage and age ≥60 years
at diagnosis were associated with a poor prognosis in patients with
HPV-positive OPC. The innovative finding in the present study is
that it is not appropriate to consider a de-escalated treatment
strategy for high-risk patients, even for HPV-positive patients
with OPC. In addition, further clinical trials using a combined
modality, such as the introduction of IC to chemoradiotherapy are
needed to improve survival.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SHC conceived and designed the study. HJB, SHL, and
SHC confirm the authenticity of all the raw data. HJB, HJK, SHL,
HJS, JEH, WKB, IJC and SHC performed the data analysis and
interpretation of the data. HJB and SHC drafted the manuscript.
HJB, HJK, SHL, HJS, JEH, WKB, IJC and SHC critically revised the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was approved (approval no.
CNUHH-2023-232) by the Institutional Review Board of Chonnam
National University Hwasun Hospital (Hwasun, Republic of
Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statics, 2024. CA Cancer J Clin. 74:12–49. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korea National Cancer CenterNational
Cancer Registration Project Annual Report (2020 Cancer Registration
Statistics), . https://ncc.re.kr/cancerStatsView.ncc?bbsnum=638&searchKey=total&searchValue=&pageNum=1May
19–2023(In Korean).
|
|
3
|
Faraji F, Rettig EM, Tsai HL, El Asmar M,
Fung N, Eisele DW and Fakhry C: The prevalence of human
papillomavirus in oropharyngeal cancer is increasing regardless of
sex or race, and the influence of sex and race on survival is
modified by human papillomavirus tumor status. Cancer. 125:761–769.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang EJ, Lee YG, Keam B, Choi JH, Kim JS,
Park KU, Lee KE, Kim HJ, Lee KW, Kim MK, et al: Characteristics and
treatment patterns in older patients with locally advanced head and
neck cancer (KCSG HN13-01). Korean J Intern Med. 37:190–200. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lechner M, Liu J, Masterson L and Fenton
TR: HPV-associated oropharyngeal cancer: Epidemiology, molecular
biology and clinical management. Nat Rev Clin Oncol. 19:306–327.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rettig EM, Fakhry C, Khararjian A and
Westra WH: Age profile of patients with oropharyngeal squamous cell
carcinoma. JAMA Otolaryngol Head Neck Surg. 144:538–539. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao X, Hou J, An Q, Assaraf YG and Wang X:
Towards the overcoming of anticancer drug resistance mediated by
p53 mutations. Drug Resist Updat. 49:1006712020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th edition.
Springer; New York, NY: 2017
|
|
10
|
Pfister DG, Spencer S, Adelstein D, Adkins
D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ,
et al: Head and Neck Cancers, Version 2.2024, NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2024.
|
|
11
|
Marur S, Li S, Cmelak AJ, Gillison ML,
Zhao WJ, Ferris RL, Westra WH, Gilbert J, Bauman JE, Wagner LI, et
al: E1308: Phase II trial of induction chemotherapy followed by
reduced-dose radiation and weekly cetuximab in patients with
HPV-associated resectable squamous cell carcinoma of the
oropharynx-ECOG-ACRIN Cancer Research Group. J Clin Oncol.
35:490–497. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seiwert TY, Foster CC, Blair EA, Karrison
TG, Agrawal N, Melotek JM, Portugal L, Brisson RJ, Dekker A,
Kochanny S, et al: OPTIMA: A phase II dose and volume de-escalation
trial for human papillomavirus-positive oropharyngeal cancer. Ann
Oncol. 30:16732019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang JJ, Yu Y, Chen L, Zakeri K, Gelblum
DY, McBride SM, Riaz N, Tsai CJ, Kriplani A, Hung TKW, et al:
Consensuses, controversies, and future directions in treatment
deintensification for human papillomavirus-associated oropharyngeal
cancer. CA Cancer J Clin. 73:164–197. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim MJ, Ki MS, Kim K, Shim HJ, Hwang JE,
Bae WK, Chung IJ, Lee DH, Lee JK, Yoon TM, et al: Different protein
expression associated with chemotherapy response in oropharyngeal
cancer according to HPV status. BMC Cancer. 14:8242014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
World Health Organization and Regional
Office for the Western Pacific, . The Asia-Pacific perspective:
redefining obesity and its treatment. Health Communications
Australia; Sydney: pp. 182000, https://iris.who.int/handle/10665/206936May
19–2023
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chow LQM: Head and Neck Cancer. N Engl J
Med. 382:60–72. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen SY, Massa S, Mazul AL, Kallogjeri D,
Yaeger L, Jackson RS, Zevallos J and Pipkorn P: The association of
smoking and outcomes in HPV-positive oropharyngeal cancer: A
systemic review. Am J Otolaryngol. 41:1025922020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai YH, Su CC, Wu SY, Hsueh WT, Wu YH,
Chen HHW, Hsiao JR, Liu CH and Tsai YS: Impact of alcohol and
smoking on outcomes of HPV-related oropharyngeal cancer. J Clin
Med. 11:65102022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferrari D, Ghi MG, Franzese C, Codeca C,
Gau M and Fayette J: The slippery role of induction chemotherapy in
head and neck cancer: Myth and reality. Front Oncol. 10:72020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cohen EE, Karrison TG, Kocherginsky M,
Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB,
Yunus F, et al: Phase III randomized trial of induction
chemotherapy in patients with N2 or N3 locally advanced head and
neck cancer. J Clin Oncol. 32:2735–2743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghi MG, Paccagnella A, Ferrari D, Foa P,
Alterio D, Codecà C, Nolè F, Verri E, Orecchia R, Morelli F, et al:
Induction TPF followed by concomitant treatment versus concomitant
treatment alone in locally advanced head and neck cancer. A phase
II–III trial. Ann Oncol. 28:2206–2212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haddad R, O'Neill A, Rabinowits G, Tishler
R, Khuri F, Adkins D, Clark J, Sarlis N, Lorch J, Beitler JJ, et
al: Induction chemotherapy followed by concurrent chemoradiotherapy
(sequential chemoradiotherapy) versus concurrent chemoradiotherapy
alone in locally advanced head and neck cancer (PARADIGM): A
randomised phase 3 trial. Lancet Oncol. 14:257–264. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdelmeguid AS, Teeramatwanich W, Roberts
DB, Amit M, Ferraroto R, Glisson BS, Kupferman ME, Su SY, Phan J,
Garden AS, et al: Neoadjuvant chemotherapy for locoregionally
advanced squamous cell carcinoma of the paranasal sinuses. Cancer.
127:1788–1795. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bossi P, Lo Vullo S, Guzzo M, Mariani L,
Granata R, Orlandi E, Locati L, Scaramellini G, Fallai C and
Licitra L: Preoperative chemotherapy in advanced resectable OCSCC:
long-term results of a randomized phase III trial. Ann Oncol.
25:462–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YG, Kang EJ, Keam B, Choi JH, Kim JS,
Park KU, Lee KE, Kim HJ, Lee KW, Kim MK, et al: Induction
chemotherapy as a prognostication index and guidance for treatment
of locally advanced head and neck squamous cell carcinoma: The
concept of chemo-selection (KCSG HN13-01). Cancer Res Treat.
54:109–117. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Q, Xu T, Shen C, Qian W, Ying H, He
X, Wang Y, Ji Q, Hu C, Zhou X and Lu X: Response to induction
chemotherapy predicts survival outcomes in oropharyngeal cancer.
Cancer Med. 12:9175–9185. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoo SH, Ock CY, Keam B, Park SJ, Kim TM,
Kim JH, Jeon YK, Chung EJ, Kwon SK, Hah JH, et al: Poor prognostic
factors in human papillomavirus-positive head and neck cancer: Who
might not be candidates for de-escalation treatment? Korean J
Intern Med. 34:1313–1323. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Broughman JR, Xiong DD, Moeller BJ,
Contrera KJ, Prendes BL, Lamarre ED, Ku JA, Burkey BB, Woody NM,
Joshi NP, et al: Rethinking the 10-pack-year rule for favorable
human papillomavirus-associated oropharynx carcinoma: A
multi-institution analysis. Cancer. 126:2784–2790. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fakhry C, Zhang Q, Nguyen-Tan PF,
Rosenthal DI, Weber RS, Lambert L, Trotti AM III, Barrett WL,
Thorstad WL, Jones CU, et al: Development and validation of
nomograms predictive of overall and progression-free survival in
patients with oropharyngeal cancer. J Clin Oncol. 35:4057–4065.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang SH, Xu W, Waldron J, Siu L, Shen X,
Tong L, Ringash J, Bayley A, Kim J, Hope A, et al: Refining
American Joint Committee on Cancer/Union for International Cancer
Control TNM stage and prognostic groups for human
papillomavirus-related oropharyngeal carcinomas. J Clin Oncol.
33:836–845. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chatsirisupachai K, Lagger C and de
Magalhaes JP: Age-associated differences in the cancer molecular
landscape. Trends Cancer. 8:962–971. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de la Iglesia JV, Slebos RJC, Martin-Gomez
L, Wang X, Teer JK, Tan AC, Gerke TA, Aden-Buie G, van Veen T,
Masannat J, et al: Effects of tobacco smoking on the tumor immune
microenvironment in head and neck squamous cell carcinoma. Clin
Cancer Res. 26:1474–1485. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khwaja SS, Baker C, Haynes W, Spencer CR,
Gay H, Thorstad W, Adkins DR, Nussenbaum B, Chernock RD, Lewis JS
Jr and Wang X: High E6 gene expression predicts for distant
metastasis and poor survival in patients with HPV-positive
oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol
Phys. 95:1132–1141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Flon CH, Haeggblom L, Holzhauser S,
Kostopoulou ON, Zupancic M, Dalianis T, Munck-Wikland E, Marklund L
and Näsman A: High levels of FGF11 correlate with poor survival in
patients with human papillomavirus (HPV)-positive oropharyngeal
squamous cell carcinoma. Cancers (Basel). 15:19542023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de Bakker T, Journe F, Descamps G, Saussez
S, Dragan T, Ghanem G, Krayem M and Van Gestel D: Restoring p53
function in head and neck squamous cell carcinoma to improve
treatments. Front Oncol. 11:7999932021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dogan S, Xu B, Middha S, Vanderbilt CM,
Bowman AS, Migliacci J, Morris LGT, Seshan VE and Ganly I:
Identification of prognostic molecular biomarkers in 157
HPV-positive and HPV-negative squamous cell carcinomas of the
oropharynx. Int J Cancer. 145:3152–3162. 2019. View Article : Google Scholar : PubMed/NCBI
|