Introduction
Malignant peripheral nerve sheath tumor (MPNST) is a
rare, biologically aggressive subtype of soft tissue sarcomas
(STS), accounting for 5–10% of all STS (1). It is a high-grade spindle-cell tumor
originating from the peripheral nerve sheaths (2), with high malignancy and poor
prognosis. A retrospective review from the Mayo Clinic Arizona by
Stucky et al (3) indicated
that high tumor grade and tumor size ≥50 mm predict undesirable
disease-specific survival for MPNST. The incidence of MPNST is low,
only 0.001% in the general population, with no gender predilection.
Neurofibromatosis type 1 (NF1) is the most important risk factor,
with ~10% of patients with NF1 developing MPNST during their
lifetime (4). Furthermore, patients
with prior radiation exposure also have a higher incidence of MPNST
than the general population (5),
and MPNST induced by radiation accounts for ~5% of all MPNSTs
(6). MPNSTs can grow throughout the
whole body, but most commonly occur in the extremities, the
proximal parts of the trunk, as well as the head and neck (7). The occurrence of intrapulmonary MPNST
is exceedingly minimal (8–12).
At present, there is still no standard treatment for
MPNST. The existing treatment options are mostly based on the
treatment of STS. Although surgery is the preferred treatment for
MPNST, it's difficult to achieve extended or complete resection due
to its high aggressiveness. The role of radiation, chemotherapy and
targeted therapy for MPNST is still limited and uncertain (13). Programmed death 1 (PD-1)/programmed
death-ligand 1 (PD-L1)-related immune checkpoint inhibitors (ICIs)
as an emerging and promising cure have been proven to be effective
for diversified cancer. However, due to the rarity of MPNST, there
are few large-scale randomized controlled trials on the
effectiveness of immunotherapy in MPNST.
The present study reported a case of intrapulmonary
MPNST in an elderly man who received sintilimab and achieved a
remarkable response. Compared with previous case reports of MPNST,
this case has several particularities. First, it is worth noting
that the primary location of MPNST in the lung is something of a
rarity. Furthermore, this patient had no pulmonary symptoms but a
large space-occupying lesion in the right upper lung lobe, which
was found due to dizziness and lower limb fatigue by coincidence.
Of note, single-agent immunotherapy was greatly effective in this
patient with intrapulmonary MPNST who had not received any
anti-tumor therapy in the past.
Case report
A 63-year-old man visited the Neurology Department
of Zhongshan Hospital of Traditional Chinese Medicine (Zhongshan,
China) in March 2023 with complaints of dizziness and weakness. The
patient had no family history of NF1 and any other cancer. The
patient had not received any radiotherapy. Computed tomography (CT)
scans of the brain, chest and abdomen were ordered as parts of the
examinations. Unexpectedly, the chest and abdominal CT examination
showed a giant mass in the right upper lung lobe invading the
adjacent chest wall and the third and fourth ribs, and its size was
91×70 mm. The primary consideration was malignancy. Multiple
metastases were also found in both lungs, mediastinal lymph nodes,
liver and bilateral iliac bone. A circular low-density mass with a
size of 54×47 mm in liver segment 8, with blurred boundaries, was
observed. No primary tumors were found in any other areas, so the
large mass in the right upper lung lobe was considered to be the
primary lesion. Various tumor markers were within the normal range.
After being seen by an oncologist, the patient was referred to the
Oncology Department of Zhongshan Hospital of Traditional Chinese
Medicine (Zhongshan, China) and underwent a percutaneous lung
puncture biopsy one week after the initial presentation.
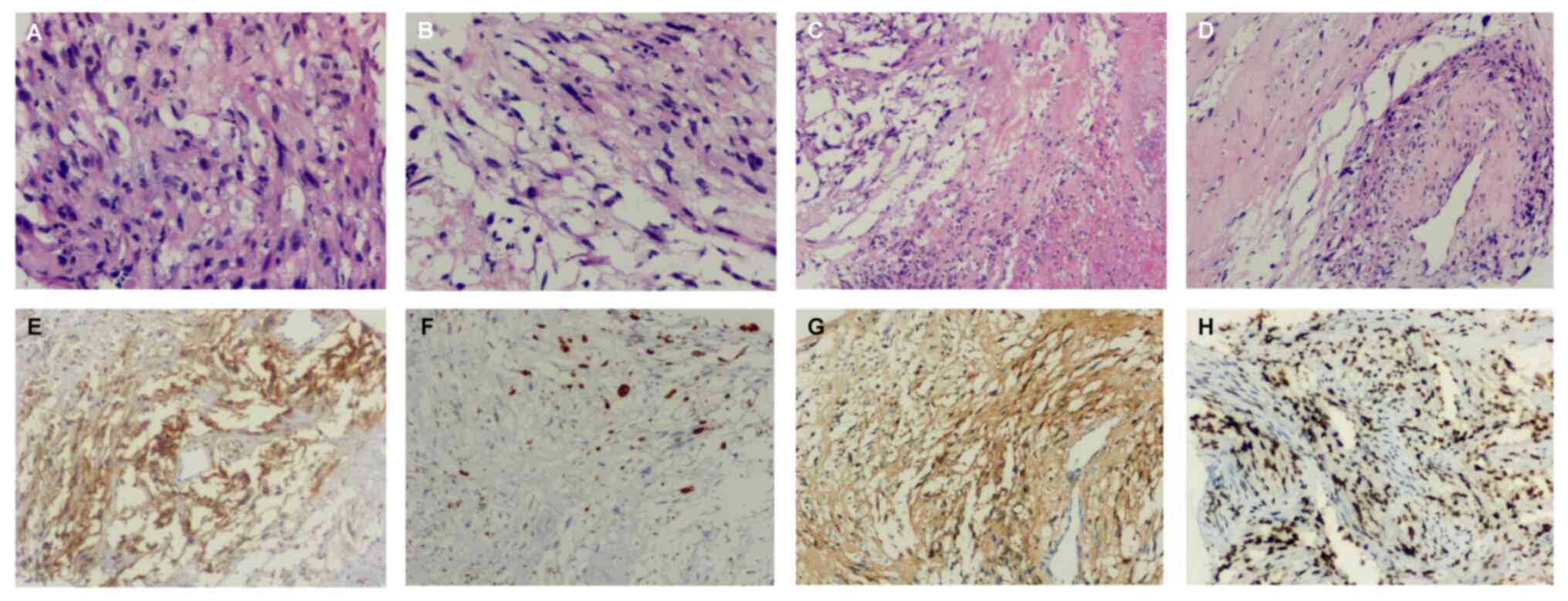
Examination of the histopathological image stained with hematoxylin
and eosin according to a standard protocol indicated the following:
The puncture tissue of the right lung mass showed a large amount of
necrosis under the microscope, and the local cells were fusiform
and oval (Fig. 1A-D). Tumor tissue
was stained according to a standard immunohistochemical protocol
(14). The final
immunohistochemical results showed that the tumor stained positive
for Vimentin (anti-Vimentin antibody: Cat. no. Kit-0019; MXB;
pre-diluted) (data not shown), SOX10 (anti-SOX10 antibody: Cat. no.
RMA-0726; MXB; pre-diluted) (data not shown), Ki-67 (20%)
(anti-Ki67 antibody: Cat. no. RMA-0542; MXB; pre-diluted) (Fig. 1F), S-100 protein (anti-S-100 protein
antibody: Cat. no. Kit-0007; MXB; pre-diluted) (Fig. 1G), histone H3 lysine 27
trimethylation (H3K27Me3) (anti-H3K27Me3 antibody: Cat. no.
RMA-0843; MXB; pre-diluted) (Fig.
1H) and the tumor proportion score of PD-L1 (anti-PD-L1
antibody: Cat. no. HY-13421; DAKO; 1:50 dilution) was 60% (Fig. 1E). Taking into account these
factors, this patient was finally diagnosed with primary
intrapulmonary MPNST.
After the diagnosis, the patient refused to undergo
surgery or chemotherapy. Considering that PD-L1 expression in 60%
of tumor cells, it was decided to use pembrolizumab for treatment
after reviewing relevant case reports. However, the patient refused
to use pembrolizumab due to its high cost, and the more affordable
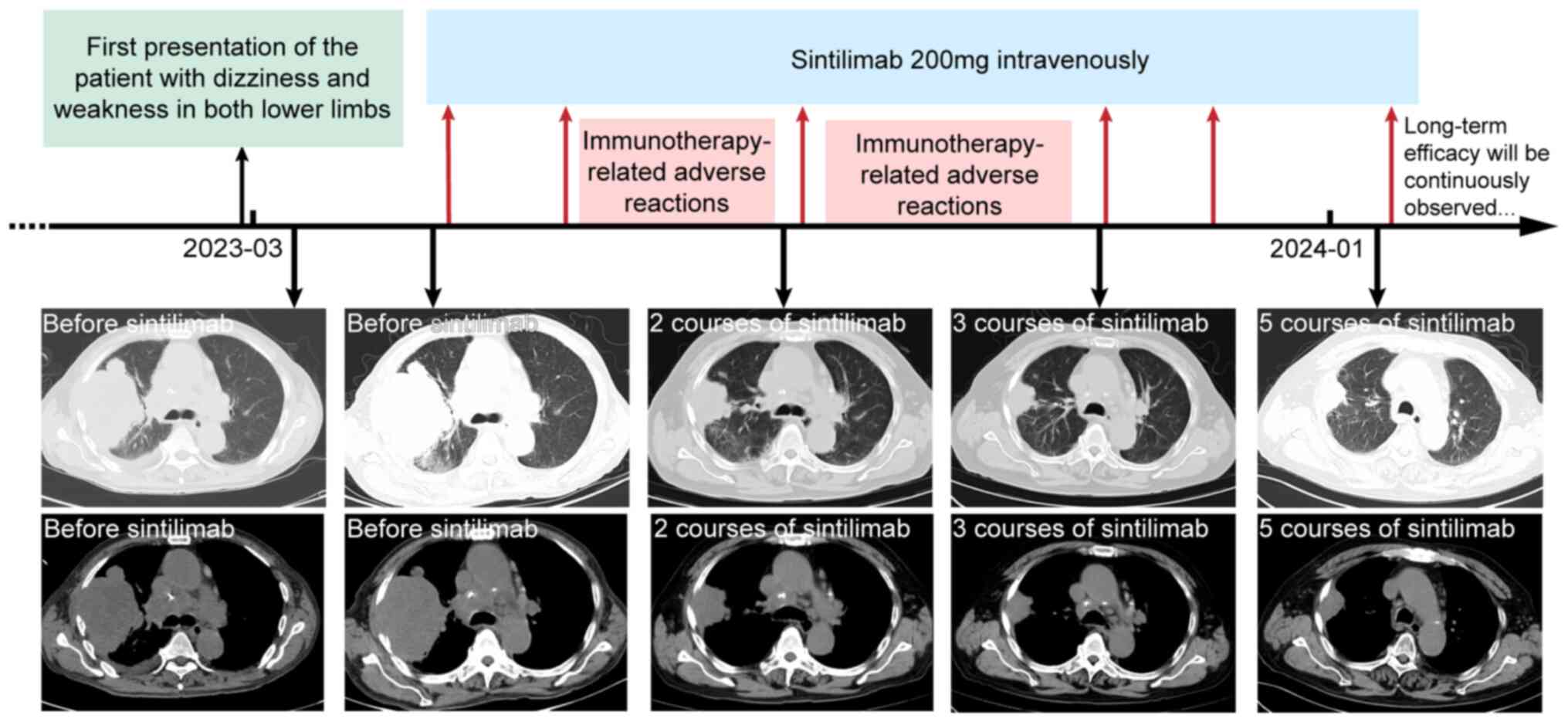
sintilimab was started at a dose of 200 mg every 21 days in late
April 2023. Initially, no immunotherapy-related adverse reactions
(irAEs) occurred. After receiving the second course of sintilimab
in late May 2023, the patient developed symptoms of generalized
skin itching. Due to the irAEs, the patient did not proceed with
the next course as scheduled. After symptomatic treatment, the
patient stabilized and received the third course in August 2023.
One week later, the patient developed symptoms of itching again and
generalized erythema appeared. After treatment with antihistamines
and glucocorticoids, the erythema gradually subsided. The patient
was then treated with three further courses of sintilimab in
October 2023, November 2023 and January 2024 without any grade 3 or
higher irAEs. Sintilimab immunotherapy was scheduled to continue
thereafter.
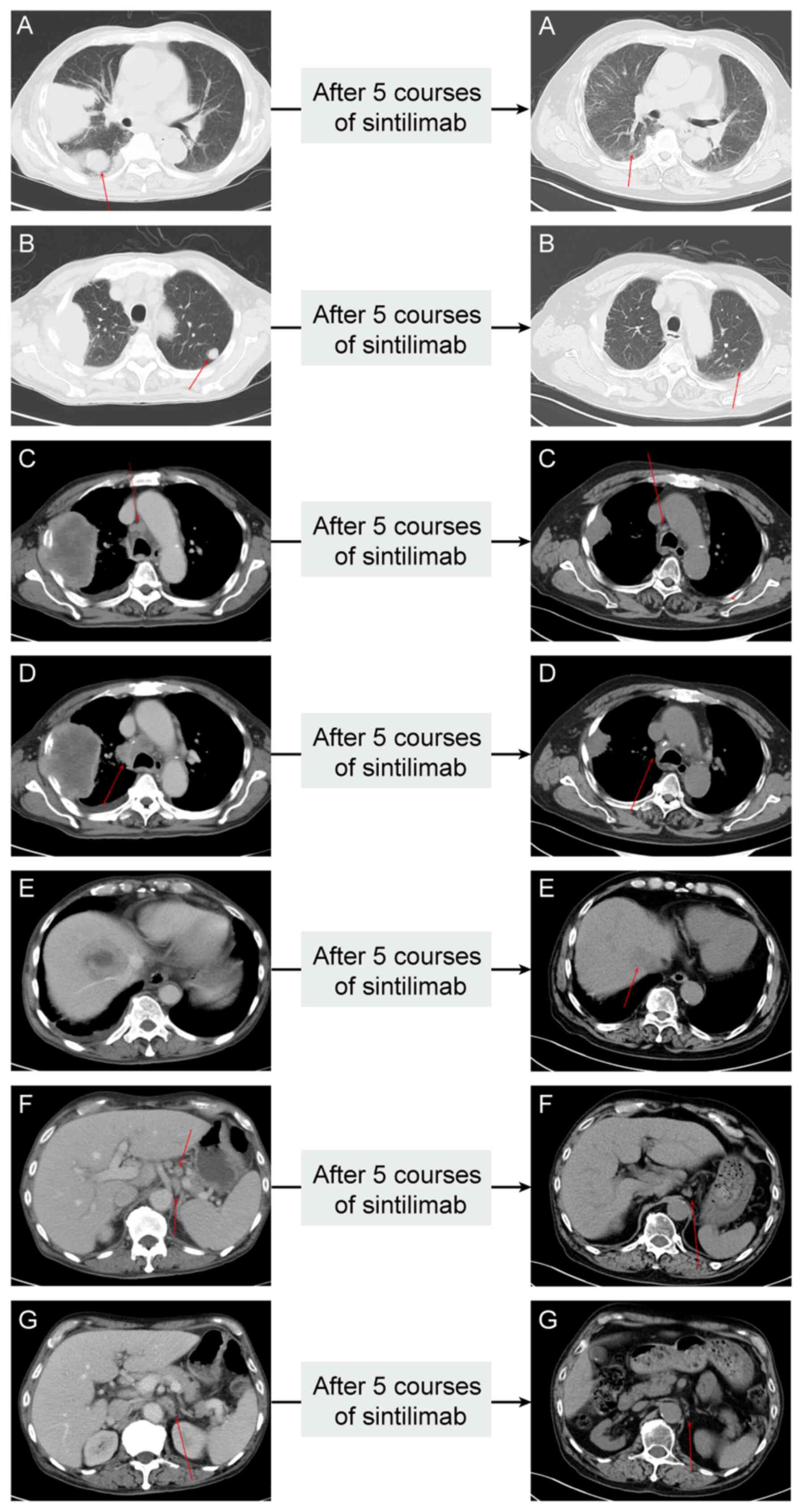
Throughout the immunotherapy period, the patient
received a CT scan nearly every three months and each imaging
review showed a significant clinical response. Specifically, the
chest CT scan from July 2023 (i.e. after having received two
courses of sintilimab) showed that the tumor in the right upper
lung lobe and multiple metastases were significantly smaller than
before. In October 2023 (i.e. after having received three courses
of sintilimab), the patient's CT scan exhibited another regression
of the tumor in the right upper lung lobe (40×30 mm). The latest
chest CT scan in January 2024 (i.e. after having received five
courses of sintilimab) revealed the tumor in the right upper lung
lobe to be 34×24 mm and the mass in the right hepatic lobe had a
diameter of 18 mm. A combination of CT images from all phases
suggested marked partial remission of all measurable primary and
metastatic lesions. CT manifestations of each metastatic lesion
before and after immunotherapy are displayed in Fig. 2. The timeline of the complete
treatment process and imaging of each stage are provided in
Fig. 3. The long-term efficacy of
sintilimab is still being observed in the patient by performing CT
examinations every three months.
Discussion
MPNST is an uncommon and growth-delayed tumor with
high occultation. Its clinical manifestations have no specificity,
and accordingly, early diagnosis of this disease is difficult. Some
patients may experience rapidly increasing masses. They may also
have corresponding motor and paresthesia neurological symptoms,
which are often caused by advanced tumor compression of the nerve
(15). However, certain patients
may be asymptomatic.
MPNST may occur throughout the whole body, with the
extremities and trunk as the most common sites, followed by deep
soft tissues, retroperitoneum and mediastinum. However, it is
rarely observed in the lung. Certain patients with intrapulmonary
MPNST may present with chest pain, cough, hemoptysis and dyspnea
because of compression of the intercostal nerve or trachea
(7). However, the patient of the
current study did not present with any pulmonary symptoms and was
diagnosed with intrapulmonary MPNST when the tumor had already
reached a considerable size. Before this case, there were already
seven reported cases of pulmonary MPNST (8–12).
Details of these cases are presented in Table I. The surgical treatment of
intrapulmonary MPNST has been highlighted in previous cases,
whereas this article is the first to report remarkable efficacy of
sintilimab in the treatment of this rare malignancy. This is
undoubtedly a reflection of the innovativeness of immunotherapy in
treating this disease.
 | Table I.List of case reports of pulmonary
malignant peripheral nerve sheath tumor. |
Table I.
List of case reports of pulmonary
malignant peripheral nerve sheath tumor.
| Age, years/sex | Self-reported
symptom | Position of
metastasis tumor | Treatment | Adverse
reactions | Outcome | (Refs.) |
|---|
| 68/male | Visual disturbances,
confusion | Liver, brain and | Imatinib 400 mg per
day | General weakness
preventing | •After five months of
treatment, | (8) |
|
| and headaches,
multiple | vertebral body | in combination
with | from walking and
disturbance | the patient's
neurological |
|
|
| cutaneous
neurofibromas all | of D3 | cerebral
radiotherapy | of consciousness
one-and-half | symptoms improved
and the |
|
|
| over the body with
the classic |
|
| months after the
start of | tumor partially
receded. |
|
|
| light brown
spots |
|
| treatment | •After 7 months of
treatment, |
|
|
|
|
|
|
| symptoms worsened
and |
|
|
|
|
|
|
| treatment was
discontinued. |
|
|
|
|
|
|
| The patient is now
lost to |
|
|
|
|
|
|
| follow-up |
|
| 69/female | Recurring intensive
hemoptysis | Lymph nodes of | •Upper
left-sided | Postoperative
worsening of | Died about two
months after | (9) |
|
| episodes, shortness
of breath on | the chest and
the | lobectomy | pain in the left
half of the | surgery |
|
|
| exertion, cough,
retrosternal | lower lobe of
the | •Radiotherapy | chest, shortness of
breath |
|
|
|
| pain and subfebrile
temperature | left lung |
| and mucous
cough |
|
|
| 66/male | Dyspnea | No | Extrapleural
right | •Postoperative
delirium | Died 22 days after
surgery | (10) |
|
|
|
| pneumonectomy | •Fungal
pneumonia |
|
|
| 67/male | Dizziness, neck
pain, nausea | Brain | •Craniotomy with
the | No | Alive without any
signs of | (10) |
|
| and vomiting |
| removal of the
tumor |
| recurrent disease
with a |
|
|
|
|
| and
postoperative |
| follow-up of 4
months |
|
|
|
|
| cranial
radiotherapy |
|
|
|
|
|
|
|
•Video-assisted |
|
|
|
|
|
|
| thoracoscopic
surgery |
|
|
|
|
|
|
| left upper
lobectomy |
|
|
|
|
|
|
| with
mediastinal |
|
|
|
|
|
|
|
lymphadenectomy |
|
|
|
| 42/female | Mild dyspnea | No | •Right upper
lobectomy | No | Alive without tumor
free with a | (10) |
|
|
|
| and mediastinal
lymph |
| follow-up of 55
months |
|
|
|
|
| node
dissection |
|
|
|
|
|
|
| •Video-assisted
thoraco- |
|
|
|
|
|
|
| scopic surgery for
the |
|
|
|
|
|
|
| presence of a
second |
|
|
|
|
|
|
| tumor in the
lingula |
|
|
|
|
|
|
| portion of the
left |
|
|
|
|
|
|
| upper lobe |
|
|
|
| 82/male | Chest pain | No | Left lower
lobectomy | No | Alive without signs
of | (11) |
|
|
|
|
|
| recurrence with a
follow-up |
|
|
|
|
|
|
| of 2 years |
|
| 33/female | Right chest
pain | No | Debulking
wedge | No | Alive without any
recurrence | (12) |
|
|
|
| resection |
|
|
|
| 63/male | Dizziness and
weakness in both | Mediastinal | Sintilimab 200
mg | Immune
dermatitis | Marked partial
remission | Curr- |
|
| lower limbs | lymph nodes, | intravenously
every |
| (sintilimab will
continue to be | ent |
|
|
| liver and | 21 days for 6
cycles |
| used) | study |
|
|
| bilateral
iliac | (due to
personal |
|
|
|
|
|
| bone | reasons, treatment
was not performed on the scheduled date) |
|
|
|
The diagnosis of MPNST is one of the most difficult
and elusive among STS. Its clinical manifestations, imaging
features and histologic features are nonspecific, and thus, the
clinical diagnosis relies on immunohistochemistry (15,16).
The most studied immunohistochemical marker is S-100 protein. S-100
is usually weakly or patchily present in MPNST cases. S-100
expression may be present in 50–60% of MPNST tumor cells. Strong
diffuse staining for S-100 nearly excludes a diagnosis of MPNST,
except for epithelioid MPNST (17).
At times, positive expression of SOX10, Ki-67, cytokeratin and
glial fibrillary acidic protein may be found in MPNST tumor cells,
but the diagnostic value of these immunohistochemical markers is
limited (17–20). H3K27me3 is a new immunohistochemical
marker for MPNST, which has better sensitivity and specificity than
S-100. Approximately 80% of high-grade MPNSTs, 60% of
intermediate-grade MPNSTs and 30% of low-grade MPNSTs showed loss
of H3K27me3 expression (21).
Several studies have assessed H3K27me3 in MPNST by
immunohistochemistry and found that a subset of MPNST retained
H3K27me3 expression (22–24). H3K27me3 loss is frequent in
radiotherapy-related, NF1-related and sporadic MPNST, but it is
less sensitive in low-grade and intermediate-grade tumors.
Therefore, H3K27me3 loss, although more specific, is not a fully
sensitive immunohistochemical marker.
At present, surgery remains the preferred treatment
for MPNST. However, not all patients with MPNST can be treated with
surgery (25). Whether MPNST can be
resected or not mainly depends on the size of the tumor, the growth
site of the tumor and the scope of nerve invasion of the tumor.
Extensive local resection is more effective for MPNST involving
distal extremities (26). However,
for MPNST in the head, neck, chest and abdomen, it is difficult to
achieve exact extensive resection because of tumors' proximity to
vital organs, blood vessels and nerves. The local recurrence rate
of MPNST following gross total resection is as high as 32–65% due
to the limitations in the extent of resection and high
aggressiveness of the tumor (13).
Radiotherapy is often used in conjunction with
surgery to improve the local control rate of MPNST, but only has a
minor effect on long-term survival and increases the risk of
radiation-induced sarcoma (15,26).
Chemotherapy regimens for MPNST are mostly based on STS. At
present, the main first-line chemotherapeutic agents are
doxorubicin and ifosfamide. When these two agents were used in
combination to treat STS, the Response Evaluation Criteria in Solid
Tumors (RECIST) response rate was ~25%; however, the RECIST
response rate for MPNST was only 21% (27). Gemcitabine, docetaxel and etoposide
can be used as second-line chemotherapeutic agents, but their
efficacy is not optimal. There is insufficient data on the roles of
radiotherapy and chemotherapy in MPNST management, and their roles
remain controversial and uncertain. At present, radiotherapy and
chemotherapy are still the main palliative treatments routinely
used to alleviate local symptoms, due to the limited treatment
options for MPNST.
With the deepening of the understanding of MPNST
pathogenesis, certain clinical trials using targeted therapy
blocking known signaling pathways that drive MPNST pathogenesis are
underway (e.g. NCT05107037 and NCT02584647) or completed (e.g.
NCT01661283 and NCT02008877). However, so far, existing research
showed that the efficacy of targeted therapy for MPNST is also
unsatisfactory (13).
PD-1 and PD-L1 can limit the killing effect of T
cells on tumors and help avoid autoimmunity (28). Therefore, blocking PD-1/PD-L1 is an
important method of tumor immunotherapy. PD-1/PD-L1-related ICIs
are ideal tumor immunotherapy agents. Furthermore, PD-L1 expression
by tumor cells has been identified as a predictive immunotherapy
biomarker for the response to PD-1/PD-L1-related ICIs (29). Although vast information about the
use of PD-1/PD-L1-related ICIs in treating common cancer has been
published, limited data on the use of immunotherapy in MPNST and
the expression of PD-L1 in MPNST are available. A study by Wang
et al (30) described
PD-1/PD-L1 axis-mediated immune escape mechanisms and revealed that
PD-L1 is expressed in NF1- and NF2-associated tumors. A study by
Farschtschi et al (31)
showed that NF1 patients with MPNST had higher serum levels of
PD-L1 compared with NF1 patients without MPNST and indicated that
PD-L1 is upregulated in patients with MPNST. Another study by Liu
et al (32) also proved
PD-L1 expression in MPNST. Furthermore, prior to this case, there
were four reports of patients with MPNST achieving significant
remission after immunotherapy (33–36).
Details of these cases are provided in Table II. Of these cases, three involved
treatment with pembrolizumab, one after two courses combination of
epirubicin, ifosfamide and mesna (33), one in combination with procarbazide
(34), and one after surgical
resection and radiation therapy (35). Furthermore, one case involved
treatment with nivolumab plus radiation (36). Overall, significant remission was
consistently seen in all five PD-L1-positive patients with MPNST
treated with immunotherapy. These clinical studies and case reports
supported the possibility of immunotherapy for MPNST and suggested
immunotherapy as a promising treatment for MPNST that needs further
exploration, particularly those ICIs aimed at inhibiting the
PD-1/PD-L1 signaling axis.
 | Table II.List of case reports of malignant
peripheral nerve sheath tumor treated with immunotherapy. |
Table II.
List of case reports of malignant
peripheral nerve sheath tumor treated with immunotherapy.
| Age, years/sex | Location of primary
tumor | Position of
metastasis tumor | Previous
treatment | Genetic change | PD-L1 expression
(assay) | Immunotherapy | Outcome | (Refs.) |
|---|
| 60/male | Primary | Left lower
lobe, | •Left
thoracotomy | Pathogenic | PD-L1 2+ 70% | •Pembrolizumab | Complete | (33) |
|
| paravertebral | liver,
peritoneum, | with resection
of | mutations in | (IHC) | 200 mg intra- | remission |
|
|
| tumor at T7-T8 | bone | the chest wall | ARID1A, |
| venously every |
|
|
|
|
|
| tumor | CDKN2A, |
| 21 days for |
|
|
|
|
|
| •Two courses | KMT2A, NF1, |
| 2 cycles |
|
|
|
|
|
| combination of | and TP53 |
| •Pembrolizumab |
|
|
|
|
|
| epirubicin,
ifos- |
|
| 400 mg intra- |
|
|
|
|
|
| famide and
mesna |
|
| venously every |
|
|
|
|
|
|
|
|
| 21 days for |
|
|
|
|
|
|
|
|
| 4 cycles |
|
|
| 48/male |
Retroperitoneum | Mesentery | •Surgery | Not | PD-L1 90%
(TPS) | Six courses of | Complete | (34) |
|
|
|
| •Six courses
of | available |
| pembrolizumab | response |
|
|
|
|
| combination of |
|
| (200 mg |
|
|
|
|
|
| doxorubicin
and |
|
| intravenously |
|
|
|
|
|
| ifosfamide |
|
| every 21 days) |
|
|
|
|
|
| •Imatinib 400
mg |
|
| combined with |
|
|
|
|
|
| per day |
|
| procarbazine |
|
|
|
|
|
| •Six courses
of |
|
| hydrochloride |
|
|
|
|
|
| Eribulin |
|
| (50
mg/m2 twice |
|
|
|
|
|
|
|
|
| a day) |
|
|
| 22/male | Head and neck | Lung and
pelvic | •Total gross | CDK6 | PD-L1 2+ 5% | Pembrolizumab | Complete | (35) |
|
| of femur | lymph node | resection with | amplification | (IHC) | 200 mg | metabolic |
|
|
|
|
| endoprosthesis |
|
| intravenously | response |
|
|
|
|
| placement |
|
| every 21 days |
|
|
|
|
|
| •Postoperative |
|
| for 21 cycles |
|
|
|
|
|
| radiotherapy |
|
|
|
|
|
| 45/male | Left calf | Lung and
pleura | •Surgery | MED12, TP53, | PD-L1 100% | •Nivolumab | Complete | (36) |
|
| (peroneal
nerve) |
| •Five courses
of | NF1, PLCG1 | (IHC) | 3 mg/kg | response |
|
|
|
|
| doxorubicin | and EP300 |
| intravenously |
|
|
|
|
|
| chemotherapy | CD274/PD-L1 |
| every 2 weeks |
|
|
|
|
|
| •Two courses
of | amplification |
| for 18 months |
|
|
|
|
|
| ifosfamide |
|
| •Radiotherapy
to |
|
|
|
|
|
|
|
|
| the bilateral |
|
|
|
|
|
|
|
|
| anterior
pleural |
|
|
|
|
|
|
|
|
| metastases |
|
|
| 63/male | The right
upper | Mediastinum, | No | Not available | PD-L1 60%
(TPS) | Sintilimab | Marked | Current |
|
| lung lobe | liver and
bilateral |
|
|
| 200 mg intra- | partial | case |
|
|
| iliac bone |
|
|
| venously every | remission |
|
|
|
|
|
|
|
| 21 days for | (sintili- |
|
|
|
|
|
|
|
| 6 cycles (due
to | mab will |
|
|
|
|
|
|
|
| personal
reasons, | be conti- |
|
|
|
|
|
|
|
| treatment was | nued) |
|
|
|
|
|
|
|
| not performed |
|
|
|
|
|
|
|
|
| on the
scheduled |
|
|
|
|
|
|
|
|
| date) |
|
|
Unlike the other four case reports, the patient with
high PD-L1 expression in the present case had not received any
prior anti-tumor therapy. The patient was treated with single-agent
sintilimab without combining it with surgery, chemotherapy,
radiotherapy or targeted therapy. For economic reasons, the patient
chose the more affordable sintilimab instead of pembrolizumab.
Sintilimab has been included in Chinese medical insurance in 2022,
so it is more affordable than pembrolizumab, and the financial
burden of patients is relatively small. Although previous case
reports have reported on the use of pembrolizumab or nivolumab
combined with chemotherapy or radiotherapy for MPNST, the efficacy
of sintilimab alone was also excellent in this case. Pembrolizumab,
nivolumab and sintilimab are humanized monoclonal IgG4 antibodies
against PD-1. They can bind to PD-1 to block the connection of PD-1
with its ligands and impede inhibitory signals in T cells. While
data from large-scale randomized clinical trials on the efficacy
and safety immunotherapy for MPNST are scarce, several clinical
trials on immunotherapy for MPNST are currently recruiting. Updated
results of a phase II trial (NCT03611868) showed that alrizomadlin
combined with pembrolizumab was well tolerated and demonstrated
preliminary anti-tumor activity in an MPNST cohort with a 40%
clinical benefit rate (37). A
phase ІІ clinical trial (NCT02691026) is underway on the efficacy
of pembrolizumab in patients with MPNST. There are also two ongoing
clinical trials (NCT02834013 and NCT04465643) on the efficacy of
nivolumab plus ipilimumab for MPNST (38,39).
However, no clinical trial has been conducted on the efficacy and
safety of sintilimab for MPNST, and it is necessary to perform this
in the future.
The present study reported for the first time that
sintilimab single-agent immunotherapy achieved a remarkable
response of intrapulmonary MPNST. From this and previous cases, it
may be speculated that single-agent immunotherapy may be a good
choice of first-line treatment for MPNST in patients with high
PD-L1 expression or in patients with an Eastern Cooperative
Oncology Group Performance Status (ECOG PS) of 3–4 who cannot
tolerate high-intensity chemotherapy. Immunotherapy in combination
with chemotherapy may be a viable treatment option for MPNST in
patients with low PD-L1 expression or in patients with an ECOG PS
of 0–2. Rational combination of immunotherapy regimens may yield
significant results. Additional prospective trials are still needed
to confirm these preliminary results.
In conclusion, the case reported in the present
study illustrates that PD-1/PD-L1-related ICIs may be an effective
therapeutic method for patients with primary intrapulmonary MPNST
with positive PD-L1 expression. Particularly for patients with high
PD-L1 expression, a remarkable response may be achieved by using
PD-1/PD-L1-related ICIs as first-line treatment. We are confident
about the outlook of immunotherapy for MPNST and expect that the
outcome of the ongoing clinical trials will contribute to the
design of personalized immunotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
Manuscript writing, literature search and
acquisition of data: YQC. Treatment and observation of the patient,
study conception and design: TC. Manuscript drafting, aggregation
of materials and analysis of data: WSZ, LZL and CTF. Manuscript
revision, manuscript reviewing for intellectual content and
interpretation of data: HTZ. All authors have read and approved the
final manuscript. HTZ and YQC have confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient to publish this report and any associated accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MPNST
|
malignant peripheral nerve sheath
tumor
|
|
PD-1
|
programmed death 1
|
|
PD-L1
|
programmed death-ligand 1
|
|
STS
|
soft tissue sarcomas
|
|
NF1
|
neurofibromatosis type 1
|
|
ICI
|
immune checkpoint inhibitor
|
|
CT
|
computed tomography
|
|
H3K27Me3
|
histone H3 lysine 27
trimethylation
|
|
irAEs
|
immunotherapy-related adverse
reactions
|
|
RECIST
|
Response Evaluation Criteria in Solid
Tumors
|
|
ECOG PS
|
Eastern Cooperative Oncology Group
Performance Status
|
References
|
1
|
Fuchs B, Spinner RJ and Rock MG: Malignant
peripheral nerve sheath tumors: An update. J Surg Orthop Adv.
14:168–174. 2005.PubMed/NCBI
|
|
2
|
Widemann BC: Current status of sporadic
and neurofibromatosis type 1-associated malignant peripheral nerve
sheath tumors. Curr Oncol Rep. 11:322–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stucky CCH, Johnson KN, Gray RJ, Pockaj
BA, Ocal IT, Rose PS and Wasif N: Malignant peripheral nerve sheath
tumors (MPNST): The Mayo Clinic experience. Ann Surg Oncol.
19:878–885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goertz O, Langer S, Uthoff D, Ring A,
Stricker I, Tannapfel A and Steinau HU: Diagnosis, treatment and
survival of 65 patients with malignant peripheral nerve sheath
tumors. Anticancer Res. 34:777–783. 2014.PubMed/NCBI
|
|
5
|
Yaga US, Shivakumar R, Kumar MA and
Sathyaprakash: Malignant peripheral nerve sheath tumor: A rarity.
Indian J Dent. 6:53–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riad S, Biau D, Holt GE, Werier J,
Turcotte RE, Ferguson PC, Griffin AM, Dickie CI, Chung PW, Catton
CN, et al: The clinical and functional outcome for patients with
radiation-induced soft tissue sarcoma. Cancer. 118:2682–2692. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kolarov V, Stanić J, Eri Z, Zvezdin B,
Kojičić M and Hromis S: Intrathoracic malignant peripheral nerve
sheath tumor with poor outcome: A case report. Bosn J Basic Med
Sci. 10:328–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maane LA, Al Bouzidi A, Damou M and
Ismaili N: Primary intrapulmonary malignant peripheral nerve sheath
tumor: A rare case. Cancer Treat Res Commun. 25:1002432020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grzywa-Celińska A, Szmygin-Milanowska K,
Emeryk-Maksymiuk J, Walczyna M, Palonka M and Siwiec J: Malignant
peripheral nerve sheath tumor in a patient without
neurofibromatosis 1 (NF1): A rare case of primary lung location. J
Educ Health Sport. 8:11–17. 2018.
|
|
10
|
Inci I, Soltermann A, Schneiter D and
Weder W: Pulmonary malignant peripheral nerve sheath tumour. Eur J
Cardiothorac Surg. 46:331–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
La Mantia E, Franco R, Cantile M, Rocco R,
De Chiara A, Martucci N and Rocco G: Primary intrapulmonary
malignant peripheral nerve sheath tumor mimicking lung cancer. J
Thorac Dis. 5:E155–E157. 2013.PubMed/NCBI
|
|
12
|
Desdiani D, Darifah S and Azali C: Giant
intrapulmonary malignant peripheral nerve sheath tumour. Respirol
Case Rep. 8:e005672020. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bradford D and Kim A: Current treatment
options for malignant peripheral nerve sheath tumors. Curr Treat
Options Oncol. 16:3282015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Committee of Consensus of
Immunohistochemistry Test on Technology, . Consensus of
immunohistochemistry test on technology. Zhonghua Bing Li Xue Za
Zhi. 48:87–91. 2019.(In Chinese). PubMed/NCBI
|
|
15
|
Farid M, Demicco EG, Garcia R, Ahn L,
Merola PR, Cioffi A and Maki RG: Malignant peripheral nerve sheath
tumors. Oncologist. 19:193–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma S, Shah JS and Bali H: Malignant
peripheral nerve sheath tumor: A rare malignancy. J Oral Maxillofac
Pathol. 24 (Suppl 1):S86–S90. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thway K and Fisher C: Malignant peripheral
nerve sheath tumor: Pathology and genetics. Ann Diagn Pathol.
18:109–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pekmezci M, Reuss DE, Hirbe AC, Dahiya S,
Gutmann DH, von Deimling A, Horvai AE and Perry A: Morphologic and
immunohistochemical features of malignant peripheral nerve sheath
tumors and cellular schwannomas. Mod Pathol. 28:187–200. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rodriguez FJ, Folpe AL, Giannini C and
Perry A: Pathology of peripheral nerve sheath tumors: Diagnostic
overview and update on selected diagnostic problems. Acta
Neuropathol. 123:295–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olsen SH, Thomas DG and Lucas DR: Cluster
analysis of immunohistochemical profiles in synovial sarcoma,
malignant peripheral nerve sheath tumor, and Ewing sarcoma. Mod
Pathol. 19:659–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schaefer IM and Fletcher CDM: Recent
advances in the diagnosis of soft tissue tumours. Pathology.
50:37–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prieto-Granada CN, Wiesner T, Messina JL,
Jungbluth AA, Chi P and Antonescu CR: Loss of H3K27me3 expression
is a highly sensitive marker for sporadic and radiation-induced
MPNST. Am J Surg Pathol. 40:479–489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schaefer IM, Fletcher CD and Hornick JL:
Loss of H3K27 trimethylation distinguishes malignant peripheral
nerve sheath tumors from histologic mimics. Mod Pathol. 29:4–13.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cleven AH, Al Sannaa GA, Briaire-De Bruijn
I, Ingram DR, van de Rijn M, Rubin BP, de Vries MW, Watson KL,
Torres KE, Wang WL, et al: Loss of H3K27 tri-methylation is a
diagnostic marker for malignant peripheral nerve sheath tumors and
an indicator for an inferior survival. Mod Pathol. 29:582–590.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grobmyer SR, Reith JD, Shahlaee A, Bush CH
and Hochwald SN: Malignant peripheral nerve sheath tumor: Molecular
pathogenesis and current management considerations. J Surg Oncol.
97:340–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gupta G, Mammis A and Maniker A: Malignant
peripheral nerve sheath tumors. Neurosurg Clin N Am. 19533–543.
(v)2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kroep JR, Ouali M, Gelderblom H, Le Cesne
A, Dekker TJA, Van Glabbeke M, Hogendoorn PCW and Hohenberger P:
First-line chemotherapy for malignant peripheral nerve sheath tumor
(MPNST) versus other histological soft tissue sarcoma subtypes and
as a prognostic factor for MPNST: An EORTC soft tissue and bone
sarcoma group study. Ann Oncol. 22:207–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chamoto K, Al-Habsi M and Honjo T: Role of
PD-1 in immunity and diseases. Curr Top Microbiol Immunol.
410:75–97. 2017.PubMed/NCBI
|
|
29
|
Dong ZY, Wu SP, Liao RQ, Huang SM and Wu
YL: Potential biomarker for checkpoint blockade immunotherapy and
treatment strategy. Tumor Biol. 37:4251–4261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang S, Liechty B, Patel S, Weber JS,
Hollmann TJ, Snuderl M and Karajannis MA: Programmed death ligand 1
expression and tumor infiltrating lymphocytes in neurofibromatosis
type 1 and 2 associated tumors. J Neurooncol. 138:183–190. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Farschtschi S, Kluwe L, Park SJ, Oh SJ,
Mah N, Mautner VF and Kurtz A: Upregulated immuno-modulator PD-L1
in malignant peripheral nerve sheath tumors provides a potential
biomarker and a therapeutic target. Cancer Immunol Immunother.
69:1307–1313. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Li H, Wei C, Li Q and Wang Z: PD-L1
expression and tumor infiltrating lymphocytes in neurofibromatosis
type 1-related benign tumors and malignant peripheral nerve sheath
tumors: An implication for immune checkpoint inhibition therapy.
Chin J Plast Reconstr Surg. 3:63–75. 2021. View Article : Google Scholar
|
|
33
|
Larson K, Russ A, Arif-Tiwari H, Mahadevan
D, Elliott A, Bhattacharyya A and Babiker H: Pembrolizumab achieves
a complete response in an NF-1 mutated, PD-L1 positive malignant
peripheral nerve sheath tumor: A case report and review of the
benchmarks. J Immunother. 45:222–226. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Payandeh M, Sadeghi M and Sadeghi E:
Complete response to pembrolizumab in a patient with malignant
peripheral nerve sheath tumor: The first case reported. J App Pharm
Sci. 7:182–184. 2017.
|
|
35
|
Davis LE, Nicholls LA, Babiker HM, Liau J
and Mahadevan D: PD-1 inhibition achieves a complete metabolic
response in a patient with malignant peripheral nerve sheath tumor.
Cancer Immunol Res. 7:1396–1400. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Özdemir BC, Bohanes P, Bisig B, Missiaglia
E, Tsantoulis P, Coukos G, Montemurro M, Homicsko K and Michielin
O: Deep response to anti-PD-1 therapy of metastatic
neurofibromatosis type 1-associated malignant peripheral nerve
sheath tumor with CD274/PD-L1 amplification. JCO Precis Oncol.
3:1–6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mckean M, Tolcher AW, Reeves JA,
Chmielowsk B, Shaheen MF, Beck JT, Orloff MM, Somaiah N, Van Tine
BA, Drabick JJ, et al: Newly updated activity results of
alrizomadlin (APG-115), a novel MDM2/p53 inhibitor, plus
pembrolizumab: Phase 2 study in adults and children with various
solid tumors. J Clin Oncol. 40 (16 Suppl):S9517–2022. View Article : Google Scholar
|
|
38
|
González-Muñoz T, Kim A, Ratner N and
Peinado H: The need for new treatments targeting MPNST: The
potential of strategies combining MEK inhibitors with
antiangiogenic agents. Clin Cancer Res. 28:3185–3195. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Paudel SN, Hutzen B and Cripe TP: The
quest for effective immunotherapies against malignant peripheral
nerve sheath tumors: Is there hope? Mol Ther Oncolytics.
30:227–237. 2023. View Article : Google Scholar : PubMed/NCBI
|