Introduction
Primary liver cancer (PLC) is one of the most common
malignant tumors worldwide. According to the International Cancer
Research Institute affiliated to the World Health Organization
(1), an estimated 906,000 new cases
of liver cancer and 830,000 deaths due to liver cancer are reported
each year. Hepatocellular carcinoma (HCC) accounts for 85–90% of
all cases of PLC. In China, the main cause of HCC is chronic
hepatitis B virus (HBV) infection (2). PLC often occurs in people aged >55
years. However, China and other East Asian countries have a
relatively higher prevalence of chronic HBV infection in young
people (age ≤18 years) due to the mother-to-child transmission
(3,4).
Young patients (age ≤35 years) with cancer differ
from elderly patients (>35 years) with respect to disease
pathogenesis, treatment and prognosis (5). Previous studies have demonstrated the
impact of age at tumor occurrence on the prognosis of gastric,
breast and colorectal cancer (6–9). Most
patients with HCC are diagnosed at an advanced stage and have lost
the opportunity for radical surgery. In addition, patients with
early liver cancer are prone to recurrence and metastasis after
liver cancer resection and transplantation due to the background of
HBV infection (10). Currently,
systemic antitumor therapy, including chemotherapy, targeted
therapy, immunotherapy and liver protection therapy, is the main
treatment method for patients with advanced liver cancer (11).
As PLC in young patients is often associated with
the mother-to-child transmission of HBV, it is characterized by
rapid disease progression and poor therapeutic efficacy (12,13).
There is a paucity of research on the treatment of young patients
with liver cancer, and the systemic antitumor therapy of this
population has not been well characterized separately. The present
study aimed to perform an in-depth analysis of the differences in
the efficacy of systemic antitumor therapy in young patients with
liver cancer. The findings may provide a clinical treatment
reference for this group of patients.
Patients and methods
Research object
The present study was a retrospective cohort study
of young patients with liver cancer (≤35 years old) who received
systemic antitumor therapy at the Nanjing Jinling Hospital
(Nanjing, China) between May 2015 and May 2023. These patients were
designated as group A. Elderly patients (≥55 years old) with liver
cancer were enrolled as the control group and designated as group
B. This study conforms to the ethical principles of the Declaration
of Helsinki (2013). Ethical approval was provided by the Ethics
Committee of Jinling Hospital (approval no. DZQH-KYLL-23-16).
The required sample size for this study was
estimated based on the cohort study design, and the comparison of
survival time (OS) between young patients and elderly patients with
liver cancer. Considering the low incidence of liver cancer in
young patients, patients in group A and group B were enrolled at a
ratio of 1:2, and the estimated HR was 2.0 (group A vs. group B).
An 80% incidence of end-point events, α=0.05 and β=0.20 were
factored in. Based on the simulation under the aforementioned
assumptions, at least 31 subjects in group A and 62 subjects in
group B were required, and 80% of patient deaths were observed
after follow-up. Considering the loss of follow-up and incomplete
data collection, 38 subjects were finally included in group A and
79 subjects in group B.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) A
pathological diagnosis of HCC or a clinical diagnosis of PLC; ii)
received systemic antitumor therapy; iii) no opportunity for
radical surgery; iv) age ≤35 years or ≥55 years; and v) provision
of informed consent.
The exclusion criteria were as follows: i)
Pathologically confirmed other types of liver cancer, such as
intrahepatic cholangiocarcinoma or mixed liver cancer; ii) no
systemic drug treatment; and c) patients with a poor general
condition and short expected survival time.
The main research population in this study consisted
of patients with advanced liver cancer, which is defined as
patients whose advanced condition is not suitable for radical
surgery and/or local regional therapy (LRT), or patients whose
condition has progressed after surgery and/or LRT. The following
systemic drug therapies were used in the patients with advanced
liver cancer included in this study: 400 mg oral sorafenib twice a
day; 12 mg/day oral lenvatinib for a bodyweight of >60 kg or 8
mg/day oral lenvatinib for a bodyweight of <60 kg; 200 mg oral
donafenib twice a day; 240 mg intravenous nivolumab once every 2
weeks; 1,200 mg intravenous atezolizumab + 15 mg/kg intravenous
bevacizumab once every 3 weeks; 200 mg intravenous tislelizumab
once every 3 weeks; and 130 mg/m2 intravenous
oxaliplatin + 200 mg/m2 intravenous leucovorin + 400
mg/m2 intravenous 5-fluorouracil once every 3 weeks.
Based on the systematic drug therapies in this study, follow-up
subgroup analysis was made, including tyrosine kinase inhibitors
(TKIs), immune checkpoint inhibitors (ICIs), chemotherapy and the
combination of the two treatments.
Data collection
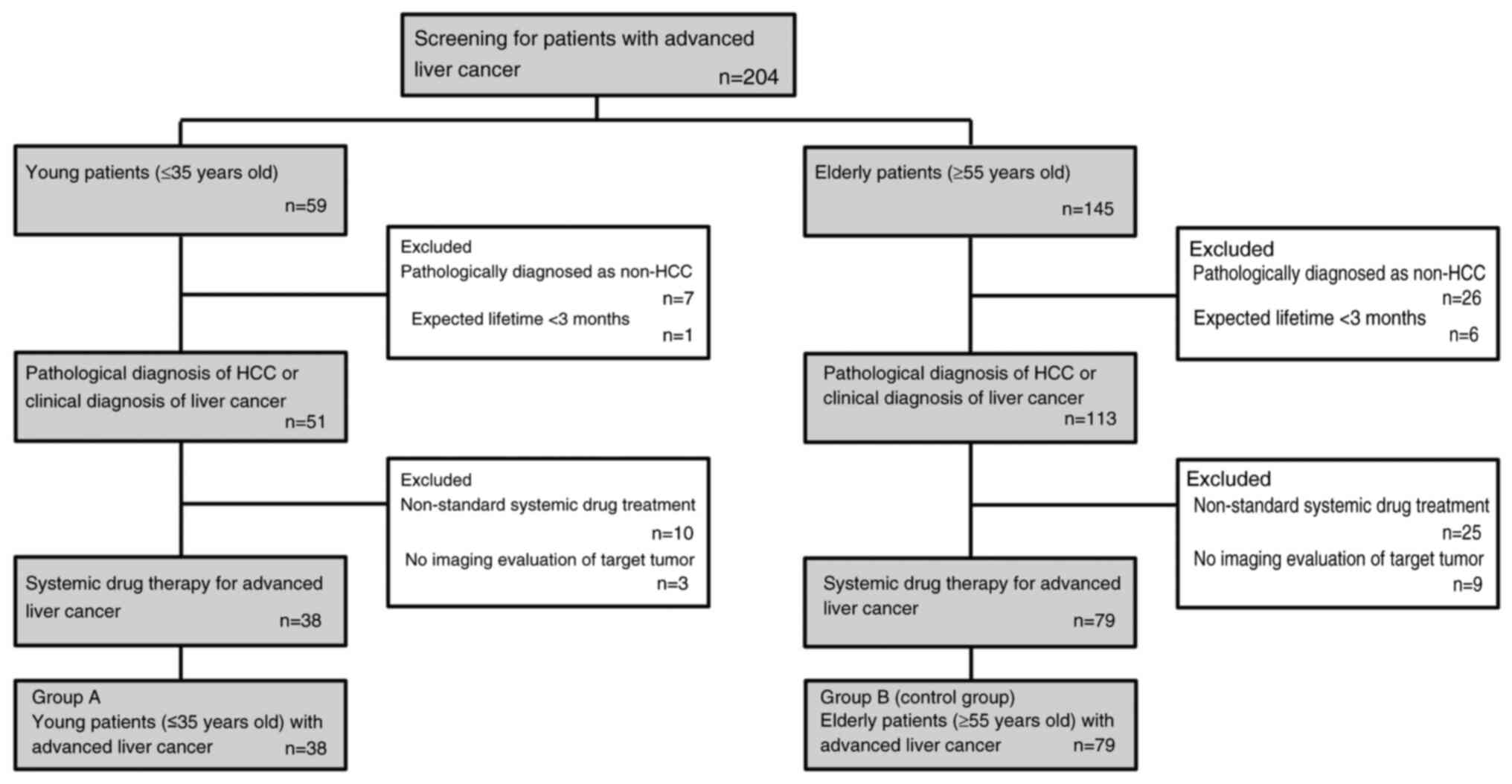
A total of 117 patients entered the final analysis
set (Fig. 1). Baseline demographic
data and clinical data, such as age, sex, medical record number,
contact information and history of liver disease were collected
from the hospital medical records. Baseline liver function indices
such as serum alanine aminotransferase (ALT), aspartate
aminotransferase (AST), total bilirubin (TBIL), Child-Pugh score
(14), and α-fetoprotein (AFP) were
collected. The indicators of HBV infection were recorded, including
HBV surface antigen (HBsAg), HBV surface antibody (HBsAb), HBeAg,
HBeAb, HBcAb and HBV DNA. Certain indicators related to liver
cancer were collected in the hospital medical record system,
namely, whether previous surgery, presence of portal vein tumor
thrombus (PVTT) and tumor stage (Barcelona Clinic Liver Cancer)
(15). Follow-up information
regarding treatment strategy and survival outcomes was also
collected. Finally, patients with advanced liver cancer were
divided into the young group (group A) and the elderly group (group
B).
Survival follow-up
Comprehensive details regarding systemic antitumor
therapy, including first-line, second-line and third-line
treatment, were collected. After the standardized systemic
antitumor therapy, the changes in target lesions in the liver were
evaluated based on the Response Evaluation Criteria in Solid Tumors
(RECIST) 1.1, such as disease progression (PD), stable disease
(SD), partial remission (PR) and complete response (CR). In
addition, follow-up information about the patient's condition was
also recorded.
Hyper-progressive disease (HPD)
HPD has no uniform standard at present, but it has
been widely used in the clinical treatment of tumors. However,
within the definition of HPD, a consensus has been reached that the
time-to-treatment failure (TTF) is <2 months after systemic drug
treatment. Others definitions have some limitations, such as the
tumor load being increased by >50% compared with the baseline
period and the tumor growth rate after treatment being more than
twice the previous rate (16).
Currently, there is no clear definition of HPD (17). In the present study, HPD was defined
as TTF <2 months.
Statistical analysis
SPSS 21.0 software (IBM Corp.) was used for data
processing and analysis. Therapeutic response was evaluated based
on RECIST 1.1 standard criteria. Continuous variables are expressed
as the mean ± standard deviation and were analyzed using an
unpaired t-test. Categorical variables are expressed as n (%) and
were analyzed using the χ2 test or Fisher's exact test.
Progression-free survival (PFS) and OS were compared using a
log-rank test. Hazard ratios (HRs) with 95% confidence intervals
(CIs) were estimated using the Cox regression model. Kaplan-Meier
curves and median survival times were estimated for each treatment
group. Landmark analysis was used to evaluate the effect of
treatment intervention at specific time points using R software
(version 4.3.1; R Core Team). The test level was α=0.05. P<0.05
was used to indicate a statistically significant difference.
Results
Study population
The present study included 117 patients with
advanced liver cancer; of these, 38 patients were in group A (≤35
years old) and 79 patients were in group B (≥55 years old). The
mean age in groups A and B was 30.1 years (range, 17–35 years) and
64.1 years (range, 55–86 years), respectively. Men accounted for
86.8% (33/38) in group A and 86.1% (68/79) in group B (P>0.05).
There was no statistical difference between group A and group B in
terms of etiology, diagnosis, PVTT or tumor stage (P>0.05).
However, the previous surgery rate in group A was higher than that
in group B (65.8 vs. 39.2%; P=0.007). Among the baseline indices,
there was no statistical difference between the two groups with
regard to the proportion of patients with increased serum AST,
albumin and AFP levels, or high Child-Pugh score (P>0.05). The
proportions of patients with increased ALT level (52.6 vs. 26.6%)
or rates of HBsAg (92.1 vs. 68.4%) and HBV DNA (36.8 vs. 32.9%)
positivity in group A were higher than those in group B
(P<0.05). However, the proportions of patients with increased
TBIL (7.9 vs. 22.8%) and ECOG score (0.0 vs. 10.1%) were lower in
group A than in group B (P<0.05). These findings suggest that
the baseline level in group A was worse than that in group B, and
that mother-to-child transmission of HBV may be the main cause in
group A (Table I).
 | Table I.Clinical information of included
patients. |
Table I.
Clinical information of included
patients.
| Factors | Group A (n=38) | Group B (n=79) | P-value |
|---|
| Age, years | 30.1±4.9 | 64.1±7.1 | – |
| Sex, n (%) |
|
|
|
|
Female | 5 (13.2) | 11 (13.9) |
|
| Male | 33 (86.8) | 68 (86.1) | 0.910 |
| Etiology, n (%) |
|
|
|
| HBV | 37 (97.4) | 65 (82.3) |
|
| HCV | 0 (0.0) | 2 (2.5) |
|
|
Alcoholic | 0 (0.0) | 3 (3.8) | 0.250 |
| Diagnosis, n (%) |
|
|
|
|
Clinical | 5 (13.2) | 18 (22.8) |
|
|
Pathological | 33 (86.8) | 61 (77.2) | 0.220 |
| Previous surgery, n
(%) |
|
|
|
| No | 13 (34.2) | 48 (60.8) |
|
| Yes | 25 (65.8) | 31 (39.2) | 0.007 |
| PVTT, n (%) |
|
|
|
| No | 25 (65.8) | 27 (34.2) |
|
|
Yes | 12 (31.6) | 30 (38.0) | 0.054 |
| Tumor stage, n
(%) |
|
|
|
| BCLC
A | 0 (0.0) | 2 (2.5) |
|
| BCLC
B | 1 (2.6) | 8 (10.1) |
|
| BCLC
C | 37 (97.4) | 67 (84.8) | 0.197 |
| ALT, n (%) |
|
|
|
| <37
U/l | 18 (47.4) | 58 (73.4) |
|
| ≥37
U/l | 20 (52.6) | 21 (26.6) | 0.006 |
| AST, n (%) |
|
|
|
| <40
U/l | 18 (47.4) | 47 (59.5) |
|
| ≥40
U/l | 20 (52.6) | 32 (40.5) | 0.216 |
| TBIL, n (%) |
|
|
|
|
<20.5 µmol/l | 35 (92.1) | 61 (77.2) |
|
| ≥20.5
µmol/l | 3 (7.9) | 18 (22.8) | 0.049 |
| Albumin, n (%) |
|
|
|
| <35
g/l | 3 (7.9) | 16 (20.3) |
|
| ≥35
g/l | 35 (92.1) | 63 (79.7) | 0.090 |
| AFP, n (%) |
|
|
|
| <20
µg/l | 7 (18.4) | 27 (34.2) |
|
| ≥20
µg/l | 31 (81.6) | 52 (65.8) | 0.079 |
| Child-Pugh, n
(%) |
|
|
|
| A | 36 (94.7) | 70 (88.6) |
|
| B | 2 (5.3) | 9 (11.4) | 0.287 |
| HBsAg, n (%) |
|
|
|
|
Negative | 2 (5.3) | 23 (29.1) |
|
|
Positive | 35 (92.1) | 54 (68.4) | 0.003 |
| HBV DNA, n (%) |
|
|
|
| <50
IU/ml | 6 (15.8) | 37 (46.8) |
|
| ≥50
IU/ml | 14 (36.8) | 26 (32.9) | 0.025 |
| ECOG performance
status, n (%) |
|
|
|
|
0-1 | 38 (100.0) | 71 (89.9) |
|
| ≥2 | 0 (0.0) | 8 (10.1) | 0.042 |
| First-line
treatment strategy, n (%) |
|
|
|
|
TKIs | 15 (39.5) | 30 (38.0) |
|
|
Chemotherapy | 12 (31.6) | 6 (7.6) |
|
|
ICIs | 0 (0.0) | 9 (11.4) |
|
| TKIs +
ICIs | 8 (21.1) | 18 (22.8) |
|
|
Chemotherapy + ICIs | 0 (0.0) | 9 (11.4) |
|
| Other
therapies | 3 (7.9) | 7 (8.8) | 0.003 |
Clinical efficacy of systemic drug
therapy for young patients with PLC
Patients were evaluated by spiral computed
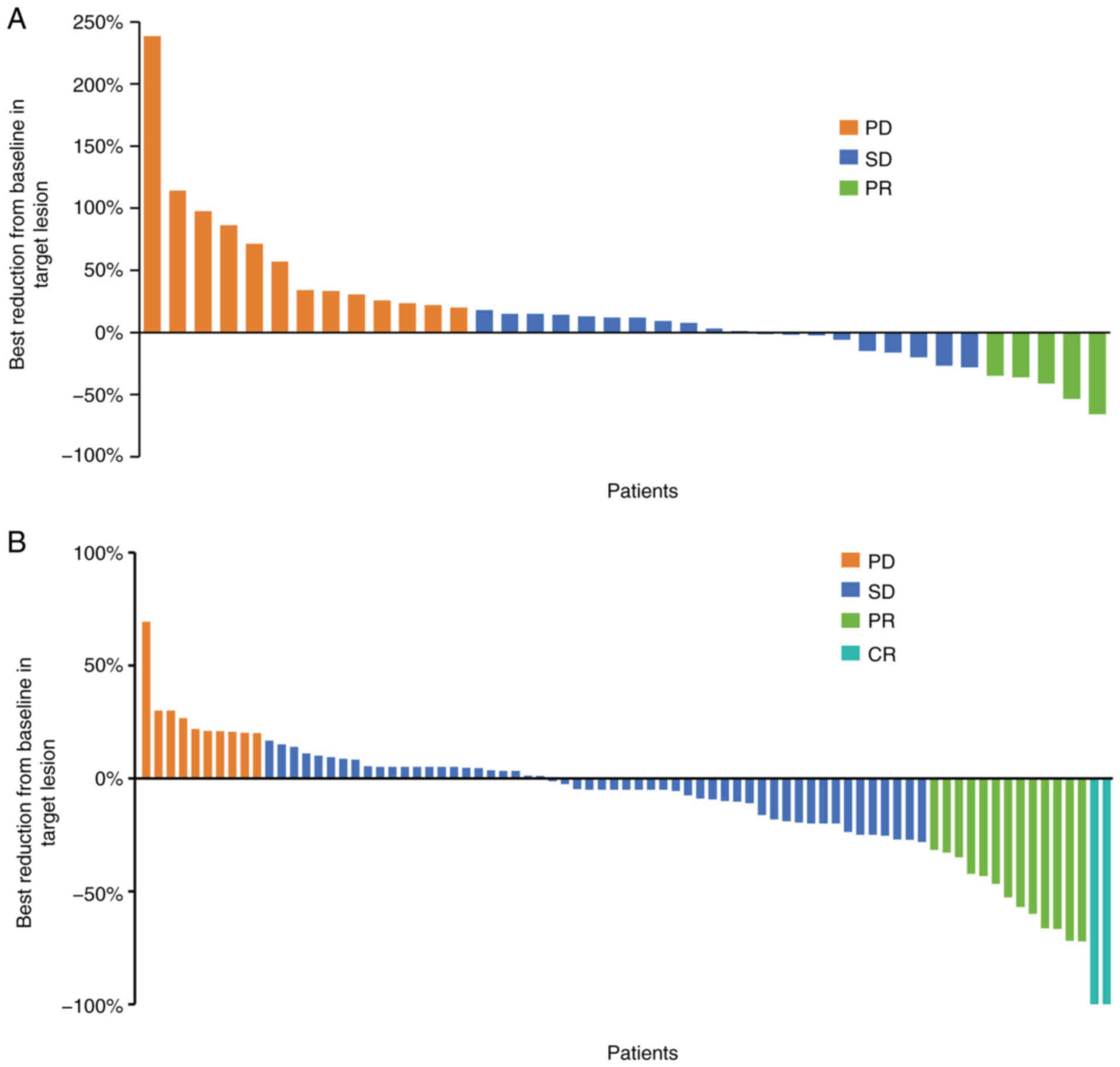
tomography every 2 months. In group A, there were 13 (34.2%)
patients with PD, 5 (13.2%) patients with PR and 20 (52.6%)
patients with SD. None of the patients was evaluated as CR
(Fig. 2A). In group B, there were
10 (12.7%) patients with PD, 13 (16.5%) patients with PR, 54
(68.4%) patients with SD, and 2 (2.5%) patients with CR (Fig. 2B). This indicated the poor efficacy
of systemic antitumor therapy in young patients with liver cancer
compared to elderly patients.
Survival analysis of young patients
with liver cancer
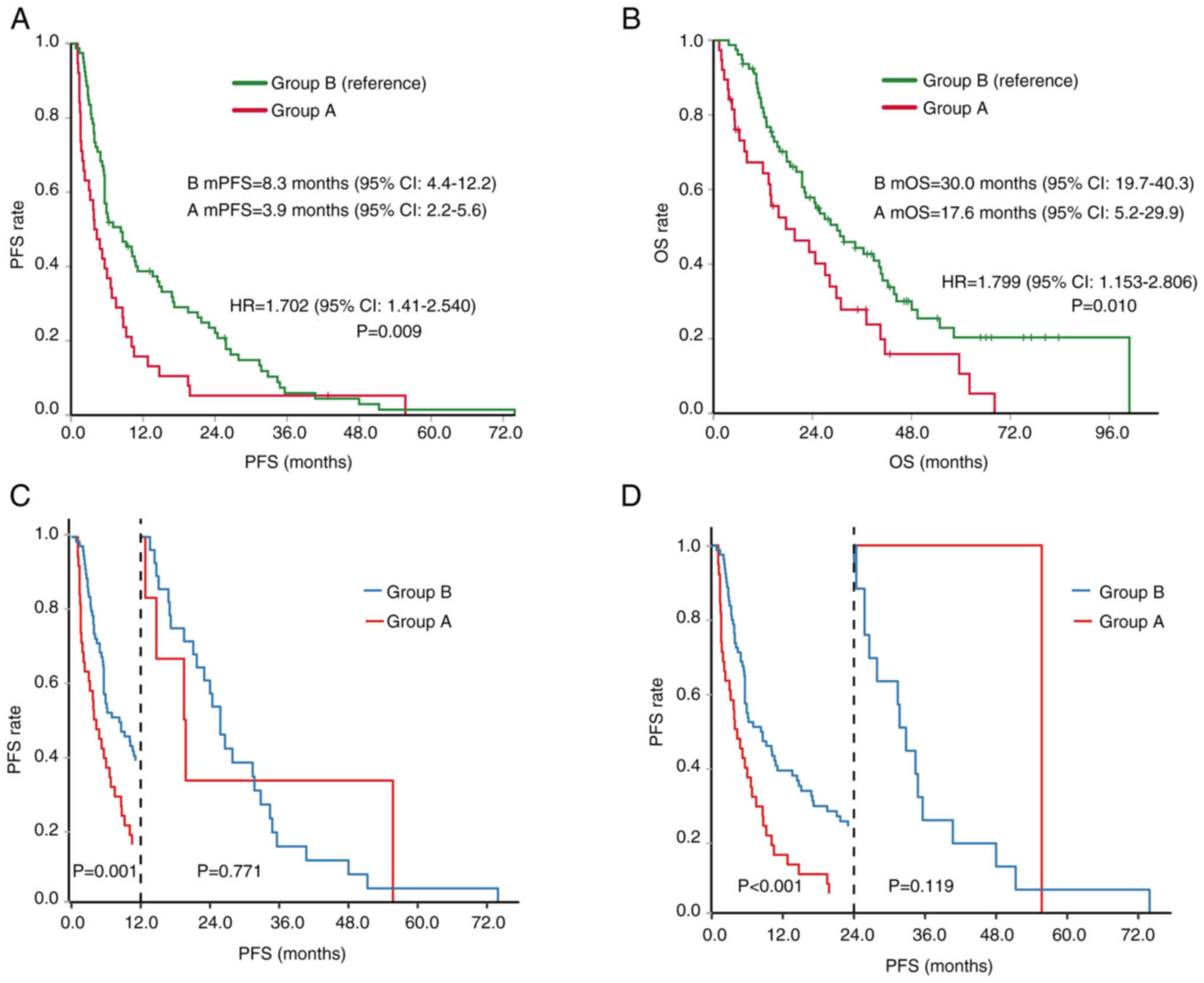
Based on the survival analysis, the median PFS
(mPFS) time of group A was 3.9 months, while that of group B was
8.3 months, which was 4.4 months shorter (HR, 1.702; P=0.009)
(Fig. 3A). A landmark analysis
showed that the PFS rate at 12 months of group A was significantly
lower than that of group B at 12 months (P=0.001) and 24 months
(P<0.001) (Fig. 3C and D).
However, there was no significant difference in the HR of PFS
between the two groups after the landmarks of 12 and 24 months
(P>0.05). The mOS time of group A was 17.6 months, while the mOS
time of group B was 30.0 months, which was 12.4 months shorter (HR,
1.799; P=0.010) (Fig. 3B). These
findings suggest that young patients with liver cancer show a poor
response to treatment and have a short survival time.
Stratified analysis of factors
influencing the survival outcomes
Next, the influence of the baseline biochemical and
virological indicators on OS was analyzed. The results of the
stratified analysis showed that male sex (HR, 1.73; 95% CI,
1.07–2.79), pathological diagnosis (HR, 1.79; 95% CI, 1.10–2.91),
previous surgical treatment (HR, 2.16; 95% CI, 1.18–3.95), no PVTT
(HR, 2.45; 95% CI, 1.22–4.93), elevated ALT (HR, 2.23; 95% CI,
1.07–4.65), elevated AST (HR, 3.22; 95% CI, 1.62–6.39), normal TBIL
(classified as <20.5 µmol/l) (HR, 1.77; 95% CI, 1.09–2.87) and
increased AFP (HR, 2.02; 95% CI, 1.19–3.41) were associated with a
shorter survival time in group A compared with group B (P<0.05)
(Fig. 4). Group A did not show
longer OS times compared with group B for any of the subgroups
(Fig. 4).
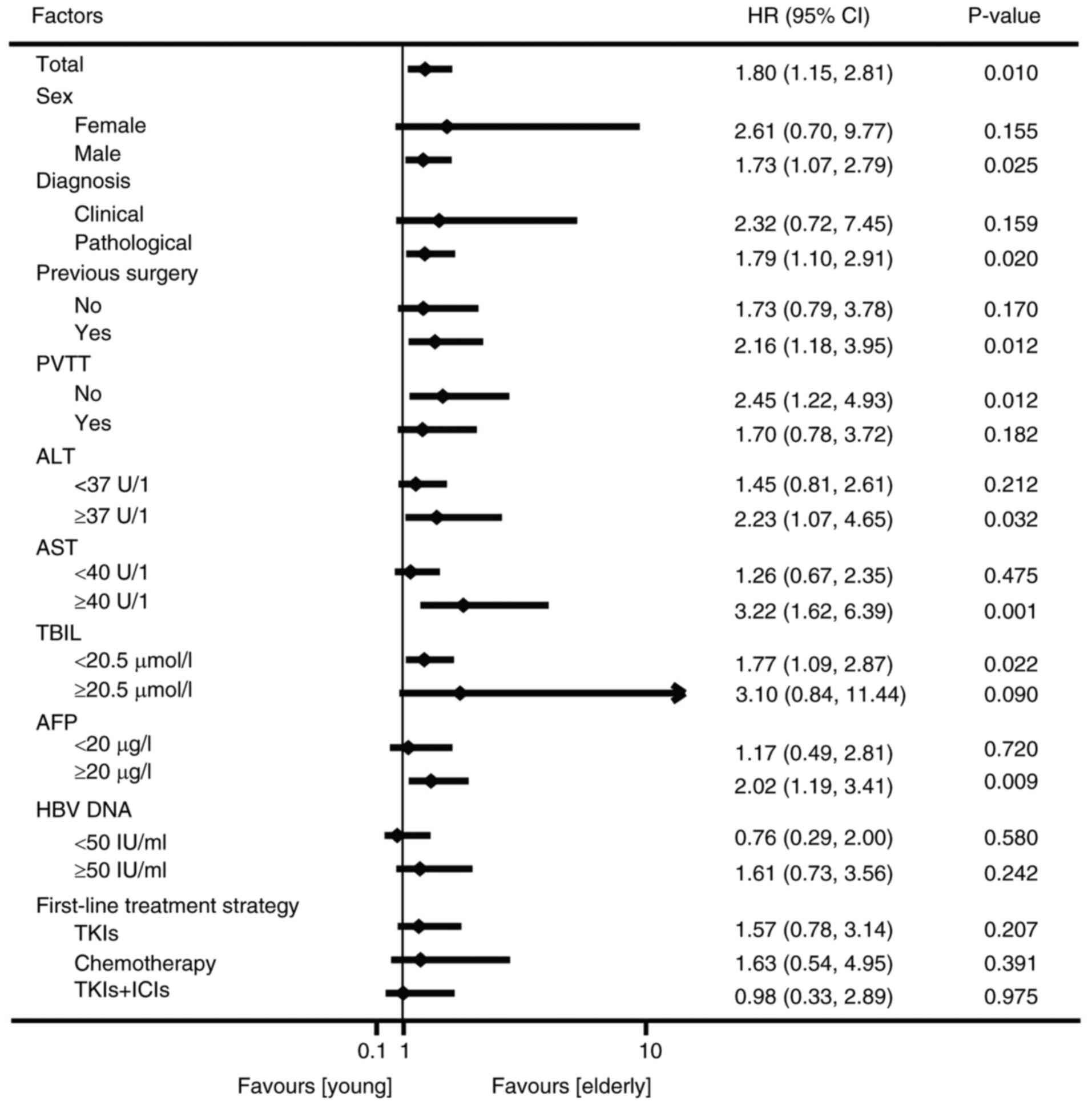 | Figure 4.Forest plot of demographic- and
biomarker-defined subgroup analyses of overall survival. PVTT,
portal vein tumor thrombus; ALT, alanine aminotransferase; AST,
aspartate aminotransferase; TBIL, total bilirubin; AFP,
α-fetoprotein; HBV, hepatitis B Virus; HR, hazard ratio; CI,
confidence interval; TKIs, tyrosine kinase inhibitors; ICIs, immune
checkpoint inhibitors. |
Sensitivity analysis
In the first-line treatment strategy, the longest
mPFS time among group B was achieved in the ICIs subgroup, followed
by the chemotherapy + ICIs and TKIs + ICIs subgroups. In group A,
TKIs + ICIs showed a longer mPFS time of 8.7 months (95% CI,
0.0–18.6 months) compared with that of group B. In the first-line
treatment strategy, the longest OS among group B was in the ICIs
subgroup, followed by the chemotherapy + ICIs and TKIs + ICIs
subgroups. In group A, TKIs + ICIs showed a longer mOS time of 30.9
months (95% CI, 21.3–40.4) compared with that of group B (Table II and Fig. S1).
 | Table II.PFS and OS values of different
treatment strategies in the two groups. |
Table II.
PFS and OS values of different
treatment strategies in the two groups.
| Therapies | Group A, months
(95% CI) | Group B, months
(95% CI) | P-value |
|---|
| PFS |
|
|
|
|
TKIs | 4.8 (1.8–7.7) | 5.6 (0.0–13.3) | 0.381 |
|
Chemotherapy | 1.6 (1.3–1.8) | 5.6 (0.7–10.4) | 0.055 |
|
ICIs | – | 16.8
(0.0–45.4) | – |
| TKIs +
ICIs | 8.7 (0.0–18.6) | 8.3 (3.5–13.0) | 0.734 |
|
Chemotherapy + ICIs | – | 8.6 (0.0–17.9) | – |
| Other
therapies | 1.4 (NA-NA) | 5.9 (5.1–6.6) | 0.164 |
| OS |
|
|
|
|
TKIs | 15.8
(12.2–19.3) | 30.0
(18.9–41.0) | 0.207 |
|
Chemotherapy | 8.1 (0.0–24.1) | 12.1
(0.0–47.6) | 0.383 |
|
ICIs | – | 43.8
(0.0–107.2) | – |
| TKIs +
ICIs | 30.9
(21.3–40.4) | 30.7
(17.6–43.7) | 0.975 |
|
Chemotherapy + ICIs | – | 40.2
(0.0–83.0) | – |
| Other
therapies | 6.3 (2.1–10.4) | 22.4
(10.5–34.2) | 0.193 |
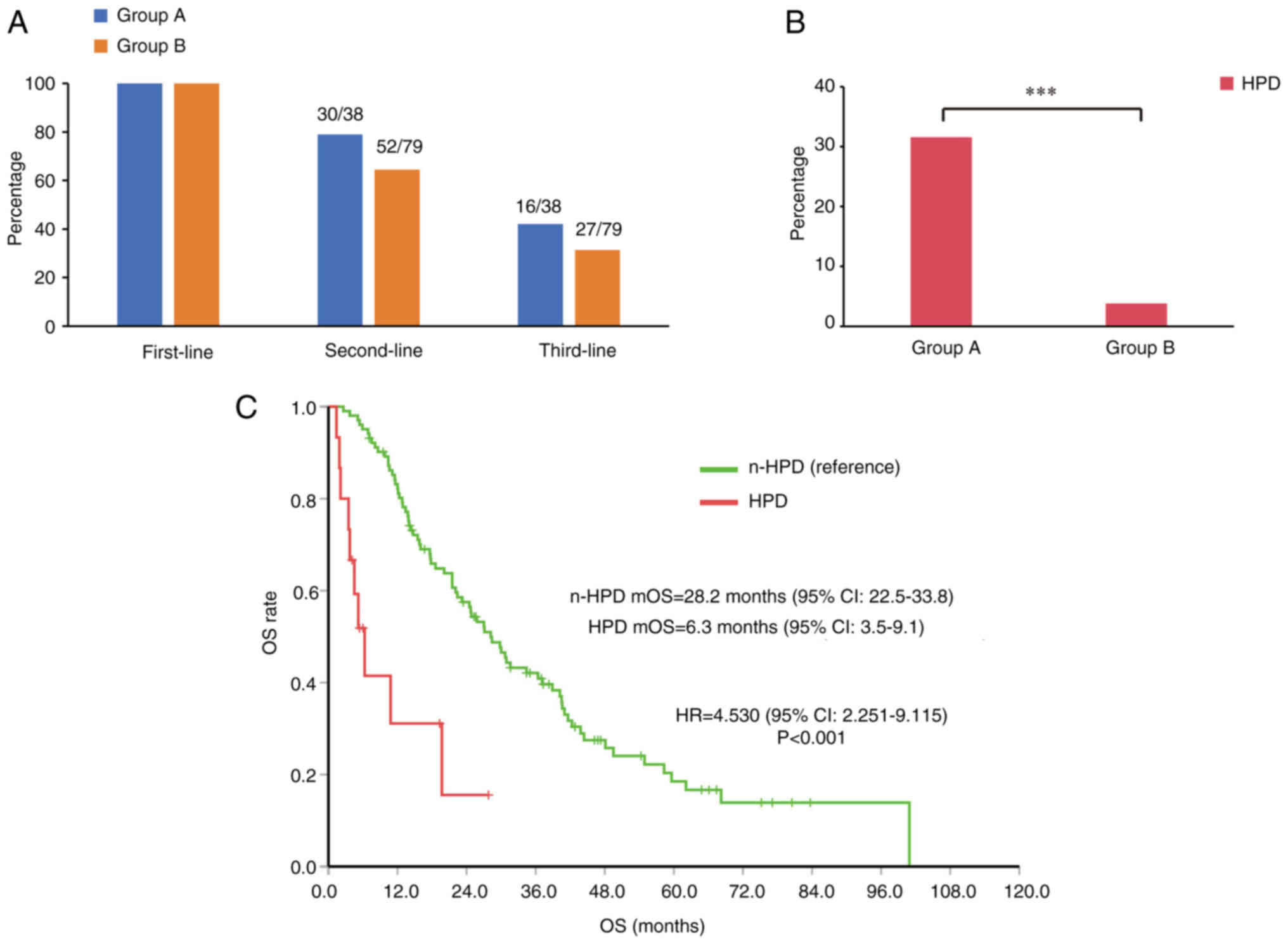
A total of 30 patients in group A and 52 patients in
group B received second-line therapy (P>0.05). The number of
patients receiving third-line treatment was also higher in group A,
but the between-group difference was not statistically significant
(P>0.05) (Fig. 5A).
HPD is a key factor affecting
survival
HPD is a phenomenon of accelerated tumor growth,
which is more common in patients with advanced tumors who use
immunosuppressants (17). Most
patients with HPD suffer from poor quality of life and a poor
prognosis. The incidence of HPD in group A was significantly higher
than that in group B (31.6 vs. 3.8%; P<0.001; Fig. 5B). In the total study population,
HPD had a significant impact on survival. Analysis showed that HPD
was a risk factor for lower mOS time in advanced liver cancer (HR,
4.530; 95% CI, 2.251–9.115; P<0.001; Fig. 5C). These findings suggest that young
patients with liver cancer are prone to HPD, and that HPD
significantly reduces the survival time of patients.
Discussion
The high prevalence of HBV infection in China has
led to a steady increase in the incidence of liver cancer among
young people (18). Young cancer
patients (≤35 years old) are essentially different from elderly
patients (≥55 years old) in terms of disease pathogenesis,
treatment and prognosis (19). In
the present study, the proportion of patients with elevated ALT
levels, HBsAg positivity and HBV DNA positivity were significantly
higher in group A. This suggests that the main cause of liver
cancer in young patients may be HBV infection. Previous studies
have shown that patients with HBV-HCC are characterized by advanced
disease at the time of diagnosis. Most of them have lost the
opportunity for radical surgery and their survival time is short.
In addition, patients with HBV-HCC are prone to recurrence and
metastasis after hepatectomy due to the background of HBV infection
(10).
For a long time, liver cancer has been considered a
disease that mainly affects the elderly, but recent research has
shown a change in the age distribution of patients. An increasing
number of young people are being diagnosed with liver cancer, which
may be related to several factors such as viral hepatitis, heavy
alcohol consumption, non-alcoholic fatty liver disease history and
excessive consumption of milk/milk substitutes (20). Most young patients with liver cancer
have clinical refractory disease (21). In the present study, the mPFS of
young patients with liver cancer was shorter by 4.4 months and the
mOS was shorter by 12.4 months compared with that of elderly
patients. The proportions of patients who received second-line
treatment and third-line treatment in group A were higher than
those in group B, but the differences were not statistically
significant.
HPD is a phenomenon of accelerated tumor growth,
which is mostly seen in patients with advanced tumors who use ICI
therapies. The incidence of HPD varies in different tumor types,
such as non-small cell lung cancer (13.8%), HCC (10.3%) and gastric
cancer (16.7%) (16,22,23).
This difference is related to the tumor type and the definitions of
HPD used in research. Referring to the commonly used research data,
the present study defined HPD as TTF <2 months (17). In the present study, the incidence
of HPD in group A was significantly higher than that in group B.
HPD was found to be a risk factor for advanced liver cancer.
Previous studies have also shown that patients with HPD have a
worse prognosis and shorter survival time than those without HPD
(24–28). Therefore, HPD is a predictor of a
poor prognosis in patients with advanced cancer.
Some limitations of the present study should be
considered while interpreting the results. This was a single-center
cohort study with a small sample size, which may have introduced an
element of bias. Moreover, this study was based on real-world data
with no standardized protocol for patient selection and treatment
follow-up. More robust prospective studies are required to obtain
more definitive evidence.
In conclusion, systemic drug therapy showed poor
efficacy in young patients with liver cancer. TKIs + ICIs are
suitable for first-line treatment. Moreover, young patients with
liver cancer are prone to HPD, suggesting the need for close
monitoring of these patients to improve the prognosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science Foundation of
Jiangsu Province (grant no. BK20200275) and the Foundation of
Jinling Hospital (grant nos. 22LCZLXJS57, 22LXZLXJS21 and
22LXYY-LH5).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JZ and XL conceived and designed the study. ZL, CC
and ZX performed data analysis and manuscript preparation. XX, PL
and YX assisted with data acquisition and statistical analysis, and
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study involving human participants was reviewed
and approved by the Ethics Committee of Nanjing Jinling Hospital
(approval no. DZQH-KYLL-23-16).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tian T, Song C, Jiang L, Dai J, Lin Y, Xu
X, Yu C, Ge Z, Ding Y, Wen Y, et al: Hepatitis B virus infection
and the risk of cancer among the Chinese population. Int J Cancer.
147:3075–3084. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Z, Hou X and Cao G: Is mother-to-infant
transmission the most important factor for persistent HBV
infection? Emerg Microbes Infect. 4:e302015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Z, Xie Z, Ni H, Zhang Q, Lu W, Yin J,
Liu W, Ding Y, Zhao Y, Zhu Y, et al: Mother-to-child transmission
of hepatitis B virus: evolution of hepatocellular carcinoma-related
viral mutations in the post-immunization era. J Clin Virol.
61:47–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fu J, Yang J, Tan Y, Jiang M, Wen F, Huang
Y, Chen H, Yi C, Zheng S and Yuan Y: Young patients (≤35 years old)
with colorectal cancer have worse outcomes due to more advanced
disease: A 30-year retrospective review. Medicine (Baltimore).
93:e1352014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kath R, Fiehler J, Schneider CP and
Höffken K: Gastric cancer in very young adults: Apropos four
patients and a review of the literature. J Cancer Res Clin Oncol.
126:233–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Wang J, Li Q, Zhang P, Yuan P, Ma
F, Luo Y, Cai R, Fan Y, Chen S, et al: Young breast cancer patients
who develop distant metastasis after surgery have better survival
outcomes compared with elderly counterparts. Oncotarget.
8:44851–44859. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng L, Chen S, Wu W, Kuo ZC, Wei Z, Meng
S, Chen C, Zhang C and He Y: Gastric cancer in young patients: A
separate entity with aggressive features and poor prognosis. J
Cancer Res Clin Oncol. 146:2937–2947. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Theuer CP, Kurosaki T, Taylor TH and
Anton-Culver H: Unique features of gastric carcinoma in the young:
A population-based analysis. Cancer. 83:25–33. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Han N, Ren X, Zhang Y and Chu X:
Effectiveness of TKI Inhibitors Combined With PD-1 in Patients With
Postoperative Early Recurrence of HCC: A Real-World Study. Front
Oncol. 12:8338842022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Selene II, Ozen M and Patel RA:
Hepatocellular Carcinoma: Advances in systemic therapy. Semin
Intervent Radiol. 41:56–62. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanaka J, Kurisu A, Ohara M, Ouoba S,
Ohisa M, Sugiyama A, Wang ML, Hiebert L, Kanto T and Akita T:
Burden of chronic hepatitis B and C infections in 2015 and future
trends in Japan: A simulation study. Lancet Reg Health West Pac.
22:1004282022.PubMed/NCBI
|
|
13
|
Mogul DB, Ling SC, Murray KF,
Schwarzenberg SJ, Rudzinski ER and Schwarz KB: Characteristics of
Hepatitis B virus-associated hepatocellular carcinoma in children:
A multi-center study. J Pediatr Gastroenterol Nutr. 67:437–440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jacob KA, Hjortnaes J, Kranenburg G, de
Heer F and Kluin J: Mortality after cardiac surgery in patients
with liver cirrhosis classified by the Child-Pugh score. Interact
Cardiovasc Thorac Surg. 20:520–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kubota Y, Yoshimura K, Hamada K, Hirasawa
Y, Shida M, Taniguchi M, Matsui H, Ariizumi H, Ishiguro T, Suzuki
N, et al: Rare Nivolumab-associated super hyper progressive disease
in patients with advanced gastric cancer. In Vivo. 35:1865–1875.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding P, Wen L, Tong F, Zhang R, Huang Y
and Dong X: Mechanism underlying the immune checkpoint
inhibitor-induced hyper-progressive state of cancer. Cancer Drug
Resist. 5:147–164. 2022.PubMed/NCBI
|
|
18
|
Ju Y, Han G, Zhang P, Xu J, Chen C, Jiang
H, Yuan D, Ye X and Zhou G: Staging and clinical characteristics of
pregnant women with chronic hepatitis B virus infection: A
retrospective cohort study from Nanjing, China. J Obstet Gynaecol
Res. 49:2427–2435. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pu JL, Chen Z, Yao LQ, Feng JY, Diao YK,
Guan MC, Li JD, Chen ZL, Zhou YH, Wang H, et al: Long-term
oncological prognosis after curative-intent liver resection for
hepatocellular carcinoma in the young versus the elderly:
Multicentre propensity score-matching study. BJS Open.
6:zrab1452022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Almohaid S and Akhtar S: Diet, lifestyle
factors, comorbidities, and hepatocellular carcinoma risk in a
middle eastern country: A case-control study. BMC Cancer.
24:6942021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Jiang R, Hou J and Sun B:
Clinicopathological features and prognostic factors of young
patients with surgically treated liver cancer. Medicine
(Baltimore). 94:e6842015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferrara R, Mezquita L, Texier M, Lahmar J,
Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau
S, Le Moulec S, et al: Hyperprogressive disease in patients with
advanced non-small cell lung cancer treated with PD-1/PD-L1
inhibitors or with single-agent chemotherapy. JAMA Oncol.
4:1543–1552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aoki T, Kudo M, Ueshima K, Morita M,
Chishina H, Takita M, Hagiwara S, Ida H, Minami Y, Tsurusaki M and
Nishida N: Incidence of hyper progressive disease in combination
immunotherapy and anti-programmed cell death protein 1/programmed
death-ligand 1 monotherapy for unresectable hepatocellular
carcinoma. Liver Cancer. 13:56–69. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka T, Koga H, Suzuki H, Iwamoto H,
Sakaue T, Masuda A, Nakamura T, Akiba J, Yano H, Torimura T and
Kawaguchi T: Anti-PD-L1 antibodies promote cellular proliferation
by activating the PD-L1-AXL signal relay in liver cancer cells.
Hepatol Int. 18:984–997. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Feng J, Kuang T, Chai D, Qiu Z,
Deng W, Dong K, Zhao K and Wang W: Blood biomarkers predict
outcomes in patients with hepatocellular carcinoma treated with
immune checkpoint Inhibitors: A pooled analysis of 44 retrospective
sudies. Int Immunopharmacol. 118:1100192023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Han J, Yang Y and Chen Y: PD-1/PD-L1
checkpoint inhibitors in advanced hepatocellular carcinoma
immunotherapy. Front Immunol. 13:10709612022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim CG, Kim C, Yoon SE, Kim KH, Choi SJ,
Kang B, Kim HR, Park SH, Shin EC, Kim YY, et al: Hyperprogressive
disease during PD-1 blockade in patients with advanced
hepatocellular carcinoma. J Hepatol. 74:350–359. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Wu L, Chen Q, Zhang B, Liu J, Liu
S, Mo X, Li M, Chen Z, Chen L, et al: Predicting hyperprogressive
disease in patients with advanced hepatocellular carcinoma treated
with anti-programmed cell death 1 therapy. EClinicalMedicine.
31:1006732020. View Article : Google Scholar : PubMed/NCBI
|