Introduction
Glioblastoma is the most aggressive and the most
frequent brain neoplasia: its incidence is about 5–8 cases per
100,000 inhabitants and represents 54% of all the diagnosed gliomas
(1,2). Recent data show a stable incidence in
the US or Canada (3), while English
and European reports indicate that the incidence is increasing
(4).
These tumors are believed to origin from neuroglial
or progenitor stem cells and are molecularly heterogeneous
(5). The brain tissue
microenvironment, including stem cells niches and blood-brain
barrier, strongly affects the low rate of glioblastoma metastasis
out of the brain, but better promotes brain-invading cancer cells
(6).
Molecular profiling has identified three subgroups
associated with TERT promoter mutation (7,8): a
molecule that plays an important role in cancer formation and in
safeguarding chromosomal steadiness by maintaining telomeres'
length and has also a role in cellular aging (9). These molecular elements impact tumor
growth, avoiding senescence and enabling immortal growth. None of
the above-mentioned subtypes are predictive for pharmacological
response to present therapies, besides the assignment to subtypes
can be difficult be-cause of the intra-tumoral heterogeneity and
also the switching subtype is possible through the evolution of
disease. Despite the advantages made in our understanding of
glioblastoma biology and the current treatment of glioblastoma,
including chemotherapy, radiotherapy and surgical approaches, the
outcome remains dismal: the median overall survival (mOS) ranging
from 14.6 to 20 months (10) and
the 5-year survival is less than 10% (11). The treatments fail mainly for the
unique molecular features of GBM, particularly due to the presence
of a population of stem-like cells called glioma stem cells (GSCs)
with ability of self-renewal, making it resistant to current
treatments, but also to the presence of blood-brain barrier (BBB)
and the privileged immune status (12). For this reason, even a little
surgical residue after resection can lead to a lethal recurrence
(13). The main weapon following
surgery, is the use of the temozolomide (TMZ)-based treatment: this
drug is an alkylating agent that better works in
methylguanine-DNA-methyltransferase (MGMT)-methylated glioblastomas
(14,15). Because of the absence of approved
healing treatments, the National Comprehensive Cancer Network
(NCCN) recommends clinical trials for eligible patients (16) in order to administer tailored
treatments basing on age, functional status, goals of care, etc.
and to present palliative care earlier in the course of disease
(3). If the patient cannot be
entered into any clinical trial, the Stupp protocol is the approved
standard treatment but roughly 70% of patients will progress within
a year and only approximately 27% will be alive at two years
(17,18). In Stupp protocol, TMZ can be
administered in its conventional schedule (6 cycles) or in its
extended schedule (more than 6 cycles). Extended duration of TMZ
has been found to be well tolerated, with a low number of major
toxicities. Many studies have demonstrated a survival benefit in
the extended schedule (mOS 24–31 months) compared to the
conventional schedule (mOS 8–16.5 months) (19–22).
However, the Spanish Group of Research in Neuro-Oncology (GEINO
group) investigated in a phase 2 prospective trial (GEINO 14–01)
the optimal duration of TMZ treatment, finding out that extending
TMZ after the sixth cycle gave more toxicities and no benefit in 6
months progression free survival (PFS) (23).
Because of these contrasting data, we decided to
conduct a bi-centric retrospective analysis to highlight the
efficacy of extending adjuvant treatment with temozolomide in
patients with glioblastoma.
Patients and methods
Study design and participants
Our study analyzed the effectiveness of extended
temozolomide as adjuvant therapy after a first phase of concomitant
chemo-radiation in 87 patients diagnosed with glioblastoma. All
data were collected retrospectively from two institutions, Azienda
Ospedaliera Universitaria Luigi Vanvitelli (Napoli, Italy) and
Ospedale Civile ‘San Giovanni di Dio’ (Frattamaggiore, Italy).
Inclusion criteria were those of clinical practice: patients should
be 18 years or older, histologically confirmed glioblastoma
diagnosis, adequate bone marrow, liver and renal function, stable
dose of glucocorticoids with a performance status according to the
Eastern Cooperative Oncology Group (ECOG) between 0 and 2.
Exclusion criteria were recurrent disease, other metachronous
malignancies, need for antiviral treatment for active hepatitis B
and C, contemporary use of strong cytochrome P3A4 inhibitors or
inducers, treatment discontinuation due to toxicity. We collected
data on Isocitrate Dehydrogenase (IDH) mutational status, although
the newer WHO classification of CNS (24) tumors define glioblastomas as
strictly IDH wild type. We decided to include also these patients
based on the initial histological report made at the time of first
diagnosis. MGMT methylation was also collected. Both were analyzed
on archived tumor tissue, stored in separate laboratories for each
center. MGMT methylation status was assessed by methylation array
by EPIC array Illumina 850k (25)
or Methylation Specific PCR (MSP/PCR) (26), while IDH mutation status was
assessed by methylation array by EPIC array Illumina 850k (25) or immunohistochemistry (27). Molecular analysis was not available
for all patients as some patients underwent surgery in different
centers and, due to the retrospective nature of our study,
information were difficult to retrieve.
Procedures
All patients underwent surgical resection or biopsy
followed by radiotherapy with concomitant temozolomide (75
mg/m2/day). After concurrent chemoradiation, treatment
was temporarily suspended for the duration of one month and then
reprised with adjuvant temozolomide as monotherapy, five days every
28 days: first cycle was administered as 150 mg/m2/day,
following cycles as 200 mg/m2/day. The choice to
administer six or more cycles was taken by the neurooncologist
responsible for the patient based on her/his experience. Brain MRI
evaluation was conducted firstly after 40–60 days the last day of
chemoradiation and then every three months since the start of
temozolomide monotherapy; in case of clinical signs suggestive of
progressive disease, brain MRI could be anticipated based on
clinician's decision. Tumor progression was defined according to
Response Assessment in Neuro-Oncology (RANO criteria). Data were
collected until 17th April 2023.
Outcomes
Primary endpoint was OS, defined as time from
treatment start to death from any cause, whereas secondary endpoint
was PFS, defined as time from treatment start to disease
progression or death. PFS2, time from second line start to disease
progression or death, was also analyzed. OS, PFS and PFS2 were
estimated with Kaplan-Meier methods. Survival data were also
stratified according to MGMT methylation status and then excluding
IDH mutant tumors. We evaluated the outcomes between 45 patients
who discontinued temozolomide therapy at 6 cycles in accordance
with the protocol outlined by Stupp et al (17) (6C group) and 42 patients wherein TMZ
therapy was continued until 12 cycles (12C group). Accordingly,
patients who stopped temozolomide before 6 cycles of therapy
because of tumor progression or death were excluded from
analysis.
Statistical analysis
Patient data were accounted as median with range of
minimum and maximum values between parentheses for continuous
variables and only percentages for categorical variables. Kaplan
Meier estimates were used to help computing survival curves, while
survival differences were analyses using the log-rank test,
significance level of P=0.05. Statistical analyses were performed
using IBM SPSS statistics v.23.0.
Results
Patient's characteristics are summarized in Table I. We included 87 patients with
glioblastoma, who received 6 or 12 cycles of temozolomide therapy
between 2012 and 2022. Around sixty-five percent (n=56) were male.
Median age was 61.6 years (range 31–75). The majority of the
patients (83.9%) presented with Eastern Cooperative Oncology Group
(ECOG) PS 0–1. Forty-five patients were in the 6C group and
forty-two patients in the 12C group. In these 87 patients, MGMT
promoter status was known in 56 patients. MGMT promoter was
methylated in 44.4% (20/45) and 23.8% (10/42) in the 6C and 12C
group respectively. In the remaining 26 patients, MGMT promoter was
unmethylated. There was no association between MGMT promoter
methylation status and the number of cycles given. As anticipated,
we included both IDH wild type and IDH mutant tumors based on
initial report made at time of first diagnosis. As expected, the
majority (70.1%) were IDH wild type tumors. In only 6 patients IDH
was mutated and in 23% the mutational status was instead
unknown.
 | Table I.Population characteristics. |
Table I.
Population characteristics.
| Variable | All patients | 6C | 12C |
|---|
| Number, n (%) | 87 (100.00) | 45 (51.7) | 42 (48.3) |
| Median age at
diagnosis, years (range) | 62 (31–75) | 63 (31–73) | 60 (32–75) |
| Male, n (%) | 56 (64.4) | 25 (55.6) | 31 (73.8) |
| PS 0–1, n (%) | 73 (83.9) | 36 (80.0) | 37 (88.1) |
| Surgery, n (%) | 73 (83.9) | 41 (91.1) | 32 (76.2) |
| MGMT-methylated, n
(%) | 30 (34.5) | 20 (44.4) | 10 (23.8) |
| MGMT-unmethylated,
n (%)a | 26 (29.9) | 14 (31.1) | 12 (28.8) |
| IDH wild-type, n
(%) | 61 (70.1) | 33 (73.3) | 28 (66.7) |
| Other genomic
alterations, n | 1 | 0 | 1 (BRAF V600E) |
| Second-line
therapy, n (%) | 72 (82.8) | 42 (93.3) | 30 (71.4) |
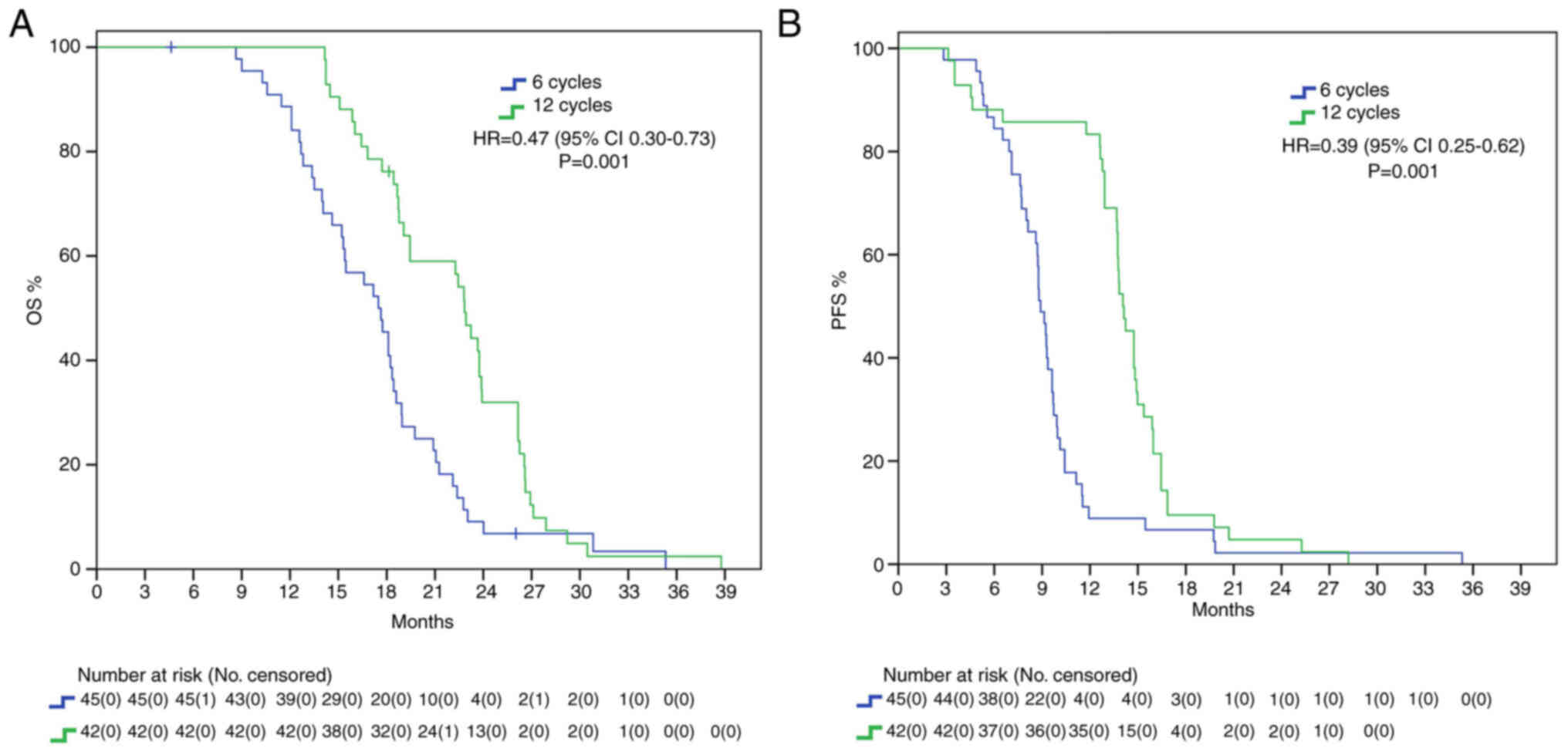
Patients whose adjuvant temozolomide therapy was
stopped at 6 cycles had a mOS of 17.5 months, whereas those that
received 12 cycles reached a mOS of 22.8 months, presenting with a
statistically significant benefit (HR 0.47, 95% IC 0.30–0.73
P=0.001). Furthermore, mPFS difference was also statistically
significant, with a delta of around 6 months between 12C group and
6C group (15.3 vs. 9 months, HR: 0.39, 95%IC 0.25–0.62, P=0.001)
(Fig. 1).
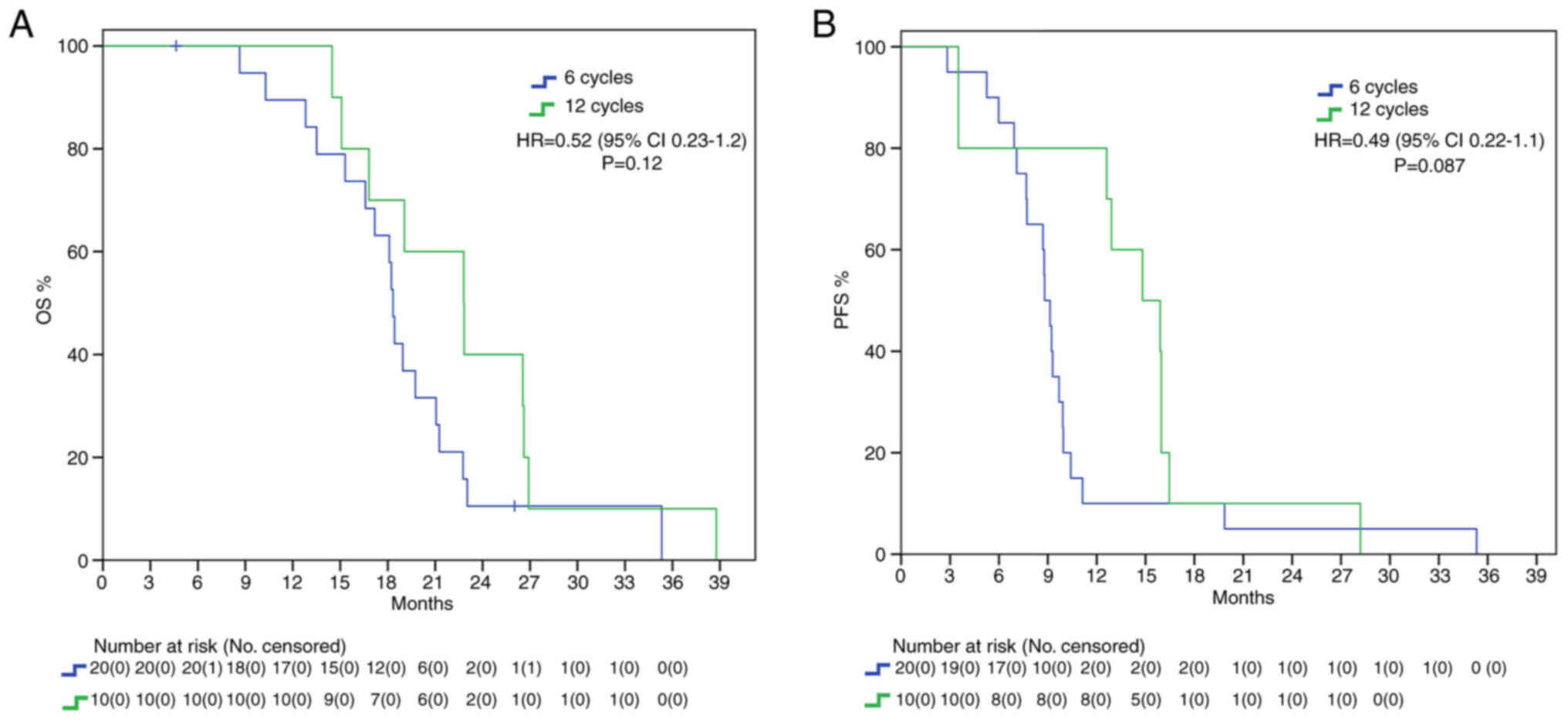
Endpoints were also evaluated in the different
subgroups. In MGMT methylated patients, there was a mOS benefit
trend in the 12C group (22.8 vs. 18.3 months, HR: 0.52, 95%IC
0.23–1.2, P=0.12) and there was also a positive trend for mPFS
(14.8 vs. 8.8 months, HR: 0.49, 95%IC 0.22–1.1, P=0.087) without
statistical significance (Fig. 2).
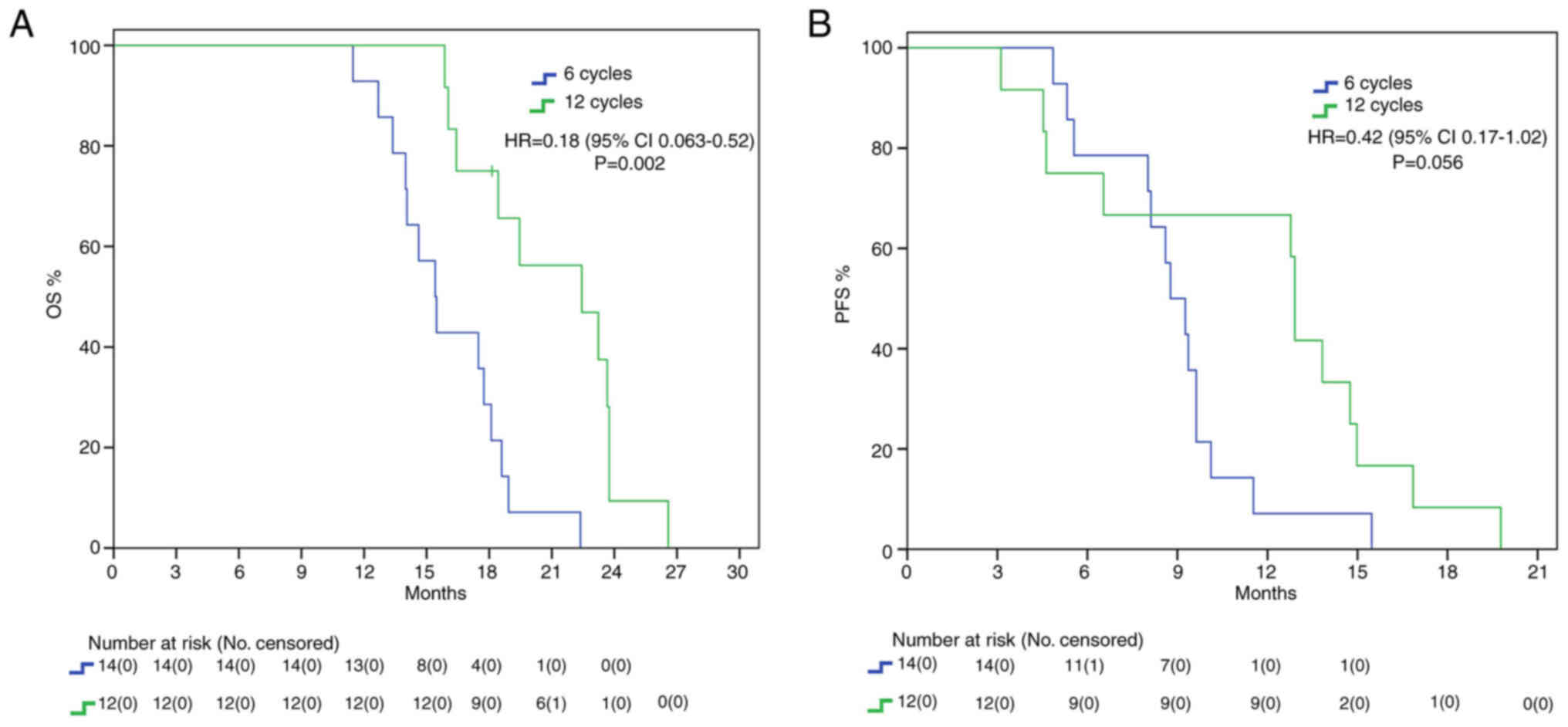
Different findings resulted from the analysis in the MGMT
unmethylated subgroup: we found a statistically significant benefit
in mOS for the 12C group (22.4 vs. 15.4 months, HR: 0.18, 95%IC
0.063–0.52, P=0.002) but no statistical benefit in mPFS (12.9 vs.
8.8 months, HR: 0.42, 95%IC 0.17–1.02, P=0.056) (Fig. 3).
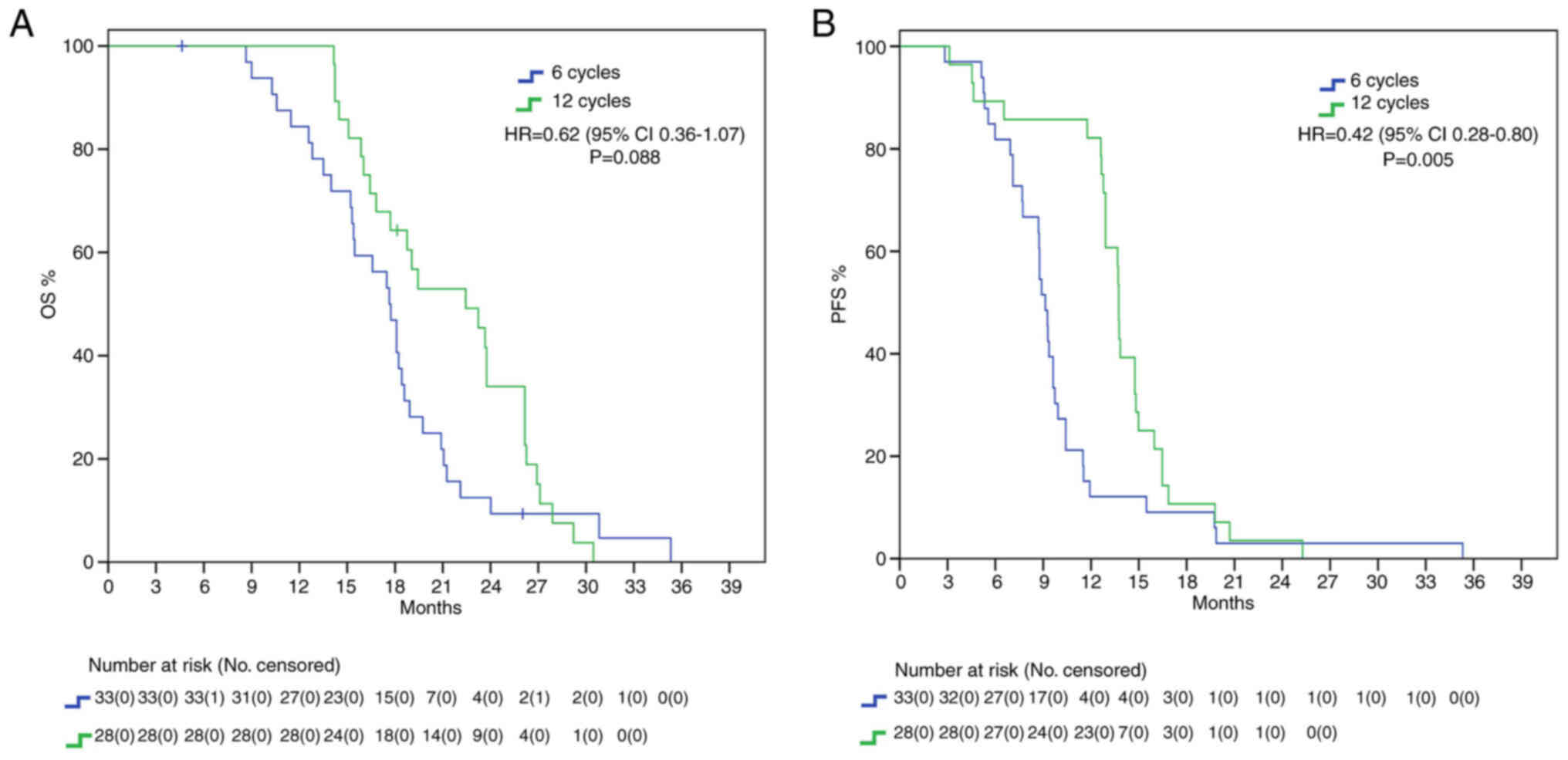
We repeated our analysis excluding IDH mutant
patients, since according to the newest WHO CNS classification they
cannot be diagnosed as glioblastomas (24). Nevertheless, here we found a
positive trend for mOS (22.4 vs. 17.6 months, HR: 0.62, 95%IC
0.36–1.07, P=0.088) and a significant difference for mPFS (13.7 vs.
9.1 months, HR: 0.42, 95%IC 0.28–0.80, P=0.005) in favor of 12C
group (Fig. 4).
Both treatments were generally well tolerated, with
a toxicity profile consistent with literature data. Leukopenia was
the most frequently observed treatment-related hematologic adverse
event, while the most frequent non-hematologic adverse event was
fatigue. Overall, in the group of patients treated with the
extended schedule of TMZ, there was an increase of adverse events,
however it was not necessary to report a statistically significant
difference (Table II).
 | Table II.Comparison of the toxicities between
patients treated with 6 or 12 cycles. |
Table II.
Comparison of the toxicities between
patients treated with 6 or 12 cycles.
| Adverse event | 6 cycles, n (%)
(n=45) | 12 cycles, n (%)
(n=42) |
|---|
| Leukopenia | 10 (22) | 11 (26) |
| Neutropenia | 9 (20) | 9 (21) |
| Anemia | 3 (7) | 3 (7) |
|
Thrombocytopenia | 4 (9) | 5 (12) |
| Fatigue | 14 (31) | 18 (43) |
| Nausea | 13 (29) | 13 (31) |
| Constipation | 4 (9) | 4 (10) |
| Pneumonia | 2 (4) | 2 (5) |
| Hepatotoxicity | 4 (9) | 3 (7) |
| Headache | 5 (11) | 7 (17) |
| Dizziness | 2 (4) | 5 (12) |
Discussion
The prognosis of patients with glioblastoma remains
poor both because, despite after gross total resection there is a
high chance of residual disease, and because of the poor efficacy
of chemotherapy and radiotherapy treatments. Furthermore, the EORTC
26981/2981/NCIC CE.3 trial (17)
allowed only 6 adjuvant cycles of temozolomide, but that was
established as an arbitrary limit. For this reason, adjuvant
temozolomide has been prolonged by many investigators, both in
everyday practice and in clinical trials, generally up until 12
cycles. Easiness of oral administration and low toxicity profile
have also favored prolonging treatment.
While many studies have investigated the benefits of
extended adjuvant TMZ (Table
III), no definitive indication has been implemented. NCCN
guidelines (2023) advice against prolonging treatment (16); European Society for Medical Oncology
(ESMO) guidelines, last updated in 2014, do not analyze the
controversy; on the contrary, Associazione Italiana di Oncologia
Medica (AIOM 2021) guidelines on brain tumor consider the
possibility of continuing adjuvant treatment until 12 cycles
(28). We found a statistically
significant benefit in the 12C group both in mOS (22.8 vs. 17.5
months, HR 0.47) and in mPFS (15.3 vs. 9 months, HR 0.39) in the
overall population, with around 5 months delta in both setting. It
must be noted that our survival data are in range with expectations
from known literature and thus do not classify as outlier. While
mPFS curves seem to cross early on, casting benefit of extended
adjuvant treatment in regard to mPFS, the curves tend to grow apart
with time, showing its survival benefit. We further analyzed our
results considering MGMT methylation status to consider the
possibility of only MGMT methylated GBMs having increased survival
from extended therapy. Our results show that this subgroup of
patients presents with a trend in increased mOS and mPFS in the 12C
group. This is in line with the well-known role of MGMT promoter
methylation as a predictive factor of increased response to
alkylating agents (29,30). It must be said that MGMT methylation
determines better prognosis, and it has been speculated that
increased survival allows patients to receive more extended
treatment (31). Furthermore,
mutational changes due to prolonged temozolomide, especially in
patients with absence of MGMT-mediated DNA repair, may promote
tumor resistance thanks to the acquisition of an alkylating
agents-resistant phenotype (32,33).
The phase II RESCUE trial on continuous dose-intense temozolomide
in recurrent GBM demonstrated worse results in those patients that
experienced progression while on extended treatment, while
increased survival was found in those with at least a 2-month
treatment free interval or experiencing progression on standard
treatment (34). In contrast with
these results, no mPFS benefit was found in the unmethylated
cohort, with only a small trend in increased survival (12.9 vs. 8.8
months). Instead, mOS was found to be statistically significantly
higher in the 12C group than in the 6C group (22.4 vs. 15.4
months). We speculated that the increase in OS may be due to second
line treatment. About 99.7% of patients in 12C group and 87.5% in
6C group underwent second line therapy. However, no difference was
found in PFS2 between the two group. IDH mutant gliomas are
characterized by increased survival and tumor response (35). However, whereas the 2016
classification allowed for IDH wild type and IDH mutant GBMs, the
newest 2021 WHO classification of CNS tumors classify GBM strictly
as IDH wild type, IDH mutant being astrocytoma or oligodendroglioma
according to 1p-19q codeletion status (24). We included in our analysis 6
patients who presented with mutation in IDH1 or IDH2 and 20 with
unknown alterations. Nevertheless, we decided to include these
patients due to the fact that, at the time of diagnosis, they were
classified as GBM. We then repeated mOS and mPFS analysis excluding
these 26 patients, limiting our scope accordingly to the newest
definition of GBM. Even stratifying according to the newest
definition, we confirmed a statistically significant difference in
mOS (22.4 vs. 17.6 months) and mPFS (13.7 vs. 9.1 months) in the
12C arm compared to the 6C arm. IDH-based classification is a
fundamental game-changer in CNS research. Many previous trials on
GBMs often included IDH mutant GBMs, misclassified and now
considered a different entity, thus limiting their interpretations.
For example, Chen et al (36) study on extended temozolomide
reported in a retrospective cohort an increased difference in mOS
between the extended adjuvant cohort and the control group, around
9.3 months (29 vs. 16.7 months). However, looking at population
characteristics, only 27.5% patients in the control group were IDH
mutant against 43.4% in the extended adjuvant group, increasing the
chance of a higher survival in the latter arm. Indeed, survival
benefit was even higher in the IDH1 mutant subgroup (+20.5 months),
while there was only a 7-months difference between the two arms in
IDH1 wild type subgroup.
 | Table III.Studies on extended adjuvant TMZ. |
Table III.
Studies on extended adjuvant TMZ.
| First author/s,
year | Treatment | Survival
results | (Refs.) |
|---|
| Chen et al,
2022 | TMZ + RT→TMZx6 or ×
>6 | ITT and methylated
improved OS and PFS; unmethylated improved OS, longer PFS; IDH1
mutant presented with bigger delta: More suitable to extended
TMZ | (36) |
| Balana et
al, 2020 | TMZ + RT→TMZx6 or
×12 | No benefit in OS
and PFS in general population, in MGMT methylated and in pts with
measurable disease | (23) |
| Hau et al,
2007 | TMZ + RT→TMZx
>6 | Increased 2-year OS
in primary GBM group with prolonged TMZ | (21) |
| Bhandari et
al, 2017 | TMZ + RT→TMZ ×6 or
×12 | Prolonged OS and
PFS, but no statistical significance | (54) |
| Skardelly et
al, 2017 | TMZ + RT→TMZ ×6 or
× >6 | Prolonged OS and
PFS, but no statistical significance. MGMT status, EOR, age were
significant covariates for survival | (42) |
| Blumenthal et
al, 2017a | TMZ +
RT→TMZb | Prolonged PFS, but
no statistical significance. Benefit enhanced in MGMT-methylated
group but lost in MGMT-unmethylated; no OS benefit | (33) |
| Refae et al,
2015 | TMZ + RT→TMZ ×6 or
× >6 | Better OS and PFS
with extended TMZ | (22) |
| Darlix et
al, 2013 | TMZ + RT→TMZ ×6 or
× >6 | Increased OS and
PFS with extended TMZ | (37) |
| Roldán Urgoiti
et al, 2012 | TMZ + RT→TMZ ×6 or
× >6 | Increased OS and
PFS in extended TMZ | (19) |
Our study is in line with previous analyses
demonstrating increasing benefit from extended therapy (Table III) (19,22,36,37).
Our results suggest, then, that extended adjuvant treatment may be
a good therapeutic opportunity in fit patients to increase survival
rates. Rigorous patient selection is of course needed, and while
MGMT methylation may take the spotlight, several other factors may
influence treatment choice. Keeping in mind the limitations of Chen
study, they showed an increased mPFS in newly diagnosed GBMs with
higher expression of Ki67 treated with extended adjuvant
temozolomide, while no such difference was found in patients with
lower Ki67 expression. No difference was found in mOS as both
groups benefitted from extended adjuvant treatment (36). A retrospective analysis by Bocangel
et al (38) evaluated the
role of p53 status, since literature reported that p53 mutational
and expression status was associated to GBM prognosis. Indeed, wild
type p53 was found to inhibit MGMT expression, potentially
increasing the response rate to alkylating agents. In Malkoun et
al (20), p53 overexpression
was associated with improved mPFS, even though contrasting results
are available in literature (39–41).
Furthermore, the study by Skardelly et al (42) published in 2017, while only
demonstrating a benefit for prolonged temozolomide only for mPFS
and not in mOS, found that MGMT status, extent of resection and age
are significant covariates for survival analysis.
Reports on toxicity with extended treatment are
contrasting. It is necessary to clearly whether prolonged therapy
impacts on toxicity and consequently on the quality of life of
patients. Quality of life still represents a primary objective to
be pursued today since we cannot yet aim for cure. Only clinical
data derived from a randomized study can disprove the common
sensation that extended treatment is accompanied by greater
toxicities, particularly hematological. In clinical practice only a
limited percentage of patients manage to have prolonged treatment
(43). The safety analysis of the
Prolonged Adjuvant Temozolomide vs. ‘Stop & Go’ in Glioblastoma
Patients (PATSGO) trial on 34 patients demonstrated that frequency
of toxicity did not increase with number of cycles (44); instead, in the GEINO trial,
lymphopenia, thrombocytopenia, nausea and vomiting were more
frequent in the extended therapy group, although few patients
experienced grade 3–4 adverse events of any kind and only three
patients (3.7%) needed to discontinue treatment (23). However, there are other several
reports of increased toxicity with prolonged temozolomide
administration: increased cumulative doses of temozolomide have
been associated with worse quality of life and fatigue (10,45),
risk of myelosuppression and immunodepression (46), myelodysplasia and even leukemia
(47).
As already mentioned before, while no consensus
exists on the benefits of additional temozolomide, most physicians
settle at a maximum of 12 cycles for maintenance therapy, trying to
find balance between possible beneficial effects and toxicities. A
single center study by Ohno et al (48) compared stopping treatment at 12
cycles or proceeding beyond 12 cycles. mPFS and mOS between the two
groups demonstrated no difference (mPFS 11.3 vs. 9.2 months, mOS
25.7 vs. 30.2 months), with only Karnosfky performance status at 12
cycles having a significant association with increased survival
(48).
Temozolamide treatment has also been associated with
induced hypermutation. No data exists on the perfect treatment
schedule or duration in order to reach the most benefit while
reducing the risk of induced hypermutation and toxicity (49). While conferring resistance to
temozolomide treatment (34), these
changes may help identify new treatment strategies for
recurrent/progressing GBM. Hypermutation seems to present with an
increased sensitivity to DNA-damaging agents (50), with preclinical trial demonstrating
improved sensitivity to lomustine in mismatch repair (MMR)
deficient MGMT methylated GBM cells resistant to temozolamide
(51). It has also been speculated
that hypermutated cancer cells may be more responsive to immune
checkpoint inhibition (52) but
results from nivolumab trials both in newly diagnosed GBM and in
recurrent GMB have demonstrated poor results. Pembrolizumab is now
under investigation in patients with recurrent gliomas with
hypermutator phenotype (NCT02658279) but a recent monocentric study
by Lombardi et al (53)
found no apparent benefit.
Of course, our study presents several limitations.
It is a retrospective analysis, with only a modest sample size (87
patients), thus limiting extrapolation of its results. The study
was of course not randomized and no information regarding treatment
choice (6 vs. 12 cycles) is available, with the possibility of
selection bias. MRIs at progression were not centrally reviewed, in
line with the nature of the study, and recorded toxicity data was
limited, not allowing for further study. OS data may also be
influenced by second-line choices (mainly fotemustine and
regorafenib). However, the number of patients enrolled and the
results obtained in our study are substantially similar with that
has already been published by Bhandari and colleagues (54).
In conclusion, our data suggests that extended
adjuvant temozolomide (12 cycles) appears to be significantly more
effective than standard treatment with only conventional six
cycles. While literature data are quite heterogeneous and do not
provide any strong evidence for stopping or continuing
temozolomide, in the absence of larger phase III trials, continuing
adjuvant temozolomide for more than six cycles may be an effective
alternative.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MF, FC and RA conceived the study. MF and RA
described the methodology to be used. Investigation was done by MP,
CCM, SF, AZ, VF, PV, IDG, CB, VC, DS, TS, LMC, PC, MC and RP. VDF,
VF, PV, IDG, CB, VC, DS, TS, LMC, PC, MC and RP analyzed the data.
Validation of data was performed by VDF, VF, PV, IDG, CB, VC, DS,
TS, LMC, PC, MC and RP. The resources were collected by MF, VF, PV,
IDG, CB, VC, DS, TS, LMC, PC, MC, RP, FC and RA. Data was curated
by VDF, VF, MP and SF. The original draft was prepared by VDF, MP,
CCM, SF and AZ. The final text was reviewed by MF, VDF and RA. Work
was supervised by MF and RA. MF and RA confirm the authenticity of
all the raw data. All authors have read and agreed to the published
version of the manuscript.
Ethics approval and consent to
participate
All subjects gave their written informed consent.
The study was conducted in accordance with the Declaration of
Helsinki. The retrospective study protocol was approved by the
institutional review board of University of Campania Luigi
Vanvitelli (Napoli, Italy; protocol no. 59;).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Crocetti E, Trama A, Stiller C, Caldarella
A, Soffietti R, Jaal J, Weber DC, Ricardi U, Slowinski J and
Brandes A; RARECARE working group, : Epidemiology of glial and
non-glial brain tumours in Europe. Eur J Cancer. 48:1532–1542.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ostrom QT, Cioffi G, Gittleman H, Patil N,
Waite K, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2012–2016. Neuro Oncol. 21 (Suppl
5):v1–v100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davis FG, Smith TR, Gittleman HR, Ostrom
QT, Kruchko C and Barnholtz-Sloan JS: Glioblastoma incidence rate
trends in Canada and the United States compared with England,
1995–2015. Neuro Oncol. 22:301–302. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Philips A, Henshaw DL, Lamburn G and
O'Carroll MJ: Brain tumours: Rise in glioblastoma multiforme
incidence in England 1995–2015 suggests an adverse environmental or
lifestyle factor. J Environ Public Health. 2018:79107542018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JH, Lee JE, Kahng JY, Kim SH, Park JS,
Yoon SJ, Um JY, Kim WK, Lee JK, Park J, et al: Human glioblastoma
arises from subventricular zone cells with low-level driver
mutations. Nature. 560:243–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lah TT, Novak M and Breznik B: Brain
malignancies: Glioblastoma and brain metastases. Semin Cancer Biol.
60:262–273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sturm D, Witt H, Hovestadt V, Khuong-Quang
DA, Jones DT, Konermann C, Pfaff E, Tönjes M, Sill M, Bender S, et
al: Hotspot mutations in H3F3A and IDH1 define distinct epigenetic
and biological subgroups of glioblastoma. Cancer Cell. 22:425–437.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Q, Hu B, Hu X, Kim H, Squatrito M,
Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, et al: Tumor
evolution of glioma-intrinsic gene expression subtypes associates
with immunological changes in the microenvironment. Cancer Cell.
32:42–56.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Daniel M, Peek GW and Tollefsbol TO:
Regulation of the human catalytic subunit of telomerase (hTERT).
Gene. 498:135–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gilbert MR, Wang M, Aldape KD, Stupp R,
Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT,
et al: Dose-dense temozolomide for newly diagnosed glioblastoma: A
randomized phase III clinical trial. J Clin Oncol. 31:4085–4091.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stupp R, Taillibert S, Kanner A, Read W,
Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K,
et al: Effect of tumor-treating fields plus maintenance
temozolomide vs maintenance temozolomide alone on survival in
patients with glioblastoma: a randomized clinical trial. JAMA.
318:2306–2316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem cells in
glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fountain DM, Bryant A, Barone DG, Waqar M,
Hart MG, Bulbeck H, Kernohan A, Watts C and Jenkinson MD:
Intraoperative imaging technology to maximise extent of resection
for glioma: A network meta-analysis. Cochrane Database Syst Rev.
1:CD0136302021.PubMed/NCBI
|
|
14
|
Cahill DP, Levine KK, Betensky RA, Codd
PJ, Romany CA, Reavie LB, Batchelor TT, Futreal PA, Stratton MR,
Curry WT, et al: Loss of the mismatch repair protein MSH6 in human
glioblastomas is associated with tumor progression during
temozolomide treatment. Clin Cancer Res. 13:2038–2045. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weller M: Where does
O6-methylguanine DNA methyltransferase promoter
methylation assessment place temozolomide in the future standards
of care for glioblastoma? Cancer. 124:1316–1318. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horbinski C, Nabors LB, Portnow J,
Baehring J, Bhatia A, Bloch O, Brem S, Butowski N, Cannon DM, Chao
S, et al: NCCN Guidelines® Insights: Central Nervous
System Cancers, Version 2.2022: Featured Updates to the NCCN
Guidelines. J Natl Comp Cancer Network. 21:12–20. 2023. View Article : Google Scholar
|
|
17
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-Year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roldán Urgoiti GB, Singh AD and Easaw JC:
Extended adjuvant temozolomide for treatment of newly diagnosed
glioblastoma multiforme. J Neurooncol. 108:173–177. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malkoun N, Chargari C, Forest F, Fotso MJ,
Cartier L, Auberdiac P, Thorin J, Pacaut C, Peoc'h M, Nuti C, et
al: Prolonged temozolomide for treatment of glioblastoma:
Preliminary clinical results and prognostic value of p53
overexpression. J Neurooncol. 106:127–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hau P, Koch D, Hundsberger T, Marg E,
Bauer B, Rudolph R, Rauch M, Brenner A, Rieckmann P, Schuth J, et
al: Safety and feasibility of long-term temozolomide treatment in
patients with high-grade glioma. Neurology. 68:688–690. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Refae AA, Ezzat A, Salem DA and Mahrous M:
Protracted adjuvant temozolomide in glioblastoma multiforme. J
Cancer Ther. 6:748–758. 2015. View Article : Google Scholar
|
|
23
|
Balana C, Vaz MA, Manuel Sepúlveda J,
Mesia C, Del Barco S, Pineda E, Muñoz-Langa J, Estival A, de Las
Peñas R, Fuster J, et al: A phase II randomized, multicenter,
open-label trial of continuing adjuvant temozolomide beyond 6
cycles in patients with glioblastoma (GEINO 14-01). Neuro Oncol.
22:1851–1861. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bady P, Sciuscio D, Diserens AC, Bloch J,
van den Bent MJ, Marosi C, Dietrich PY, Weller M, Mariani L,
Heppner FL, et al: MGMT methylation analysis of glioblastoma on the
Infinium methylation BeadChip identifies two distinct CpG regions
associated with gene silencing and outcome, yielding a prediction
model for comparisons across datasets, tumor grades, and
CIMP-status. Acta Neuropathol. 124:547–560. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vlassenbroeck I, Califice S, Diserens AC,
Migliavacca E, Straub J, Di Stefano I, Moreau F, Hamou MF, Renard
I, Delorenzi M, et al: Validation of real-time methylation-specific
PCR to determine O6-methylguanine-DNA methyltransferase gene
promoter methylation in glioma. J Mol Diagn. 10:332–337. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Capper D, Weissert S, Balss J, Habel A,
Meyer J, Jäger D, Ackermann U, Tessmer C, Korshunov A, Zentgraf H,
et al: Characterization of R132H mutation-specific IDH1 antibody
binding in brain tumors. Brain Pathol. 20:245–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Franceschi E, Lombardi G, Balestrini D,
Buglione M, Caranci F, Castellano A, Ferreri A, Franchino F,
Giangaspero F and Mascarin M: Guidelines for brain neoplasies|AIOM.
https://www.aiom.it/linee-guida-aiom-2021-neoplasie-cerebrali/June
23–2023
|
|
29
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weller M, van den Bent M, Preusser M, Le
Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven
L, et al: EANO guidelines on the diagnosis and treatment of diffuse
gliomas of adulthood. Nat Rev Clin Oncol. 18:170–186. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Balañá C, Vaz MA, Lopez D, de la Peñas R,
García-Bueno JM, Molina-Garrido MJ, Sepúlveda JM, Cano JM, Bugés C,
Sanz SM, et al: Should we continue temozolomide beyond six cycles
in the adjuvant treatment of glioblastoma without an evidence of
clinical benefit? A cost analysis based on prescribing patterns in
Spain. Clin Transl Oncol. 16:273–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Attarian F, Taghizadeh-Hesary F,
Fanipakdel A, Javadinia SA, Porouhan P, PeyroShabany B and
Fazilat-Panah D: A systematic review and meta-analysis on the
number of adjuvant temozolomide cycles in newly diagnosed
glioblastoma. Front Oncol. 11:7794912021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Blumenthal DT, Gorlia T, Gilbert MR, Kim
MM, Burt Nabors L, Mason WP, Hegi ME, Zhang P, Golfinopoulos V,
Perry JR, et al: Is more better? The impact of extended adjuvant
temozolomide in newly diagnosed glioblastoma: A secondary analysis
of EORTC and NRG oncology/RTOG. Neuro Oncol. 19:1119–1126. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Perry JR, Bélanger K, Mason WP, Fulton D,
Kavan P, Easaw J, Shields C, Kirby S, Macdonald DR, Eisenstat DD,
et al: Phase II trial of continuous dose-intense temozolomide in
recurrent malignant glioma: RESCUE study. J Clin Oncol.
28:2051–2057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van den Bent MJ, Dubbink HJ, Marie Y,
Brandes AA, Taphoorn MJ, Wesseling P, Frenay M, Tijssen CC, Lacombe
D, Idbaih A, et al: IDH1 and IDH2 mutations are prognostic but not
predictive for outcome in anaplastic oligodendroglial tumors: A
report of the European organization for research and treatment of
cancer brain tumor group. Clin Cancer Res. 16:1597–1604. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Wang T, Liu W, Qiu H, Zhang N,
Chen X, Ding X and Zhang L: Extended adjuvant temozolomide in newly
diagnosed glioblastoma: A single-center retrospective study. Front
Oncol. 12:10005012022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Darlix A, Baumann C, Lorgis V,
Ghiringhelli F, Blonski M, Chauffert B, Zouaoui S, Pinelli C, Rech
F, Beauchesne P and Taillandier L: Prolonged administration of
adjuvant temozolomide improves survival in adult patients with
glioblastoma. Anticancer Res. 33:3467–3474. 2013.PubMed/NCBI
|
|
38
|
Bocangel D, Sengupta S, Mitra S and Bhakat
KK: p53-Mediated down-regulation of the human DNA repair gene
O6-methylguanine-DNA methyltransferase (MGMT) via interaction with
Sp1 transcription factor. Anticancer Res. 29:3741–3750.
2009.PubMed/NCBI
|
|
39
|
Weller M, Felsberg J, Hartmann C, Berger
H, Steinbach JP, Schramm J, Westphal M, Schackert G, Simon M, Tonn
JC, et al: Molecular predictors of progression-free and overall
survival in patients with newly diagnosed glioblastoma: a
prospective translational study of the German glioma network. J
Clin Oncol. 27:5743–5750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pollack IF, Finkelstein SD, Burnham J,
Holmes EJ, Hamilton RL, Yates AJ, Finlay JL and Sposto R;
Children's Cancer Group, : Age and TP53 mutation frequency in
childhood malignant gliomas: Results in a multi-institutional
cohort. Cancer Res. 61:7404–7407. 2001.PubMed/NCBI
|
|
41
|
Miyagami M, Tazoe M and Nakamura S:
Expression of vascular endothelial growth factor and p53 protein in
association with neovascularization in human malignant gliomas.
Brain Tumor Pathol. 15:95–100. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Skardelly M, Dangel E, Gohde J, Noell S,
Behling F, Lepski G, Borchers C, Koch M, Schittenhelm J, Bisdas S,
et al: Prolonged temozolomide maintenance therapy in newly
diagnosed glioblastoma. Oncologist. 22:570–575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Seiz M, Krafft U, Freyschlag CF, Weiss C,
Schmieder K, Lohr F, Wenz F, Thomé C and Tuettenberg J: Long-term
adjuvant administration of temozolomide in patients with
glioblastoma multiforme: Experience of a single institution. J
Cancer Res Clin Oncol. 136:1691–1695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hammouch F, Boterberg T, Clement P,
Joosens E, Whenham N, Verschaeve V, Devriendt D, Renard L and
Baurain JFR: 8744 POSTER extended use of adjuvant TMZ in newly
diagnosed GBM patients is safe-results from the safety analysis of
the PATSGO trial. Eur J Cancer. 47 (Suppl 1):S5872011. View Article : Google Scholar
|
|
45
|
Pouratian N, Gasco J, Sherman JH, Shaffrey
ME and Schiff D: Toxicity and efficacy of protracted low dose
temozolomide for the treatment of low grade gliomas. J Neurooncol.
82:281–288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Su YB, Sohn S, Krown SE, Livingston PO,
Wolchok JD, Quinn C, Williams L, Foster T, Sepkowitz KA and Chapman
PB: Selective CD4+ lymphopenia in melanoma patients treated with
temozolomide: A toxicity with therapeutic implications. J Clin
Oncol. 22:610–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Momota H, Narita Y, Miyakita Y and Shibui
S: Secondary hematological malignancies associated with
temozolomide in patients with glioma. Neuro Oncol. 15:14452013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ohno M, Miyakita Y, Takahashi M,
Yanagisawa S, Tamura Y and Narita Y: Continuing maintenance
temozolomide therapy beyond 12 cycles confers no clinical benefit
over discontinuation at 12 cycles in patients with IDH1/2-wildtype
glioblastoma. Jpn J Clin Oncol. 52:1134–1142. 2022.PubMed/NCBI
|
|
49
|
Johnson BE, Mazor T, Hong C, Barnes M,
Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et
al: Mutational analysis reveals the origin and therapy-driven
evolution of recurrent glioma. Science. 343:189–193. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schlesner M and Eils R: Hypermutation
takes the driver's seat. Genome Med. 7:312015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Stritzelberger J, Distel L, Buslei R,
Fietkau R and Putz F: Acquired temozolomide resistance in human
glioblastoma cell line U251 is caused by mismatch repair deficiency
and can be overcome by lomustine. Clin Transl Oncol. 20:508–516.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Snyder A, Makarov V, Merghoub T, Yuan J,
Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et
al: Genetic basis for clinical response to CTLA-4 blockade in
melanoma. N Engl J Med. 371:2189–2199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lombardi G, Barresi V, Indraccolo S,
Simbolo M, Fassan M, Mandruzzato S, Simonelli M, Caccese M, Pizzi
M, Fassina A, et al: Pembrolizumab activity in recurrent high-grade
gliomas with partial or complete loss of mismatch repair protein
expression: A monocentric, observational and prospective pilot
study. Cancers (Basel). 12:22832020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bhandari M, Gandhi AK, Devnani B, Kumar P,
Sharma DN and Julka PK: Comparative study of adjuvant temozolomide
six cycles versus extended 12 cycles in newly diagnosed
glioblastoma multiforme. J Clin Diagn Res. 11:XC04–XC08.
2017.PubMed/NCBI
|