Introduction
The term pituitary apoplexy (PA) was initially
introduced by Brougham in 1950 (1),
defined as an emergency condition caused by hemorrhage or
infarction of the pituitary gland. However, PA can be generally
neglected in 25% of pituitary tumors and there may only be
radiological or histopathological evidence of infarction and/or
hemorrhage without any clinical manifestation (2). In the present article, PA refers to
clinically diagnosed PA with classical symptoms.
The prevalence of PA reported in different studies
ranges from 0.6 to 7% (3–7), suggesting that numerous cases were
undiagnosed and did not receive any clinical attention (8). While the pathophysiology of PA remains
elusive, several risk factors have been identified, such as
fluctuation of blood pressure (BP), use of anticoagulant drugs,
major surgeries, pregnancy and pituitary function test (9–11).
Clinical symptoms vary from person to person, commonly manifesting
as sudden onset of headache, visual field defect, diplopia,
ophthalmoplegia, decreased consciousness, increased urine volume,
nausea and vomiting (12–14). Dysfunctions in the
hypothalamic-pituitary hormone axis can result in a lack of various
pituitary-related hormones, which may lead to physiological
disorders in several ways (Fig. 1).
Hypothyroidism and adrenal insufficiency secondary to PA may weaken
the body's tolerance to surgical trauma. Glucocorticoid and thyroid
hormones both have a role in post-operative stress. Glucocorticoids
help maintain BP and blood sugar, facilitate fat mobilization,
combat cellular damage and suppress inflammatory responses
(15), and thyroid hormones can
speed up the metabolism and increase peripheral cells' utilization
of glucose (16). The choice of
treatment between hormonal replacement therapy and trans-sphenoidal
resection is determined based on the severity of neuro-ophthalmic
symptoms and the patient's capacity to undergo a second surgery
(2).
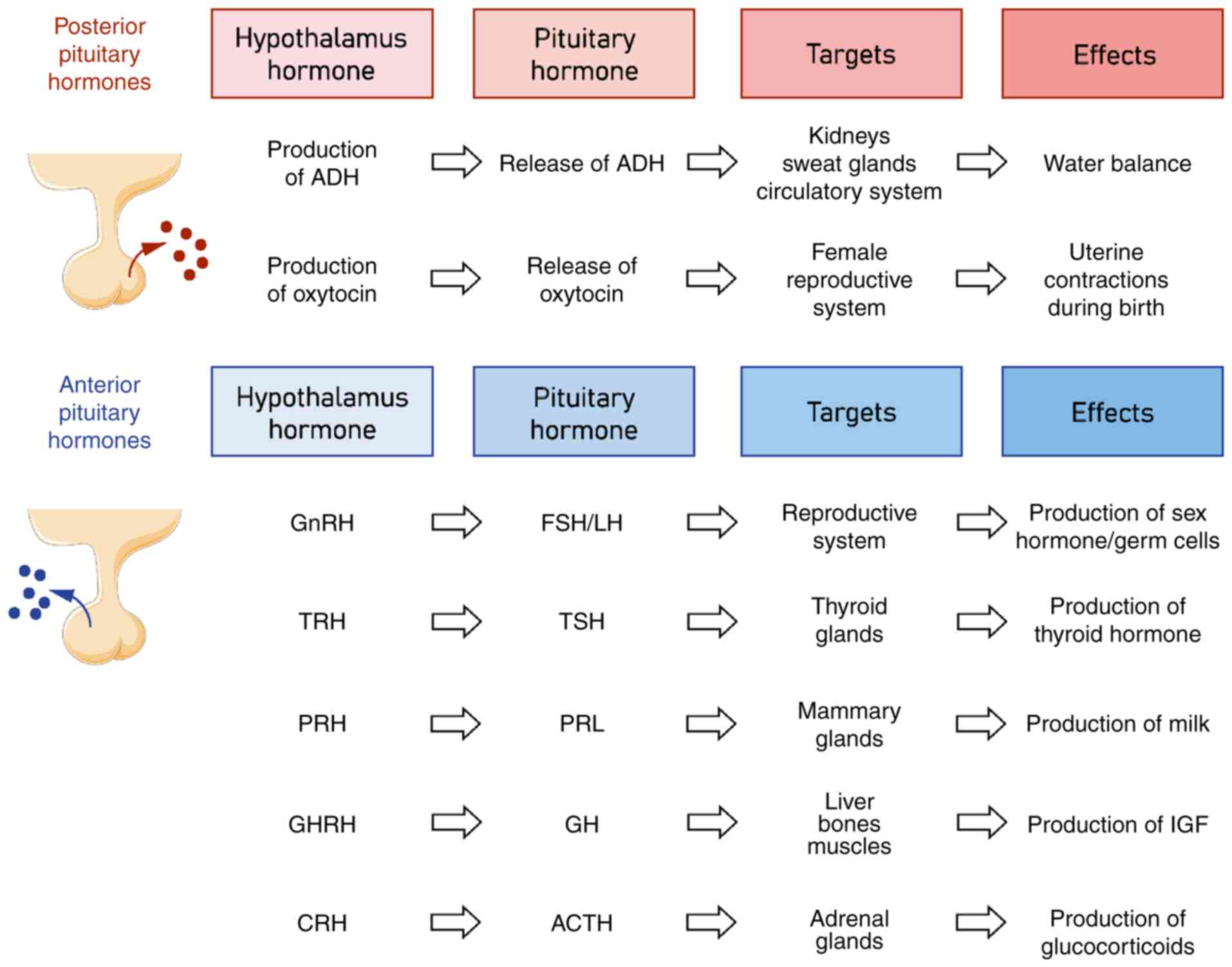 | Figure 1.Hypothalamic-pituitary hormone axis
and its main functions. ACTH, adrenocorticotropic hormone; ADH,
antidiuretic hormone; CRH, corticotropin-releasing hormone; FSH,
follicle-stimulating hormone; GH, growth hormone; GHRH, growth
hormone releasing hormone; GnRH, gonadotropin-releasing hormone;
LH, luteinizing hormone; PRH, prolactin releasing hormone; PRL,
prolactin; TRH, thyrotropin-releasing hormone; TSH, thyroid
stimulating hormone. |
However, presenting as the initial sign of unknown
pituitary tumors, it can be a challenging task to diagnose PA
post-surgery due to its symptomatic overlap with postoperative
complications. It is a rare postoperative complication that may
have severe consequences if not treated timely and properly. Due to
the lack of reviews on PA after surgery, the current article
presented a clinical case and summed up the characteristics of
relevant cases published over the years.
Case report
A 64-year-old female with a history of subtotal
hysterectomy 20 years prior presented with vaginal bleeding
persisting for three months and was admitted in Shengjing Hospital
of China Medical University (Shenyang, China) in March 2023. The
patient's body mass index was 24.2 kg/m2 (body height,
164 cm; body weight, 65 kg). The obstetric history included two
pregnancies-one ending in abortion and the other in a vaginal
birth. At the age of 44 years, the patient underwent a subtotal
hysterectomy and left adnexectomy due to multiple uterine
leiomyomas and a lateral ovarian cyst, with postoperative pathology
confirming benignity. Irregular human papillomavirus (HPV) and
thinprep cytology test (TCT) screening were conducted post-surgery,
and the last screening was 2 years prior and the results remained
negative. After undergoing minor but persistent vaginal bleeding
for 3 months, the patient tested HPV-16 positive and negative for
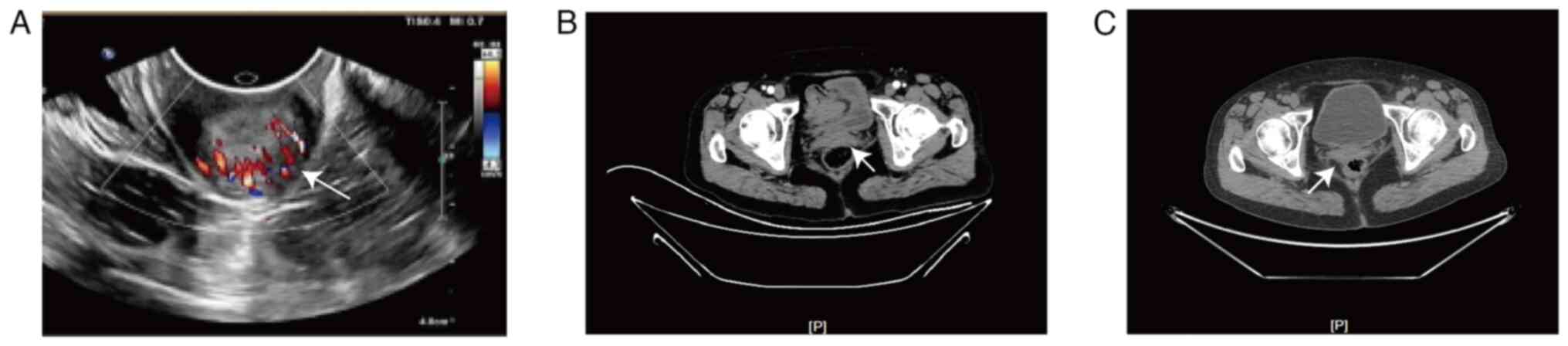
intraepithelial lesion or malignancy on TCT. The pelvic ultrasound,
computerized tomography (CT) and positron emission
tomography-computed tomography (PET-CT) identified a moderately
hyperechoic mass in the cervical stump region, which was highly
suggestive of cancer (Fig. 2).
Gynecological examination revealed normal vulvar development with
signs of aging and a smooth vaginal canal. An exogenous lesion with
a diameter of ~2.5 cm was observed at the stump of the cervix,
exhibiting lesion contact bleeding. The anterior fornix was shallow
and the pelvic cavity was with no obvious abnormalities in the
adnexal areas. The patient reported no comorbidities, aside from a
sulfonamide allergy. General examination was unremarkable, except
for admission BP of 148/96 mmHg. According to the latest guideline
of hypertension in China (17),
hypertension was defined as systolic BP ≥140 mmHg/or diastolic BP
≥90 mmHg. The patient's BP was monitored and a cardiologist was
consulted. During hospitalization, the patient's BP was stable,
ranging from 120/80 to 140/90 mmHg. The patient cooperated well in
the physical examination. The bilateral pupils were equally large
and round with a diameter of 3 mm and had no limitation of eye
movement or visual field defect. The patient exhibited full
mobility in all four limbs with normal muscle force and strength
and neither had a history of pituitary adenoma nor manifested any
related symptoms. The patient underwent tissue biopsy and cervical
stump adenocarcinoma was diagnosed. After comprehensive
pre-operative evaluations, including PET-CT, the patient underwent
open extensive stump cervicectomy, pelvic lymph node dissection and
transcystoscopic bilateral ureteral stenting. Pelvic drainage and
vaginal drainage were used. The surgery proceeded smoothly with an
intraoperative bleeding volume of 100 ml. Intraoperative anesthesia
and medication details are provided in Fig. S1 [Illustrator 27.7 (Adobe, Inc.)
was used to generate the translated version of this image]. The
patient's BP remained stable during the operation and no
hypotension was detected prior to or after the surgery. After the
gynecological operation, the patient was treated with intravenous
cefazolin sodium (Sinopharm CNBG Zhongnuo Pharmaceuticals Co.,
Ltd.) 0.5 g per 8 h, intramuscular enoxaparin sodium [Sanofi
Aventis (Beijing) Pharmaceutical Co., Ltd.] 40 mg per day,
intravenous methylprednisolone (Pharmacia & Upjohn Co., Ltd.)
20 mg per day and other supportive care (methylprednisolone is
administered as a common treatment at the Enhanced Recovery After
Surgery ward to alleviate peri-operative stress and inflammation).
From postoperative day 1 (POD1), the patient complained of the
sudden onset of a headache [pain visual analogue scale (VAS) score
(18), 4/10] and drowsiness. Since
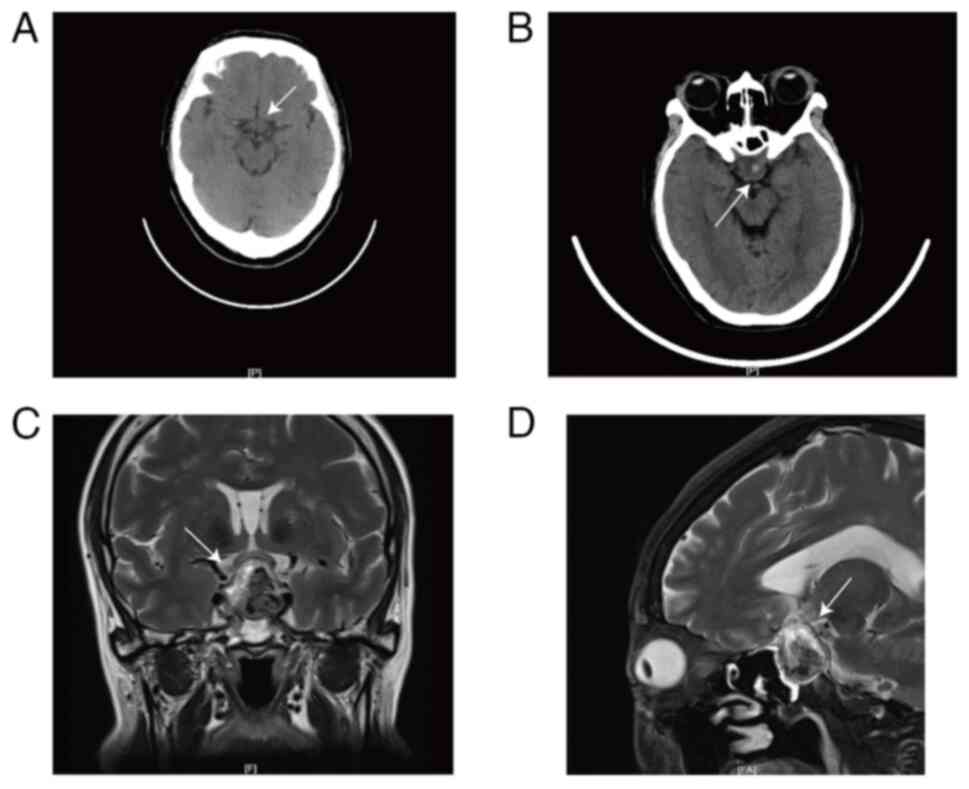
the pre-operative PET-CT did not indicate any pituitary tumor
(Fig. 3A), it was inferred that the
patient's symptoms may be due to general anesthesia and the
postoperative analgesia pump and the pump was turned off
immediately. On POD2, the patient still reported headaches and a
neurosurgery consultation was started. The neurosurgery doctor
suggested recording a head CT but the patient perceived her
headache to be of mild severity and signed to refuse relevant
tests. On POD3, the patient's BP fluctuated between 120/70 and
145/85 mmHg. It was not until the patient's headache worsened (pain
VAS 7/10) and a new complaint of blurred vision and blepharoptosis
of the left eye occurred on POD4 that she consented to further
examination. The patient's Glasgow coma scale score was 3-5-6
points (19). A head CT scan and an
ophthalmic consultation were carried out immediately, revealing
multiple lacunar infarctions and local density increases in the
sella turcica and suprasellar regions (Fig. 3B). Enhanced pituitary MRI showed a
2.4×1.7×2.6 cm occupation in the sellar area with a heterogeneous
signal, indicating a pituitary macroadenoma with apoplexy (Fig. 3C and D). Ophthalmic assessments
showed bitemporal hemianopsia and abnormal findings in the fundus
photography and visual pathway images (data not shown). Laboratory
tests indicated panhypopituitarism (Table I). The patient was promptly
transferred to the neurosurgery ward. Considering the visual field
defect was stable, there was no indication of an emergency surgery
and hydrocortisone (Tianjin Jinyao Amino Acid Co., Ltd.; 100
mg/day) replacement therapy was used to complete enhanced head CT
and arterial angiography, and transsphenoidal hypophysical lesion
resection through the neuroendoscope under general anesthesia was
carried out successfully on POD12. After the neurosurgery
operation, the patient took desmopressin acetate tablets [Huilin
(Sweden) Pharmaceuticals Co., Ltd.] 0.1 mg to treat postoperative
diabetes insipidus, sustained-release potassium tablets (Shenzhen
Zhonglian Pharmaceutical Co., Ltd.) 1 g three times per day to
treat hypokalemia and prednisolone acetate tablets (Tianjin Tianyao
Pharmaceuticals Co., Ltd.) 10 mg at 8:00 a.m. and 5 mg at 4:00 p.m.
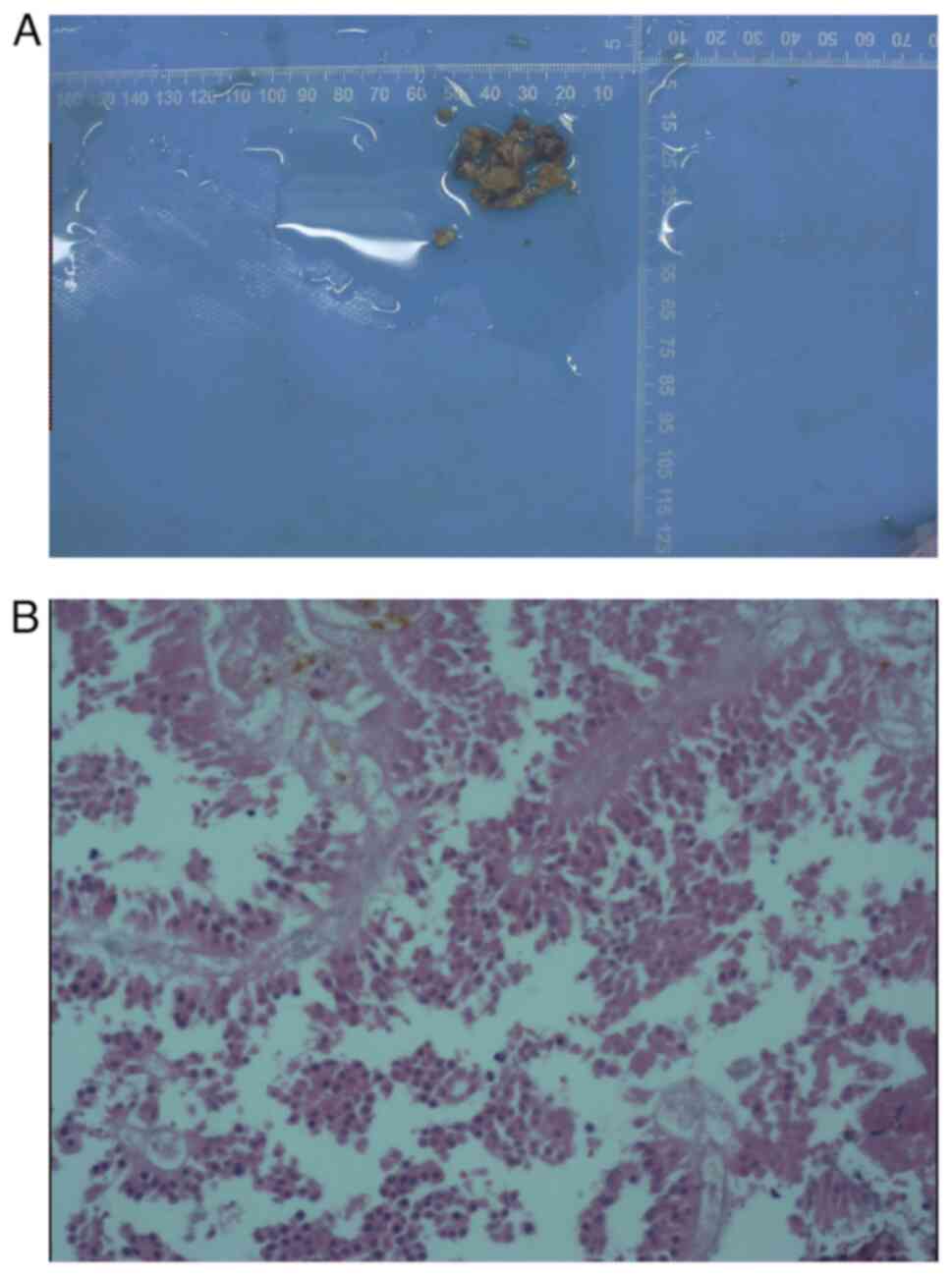
each day. Postoperative pathology was performed by reticulin fiber
staining with kit no. G3535 from Solarbio Science and Technology
(Beijing) Co., Ltd (20), and it
indicated hemorrhage of pituitary tumor (Fig. 4). The patient reported complete
resolution of headaches, bitemporal hemianopia and visual field
improvement the day after the operation and was discharged from the
hospital a week later. The patient reported mild blurry vision
during the follow-up of six weeks after the neurosurgery operation.
After discharge, the patient underwent follow-up every three
months, and her hormone levels completely returned to normal. The
patient was advised to undergo hormone level assessments and
pituitary imaging every six months, with continued lifelong
follow-up.
 | Table I.Hormone and ion levels of the patient
at different stages. |
Table I.
Hormone and ion levels of the patient
at different stages.
| Time-point | K+,
mmol/l | Na+,
mmol/l | FT3, pmol/l | FT4, pmol/l | TSH, µmol/l | ACTH-8:00 a.m.,
pg/ml | Cortisol-8:00 a.m.,
µg/dl | FSH, µIU/ml | LH, µIU/ml | Prolactin,
ng/ml |
|---|
| Pre-operation | 3.78 | 139 | 4 | 9.42 | 1.75 | 16.74 | 15.6 | 23.45 | 6.09 | 38.28 |
| Between two
operations | 3.27↓ | 135↓ | 2.48 | 6.81↓ | 0.19↓ | 3.83↓ | 5.63↓ | 3.77↓ | <0.2↓ | 1.13↓ |
| Post-operation | 3.24↓ | 137 | <1.54↓ | 8.48↓ | 0.2↓ | 2.25↓ | 11.94 | 2.49↓ | <0.2↓ | 1.57↓ |
| 6 weeks after the
operation | 4.49 | 141 | 3.24 | 12.22 | 0.9 | 11.9 | 10.11 | 2.41↓ | 0.62↓ | 3.75 |
| Normal ranges | 3.5–5.5 | 136-145 | 2.43–6.01 | 9.01–19.05 | 0.3–4.8 | 7.2–66.3 | 6.02–18.4 | 6.74–113.59 | 10.87–58.64 | 2.74–19.64 |
Discussion
PA is a rare postoperative complication that may be
life-threatening if not diagnosed and treated properly. It is
usually caused by a sudden ischemic or hemorrhagic infarction of a
preexisting pituitary adenoma, while only pituitary apoplexy rarely
occurs in normal pituitary glands. Bonicki et al (3) reported that PA occurs in 5% of
patients with pituitary adenomas; however, >40% of PA cases have
never been diagnosed with a pituitary tumor prior to onset
(21). PA is related to a variety
of inducible factors, but its exact pathogenesis remains elusive.
Due to the specific features of the pituitary vascular system,
pituitary tissue is more susceptible to hypoperfusion, ischemia and
intraoperative embolism, particularly during pump-on surgery.
During the literature review for the current study, it was found
that predisposing factors of PA included not only transient
hypertension or hypotension, but also diabetes, angiographic test,
cardiac surgery, hemodialysis, pituitary dynamic function test,
radiation therapy, positive pressure mechanical ventilation and
anticoagulant therapy (22).
To the best of our knowledge, the present study was
the first case report of PA after gynecological malignancy. Since
the pre-operative PET-CT did not indicate any pituitary tumor,
postoperative symptoms such as headache, visual field defect,
ptosis and hypopituitarism were confused with common postoperative
complications and PA was not detected in the initial stage. This
may be for the following two primary reasons. First, PET-CT lacks
specificity and sensitivity in the hypothalamic-pituitary region,
potentially resulting in undetected pituitary microadenomas.
Furthermore, the pituitary adenoma became enlarged due to
hemorrhage post-surgery, thereby facilitating detection. However,
headaches likely occurred due to extravasation of blood into the
subarachnoid space, causing meningeal irritation (22). Bitemporal hemianopia is the most
common type of visual field defect caused by a pituitary tumor,
which occurs due to the PA pressing on the middle of the optic
chiasma (14). Ptosis presented as
a result of oculomotor nerve compression and hypopituitarism was a
sign of pituitary dysfunction. Besides, severe hypoglycemia and
hyponatremia may occur due to a reduced glucocorticoid effect
because of low cortisol response, as well as water overload caused
by adrenocorticotropic hormone deficiency.
A total of four primary factors contributing to the
occurrence of PA were identified for the present case. First,
general anesthesia carries a greater risk of low BP than local
anesthesia. General anesthesia may also lead to reduced cerebral
perfusion. Second, surgery can cause intravascular fluid to spread
into the interstitial space, leading to edema and a drop in BP.
Third, blood loss may also be a contributing factor. Although only
100 ml of blood loss was stated in the surgical record, the patient
underwent a major resection and lymph node dissection with
postoperative nausea and vomiting, which indicated the possibility
of local bleeding after abdominal closure and blood loss may be
difficult to estimate accurately. Fourth, anticoagulant therapy is
another risk factor, as it increases the risk of bleeding from
damaged pituitary tissue.
PA can also occur in various types of surgery,
particularly in surgery of the circulatory system. A literature
search was conducted through PubMed, using ‘pituitary apoplexy’ and
‘surgery or operation’ as key terms to identify relevant articles
published between 1984 and 2023. The search was limited to articles
in English and only studies with sufficient information were
included in the literature review (Table II). Based on the literature review,
PA after surgery mostly occurred in males (76%), with an average
age of 53 years for women and 68 years for men. Only 8% of cases
had known pituitary disease (23,24).
Clinical symptoms usually occur on the operation day or on POD1
(72%) and headache (76%) was the main and the earliest complaint in
most cases. This symptom was possibly triggered by dural stretching
and meningeal irritation caused by extravasation of blood and
necrotic tissue into the subarachnoid space (25). Further examination of the literature
indicated that visual disturbances were mentioned in 64% (visual
deterioration in 24%, diplopia in 24%, visual defects in 20% and
loss of light reflex in 20%), which was caused by pressure on
different parts of the optic nerve and oculomotor nerve involvement
may present as ptosis (44%). In addition, adrenal insufficiency may
reduce the level and the efficiency of glucocorticoids and
eventually cause arterial hypotension and/or hypoglycemia, as well
as varying degrees of consciousness change, which was noted in 12%
of cases in the present review.
 | Table II.Summary of information on PA cases
during and after surgery from the literature review and the present
case. |
Table II.
Summary of information on PA cases
during and after surgery from the literature review and the present
case.
| Authors, year | Age, years/sex | Prior lesion | Operation
method | Clinical
presentation | Onset time | Pituitary imaging
MRI/CT | Potential risk
factors | Treatment hormone
replacement therapy | Surgery | Prognosis | (Refs.) |
|---|
| Mura et
al, | 85/male | – | Laparoscopic | Left palpebral | During the | CT: Pituitary
gland | Preoperative | Dexamethasone | – | – | (34) |
| 2014 |
|
| colorectal | ptosis, | operation | increase; | anticoagulant | 4 mg ×2/day |
|
|
|
|
|
|
| resection | anisocoria, |
| MRI: Pituitary
gland | therapy, |
|
|
|
|
|
|
|
|
| divergent |
| increase with | intraoperative |
|
|
|
|
|
|
|
|
| strabismus, |
| hematoma | BP fluctuation |
|
|
|
|
|
|
|
|
| mydriasis |
|
|
|
|
|
|
|
|
|
|
|
| without photo- |
|
|
|
|
|
|
|
|
|
|
|
| motor reflex |
|
|
|
|
|
|
|
| McClain et
al, | 75/female | – | Elective | Headache, | POD1 | CT: A large
sellar | History of | – | Urgent | Ptosis and | (35) |
| 2022 |
|
| rotator | vomiting, |
| mass; | essential |
|
transsphenoidal | ophthal- |
|
|
|
|
| cuff repair | diplopia, |
| MRI: A sellar
and | hypertension |
| endoscopic | moplegia |
|
|
|
|
|
| inability to |
| suprasellar
mass |
|
| resection of
the | completely |
|
|
|
|
|
| open the |
| effect
compressing |
|
| pituitary mass | recovered |
|
|
|
|
|
| right eye |
| the optic
chiasm |
|
|
| and visual |
|
|
|
|
|
|
|
|
|
|
|
| field deficits |
|
|
|
|
|
|
|
|
|
|
|
| stabilized |
|
| Liberale et
al, | 73/male | – | Subrenal | Diplopia,
right | On the | MRI: A large
sellar | Preoperative | Cortisone |
Transsphenoidal | Partially | (36) |
| 2006 |
|
| aortic | palpebral | operation | mass
compressing | anticoagulant | acetate 50 mg/ | adenectomy | recovered the |
|
|
|
|
| abdominal | ptosis, | day | back carotid
arteries | therapy | morning and |
| right third |
|
|
|
|
| aneurysm | mydriasis, |
| and the
optical |
| 25 mg/evening, |
| oculomotor |
|
|
|
|
| repair by | divergent |
| chiasma |
| sodique |
| palsy and |
|
|
|
|
| subcostal | strabism |
|
|
| levothyroxin, |
| remained |
|
|
|
|
| bilateral |
|
|
|
| 50 g/day |
| stable during |
|
|
|
|
| laparotomy |
|
|
|
|
|
| the 4-year |
|
|
|
|
|
|
|
|
|
|
|
| follow-up |
|
| Naito et
al, | 14/female | – | Recurrent | Headache | POD1 | CT: A tumor | Hemodynamic | l-T4 treatment | – | Improved | (37) |
| 2019 |
|
| cardiac | and visual |
| surrounding
the | instability | for 3 months |
| visual field |
|
|
|
|
| myxoma | impairment |
|
hypothalamopituitary | during
surgery, |
|
| of both eyes |
|
|
|
|
| resection |
|
| lesion; | use of |
|
| before |
|
|
|
|
| surgery |
|
| MRI: An intra-
and | anticoagulant |
|
| discharge |
|
|
|
|
|
|
|
| supra-sellar
tumor |
|
|
|
|
|
|
|
|
|
|
|
| compressing
optic |
|
|
|
|
|
|
|
|
|
|
|
| chiasma and
bilateral |
|
|
|
|
|
|
|
|
|
|
|
| optic nerves |
|
|
|
|
|
| Hidiroglu et
al, | 47/male | – | Coronary | Ptosis of both | POD2 | CT: A solid
mass | – | – |
Transsphenoidal | – | (38) |
| 2010 |
|
| artery bypass | eyes, headache |
| compressing the
optic |
|
| adenectomy |
|
|
|
|
|
| grafting |
|
| chiasm |
|
|
|
|
|
|
|
|
| operation |
|
|
|
|
|
|
|
|
| Yakupoglu et
al, | 74/male | – | Open three- | Right ophtha- | 6 h after | CT: A mass on
the | Hemodynamic | Intravenous | Transcranial | Full recovery | (39) |
|
2010 |
|
| vessel | lmoplegia with | the | pituitary
gland; | changes | hydrocortisone | adenoma | of ptosis, |
|
|
|
|
| CABG and | ptosis, right | operation | MRI: Pituitary |
| 50 mg/day,
oral | excision | visual field |
|
|
|
|
| insertion of | mydriasis, |
| macroadenoma
with |
| levothyroxine |
| deficits and |
|
|
|
|
| a saphenous | headache |
| haemorrhage
and |
| 0.2 mg/day |
| mental |
|
|
|
|
| vein graft |
|
| infarction |
|
|
| changes |
|
|
|
|
|
|
|
|
|
|
|
| within |
|
|
|
|
|
|
|
|
|
|
|
| 2 weeks of |
|
|
|
|
|
|
|
|
|
|
|
| surgery |
|
| Yoshino et
al, | 78/male | – | Right upper | Headache, | POD6 | MRI: A high- | – | Hydrocortisone | – | Complete | (40) |
| 2014 |
|
| and middle | sudden |
| intensity area
inside |
| 200 mg/day |
| recovery |
|
|
|
|
| lobectomy | increase in |
| the pituitary
gland |
|
|
|
|
|
|
|
|
| and lymph | urine volume |
|
|
|
|
|
|
|
|
|
|
| node |
|
|
|
|
|
|
|
|
|
|
|
| dissection |
|
|
|
|
|
|
|
|
| Joo et al,
2018 | 73/male | – | Lumbar | Severe | POD2 | CT and MRI: | Intraoperative | Hydrocortisone |
Transsphenoidal | Improved | (41) |
|
|
|
| fusion | headache, |
| A mass in the
sellar | BP fluctuation | 300 mg/day | hypophysectomy | ptosis and |
|
|
|
|
| surgery | ophthalmalgia |
| fossa and
suprasellar |
|
|
| anisocoria |
|
|
|
|
| in prone | and ptosis |
| region,
compressing |
|
|
|
|
|
|
|
|
| position | on right eye |
| the optic
chiasm |
|
|
|
|
|
| Goel et al,
2009 | 76/male | – | Elective left | Sudden | POD1 | CT: A mass in
the | Transient | Dexamethasone | Transnasal | Complete | (42) |
|
|
|
| total hip | headache, |
| left pituitary
fossa; | episode of | 2 mg/6 h | transphenoidal | recovery |
|
|
|
|
| athroplasty | total left
vision |
| MRI: Intersellar
mass | hypotension in |
| decompression |
|
|
|
|
|
|
| loss and |
| with a
suprasellar | the
postoperative |
| of the
pituitary |
|
|
|
|
|
|
| temporal right |
| extension on
the | period |
| tumor |
|
|
|
|
|
|
| hemianopia |
| left side,
compressing |
|
|
|
|
|
|
|
|
|
|
|
| the optic
chiasma |
|
|
|
|
|
|
|
|
|
|
|
| and cavernous
sinus |
|
|
|
|
|
| Goel et al,
2009 | 61/male | – | Elective left | Sudden | POD1 | CT: An
intersellar | Microembolism | High-dose | Craniotomy and | Complete | (42) |
|
|
|
| total knee | headache, |
| tumor |
| dexamethasone | decompression | recovery |
|
|
|
|
| athroplasty | nausea, |
|
|
| intravenously | of pituitary |
|
|
|
|
|
|
| vomiting, |
|
|
|
| adenoma |
|
|
|
|
|
|
| right ptosis |
|
|
|
|
|
|
|
| Kim et al,
2015 | 69/male | – | Open heart | Severe | After the | MRI: A sellar
mass | Excessive | High-dose |
Transsphenoidal | Complete | (43) |
|
|
|
| mitral | headache, | operation | with
hemorrhage |
anticoagulation, | steroids | resection of
the | recovery |
|
|
|
|
| valvuloplasty | visual field |
| pituitary | hemodynamic |
| tumor |
|
|
|
|
|
|
| defects, |
| macroadenoma | instability |
|
|
|
|
|
|
|
|
| double vision |
|
|
|
|
|
|
|
| Mizuno et
al, | 73/male | – | Elective | Right ptosis | 4 h after | CT and MRI: | Strong | – | Endonasal | Complete | (44) |
| 2011 |
|
| coronary | with
completely | the surgery | A large |
heparinization, |
|
transsphenoidal | recovery |
|
|
|
|
| artery bypass | dilated
pupils, |
| suprasellar
mass | BP fluctuation |
| resection of
the |
|
|
|
|
|
| grafting | light reflex |
| with bleeding | during CPB |
| pituitary
gland |
|
|
|
|
|
|
| loss, headache |
|
|
|
|
|
|
|
| Thurtell et
al, | 79/male | – | Coronary | Blindness, | Following | CT: A large
pituitary | Hemodilution, | Intravenous |
Transsphenoidal | Remained | (45) |
| 2008 |
|
| artery bypass | no light | extubation | mass; | hypotension, | dexamethasone | decompression | blind with |
|
|
|
|
| grafting | perception, |
| MRI: The mass |
anticoagulation | sodium |
| no light |
|
|
|
|
|
| miosis |
| extended into
the |
| phosphate 8 mg |
| perception |
|
|
|
|
|
|
|
| suprasellar
cistern |
|
|
| on follow-up |
|
|
|
|
|
|
|
| and compressed
the |
|
|
|
|
|
|
|
|
|
|
|
| optic chiasm |
|
|
|
|
|
| Thurtell et
al, | 64/male | – | Coronary | Blindness, | Following | CT: A large
pituitary | Hemodilution, | Intravenous |
Transsphenoidal | Remained | (45) |
| 2008 |
|
| artery bypass | no light | extubation | mass; | hypotension, | dexamethasone | decompression | blind with |
|
|
|
|
| grafting | perception, |
| MRI: The mass |
anticoagulation | sodium |
| no light |
|
|
|
|
|
| miosis |
| extended into
the |
| phosphate |
| perception |
|
|
|
|
|
|
|
| suprasellar
cistern |
| 12 mg |
| on follow-up |
|
|
|
|
|
|
|
| and compressed |
|
|
|
|
|
|
|
|
|
|
|
| the optic
chiasm |
|
|
|
|
|
| Matsusaki et
al, | 56/female | – | Living donor | Headache, | POD10 | CT: A
high-density | Intraoperative | Prednisolone, | – | Complete | (46) |
| 2011 |
|
| liver trans- | thirst,
frequent |
| area in the
pituitary | hypotension, | 20 mg/days |
| recovery |
|
|
|
|
| plantation | urination |
| gland; | coagulopathy, |
|
|
|
|
|
|
|
|
|
|
| MRI: A
suspicious | transient |
|
|
|
|
|
|
|
|
|
|
| area between
the | hypertension, |
|
|
|
|
|
|
|
|
|
|
| anterior and
posterior | dopamine |
|
|
|
|
|
|
|
|
|
|
| of the pituitary
gland | agonist
therapy |
|
|
|
|
| Telesca et
al, | 70/male | – | Elective | Headache, | Following | CT and MRI: A
sellar | – | High-dose | – | Complete | (47) |
| 2009 |
|
| coronary | visual field | extubation | mass with
suprasellar |
| steroids |
| recovery |
|
|
|
|
| bypass | defects, |
| extension |
|
|
|
|
|
|
|
|
| surgery | diplopia |
|
|
|
|
|
|
|
| Fyrmpas et
al, | 67/male | Non- | Bilateral | Reduced | POD2 | CT and MRI: | Hypertension, | Corticosteroid | Microscopic | Regained | (23) |
| 2010 |
| secreting | endoscopic | vision, |
| Haemorrhage
within | diabetes, | replacement | endoscopic | vision and |
|
|
|
| pituitary | middle | diplopia, |
| the pituitary
tumor |
anticoagulation | therapy |
transsphenoidal | oculomotor |
|
|
|
| macro- | meatal | headache |
|
| therapy, |
| resection | nerve |
|
|
|
| adenoma | antrostomy, |
|
|
| prolonged |
|
| function |
|
|
|
|
| ethmoidec- |
|
|
| intraoperative |
|
| partly |
|
|
|
|
| tomy and |
|
|
| hypotension |
|
|
|
|
|
|
|
| polypectomy |
|
|
|
|
|
|
|
|
| Absalom et
al, | 61/male | Non- | Coronary | Sudden onset | 40 h after | CT: A 3-cm | Preoperative | Mannitol 80 g | Craniotomy,
de- | Dead of | (24) |
| 1993 |
| secreting | artery bypass | of headache, | the surgery | suprasellar
mass | anticoagulant | and | compression of | acute |
|
|
|
| pituitary | grafting | nausea, |
| with a large | therapy,
sudden | dexamethasone | the optic
nerves, | myocardial |
|
|
|
| tumor |
| vomiting |
| bleeding area
in | coronary | 10 mg iv | intracapsular | infarction |
|
|
|
|
|
|
|
| the pituitary |
revascularization |
| removal of |
|
|
|
|
|
|
|
|
|
|
|
| pituitary
tumor |
|
|
| Madhusudhan | 62/male | – | Right total | Bilateral | POD5 | CT: A low | Preoperative | Hydrocortisone | – | Complete | (48) |
| et al,
2011 |
|
| shoulder | frontal |
| attenuation
signal | anticoagulant | and thyroxine |
| recovery |
|
|
|
|
| replacement | headaches, |
| in the
pituitary | therapy, | supplements, |
|
|
|
|
|
|
|
| binocular |
| fossa; | postoperative | testosterone |
|
|
|
|
|
|
|
| diplopia, |
| MRI: The
pituitary | hypoperfusion | replacement |
|
|
|
|
|
|
|
| increased |
| stalk was
markedly |
| therapy |
|
|
|
|
|
|
|
| urinary
output, |
| deviated to the
right |
|
|
|
|
|
|
|
|
|
| confusion, |
| with an
enhancing |
|
|
|
|
|
|
|
|
|
| drowsiness |
| area in the
pituitary |
|
|
|
|
|
|
|
|
|
|
|
| fossa, suggesting
an |
|
|
|
|
|
|
|
|
|
|
|
| adenoma |
|
|
|
|
|
| Cohen et
al, | 50/female | – | Liposuction | Persistent | After the | MRI: An
intrasellar | Large dose of | – |
Transsphenoidal | Complete | (49) |
| 2004 |
|
| on abdomen, | headache, | surgery | and suprasellar
mass | local
anesthetic, |
| resection of
the | recovery |
|
|
|
|
| hips and | nausea, |
| extending into
the | hypovolemia, |
| pituitary mass |
|
|
|
|
|
| thighs | vomiting |
| right cavernous
sinus | fluid overload |
|
|
|
|
| Shapiro, 1990 | 60/female | – | Coronary | Headache, | POD1 | CT: A
right-sided | Reduced | Hydrocortisone |
Transsphenoidal | Third nerve | (50) |
|
|
|
| artery bypass | severe right |
| sellar mass
with | perfusion | 50 mg every | surgery | palsy |
|
|
|
|
| surgery | ptosis, |
| extension into
the | pressure
during | 6 h |
| persisted |
|
|
|
|
|
| unresponsive |
| sphenoid
sinus; |
cardiopulmonary |
|
| post- |
|
|
|
|
|
| pupil on the |
| MRI: A
pituitary | bypass, |
|
| operatively |
|
|
|
|
|
| right side |
| tumor with
surroun- | anticoagulant |
|
|
|
|
|
|
|
|
|
|
| ding
hemorrhage | therapy |
|
|
|
|
| Tansel et
al, | 60/male | – | Coronary | Unexplained | Following | MRI: Pituitary | Protamine |
Hydrocortisone, | – | In good | (51) |
| 2010 |
|
| artery bypass | episodes of | extubation | infarction |
hypersensitivity | testosterone, |
| condition |
|
|
|
|
| grafting | hypotension, |
|
|
| thyroxin |
| except for |
|
|
|
|
|
| dysrhythmia, |
|
|
|
|
| a certain |
|
|
|
|
|
| electrolyte |
|
|
|
|
| degree of |
|
|
|
|
|
| imbalances, |
|
|
|
|
| visual |
|
|
|
|
|
| somnolence, |
|
|
|
|
| disturbance |
|
|
|
|
|
| agitation, |
|
|
|
|
|
|
|
|
|
|
|
| respiratory |
|
|
|
|
|
|
|
|
|
|
|
| distress, high |
|
|
|
|
|
|
|
|
|
|
|
| fever |
|
|
|
|
|
|
|
| Slavin and | 57/male | – | Three-vessel | Mild
periorbital | Awakening | CT: An
intrasellar | Intraoperative |
Corticosteroids |
Transsphenoidal | Complete | (52) |
| Budabin, 1984 |
|
| coronary | pain, unable | from | mass with
right | or
postoperative |
| hypophysectomy | recovery |
|
|
|
|
| bypass | to open right | anesthesia | parasellar
extension | hypotension, |
|
| except for a |
|
|
|
|
| surgery | eye, headache |
|
|
anticoagulation, |
|
| mild visual |
|
|
|
|
|
|
|
|
| positive
pressure |
|
| field defect |
|
|
|
|
|
|
|
|
| ventilation |
|
|
|
|
| Slavin and | 55/male | – | Mitral valve | Bilateral | After the | CT: An
intrasellar | Intraoperative |
Corticosteroids |
Transsphenoidal | Complete | (52) |
| Budabin, 1984 |
|
| replacement | blepharoptosis | surgery | mass with
large | or
postoperative |
| hypophysectomy | recovery |
|
|
|
|
| under cardio- | and partial
oph- |
| radiolucent
areas | hypotension, |
|
| except for |
|
|
|
|
| pulmonary | thalmoplegia |
| encroaching on
the |
anticoagulation, |
|
| a mild right |
|
|
|
|
| bypass | on each side, |
| right cavernous
sinus | positive
pressure |
|
| abduction |
|
|
|
|
|
| bilateral |
|
| ventilation |
|
| defect |
|
|
|
|
|
| confrontation |
|
|
|
|
|
|
|
|
|
|
|
| visual fields |
|
|
|
|
|
|
|
|
|
|
|
| disclosed
nasal |
|
|
|
|
|
|
|
|
|
|
|
| field defects |
|
|
|
|
|
|
|
| Present case, | 64/female | – | Extensive | Sudden onset | POD1 | CT: Multiple | Reduced
cerebral | Hormone | Microscopic | Complete | – |
| 2024 |
|
| stump | of headache, |
| lacunar
infarctions | perfusion, | replacement | endoscopic | recovery |
|
|
|
|
| cervicectomy, | drowsiness |
| and local
density | anticoagulant | therapy |
transsphenoidal | except for a |
|
|
|
|
| pelvic lymph |
|
| increase in
saddle | therapy |
| resection | mild blurry |
|
|
|
|
| node |
|
| and
suprasellar |
|
|
| vision |
|
|
|
|
| dissection |
|
| region; |
|
|
|
|
|
|
|
|
|
|
|
| MRI: The
pituitary |
|
|
|
|
|
|
|
|
|
|
|
| was enlarged
and |
|
|
|
|
|
|
|
|
|
|
|
| mixed signals
were |
|
|
|
|
|
|
|
|
|
|
|
| seen |
|
|
|
|
|
The diagnosis of PA is based on imaging evaluation,
mainly by MRI, which is more sensitive than CT. Pituitary MRI is
the radiological examination of choice (26). It can identify areas of bleeding and
necrosis and determine the relationship between the tumor and
neighboring structures, such as the optic chiasm, cavernous sinuses
and hypothalamus (27). However, CT
is also an examination that cannot be ignored, which can exclude
headaches caused by subarachnoid hemorrhage and make a tentative
diagnosis of intrasellar mass in most cases (28). In the present review, 80% of cases
were detected by CT and 80% by MRI.
Endocrine deficiencies can exist at the onset and
urgent evaluation of hormonal levels is suggested. According to the
latest guidelines from Oxford and Royal College of Physicians
(29), empirical hormonal
replacement is indicated in each patient with secondary adrenal
insufficiency no matter whether to perform a surgery or not.
Applying hydrocortisone 100–200 mg intravenously and then applying
either continuous intravenous infusion 2–4 mg/h or intramuscular
injection 50–100 mg/6 h are suggested. Reviewing the series of
patients with PA, 84% of cases received hormonal replacement
therapy regardless of whether surgery was performed, while 72% of
cases ended up receiving neurosurgical intervention. Applying
exogenous hormones alone has certain inherent imperfections, as
different hormones can influence the regulation of each other to a
certain extent (30). The
indications for surgery following hormonal replacement are as
follows: i) Evidence of worsening or persistent neurological
symptoms, such as visual impairment and ophthalmoplegia (paralysis
or weakness of the eye muscles); ii) altered mental state; iii)
patient is stable (no progressive deterioration in visual or mental
state) and shows improvement with conservative treatment (26). Most cases (84%) achieved partial or
complete remission in the visual field and ophthalmoplegia after
prompt treatment. However, most studies demonstrate that surgical
treatment, usually within 7 days of the event, leads to a higher
rate of recovery from visual impairment (31). Nevertheless, certain retrospective
studies confirm that there is no significant difference in the
recovery of vision and endocrine function between patients with
pituitary tumors treated conservatively and those undergoing
surgical decompression (32,33).
Currently, there is a lack of high-level evidence-based medical
evidence for choosing a treatment approach. The UK guidelines for
the management of pituitary tumor apoplexy recommend that the
treatment plan should be determined through multidisciplinary
collaboration, considering emergency surgical treatment based on
the patient's pituitary apoplexy score evaluation (9).
A limitation of this study was the omission of
ophthalmic assessment figures. These results were not included in
our hospital's electronic medical records. Consequently, only
copies of photographs of these results are available. Additionally,
paper reports were not preserved, precluding the possibility of
scanning them for enhanced clarity.
In conclusion, this review emphasized that even as
an uncommon postoperative complication, PA is potentially
life-threatening. It may occur in postoperative patients either
with diagnosed or undiagnosed prior pituitary adenoma. Early
diagnosis is essential for the timely treatment of hypopituitarism
and prevention of serious neurological complications. In short, the
wise surgeon should: i) Recognize PA after surgery in a timely
manner by obtaining early neuroimaging tests and pituitary-related
hormone tests and remember MRI is more sensitive than CT in
observing early changes of hemorrhage or infarction. ii) Take
initial action, such as applying intravenous glucocorticoids and
mannitol. Transsphenoidal surgery should be considered and
performed at the early stage of PA, if possible, to achieve better
recovery. iii) If vision and the visual field are not affected, or
vision defects are stable or temporary, hormonal replacement
therapy alone may be considered, which is more appropriate for
patients with surgical contraindications and may also spare
patients from unnecessary surgery.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KL and XY conceived the study and revised the
manuscript. CS made substantial contributions to the acquisition
and analysis of the data and drafted the tables of the manuscript.
LJ drafted the figures of the manuscript and interpreted the data.
All authors read and approved the final manuscript. XY and KL
checked and confirmed the authenticity of the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACTH
|
adrenocorticotropic hormone
|
|
BP
|
blood pressure
|
|
CT
|
computerized tomography
|
|
FSH
|
follicle-stimulating hormone
|
|
FT3
|
free triiodothyronine
|
|
FT4
|
free thyroxine
|
|
HPV
|
human papillomavirus
|
|
K+
|
potassium ion
|
|
LH
|
luteinizing hormone
|
|
MRI
|
magnetic resonance imaging
|
|
Na+
|
sodium
|
|
PA
|
pituitary apoplexy
|
|
PET-CT
|
positron emission tomography-computed
tomography
|
|
POD
|
postoperative day
|
|
TCT
|
thinprep cytology test
|
|
TSH
|
thyroid stimulating hormone
|
References
|
1
|
Brougham M, Heusner AP and Adams RD: Acute
degenerative changes in adenomas of the pituitary body-with special
reference to pituitary apoplexy. J Neurosurg. 7:421–439. 1950.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muthukumar N: Pituitary apoplexy: A
comprehensive review. Neurol India. 68:S72–S78. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonicki W, Kasperlik-Załuska A, Koszewski
W, Zgliczyński W and Wisławski J: Pituitary apoplexy: Endocrine,
surgical and oncological emergency. Incidence, clinical course and
treatment with reference to 799 cases of pituitary adenomas. Acta
Neurochir (Wien). 120:118–122. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verrees M, Arafah BM and Selman WR:
Pituitary tumor apoplexy: Characteristics, treatment, and outcomes.
Neurosurg Focus. 16:E62004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sibal L, Ball SG, Connolly V, James RA,
Kane P, Kelly WF, Kendall-Taylor P, Mathias D, Perros P, Quinton R
and Vaidya B: Pituitary apoplexy: A review of clinical
presentation, management and outcome in 45 cases. Pituitary.
7:157–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lubina A, Olchovsky D, Berezin M, Ram Z,
Hadani M and Shimon I: Management of pituitary apoplexy: Clinical
experience with 40 patients. Acta Neurochir (Wien). 147:151–157.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Capatina C, Inder W, Karavitaki N and Wass
JA: Management of endocrine disease: Pituitary tumour apoplexy. Eur
J Endocrinol. 172:R179–R190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bills DC, Meyer FB, Laws ER Jr, Davis DH,
Ebersold MJ, Scheithauer BW, Ilstrup DM and Abboud CF: A
retrospective analysis of pituitary apoplexy. Neurosurgery.
33:602–609. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bujawansa S, Thondam SK, Steele C,
Cuthbertson DJ, Gilkes CE, Noonan C, Bleaney CW, Macfarlane IA,
Javadpour M and Daousi C: Presentation, management and outcomes in
acute pituitary apoplexy: A large single-centre experience from the
United Kingdom. Clin Endocrinol (Oxf). 80:419–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dubuisson AS, Beckers A and Stevenaert A:
Classical pituitary tumour apoplexy: Clinical features, management
and outcomes in a series of 24 patients. Clin Neurol Neurosurg.
109:63–70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nawar RN, AbdelMannan D, Selman WR and
Arafah BM: Pituitary tumor apoplexy: A review. J Intensive Care
Med. 23:75–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Randeva HS, Schoebel J, Byrne J, Esiri M,
Adams CB and Wass JA: Classical pituitary apoplexy: Clinical
features, management and outcome. Clin Endocrinol (Oxf).
51:181–188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ranabir S and Baruah MP: Pituitary
apoplexy. Indian J Endocrinol Metab. 15 (Suppl 3):S188–S196. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Briet C, Salenave S, Bonneville JF, Laws
ER and Chanson P: Pituitary apoplexy. Endocr Rev. 36:622–645. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pivonello R, De Leo M, Cozzolino A and
Colao A: The treatment of cushing's disease. Endocr Rev.
36:385–486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mullur R, Liu YY and Brent GA: Thyroid
hormone regulation of metabolism. Physiol Rev. 94:355–382. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Chen Z, Zhang L, Wang X, Hao G,
Zhang Z, Shao L, Tian Y, Dong Y, Zheng C, et al: Status of
hypertension in China: Results from the China hypertension survey,
2012–2015. Circulation. 137:2344–2356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kelly AM: The minimum clinically
significant difference in visual analogue scale pain score does not
differ with severity of pain. Emerg Med J. 18:205–207. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reith FC, Van den Brande R, Synnot A,
Gruen R and Maas AI: The reliability of the Glasgow Coma Scale: A
systematic review. Intensive Care Med. 42:3–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noh S and Kim SH, Cho NH and Kim SH: Rapid
reticulin fiber staining method is helpful for the diagnosis of
pituitary adenoma in frozen section. Endocr Pathol. 26:178–184.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shaikh AA, Williams DM, Stephens JW,
Boregowda K, Udiawar MV and Price DE: Natural history of pituitary
apoplexy: A long-term follow-up study. Postgrad Med J. 99:595–598.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnston PC, Hamrahian AH, Weil RJ and
Kennedy L: Pituitary tumor apoplexy. J Clin Neurosci. 22:939–944.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fyrmpas G, Constantinidis J, Foroglou N
and Selviaridis P: Pituitary apoplexy following endoscopic sinus
surgery. J Laryngol Otol. 124:677–679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Absalom M, Rogers KH, Moulton RJ and Mazer
CD: Pituitary apoplexy after coronary artery surgery. Anesth Analg.
76:648–649. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suri H and Dougherty C: Presentation and
management of headache in pituitary apoplexy. Curr Pain Headache
Rep. 23:612019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barkhoudarian G and Kelly DF: Pituitary
apoplexy. Neurosurg Clin N Am. 30:457–463. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong CS and Omay SB: Pituitary Apoplexy. N
Engl J Med. 387:23662022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goyal P, Utz M, Gupta N, Kumar Y, Mangla
M, Gupta S and Mangla R: Clinical and imaging features of pituitary
apoplexy and role of imaging in differentiation of clinical mimics.
Quant Imaging Med Surg. 8:219–231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rajasekaran S, Vanderpump M, Baldeweg S,
Drake W, Reddy N, Lanyon M, Markey A, Plant G, Powell M, Sinha S
and Wass J: UK guidelines for the management of pituitary apoplexy.
Clin Endocrinol (Oxf). 74:9–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feldt-Rasmussen U, Klose M and Benvenga S:
Interactions between hypothalamic pituitary thyroid axis and other
pituitary dysfunctions. Endocrine. 62:519–527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abdulbaki A and Kanaan I: The impact of
surgical timing on visual outcome in pituitary apoplexy: Literature
review and case illustration. Surg Neurol Int. 8:162017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ayuk J, McGregor EJ, Mitchell RD and
Gittoes NJ: Acute management of pituitary apoplexy-surgery or
conservative management? Clin Endocrinol (Oxf). 61:747–752. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gruber A, Clayton J, Kumar S, Robertson I,
Howlett TA and Mansell P: Pituitary apoplexy: Retrospective review
of 30 patients-is surgical intervention always necessary? Br J
Neurosurg. 20:379–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mura P, Cossu AP, Musu M, De Giudici LM,
Corda L, Zucca R and Finco G: Pituitary apoplexy after laparoscopic
surgery: A case report. Eur Rev Med Pharmacol Sci. 18:3524–3527.
2014.PubMed/NCBI
|
|
35
|
McClain IJ and Skidd PM: Case of pituitary
apoplexy after surgery. J Neuroophthalmol. 42:e385–e386. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liberale G, Bruninx G, Vanderkelen B,
Dubois E, Vandueren E and Verhelst G: Pituitary apoplexy after
aortic abdominal aneurysm surgery: A case report. Acta Chir Belg.
106:77–80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Naito Y, Mori J, Tazoe J, Tomida A, Yagyu
S, Nakajima H, Iehara T, Tatsuzawa K, Mukai T and Hosoi H:
Pituitary apoplexy after cardiac surgery in a 14-year-old girl with
Carney complex: A case report. Endocr J. 66:1117–1123. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hidiroglu M, Kucuker A, Ucaroglu E,
Kucuker SA and Sener E: Pituitary apoplexy after cardiac surgery.
Ann Thorac Surg. 89:1635–1637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yakupoglu H, Onal MB, Civelek E, Kircelli
A and Celasun B: Pituitary apoplexy after cardiac surgery in a
patient with subclinical pituitary adenoma: Case report with review
of literature. Neurol Neurochir Pol. 44:520–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoshino M, Sekine Y, Koh E, Hata A and
Hashimoto N: Pituitary apoplexy after surgical treatment of lung
cancer. Ann Thorac Surg. 98:1830–1832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Joo C, Ha G and Jang Y: Pituitary apoplexy
following lumbar fusion surgery in prone position: A case report.
Medicine (Baltimore). 97:e06762018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goel V, Debnath UK, Singh J and Brydon HL:
Pituitary apoplexy after joint arthroplasty. J Arthroplasty.
24:826.e7–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim YH, Lee SW, Son DW and Cha SH:
Pituitary apoplexy following mitral valvuloplasty. J Korean
Neurosurg Soc. 57:289–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mizuno T: Pituitary apoplexy with third
cranial nerve palsy after off-pump coronary artery bypass grafting.
Interact Cardiovasc Thorac Surg. 13:240–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thurtell MJ, Besser M and Halmagyi GM:
Pituitary apoplexy causing isolated blindness after cardiac bypass
surgery. Arch Ophthalmol. 126:576–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matsusaki T, Morimatsu H, Matsumi J,
Matsuda H, Sato T, Sato K, Mizobuchi S, Yagi T and Morita K:
Pituitary apoplexy precipitating diabetes insipidus after living
donor liver transplantation. J Anesth. 25:108–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Telesca M, Santini F and Mazzucco A:
Adenoma related pituitary apoplexy disclosed by ptosis after
routine cardiac surgery: Occasional reappearance of a dismal
complication. Intensive Care Med. 35:185–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Madhusudhan S, Madhusudhan TR, Haslett RS
and Sinha A: Pituitary apoplexy following shoulder arthroplasty: A
case report. J Med Case Rep. 5:2842011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cohen A, Kishore K, Wolansky L and Frohman
L: Pituitary apoplexy occurring during large volume liposuction
surgery. J Neuroophthalmol. 24:31–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shapiro LM: Pituitary apoplexy following
coronary artery bypass surgery. J Surg Oncol. 44:66–68. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tansel T, Ugurlucan M and Onursal E:
Pituitary apoplexy following coronary artery bypass grafting:
Report of a case. Acta Chir Belg. 110:484–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Slavin ML and Budabin M: Pituitary
apoplexy associated with cardiac surgery. Am J Ophthalmol.
98:291–296. 1984. View Article : Google Scholar : PubMed/NCBI
|