Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-related deaths and has become a global health
issue, with an increasing incidence worldwide (1,2), as
HCC is often diagnosed at an advanced stage (3). Although various systemic therapies
such as molecular-targeted agents have been developed for patients
with unresectable HCC (u-HCC), few patients achieve durable
benefits, and long-term survival rates remain unsatisfactory
(4). Recently, immune checkpoint
inhibitors (ICIs) have revolutionized the treatment strategy for
u-HCC, and remarkable changes have occurred in systemic treatment
settings (5,6). After the combination of atezolizumab
and bevacizumab (Atez/Bev) was established for u-HCC patients,
durvalumab plus tremelimumab (Dur/Tre) was recently approved as a
first-line therapy for dual ICIs (7).
The Single Tremelimumab Regular Interval Durvalumab
(STRIDE) regimen is a combination immunotherapy with tremelimumab,
an anti-cytotoxic T-lymphocyte-associated antigen 4 inhibitor, and
durvalumab, an anti-programmed cell death ligand-1 inhibitor
(5). Based on the findings of the
HIMALAYA phase 3 clinical trial, this first immunotherapy
combination showed non-inferior progression-free survival (PFS) but
improved overall survival (OS) compared to sorafenib (7) for patients with u-HCC. Following the
results of this clinical trial, the STRIDE regimen has become the
preferred first-line treatment along with Atez/Bev for u-HCC
(8). Compared with other clinical
trials for u-HCC, the STRIDE regimen is characterized by a longer
observation period, where favorable results have been reported,
with a 3-year survival rate of 30.7%. Furthermore, When compared to
patients treated with sorafenib, patients treated with the STRIDE
regimen had a lower relative risk of reduced quality of life
(9). Several phase 3 trials have
also reported the efficacy and safety of the STRIDE regimen in
other cancers (10,11). However, ICIs largely affect all
internal organs, and their use can lead to immune-related adverse
events (irAEs) that require treatment with immunosuppressive
medications in moderate to severe cases (12,13).
In fact, the rate of cases with irAE requiring high-dose steroids
(≥ prednisolone 40 mg/day) was 20.1% in the HIMALAYA phase 3
clinical trial. However, the efficacy and safety of Dur/Tre for
u-HCC in real-world clinical practice remains unknown.
This study aimed to evaluate the overall therapeutic
outcomes and safety with the initial use of Dur/Tre for u-HCC
treatment.
Patients and methods
Study design and patients
The present retrospective study evaluated 139
patients who were treated with Dur/Tre between March 2023 and
January 2024 at 16 Japanese institutions: Kurume University
Hospital (Kurume, Japan), Yamaguchi University Hospital (Yamaguchi,
Japan), Tokushima University Hospital (Tokushima, Japan), Nagoya
University Hospital (Nagoya, Japan), Kagawa University Hospital
(Kagawa, Japan), Okayama University Hospital (Okayama, Japan),
Japanese Red Cross Nagoya Daiichi Hospital (Nagoya, Japan),
Toyohashi Municipal Hospital (Aichi, Japan), Anjo Kosei Hospital
(Anjo, Japan), National Hospital Organization Takasaki General
Medical Center (Gunma, Japan), Gunma Saiseikai Maebashi Hospital
(Gunma, Japan), Kawasaki Medical School (Okayama, Japan), Fukuyama
City Hospital (Okayama, Japan), Fukuyama Medical Center (Okayama,
Japan), Iwamoto Internal Medical Clinic (Kitakyushu, Japan), and
Kurume Central Hospital (Kurume, Japan). The following eligibility
criteria were used: i) age >18 years, ii) Eastern Cooperative
Oncology Group performance status (PS) <3, and iii) available
clinical data and followed-up until study cessation (January 2024)
or death. The exclusion criteria were: i) Child-Pugh class C and
ii) a history of autoimmune disease, and iii) Barcelona Clinic
Liver Cancer stage 0 or A. Therefore, 19 patients were excluded
from the study. In total, 120 patients were enrolled in this study
(Fig. S1). This study was
conducted in accordance with the Declaration of Helsinki and
approved by the Ethics Committee of the Kurume University School of
Medicine (approval code: 23153), and an implementation permit was
obtained from each of the 15 other institutions. An opt-out
approach was used to obtain informed consent from patients. The
cutoff date for this analysis was January 2024.
Durvalumab plus tremelimumab treatment
and safety evaluation
The patients were administered tremelimumab for one
dose of 300 mg intravenously and durvalumab at a dose of 1,500 mg
once every 4 weeks. The treatment was continued until unacceptable
AEs or progressive disease occurred. AEs were assessed using the
National Cancer Institute Common Terminology Criteria for Adverse
Events (version 5.0).
Assessment of hepatic reserve
function
Liver function was evaluated using Child-Pugh class
(14) and albumin-bilirubin (ALBI)
scores (15). ALBI score was
calculated based on serum albumin and total bilirubin levels: ALBI
score=[log10 bilirubin (µmol/l) ×0.66] + [albumin (g/l) × −0.085],
and was graded as follows: ≤-2.60=ALBI grade 1; >-2.60 to
≤-1.39=ALBI grade 2; >-1.39=ALBI grade 3. Based on the ALBI
score, we also assessed liver function using the modified ALBI
grade (mALBI grade) (16). We
evaluated the change in the ALBI score from baseline at 4, 8, and
12 weeks.
Assessment of therapeutic
response
The therapeutic response of HCC was assessed at the
time of best response using dynamic computed tomography or magnetic
resonance imaging every 4–8 weeks based on the Response Evaluation
Criteria In Solid Tumors version 1.1 (RECIST v1.1) (17).
Statistical analysis
All categorical variables are presented as numbers
or medians (ranges). Progression-free survival (PFS) was calculated
using the Kaplan-Meier method and analyzed using the log-rank test.
Continuous variables were compared using a one-way analysis of
variance with Scheffe's post-hoc test, and categorical variables
were compared between groups using χ2 test or Fisher's
exact analysis. We also performed a decision tree analysis to
identify factors associated with discontinuation owing to AEs.
Statistical significance was defined as a two-tailed P-value of
<0.05. All statistical analyses were performed using JMP
Pro® version 15 (SAS Institute Inc.).
Results
Clinical characteristics
Patient characteristics are presented in Table I. The median age was 73 years, 96
patients (80.0%) were men, and 100 patients (83.3%) had a
performance status of 0. Child-Pugh classes A and B were observed
in 110 (91.7%) and 10 (8.4%) patients, respectively. The median
ALBI score was −2.27, and m-ALBI grade 1 was observed in 29
patients (24.1%). Barcelona Clinic Liver Cancer stage B was
observed in 67 patients (55.8%). Macrovascular invasion and
extrahepatic spread were observed in 22 (18.3%) and 36 (30.0 %)
patients, respectively. Dur/Tre treatment was introduced as the
1st-line, 2nd, 3rd, 4th, 5th, 6th, and 7th treatment in 44 (36.6%),
36 (30.0%), 18 (15.0%), 11 (9.2%), 5 (4.2%), 5 (4.2%), and 1 (0.8
%) patients, respectively. The median follow-up was 5.6 (1.0–9.0)
months.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| N | 120 |
| Median age, years
(range) | 73 (42–92) |
| Sex,
female/male | 24/96 |
| PS, 0/1/2 | 100/18/2 |
| Median body mass
index, kg/m2 (range) | 23.3
(15.9–38.8) |
| Cause of HCC,
HBV/HCV/Non-B,C | 23/46/51 |
| Child-Pugh
class/score |
|
| A
(5/6) | 110 (54/56) |
| B
(7/8) | 10 (7/3) |
| Median AST, U/l
(range) | 34 (10–274) |
| Median ALT, U/l
(range) | 24 (4–208) |
| Median serum
albumin, g/dl (range) | 3.6 (2.4–4.6) |
| Median total
bilirubin, mg/dl (range) | 0.8 (0.3–2.0) |
| Median ALBI score
(range) | −2.27 |
|
| (−3.19 to
−1.04) |
| mALBI grade,
1/2a/2b/3 | 29/33/57/1 |
| BCLC stage,
B/C | 67/53 |
| Macrovascular
invasion, yes/no | 22/98 |
| Extrahepatic
spread, yes/no | 36/84 |
| Median AFP, ng/ml
(range) | 56.5 |
|
|
(1.6–2580,000.0) |
| Treatment line |
|
|
First-line | 44 |
|
Later-line
(2nd/3rd/4th/5th/6th/7th) | 76 |
|
|
(36/18/11/5/5/1) |
| Pre-ICI treatment,
yes/no | 67/53 |
| HIMALAYA trial
exclusion criteria, yes/no | 82/38 |
|
Later-line | 65 |
|
Later-line + Vp 4 | 3 |
|
Later-line + Child-Pugh class
B | 6 |
|
Later-line + Increased AST or
ALT | 2 |
|
Child-Pugh class B | 4 |
|
Increased AST or ALT | 2 |
| Median follow-up
duration, months (range) | 5.6 (1.0–9.0) |
Clinical therapeutic outcomes of
durvalumab plus tremelimumab
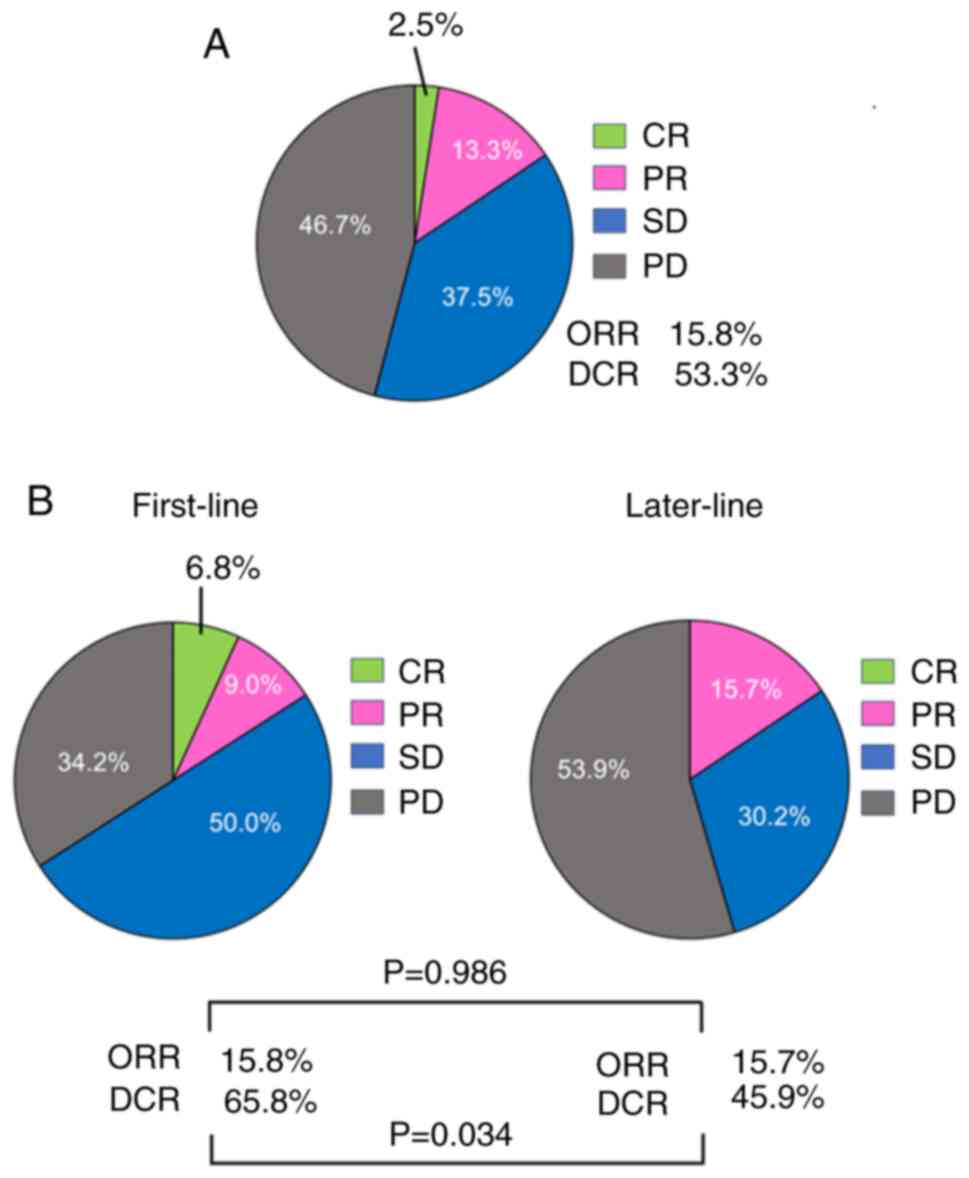
The therapeutic responses are presented in Fig. 1A. Complete response (CR) was
observed in three patients (2.5%), and partial response (PR) was
observed in 16 patients (13.3%). The objective response rate (ORR)
was 15.8%. Stable disease (SD) was observed in 45 patients (37.5%),
and progressive disease (PD) was observed in 56 patients (46.7%).
The overall disease control rate (DCR) was 53.3%. The median
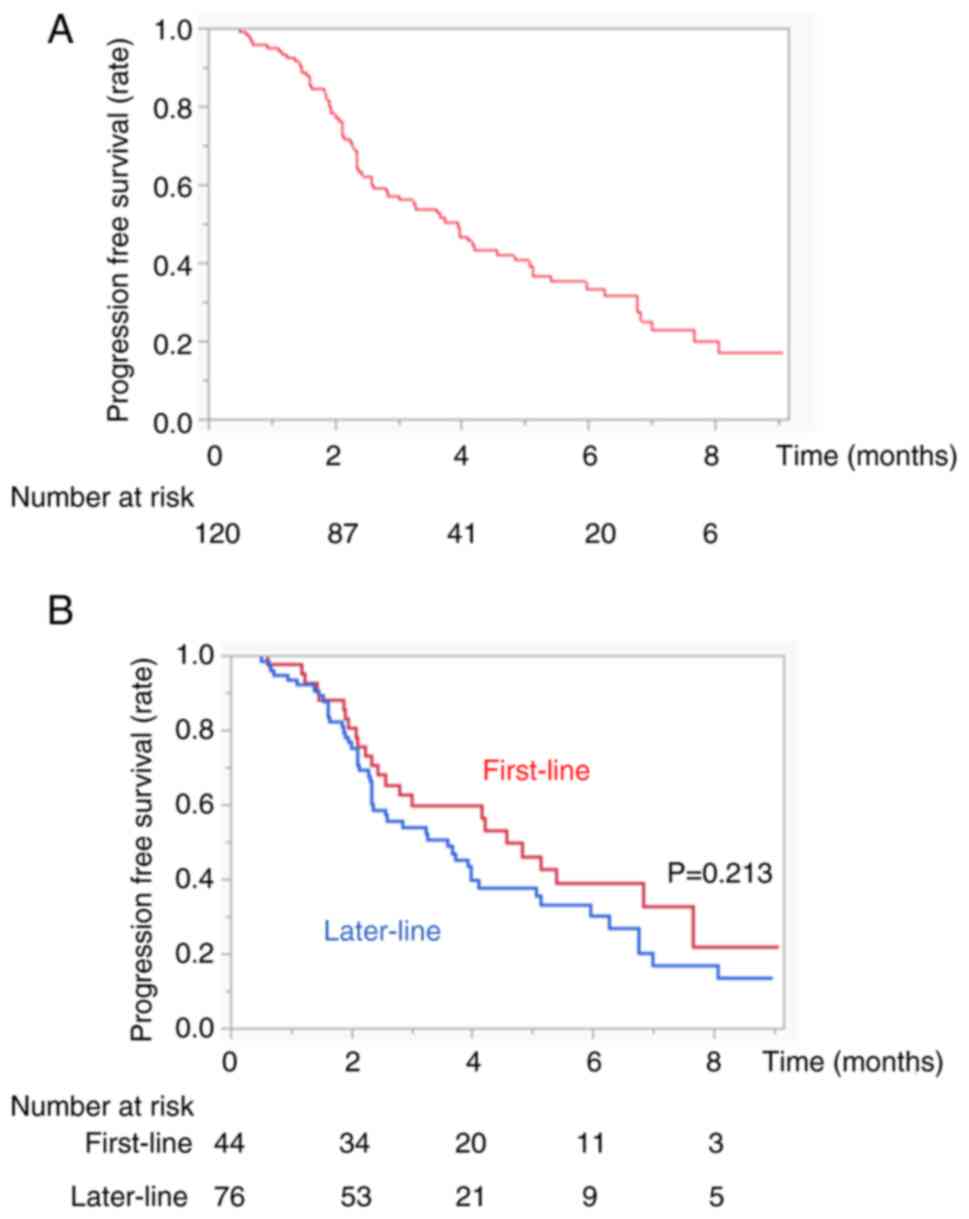
overall PFS was 3.9 months (Fig.
2A). There were no cases of pseudoprogression.
Clinical safety
The AEs that occurred during Dur/Tre treatment are
shown in Table II. The overall
incidence rates of any grade and grade 3 or higher were 83.3 and
36.7%, respectively. Among them, 61 patients (50.8%) had increased
aspartate aminotransferase (AST) levels, 45 (37.5%) had increased
alanine aminotransferase (ALT) levels, 45 patients (37.5%)
experienced rash, 27 (22.5%) experienced fever, 22 (18.3%) had
diarrhea, 6 (5.0%) had pituitary or adrenal insufficiency and
drug-induced pneumonia, 26 (21.6%) had fatigue, and 29 (24.1%)
experienced decreased appetite. Grade 3 or higher AEs included
elevated AST levels (13.3%), elevated ALT levels (10.8%), diarrhea
(10.0%), and rash (8.3%). High-dose steroids were administered to
27 patients (22.5%).
 | Table II.Adverse events associated with
Dur/Tre (n=120). |
Table II.
Adverse events associated with
Dur/Tre (n=120).
| Adverse event | Any, n (%) | Grade 3 or higher,
n (%) |
|---|
| Total adverse
events | 100 (83.3) | 44 (36.7) |
| AST evaluation | 61 (50.8) | 16 (13.3) |
| ALT evaluation | 45 (37.5) | 13 (10.8) |
| Rash | 45 (37.5) | 10 (8.3) |
| Fever | 27 (22.5) | 1 (0.8) |
| Diarrhea | 22 (18.3) | 12 (10.0) |
| Abdominal pain | 8 (6.7) | 2 (1.6) |
| Pituitary or
adrenal insufficiency | 6 (5.0) | 6 (5.0) |
| Drug-induced
pneumonia | 6 (5.0) | 4 (3.3) |
| Pancreatitis | 3 (2.4) | 2 (1.6) |
| Infusion
reaction | 4 (3.3) | 1 (0.8) |
| Other irAEs | 8 (6.7) | 4 (3.3) |
| Fatigue | 26 (21.6) | 5 (4.1) |
| Decreased
appetite | 29 (24.1) | 1 (0.8) |
| Factors |
|
|
| Requiring high-dose
steroid |
| 27 (22.5) |
Comparison of therapeutic outcomes and
safety according to first or later lines of durvalumab plus
tremelimumab
The therapeutic responses to the first or later
lines are shown in Fig. 1B.
Although there was no significant difference in the ORR between the
first- or later-line treatment groups (ORR, 15.8 vs. 15.7%,
P=0.986), there was a significant difference in the DCR between the
two groups (65.8 vs. 45.9%, P=0.034). There was no significant
difference in PFS between the two groups (PFS 4.5 vs. 3.6 months,
P=0.213) (Fig. 2B). Differences in
treatment-related AEs between the first- or later-line treatments
are shown in Table III. There was
no significant difference in the frequency of AEs between the
first- and later-line treatment groups at any grade or grade 3 or
higher (Table III).
 | Table III.The difference in adverse events
associated with first-line and later-line groups. |
Table III.
The difference in adverse events
associated with first-line and later-line groups.
| Characteristic | First-line
group | Later-line
group | P-value |
|---|
| n | 44 | 76 |
|
| Total |
|
|
|
| Any
grade | 37 (84.1%) | 63 (82.8%) | 0.865 |
| Grade
≥3 | 15 (34.0%) | 29 (38.1%) | 0.655 |
| AST evaluation |
|
|
|
| Any
grade | 22 (50.0%) | 39 (51.3%) | 0.889 |
| Grade
≥3 | 5 (11.3%) | 11 (14.4%) | 0.625 |
| ALT evaluation |
|
|
|
| Any
grade | 14 (31.8%) | 31 (40.7%) | 0.325 |
| Grade
≥3 | 4 (9.1%) | 9 (11.8%) | 0.635 |
| Rash |
|
|
|
| Any
grade | 17 (38.6%) | 28 (36.8%) | 0.619 |
| Grade
≥3 | 5 (11.3%) | 5 (6.5%) | 0.368 |
| Fever |
|
|
|
| Any
grade | 11 (25.0%) | 16 (21.0%) | 0.521 |
| Grade
≥3 | 0 (0.0%) | 1 (1.3%) | 0.337 |
| Diarrhea |
|
|
|
| Any
grade | 12 (27.2%) | 10 (13.1%) | 0.058 |
| Grade
≥3 | 7 (15.9%) | 5 (6.6%) | 0.107 |
| Abdominal pain |
|
|
|
| Any
grade | 2 (4.5%) | 6 (7.8%) | 0.466 |
| Grade
≥3 | 1 (2.2%) | 1 (1.3%) | 0.698 |
| Pituitary or
adrenal insufficiency |
|
|
|
| Any
grade | 3 (6.8%) | 3 (3.9%) | 0.486 |
| Grade
≥3 | 3 (6.8%) | 3 (3.9%) | 0.486 |
| Drug-induced
pneumonia |
|
|
|
| Any
grade | 2 (4.5%) | 4 (5.2%) | 0.862 |
| Grade
≥3 | 1 (2.2%) | 3 (3.9%) | 0.612 |
| Pancreatitis |
|
|
|
| Any
grade | 2 (4.5%) | 1 (1.3%) | 0.274 |
| Grade
≥3 | 1 (2.2%) | 1 (1.3%) | 0.693 |
| Infusion
reaction |
|
|
|
| Any
grade | 2 (4.5%) | 2 (2.6%) | 0.580 |
| Grade
≥3 | 1 (2.2%) | 0 (0.0%) | 0.186 |
| Other irAEs |
|
|
|
| Any
grade | 4 (4.5%) | 4 (2.6%) | 0.623 |
| Grade
≥3 | 2 (4.5%) | 2 (2.6%) | 0.580 |
| Fatigue |
|
|
|
| Any
grade | 9 (20.4%) | 17 (22.3%) | 0.805 |
| Grade
≥3 | 1 (2.2%) | 4 (5.2%) | 0.402 |
| Decreased
appetite |
|
|
|
| Any
grade | 10 (22.7%) | 19 (25.0%) | 0.779 |
| Grade
≥3 | 0 (0.0%) | 1 (1.3%) | 0.429 |
| High-dose
steroid | 12 (27.2%) | 15 (19.8%) | 0.340 |
Analyses of changes in ALBI score
using durvalumab plus tremelimumab
Fig. S2 shows the
changes in ALBI scores at 4, 8, and 12 weeks from baseline after
Dur/Tre treatment. The median ALBI scores at baseline, 4, 8, and 12
weeks after introducing Dur/Tre were −2.28, −2.10, −2.14, and
−2.11, respectively. Although the deterioration of ALBI score was
significant at 4 weeks compared to baseline (−2.28 vs. −2.10, P=
0.012), there were no significant differences at 8 weeks compared
to baseline (−2.28 vs. −2.14, P=0.056). No deterioration in the
ALBI score was observed during subsequent treatment.
Decision-tree analysis for
discontinuation of durvalumab plus tremelimumab due to AEs
In this study, discontinuation due to AEs in all
subjects was 31.8% at the time of study discontinuation (Fig. 3). The Child-Pugh score was
identified as the first splitting variable for the rate of
discontinuation owing to AEs. Although the discontinuation rate due
to AEs was only 27.9% in patients in Child-Pugh class A, it was
63.2% in patients in Child-Pugh class B. The second splitting
variable was age in Child-Pugh class A patients. In patients with
Child-Pugh class A aged <70 years, the discontinuation rate due
to AEs was only 16.5%.
Comparison of safety according to Age
or Child-Pugh classification
Differences in treatment-related AEs between the age
<70 and age ≥70 groups are shown in Table IV. There was no significant
difference in each frequency of AEs between age <70 and age ≥70
patients. Also, there was no significant difference in each
frequency of AEs between Child-Pugh A and Child-Pugh B (Table V).
 | Table IV.Differences in AEs associated with
age. |
Table IV.
Differences in AEs associated with
age.
| AE | Age <70 | Age ≥70 | P-value |
|---|
| n | 38 | 82 |
|
| Total |
|
|
|
| Any
grade | 32 (84.2%) | 68 (82.9%) | 0.860 |
| Grade
≥3 | 17 (44.7%) | 27 (32.9%) | 0.211 |
| AST evaluation |
|
|
|
| Any
grade | 20 (52.6%) | 41 (50.0%) | 0.788 |
| Grade
≥3 | 7 (18.4%) | 9 (10.9%) | 0.264 |
| ALT evaluation |
|
|
|
| Any
grade | 16 (42.1%) | 29 (35.3%) | 0.478 |
| Grade
≥3 | 5 (13.1%) | 8 (9.7%) | 0.577 |
| Rash |
|
|
|
| Any
grade | 14 (36.8%) | 31 (37.8%) | 0.919 |
| Grade
≥3 | 4 (10.5%) | 6 (7.3%) | 0.554 |
| Fever |
|
|
|
| Any
grade | 10 (26.3%) | 17 (20.7%) | 0.495 |
| Grade
≥3 | 1 (2.6%) | 0 (0.0%) | 0.127 |
| Diarrhea |
|
|
|
| Any
grade | 8 (21.1%) | 14 (17.0%) | 0.600 |
| Grade
≥3 | 7 (18.4%) | 5 (6.1%) | 0.059 |
| Abdominal pain |
|
|
|
| Any
grade | 3 (7.8%) | 5 (6.1%) | 0.717 |
| Grade
≥3 | 1 (2.6%) | 1 (1.2%) | 0.574 |
| Pituitary or
adrenal insufficiency |
|
|
|
| Any
grade | 3 (7.8%) | 3 (3.6%) | 0.321 |
| Grade
≥3 | 3 (7.8%) | 3 (3.6%) | 0.321 |
| Drug-induced
pneumonia |
|
|
|
| Any
grade | 0 (0.0%) | 6 (7.3%) | 0.087 |
| Grade
≥3 | 0 (0.0%) | 4 (4.8%) | 0.161 |
| Pancreatitis |
|
|
|
| Any
grade | 2 (5.2%) | 1 (1.2%) | 0.186 |
| Grade
≥3 | 1 (2.6%) | 1 (1.2%) | 0.574 |
| Infusion
reaction |
|
|
|
| Any
grade | 3 (7.8%) | 1 (1.2%) | 0.070 |
| Grade
≥3 | 1 (2.6%) | 0 (0.0%) | 0.140 |
| Other irAEs |
|
|
|
| Any
grade | 3 (7.8%) | 5 (6.1%) | 0.717 |
| Grade
≥3 | 0 (0.0%) | 4 (4.8%) | 0.161 |
| Fatigue |
|
|
|
| Any
grade | 9 (23.6%) | 17 (20.7%) | 0.715 |
| Grade
≥3 | 2 (5.2%) | 3 (3.6%) | 0.682 |
| Decreased
appetite |
|
|
|
| Any
grade | 10 (26.3%) | 19 (22.3%) | 0.709 |
| Grade
≥3 | 1 (2.6%) | 0 (0.0%) | 0.140 |
| High-dose
steroid | 12 (31.5%) | 15 (18.2%) | 0.117 |
 | Table V.Differences in AEs associated with
Child-Pugh classification. |
Table V.
Differences in AEs associated with
Child-Pugh classification.
| AE | Child-Pugh A
(n=110) | Child-Pugh B
(n=10) | P-value |
|---|
| Total |
|
|
|
| Any
grade | 92 (83.6%) | 8 (80.0%) | 0.767 |
| Grade
≥3 | 39 (35.4%) | 5 (50.0%) | 0.360 |
| High-dose
steroid | 23 (20.9%) | 4 (40.0%) | 0.166 |
Discussion
This multicenter study showed that Dur/Tre therapy
was effective and safe for patients with u-HCC under real-world
conditions. Patients who received durvalumab plus tremelimumab as
1st-line therapy had better disease control responses than those
who received durvalumab plus tremelimumab as later lines. However,
the STRIDE regimen should be carefully administered to patients
with deteriorating hepatic function or advanced age.
The ORR, DCR, and PFS in the HIMALAYA study were
20.1%, 60.1%, and 3.78 months, respectively. Under real-world
conditions in this study, the ORR, DCR, and PFS were 15.0%, 53.3%,
and 3.7 months, respectively, similar to those observed in the
HIMALAYA study (7). In this study,
although 38.4% of patients received Dur/Tre treatment as first-line
therapy, Dur/Tre treatment presented promising results. However,
Dur/Tre treatment as first-line therapy had a better DCR than
later-line therapy. A previous study reported that patients who
received immunotherapy as first-line therapy had better clinical
outcomes than those who received immunotherapy as a later-line
therapy (18). Also, all patients
in this study who received late-line therapy received anti-VEGF
therapy including Atez/Bev during previous systemic treatment. Yang
et al previously reported that discontinuation of anti-VEGF
therapy promoted metastasis through a liver revascularization
mechanism (19). Therefore, we
cannot deny the possibility that these factors influenced the
efficacy of patients with later-line therapy. However, it has been
reported that patients who achieved DCR during Dur/Tre treatment
had longer median survival (20).
Thus, achieving DCR during immunotherapy is important for longer
survival (21). Although there was
no significant difference in PFS between the first-line and
later-line therapies in this study, this result may change if the
observation period is extended. Therefore, in the future, it will
be necessary to establish biomarkers related to treatment responses
in patients receiving Dur/Tre treatment.
Several phase 3 trials have also reported the
efficacy and safety of the STRIDE regimen in other cancers
(10,11). Currently, although patients with
higher levels of tumor PD-L1 expression received a clinical benefit
from anti-PD-L1 therapy, it has become considered that patients
with a low PD-L1 expression or PD-L1 negative are resistant to
anti-PD-L1 therapy (22). Thus, new
therapeutic strategies with immunotherapy combinations are needed
for these patients. Tremelimumab enhances the binding of CD80 and
CD86 to CD28, and it causes diversified T-cell responses and leads
to increased tumor infiltration (23,24).
Given their mechanisms, the addition of tremelimumab to a
durvalumab-based regimen is expected to overcome resistance to
PD-L1 therapy. In this study, although even with the high
prevalence of later-line, Dur/Tre therapy was effective and safe
for patients with u-HCC, we should compare to durvalumab alone
therapy to build a more robust of efficacy of Dur/Tre therapy in
real-world practice.
In this study, liver injury was the most common AE,
and irAEs caused by Dur/Tre treatment were more common than in the
clinical trial results. The higher rate of liver function
deterioration and later-line therapy included in this study
compared with clinical trials might have increased the frequency of
liver injury and other AEs. Deterioration of liver function has
been reportedly associated with a higher prevalence of AEs
(25). Moreover, patients with
prior irAEs due to ICIs are considered at risk of irAEs, even with
a different ICI (26,27). In fact, 88% (67/76) of patients who
received late-line therapy received Atez/Bev therapy during
previous systemic treatment. In immunotherapy, liver injury is
thought to be caused by the infiltration of activated T cells
(28,29). Most HCCs also develop due to chronic
liver disease or cirrhosis (30),
making them susceptible to liver injury. These factors may explain
the high rate of AEs, including liver damage. However, regarding
treatment safety with Dur/Tre, there were no significant
differences in AEs, even for the later-line cases, compared with
first-line therapy. Thus, a detailed analysis of the AEs that occur
with Dur/Tre treatment requires data accumulation and a longer
observation period.
Currently, sequential therapy involving switching
across systemic therapies is the primary evidence-based treatment
strategy for u-HCC patients (31).
Preserved hepatic function is a significant independent factor for
sequential therapy in HCC (32,33).
Furthermore, discontinuation due to AEs should be avoided during
sequential therapy (34).
Discontinuation due to AEs hampers the uptake of subsequent lines
of systemic treatment and is associated with poorer prognosis
(35). In this study, we found that
Child-Pugh Class was the initial splitting variable for
discontinuation due to AEs in patients with HCC treated with
Dur/Tre, followed by age in the decision tree analysis.
Additionally, there was no significant difference in the frequency
of AEs between Child-Pugh A and Child-Pugh B or age <70 and age
≥70 patients, it results show that Child-Pugh and age are important
factors related to discontinuation due to AEs. Particularly, it has
been reported that advanced age is an independent factor that
causes the discontinuation of AEs in systemic therapy (36). One reason for this may be that older
patients are more vulnerable to the toxicity of anticancer drugs
(37). Therefore, advanced age may
be an important factor associated with treatment discontinuation
due to AEs. However, discontinuation due to AEs in Dur/Tre
treatment remains unclear. Therefore, Clinicians should be vigilant
in monitoring AEs during treatment with Dur/Tre. Further studies
are needed to establish a grading system, to predict
discontinuation due to AEs.
This study had several limitations. First, this was
a retrospective study. Second, although this was a multicenter
study, the treatment observation period was relatively short and
could not evaluate OS. Despite its limitations, to our knowledge,
this study is the first to evaluate the efficacy and safety of
Dur/Tre for the treatment of unresectable HCC in real-world
clinical practice. Additional studies with larger sample sizes and
longer observation periods are needed to further evaluate the
efficacy and safety of Dur/Tre.
In conclusion, we demonstrated that patients who
received durvalumab plus tremelimumab as first-line therapy had a
better tumor response than those who received durvalumab plus
tremelimumab in later lines. Although there were no significant
differences in AEs between the first- and later-line groups, this
regimen should be carefully administered to patients with
deteriorating hepatic function or advanced age to prevent
discontinuation due to AEs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SS participated in study conception and design, data
acquisition, data interpretation and manuscript drafting. SS and IS
confirm the authenticity of all the raw data. IS, TeTo, TI, JT, YT,
NY, TN, MT, SK, TH, KS, TeY, MS, HI, SI, TS, NT, TaY and AN
participated in data acquisition. SN, MO, HK, TeTa, TaTa and TK
participated in data analysis, data interpretation, and manuscript
drafting. All authors participated in the critical revision of the
manuscript, and read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Kurume University School of Medicine (approval code: 23153),
and an implementation permit was obtained from each of the 15 other
institutions. An opt-out approach was used to obtain informed
consent from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALBI
|
albumin-bilirubin
|
|
DCR
|
disease control rate
|
|
Dur/Tre
|
durvalumab plus tremelimumab
|
|
HCC
|
hepatocellular carcinoma
|
|
ICIs
|
immune checkpoint inhibitors
|
|
irAEs
|
immune-related adverse events
|
|
ORR
|
objective response rate
|
|
PFS
|
progression-free survival
|
|
STRIDE
|
Single Tremelimumab Regular Interval
Durvalumab
|
References
|
1
|
Rumgay H, Arnold M, Ferlay J, Lesi O,
Cabasag CJ, Vignat J, Laversanne M, McGlynn KA and Soerjomataram I:
Global burden of primary liver cancer in 2020 and predictions to
2040. J Hepatol. 77:1598–1606. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vogel A, Meyer T, Sapisochin G, Salem R
and Saborowski A: Hepatocellular carcinoma. Lancet. 400:1345–1362.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo M: Recent advances in systemic
therapy for hepatocellular carcinoma in an aging society: 2020
update. Liver Cancer. 9:640–662. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rimassa L, Finn RS and Sangro B:
Combination immunotherapy for hepatocellular carcinoma. J Hepatol.
79:506–515. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singal AG, Llovet JM, Yarchoan M, Mehta N,
Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA,
et al: AASLD practice guidance on prevention, diagnosis, and
treatment of hepatocellular carcinoma. Hepatology. 78:1922–1965.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abou-Alfa GK, Lau G, Kudo M, Chan SL,
Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Van Dao T, De
Toni EN, et al: Tremelimumab plus durvalumab in unresectable
hepatocellular carcinoma. NEJM Evid. 1:EVIDoa21000702022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abou-Alfa GK, Lau G, Kudo M, Chan SL,
Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Dao TV, De Toni
EN, et al: Plain language summary of the HIMALAYA study:
Tremelimumab and durvalumab for unresectable hepatocellular
carcinoma (liver cancer). Future Oncol. 19:2505–2516. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson ML, Cho BC, Luft A,
Alatorre-Alexander J, Geater SL, Laktionov K, Kim SW, Ursol G,
Hussein M, Lim FL, et al: Durvalumab with or without tremelimumab
in combination with chemotherapy as first-line therapy for
metastatic non-small-cell lung cancer: The phase III POSEIDON
study. J Clin Oncol. 41:1213–1227. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Psyrri A, Fayette J, Harrington K,
Gillison M, Ahn MJ, Takahashi S, Weiss J, Machiels JP, Baxi S,
Vasilyev A, et al: Durvalumab with or without tremelimumab versus
the EXTREME regimen as first-line treatment for recurrent or
metastatic squamous cell carcinoma of the head and neck: KESTREL, a
randomized, open-label, phase III study. Ann Oncol. 34:262–274.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Horvat TZ, Adel NG, Dang TO, Momtaz P,
Postow MA, Callahan MK, Carvajal RD, Dickson MA, D'Angelo SP, Woo
KM, et al: Immune-related adverse events, need for systemic
immunosuppression, and effects on survival and time to treatment
failure in patients with melanoma treated with ipilimumab at
memorial sloan kettering cancer center. J Clin Oncol. 33:3193–3198.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cholongitas E, Papatheodoridis GV, Vangeli
M, Terreni N, Patch D and Burroughs AK: Systematic review: The
model for end-stage liver disease-should it replace Child-Pugh's
classification for assessing prognosis in cirrhosis? Aliment
Pharmacol Ther. 22:1079–1089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson PJ, Berhane S, Kagebayashi C,
Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A,
Palmer D, et al: Assessment of liver function in patients with
hepatocellular carcinoma: A new evidence-based approach-the ALBI
grade. J Clin Oncol. 33:550–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hatanaka T, Kakizaki S, Hiraoka A, Tada T,
Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi
E, et al: Comparing the impact of atezolizumab plus bevacizumab and
lenvatinib on the liver function in hepatocellular carcinoma
patients: A mixed-effects regression model approach. Cancer Med.
12:21680–21693. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayakawa Y, Tsuchiya K, Kurosaki M, Yasui
Y, Kaneko S, Tanaka Y, Ishido S, Inada K, Kirino S, Yamashita K, et
al: Early experience of atezolizumab plus bevacizumab therapy in
Japanese patients with unresectable hepatocellular carcinoma in
real-world practice. Invest New Drugs. 40:392–402. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Zhang Y, Iwamoto H, Hosaka K, Seki
T, Andersson P, Lim S, Fischer C, Nakamura M, Abe M, et al:
Discontinuation of anti-VEGF cancer therapy promotes metastasis
through a liver revascularization mechanism. Nat Commun.
7:126802016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sangro B, Chan SL, Kelly RK, Lau G, Kudo
M, Sukeepaisarnjaroen W, Yarchoan M, De Toni EN, Furuse J, Kang YK,
et al: Four-year overall survival update from the phase 3 HIMALAYA
study of tremelimumab plus durvalumab in unresectable
hepatocellular carcinoma. Ann Oncol. 35:448–457. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanabe N, Saeki I, Aibe Y, Matsuda T,
Hanazono T, Nishi M, Hidaka I, Kuwashiro S, Shiratsuki S, Matsuura
K, et al: Early prediction of response focused on tumor markers in
atezolizumab plus bevacizumab therapy for hepatocellular carcinoma.
Cancers (Basel). 15:29272023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waterhouse D, Lam J, Betts KA, Yin L, Gao
S, Yuan Y, Hartman J, Rao S, Lubinga S and Stenehjem D: Real-world
outcomes of immunotherapy-based regimens in first-line advanced
non-small cell lung cancer. Lung Cancer. 156:41–49. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
pathways: Similarities, differences, and implications of their
inhibition. Am J Clin Oncol. 39:98–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robert L, Tsoi J, Wang X, Emerson R, Homet
B, Chodon T, Mok S, Huang RR, Cochran AJ, Comin-Anduix B, et al:
CTLA4 blockade broadens the peripheral T-cell receptor repertoire.
Clin Cancer Res. 20:2424–2432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimose S, Hiraoka A, Tanaka M, Iwamoto H,
Tanaka T, Noguchi K, Aino H, Yamaguchi T, Itano S, Suga H, et al:
Deterioration of liver function and aging disturb sequential
systemic therapy for unresectable hepatocellular carcinoma. Sci
Rep. 12:170182022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delire B, De Martin E, Meunier L, Larrey D
and Horsmans Y: Immunotherapy and gene therapy: New challenges in
the diagnosis and management of drug-induced liver injury. Front
Pharmacol. 12:7861742021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ito T, Ishigami M, Yamamoto T, Mizuno K,
Yamamoto K, Imai N, Ishizu Y, Honda T, Kawashima H, Yasuda S, et
al: Clinical course of liver injury induced by immune checkpoint
inhibitors in patients with advanced malignancies. Hepatol Int.
15:1278–1287. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dudek M, Pfister D, Donakonda S, Filpe P,
Schneider A, Laschinger M, Hartmann D, Hüser N, Meiser P, Bayerl F,
et al: Auto-aggressive CXCR6+ CD8 T cells cause liver
immune pathology in NASH. Nature. 592:444–449. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pfister D, Núñez NG, Pinyol R, Govaere O,
Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, et
al: NASH limits anti-tumour surveillance in immunotherapy-treated
HCC. Nature. 592:450–456. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tani J, Senoh T, Moriya A, Ogawa C,
Deguchi A, Sakamoto T, Takuma K, Nakahara M, Oura K, Tadokoro T, et
al: Long-term outcomes and evaluation of hepatocellular carcinoma
recurrence after hepatitis C virus eradication by direct-acting
antiviral treatment: All kagawa liver disease group (AKLDG) study.
Cancers (Basel). 13:22572021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Persano M, Rimini M, Tada T, Suda G,
Shimose S, Kudo M, Cheon J, Finkelmeier F, Lim HY, Presa J, et al:
Sequential therapies after atezolizumab plus bevacizumab or
lenvatinib first-line treatments in hepatocellular carcinoma
patients. Eur J Cancer. 189:1129332023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takeuchi Y, Nouso K, Fujioka SI, Kariyama
K, Kobashi H, Uematsu S, Moriya A, Hagihara H, Takabatake H,
Nakamura S, et al: The prediction of early progressive disease in
patients with hepatocellular carcinoma receiving atezolizumab plus
bevacizumab. Cancer Med. 12:17559–17568. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tomonari T, Tani J, Sato Y, Tanaka H,
Tanaka T, Taniguchi T, Asahiro M, Okamoto K, Sogabe M, Miyamoto H,
et al: Initial therapeutic results of atezolizumab plus bevacizumab
for unresectable advanced hepatocellular carcinoma and the
importance of hepatic functional reserve. Cancer Med. 12:2646–2657.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hatanaka T, Kakizaki S, Hiraoka A, Tada T,
Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi
E, et al: Comparative analysis of the therapeutic outcomes of
atezolizumab plus bevacizumab and lenvatinib for hepatocellular
carcinoma patients aged 80 years and older: Multicenter study.
Hepatol Res. 54:382–391. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iavarone M, Cabibbo G, Biolato M, Corte
CD, Maida M, Barbara M, Basso M, Vavassori S, Craxì A, Grieco A, et
al: Predictors of survival in patients with advanced hepatocellular
carcinoma who permanently discontinued sorafenib. Hepatology.
62:784–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimose S, Iwamoto H, Niizeki T, Shirono
T, Noda Y, Kamachi N, Okamura S, Nakano M, Suga H, Kuromatsu R, et
al: Clinical significance of adverse events for patients with
unresectable hepatocellular carcinoma treated with lenvatinib: A
multicenter retrospective study. Cancers (Basel). 12:18672020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dale W, Mohile SG, Eldadah BA, Trimble EL,
Schilsky RL, Cohen HJ, Muss HB, Schmader KE, Ferrell B, Extermann
M, et al: Biological, clinical, and psychosocial correlates at the
interface of cancer and aging research. J Natl Cancer Inst.
104:581–589. 2012. View Article : Google Scholar : PubMed/NCBI
|