Introduction
According to the 2022 Global Cancer Statistics,
there were 661,021 new cases of cervical cancer and 348,189 deaths
attributed to the disease. This ranks cervical cancer as the fourth
most common cancer amongst women globally and the fourth leading
cause of cancer-associated deaths amongst women worldwide (1). For patients diagnosed with stage
IB1-IIA cervical cancer, as classified by the 2008 Federation of
Gynecology and Obstetrics (FIGO) staging system (2), the recommended primary treatment
involves radical hysterectomy accompanied by pelvic
lymphadenectomy, which is endorsed by the guidelines of the
National Comprehensive Cancer Network (3) and the Japan Society of Gynecologic
Oncology (4). The guidelines of the
Japan Society of Gynecologic Oncology suggest that patients
diagnosed with stage IIB cervical cancer may also be considered for
treatment with radical hysterectomy accompanied by pelvic
lymphadenectomy (4). After surgery,
adjuvant treatments such as irradiation or concurrent
chemoradiation are employed based on risk factors for recurrence
evaluated from the resected specimens. These risk factors include
lymphovascular space invasion, a larger tumor size, deep cervical
interstitial infiltration, parametrial invasion, and lymph node
metastasis (5). However,
irradiation or concurrent chemoradiation following radical surgery
can lead to adverse events, including urinary disturbance,
lower-limb lymphedema, bowel obstruction, sexual dysfunction,
ovarian dysfunction, and mental health issues associated with these
lasting adverse effects (6–8). Given these concerns, efforts have been
made globally to introduce postoperative adjuvant chemotherapy for
patients at intermediate or high risk of recurrence (6,7,9,10).
However, the current evidence supporting chemotherapy as
postoperative adjuvant therapy is limited, and careful
consideration is warranted when determining its suitability. Thus,
the ability to predict the effectiveness of adjuvant chemotherapy
may significantly assist gynecologic oncologists in selecting the
appropriate adjuvant treatment, whether it be irradiation or
chemotherapy.
T-box (TBX)2, a transcription factor, belongs to the
TBX gene family, which plays a critical role in organogenesis and
pattern formation across vertebrate and invertebrate species
(11). TBX2 is involved in
development, cell cycle regulation, and oncogenesis (12,13).
Elevated levels of TBX2 expression have been observed in various
types of cancer, including esophageal squamous cell (14), endometrial (15), melanoma (16), prostate, breast (17), laryngeal squamous cell carcinoma
(18), non-small cell lung
(19), gastric (20), and pancreatic cancer (21). Additionally, elevated expression of
TBX2 has been associated with resistance to DNA-damaging
chemotherapy agents such as cisplatin, carboplatin, and doxorubicin
(22–26).
In the present study, the relationship between TBX2
expression and recurrence was investigated in patients with
intermediate- and high-risk stage IB-IIB cervical cancer who
received adjuvant cisplatin and paclitaxel (TP) chemotherapy
following radical hysterectomy. Additionally, the impact of TBX2
knockdown on the sensitivity of cervical cancer cells to cisplatin
in vitro was assessed.
Materials and methods
Patients
This retrospective analysis included 100 patients
who underwent radical hysterectomy for cervical cancer stages
IB-IIB (FIGO 2008) and received postoperative adjuvant TP
chemotherapy (paclitaxel at 135 mg/m2 and cisplatin at
50 mg/m2 every 3 weeks for 3–6 cycles) between January
1, 2014 and December 31, 2020, at Osaka City University and Osaka
Metropolitan University. Clinical data including age, FIGO stage,
histology, results of blood sample laboratory analysis, date of
primary surgery, initiation and completion of chemotherapy,
recurrence, and death from any cause were recorded. Assessment of
recurrence during postoperative adjuvant chemotherapy was conducted
every 3 cycles using computed tomography (CT) or magnetic resonance
imaging (MRI), complemented by physical examinations after each
cycle. Upon completion of the treatment regimen, outpatient
evaluations, including physical examinations, ultrasonography, and
tumor marker measurements, were scheduled every 3 months.
Furthermore, in the second postoperative year, recurrence
evaluation was performed using CT or MRI. In cases where abnormal
clinical findings or elevated tumor markers were observed, imaging
assessments were performed without waiting for the 2-year
postoperative period. Overall survival was calculated from the date
of surgery to the date of death from any cause, while disease-free
survival was defined as the time from surgery to the detection of
recurrence. For patients still alive at the end of the assessment
period, data were censored as of the last confirmed survival
date.
To explore the association between TBX2 expression
and recurrence, patients were categorized into two groups: Those
who did not experience recurrence within 2 years after primary
surgery (non-recurrent group), and those who experienced a
recurrence within 2 years (recurrent group).
The research was conducted at Osaka City University
and Osaka Metropolitan University. All participating patients
provided written informed consent for the treatment regimen and the
use of their samples in future research endeavors, including the
present study. The present study was approved by the Institutional
Review Board of Osaka Metropolitan University Hospital (approval
no. 2022-102). Osaka Metropolitan University was established in
April 2022 through the merger of Osaka City University and Osaka
Prefecture University. Currently, only Osaka Metropolitan
University exists; its approval in 2022 encompasses the approval
for Osaka City University.
Immunohistochemical staining and
scoring
To assess the expression of TBX2 in cervical cancer
surgical specimens, immunohistochemical staining was performed
followed by scoring of the tissues. Initially, 4-µm
paraffin-embedded sections were prepared from tissue blocks
obtained during surgery of patients with cervical cancer. These
sections underwent deparaffinization and rehydration using
autoclaving, which involved heating at 121°C for 20 min. The
sections were then incubated overnight at 4°C with a TBX2 antibody
(cat. no. LS-C402301; LifeSpan BioSciences, Inc.) diluted 1:500.
This was followed by application of the Dako REAL EnVision
Detection System Peroxidase/DAB+, Rabbit/Mouse (cat. no. K5007;
Agilent Technologies, Inc.) at room temperature for 3 min for
visualization of antibody staining. Tissue sections were
counterstained with hematoxylin for 1 min at room temperature to
enhance the visibility of the structures. Scoring for TBX2
expression utilized a weighted scoring system as described by
Sinicrope et al (27) based
on the percentage of stained tumor cells and staining intensity.
Percentage stained was scored as follows: 0, <5% coverage; 1,
5–25%; 2, 25–50%; 3, 50–75%; and 4, >75%. Intensity was
categorized as 1 for weak, 2 for moderate, and 3 for intense. This
scoring system allows for a quantifiable assessment of TBX2
expression levels in the tumor tissues
Cell culture
The TCS cell line, which comprises human cells
derived from uterine cervical squamous carcinoma, were sourced from
the RIKEN BioResource Center. These cells were cultured in Minimum
Essential Medium from Gibco; Thermo Fisher Scientific, Inc.,
supplemented with 10% FBS and 1% penicillin-streptomycin solution.
Cultures were maintained in a humidified incubator at 37°C with an
atmosphere of 5% CO2.
TBX2 knockdown and cell survival
assays
For siRNA transfections, Lipofectamine®
RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.) was used.
TBX2-specific siRNA (cat. no. SASI_Hs01_00169003; MilliporeSigma)
and control siRNA (cat. no. SIC001_10NMOL; MilliporeSigma) were
used. The si-TBX2 sense sequence was CGCUAUAAGUUCCACAACUdTdT and
the antisense sequence was AGUUGUGGAACUUAUAGCGdTdT; the sequence
for the control siRNA was not disclosed by the manufacturer. TCS
cells were seeded at a density of 1×104 cells/well in
96-well tissue culture plates. Post-seeding, the cells were
incubated with media containing either TBX2 siRNA or control siRNA
at 37°C for 24 h. This was followed by exposure to various
concentrations of cisplatin (3.125, 6.25, 12.5, or 25 µM) at 37°C
for an additional 24 h. Subsequently, 10 µl Cell Counting Kit-8
solution (Dojindo Molecular Technologies, Inc.) was added to each
well, and the cells were incubated at 37°C for 1 h. The absorbance
at 450 nm was then measured using a microplate reader (Corona
Electric, Co., Ltd.). All procedures were performed in strict
accordance with the manufacturer's protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
Following TBX2 siRNA transfection, TBX2 mRNA
expression knockdown was confirmed using RT-qPCR. TaqMan primer and
probes for TBX2 (cat. no. Hs00911929_m1) and hypoxanthine
phosphoribosyl-transferase 1 (HPRT1) (cat. no. Hs02800695_ml), both
sourced from Thermo Fisher Scientific, Inc., were utilized as per
the manufacturer's instructions. HRPT1 was used as a housekeeping
gene as a reference for mRNA expression. Total RNA extraction from
TCS cells was performed using a RNeasy Mini Kit according to the
manufacturer's protocol (Qiagen GmbH). Subsequently, 1 µg total RNA
was reverse transcribed into cDNA using the High-Capacity cDNA
Reverse Transcription Kit (Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. For qPCR analysis, TaqMan
Fast Universal PCR MasterMix (Thermo Fisher Scientific, Inc.) was
employed. The thermocycling conditions consisted of an initial
denaturation step at 95°C for 20 sec, followed by 40 cycles of
denaturation at 95°C for 3 sec and annealing/extension at 60°C for
30 sec. The relative changes in gene expression were calculated
using the 2−ΔΔCq method (28).
Statistical analysis
The data were analyzed using GraphPad Prism version
9 (GraphPad Software, Inc.), and the data are presented as the
median and range. Differences between two groups were assessed
using a Fisher's exact test or Mann-Whitney U-test. The receiver
operating characteristic (ROC) curve was used to establish the
cut-off value for weighted scores of TBX2 expression. Prognostic
analysis was performed using the Kaplan-Meier method alongside
log-rank tests. To identify independent risk factors for
recurrence, detected through univariate analysis with a Fisher's
exact test, multivariate analysis was used for the logistic
regression analysis. P<0.05 was considered to indicate a
statistically significant difference. RT-qPCR was performed using
five replicates, while cell survival assays were performed using 10
replicates.
Results
Patient characteristics
In the present study, 85 patients were included in
the non-recurrent group and 15 patients were included in the
recurrent group. There were no significant differences in the age
and FIGO stage between the two groups. The recurrent group had a
significantly higher proportion of cases exhibiting non-squamous
cell carcinoma (non-SCC), positive parametrium invasion, and
positive lymph node metastasis (P=0.01, P=0.024, and P=0.026,
respectively; Table I). This
suggests that non-SCC histology, positive parametrium invasion, and
positive lymph node metastasis are risk factors for recurrence
during univariate analysis. Specifically, non-SCC histology
consisted of eight usual type endocervical adenocarcinoma cases,
two adenosquamous carcinoma cases, two endometrioid carcinoma
cases, and one clear cell carcinoma case in the non-recurrent
group, whereas in the recurrent group, it consisted of four usual
type endocervical adenocarcinoma cases, two large cell
neuroendocrine carcinoma cases, and one poorly differentiated
adenocarcinoma case.
 | Table I.Clinicopathological characteristics of
the patients. |
Table I.
Clinicopathological characteristics of
the patients.
| Characteristic | Non-recurrent
group | Recurrent group | P-value |
|---|
| No. of
patients | 85 | 15 |
|
| Age,
yearsa | 55 (27–78) | 55 (39–76) | 0.915b |
| FIGO stage |
|
| 0.159c |
| I | 52 | 6 |
|
| II | 33 | 9 |
|
| Histology |
|
| 0.01c,d |
|
SCC | 72 | 8 |
|
|
Non-SCC | 13 | 7 |
|
| Parametrium
invasion |
|
| 0.024c,e |
| + | 19 | 8 |
|
| − | 66 | 7 |
|
| Lymph node
metastasis |
|
| 0.026c,e |
| + | 35 | 11 |
|
| − | 50 | 4 |
|
TBX2 weighted score and cutoff value
to predict recurrence
Immunohistochemical staining demonstrated that TBX2
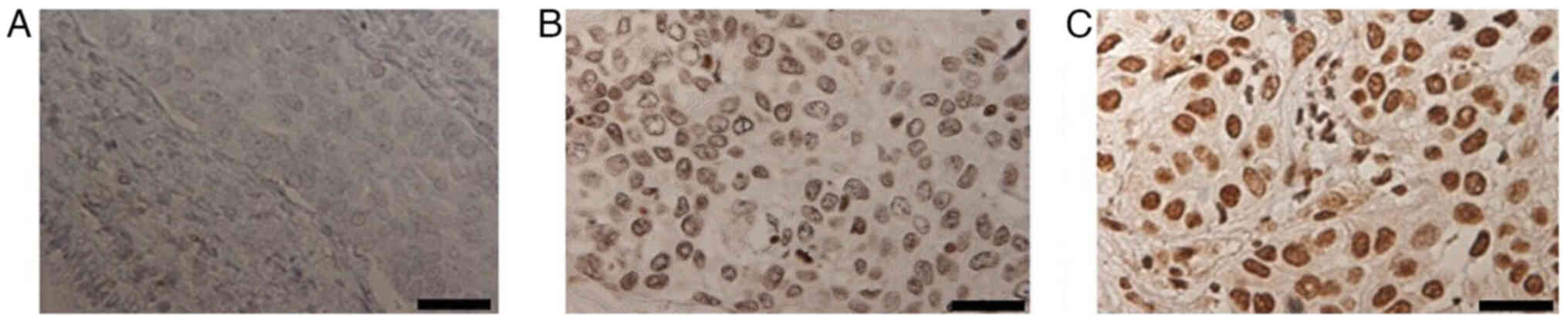
was predominantly localized in the cell nuclei. Representative
images with weighted scores of 0, 6, and 12 are shown in Fig. 1. TBX2 expression was found to be
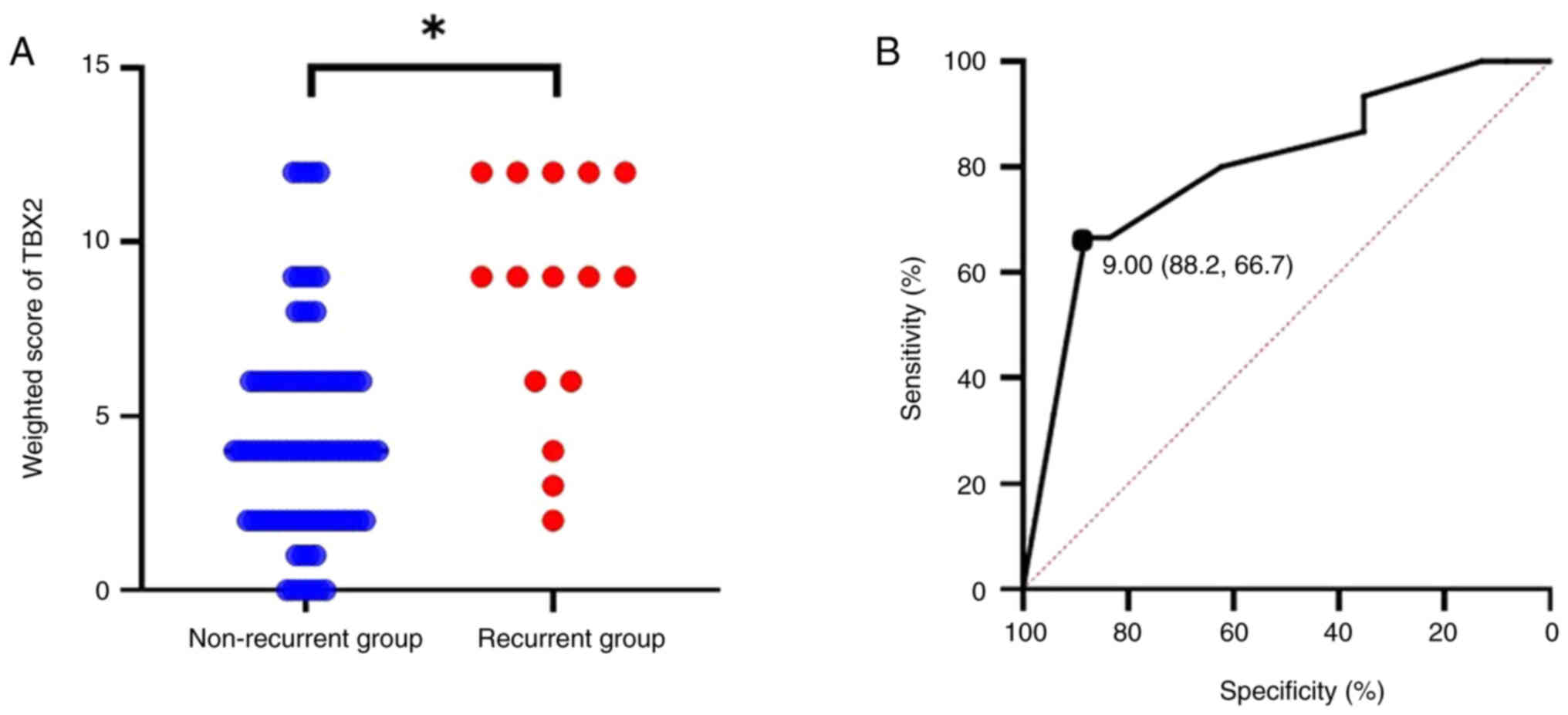
significantly higher in the recurrence group (P<0.01, Fig. 2A). To determine the cutoff value of
TBX2 expression for predicting recurrence, ROC curve analysis was
performed. Setting the cutoff value for the weighted score at 9
yielded a sensitivity of 66.7% and a specificity of 88.2%.
Additionally, the area under the curve was 0.797, with a 95%
confidence interval ranging from 0.666 to 0.928 (Fig. 2B).
TBX2 expression as a predictor of
recurrence and overall survival
After applying a cutoff value of 9, the study
population was divided into two groups based on TBX2 expression
levels: the low expression group (score ≤8; 80 patients) and the
high expression group (score ≥9; 20 patients) of the weighted
score. No significant differences were observed between the two
groups in terms of age, histology, parametrium invasion, and lymph
node metastasis. However, there was a significant difference in the
FIGO stage between the groups (P=0.024; Table II). In the low TBX2 expression
group, 5 cases (6.25%) experienced recurrence, whereas in the high
expression group, 10 cases (50%) exhibited recurrence, and the
difference in the incidence of recurrence was significant
(P<0.01; Table III).
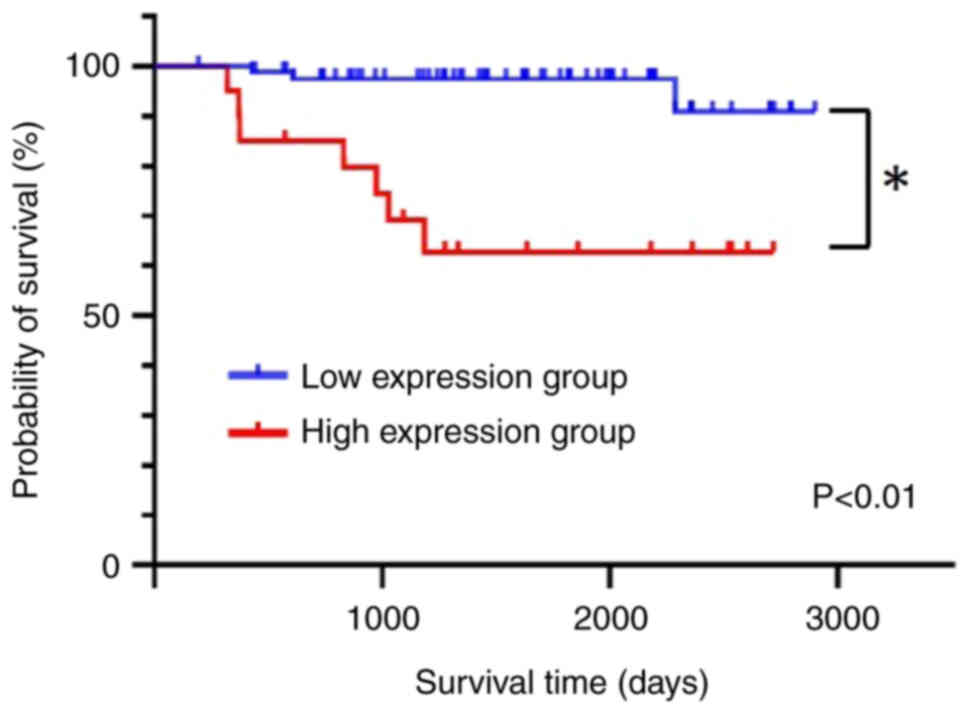
Furthermore, overall survival was significantly longer in the low
TBX2 expression group (P<0.01; Fig.
3), suggesting that TBX2 expression can be used to predict
overall survival.
 | Table II.Characteristics of the patients
according to T-box 2 expression. |
Table II.
Characteristics of the patients
according to T-box 2 expression.
|
Characteristics | Low expression
group | High expression
group | P-value |
|---|
| No. of
patients | 80 | 20 |
|
| Age,
yearsa | 54.5 (29–76) | 61.0 (37–76) | 0.93b |
| FIGO stage |
|
| 0.024c,d |
| I | 51 | 7 |
|
| II | 29 | 13 |
|
| Histology |
|
| 0.114d |
|
SCC | 67 | 13 |
|
|
Non-SCC | 13 | 7 |
|
| Parametrium
invasion |
|
| 0.053d |
| + | 18 | 9 |
|
| − | 62 | 11 |
|
| Lymph node
metastasis |
|
| 0.454d |
| + | 35 | 11 |
|
| − | 45 | 9 |
|
 | Table III.Association between T-box 2
expression and recurrence. |
Table III.
Association between T-box 2
expression and recurrence.
| expression, n
(%) | TBX2 recurrence
(%) | No Recurrence
(%) | P-value |
|---|
| Low
expressiona | 75 (93.8) | 5 (6.25) |
<0.01b,c |
| High
expressiond | 10 (50.0) | 10 (50.0) |
|
Identification of independent risk
factors for recurrence through multivariate analysis
Multivariate analysis was used to identify
independent predictors of recurrence in patients with intermediate-
and high-risk stage IB-IIB cervical cancer who received adjuvant TP
chemotherapy following radical hysterectomy. According to the
analysis, TBX2 expression and histological type were significant
risk factors for recurrence (Table
IV). The odds ratios associated with high TBX2 expression and
non-SCC histological types were 12.3 and 5.01, respectively. High
TBX2 expression was identified as the most significant independent
risk factor for recurrence in these patients, demonstrating the
highest odds ratio.
 | Table IV.Multivariate analysis for detecting
independent risk factors for recurrence. |
Table IV.
Multivariate analysis for detecting
independent risk factors for recurrence.
| Variable | Odds ratio | 95% confidence
interval | P-value |
|---|
| T-box 2 expression,
high/low | 12.3 | 3.0–50.5 |
<0.01a,b |
| Histology,
SCC/non-SCC | 5.01 | 1.06–20.50 | 0.038b,c |
| Parametrium
invasion, +/- | 1.99 | 0.471–8.390 | 0.350b |
| Lymph node
metastasis, +/- | 4.58 | 0.985–21.300 | 0.052b |
Enhancing sensitivity of cervical
cancer cells to cisplatin through TBX2 knockdown
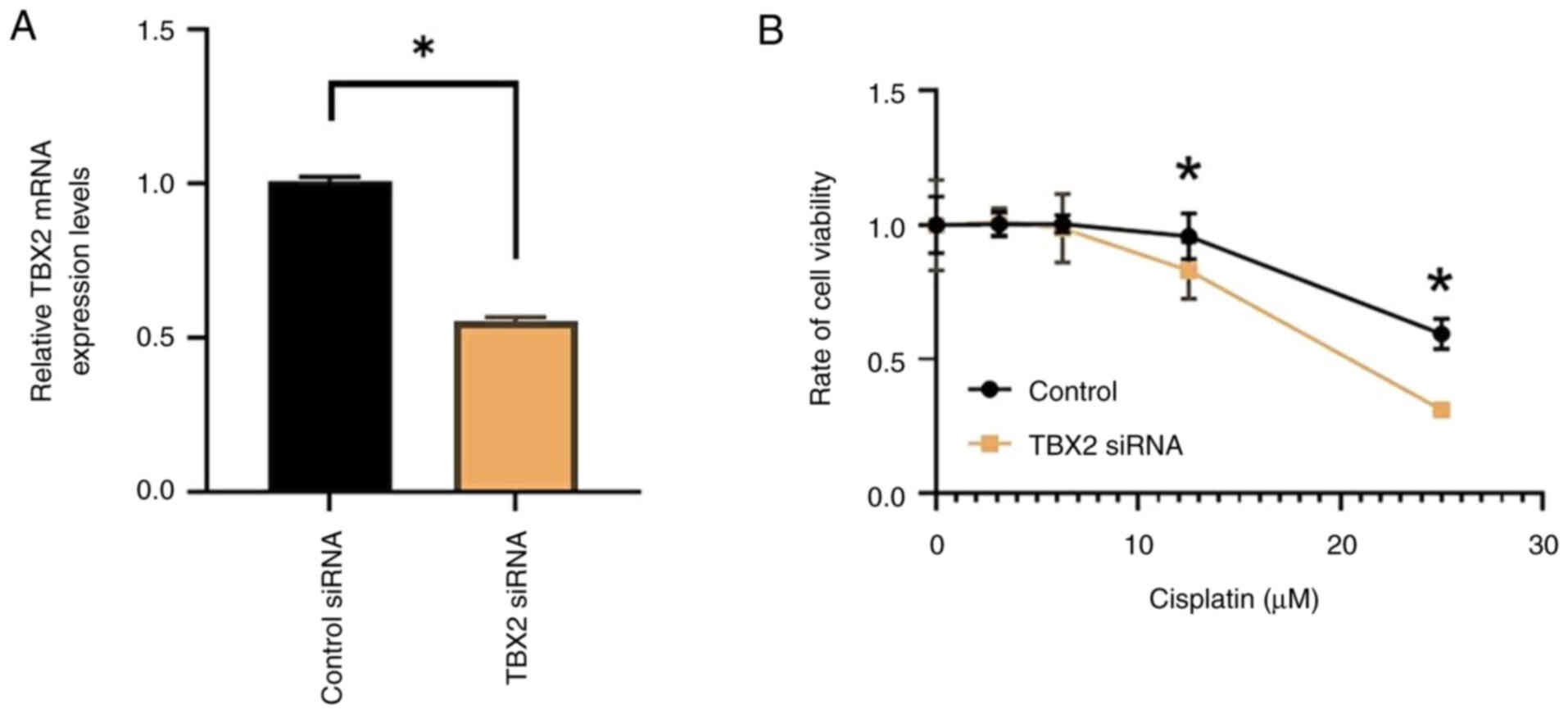
To assess the impact of TBX2 expression knockdown on
chemotherapy sensitivity, cervical cancer cell lines were utilized
in vitro. Transfection of si-TBX2 successfully decreased
TBX2 expression in these cells, as evidenced by RT-qPCR analysis,
which demonstrated a significant reduction in TBX2 mRNA levels
compared to cells transfected with control siRNA (P<0.01;
Fig. 4A). After confirming the
successful knockdown of TBX2, the viability of cervical cancer
cells with TBX2 expression knocked down was compared to that of the
control-transfected cells. Notably, at concentrations of ≥12.5 µM
cisplatin, the viability of tumor cells with TBX2 knockdown was
significantly lower than that of the corresponding control cells
(P<0.05; Fig. 4B). These
findings demonstrate that TBX2 knockdown increased the sensitivity
of cells to cisplatin.
Discussion
Despite the introduction of the HPV vaccine, which
can decrease the risk of developing cervical cancer by up to 90%
with complete vaccine coverage (29), cervical cancer remains a significant
global health threat for women. The current standard of care for
patients with intermediate- and high-risk stage IB-IIB cervical
cancer post-radical hysterectomy involves either irradiation or
concurrent chemoradiation, determined by the risk factors of
recurrence assessed using resected specimens (3,4).
However, these treatments, particularly following highly invasive
gynecologic surgery, can lead to severe side effects that
significantly impair a patient's quality of life. These include
urinary disturbances, lower-limb lymphedema, bowel obstruction,
sexual and ovarian dysfunction, and mental health issues stemming
from these chronic conditions (6–8). Given
these complications, there has been a global shift towards
exploring adjuvant chemotherapy as an alternative, with promising
results reported (6,7,9,10). A
notable randomized non-inferiority multicenter trial showed no
significant difference in 3-year progression-free survival rates,
which were 91.9% in both the adjuvant chemotherapy and concurrent
chemoradiation therapy (CCRT) groups. Similarly, 5-year overall
survival rates were 90.6% for chemotherapy and 90.0% for CCRT, with
the chemotherapy group displaying a trend towards a better quality
of life (9). Nevertheless, adjuvant
chemotherapy is not yet a standard treatment following radical
hysterectomy. If the likelihood of recurrence could be more
accurately predicted following adjuvant chemotherapy, this would
provide valuable guidance for gynecological oncologists in choosing
between chemotherapy and radiation therapy as adjuvant
treatments.
The TBX gene family consists of five distinct
subfamilies: T, Tbx1, Tbx2, Tbx6, and Tbr1. Within the T subfamily,
the T subfamily encompasses T and TBX19; the Tbx1 subfamily
encompasses TBX1, TBX10, TBX15, TBX18, TBX20, and TBX22; the Tbx2
subfamily includes TBX2, TBX3, TBX4, and TBX5; the Tbx6 subfamily
contains TBX6 and Mga; and the Tbr1 subfamily consists of TBR1,
TBR2, and TBX21 (11). TBX genes
play pivotal roles in organogenesis and pattern formation across
vertebrate and invertebrate species (11). TBX2 is a transcription factor that
was found to be involved in the regulation of cell cycle
progression during cancer and embryonic development (12,13).
TBX2 has been shown to facilitate the evasion of senescence by
down-regulating the cell cycle regulators p21 and p14 (16,30,31).
In addition to its role in cell cycle regulation, TBX2 also
mediates apoptotic signaling pathways through p21 (32). Suppression of p21 reportedly leads
to chemoresistance via modulation of the G1/S cell cycle transition
and inhibiting apoptosis induced by chemotherapy in lung cancer
(33). Additionally, knockdown of
TBX2 sensitized cisplatin-resistant breast cancer cells to
cisplatin (23), which is
consistent with the results of the present study. However, to the
best of our knowledge, the association between TBX2 expression and
the efficacy of platinum-based adjuvant chemotherapy in patients
with intermediate to high-risk stage IB-IIB cervical cancer
following radical hysterectomy remains largely unclear.
In the present study, it was found that TBX2
expression levels were associated with both recurrence and overall
survival rates among patients with intermediate- to high-risk stage
IB-IIB cervical cancer who underwent adjuvant TP chemotherapy
following radical hysterectomy. Specifically, the high expression
group (score ≥9; consisting of 20 patients) exhibited a higher
likelihood of recurrence and poorer overall survival outcomes.
Multivariate analysis aimed at identifying independent predictors
of recurrence in this patient subset revealed that high TBX2
expression emerged as the most significant risk factor,
demonstrating the highest odds ratio. Furthermore, the in
vitro experiments confirmed that TBX2 knockdown using siRNA
enhanced the effectiveness of cisplatin against cervical cancer
cells.
This study has several limitations. Firstly, its
retrospective design may introduce biases that affect the
collection, analysis, and interpretation of data. Secondly, it
includes a relatively small cohort of 100 patients, which could
restrict the generalizability of the findings and reduce the
statistical power. Thirdly, the study participants were drawn from
specific institutions within a limited geographical area, which may
further constrain the applicability of the findings to broader
populations or regions. Fourthly, TBX2 expression was assessed
using only immunohistochemical techniques and a single scoring
system. Fifthly, the study did not examine interactions with other
biomarkers, such as CLPTM1L, which is also reported to be
associated with the effectiveness of TP chemotherapy (34). Sixthly, the study did not
investigate the underlying mechanisms by which TBX2 contributes to
chemoresistance. To validate these results, larger, multicenter
prospective studies are necessary. Standardizing the techniques for
assessing TBX2 expression is crucial for reliable clinical
implementation. Further research into the biological mechanisms by
which TBX2 contributes to chemoresistance and tumor progression
could uncover additional therapeutic targets. Additionally, further
research should also investigate additional factors that may
interact with TBX2 expression levels to improve the accuracy of
predictions regarding recurrence.
To the best of our knowledge, this study is the
first to show the correlation between TBX2 expression and
recurrence among cervical cancer patients treated with TP as
adjuvant chemotherapy. TBX2 shows promise as a valuable clinical
marker for gauging the efficacy of TP in this patient cohort,
characterized by intermediate- to high-risk stage IB-IIB disease
following radical hysterectomy. While adjuvant chemotherapy is
gaining traction due to growing evidence of its clinical benefits,
it has yet to become a standard guideline-recommended treatment.
Therefore, it is crucial to identify reliable indicators that
gynecological oncologists can utilize to assess the sensitivity of
adjuvant chemotherapy. By stratifying patients based on TBX2
expression levels, gynecological oncologist can tailor adjuvant
treatment plans more effectively. For instance, patients with high
TBX2 expression might benefit from more aggressive monitoring and
conventional therapeutic strategies including irradiation or
concurrent chemoradiation. Conversely, patients with low TBX2
expression, who are at a lower risk of recurrence with adjuvant TP
chemotherapy, could choose TP chemotherapy as adjuvant therapies
and avoid the side effects related to irradiation or concurrent
chemoradiation, thereby improving their quality of life. This study
may contribute significantly to the ongoing effort to identify
optimal candidates for adjuvant chemotherapy among patients with
cervical cancer.
In conclusion, the present study suggests that TBX2
expression may potentially function as a predictive biomarker for
recurrence in patients with intermediate- and high-risk stages
IB-IIB cervical cancer treated with adjuvant TP chemotherapy
post-radical hysterectomy.
Acknowledgements
The authors would like to thank Dr Yukimi Kira
(Research Support Platform, Osaka Metropolitan University, Graduate
School of Medicine in Osaka, Japan) for their technical
assistance.
Funding
This study received financial support from the JSPS KAKENHI
(grant no. 19K09808).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TN, TF and TS conceived and designed the research.
TN, EU, YA, TW, RT and MY performed the experiments and collected
the data. TN, TF, TY and TS were responsible for data analysis. TN
and TF drafted the manuscript. TN and TF confirm the authenticity
of all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Osaka Metropolitan
University Hospital (Osaka, Japan) approved the study protocol
(approval no. 2022-102). All participants provided written informed
consent to take part in this study.
Patient consent for publication
Written informed consent was obtained from all
participants for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
FIGO Committee on Gynecologic Oncology, .
FIGO staging for carcinoma of the vulva, cervix, and corpus uteri.
Int J Gynaecol Obstet. 125:97–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abu-Rustum NR, Yashar CM, Arend R, Barber
E, Bradley K, Brooks R, Campos SM, Chino J, Chon HS, Crispens MA,
et al: NCCN guidelines® insights: Cervical cancer,
version 1.2024. J Natl Compr Canc Netw. 21:1224–1233. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ebina Y, Mikami M, Nagase S, Tabata T,
Kaneuchi M, Tashiro H, Mandai M, Enomoto T, Kobayashi Y, Katabuchi
H, et al: Japan society of gynecologic oncology guidelines 2017 for
the treatment of uterine cervical cancer. Int J Clin Oncol.
24:1–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peters WA III, Liu PY, Barrett RJ II,
Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W Jr and
Alberts DS: Concurrent chemotherapy and pelvic radiation therapy
compared with pelvic radiation therapy alone as adjuvant therapy
after radical surgery in high-risk early-stage cancer of the
cervix. J Clin Oncol. 18:1606–1613. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hosaka M, Watari H, Kato T, Odagiri T,
Konno Y, Endo D, Mitamura T, Kikawa S, Suzuki Y and Sakuragi N:
Clinical efficacy of paclitaxel/cisplatin as an adjuvant
chemotherapy for patients with cervical cancer who underwent
radical hysterectomy and systematic lymphadenectomy. J Surg Oncol.
105:612–616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matoda M, Takeshima N, Michimae H, Iwata
T, Yokota H, Torii Y, Yamamoto Y, Takehara K, Nishio S, Takano H,
et al: Postoperative chemotherapy for node-positive cervical
cancer: Results of a multicenter phase II trial (JGOG1067). Gynecol
Oncol. 149:513–519. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li S, Hu T, Chen Y, Zhou H, Li X, Cheng X,
Yang R, Wang S, Xie X and Ma D: Adjuvant chemotherapy, a valuable
alternative option in selected patients with cervical cancer. PLoS
One. 8:e738372013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weng D, Xiong H, Zhu C, Wan X, Chen Y,
Wang X, Zhang Y, Jiang J, Zhang X, Gao Q, et al: Adjuvant
chemotherapy versus adjuvant concurrent chemoradiotherapy after
radical surgery for early-stage cervical cancer: A randomized,
non-inferiority, multicenter trial. Front Med. 17:93–104. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Yu R, Zhang L, Wang R and Xiao L:
Chemotherapy versus chemoradiotherapy for FIGO stages IB1 and IIA1
cervical squamous cancer patients with lymphovascular space
invasion: A retrospective study. BMC Cancer. 22:2022022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang F, Xing P, Song F, Du X, Wang G,
Chen K and Yang J: The role of T-box genes in the tumorigenesis and
progression of cancer. Oncol Lett. 12:4305–4311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bilican B and Goding CR: Cell cycle
regulation of the T-box transcription factor tbx2. Exp Cell Res.
312:2358–2366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abrahams A, Parker MI and Prince S: The
T-box transcription factor Tbx2: Its role in development and
possible implication in cancer. IUBMB Life. 62:92–102. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu R, Deng J, Li C, Xu C, Cui ZH and Zhao
J: Clinical significance of TBX2 in esophageal squamous cell
carcinomas and its role in cell migration and invasion. Eur Rev Med
Pharmacol Sci. 24:3062–3068. 2020.PubMed/NCBI
|
|
15
|
Ding N, Zhang T, Yu X and Zhuang S: T-box
transcription factor 2 enhances chemoresistance of endometrial
cancer by mediating NRF2 expression. Curr Protein Pept Sci.
23:563–570. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vance KW, Carreira S, Brosch G and Goding
CR: Tbx2 is overexpressed and plays an important role in
maintaining proliferation and suppression of senescence in
melanomas. Cancer Res. 65:2260–2268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nandana S, Tripathi M, Duan P, Chu CY,
Mishra R, Liu C, Jin R, Yamashita H, Zayzafoon M, Bhowmick NA, et
al: Bone metastasis of prostate cancer can be therapeutically
targeted at the TBX2-WNT signaling axis. Cancer Res. 77:1331–1344.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Y, Li Z, Zhong Q, Li G, Zhang Y and
Huang Z: Association of TBX2 and P21 expression with
clinicopathological features and survival of laryngeal squamous
cell carcinoma. Int J Clin Exp Med. 7:5394–5402. 2014.PubMed/NCBI
|
|
19
|
Zhang Z and Guo Y: High TBX2 expression
predicts poor prognosis in non-small cell lung cancer. Neoplasma.
61:476–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu H, Liu BO, Liu A, Li K and Zhao H:
T-box 2 expression predicts poor prognosis in gastric cancer. Oncol
Lett. 10:1689–1693. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahlamäki EH, Bärlund M, Tanner M,
Gorunova L, Höglund M, Karhu R and Kallioniemi A: Frequent
amplification of 8q24, 11q, 17q, and 20q-specific genes in
pancreatic cancer. Genes Chromosomes Cancer. 35:353–358. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davis E, Teng H, Bilican B, Parker MI, Liu
B, Carriera S, Goding CR and Prince S: Ectopic Tbx2 expression
results in polyploidy and cisplatin resistance. Oncogene.
27:976–984. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wansleben S, Davis E, Peres J and Prince
S: A novel role for the anti-senescence factor TBX2 in DNA repair
and cisplatin resistance. Cell Death Dis. 4:e8462013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ismail A and Bateman A: Expression of TBX2
promotes anchorage-independent growth and survival in the
p53-negative SW13 adrenocortical carcinoma. Cancer Lett.
278:230–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inoue Y, Fukuda T, Nanno S, Awazu Y,
Shimomura M, Matsubara H, Yamauchi M, Yasui T and Sumi T: T-box 2
expression is a useful indicator of the response to neoadjuvant
chemotherapy for patients with locally advanced uterine cervical
squamous cell carcinoma. Oncol Lett. 22:7552021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tasaka R, Fukuda T, Shimomura M, Inoue Y,
Wada T, Kawanishi M, Yasui T and Sumi T: TBX2 expression is
associated with platinum-sensitivity of ovarian serous carcinoma.
Oncol Lett. 15:3085–3090. 2018.PubMed/NCBI
|
|
27
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Sanjose S, Quint WG, Alemany L, Geraets
DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin
HR, et al: Human papillomavirus genotype attribution in invasive
cervical cancer: A retrospective cross-sectional worldwide study.
Lancet Oncol. 11:1048–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peres J, Davis E, Mowla S, Bennett DC, Li
JA, Wansleben S and Prince S: The highly homologous T-box
transcription factors, TBX2 and TBX3, have distinct roles in the
oncogenic process. Genes Cancer. 1:272–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jacobs JJ, Keblusek P, Robanus-Maandag E,
Kristel P, Lingbeek M, Nederlof PM, van Welsem T, van de Vijver MJ,
Koh EY, Daley GQ and van Lohuizen M: Senescence bypass screen
identifies TBX2, which represses Cdkn2a (p19(ARF)) and is amplified
in a subset of human breast cancers. Nat Genet. 26:291–299. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujiwara K, Daido S, Yamamoto A, Kobayashi
R, Yokoyama T, Aoki H, Iwado E, Shinojima N, Kondo Y and Kondo S:
Pivotal role of the cyclin-dependent kinase inhibitor p21WAF1/CIP1
in apoptosis and autophagy. J Biol Chem. 283:388–397. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Zhu LJ, Yang YC, Wang ZX and Wang
R: MiR-224 promotes the chemoresistance of human lung
adenocarcinoma cells to cisplatin via regulating G1/S transition
and apoptosis by targeting p21(WAF1/CIP1). Br J Cancer.
111:339–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Awazu Y, Fukuda T, Noda T, Uchikura E,
Nanno S, Imai K, Yamauchi M, Yasui T and Sumi T: CLPTM1L expression
predicts recurrence of patients with intermediate- and high-risk
stage IB-IIB cervical cancer undergoing radical hysterectomy
followed by TP as adjuvant chemotherapy. Oncol Lett. 26:3532023.
View Article : Google Scholar : PubMed/NCBI
|