Introduction
Colorectal cancer (CRC) is a global health concern,
being the third most diagnosed type of cancer and the second
leading cause of cancer-related deaths worldwide, with 1,926,118
new cases and 903,859 deaths reported in 2022 (1). The treatment of advanced CRC,
particularly when accompanied by liver metastases (LMs), which
affect ~50% of patients with CRC during the course of the disease,
presents a substantial challenge worldwide (2). Notably, there has been a shift towards
novel treatment strategies, with ramucirumab (RAM), a human IgG1
monoclonal antibody that targets the extracellular domain of VEGF
receptor (VEGFR)2, attracting considerable attention as a potential
treatment for various malignancies, including CRC (3).
The therapeutic efficacy of RAM has been extensively
examined in patients with advanced CRC and other types of cancer,
and has shown promising results (4–6).
Notably, in a randomized, double-blind, multicenter phase III
study, RAM in combination with folinic acid, fluorouracil and
irinotecan (FOLFIRI) demonstrated a median overall survival (OS)
time of 13.3 months and a median progression-free survival (PFS)
time of 5.7 months when used as second-line treatment, suggesting
that this may be a promising option for patients with metastatic
CRC (mCRC) (4).
Current research has also assessed the effects of
RAM on LMs derived from CRC, with evidence suggesting that RAM is
more favorable for patients without liver-only metastases (4). Notably, unlike other anti-VEGF
antibodies, RAM is effective against advanced hepatocellular
carcinoma (5); however, the
understanding of the benefits patients at different disease stages
could obtain from treatment with RAM, and the exact mechanisms
through which RAM affects the pathology, especially of the liver,
remains limited.
Addressing these issues, the present study aimed to
assess the potential role of RAM in second-line or salvage
treatments in patients with advanced CRC and LMs. This study
focused on a specific patient population, those with advanced CRC
and LMs receiving RAM and FOLFIRI as second-line or salvage
treatments, and aimed to evaluate the effectiveness of this
treatment strategy based on PFS, OS, overall response rate (ORR)
and LM resection rate, parameters that have yet to be adequately
investigated in previous studies involving RAM (7,8). In
addition, the present study aimed to elucidate the impact of RAM on
the pathology of LMs, specifically on platelet aggregation in liver
sinusoids. This study may contribute to the literature, and has the
potential to influence future research and clinical practice in the
treatment of patients with advanced CRC.
Materials and methods
Patients
The present retrospective cohort study included 36
patients who received RAM plus FOLFIRI treatment for unresectable
CRC as second-line or salvage chemotherapy. The study was conducted
between September 15, 2016, and May 5, 2019, at the Kawasaki
Medical School Hospital (Kurashiki, Japan) and Okayama University
Hospital (Okayama, Japan). Relevant clinical data, including
patient demographics, laboratory parameters, imaging findings,
treatment toxicities and prognostic outcomes, were retrospectively
collected.
Treatment
The RAM plus FOLFIRI regimen was administered
intravenously every 2 weeks. RAM was administered at a dose of 8
mg/kg over 1 h on day 1, folinic acid was administered at a dose of
400 mg/m2 over 2 h on day 1, irinotecan was administered
at 150 mg/m2 over 150 min on day 1 of each cycle;
fluorouracil was administered intravenously as a bolus of 400
mg/m2 on day 1, followed by continuous infusion of
fluorouracil at a dosage of 2,400 mg/m2 over 46 h on
days 1–3. Dose adjustments or interruptions were made in accordance
with the institutional clinical practice guidelines. Treatment
response was evaluated using the Response Evaluation Criteria in
Solid Tumors (RECIST) version 1.1 (9). Regular imaging evaluations,
predominantly using computed tomography, were performed every 2
months, corresponding to once every four treatment cycles, as per
standard institutional practice.
Calculation of the duration of
bevacizumab (BEV) administration
The patients were divided into two treatment groups:
The second-line group consisted of 58% (21 patients) of the
patients and included patients who received RAM plus FOLFIRI as a
second-line treatment, whereas the salvage-line group consisted of
42% (15 patients) of the patients, who were treated with RAM plus
FOLFIRI as salvage therapy (third-line or later). The duration of
BEV administration was calculated before the administration of RAM,
excluding the BEV withdrawal period. In the second-line group,
among 21 patients, 4 did not use BEV in the first-line treatment,
whereas the remaining 17 received BEV plus oxaliplatin with a
fluoropyrimidine (5-FU or capecitabine). In the salvage-line group,
the duration of BEV was calculated from the treatment history.
Among the 15 patients in the salvage-line group, 13 were treated
with BEV plus oxaliplatin with a fluoropyrimidine (5-FU,
capecitabine or S1) as first-line therapy. Of the remaining 2
patients, 1 patient received BEV plus irinotecan with capecitabine;
the other received panitumumab plus oxaliplatin with 5-FU. BEV was
used in 8 of the 15 patients in the second-line treatment and was
administered again in 3 patients in the third-line or later
chemotherapy before RAM administration.
Criteria for hepatic resection of
LMs
The criteria for hepatic resection were as follows:
i) LMs must be considered surgically resectable (regardless of the
number of LMs); ii) the disease must be controlled by chemotherapy
[partial response (PR) to stable disease (SD) for ≥2 months]; iii)
the only other organ with metastasis, other than the liver, was the
lungs.
Immunohistochemistry for CD42b
Formalin-fixed paraffin-embedded tissue specimens
were used for immunohistochemical (IHC) staining. Serial 3-µm
tissue sections were prepared and subjected to either hematoxylin
and eosin (H&E) staining or IHC staining using mouse monoclonal
anti-CD42b (cat. no. NBP1-28457; clone MM2/174; Novocastra; Leica
Biosystems). Immunostaining was performed using an automated
Bond-III Stainer (Leica Microsystems GmbH) according to the
manufacturer's instructions.
Briefly, tissue specimens were fixed in 10%
neutral-buffered formalin at room temperature for 24–48 h. The
sections were first dewaxed with BOND Dewax Solution, after which,
antigen retrieval was performed by heating the tissue sections in
BOND Epitope Retrieval ER1 Solution (citrate-based buffer, pH 6.0)
at 95–100°C for 20 min. Endogenous peroxidase activity was quenched
using the Refine Detection Kit Peroxide Block (Leica Biosystems)
for 5 min at room temperature and blocking was performed using
normal goat serum (cat. no. 5425; Cell Signaling Technology, Inc.)
at room temperature for 20 min. Subsequently, the sections were
incubated with anti-CD42b (1:100) at room temperature for 30 min.
Secondary detection was performed using the Refine Detection Kit
Polymer (cat. no. DS9800; Leica Biosystems) at room temperature for
10 min. Chromogen detection was carried out using the Refine
Detection Kit Mixed DAB Refine (Leica Biosystems), according to the
manufacturer's instructions. For H&E staining, the sections
were automatically stained using the Bond-III Stainer with 0.1%
hematoxylin for 5 min at room temperature, followed by 0.5% eosin
staining for 2 min at room temperature.
Images were captured using a light microscope
(Olympus BX53; Olympus Corporation) with a 40× objective lens.
CD42b immunostaining results were categorized into four stages
based on the observed staining pattern: Score 0, absence of
deposition; score 1, presence of fine granular deposition; score 2,
presence of coarse granular deposition; and score 3, presence of
linear deposition.
Statistical analysis
Statistical analyses were performed using JMP Pro 17
(SAS Institute, Inc.). PFS was defined as the time from the
initiation of RAM plus FOLFIRI treatment to disease progression. OS
was defined as the time from the initiation of RAM plus FOLFIRI
treatment to death. Cumulative survival probabilities were
estimated using the Kaplan-Meier method. The ORR was calculated as
the proportion of patients achieving a complete response (CR) or
PR, and the disease control rate (DCR) was defined as the
proportion of patients achieving CR, PR or SD. In cases where the
RECIST evaluation differed from the attending physician's
assessment, the latter's judgment was included in the evaluation.
Differences in CD42b scores, presented as median (IQR), and the
mean duration of anti-VEGF antibody treatment before liver
resection between the groups were evaluated using a Mann-Whitney U
test. The correlation between the CD42b score and the duration of
anti-VEGF antibody treatment (months) before liver resection was
examined using logistic regression analysis with Spearman's
coefficient (ρ). Categorical variables were examined by Fisher's
exact test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients and clinical responses
This study evaluated 36 patients with mCRC who were
treated with RAM plus FOLFIRI. The characteristics of the patients
enrolled in the present study are shown in Table I. Of these, 58% were female. With
respect to primary tumor location, 31% of primary tumors were on
the right side. Additionally, 67% presented with LMs and 94% had an
Eastern Cooperative Oncology Group (ECOG) performance status (PS)
of 0–1 (10). Moreover, 64% of the
patients had a mutant RAS status.
 | Table I.Patient characteristics and clinical
responses. |
Table I.
Patient characteristics and clinical
responses.
| Characteristic | Total (n=36) | Second-line treatment
group (n=21) | Salvage treatment
group (n=15) |
|---|
| Median age, years
(range) | 58 (16–76) | 57 (16–73) | 63 (37–76) |
| Female sex, n
(%) | 21 (58%) | 12 (57%) | 9 (60%) |
| Right primary tumor
location, n (%) | 11 (31%) | 6 (29%) | 5 (33%) |
| With liver
metastases, n (%) | 24 (67%) | 13 (62%) | 11 (73%) |
| ECOG PS 0–1, n
(%) | 34 (94%) | 21 (100%) | 13 (87%) |
| RAS mutant, n
(%) | 23 (64%) | 14 (67%) | 9 (60%) |
| BRAF mutant, n
(%) | 1 (3%) | 0 (0) | 1 (7%) |
| MSI-high, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Mean no. of cycles of
RAM plus | 7.4 (5.3–9.5) | 8.2 (5.0–11.4) | 6.2 (3.7–8.7) |
| FOLFIRI (95% CI) |
|
|
|
| Duration of BEV
treatment before |
|
|
|
| RAM induction, n
(%) |
|
|
|
| No
use | 4 (11%) | 4 (19%) | 0 (0) |
| <6
months | 9 (25%) | 6 (29%) | 3 (20%) |
| 6 to 12
months | 7 (19%) | 6 (29%) | 1 (7%) |
| ≥13
months | 16 (44%) | 5 (24%) | 11 (73%) |
| No. of
chemotherapeutic lines after |
|
|
|
| RAM therapy, n
(%) |
|
|
|
| 0 | 11 (31%) | 6 (29%) | 5 (33%) |
| 1 | 13 (36%) | 6 (29%) | 7 (47%) |
| 2 | 5 (14%) | 2 (10%) | 3 (20%) |
| 3 or
more | 4 (11%) | 4 (19%) | 0 (0) |
|
Unknown | 3 (8%) | 3 (14%) | 0 (0) |
| Response, n
(%) |
|
|
|
|
Complete response | 0 (0) | 0 (0) | 0 (0) |
| Partial
response | 3 (8%) | 2 (10%) | 1 (7%) |
| Stable
disease | 21 (58%) | 15 (71%) | 6 (40%) |
|
Progressive disease | 11 (31%) | 3 (14%) | 8 (53%) |
| Not
evaluated | 1 (3%) | 1 (5%) | 0 |
| Overall response
rate, n (%) | 3 (8%) | 2 (10%) | 1 (7%) |
| Disease control
rate, n (%) | 24 (66%) | 17 (81%) | 7 (47%) |
In the second-line group, the median age was 57
years (range, 16–73 years) and 57% of patients were female. All
patients in this group had an ECOG PS of 0–1. Of the patients in
this group, 29% had right-sided primary tumors located from the
cecum to the transverse colon, 62% presented with LMs and 67% had a
mutant RAS status. In the salvage group, the median age was 63
years (range, 37–76 years) and 60% were female. Among these
patients, 87% had an ECOG PS of 0–1. Additionally, 33% had
right-sided primary colon tumors, 60% had a mutant RAS status and
73% had LMs.
The present study also reviewed the duration of BEV
treatment prior to RAM administration, categorizing it into the
following: No use, <6 months, 6–12 months, and ≥13 months.
Across the entire cohort, 4 patients had not been treated with BEV
prior to RAM, 9 were treated for <6 months, 7 for 6–12 months
and 16 for ≥13 months. In the second-line group, 4 patients had no
prior BEV treatment, 6 were treated for <6 months, 6 for 6–12
months and 5 for ≥13 months. In the salvage group, all had received
prior BEV treatment, with 3 treated for <6 months, 1 for 6–12
months and 11 for ≥13 months.
On average, patients underwent 7.4 cycles of RAM
plus FOLFIRI treatment [95% confidence interval (CI), 5.3–9.5].
Those in the second-line group averaged 8.2 cycles (95% CI,
5.0–11.4), whereas the salvage group averaged 6.2 cycles (95% CI,
3.7–8.7). The number of chemotherapy lines after RAM administration
was not significantly different between the second-line and salvage
groups for 0–2 lines (excluding cases with unknown data), although
there were slightly different percentages of patients undergoing 1
or 2 lines of chemotherapy in each group. Notably, only patients in
the second-line group had ≥3 chemotherapy lines.
Finally, clinical responses were assessed. Neither
the second-line nor salvage treatment groups exhibited CR. The ORR
was 10% in the second-line group and 7% in the salvage group. The
DCR was notably higher in the second-line group at 81% compared to
47% in the salvage group. In the second-line group, 71% of patients
displayed SD, whereas in the salvage group, this was observed in
40%. PD was observed in 14% of the second-line group and 53% of the
salvage group.
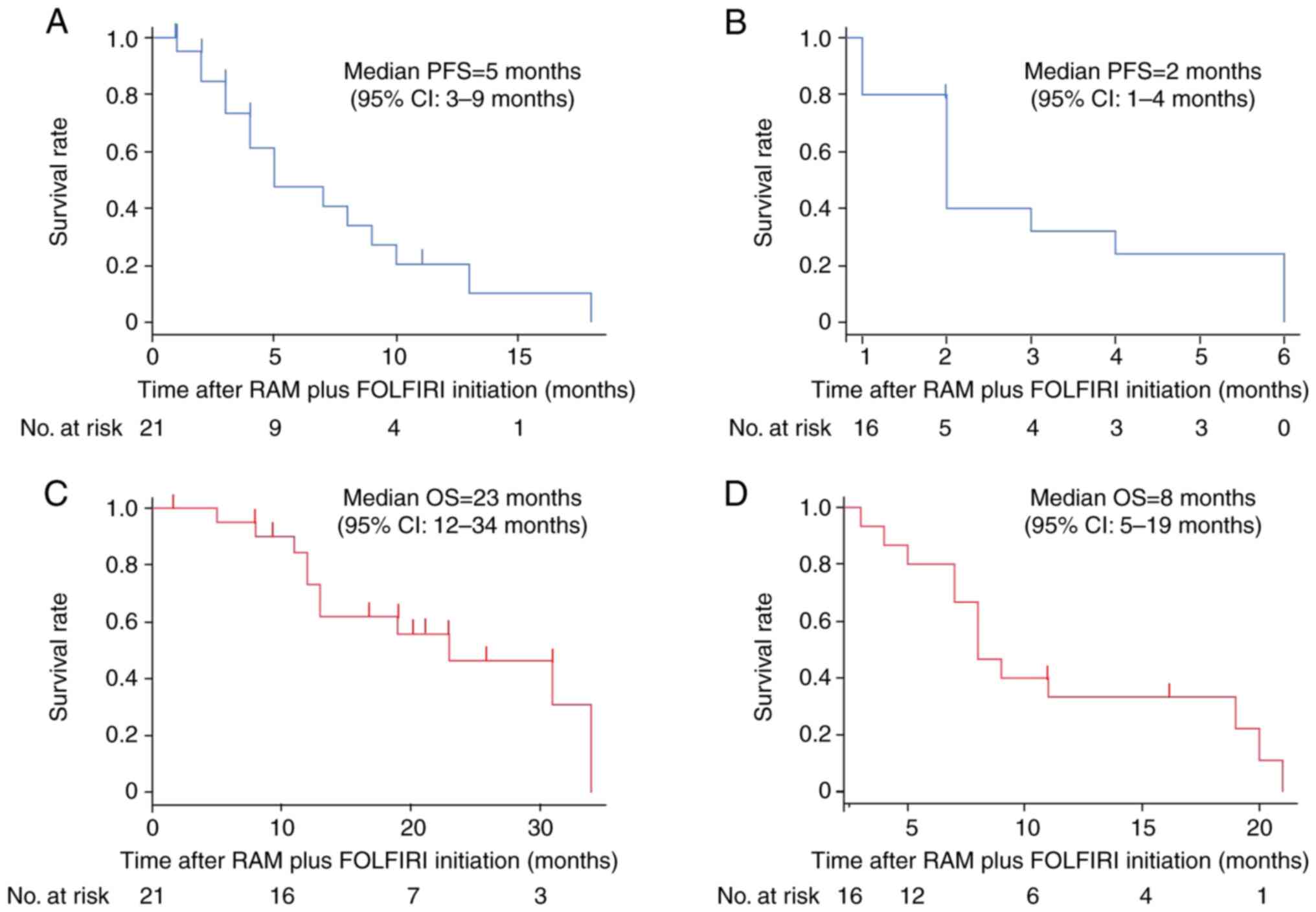
PFS and OS
PFS and OS were evaluated following the initiation
of RAM plus FOLFIRI treatment. For the second-line group, the
median PFS was 5 months (95% CI, 3–9 months; Fig. 1A). Conversely, the median PFS in the
salvage group was shorter, at 2 months (95% CI, 1–4 months;
Fig. 1C). The median OS in the
second-line group was 23 months (95% CI, 12–34 months; Fig. 1B), which is in contrast to the
salvage group, where the median OS was 8 months (95% CI, 5–19
months; Fig. 1D).
Characteristics of patients undergoing
hepatic resection for LMs
Of the 36 patients, 14 (39%) underwent surgical
resection of LMs during chemotherapy. In this subset, 6 patients
underwent their first surgical resection for LMs during second-line
RAM plus FOLFIRI treatment, forming the RAM-LM group. Of the
remaining 8 patients, 2 underwent their first resection during
first-line chemotherapy before BEV initiation and thus these cases
were excluded, and 6 underwent their first resection during
BEV-based first-line chemotherapy, constituting the BEV-LM group
(Fig. 2A). The details of patients
who underwent LM resection can be found in Table II. The RAM-LM group showed a trend
toward a shorter duration of BEV administration, except in one
case; this was due to the early failure of front-line BEV plus
oxaliplatin-based chemotherapy. Notably, 1 patient did not receive
BEV but was treated with RAM plus FOLFIRI as a second-line regimen
because of relapse during oxaliplatin-based adjuvant
chemotherapy.
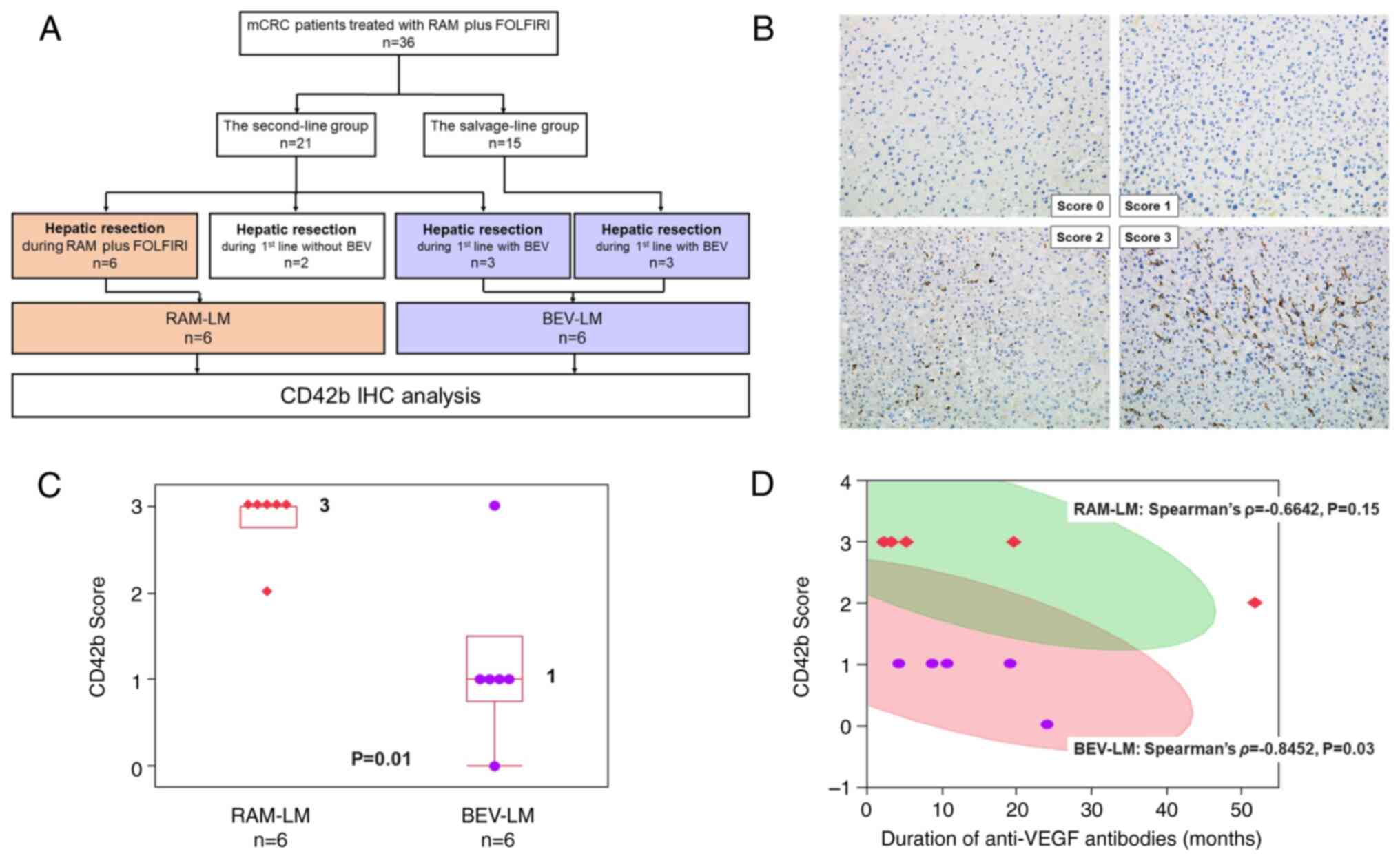 | Figure 2.Features of patients undergoing
hepatic resection for LMs. (A) Study flow chart. (B) Examples of
CD42 IHC staining and scoring. Images show examples of CD42
staining with corresponding scores: 0 (absence of deposition), 1
(presence of fine granular deposition), 2 (presence of coarse
granular deposition) and 3 (presence of linear deposition).
Magnification, ×400. (C) CD42 score distribution. Each box
represents a quartile range, and whiskers represent the maximum and
minimum values. The number to the right of each box represents the
median. The P-value was calculated using a Mann-Whitney U test. (D)
Correlation between CD42 score and the duration of anti-VEGF
antibody treatment. Each red square represents a case in the RAM-LM
group, while each purple dot represents a case in the BEV-LM group.
The green-shaded area denotes the 95% CI for the scatterplot matrix
of the RAM-LM group. Similarly, the red-shaded area represents the
95% CI for the scatterplot matrix of the BEV-LM group. BEV,
bevacizumab; CI, confidence interval; FOLFIRI, folinic acid,
fluorouracil and irinotecan; IHC, immunohistochemical; LM, liver
metastasis; mCRC, metastatic colorectal cancer; RAM,
ramucirumab. |
 | Table II.Detailed information of patients
undergoing hepatic resection for LMs. |
Table II.
Detailed information of patients
undergoing hepatic resection for LMs.
| A, BEV-LM |
|---|
|
|---|
| Sex | Age, years | Primary tumor
location | Stage at
diagnosis | Number of LMs at
resection | Surgical
procedure | CD42b score | Duration of
anti-VEGF treatment, months |
|---|
|
|---|
| BEV | RAM | Total | Mean (range) |
|---|
| M | 66 | Sigmoid colon | IV | 4 | Anatomical
subsegmentectomy (S5) and lateral segmentectomy (S2+3) | 1 | 10.5 | - | 10.5 | 11.3 (2–24)a |
| F | 70 | Ascending
colon | IV | 3 | Posterior
segmentectomy (S6+7) | 1 | 4 | - | 4 |
|
| F | 76 | Sigmoid colon | IV | 3 | Right lobectomy
(S5+6+7+8) | 1 | 19 | - | 19 |
|
| F | 70 | Ascending
colon | IV | 2 | Partial
resection | 1 | 8.5 | - | 8.5 |
|
| M | 67 | Rectum | II | 1 | Partial
resection | 0 | 24 | - | 24 |
|
| F | 62 | Ascending
colon | IV | 9 | Anatomical
subsegmentectomy (S8) and partial resection ×2 | 3 | 2 | - | 2 |
|
|
| B,
RAM-LM |
| Sex | Age,
years | Primary tumor
location | Stage at
diagnosis | Number of LMs at
resection | Surgical
procedure | CD42b
score | Duration of
anti-VEGF treatment, months |
|
| BEV | RAM | Total | Mean
(range) |
|
| M | 58 | Transverse
colon | pIIIa | 8 | Anatomical
segmentectomy (S3+4+8) and partial resection ×4 | 3 | 2.5 | 2.5 | 5 | 14.4 (2–52)a |
| F | 52 | Ascending
colon | pIIIb | 6 | Anatomical
subsegmentectomy (S5) and partial resection ×2 | 3 | 0 | 2 | 2 |
|
| F | 71 | Cecum | pIIIa | 1 | Anterior
segmentectomy (S5+8) | 3 | 1.5 | 1.5 | 3 |
|
| M | 58 | Sigmoid colon | IV | 20 | Anterior
segmentectomy (S5+8) + partial resection ×3 | 3 | 13 | 6.5 | 19.5 |
|
| F | 38 | Sigmoid colon | IV | 2 | Left lobectomy
(S2+3+4) | 3 | 4 | 1 | 5 |
|
| F | 62 | Rectum | IV | 8 | Anatomical
subsegmentectomy (S2) and partial resection ×5 | 2 | 40 | 12 | 52 |
|
Since H&E staining showed no hepatic sinusoid
injury in any LM samples resected after BEV treatment or those
resected after BEV followed by RAM administration (data not shown),
the association between anti-VEGF antibody exposure duration and
platelet aggregation (CD42b) score was subsequently assessed. While
CD42b score in the liver sinusoids of the BEV-LM group was
influenced by the duration of BEV treatment only, 1 of the 6
patients in the RAM-LM group was BEV-naive (CD42b score=3), while
the remaining 5 patients received BEV prior to RAM; therefore, the
CD42b score of the RAM-LM group was influenced by the duration of
both BEV and RAM treatment. Although there was no significant
difference in the mean duration of anti-VEGF antibody treatment
between the BEV-LM and RAM-LM groups (Table II), the RAM-LM group had a notably
higher median platelet aggregation (CD42b) score (median value, 3;
range, 0–3) compared with the BEV-LM group (median value, 1; range,
0–3; P=0.01; Fig. 2B and C).
Additionally, a tendency for a negative correlation between the
duration of anti-VEGF antibody exposure and the CD42b score was
detected in both the BEV-LM and RAM-LM groups (Fig. 2D).
Discussion
The present study provides a comprehensive
exploration of the therapeutic outcomes in relation to the
administration of RAM and FOLFIRI in patients with mCRC. Most
patients in the present cohort had RAS mutations, reflecting the
frequency of this mutation in CRC (11). Additionally, right-sided primary
tumors were frequently observed in the present cohort, a
characteristic associated with poorer prognosis in CRC (12).
Despite the modest ORR observed in the present
study, this aligned with the results of previous studies assessing
the efficacy of RAM and FOLFIRI combination therapy in patients
with mCRC (4,13). Notably, the DCR was markedly higher
in the group receiving second-line treatment, implying a
potentially superior efficacy of RAM plus FOLFIRI as second-line
therapy. Conversely, disease progression was observed more
frequently in the salvage group, suggesting a possible decline in
treatment efficacy in the advanced therapy stages.
The present findings regarding median PFS and OS
were congruent with the outcomes reported in the RAISE study, a
phase III trial examining RAM plus FOLFIRI in patients with mCRC
(4). The relatively lower survival
rates of the salvage group in the present study highlight the
inherent challenges of treating mCRC in its advanced stages; this
predicament has been well-documented in other studies (13,14).
The present study further explored the pivotal role
of platelets in tumor angiogenesis, growth and metastasis, and
assessed the interactions between anti-VEGF antibodies, platelet
aggregation and the liver microenvironment in patients with mCRC.
Notably, the selective binding of RAM to VEGFR2 inhibits its ligand
interaction, thus blocking the angiogenesis pathway (15). This mechanism is distinct from BEV,
which sequesters VEGF-A itself, preventing the interaction between
VEGF-A, VEGFR1 and VEGFR2 on the cell surface (16). Building on the current
understanding, the present study was initiated by examining the
pathological characteristics of LMs resected after the
administration of BEV and those following RAM administration.
Initially, H&E staining showed no hepatic sinusoid injury in
any LM samples resected after BEV treatment or those resected after
BEV followed by RAM administration. As a result, the focus was
shifted to platelet behavior, which could not be discerned by
H&E staining. Notably, the CD42b score tended to decrease as
the duration of anti-VEGF antibody administration increased in both
the BEV-LM group and the RAM-LM group. In addition, it was
indicated that the mean CD42b score was significantly higher in the
RAM group than that in the BEV group, despite no difference in the
mean duration of anti-VEGF antibody administration between the two
groups. Although the number of cases was small, among the
second-line group, 13 patients had LMs, of which 6 patients
underwent hepatic resection. This observation led to the hypothesis
that platelet aggregation within hepatic sinusoids, observed in
patients who underwent resection of LMs after RAM plus FOLFIRI,
might have an antitumor effect on LMs. This finding suggests that
upfront use of RAM could enhance the resection rate of LMs in
patients with mCRC, potentially offering a new therapeutic strategy
for these patients, especially those with RAS mutations (4).
In the context of hepatic injury associated with
chemotherapy for mCRC treatment, oxaliplatin-induced hepatic
sinusoidal injury (HSI) is a recognized condition.
Oxaliplatin-induced HSI reveals specific pathological changes,
including edema of hepatic sinusoidal epithelial cells, expansion
of the intercellular space and ongoing disintegration of the
hepatic sinusoid wall, leading to necrosis, detachment of the sinus
endothelium, and sinus expansion and rupture during the early and
middle stages of HSI (17).
Although the precise mechanism underlying this toxicity remains
unclear, it appears that oxaliplatin-induced reactive oxygen
species and the subsequent increase in VEGF-A levels may serve a
critical role (18,19). In rat models of HSI induced by
monocrotaline, a pyrrolizidine alkaloid, VEGF inhibition with
either sorafenib or regorafenib protected against this injury
(20,21).
In contrast, the association between platelet
aggregation and HSI is unclear. Given the known mitigating effect
of BEV on HSI (22), the present
study aimed to investigate the correlation between the duration of
anti-VEGF antibody treatment and CD42b score. A negative
correlation was observed between the duration of anti-VEGF antibody
exposure and the CD42b score in both the BEV-LM and RAM-LM groups.
Pathological examination using H&E staining revealed no markers
of oxaliplatin-induced HSI, despite both groups receiving
oxaliplatin regimens. In addition, the RAM-LM group, treated with
irinotecan following first-line oxaliplatin-based chemotherapy,
showed no signs of irinotecan-induced fatty liver. This suggests
that RAM itself may have an effect on the increasing CD42b scores,
i.e., by promoting platelet aggregation.
It is widely recognized that patients with
RAS-mutant CRC lack effective targeted therapy, as anti-EGFR
antibodies have demonstrated limited efficacy in this subtype
(23). Therefore, the present
findings underscore the potential of RAM as a promising therapeutic
strategy for such cases. However, it is essential to note that
these interpretations, although promising, remain largely
speculative at this stage, and necessitate further investigation
for their validation and potential clinical application.
The present study is not without limitations. The
modest sample size could have influenced both the statistical power
and the broader applicability of the results. Given the
retrospective design of the present study, there is an inherent
risk of selection and information biases. Additionally, the effect
of RAM plus FOLFIRI on quality of life, a pivotal factor when
considering treatment options for advanced CRC, was not evaluated.
Moreover, the present data did not encompass potential molecular
markers beyond RAS and BRAF, which might have a role in determining
the response to RAM plus FOLFIRI.
Nevertheless, the results of the present study
indicated that the unique pattern of intrasinusoidal platelet
aggregation observed could act as a potential biomarker, signaling
a favorable response to RAM treatment. This hypothesis might be
attributed to the specific mode of action of RAM related to VEGFR2.
While these insights are encouraging, they remain conjectural, and
require further scrutiny and confirmation.
Future research must address these shortcomings,
evaluating the precise mechanisms underlying hepatic sinusoidal
platelet aggregation in patients receiving anti-VEGF antibodies,
and comprehensively investigating its prospects as a predictive
biomarker for treatment outcomes. Such endeavors may corroborate
the present conclusions and provide pivotal insights into the
broader context of advanced CRC diagnosis and management.
Acknowledgements
The authors would like to thank Mr. Toru Nakai
(Department of Gastroenterological Surgery, Okayama University
Graduate School of Medicine, Dentistry and Pharmaceutical
Sciences), Mrs. Tae Yamanishi (Department of Gastroenterological
Surgery, Okayama University Graduate School of Medicine, Dentistry
and Pharmaceutical Sciences) and Mrs. Kikue Tokuda (Department of
Clinical Oncology, Kawasaki Medical School) for their technical
assistance.
Funding
This study was supported by KAKENHI (grant nos. 15H03034,
18K18464 and 18H03554 to TN).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KK and YK conducted all analyses and drafted the
manuscript. YU and KY provided a summary of patients who underwent
hepatic resection, and performed analysis and interpretation of
clinical data regarding patients that underwent hepatic resection.
TT performed the immunohistochemical analysis and provided a
pathological diagnosis. SY, TF and YM supplied patient samples, and
analyzed and interpreted the clinicopathological data. TY assisted
with all the statistical analyses. TN designed the project,
assisted with the interpretation of all the data, secured funding
and drafted the manuscript. KK, YK, TY and TN confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Kawasaki Medical School (Institutional Review Board
nos. 3214 and 3196). The opt-out method was applied with regard to
consent for this study, which complies with the ethical guidelines
of the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manfredi S, Lepage C, Hatem C, Coatmeur O,
Faivre J and Bouvier AM: Epidemiology and management of liver
metastases from colorectal cancer. Ann Surg. 244:254–259. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spratlin JL, Mulder KE and Mackey JR:
Ramucirumab (IMC-1121B): A novel attack on angiogenesis. Future
Oncol. 6:1085–1094. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tabernero J, Yoshino T, Cohn AL,
Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy
DC, Van Cutsem E, Grothey A, et al: Ramucirumab versus placebo in
combination with second-line FOLFIRI in patients with metastatic
colorectal carcinoma that progressed during or after first-line
therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine
(RAISE): A randomised, double-blind, multicentre, phase 3 study.
Lancet Oncol. 16:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R,
Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, et al:
Ramucirumab versus placebo as second-line treatment in patients
with advanced hepatocellular carcinoma following first-line therapy
with sorafenib (REACH): A randomised, double-blind, multicentre,
phase 3 trial. Lancet Oncol. 16:859–870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arnold D, Lueza B, Douillard JY, Peeters
M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP,
Tabernero J, et al: Prognostic and predictive value of primary
tumour side in patients with RAS wild-type metastatic colorectal
cancer treated with chemotherapy and EGFR directed antibodies in
six randomized trials. Ann Oncol. 28:1713–1729. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen CP, Ke TW, Cheng R and Wang JY:
Ramucirumab in the second-line treatment of metastatic colorectal
cancer: A narrative review of literature from clinical trials.
Transl Cancer Res. 9:5645–5654. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cancer Genome Atlas Network, .
Comprehensive molecular characterization of human colon and rectal
cancer. Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tejpar S, Stintzing S, Ciardiello F,
Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ and Heinemann
V: Prognostic and predictive relevance of primary tumor location in
patients with RAS wild-type metastatic colorectal cancer:
Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA
Oncol. 3:194–201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshihiro T, Kusaba H, Makiyama A,
Kobayashi K, Uenomachi M, Ito M, Doi Y, Mitsugi K, Aikawa T,
Takayoshi K, et al: Efficacy and safety of ramucirumab plus
modified FOLFIRI for metastatic colorectal cancer. Int J Clin
Oncol. 24:508–515. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Holmes K, Roberts OL, Thomas AM and Cross
MJ: Vascular endothelial growth factor receptor-2: Structure,
function, intracellular signalling and therapeutic inhibition. Cell
Signal. 19:2003–2012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jayson GC, Kerbel R, Ellis LM and Harris
AL: Antiangiogenic therapy in oncology: Current status and future
directions. Lancet. 388:518–529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu C, Ren X, Liu D and Zhang C:
Oxaliplatin-induced hepatic sinusoidal obstruction syndrome.
Toxicology. 460:1528822021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fontana RJ: Pathogenesis of idiosyncratic
drug-induced liver injury and clinical perspectives.
Gastroenterology. 146:914–928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mudd TW and Guddati AK: Management of
hepatotoxicity of chemotherapy and targeted agents. Am J Cancer
Res. 11:3461–3474. 2021.PubMed/NCBI
|
|
20
|
Björnsson ES: Hepatotoxicity by drugs: The
most common implicated agents. Int J Mol Sci. 17:2242016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okuno M, Hatano E, Nakamura K,
Miyagawa-Hayashino A, Kasai Y, Nishio T, Seo S, Taura K and Uemoto
S: Regorafenib suppresses sinusoidal obstruction syndrome in rats.
J Surg Res. 193:693–703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Overman MJ, Ferrarotto R, Raghav K, George
B, Qiao W, Machado KK, Saltz LB, Mazard T, Vauthey JN, Hoff PM, et
al: The addition of bevacizumab to oxaliplatin-based chemotherapy:
Impact upon hepatic sinusoidal injury and thrombocytopenia. J Natl
Cancer Inst. 110:888–894. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|