Introduction
Upper tract urothelial carcinoma (UTUC) represents
only 5% of all urothelial carcinoma cases globally (1); however, UTUC is more prevalent in
Taiwan, specifically in the Southwest region (2,3). As
UTUC has a high risk of recurrence and progression, radical
nephroureterectomy (RNU) with bladder cuff excision has served as
the standard treatment for localized disease (4,5). In
high-risk patients with UTUC, including those with an advanced
tumor stage, regional lymph node (LN) metastasis and lymphovascular
invasion (LVI) positivity, neoadjuvant or adjuvant systemic therapy
has shown promising results (6–9).
Hence, identification of pre-and postoperative prognostic factors
for high-risk disease progression is a major focus of numerous
studies, which will aid in conducting accurate pretreatment
assessments of UTUC.
Tumor multifocality, defined as the simultaneous
occurrence of multiple (>1) tumors, in the renal pelvis and/or
ureter has been considered a potential risk biomarker for UTUC
(10–12). Some studies have suggested that
multifocal UTUC is associated with a more aggressive biological
behavior and poorer clinical outcome (10,13,14).
Some evidence showed that multifocal tumors, regardless of the
tumor location, are only significantly associated with bladder
tumor recurrence and not cancer-specific survival (CSS) (15–17).
In addition, emerging research has highlighted the role of
inflammatory markers in tumor development and progression (18–23).
Previous studies have shown that the systemic inflammation response
index (SIRI) is a useful inflammation biomarker associated with
poorer urologic outcomes (24–26).
However, whether the systemic inflammation state influences the
prognostic significance of tumor multifocality remains unclear.
Therefore, the aim of the present study was to investigate the
effect of tumor multifocality with different inflammation states
(high vs. low-level SIRI) on clinical outcomes in patients with
UTUC after RNU.
Materials and methods
Patient population and selection
The present single-center retrospective study
included 645 patients with UTUC who received RNU at National
Chen-Kung University Hospital (Tainan, Taiwan) from January-2008 to
December-2020. The exclusion criteria included: i) Patients who did
not undergo RNU; ii) patients with an active infection status; iii)
lack of differential count information from preoperative complete
blood counts (CBCs) 30 days before surgery; iv) visceral or bone
metastasis at the time of diagnosis; v) other concurrent cancer;
vi) the use of immunosuppressive drugs; vii) a follow-up duration
of <30 days; and viii) treatment with neo-adjuvant systemic
therapy or radiotherapy. Multifocal UTUC was defined as the
synchronous presence of pathologically confirmed >1 tumor in the
upper urinary tract. All enrolled patients were divided into the
multifocal and non-multifocal groups, and clinicopathological
parameters collected from medical records were compared, including
age, sex, renal function, hemodialysis, comorbidities (hypertension
or diabetes mellitus), associated symptoms (gross hematuria and
hydronephrosis), prior or concomitant bladder cancer, pathological
tumor (pT) stage, LN status, tumor grade, tumor size, tumor
necrosis, LVI, adjuvant chemotherapy, white blood cell count (WBC),
absolute neutrophil count (ANC) and SIRI. SIRI was calculated as
follows: Neutrophil count × monocyte count/lymphocyte count. The
present study was approved by The Cheng Kung University Hospital
Institutional Review Board (IRB no. B-ER-112-216).
Collection of tumor pathological
characteristics
All tumor characteristics were collected from the
medical records. All slides from surgical specimens were reviewed
by genitourinary pathologists based on the same criteria. Tumors
were staged according to the American Joint Committee on Cancer TNM
Classification (7th edition) (27)
and graded according to the 2004 World Health
Organization/International Society of Urological Pathology
consensus classification (28).
Follow-up schedule
Generally, patients were scheduled for postoperative
follow-up every 3–6 months in the first year following RNU, every 6
months in the second to fifth year and annually thereafter. During
each visit, clinical evaluations included updating the medical
history, physical examination, blood tests, urinary cytology and
imaging studies. Disease recurrence was defined as distant
metastasis or local recurrence in the tumor bed or regional LNs.
The cause of death was determined by the death certificate, medical
notes or the attending doctor. The primary endpoints were overall
survival (OS), CSS and recurrence-free survival (RFS). OS was
defined as the interval from RNU until death; CSS was defined as
the interval from RNU until UTUC-related death; RFS was defined as
the duration from RNU until local recurrence or distant metastases
(did not include intravesical or contralateral upper tract
recurrence).
Statistical analysis
Descriptive statistics were performed for all
variables. Categorical variables were compared using the
χ2 test. Continuous variables were evaluated using the
unpaired Student's t-test or the Mann-Whitney U test, depending on
the data distribution. The optimal SIRI cut-off value was
determined as 1.95 using receiver operating characteristic (ROC)
curve analysis with Youden's index based on CSS (Fig. S1). Specifically, a SIRI of ≥1.95 or
<1.95 were defined as high or low level in the setting. The
cut-off values for WBC and ANC were set based on the median counts.
Survival curves were plotted using the Kaplan-Meier method, and the
differences were assessed using the log-rank test. Univariate and
multivariate Cox regression analyses were performed to identify
significant prognostic factors for OS, CSS and RFS. All statistical
analyses were performed using SPSS version 26.0 (IBM Corp.), and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Demographic and clinicopathological
features of the study population
In the population of 645 patients, tumor
multifocality was absent in 482 and present in 163 patients. The
mean age was 69.2±11.0 years and the median follow-up time was
66.0±45.0 months. No significant differences were noted in age,
sex, hemodialysis, DM or HTN, gross hematuria, pT stage, LN status,
LVI, tumor grade and adjuvant chemotherapy between the two
multifocality groups. Conversely, the presence of tumor
multifocality was significantly associated with higher rates of
renal function impairment, hydronephrosis, prior or concomitant BC,
larger tumor size, tumor necrosis, WBC, ANC and SIRI (Table I).
 | Table I.Association between
clinicopathological characteristics and multifocal tumors in 645
patients with upper tract urothelial carcinoma. |
Table I.
Association between
clinicopathological characteristics and multifocal tumors in 645
patients with upper tract urothelial carcinoma.
|
|
| Multifocality |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | All patients,
n=645 | Absence, n=482 | Presence,
n=163 | P-value |
|---|
| Mean age ± SD,
years | 69.2±11.0 | 69.2±10.8 | 69.2±11.5 |
|
| Mean follow-up
after surgery ± SD, months | 66.0±45.0 | 68.1±44.6 | 60.2±45.9 |
|
| Age, n (%) |
|
|
| 0.867 |
| ≤69
years | 305 (47) | 227 (47) | 78 (48) |
|
| >69
years | 340 (53) | 255 (53) | 85 (52) |
|
| Sex, n (%) |
|
|
| 0.926 |
|
Male | 279 (43) | 209 (43) | 70 (43) |
|
|
Female | 366 (57) | 273 (57) | 93 (57) |
|
| Hemodialysis, n
(%) |
|
|
| 0.180 |
| No | 529 (82) | 401 (83) | 128 (79) |
|
|
Yes | 116 (18) | 81 (17) | 35 (21) |
|
| eGFRa, n (%) |
|
|
| 0.019 |
| ≥60
ml/min/1.73 m2 | 256 (40) | 204 (42) | 52 (32) |
|
| <60
ml/min/1.73 m2 | 389 (60) | 278 (58) | 111 (68) |
|
| DM or HTN, n
(%) |
|
|
| 0.715 |
|
Absent | 273 (42) | 206 (43) | 67 (41) |
|
|
Present | 372 (58) | 276 (57) | 96 (59) |
|
| Hematuria (%) |
|
|
| 0.373 |
| No | 82 (13) | 58 (12) | 24 (15) |
|
|
Yes | 563 (87) | 424 (88) | 139 (85) |
|
| Hydronephrosis, n
(%) |
|
|
| 0.005 |
| No | 137 (21) | 115 (24) | 22 (13) |
|
|
Yes | 508 (79) | 367 (76) | 141 (87) |
|
| Prior BC, n
(%) |
|
|
| <0.001 |
| No | 540 (84) | 422 (88) | 118 (72) |
|
|
Yes | 105 (16) | 60 (12) | 45 (28) |
|
| Concomitant BC
(%) |
|
|
| <0.001 |
| No | 515 (80) | 402 (83) | 113 (69) |
|
|
Yes | 130 (20) | 80 (17) | 50 (31) |
|
| Pathological T
stage, n (%) |
|
|
| 0.057 |
|
Tis/a/1 | 238 (37) | 189 (39) | 49 (30) |
|
| T2 | 126 (20) | 86 (18) | 40 (25) |
|
|
T3/T4 | 281 (43) | 207 (43) | 74 (45) |
|
| LN status, n
(%) |
|
|
| 0.091 |
|
N0/x | 607 (94) | 458 (95) | 149 (91) |
|
| N+ | 38 (6) | 24 (5) | 14 (9) |
|
| Tumor grade, n
(%) |
|
|
| 0.058 |
|
Low | 29 (5) | 26 (5) | 3 (2) |
|
|
High | 616 (95) | 456 (95) | 160 (98) |
|
| Tumor size, n
(%) |
|
|
| 0.014 |
| ≤2
cm | 183 (28) | 149 (31) | 34 (21) |
|
| >2
cm | 462 (72) | 333 (69) | 129 (79) |
|
| Tumor necrosis, n
(%) |
|
|
| 0.046 |
| No | 521 (81) | 398 (83) | 123 (75) |
|
|
Yes | 124 (19) | 84 (17) | 40 (25) |
|
| LVI, n (%) |
|
|
| 0.104 |
| No | 452 (70) | 346 (72) | 106 (65) |
|
|
Yes | 193 (30) | 136 (28) | 57 (35) |
|
| Adjuvant
chemotherapy, n (%) |
|
|
| 0.526 |
| No | 582 (90) | 437 (91) | 145 (89) |
|
|
Yes | 63 (10) | 45 (9) | 18 (11) |
|
| WBC, n (%) |
|
|
| 0.001 |
|
≤6.6109/l | 317 (49) | 255 (53) | 62 (38) |
|
|
>6.6109/l | 328 (51) | 227 (47) | 101 (62) |
|
| ANC, n (%) |
|
|
| 0.044 |
|
≤4,235/mm3 | 321 (50) | 251 (52) | 70 (43) |
|
|
>4,235/mm3 | 324 (50) | 231 (48) | 93 (57) |
|
| SIRI, n (%) |
|
|
| <0.001 |
|
≤1.95 | 428 (66) | 338 (70) | 90 (55) |
|
|
>1.95 | 217 (34) | 144 (30) | 73 (45) |
|
Prognostic impact of tumor
multifocality and SIRI
Kaplan-Meier analysis revealed that multifocal UTUC
was significantly associated with a poorer OS, CSS and RFS compared
with non-multifocal UTUC (all P<0.05; Fig. S2). ROC analysis showed SIRI was
preferred over WBC or ANC for predictive ability on oncological
outcomes (Fig. S3). Also in the
multivariate analysis, SIRI, pT stage, LN stage, LVI, and tumor
multifocality were independent factors for OS, CSS and RFS
(Table SI). SIRI was used as
systemic inflammation state as appropriate. To further investigate
the effect of the systemic inflammation state on tumor
multifocality, patients were categorized into four groups based on
tumor multifocality and SIRI, including multifocal with a
high-level SIRI, non-multifocal with a high-level SIRI, multifocal
with a low-level SIRI and non-multifocal with a low-level SIRI.
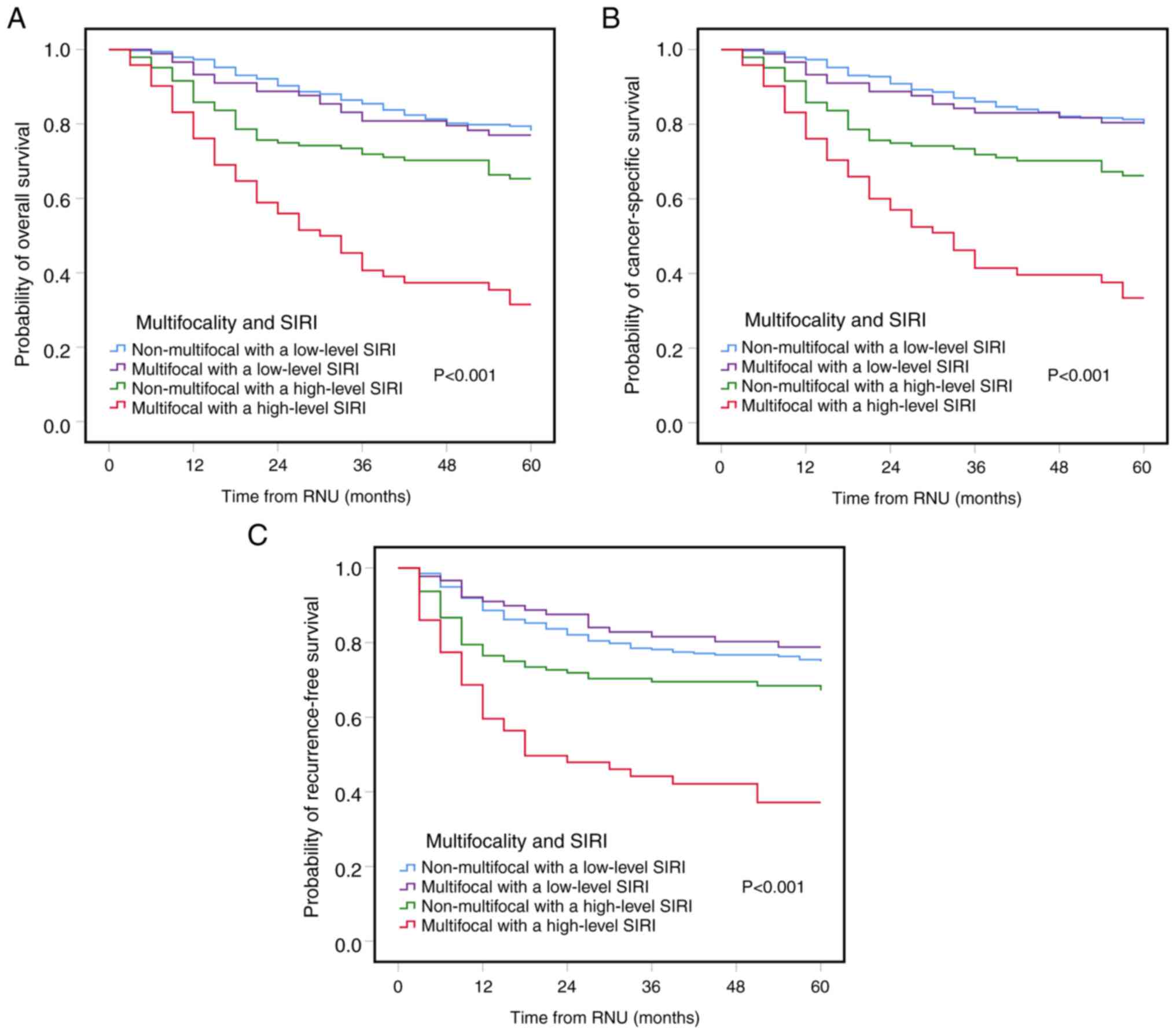
Kaplan-Meier analysis revealed that multifocal UTUC coupled with a
high-level SIRI was significantly associated with a worse OS, CSS
and RFS compared with the other groups (Fig. 1).
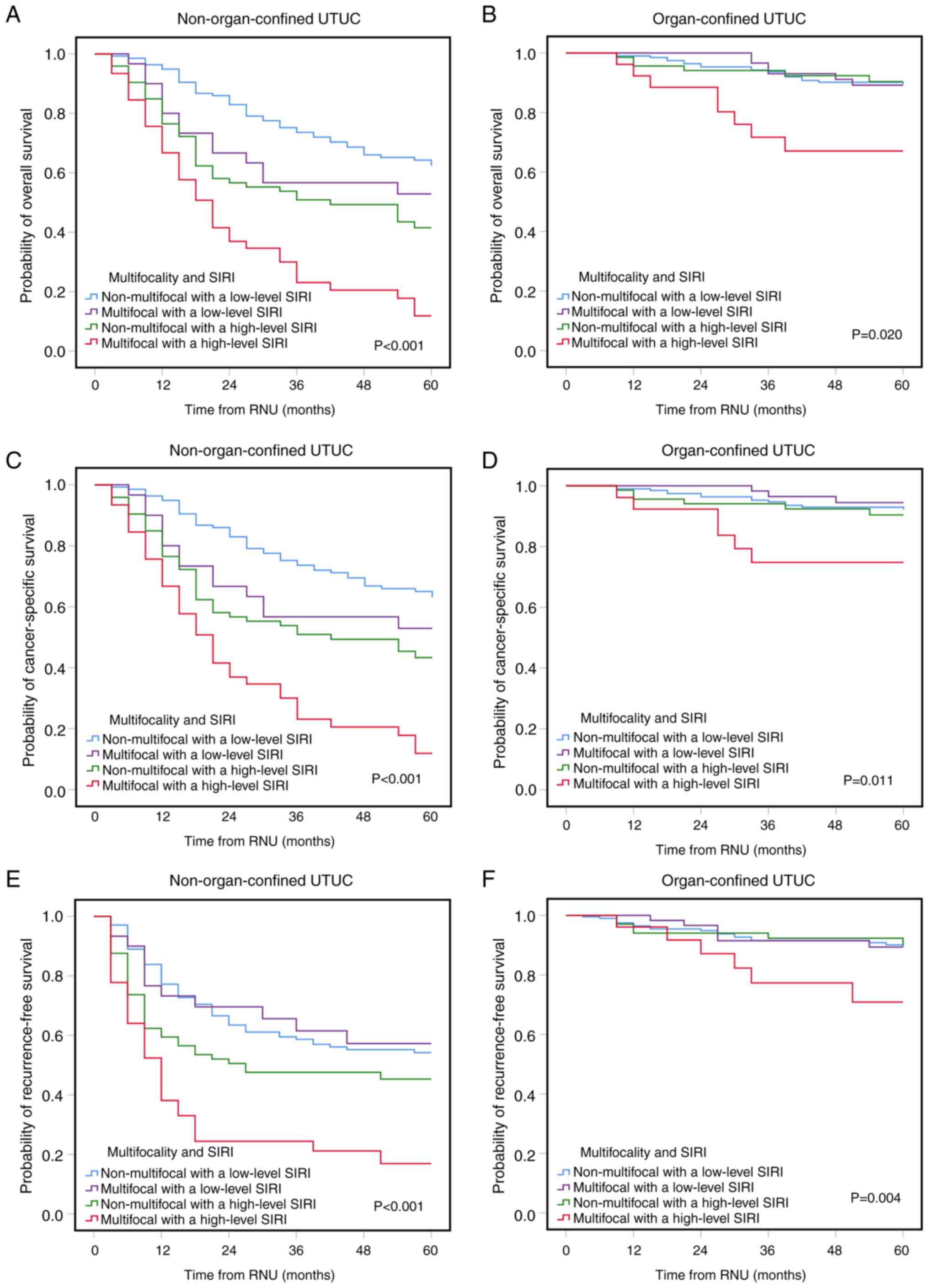
Additionally, as non-organ-confined (NOC) and OC)
diseases have different pathophysiological characteristics, all
patients were stratified into the NOC and OC groups, before
evaluating the combinatorial impact of tumor multifocality and SIRI
on OS, CSS and RFS. Kaplan-Meier analyses showed that multifocal
UTUC with a high-level SIRI was significantly associated with a
poorer OS, CSS and RFS in both OC and NOC UTUC compared with the
other three groups (Fig. 2). Thus,
multifocal UTUC with a high-level SIRI was considered a reliable
and feasible risk factor for discriminating survival outcomes.
Multifocal UTUC with a high-level SIRI
as an independent factor
In the univariate analyses for OS and CSS, variables
including sex, age, pT stage, LN stage, LVI, tumor size, tumor
necrosis, adjuvant chemotherapy and multifocal tumor with a
high-level SIRI, were potential prognostic factors. After
controlling for confounding variables, the multivariate analysis
confirmed that sex, age, pT stage, LN stage, LVI, adjuvant
chemotherapy, non-multifocal tumor with a high SIRI, and multifocal
tumor with a high-level SIRI were indicated as independent factors
for predicting OS and CSS (Table
II). Regarding RFS, the univariate analysis showed significant
variables, including sex, hemodialysis, pT stage, LN stage, tumor
grade, LVI, tumor size, tumor necrosis, adjuvant chemotherapy and
multifocal tumor with a high-level SIRI. The multivariate analysis
revealed that advanced pT stage, LN stage, LVI and multifocal tumor
with a high-level SIRI were independent prognostic factors for RFS
(Table II).
 | Table II.Multivariate cox regression analyses
for predicting overall survival, cancer-specific survival, and
recurrence-free survival in patients with UTUC who underwent
radical nephroureterectomy. |
Table II.
Multivariate cox regression analyses
for predicting overall survival, cancer-specific survival, and
recurrence-free survival in patients with UTUC who underwent
radical nephroureterectomy.
|
| Overall
survival | Cancer-specific
survival | Recurrence-free
survival |
|---|
|
|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex, female vs.
male | 0.793
(0.598–1.053) | 0.109 | 0.780
(0.586–1.039) | 0.089 | 0.782
(0.584–1.047) | 0.098 |
| Age at RNU |
|
|
|
|
|
|
| >69
vs. ≤69 year | 1.773
(1.320–2.238) | 0.001 | 1.713
(1.265–2.320) | 0.001 | 1.209
(0.895–1.634 | 0.216 |
| Pre-eGFR
(ml/min/1.73 m2) |
|
|
|
|
|
|
| ≥60 vs.
<60 | - |
| - |
| - |
|
| Hemodialysis |
|
|
|
|
|
|
| Yes vs.
no | - |
| - |
| 0.672
(0.422–1.071) | 0.094 |
| DM or HTN |
|
|
|
|
|
|
| Present
vs. absent | - |
| - |
| - |
|
| Prior BC |
|
|
|
|
|
|
| Yes vs.
no | - |
| - |
| - |
|
| Concomitant BC |
|
|
|
|
|
|
| Yes vs.
no | - |
| - |
| - |
|
| Hydronephrosis |
|
|
|
|
|
|
| Present
vs. absent | - |
| - |
| - |
|
| Hematuria |
|
|
|
|
|
|
| Present
vs. absent | - |
| - |
| - |
|
| Pathological T
stage |
|
|
|
|
|
|
| T2 vs.
Tis/a/1 | 1.871
(1.049–3.339) | 0.034 | 3.031
(1.537–7.860) | 0.001 | 1.780
(0.956–3.312) | 0.069 |
| T3/4
vs. Tis/a/1 | 5.481
(3.393–8.853) | 0.001 | 9.253
(5.124–16.709) | <0.001 | 5.695
(3.461–9.371) | <0.001 |
| Lymph node
stage |
|
|
|
|
|
|
| N+ vs.
Nx/0 | 2.106
(1.336–3.321) | 0.001 | 2.170
(1.370–3.436) | 0.001 | 2.073
(1.301–3.305) | 0.002 |
| Tumor grade |
|
|
|
|
|
|
| High
vs. low | 0.992
(0.304–3.237) | 0.989 | 0.687
(0.208–2.271) | 0.538 | 0.873
(0.398–1.913) | 0.734 |
| LVI, yes vs.
no | 1.814
(1.305–2.522) | 0.001 | 1.729
(1.232–2.427) | 0.002 | 1.920
(1.363–2.706) | <0.001 |
| Tumor size |
|
|
|
|
|
|
| >2
cm vs. ≤2 cm | 1.117
(0.755–1.652) | 0.579 | 1.113
(0.741–1.673) | 0.606 | 1.316
(0.866–2.001) | 0.199 |
| Tumor necrosis |
|
|
|
|
|
|
| Yes vs.
no | 1.027
(0.742–1.423) | 0.872 | 1.015
(0.727–1.417) | 0.932 | 1.035
(0.738–1.451) | 0.842 |
| Adjuvant
chemotherapy |
|
|
|
|
|
|
| Yes vs.
no | 0.532
(0.337–0.838) | 0.007 | 0.533
(0.337–843) | 0.007 | 0.838
(0.549–1.277) | 0.410 |
|
Non-multifocal with high
SIRI | 1.611
(1.124–2.307) | 0.009 | 1.680
(1.163–2.426) | 0.006 | 1.296
(0.894–1.879) | 0.171 |
|
Multifocal with low SIRI | 1.253
(0.789–1.992) | 0.339 | 1.199
(0.734–1.958) | 0.468 | 0.960
(0.584–1.578) | 0.873 |
|
Multifocal with high SIRI | 3.364
(2.432–6.822) | <0.001 | 3.581
(2.411–5.318) | <0.001 | 2.634
(1.753–3.957) | <0.001 |
Overall, the findings indicated a strong association
between prognoses and the integration of tumor multifocality. In
particular, multifocal tumors coupled with a high-level SIRI were
significantly associated with poorer OS, CSS and RFS.
Combining multifocal UTUC and SIRI
increased the prediction ability of survival outcomes
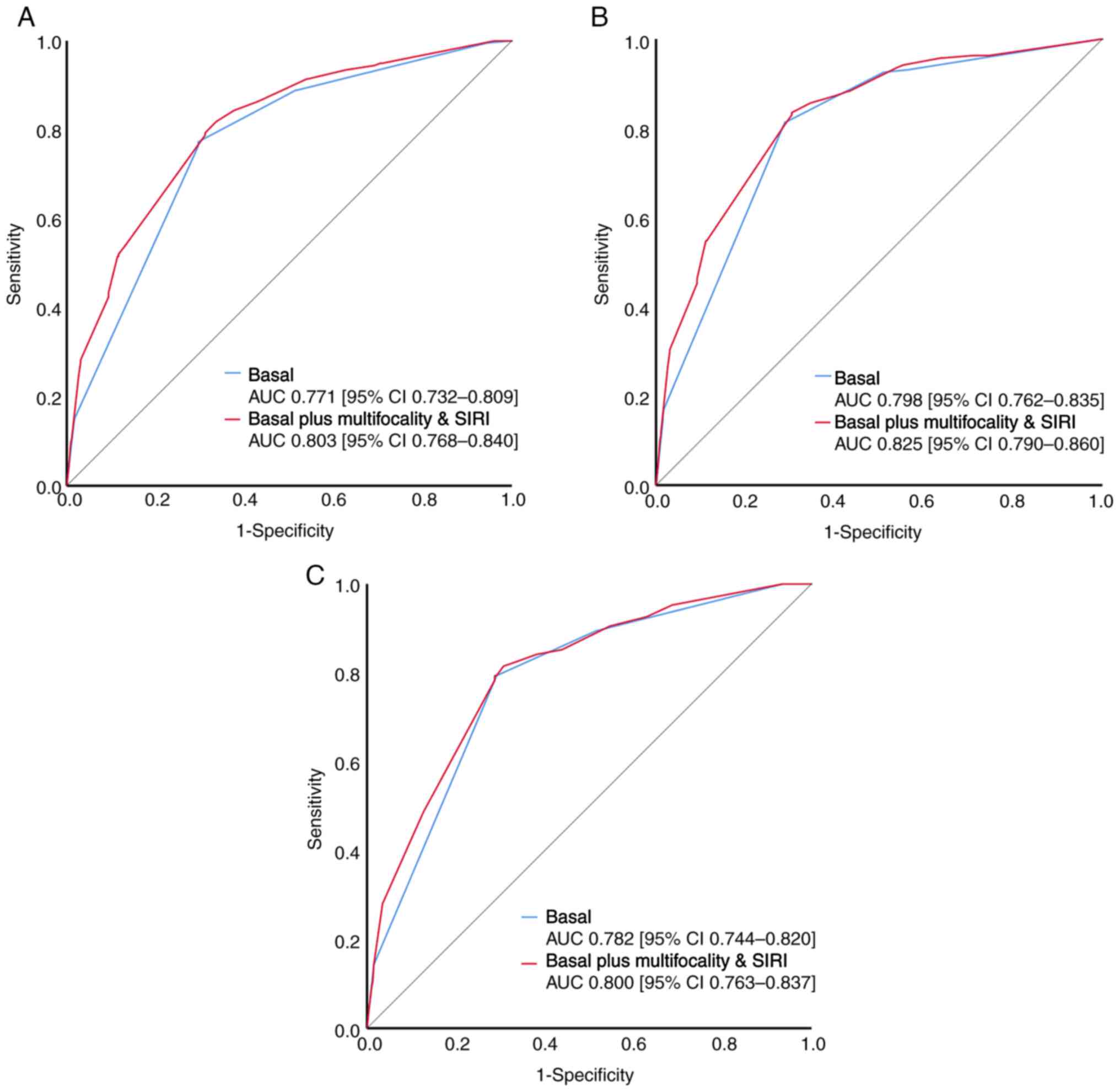
Further ROC analyses were conducted to evaluate
predictive ability of a combination of tumor multifocality and SIRI
including the basal model (consisting of the relevant prognostic
factors pT stage, LN status and tumor grade) for OS, CSS and RFS in
UTUC, compared with the basal model. The predictive model combining
tumor multifocality and SIRI showed AUCs of 0.803, 0.825 and 0.800
for OS, CSS and RFS, respectively (all P<0.001) (Fig. 3), which was further demonstrated to
have an improved predictive accuracy of survival than the basal
model (all P<0.05; Fig. 3).
Discussion
Multifocal UTUC tends to correlate with a poorer
survival, possibly indicating a more extensive disease burden
(14). Moreover, profound systemic
inflammation has been reported to be closely associated with a
higher tumor burden (29).
Accordingly, the present study assessed the influence of the
preoperative SIRI on the prognostic relevance of tumor
multifocality among patients with UTUC following RNU. The results
demonstrated that patients with multifocal UTUC and a high
preoperative SIRI exhibited shorter survival rates and a more
unfavorable disease progression compared with those with multifocal
UTUC and a low-level SIRI or non-multifocal UTUC. More notably,
SIRI was found to increase the clinical value of tumor
multifocality in predicting OS, CSS and RFS. Furthermore,
incorporating SIRI into tumor multifocality assessment proved to be
an independent prognostic indicator of OS, CSS and RFS, potentially
aiding adjuvant systemic treatment planning after RNU.
Although most studies demonstrate that multifocal
tumors are linked to a poorer outcome in UTUC, there remains room
for discussion. Previous studies observed a trend towards decreased
survival rates and worse disease extension in patients with
multifocal UTUC after surgery, compared with their non-multifocal
counterparts (10,13,14).
However, the association between tumor multifocality with
oncological outcomes has been controversial in UTUC. Milojevic
et al (16) highlighted a
significant association between multifocal UTUC and disease
recurrence but not CSS. Moreover, Sheu et al (17) found that multifocal tumors in
different locations of the upper urinary tract did not influence
survival or disease progression, but synchronous involvement of the
ureter and pelvis resulted in a significantly higher rate of
subsequent bladder cancer recurrence compared with multiple renal
pelvic/ureter tumors. These findings indicated that tumor number
had a significant impact on survival outcomes independent of tumor
distribution. Consistent with previous studies, the present study
demonstrated that multifocal UTUC predicted poorer outcomes
following RNU.
Previous studies suggest that inflammation is widely
recognized for promoting cancer development/growth, progression and
metastasis (30,31). Inflammatory responses triggered by
tumor-stimulated immune cells and mediators can foster an
inflammatory microenvironment inside/around tumors that benefits
tumor growth, angiogenesis and metastasis (32,33).
Recently, an increasing number of studies have indicated that
various blood-based immune cell parameters may be indicators of the
tumor-related inflammation state for predicting oncological
outcomes in a number of solid malignancies (34–36).
Furthermore, in the multivariate analyses for OS, CSS and RFS, only
SIRI retained prognostic significance, whereas WBC and ANC did not.
Accordingly, SIRI was adopted as an optimal systemic inflammation
marker to predict the prognosis of patients after surgery. The
results indicated that a higher SIRI led to a lower survival rate.
An elevated SIRI represented an increase in serum neutrophils
and/or monocytes and a decrease in serum lymphocytes. Neutrophils
have been identified as key contributors to this process by
enhancing tumor angiogenesis and metastasis (32,31).
Tumor-activated macrophages, differentiated from circulating
monocytes, may also advance tumor growth, invasion and migration
(37,38). By contrast, lymphocytes serve as
protective prognostic factors for patients with cancer as they
inhibit tumor cell proliferation and metastasis (33). Therefore, a higher SIRI indicated a
systemic inflammation state and implied a weak antitumor potential
or a build-up of immunosuppression.
Previously, Zheng et al (26) attempted to incorporate SIRI with
platelet-to-lymphocyte ratio (PLR) and demonstrated that the
combination of these two preoperative inflammation markers had an
improved prognostic significance in UTUC after surgery than SIRI or
PLR alone. The present study emphasized that preoperative SIRI can
be employed to improve the prognostic value of postoperative
pathological character such as tumor multifocality. Next, tumor
multifocality was defined as a tumor number of >1 regardless of
tumor location. The results of the present study demonstrated that
high-level SIRI (>1.95) significantly influenced survival and
disease progression in multifocal cases, particularly in locally
advanced UTUC. Conversely, without significant immune inflammation,
indicated by low-level SIRI (<1.95), tumor multifocality lost
its prognostic value. Notably, integrating SIRI and multifocality
improved the predictive accuracy for OS, CSS and RFS with
corresponding AUC values of 0.803, 0.827 and 0.800 (all
P<0.001). These findings underscored the compelling utility of
combining SIRI and multifocality as a new prognostic tool,
indicative of heightened predictive accuracy for OS, CSS and RFS.
This strategic combination of SIRI and tumor multifocality holds
promise in identifying high-risk patients, thereby necessitating
close surveillance and potential consideration for adjunctive
therapeutic interventions. We suggest that a higher SIRI could
represent the active immune-inflammation state, which may
positively affect the aggressiveness of UTUC tumors. When
multifocal tumors are under relatively energetic
immune-inflammatory circumstances, more inflammatory immune cells
participate in the tumor microenvironment and facilitate the
invasiveness/dissemination and viability of tumor cells (39,40).
Increase in SIRI signifies a diminished anticancer immune response,
possibly due to the increased involvement of neutrophils and
macrophages, decreased cytotoxic effect of lymphocytes and an
accumulation of cytokines and growth factors. However, further
studies are needed to elucidate the underlying mechanisms involved.
Therefore, in multifocal UTUC, SIRI may be considered for
identifying high-risk patients with unfavorable outcomes.
Chemotherapy and immunotherapy as optimal systemic treatments for
high risk UTUC. Immunotherapy response is warranted based on PD-L1
expression of tumor now, but this method is still not sufficient.
Thereafter, except for PD-L1 expression, this combination marker
may be employed to assess response to immunotherapy. To the best of
our knowledge, the present study is the first to demonstrate that
incorporating SIRI with tumor multifocality could be a useful
prognostic indicator of worse clinical outcomes in UTUC. The SIRI
is a cost-effective and easily obtainable method for assessing
inflammation derived from a CBC with differential count. However,
there were several limitations in the present study. First, this
retrospective study of reviewing medical records was from a
single-center institution and all enrolled patients were Taiwanese.
The incidence of UTUC in Taiwan is relatively higher than U.S. and
European. Second, the optimal SIRI cut-off value that adequately
corresponds to systemic inflammation has yet to be determined in
UTUC. Therefore, further studies with external validation are
required to establish the optimal SIRI cut-off value. Third,
relevant inflammation markers as a reference, such as C-reactive
protein andIL-6, were also missing from the present study. Lastly,
the present study could not evaluate the effects of dynamic changes
of SIRI on survival due to incomplete data. Therefore, future
studies are warranted to explore this issue. In addition, combining
multifocality and SIRI may be applied as a complementary tool to
evaluate immunotherapy responses when integrating programmed cell
death protein 1/programmed death ligand 1 expression in tumor
specimens.
In conclusion, the present study provided an insight
into the significant impact of tumor multifocality and SIRI on the
prognosis of patients with UTUC after RNU. Specifically, multifocal
tumors with a high-level SIRI independently predicted worse
oncological outcomes. Combining tumor multifocality and SIRI maybe
a potentially useful marker and assist with planning therapeutic
strategies, such as in adjuvant systemic treatment, for improving
outcomes.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Sheng-Hsiang Lin
and Ms. Wan-Ni Chen (Biostatistics Consulting Center, Clinical
Medicine Research Center, National Cheng Kung University Hospital,
Tainan, Taiwan, R.O.C) for providing statistical consulting
services.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LCY and HCJ conceived the study and analyzed data.
CYH, KCL, CHO and HCJ collected data. CAW, CYH, KCL and CHO
interpreted data. LCY and CAW wrote the manuscript. CHO and HCJ
supervised the study and reviewed and edited the manuscript. LCY,
CAW, CHO and HCJ confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of National Cheng Kung University Hospital (Tainan,
Taiwan; approval no. B-ER-112-216), who waived the requirement for
informed consent from participants and allowed access to the
follow-up clinical records. The present study was conducted based
on the guidelines of the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raman JD, Messer J, Sielatycki JA and
Hollenbeak CS: Incidence and survival of patients with carcinoma of
the ureter and renal pelvis in the USA, 1973–2005. BJU Int.
107:1059–1064. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chou YH and Huang CH: Unusual clinical
presentation of upper urothelial carcinoma in Taiwan. Cancer.
85:1342–1344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rouprêt M, Babjuk M, Compérat E, Zigeuner
R, Sylvester RJ, Burger M, Cowan NC, Böhle A, Van Rhijn BW,
Kaasinen E, et al: European association of urology guidelines on
upper urinary tract urothelial cell carcinoma: 2015 Update. Eur
Urol. 68:868–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Margulis V, Youssef RF, Karakiewicz PI,
Lotan Y, Wood CG, Zigeuner R, Kikuchi E, Weizer A, Raman JD, Remzi
M, et al: Preoperative multivariable prognostic model for
prediction of nonorgan confined urothelial carcinoma of the upper
urinary tract. J Urol. 184:453–458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang MH, Chen KK, Yen CC, Wang WS, Chang
YH, Huang WJS, Fan FS, Chiou TJ, Liu JH and Chen PM: Unusually high
incidence of upper urinary tract urothelial carcinoma in Taiwan.
Urology. 59:681–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liao RS, Gupta M, Schwen ZR, Patel HD,
Kates M, Johnson MH, Hahn NM, McConkey D, Bivalacqua TJ and
Pierorazio PM: Comparison of pathological stage in patients treated
with and without neoadjuvant chemotherapy for high risk upper tract
urothelial carcinoma. J Urol. 200:68–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leow JJ, Chong YL, Chang SL, Valderrama
BP, Powles T and Bellmunt J: Neoadjuvant and adjuvant chemotherapy
for upper tract urothelial carcinoma: A 2020 systematic review and
meta-analysis, and future perspectives on systemic therapy. Eur
Urol. 79:635–654. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stangl-Kremser J, Muto G, Grosso AA,
Briganti A, Comperat E, Di Maida F, Montironi R, Remzi M, Pradere
B, Soria F, et al: The impact of lymphovascular invasion in
patients treated with radical nephroureterectomy for upper tract
urothelial carcinoma: An extensive updated systematic review and
meta-analysis. Urol Oncol. 40:243–261. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Birtle A, Johnson M, Chester J, Jones R,
Dolling D, Bryan RT, Harris C, Winterbottom A, Blacker A, Catto
JWF, et al: Adjuvant chemotherapy in upper tract urothelial
carcinoma (the POUT trial): A phase 3, open-label, randomised
controlled trial. Lancet. 395:1268–1277. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chromecki TF, Cha EK, Fajkovic H, Margulis
V, Novara G, Scherr DS, Lotan Y, Raman JD, Kassouf W, Bensalah K,
et al: The impact of tumor multifocality on outcomes in patients
treated with radical nephroureterectomy. Eur Urol. 61:245–253.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zou L, Zhang L, Zhang H, Jiang H and Ding
Q: Comparison of post-operative intravesical recurrence and
oncological outcomes after open versus laparoscopic
nephroureterectomy for upper urinary tract urothelial carcinoma.
World J Urol. 32:565–570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang D, Xiong GY, Li XS, Chen XP, Zhang L,
Yao L, He ZS and Zhou LQ: Pattern and risk factors of intravesical
recurrence after nephroureterectomy for upper tract urothelial
carcinoma: A large Chinese center experience. J Formos Med Assoc.
113:820–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ouzzane A, Colin P, Xylinas E, Pignot G,
Ariane MM, Saint F, Hoarau N, Adam E, Azemar MD, Bensadoun H, et
al: Ureteral and multifocal tumours have worse prognosis than renal
pelvic tumours in urothelial carcinoma of the upper urinary tract
treated by nephroureterectomy. Eur Urol. 60:1258–1265. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams AK, Kassouf W, Chin J, Rendon R,
Jacobsen N, Fairey A, Kapoor A, Black P, Lacombe L, Tanguay S, et
al: Multifocality rather than tumor location is a prognostic factor
in upper tract urothelial carcinoma. Urol Oncol. 31:1161–1165.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen CS, Li JR, Wang SS, Yang CK, Cheng
CL, Yang CR, Ou YC, Ho HC, Lin CY, Hung SC, et al: Tumor
multifocality is a significant risk factor of urinary bladder
recurrence after nephroureterectomy in patients with upper tract
urothelial carcinoma: A single-institutional study. Diagnostics
(Basel). 10:2012020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Milojevic B, Bumbasirevic U, Santric V,
Kajmakovic B, Dragicevic D, Radisavcevic D, Sretenovic M and
Grujicic SS: Prognostic significance of tumor multifocality on
outcomes in patients with upper tract urothelial carcinoma after
radical nephroureterectomy: A cohort study. Curr Probl Cancer.
45:1007472021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sheu ZL, Huang CP, Chang CH, Chen CH, Hong
JH, Weng HY, Tai TY, Chung SD, Lo CW, Hsueh TY, et al: Tumor
distribution affects bladder recurrence but not survival outcome of
multifocal upper tract urothelial carcinoma treated with radical
nephroureterectomy. Sci Rep. 11:190592021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nøst TH, Alcala K, Urbarova I, Byrne KS,
Guida F, Sandanger TM and Johansson M: Systemic inflammation
markers and cancer incidence in the UK Biobank. Eur J Epidemiol.
36:841–848. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Q, Su S, You W, Wang T, Ren T and Zhu
L: Systemic inflammation response index as a prognostic marker in
cancer patients: A systematic review and meta-analysis of 38
cohorts. Dose Response. 19:155932582110647442021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chao B, Ju X, Zhang L, Xu X and Zhao Y: A
novel prognostic marker systemic inflammation response index (SIRI)
for operable cervical cancer patients. Front Oncol. 10:7662020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang T, Zhang D, Tang D, Heng Y, Lu LM and
Tao L: The role of systemic inflammatory response index (SIRI) and
tumor-infiltrating lymphocytes (TILs) in the prognosis of patients
with laryngeal squamous cell carcinoma. J Cancer Res Clin Oncol.
149:5627–5636. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Zhou Y, Xia S, Lu L, Dai T, Li A,
Chen Y and Gao E: Prognostic value of the systemic inflammation
response index (SIRI) before and after surgery in operable breast
cancer patients. Cancer Biomark. 28:537–547. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Min L, Ziyu D, Xiaofei Z, Shunhe X and
Bolin W: Analysis of levels and clinical value of CA19-9, NLR and
SIRI in patients with pancreatic cancer with different clinical
features. Cell Mol Biol (Noisy-le-grand). 67:302–308. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jan HC, Yang WH and Ou CH: Combination of
the preoperative systemic immune-inflammation index and
monocyte-lymphocyte ratio as a novel prognostic factor in patients
with upper-tract urothelial carcinoma. Ann Surg Oncol. 26:669–684.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chien TM, Li CC, Lu YM, Chou YH, Chang HW
and Wu WJ: The predictive value of systemic immune-inflammation
index on bladder recurrence on upper tract urothelial carcinoma
outcomes after radical nephroureterectomy. J Clin Med. 10:52732021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng Y, Chen Y, Chen J, Chen W, Pan Y,
Bao L and Gao X: Combination of systemic inflammation response
index and platelet-to-lymphocyte ratio as a novel prognostic marker
of upper tract urothelial carcinoma after radical
nephroureterectomy. Front Oncol. 9:9142019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eble JN, Sauter G, Epstein J and
Sesterhenn I: Pathology and genetics of tumours of the urinary
system and male genital organs. WHO Classification of Tumours. Vol
7. 3rd edition. IARC; 2004
|
|
29
|
Pacheco-Barcia V, Mondéjar Solis R, France
T, Asselah J, Donnay O, Zogopoulos G, Bouganim N, Guo K, Rogado J,
Martin E, et al: A systemic inflammation response index (SIRI)
correlates with survival and predicts oncological outcome for
mFOLFIRINOX therapy in metastatic pancreatic cancer. Pancreatology.
20:254–264. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Greten FR and Grivennikov SI: Inflammation
and cancer: Triggers, mechanisms, and consequences. Immunity.
51:27–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McFarland DC, Doherty M, Atkinson TM,
O'Hanlon R, Breitbart W, Nelson CJ and Miller AH: Cancer-related
inflammation and depressive symptoms: Systematic review and
meta-analysis. Cancer. 128:2504–2519. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moses K and Brandau S: Human neutrophils:
Their role in cancer and relation to myeloid-derived suppressor
cells. Semin Immunol. 28:187–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mori K, Resch I, Miura N, Laukhtina E,
Schuettfort VM, Pradere B, Katayama S, D'Andrea D, Kardoust Parizi
M, Abufaraj M, et al: Prognostic role of the systemic
immune-inflammation index in upper tract urothelial carcinoma
treated with radical nephroureterectomy: Results from a large
multicenter international collaboration. Cancer Immunol Immunother.
70:2641–2650. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang C, Wang M, Chen L, Wang H, Huang D,
Shi J, Zhang W, Tian Y and Zhu Y: The pretherapeutic systemic
inflammation score is a prognostic predictor for elderly patients
with oesophageal cancer: A case control study. BMC Cancer.
23:5052023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bruno M, Bizzarri N, Teodorico E, Certelli
C, Gallotta V, Pedone Anchora L, Fagotti A, Fanfani F, Scambia G
and Ferrandina G: The potential role of systemic inflammatory
markers in predicting recurrence in early-stage cervical cancer.
Eur J Surg Oncol. 50:1073112024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hamilton G, Rath B, Klameth L and Hochmair
MJ: Small cell lung cancer: Recruitment of macrophages by
circulating tumor cells. Oncoimmunology. 5:e10932772015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim J and Bae JS: Tumor-associated
macrophages and neutrophils in tumor microenvironment. Mediators
Inflamm. 2016:60581472016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|