Introduction
Although the number of gastric cancer (GC) cases is
gradually decreasing, GC is the third leading cause of cancer death
worldwide (1,2). Overall, >40% of patients who
undergo radical resection for GC experience recurrence within 2
years of surgery (3). The treatment
of GC has gradually advanced with the development of minimally
invasive surgical treatments, such as robotic technology (4), and anticancer drugs, and the
introduction of immune checkpoint inhibitors; however, treatment
outcomes are not yet optimal. The incidence of intestinal-type GC
in the West has decreased; however, the proportion of diffuse-type
GC according to the Lauren classification has increased (5–7).
Furthermore, the incidence of poorly cohesive carcinoma (PCC),
including cases comprising signet ring cell (SRC) histology and
non-solid cases [not otherwise specified (NOS)] according to the
World Health Organization (WHO) classification, has increased
(6,8). Therefore, the interest in PCC has
increased worldwide. The fifth edition of the WHO classifies PCC
into SRC and NOS (8). In clinical
practice, PCC is considered to comprise a combination of SRC and
NOS tumors (9). According to the
Japanese classification of GC, PCC corresponds to poorly
differentiated non-solid-type adenocarcinoma (por2) and SRC
carcinoma cases (10). In the
intestinal type, frequent genetic abnormalities include loss of
heterozygosity and mutations in tumor protein p53 (11), as well as APC regulator of WNT
signaling pathway (12). By
contrast, the importance of the cadherin 1 (CDH1) gene has been
recognized in the diffuse type due to the high frequency of cases
with decreased E-cadherin expression and hypermethylation of its
promoter (13). However, treatment
outcomes and clinicopathological characteristics of PCC in Japan
have not been adequately studied.
Epithelial-mesenchymal transition (EMT) is the
process by which epithelial cells acquire mesenchymal properties,
and it is involved in the metastasis, invasion and proliferation of
cancer cells (14). Helicobacter
pylori infection is considered to have a significant effect on
the gastric microenvironment by inducing several inflammatory
responses via the infiltration of macrophages, neutrophils,
regulatory T cells and natural killer cells, which are associated
with promoting EMT (15).
Transforming growth factor β (TGF-β) in gastric cancer is a
representative signal of the promotion of EMT (16). PCC and its microenvironmental
changes, including EMT, have not been adequately assessed.
Therefore, it is important to examine the characteristics of PCC by
focusing on intratumor-infiltrating CD8-positive T cells, protein
wnt3a (wnt3a) and phosphorylated serine/threonine-protein kinase
mTOR (p-mTOR) signaling, cancer stemness markers [leucine-rich
repeat-containing G-protein coupled receptor 5 (LGR5)] contributing
to drug resistance, EMT markers (E-cadherin) and TGF-β
signaling.
Therefore, the present novel study examined the
surgical outcome of PCC and its prognosis, and further
characterized the molecular pathological factors of PCC, EMT and
interstitial signals.
Patients and methods
Patients
The present study included 281 patients who
underwent surgery, including total, proximal and distal resection,
for GC between April 2015 and August 2020 at Gunma University
Hospital (Maebashi, Japan). Furthermore, samples from 197 patients
with GC who underwent surgery, including total, proximal and distal
resection, between 1999 and 2003 were evaluated using a tissue
microarray (TMA). Preoperatively treated cases and endoscopic
submucosal dissection cases were excluded, and multiple blocks
containing the invasion region were collected for TMA. PCC was
defined as cases in which the main component of the cancer was
poorly differentiated non-solid-type adenocarcinoma (por2) and SRC
carcinoma. For surgical case analysis, clinicopathological factors,
including age, sex, body mass index (BMI), tumor size, tumor depth,
presence of lymph node metastasis, number of lymph node metastases,
peritoneal washing cytology, presence of distant metastasis, stage,
surgical method (such as open and laparoscopic surgery), lymph node
dissection, resection method, operative time and blood loss, were
collected. Additionally, for TMA analysis, clinicopathological
factors, including age, sex, tumor size, depth of tumor, presence
of lymph node metastasis, number of lymph node metastases and
stage, were collected. In terms of TMA analysis, existing
immunohistochemistry staining data was used to analyze PCC
characteristics (17–21). Wnt3a (representative of Wnt
signaling), LGR5 (a cancer stemness marker), TGF-β-induced (TGFBI)
(a representative of downstream genes of TGF-β signaling), p-mTOR
signaling, and E-cadherin as an EMT marker were assessed. This
study was approved by the Institutional Review Board (IRB) of Gunma
University (Gunma, Japan; approval no. HS2022-153) and has
therefore been performed in accordance with the ethical standards
laid down in the 1964 Declaration of Helsinki and its later
amendments. As this was a retrospective study, the requirement to
obtain informed consent was waived by the IRB of Gunma University.
An opt-out method was used to obtain consent from the participants.
The data obtained were all collected from medical records or
existing immunohistochemistry staining. Both the application for
the waiver of informed consent and the document of the opt-out
consent were posted on the hospital's website for viewing.
Immunohistochemistry
All paraffin-embedded specimens were cut into 4-µm
thick sections and mounted on glass slides. Sections were
deparaffinized with xylene, hydrated and incubated with 0.3%
hydrogen peroxide for 30 min at room temperature to block
endogenous peroxidase activity. Non-specific binding sites were
blocked by incubation with Protein Block Serum-Free (Dako; Agilent
Technologies, Inc.) for 30 min at room temperature. The primary
antibodies were diluted with REAL Antibody Diluent (Dako; Agilent
Technologies, Inc.) and incubated overnight at 4°C. Wnt3a
polyclonal antibodies (1:200 dilution; cat. no. bs-1700R; BIOSS),
LGR5 antibodies (1:200 dilution; cat. no. ab75850; Abcam), TGFBI
antibodies (1:200 dilution; anti-TGFBI/BIGH3 antibody; cat. no.
10188-1-AP; Proteintech Group, Inc.), p-mTOR antibodies (1:80
dilution; anti-rabbit monoclonal antibody; cat. no. #2976; Cell
Signaling Technology, Inc.) and E-cadherin antibodies (1:500
dilution; HECD-1; mouse monoclonal; cat. no. M108; Takara Bio,
Inc.) were used as the primary antibodies. For Wnt3a, the citric
acid method was adopted as the antigen activation method, and
specimens were immersed in hot water at 98°C for 30 min using
sodium citrate buffer (LSI Medience Corporation), followed by
immersion in hot water at 75°C for 10 min. For TGFBI, antigen
retrieval was performed using ImmunoSaver (Nisshin EM, Co., Ltd.)
at 98°C for 45 min. Antigen activation of LGR5 was performed by
heating citrate buffer (pH 6.0) in an autoclave for 5 min. To
enable E-cadherin activation, the sections were boiled in 10 mM
citrate buffer (pH 6.0) at 98°C for 30 min. Antigen retrieval of
p-mTOR was not conducted. The secondary antibody from the Histofine
Simple Stain MAX-PO (Multi) Kit (cat. no. 414152F; Nichirei
Biosciences, Inc.) was used, which was incubated for 30 min at room
temperature. Staining with 3,3-diaminobenzidine tetrahydrochloride
was performed in a 0.02% solution in 50 mM ammonium acetate-citrate
buffer (pH 6.0) containing 0.005% hydrogen peroxide. Sections were
stained with hematoxylin at room temperature for 1 min, placed on a
cover glass and observed under a light microscope. The evaluation
methods were as previously described (17–21).
Wnt3a and TGFBI were evaluated using intensity scores. No staining
and weak staining were classified as low expression, and moderate
staining and strong staining as high expression. These evaluations
were conducted manually as described previously (17,19).
For LGR5, the percentage of stained cells was classified into four
categories (0, negative; 1, 1–25% positive; 2, 25–50% positive; and
3, >50% positive) and staining intensity into four categories
(0, no staining; 1, weak staining; 2, moderate staining; and 3,
strong staining). Percentage and intensity scores were added
(0–6) and ranks of 3 or higher were
classified as high expression (18). p-mTOR was evaluated using the
following intensity scores: 0, no tumor cells are stained, only
cytoplasm; 1, 0–10% of tumor cells show weak to moderate staining;
2, >10% of tumor cells show moderate staining or 1–10% of tumor
cells show strong staining; and 3, >10% of tumor cells show
strong staining. Scores 2 and 3 were classified as positive
(20). E-cadherin positive cases
were defined as those in which at least 50% of the cancer cells had
moderate or higher staining intensity (21).
Statistical analysis
Normally distributed data are presented as the mean
± standard deviation and were analyzed using an unpaired t-test,
and non-normally distributed data are presented as the median
(interquartile range) and were analyzed using the Mann-Whitney U
test. The χ2 test was used for categorical data, with
the exception of distant metastasis and resection method data,
which were analyzed using Fisher's exact test. Overall survival
(OS) and progression-free survival (PFS) from the date of surgery
were determined and plotted using the Kaplan-Meier method. The
log-rank test was employed for comparison. P<0.05 was used to
indicate a statistically significant difference. All statistical
analyses were performed using the JMP Pro software (version 15.0;
SAS Institute, Inc.).
Results
Association between PCC and
clinicopathological factors and prognosis in surgical cases
The associations between PCC and clinicopathological
factors are shown in Table I. PCC
occurred in 77 (27.4%) patients, with a higher proportion of
younger (P<0.0001) and female (P=0.002) patients compared with
the non-PCC group. There was no difference in BMI; however, the
tumor was significantly deeper (P=0.048) in patients with PCC than
in those without. There was no significant difference in the
presence of lymph node metastasis. No significant associations were
observed for cytology, distant metastasis and stage. Additionally,
no significant associations were found for surgical methods, such
as open and laparoscopic surgery, lymph node dissection, resection
method, operative time and blood loss. There was no significant
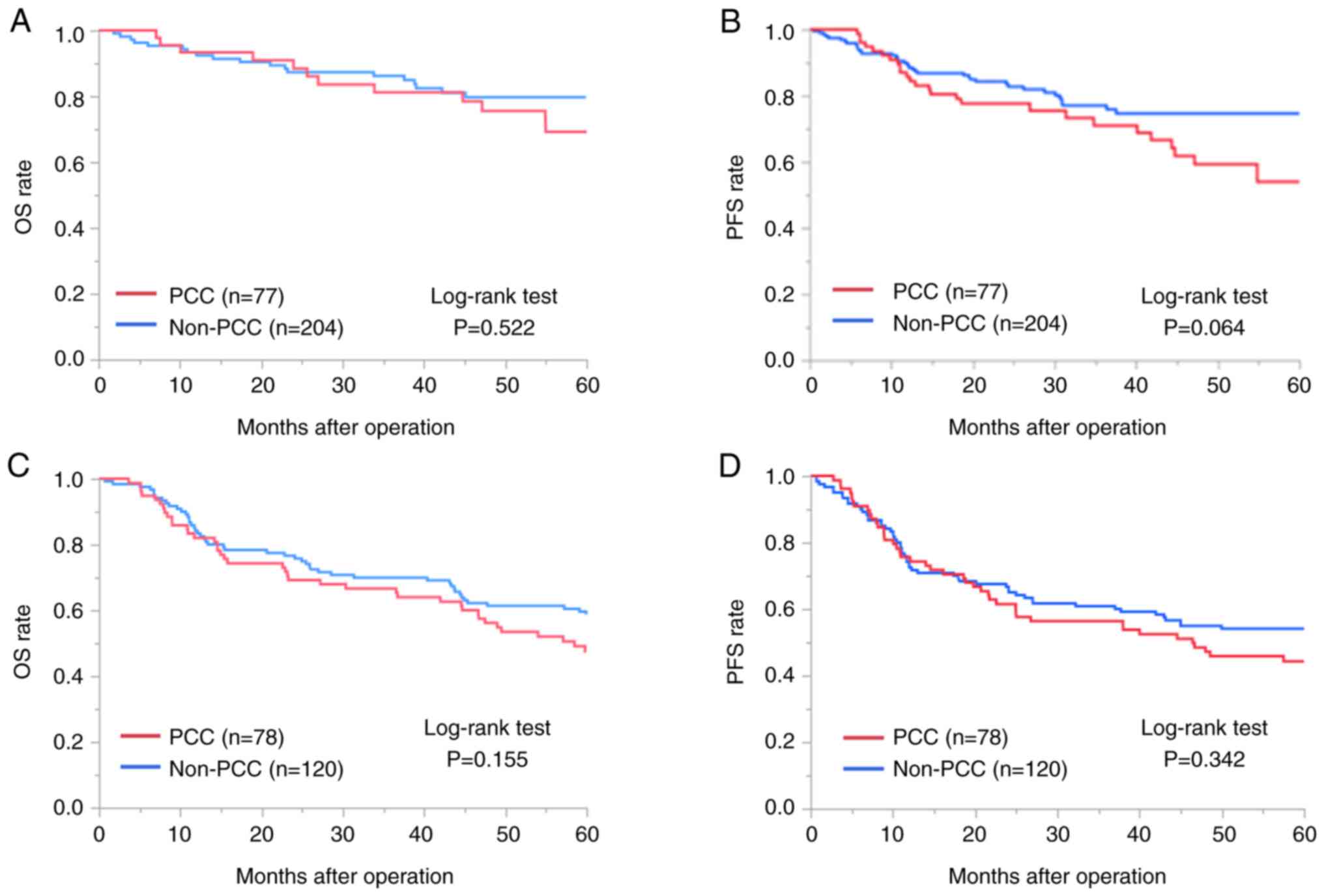
difference in prognosis for PCC in terms of OS (P=0.522; Fig. 1A) and PFS (P=0.064; Fig. 1B).
 | Table I.Association between PCC and
clinicopathological factors in surgical cases. |
Table I.
Association between PCC and
clinicopathological factors in surgical cases.
| Factor | PCC (n=77) | Non-PCC (n=204) | P-value |
|---|
| Age, years | 66±11 | 71±9 |
<0.0001a |
| Sex |
|
| 0.0015a |
| Male | 42 | 152 |
|
|
Female | 35 | 52 |
|
| BMI,
kg/m2 | 22.08±0.26 | 21.70±0.43 | 0.448 |
| Tumor size, mm | 45.0 (27.3–76.5) | 41.0 (25.0–61.8) | 0.265a |
| Depth |
|
| 0.048a |
|
M,SM,MP | 39 | 128 |
|
|
SS,SE,SI | 38 | 73 |
|
| Lymph node
metastasis |
|
| 0.443 |
|
Absent | 42 | 121 |
|
|
Present | 35 | 82 |
|
| Number of lymph
node metastases | 0 (0–4.8) | 0 (0–3.0) | 0.303a |
| Cy |
|
| 0.191 |
| 0 | 72 | 198 |
|
| 1 | 5 | 6 |
|
| Distant
metastasis |
|
| >0.999 |
|
Absent | 74 | 194 |
|
|
Present | 3 | 8 |
|
| Stage |
|
| 0.098 |
|
I/II | 50 | 153 |
|
|
III/IV | 27 | 51 |
|
| Surgical
method |
|
| 0.906 |
|
Open | 27 | 70 |
|
|
Laparoscopic | 50 | 134 |
|
| Lymph node
dissection |
|
| 0.402 |
|
D0,D1,D1+ | 38 | 111 |
|
|
D2,D3 | 39 | 91 |
|
| Resection
method |
|
| 0.229 |
| Total
gastrectomy | 27 | 61 |
|
|
Proximal gastrectomy | 5 | 23 |
|
| Distal
gastrectomy | 43 | 119 |
|
|
Other | 2 | 1 |
|
| Operation time,
min | 299±9 | 301±5 | 0.849 |
| Blood loss, ml | 76 (18–230) | 103 (16–253) | 0.510 |
Association between PCC and
clinicopathological factors and prognosis in TMA cases
The associations between PCC and clinicopathological
factors are shown in Table II. In
the present TMA study, 78 cases of PCC were included (39.4% of the
total). The patients with PCC were younger (P=0.0015), more often
women (P=0.045) and had larger tumors (P=0.0199) compared with the
patients without PCC. Although there were no associations with
tumor depth and lymph node metastasis, the results of this study
indicated that stage III–IV cases were significantly more common in
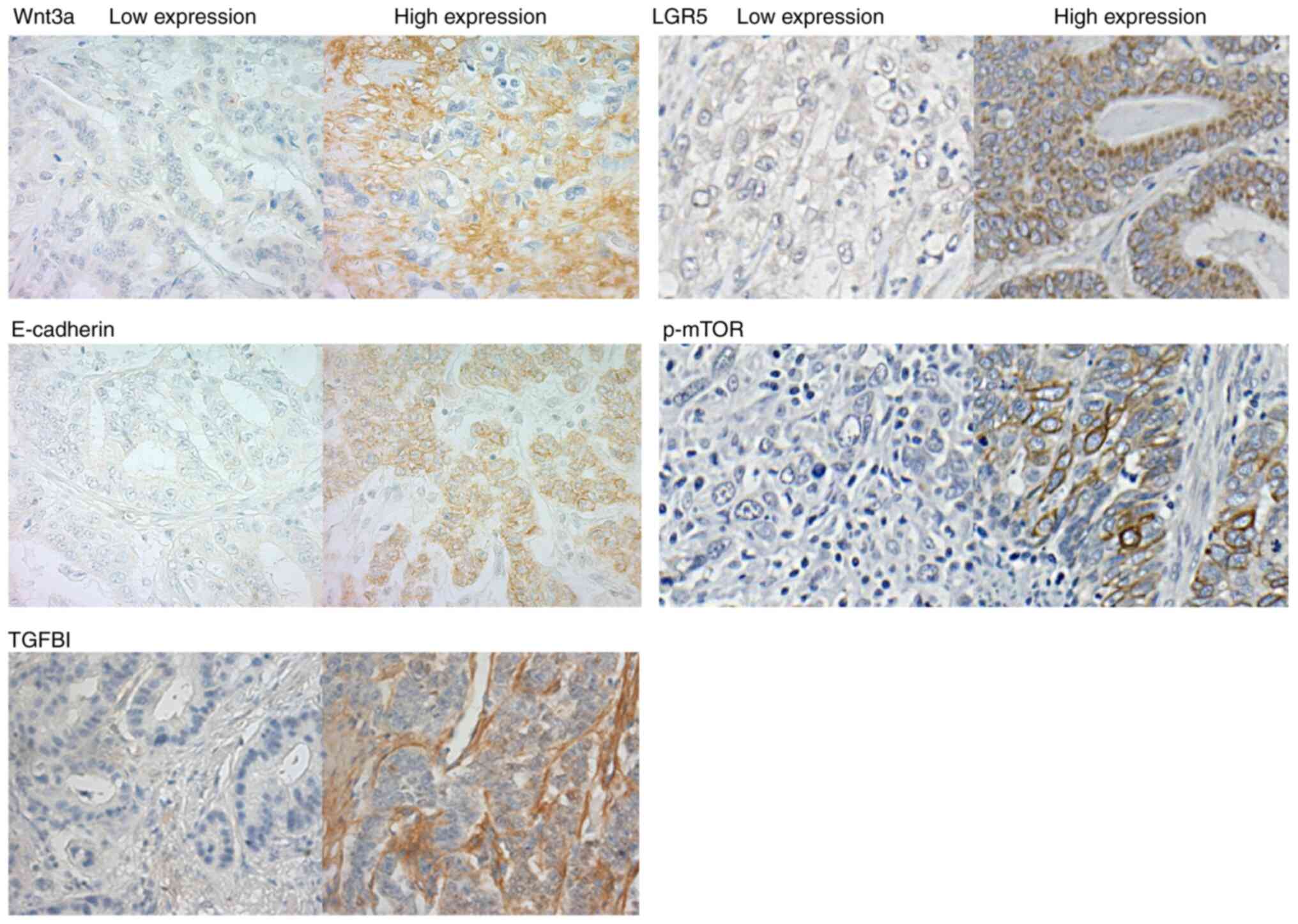
patients with PCC (P=0.0041). The representative
immunohistochemistry staining images of low and high expression
levels of Wnt3a, LGR5, E-cadherin, p-mTOR and TGFBI are presented
in Fig. 2. Examination of the
expression of the various proteins revealed that wnt3a expression
was significantly upregulated in PCC (P=0.0078), whereas E-cadherin
(P=0.022) was significantly downregulated. No significant
associations were observed with the expression of LGR5, TGFBI and
p-mTOR. Furthermore, there was no significant difference in
prognosis for PCC in terms of OS (P=0.155; Fig. 1C) and PFS (P=0.342; Fig. 1D).
 | Table II.Association between PCC and
clinicopathological factors in tissue microarray cases. |
Table II.
Association between PCC and
clinicopathological factors in tissue microarray cases.
| Factor | PCC (n=78) | Non-PCC
(n=120) | P-value |
|---|
| Age, years | 62±12 | 67±10 | 0.0015a |
| Sex |
|
| 0.045a |
|
Male | 48 | 90 |
|
|
Female | 30 | 30 |
|
| Tumor size, mm | 62.5
(41.5–108.3) | 54.5
(40.0–71.5) | 0.0199a |
| Depth |
|
| 0.111 |
|
M,SM,MP | 15 | 35 |
|
|
SS,SE,SI | 63 | 85 |
|
| Lymph node
metastasis |
|
| 0.798 |
|
Absent | 24 | 39 |
|
|
Present | 54 | 81 |
|
| Stage |
|
| 0.0041a |
|
I/II | 28 | 68 |
|
|
III/IV | 50 | 52 |
|
| Wnt3a |
|
| 0.0078a |
| Low
expression | 23 | 58 |
|
| High
expression | 55 | 62 |
|
| LGR5 |
|
| 0.855 |
| Low
expression | 22 | 35 |
|
| High
expression | 56 | 84 |
|
| E-cadherin |
|
| 0.022a |
| Low
expression | 48 | 54 |
|
| High
expression | 30 | 66 |
|
| TGFBI |
|
| 0.786 |
| Low
expression | 47 | 74 |
|
| High
expression | 31 | 45 |
|
| p-mTOR |
|
| 0.770 |
| Low
expression | 35 | 52 |
|
| High
expression | 42 | 68 |
|
Expression of E-cadherin and TGFBI
associated with EMT and OS in PCC TMA cases
As the immunohistochemistry results revealed that
PCC cells exhibited increased EMT, as determined based on
E-cadherin levels (Table II), a
focus was placed on the expression of E-cadherin as an EMT marker.
Furthermore, TGFBI is a representative downstream gene of TGF-β
signaling that is known to be associated with EMT (22). Therefore, the significance of
E-cadherin and TGFBI expression levels in PCC was investigated with
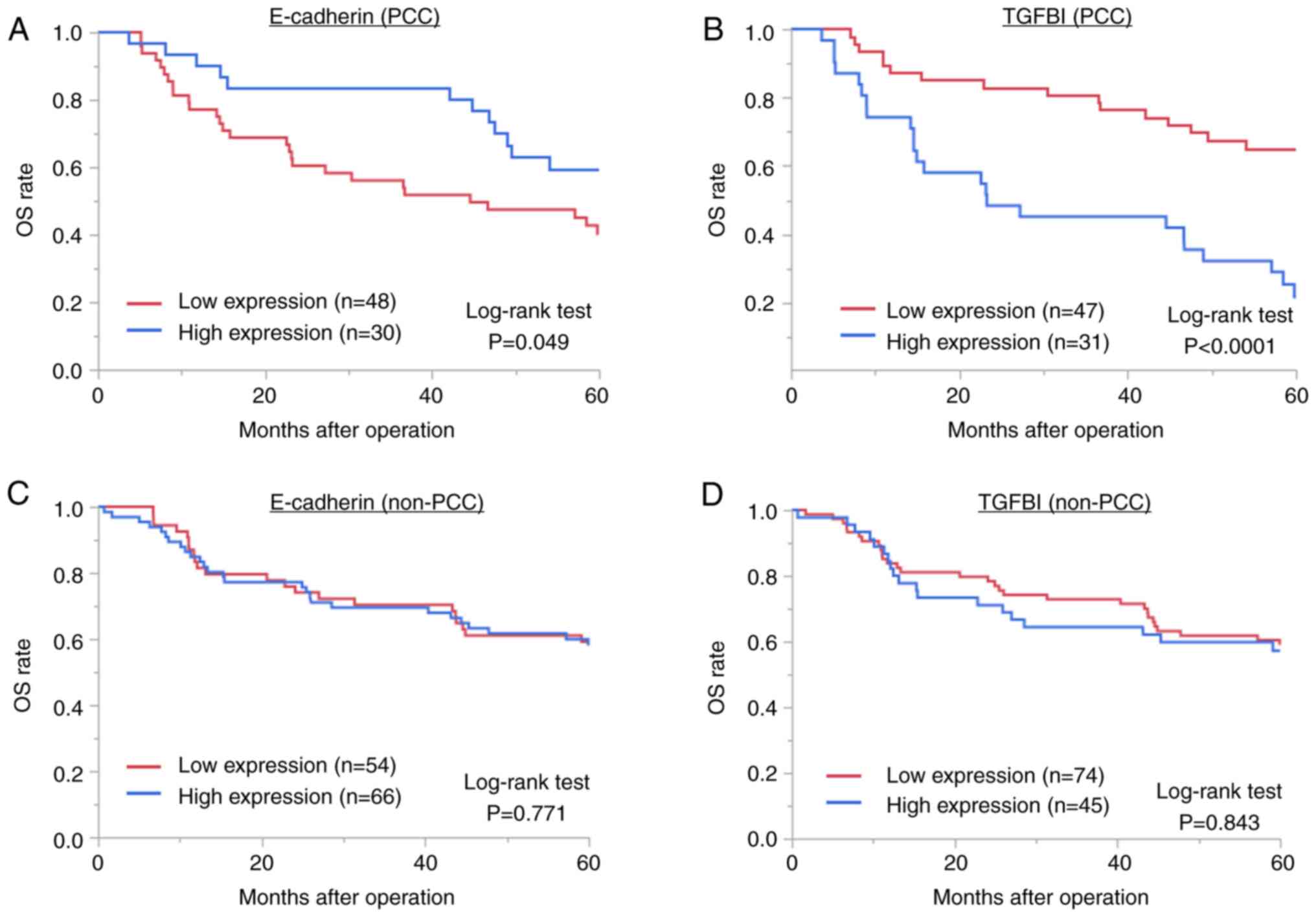
regard to survival (Fig. 3). Among
patients with PCC, low E-cadherin expression (P=0.049; Fig. 3A) and high TGFBI expression
(P<0.0001; Fig. 3B), which are
involved in EMT, were associated with a significantly poorer
prognosis. Furthermore, during the evaluation of PFS, low
expression of E-cadherin indicated a non-significant tendency for a
poor prognosis (P=0.076), and high expression of TGFBI was
significantly associated with a poor prognosis (P<0.0001)
(Fig. S1). However, there was no
association between E-cadherin expression level (P=0.771; Fig. 3C) or TGFBI expression level
(P=0.843; Fig. 3D) and a poor
prognosis in non-PCC cases.
Discussion
The present study evaluated surgical cases using a
TMA and revealed that PCC was more common in younger patients and
women, as the ratio of women to men was higher in the PCC group
compared with that in the non-PCC group. However, none of the
results showed that the prognosis was worse for patients with PCC.
Furthermore, in the TMA study, PCC was associated with both
decreased expression of EMT markers, such as E-cadherin, and the
activation of wnt3a signaling. In the TMA study, patients with
elevated EMT in the PCC group had a significantly poorer prognosis
compared with patients without PCC.
The prognosis for PCC varies from study to study and
remains controversial (23,24). In the present study of 478 cases, a
poor prognosis of PCC compared with non-PCC was not observed. The
proportion of SRC in PCC is inversely associated with tumor
invasiveness and has been reported to be an independent predictor
of survival (25). We hypothesized
that the proportion of SRC may contribute to these controversial
results.
Previous studies have indicated that PCC with
increased EMT has a poorer prognosis (26,27).
The present study focused on E-cadherin and TGF-β signaling as
indicators of EMT. PCC patients with low E-cadherin or high TGFBI
expression had a significantly poorer prognosis, indicating that
E-cadherin and TGFBI may be prognostic factors for PCC. The
wnt/β-catenin pathway, TGF-β signaling, hypoxia, neurogenic locus
notch homolog protein 3 signaling and matrix metalloproteinases are
known to induce EMT (28). EMT
contributes to resistance to chemotherapy and immune checkpoint
inhibitors (29). A previous study
reported that PCC was associated with low PD-L1 expression
(30). Furthermore, we previously
reported that high TGFBI expression contributes to EMT and
treatment resistance in nivolumab-treated patients with lung cancer
(19). Hence, the possibility of
immunotherapy resistance has also been considered in PCC, and
further development of chemotherapy treatment selection strategies
is mandatory.
The present study evaluated both surgical cases and
TMA results, and these results indicated that PCC was more common
in younger patients and women. Koseki et al (31) also reported that PCC was
significantly more common in younger women. Furthermore, the study
suggested that mutations in CDH1 and ras homolog family member A
(RHOA) are more frequent in PCC. GC is classified as Epstein-Barr
virus-CpG island methylator phenotype, hypermutated (microsatellite
instability), genomically stable (GC) or chromosomal instability
based on its pathological features (32), while PCC is classified as a GC and
has frequent mutations in CDH1 and RHOA.
The present study showed that TGFBI expression was
associated with prognosis in PCC (high expression was associated
with poor OS), but that there was no significant difference in
terms of prognosis between PCC and non-PCC patients with different
expression levels of TGFBI. Mizoi et al (33) reported that TGF-β signaling is
upregulated in the GC stroma, and we previously reported (34) that TGFBI, a representative
downstream gene of TGF-β signaling, is secreted by
cancer-associated fibroblasts in the cancer stroma and that
suppressing TGFBI inhibits cancer cell invasion. The reason for the
poor prognosis in the PCC group with high TGFBI expression may be
due to the fact that PCC is rich in cancer stroma and contains many
cancer-associated fibroblasts, which mediated the activation of
TGF-β signaling.
The present study had several limitations. First,
this was a single-center, retrospective study. Therefore,
large-scale, multicenter prospective studies are required for a
detailed analysis of the pathological characteristics and prognosis
of PCC. Second, this study focused on immunohistochemical staining
and did not include cellular experiments.
In conclusion, PCC was more common in younger
patients and women. Furthermore, PCC was associated with the
absence of cell adhesion molecules and the activation of wnt
signaling. In the present study, there was no clear association
between PCC and prognosis. However, PCC with increased EMT was
associated with a significantly poorer prognosis than PCC without
EMT.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by a Japan Society for the Promotion of
Science KAKENHI grant (no. 23K15513).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NN was responsible for study conception and design.
Acquisition of data was performed by NN, MI and YS. NN, MSo, AS,
MSa, TO, KS and HS analyzed and interpreted the data. Writing,
review and/or revision of the manuscript was completed by NN, MSo,
KS and HS. TO, KS and HS supervised the study. NN and MSo confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Gunma University (Gunma, Japan; approval no. HS2022-153).
As this was a retrospective study, the requirement to obtain
informed consent was waived by the Institutional Review Board of
Gunma University. An opt-out method was used to obtain the
participant's consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marubashi S, Takahashi A, Kakeji Y,
Hasegawa H, Ueno H, Eguchi S, Endo I, Goi T, Saiura A, Sasaki A, et
al: Surgical outcomes in gastroenterological surgery in Japan:
Report of the national clinical database 2011–2019. Ann
Gastroenterol Surg. 5:639–658. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu D, Lu M, Li J, Yang Z, Feng Q, Zhou M,
Zhang Z and Shen L: The patterns and timing of recurrence after
curative resection for gastric cancer in China. World J Surg Oncol.
14:3052016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kikuchi K, Suda K, Shibasaki S, Tanaka T
and Uyama I: Challenges in improving the minimal invasiveness of
the surgical treatment for gastric cancer using robotic technology.
Ann Gastroenterol Surg. 5:604–613. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marrelli D, Pedrazzani C, Morgagni P, de
Manzoni G, Pacelli F, Coniglio A, Marchet A, Saragoni L, Giacopuzzi
S and Roviello F; Italian Research Group for Gastric Cancer, :
Changing clinical and pathological features of gastric cancer over
time. Br J Surg. 98:1273–1283. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Henson DE, Dittus C, Younes M, Nguyen H
and Albores-Saavedra J: Differential trends in the intestinal and
diffuse types of gastric carcinoma in the United States, 1973–2000:
Increase in the signet ring cell type. Arch Pathol Lab Med.
128:765–770. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laurén PA and Nevalainen TJ: Epidemiology
of intestinal and diffuse types of gastric carcinoma. A time-trend
study in Finland with comparison between studies from high- and
low-risk areas. Cancer. 71:2926–2933. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board, : The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mariette C, Carneiro F, Grabsch HI, van
der Post RS, Allum W and de Manzoni G; European Chapter of
International Gastric Cancer Association, : Consensus on the
pathological definition and classification of poorly cohesive
gastric carcinoma. Gastric Cancer. 22:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Japanese Gastric Cancer Association.
Japanese classification of gastric carcinoma, . 15th edition.
Tokyo: Kanehara Shuppan; 2017
|
|
11
|
Joypaul BV, Newman EL, Hopwood D, Grant A,
Qureshi S, Lane DP and Cuschieriet A: Expression of p53 protein in
normal, dysplastic, and malignant gastric mucosa: An
immunohistochemical study. J Pathol. 170:279–283. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Horii A, Nakatsuru S, Miyoshi Y, Ichii S,
Nagase H, Ando H, Yanagisawa A, Tsuchiya E, Kato Y and Nakamuraet
Y: Frequent somatic mutations of the APC gene in human pancreatic
cancer. Cancer Res. 52:6696–6698. 1992.PubMed/NCBI
|
|
13
|
Tamura G, Yin J, Wang S, Fleisher AS, Zou
T, Abraham JM, Kong D, Smolinski KN, Wilson KT, James SP, et al:
E-Cadherin gene promoter hypermethylation in primary human gastric
carcinomas. J Natl Cancer Inst. 92:569–573. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ribatti D: Epithelial-mesenchymal
transition in morphogenesis, cancer progression and angiogenesis.
Exp Cell Res. 353:1–5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma HY, Liu XZ and Liang CM: Inflammatory
microenvironment contributes to epithelial-mesenchymal transition
in gastric cancer. World J Gastroenterol. 22:6619–6628. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Zhang P, Shao M, Zang X, Zhang J,
Mao F, Qian H and Xu W: SALL4 activates TGF-β/SMAD signaling
pathway to induce EMT and promote gastric cancer metastasis. Cancer
Manag Res. 10:4459–4470. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakazawa N, Sohda M, Yokobori T, Gombodorj
N, Sano A, Sakai M, Oyama T, Kuwano H, Shirabe K and Saeki H:
Cytoplasmic localization of connexin 26 suppresses transition of
β-catenin into the nucleus in intestinal- and mix-type gastric
cancer. Ann Gastroenterol Surg. 6:505–514. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakazawa N, Sohda M, Ide M, Shimoda Y,
Tateno K, Watanabe T, Sano A, Sakai M, Yokobori T, Ogawa H, et al:
PROX1 was associated with LGR5 and wnt signaling and contributed to
poor prognosis in gastric cancer. Oncology. 100:569–575. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakazawa N, Yokobori T, Kaira K, Turtoi A,
Baatar S, Gombodorj N, Handa T, Tsukagoshi M, Ubukata Y, Kimura A,
et al: High stromal TGFBI in lung cancer and intratumoral
CD8-positive T cells were associated with poor prognosis and
therapeutic resistance to immune checkpoint inhibitors. Ann Surg
Oncol. 27:933–942. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakazawa N, Sohda M, Ide M, Shimoda Y,
Ubukata Y, Kuriyama K, Hara K, Sano A, Sakai M, Yokobori T, et al:
High L-type amino acid Transporter 1 levels are associated with
chemotherapeutic resistance in gastric cancer patients. Oncology.
99:732–739. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakazawa N, Yokobori T, Sohda M, Hosoi N,
Watanabe T, Shimoda Y, Ide M, Sano A, Sakai M, Erkhem-Ochir B, et
al: Significance of lipopolysaccharides in gastric cancer and their
potential as a biomarker for nivolumab sensitivity. Int J Mol Sci.
24:117902023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Xue M, Du S, Feng W, Zhang K, Zhang
L, Liu H, Jia G, Wu L, Hu X, et al: Competitive endogenous RNA is
an intrinsic component of EMT regulatory circuits and modulates
EMT. Nat Commun. 10:16372019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamura K, Eto K, Iwagami S, Ogawa K,
Sawayama H, Ishimoto T, Iwatsuki M, Baba Y, Miyamoto Y, Yoshida N
and Baba H: Clinicopathological characteristics and prognosis of
poorly cohesive cell subtype of gastric cancer. Int J Clin Oncol.
27:512–519. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sarriugarte Lasarte A, García Alberdi E,
Martínez Indart L, Gutiérrez Grijalba O, Álvarez Abad I, Guerra
Lerma M, Calle Baraja M and Colina Alonso A: From Lauren's diffuse
gastric cancer to WHO's poorly cohesive carcinoma.
Clinicopathological and prognostic characteristics. Rev Esp Enferm
Dig. 113:324–331. 2021.PubMed/NCBI
|
|
25
|
Roviello F, Marano L, Ambrosio MR, Resca
L, D'Ignazio A, Petrelli F, Petrioli R, Costantini M, Polom K,
Macchiarelli R, et al: Signet ring cell percentage in poorly
cohesive gastric cancer patients: A potential novel predictor of
survival. Eur J Surg Oncol. 48:561–569. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bencivenga M, Simbolo M, Ciaparrone C,
Vicentini C, Torroni L, Piredda ML, Sacco M, Alloggio M, Castelli
C, Tomezzoli A, et al: Poorly cohesive gastric cancers showing the
transcriptomic hallmarks of epithelial-mesenchymal transition
behave aggressively. Ann Surg. 276:822–829. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bencivenga M, Treppiedi E, Dal Cero MD,
Torroni L, Verlato G, Iglesias M, Renaud F, Tomezzoli A, Castelli
C, Piessen G, et al: The amount of signet ring cells is
significantly associated with tumour stage and survival in gastric
poorly cohesive tumours. J Surg Oncol. 121:1084–1089. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong STC, Choi H, Rayes TE, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim H, Heo YJ, Cho YA, Kang SY, Ahn S and
Kim KM: Tumor immune microenvironment is influenced by frameshift
mutations and tumor mutational burden in gastric cancer. Clin
Transl Oncol. 24:556–567. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koseki Y, Hatakeyama K, Terashima M,
Nagashima T, Urakami K, Ohshima K, Aizawa D, Sugino T, Furukawa K,
Fujiya K, et al: Molecular profile of poorly cohesive gastric
carcinoma with special reference to survival. Gastric Cancer.
26:553–564. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mizoi T, Ohtani H, Miyazono K, Miyazawa M,
Matsuno S and Nagura H: Immunoelectron microscopic localization of
transforming growth factor beta 1 and latent transforming growth
factor beta 1 binding protein in human gastrointestinal carcinomas:
Qualitative difference between cancer cells and stromal cells.
Cancer Res. 53:183–190. 1993.PubMed/NCBI
|
|
34
|
Suzuki M, Yokobori T, Gombodorj N, Yashiro
M, Turtoi A, Handa T, Ogata K, Oyama T, Shirabe K and Kuwano H:
High stromal transforming growth factor β-induced expression is a
novel marker of progression and poor prognosis in gastric cancer. J
Surg Oncol. 118:966–974. 2018. View Article : Google Scholar : PubMed/NCBI
|