Introduction
Given the infiltrative characteristics and molecular
heterogeneity of glioblastoma (GBM), the ongoing difficulty in
discovering a cure persists. Despite advancements in technology and
biological research, the median overall survival (OS) time of
patients with GBM remains low, with a median OS time of 14–18
months (1–3). Even with advances in genetics and
surgical techniques, neuro-oncology research has shown generally
disappointing outcomes since the Stupp regimen was implemented.
Although there have been advancements in surgical techniques,
imaging protocols and radiotherapy (RT), as well as the use of
intraoperative mapping to guide macroscopic complete resection, the
median survival time has only increased by a few months (4). The current gold standard treatment
after surgery for newly diagnosed patients with GBM dates back to
2005, with the EORTC/NCIC 26981 study demonstrating the survival
benefits of combining radiation with temozolomide (TMZ) over
radiation alone (2). Despite
subsequent efforts, progress has remained constrained, as therapies
including bevacizumab, everolimus and dose-dense TMZ have not
demonstrated a significant survival benefit in randomized clinical
trials when compared with the standard regimen of radiation and
adjuvant TMZ. Challenges in developing new therapies include the
need to penetrate the blood-brain barrier (BBB), tumor
heterogeneity, and the widespread distribution of microscopic
disease (1–4).
The resistance of GBM to treatment is widely known
and can be explained by numerous different aspects of the tumor: i)
GBM spatial heterogeneity limits the options and efficacy of target
therapies; ii) GBM resistance to radiation and chemotherapy is
facilitated by the pro-tumorigenic activity of the tumor
microenvironment (TME); and iii) the limited immunogenicity of GBM
inhibits a robust immunological reaction (5–7). In
total, ~80% of GBM recurrences manifest within or on the periphery
of the radiation field, highlighting the significant influence of
local variables on the recurrence of the tumor. The localized
recurrence significantly correlates with a notable reduction in
progression-free survival (PFS), underscoring the imperative need
for effective local treatment interventions (8). Additionally, the isolation of the
brain by the BBB provides a distinctive chance for aggressive local
treatment with minimal risk of systemic toxicity. In recent
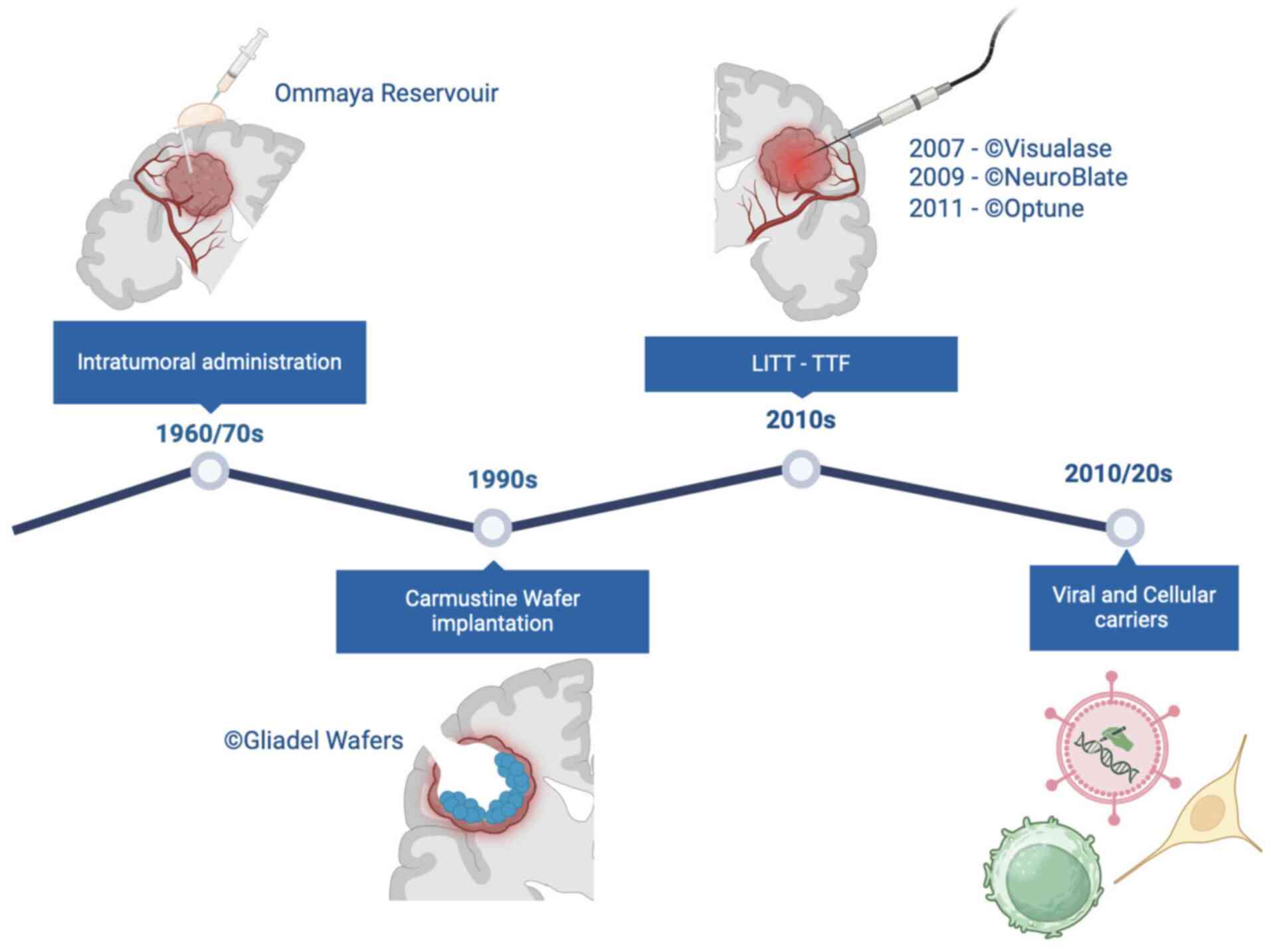
decades, several local therapy (LT) strategies have been developed.
These include local thermal or laser therapy, local injection of
immunotoxins via convection-enhanced delivery systems and
implantation of Carmustine Wafers (CWs) into the resection cavity
(9,10). Intracavitary radioimmunotherapy and
Tumor Treating Fields (TTF) represent other examples of localized
therapeutic strategy aimed at delaying or potentially preventing
local tumor recurrence (11).
The 2021 World Health Organization (WHO)
classification (12) of central
nervous system (CNS) tumors brought significant revisions,
particularly concerning GBM. These updates involved molecular
parameters, refining GBM subtypes such as isocitrate dehydrogenase
[NADP(+)] (IDH)-wild-type and IDH-mutant. It is crucial to note
that each of these subtypes has distinct clinical and prognostic
implications. For instance, IDH-wild-type GBM is associated with a
more aggressive disease course and poorer prognosis compared with
IDH-mutant GBM. In addition, the emergence of new entities, such as
diffuse midline glioma with histone H3 K27M mutation further
complicates historical comparisons, emphasizing the need for
updated diagnostic criteria and standardized reporting for
consistency across studies (12).
The new classification represents a significant temporal landmark,
which can make it challenging and potentially misleading to compare
studies conducted before and after this time point. Therefore, it
is crucial to be aware of these complexities when interpreting and
applying research findings.
Despite the limited or marginal improvements
demonstrated by LT over current standard radio-chemotherapy, its
future potential is promising. This is particularly true in light
of recent advancements in gene-editing technologies and novel
molecular and genetics discoveries, which are opening up new
possibilities and avenues for treatment. The present review aimed
to provide a historical review of LT and summarize the LTs that are
currently being investigated or explored in GBM management.
Study selection
Study design and search strategy
The present review failed to satisfy the inclusion
prerequisites set forth by the International Prospective Register
of Systematic Reviews (PROSPERO) due to its specific focus on the
evolution of LT for glioma over the past two decades. Consequently,
the study protocol was not recorded within the PROSPERO
database.
The present review constituted a systematic review
of existing literature, conducted in accordance with the Preferred
Reporting Items for Systematic Reviews (PRISMA) statement
guidelines (13). Both prospective
and retrospective clinical studies were considered. The search
strategy of the pertinent literature was conducted by screening
four distinct medical databases: Mendeley (https://www.mendeley.com/reference-management/mendeley-cite),
Cochrane Library (https://www.cochranelibrary.com/library), EMBASE
(https://www.elsevier.com/products/embase) and MEDLINE
(https://pubmed.ncbi.nlm.nih.gov/), from
January 1990 to December 2023. The following keywords, either
singularly or in combination (using the Boolean operator ‘AND’),
were examined in all abstracts of English-language publications:
‘glioma’, ‘gliomas’, ‘high grade glioma’, ‘glioblastoma’,
‘surgery’, ‘Intracavity Therapy’, ‘brachytherapy’, ‘immunotherapy’,
‘thermotherapy’, ‘laser interstitial thermal therapy’, ‘magnetic
hyperthermia’, ‘magnetic field’, ‘nanomaterial’, ‘focused
ultrasound therapy’, ‘gene therapy’, ‘tumor treating fields’,
‘virotherapy’, ‘oncolytic’, ‘t-cell’, ‘engineered cell’ and ‘local
treatment’. In the present study, two authors conducted the initial
review of titles and abstracts, with any discrepancies resolved by
consensus among three senior authors. Additionally, the references
cited in each paper were scrutinized for relevant articles. The
accuracy and completeness of all extracted data were verified by
two independent authors. For ongoing clinical trials, a search of
ClinicalTrials.gov was conducted,
specifically for trials related to GBM with statuses such as ‘not
yet recruiting’, ‘recruiting’, ‘enrolling by invitation’, ‘active,
not recruiting’ or ‘available’.
Inclusion and exclusion criteria
All Studies were included based on the following
criteria: i) Published in the English language; ii) clinical
trials, encompassing single-arm or double-arm studies, with a focus
on both randomized controlled and non-randomized controlled trials;
iii) investigations on immunotherapy strategies for GBM, whether as
stand-alone or combined therapies with chemotherapy and/or RT; and
iv) studies incorporating OS and PFS among the analyzed LT options.
The exclusion criteria comprised: i) Editorials, case reports, case
series, cohort studies, literature reviews and meta-analyses; ii)
studies lacking clear definition of methods and/or results; and
iii) studies without reported data on PFS or OS. The identified
studies were imported into Endnote X9 (https://support.clarivate.com/Endnote/s/article/EndNote-X9)and
duplicates were removed. In the present study, two independent
authors assessed the results against the inclusion and exclusion
criteria, with any disagreements resolved by a third author.
Eligible articles then underwent full-text screening.
Data extraction
The following details were extracted for each study:
Author information, publication year and journal, title, clinical
trial name and phase, patient count, diagnosis, duration of
follow-up, treatment type and outcomes. The primary outcomes
assessed were OS and PFS following LT for glioma.
Risk of bias assessment
The Newcastle-Ottawa Scale was employed to evaluate
the quality of the studies included in the present review (14). Quality assessment included the
evaluation of selection criteria, comparability among studies and
outcome assessment. A maximum score of 9 was considered ideal, with
higher scores indicating greater study quality. Studies that
received ≥7 points were classified as high-quality and included in
the present review. The quality assessment was independently
conducted by two authors. In cases of discrepancies, a third author
re-examined the study.
Study selection process
Using a combination of keywords, MeSH and Emtree
hierarchical terms, the authors found 2,711 potentially relevant
articles, which were saved in a unique PubMed (.nbib) file and
imported into Endnote to identify possible duplicates. For ongoing
clinical trials, the same keywords were searched on ClinicalTrials.gov for the disease, ‘glioblastoma’ and
‘glioma’, leading to 171 potentially relevant trials. After the
removal of duplicates and studies published before 2010, 619
studies were deleted. The remaining 2,263 studies were screened by
title and abstract, leading to the exclusion of another 2,041
studies (Cohen's κ coefficient=0.92). Finally, 222 studies were
sought for retrieval and fully assessed for eligibility leading to
the final inclusion of 46 studies. The study selection process is
outlined in Fig. 1, Fig. 2, Fig.
3, adhering to the PRISMA guidelines.
Study characteristics
The selected reviewed studies were subsequently
categorized into three groups according to the specific type of LT
utilized: 1) Intraoperative LT modalities; ii) local RT; and iii)
local immunotherapy. Following the review of studies, it emerged
that 25 (15–39), 10 (40–49)
and 11 (50–55) clinical studies explored the
efficiency of intraoperative LT modalities, local RT and local
immunotherapy, respectively. Table
I provides a summary of the primary clinical studies involving
patients who underwent treatment with intraoperative LT modalities,
Table II outlines clinical
investigations based on local RT and Table III presents clinical studies
focusing on advancements in local immunotherapy.
 | Table I.Clinical studies assessing
intraoperative local treatment modalities (LITT, FUS, MH, TTF and
CWs) in patients with GBM. |
Table I.
Clinical studies assessing
intraoperative local treatment modalities (LITT, FUS, MH, TTF and
CWs) in patients with GBM.
| A, LITT |
|---|
|
|---|
| First author,
year | Study | No. of
patients | Pathology | Pre-treatment | Post-treatment | Complications | OS | (Refs.) |
|---|
| Mohammadi | Multicenter | 34 (35 | 24 GBM; 10 | 18 RT + CT | 14 RT or CT;
13 | 7 worsening of
preoperative motor | 1-year estimate of
OS was | (15) |
| et al,
2014 | retrospective | procedures) | anaplastic |
| RT + CT | deficit; 1 seizure;
1 postoperative | 68%. Median PFS was
5.1 |
|
|
|
|
|
oligodendroglioma |
|
| hyponatremia; 1
bilateral deep vein | months |
|
|
|
|
|
|
|
| thrombosis; 1
superficial wound |
|
|
|
|
|
|
|
|
| infection; 1
ventriculitis |
|
|
| Thomas | Single- | 21 (8 newly | GBM | 6 GTR; 5 | 5 adjuvant | 1 functional
decline; 1 status | Median, 8 months
in | (16) |
| et al,
2016 | institution | diagnosed; |
| STR; 12 RT; | combination of | epilepticus | newly diagnosed
group; 7 |
|
|
| retrospective | 13 recurrent) |
| 12 TMZ; 2 | CCNU and |
| months in recurrent
group |
|
|
|
|
|
| additional | bevacizumab |
|
|
|
|
|
|
|
| bevacizumab; |
|
|
|
|
|
|
|
|
| 1 stereotactic |
|
|
|
|
|
|
|
|
| radiosurgery |
|
|
|
|
|
|
|
|
| previously |
|
|
|
|
| Beaumont | Multicenter | 15 (9 newly | 13 GBM; 2 LGG | 4 craniotomy | 11 CT + RT | 3 hemiparesis; 1
edema and | 18.2 months | (17) |
| et al,
2018 | retrospective | diagnosed; |
| + RT + CT; 1 |
| herniation; 1
ventriculitis; 1 |
|
|
|
|
| 6 recurrent) |
| biopsy +RT + |
| weakness; 1
hydrocephalus |
|
|
|
|
|
|
| craniotomy + |
|
|
|
|
|
|
|
|
| CT; 1 RT + CT |
|
|
|
|
| Kamath | Single- | 54 (58 | GBM | 35 resection + | 53 CT; 15 RT;
3 | 3 cerebral edema; 3
seizures; 1 | 11 months | (18) |
| et al,
2019 | institution | procedures; |
| CT; 17 biopsy; | surgery; 10 | hydrocephalus; 1
hyponatremia; 1 |
|
|
|
| retrospective | 17 newly |
| 5 CT | other (tumor- | infection; 2
mortalities |
|
|
|
|
| diagnosed; |
|
| treating
field; |
|
|
|
|
|
| 41 recurrent) |
|
| vaccines) |
|
|
|
| O'Connor | Single- | 43 | recurrent GBM | - | - | 2 intracranial
hypertension; 1 | - | (19) |
| et al,
2020 | institution |
|
|
|
| weakness; 1 skin
dehiscence; 1 |
|
|
|
| retrospective |
|
|
|
| CSF leak |
|
|
| Traylor | Single- | 69 (20 newly | GBM | 28 GTR; 41 | 47 CT; 19 RT | 17 neurological
complication; 4 | Mean, 12
months | (20) |
| et al,
2021 | institution | diagnosed; |
| not GTR |
| seizure; 3 impaired
cognition; 1 |
|
|
|
| retrospective | 49 recurrent) |
|
|
| urinary tract
infection; 1 |
|
|
|
|
|
|
|
|
| pneumonia; 1
hyponatremia; 1 |
|
|
|
|
|
|
|
|
| pulmonary embolism;
1 acute |
|
|
|
|
|
|
|
|
| kidney injury |
|
|
| de Groot | Multicenter | 89 (29 newly | GBM | 53 resection; | 57 CT; 19 RT;
9 | 5 neurological
deficits; 4 edema; 1 | Median, 9.7 months
in | (21) |
| et al,
2022 | prospective | diagnosed; |
| 54 CT; 53 RT | immunotherapy | seizure; 1
hemorrhage; 1 deep vein | newly diagnosed
group; |
|
|
|
| 60 recurrent) |
|
|
| thrombosis | 8.9 months in
recurrent |
|
|
|
|
|
|
|
|
| group |
|
| Johnson | Single- | 22 | IDH 1/2 mutant | 14 resection | 9 RT + CT; 4 | 1 edema; 1 seizure;
1 deep vein | Only PFS
reported | (22) |
| et al,
2022 | institution |
| gliomas | (GTR/STR); | CT; 1 STR + | thrombosis | (mean 5.2
months) |
|
|
| retrospective |
|
| 12 RT; 10 CT; | RT + CT; 1 CT |
|
|
|
|
|
|
|
| 4 biopsy; 1 | + γ knife |
|
|
|
|
|
|
|
| radiosurgery |
|
|
|
|
| Muir et
al, | Single- | 20 | GBM | 4 RT; 3 CT | 13 RT; 12 CT | 12 new or worsened
motor deficit; | 11 months | (23) |
| 2022 | institution |
|
|
|
| 2 seizure |
|
|
|
| retrospective |
|
|
|
|
|
|
|
| Kaisman- | Single- | 56 | GBM multiforme | - | 50 CT; 39 | 1 mortality; 4
permanent | - | (24) |
| Elbaz | institution |
|
|
| additional | neurological
deficit |
|
|
| et al,
2023 | retrospective |
|
|
| oncological |
|
|
|
|
|
|
|
|
| treatment |
|
|
|
| Jubran et
al, | Single- | 49 | 31 recurrent
GBM | 20
stereotactic | - | 3 worsening
aphasia; 1 seizure; 1 | 20 months | (25) |
| 2023 | institution |
| multiforme; 18 | radiosurgery; |
| epidural hematoma;
1 |
|
|
|
| retrospective |
| radiation
necrosis | 37 RT; 49 CT |
| intraparenchymal
hemorrhage |
|
|
|
| B, FUS |
|
| First author,
year | Study | No. of
patients |
Pathology |
Pre-treatment |
Post-treatment |
Complications | OS | (Refs.) |
|
| Guthkelch | Retrospective | 15 | 11 GBM | Craniectomy | 10 resection + | 4 hemorrhage; 2
thermal lesion, 2 | 11.8 months | (26) |
| et al,
1991 |
|
| multiforme; 4 |
| RT; 5 RT | CSF leak |
|
|
|
|
|
| anaplastic |
|
|
|
|
|
|
|
|
| astrocytoma |
|
|
|
|
|
| Carpentier | Prospective | 15 | recurrent GBM | 15 RT + TMZ; | 15 CT | 1 facial pain; 1
vagal episode with | - | (27) |
| et al,
2016 | phase I/IIa |
|
| 9 resection | (carboplatin- | bradycardia
followed by |
|
|
|
| clinical trial |
|
|
| based | tachycardia; 1
edema; 2 cerebellar |
|
|
|
|
|
|
|
| chemotherapy) | vascular
stroke |
|
|
|
| C, MH |
|
| First author,
year | Study | No. of
patients |
Pathology |
Pre-treatment |
Post-treatment |
Complications | OS | (Refs.) |
|
| Stea et
al, | Prospective | 33 | 22 GBM (17 | Surgical | RT: 48.4 Gy, | 11 (33.3%) cerebral
edema, 11 | Primary, 23.5
months | (28) |
| 1994 | phase I/II |
| primary, 5 | debulking | interstitial | (33.3%) focal
seizures, 2 (6.1%) | (control, 13.5
months); |
|
|
| Trial |
| recurrent); 11 |
| brachytherapy: | pulmonary
embolism | recurrent, 12
months |
|
|
|
|
| anaplastic |
| 13.9–50 |
| (control, 12
months) |
|
|
|
|
| astrocytomas
(8 |
|
|
|
|
|
|
|
|
| primary, 3 |
|
|
|
|
|
|
|
|
| recurrent) |
|
|
|
|
|
| Maier-Hauff | Prospective | 59 | Recurrent GBM | Stereotactic | Stereotactic | 14 (21.2%) motor
disturbances, 15 | From tumor
recurrence, | (29) |
| et al,
2011 | phase II |
|
| beam RT | beam RT (30 | (22.7%) focal
convulsions; 6 | 13.4 months (95%
CI, |
|
|
| Trial |
|
| (30 Gy) | Gy) | (9.1%) grade 1–3
thermal stress, | 10.6–16.2) |
|
|
|
|
|
|
|
| temperature
>38°C; 9 (13.6%) |
|
|
|
|
|
|
|
|
| headaches |
|
|
|
| D, TFF |
|
| First author,
year | Study | No. of
patients |
Pathology |
Pre-treatment |
Post-treatment |
Complications | OS | (Refs.) |
|
| Stupp et
al, | Randomized | 237 TTF | Recurrent GBM | Surgery and | - | The TTF-related
adverse events | Median survival was
6.6 | (30) |
| 2012 | phase III | alone |
| different |
| were mild (14%) to
moderate (2%) | vs. 6.0 months (HR,
0.86; |
|
|
| trial | (n=120) or |
| postoperative |
| skin rash beneath
the transducer | 95% CI,
0.66–1.12; |
|
|
|
| active |
| treatments. |
| arrays. Severe
adverse events | P=0.27), 1-year
survival |
|
|
|
| chemotherapy |
| Number of |
| occurred in 6 and
16% (P=0.022) | rate was 20%
respectively |
|
|
|
| control |
| prior |
| of patients treated
with TTF and | in TTF and active
control |
|
|
|
| (n=117) |
| treatments was |
| chemotherapy,
respectively. | groups |
|
|
|
|
|
| two (range
1–6) |
| Quality of life
analyses favored |
|
|
|
|
|
|
|
|
| TTF therapy in most
domains |
|
|
|
| C, MH |
|
| First author,
year | Study | No. of
patients |
Pathology |
Pre-treatment |
Post-treatment |
Complications | OS | (Refs.) |
|
| Stupp et
al, | Randomized | After | GM newly | All patients | - | The overall
incidence, distribution | Median overall
survival in | (31) |
| 2012 | controlled | completion of | diagnosed | underwent |
| and severity of
adverse events were | the
per-protocol |
|
|
| trial |
chemoradiotherapy, |
| surgery and |
| similar in patients
treated with TTF | population was
20.5 |
|
|
|
| patients with |
| had completed |
| + TMZ and in those
treated with | months (95% CI,
16.7- |
|
|
|
| GBM were |
| radiotherapy |
| TMZ alone. Mild to
moderate skin | 25.0) in the TTF +
TMZ |
|
|
|
| randomized |
| and
concomitant |
| irritation was
observed in 43% of | group (n=196) and
15.6 |
|
|
|
| (2:1) to
receive |
| TMZ as per |
| patients treated
with TTF + TMZ | months (95% CI,
13.3- |
|
|
|
| maintenance |
| local practice |
| and severe skin
reaction (grade 3) | 19.1) in the TMZ
alone |
|
|
|
| treatment with |
|
|
| in 2% | group (n=84; HR,
0.64; |
|
|
|
| either TTF +
TMZ |
|
|
|
| 99.4% CI,
0.42–0.98; |
|
|
|
| (n=466) or TMZ |
|
|
|
| P=0.004) |
|
|
|
| alone (n=229) |
|
|
|
|
|
|
|
| E, CWs |
|
| First author,
year | Study | No. of
patients |
Pathology |
Pre-treatment |
Post-treatment |
Complications | OS | (Refs.) |
|
| Vinjamuri | Randomized | 48 with CW | GBM | - | Chemoradiation | - | Median OS was
superior in | (32) |
| et al,
2009 | controlled | 32 controls |
|
| standard |
| the TMZ vs. the
BCNU |
|
|
| trial |
|
|
| protocol |
| group (15.9 vs.
11.5 months) |
|
|
|
|
|
|
|
|
| and the curves were
judged |
|
|
|
|
|
|
|
|
| to be significantly
different |
|
|
|
|
|
|
|
|
| by the log-rank
test (P<0.02) |
|
| De Bonis | Randomized | 10 with CW | IV | - | Adjuvant | The toxicity after
CW use was | Adding CWs to
standard | (33) |
| et al,
2012 | controlled | 67 controls |
|
| therapy with | significantly
higher, both for patients | treatment did
not |
|
|
| trial |
|
|
| TMZ | with newly
diagnosed GBM and | significantly
improve the |
|
|
|
|
|
|
|
| patients with
recurrent GBM. | outcome |
|
|
|
|
|
|
|
| Patients with 8
Gliadel wafers |
|
|
|
|
|
|
|
|
| implanted had a
3-fold increased |
|
|
|
|
|
|
|
|
| risk of adverse
events. Patients |
|
|
|
|
|
|
|
|
| with recurrent
tumor had a 2.8-fold |
|
|
|
|
|
|
|
|
| increased risk of
adverse events |
|
|
| Catalán- | Randomized | 55 with CW | HGG | - | Chemoradiation | - | CWs group had a
median | (34) |
| Uribarrena | controlled | 55 controls |
|
| standard |
| survival of 13.414
months |
|
| et al,
2012 | trial |
|
|
| protocol |
| compared with
11.047 |
|
|
|
|
|
|
|
|
| months in the group
without |
|
|
|
|
|
|
|
|
| implants
(P=0.856) |
|
| Noël et
al, | Randomized | 28 with CW | HGG | - | Chemoradiation | 4 cases of grade 3
thrombopenia | For patients
treated with | (35) |
| 2012 | controlled | 37 controls |
|
| standard | occurred, all in
the CW group | and without CWs,
median |
|
|
| trial |
|
|
| protocol |
| and 1-year OS were
20.6 |
|
|
|
|
|
|
|
|
| months and 78.6%
vs. 20.8 |
|
|
|
|
|
|
|
|
| months and
78.4%, |
|
|
|
|
|
|
|
|
| respectively |
|
| Pallud et
al, | Randomized | 354 with | IV | - | Chemoradiation | The rate of
postoperative non- | The median overall
survival | (36) |
| 2015 | controlled | CW |
|
| standard | infectious adverse
events did not | was 20.4 and 18.0
months |
|
|
| trial | 433 controls |
|
| protocol | differ
significantly between the | in the CWs and
non-CWs |
|
|
|
|
|
|
|
| implantation group
and the | groups,
respectively |
|
|
|
|
|
|
|
| standard group,
except |
|
|
|
|
|
|
|
|
| for raised
intracranial pressure |
|
|
|
|
|
|
|
|
| (P=0.004). The rate
of |
|
|
|
|
|
|
|
|
| postoperative
overall infections |
|
|
|
|
|
|
|
|
| was significantly
higher in the |
|
|
|
|
|
|
|
|
| implantation group
(7.1%) than in |
|
|
|
|
|
|
|
|
| the standard group
(1.5%) |
|
|
|
|
|
|
|
|
| (P=0.001) |
|
|
| Roux et
al, | Randomized | 123 with | IV | - | Standard | CWs did not show a
significant rise | CWs implantation
was | (37) |
| 2017 | controlled | CW |
|
| combined | in postoperative
complications, | independently
associated |
|
|
| trial | 217 controls |
|
|
chemoradiotherapy | including
postoperative infections | with a longer
OS |
|
|
|
|
|
|
|
|
| (P=0.0290) |
|
| Akiyama | Randomized | 25 with CW | IV | - | Standard | The occurrence of
adverse events | The median OS in
the CWs | (38) |
| et al,
2018 | controlled | 29 controls |
|
| combined | was comparable
among the | and non-CWs groups
were |
|
|
| trial |
|
|
|
chemoradiotherapy | treatment groups,
except for | 24.2 and 15.30
months, |
|
|
|
|
|
|
|
| infections, which
were more | respectively
(P=0.027) |
|
|
|
|
|
|
|
| prevalent in the
CWs group (3.5 |
|
|
|
|
|
|
|
|
| vs. 0%) |
|
|
| Bos et
al, | Human | 15 recurrent | Recurrent GBM | - | Surgery and | Second surgery and
intra- and | 1 patient showed
partial | (39) |
| 2023 | phase I study | GBM |
|
| various | peritumoral
infusion of hrBMP4 | response and 2
patients a |
|
|
|
|
|
|
| treatments |
| complete (local)
tumor |
|
|
|
|
|
|
|
|
| response, which
was |
|
|
|
|
|
|
|
|
| maintained until
the most |
|
|
|
|
|
|
|
|
| recent follow-up,
57 and |
|
|
|
|
|
|
|
|
| 30 months
post-hrBMP4. |
|
|
|
|
|
|
|
|
| Tumor growth was
inhibited |
|
|
|
|
|
|
|
|
| in areas permeated
by |
|
|
|
|
|
|
|
|
| hrBMP4 |
|
 | Table II.Clinical investigations assessing
local radiotherapy in patients with GBM. |
Table II.
Clinical investigations assessing
local radiotherapy in patients with GBM.
| First author,
year | Design | Sample | Age, years | Setting | Dose | Outcome | Toxicity | (Refs.) |
|---|
| Chan et
al, | Monocentric, | 24 | 48.1 | Recurrent | 53.1 Gy | Median
Survival | 1 patients | (40) |
| 2005 | retrospective |
|
| GBM |
| from diagnosis,
23.3 | wound |
|
|
|
|
|
|
|
| months; after
BCT | infection, 2 |
|
|
|
|
|
|
|
| 9.1 months | symptomatic |
|
|
|
|
|
|
|
|
| radiation |
|
|
|
|
|
|
|
|
| necrosis |
|
| Schueller | Monocentric, | 45 | 56 | Newly | 20 Gy | Median OS,
14.2 | 2.8% | (41) |
| et al,
2005 | retrospective |
|
| diagnosed |
| months; time
to | radiation |
|
|
|
|
|
| GBM |
| local failure,
9.9 | necrosis, 5.6% |
|
|
|
|
|
|
|
| months | hemorrhage |
|
| Gabayan | Multicenter, | 95 | 51 | Recurrent | Median, | Median
survival | 8 RTOG | (42) |
| et al,
2006 | retrospective |
|
| grade 3 and | 60 Gy | from BCT, 36.3 | grade 2 |
|
|
|
|
|
| 4 gliomas |
| weeks | toxicity, 2 |
|
|
|
|
|
|
|
|
| grade 3 who |
|
|
|
|
|
|
|
|
| required |
|
|
|
|
|
|
|
|
| reoperation
for |
|
|
|
|
|
|
|
|
| symptomatic |
|
|
|
|
|
|
|
|
| radiation |
|
|
|
|
|
|
|
|
| necrosis |
|
| Chen et
al, | Phase 1 | 18 | 50 | Newly | 400 Gy | OS, 114 weeks;
PFS, | 11 patients | (43) |
| 2007 |
|
|
| diagnosed |
| 57 weeks | underwent |
|
|
|
|
|
| GBM |
|
| reoperations |
|
|
|
|
|
|
|
|
| for radiation |
|
|
|
|
|
|
|
|
| necrosis |
|
| Welsh et
al, | Multicenter, | 20 | 59 | Newly | Median | Average
survival, | 3 patients | (44) |
| 2007 | retrospective |
|
| diagnosed | dose, 50 | 11.4 months | (14%) grade 3 |
|
|
|
|
|
| GBM | Gy |
| CNS toxicity |
|
| Chino et
al, | Monocentric, | 32 | - | Recurrent | Median 60 | Average
survival | 1 leak | (45) |
| 2008 | Retrospective |
|
| and newly | Gy | after BCT,
12.5 | from BCT |
|
|
|
|
|
| diagnosed |
| months | balloon |
|
|
|
|
|
| GBM |
|
|
|
|
| Fabrini | Monocentric, | 21 | 60 | Recurrent | 18 Gy | Median OS,
21.7 | 1 patients | (46) |
| et al,
2009 | retrospective |
|
| GBM |
| months; median | had a fatal |
|
|
|
|
|
|
|
| survival after | venous |
|
|
|
|
|
|
|
| recurrence,
8.0 | hemorrhage, |
|
|
|
|
|
|
|
| months | 2 patients |
|
|
|
|
|
|
|
|
| had
asymptomatic |
|
|
|
|
|
|
|
|
| radionecrosis |
|
| Usychkin | Monocentric, | 12 | 48 | Newly | Median | Median OS, 13 | Radiation | (47) |
| et al,
2013 | retrospective |
|
| diagnosed | dose, 12.5 | months | necrosis,
9.4%; |
|
|
|
|
|
| GBM | Gy |
| hemorrhage,
3.1% |
|
| Schwartz | Monocentric, | 40 | 57.6 | Recurrent | 50 Gy | Median OS,
41.8 | 3 patients | (48) |
| et al,
2015 | retrospective |
|
| GBM |
| months; PFS,
8.3 | grade I, 1 grade
II |
|
|
|
|
|
|
|
| months | and 2 grade IV |
|
|
|
|
|
|
|
|
|
edema-associated |
|
|
|
|
|
|
|
|
| toxicity |
|
| Sarria et
al, | Multicenter, | 51 | 55 | Newly | Median | Median OS, 18 | 25.5% | (49) |
| 2020 | retrospective |
|
| diagnosed | dose, 10 | months; PFS,
11.4 | radiation |
|
|
|
|
|
| GBM | Gy | months | necrosis |
|
 | Table III.Local immunotherapy clinical trials
in patients with GBM. |
Table III.
Local immunotherapy clinical trials
in patients with GBM.
| Immunotherapy | Target | Clinical trial
identifier | Study phase | No. of
patients | Setting | Trial design | Outcomes | Status |
|---|
| CAR-T cells | IL-13Rα2 | NCT01082926 | 1 | 6 | rGBM | Intratumoral | OS, 19.7 | Completed |
|
|
| Keu et
al, |
|
|
| infusion of | months |
|
|
|
| 2017 (50) |
|
|
| GRm13Z40-2 |
|
|
|
|
| NCT02208362 | 1 | 1 | rGBM | Intracavitary | PFS, 7.6 | Not |
|
|
| Brown et
al, |
|
|
| infusion CAR-T | months | recruiting |
|
|
| 2016 (51) |
|
|
| cells
targeting |
|
|
|
|
|
|
|
|
| IL-13Rα2 |
|
|
|
| B7-H3 | NCT04670068 | 1/2 | 40 | rGBM | Intratumoral | NA | Recruiting |
|
|
|
|
|
|
| injection of
B7- |
|
|
|
|
|
|
|
|
| H3 CAR-T cells |
|
|
|
|
|
|
|
|
| between TMZ |
|
|
|
|
|
|
|
|
| cycle |
|
|
|
| EGFRvIII | NCT03283631 | 1 | 24 | rGBM | CAR-T | NA | Recruiting |
|
|
|
|
|
|
| intracerebral |
|
|
|
|
|
|
|
|
| with dose |
|
|
|
|
|
|
|
|
| escalation |
|
|
| Checkpoint | CAR-T | NCT04003649 | 1 | 60 | rGBM | Intracranial | NA | Recruiting |
| inhibitors | (IL-13Rα2) |
|
|
|
| infusion of |
|
|
|
| + PD1 |
|
|
|
| CAR-T |
|
|
|
|
|
|
|
|
| [IL-13Rα2] + |
|
|
|
|
|
|
|
|
| EV nivolumab |
|
|
|
|
|
|
|
|
| and ipilimumab |
|
|
|
| DNX-2401 | NCT02798406 | 1/2 | 49 | rGBM | Intratumoral | OS, 12.3 | Completed |
|
| + PD1 | Nassiri et
al, |
|
| or | DNX-2401 | months; |
|
|
|
| 2023 (52) |
|
| gliosarcoma | followed by | ORR, 10.4 |
|
|
|
|
|
|
|
| pembrolizumab | months |
|
|
|
|
|
|
|
| (anti-PD1) |
|
|
| Oncolytic | Toca 511 | NCT02414165 | 2/3 | 403 | rGBM | Toca511 | Median | Completed |
| viruses |
| Cloughesy et
al, |
|
|
| intracavitary | OS ITT, |
|
|
|
| 2020 (53) |
|
|
| followed by | 11.1 (E) |
|
|
|
|
|
|
|
| TocaFC | vs. 12.2 |
|
|
|
|
|
|
|
| administration | (S) |
|
|
|
|
|
|
|
| vs. SOC | months |
|
|
| DNX-2401 | NCT01956734 | 1 | 31 | rGBM | DNX-2401 | NA | Completed |
|
|
|
|
|
|
| intratumoral |
|
|
|
|
|
|
|
|
| injection +
TMZ |
|
|
|
| HSV | 2004-000464- | 3 | 250 | New | Surgical | Median | Completed |
|
|
| 28 (ASPECT) |
|
| GBM | resection + | OS, 16 (E) |
|
|
|
| Westphal et
al, |
|
| diagnosis | intraoperative | vs. 14 (S) |
|
|
|
| 2013 (54) |
|
|
| perilesional | months |
|
|
|
|
|
|
|
| injection of
HSV- |
|
|
|
|
|
|
|
|
| thymidine
kinase |
|
|
|
|
|
|
|
|
| followed by |
|
|
|
|
|
|
|
|
| ganciclovir + |
|
|
|
|
|
|
|
|
| SOC vs.
resection |
|
|
|
|
|
|
|
|
| and SOC alone |
|
|
|
| Parvovirus | NCT01301430 | 1/2 | 18 | New | Parvovirus
either | NA | Completed |
|
|
|
|
|
| GBM | intratumoral
or |
|
|
|
|
|
|
|
| diagnosis | EV + surgical |
|
|
|
|
|
|
|
| or rGBM | resection 10
days |
|
|
|
|
|
|
|
|
| later |
|
|
|
| Poliovirus | NCT0149189 | 1 | 61 | rGBM | Sabin type 1 | Median | Completed |
|
|
| Desjardins et
al, |
|
|
| poliovirus | OS, 12.5 |
|
|
|
| 2018 (55) |
|
|
| (PVSRIPO) | months |
|
|
|
|
|
|
|
| intratumoral |
|
|
BBB
GBM persists as one of the most resistant malignant
tumors in the CNS, characterized by inevitable recurrence despite
progressions in neurosurgery, chemotherapy and RT. Recurrences
predominantly occur within or proximal to the resection cavity,
typically within regions exposed to the highest radiation doses
(2,3,9,10).
There is thus an urgent requirement for novel therapeutic
techniques to improve patient outcomes. In this clinical setting,
LTs have gained significant interest as a developing approach. This
is due to their potential to overcome the constraints associated
with conventional glioma treatment protocols.
The brain presents a substantial challenge for the
efficient delivery of pharmacological compounds owing to the
presence of specialized interfaces governing the exchange between
the peripheral blood circulation and the cerebrospinal fluid (CSF)
circulatory system. These interfaces include the choroid plexus
epithelium (regulating blood-ventricular CSF), the arachnoid
epithelium (regulating blood-subarachnoid CSF) and the blood-brain
interstitial fluid. The BBB, formed by endothelial cells, limits
the paracellular flux of hydrophilic molecules through tight
junctions (TJs). The endothelial cells are surrounded by a basal
lamina, and astrocytic glial cells provide biochemical support,
regulate blood flow, supply nutrients, maintain ion balance and
contribute to repair processes. Essential elements of the BBB
supporting system include brain capillary endothelial cells,
extracellular base membrane, pericytes, astrocytes and microglia.
In detail, TJs are complex networks of transmembrane and
cytoplasmic strands that are found on the apical portion of
endothelial cells. They are made up of integral membrane proteins
termed claudin, occludin and junction adhesion molecules. In
addition, adherens junctions (AJs), situated below TJs in the basal
region of the lateral plasma membrane, involve transmembrane
glycoproteins (cadherins) linked to the cytoskeleton, enhancing the
structural integrity between adjacent endothelial cells at the BBB.
The ability of the BBB to control the passage of solutes and other
substances is thus greatly supported by both TJs and AJs (26,54,56,57).
Overall, the BBB is a specialized structure that
tightly regulates the molecular transit into the CNS. Typically,
the relative impermeability of the BBB protects the brain from
circulating toxins, maintaining an optimal microenvironment for
neuronal function. Nevertheless, these obstacles impede efficient
medication administration in disorders of the CNS, such as tumors
(56,57). Hence, contemporary therapeutic
strategies emphasize the endeavor to overcome BBB obstacles by
administering treatments directly into or in close proximity to the
tumor cavity. The present review explores the spectrum of currently
accessible and emerging techniques, including both LTs and the
latest advancements in immunotherapeutic drugs and genetically
engineered cell therapies (Fig.
4).
Local treatments
Over time, a multitude of instruments have been
developed to aid brain tumor surgery (9,10).
Instruments such as surgical microscopes, high-resolution imaging,
fluorescence-guided surgery and neuro-navigation are extensively
employed in neurosurgery but fall outside the scope of the present
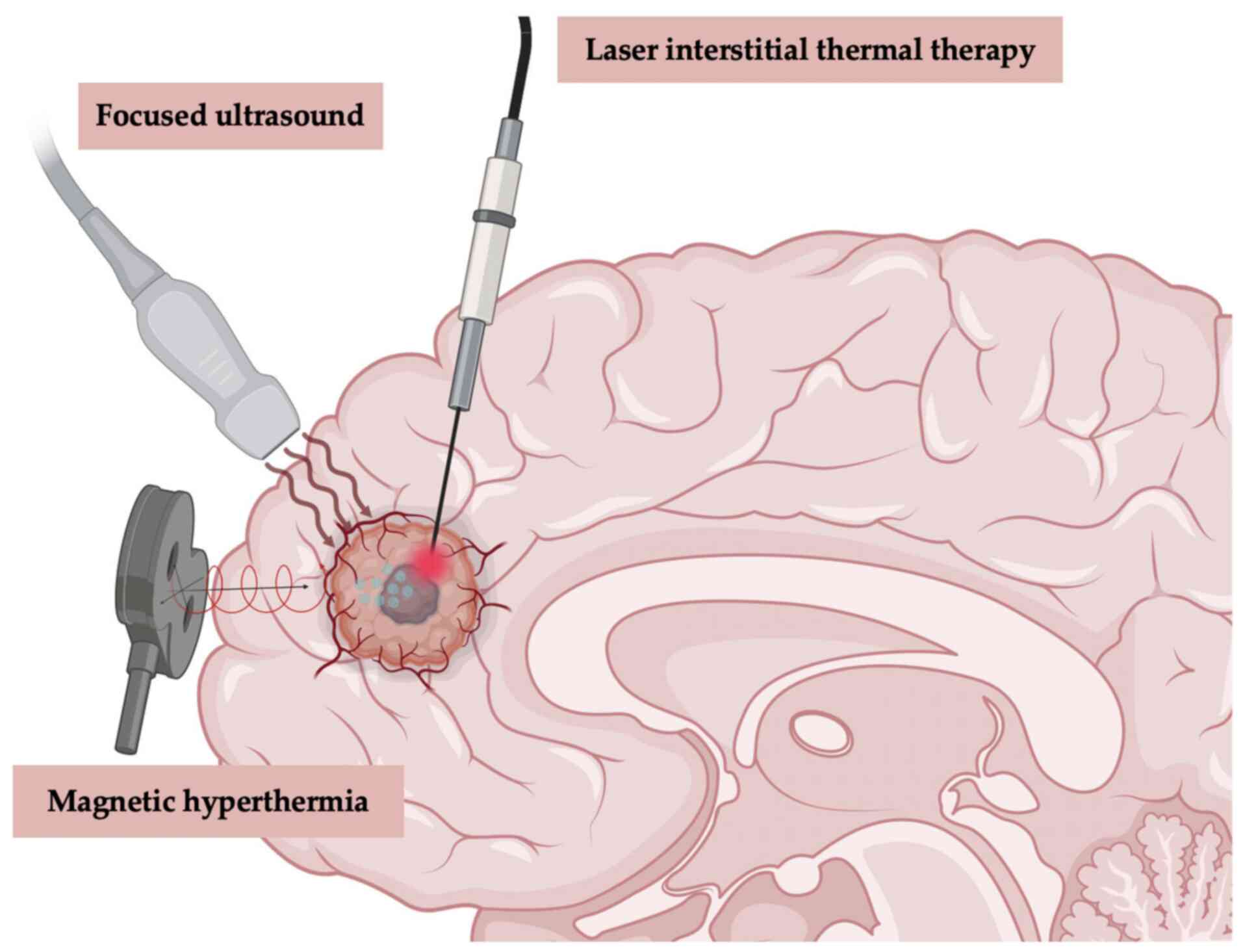
review. At present, four main approaches, laser interstitial
thermal therapy (LITT), magnetic hyperthermia (MH), (TTF) and
focused ultrasound (FUS), are undergoing regulatory approval at
different stages for the LT of GBM (Fig. 5).
Local thermal therapy (LTT)
LTT, known since the 1890s, has a recognized ability
to disrupt the BBB (58). The
effectiveness of LTT is primarily ascribed to its ability to
utilize heat, thus triggering programmed cell death and tissue
necrosis in GBM cells. In addition to directly inducing apoptosis
and necrosis, hyperthermia elicits supplementary outcomes,
including the activation of immune responses, heightened
susceptibility of GBM cells to RT and chemotherapy and temporary
disruption of the BBB (9). The
current use of LTT in patients with glioma reflects a dynamic
landscape of research and clinical applications, with ongoing
efforts aimed at refining techniques, optimizing treatment
protocols and expanding their integration into comprehensive
treatment strategies for brain tumors. Due to the inadequate
quality of the existing literature, it is not possible to offer
conclusive findings about the cost-effectiveness of LTT for
patients with GBM.
LITT
Recent technological advancements have facilitated
the emergence of LITT as a promising treatment modality,
particularly advantageous in situations where traditional open
surgical approaches are deemed suboptimal, either due to surgical
complexities or the frail condition of the patient (15–25).
These technological advancements include laser probe design,
cooling mechanisms, stereotactic targeting hardware and real-time
thermography (18,59,60).
Furthermore, two extensive studies comparing patients who underwent
LITT for primary GBM with a control group found that the overall
risk of complications was ~15%. However, there were no significant
differences in PFS or OS between the two groups (18,60). A
subsequent analysis revealed that LITT is a valid and effective
choice for treating unifocal, lobar and recurrent GBM compared with
a similar group of patients who underwent a second surgery
(61). In addition, a recent study
comparing surgically accessible recurrent GBM found no significant
differences in survival outcomes or morbidity between LITT and
repeat surgery. However, LITT was associated with shorter hospital
stays and more efficient postoperative care (61). Similar to other clinical studies
conducted on patients with brain tumors, research on LITT often
consists of retrospective observational studies with a limited
number of patients (15–25). However, to obtain more reliable
results, it is necessary to conduct a meticulously planned
prospective multicenter randomized controlled study.
FUS
Recent developments in FUS technology have increased
its viability and safety for the treatment of numerous intracranial
diseases (62). In summary, FUS
technology precisely guides ultrasound beams to specific areas of
the brain, targeting tumor cells while minimizing damage to healthy
tissue (26,27). The FUS technology is extensive,
primarily categorized into high-intensity FUS (HIFUS) and
low-intensity FUS (LIFUS) according to frequency (63–65).
The two main categories of FUS infer thermal and non-thermal
effects. The goal of HIFUS treatment in GBM is to employ heat to
thermally ablate the tumor and the peritumoral surrounding tissue.
HIFUS causes tissue heating, DNA fragmentation and protein
denaturation by molecular vibration. (66). However, LIFUS is mostly dependent on
non-thermal phenomena such as sonic cavitation and mechanical
disturbance. When administered intravenously to the target location
in conjunction with LIFUS, microbubbles enhance the administration
of therapeutic drugs and facilitate liquid biopsies (63–65).
During FUS treatment for intracranial malignancies,
steady cavitation is thus utilized to increase the permeability of
the BBB, thereby facilitating drug delivery by loosening tight
junctions. However, inertial cavitation causes direct harm to
tissues by temporarily breaking the BBB (64,65,67).
LIFUS can also induce the release of pro-inflammatory cytokines and
stress responses in intra-tumoral immune cell populations and
increase dendritic cell activity (68). When combined, these actions hold the
promise of overcoming immune evasion mechanisms initiated by GBM,
potentially triggering an antitumor immune response that is not
just relevant but could be a significant breakthrough in the field
(68).
MH
MH is a form of targeted thermal therapy where
electromagnetic energy is transformed into heat by activating
magnetic nanoparticles or mediators within the tumor or resection
cavity using an external alternating magnetic field. Apoptotic and
necrotic cell deaths are directly caused by thermal energy
delivered to the tumor site (69),
which also indirectly triggers an immunological response in a
‘cold’ immunological site such as GBM (70). Following MH, heat shock proteins,
which attach to antigen-presenting cells directly and then
secondarily trigger an immune response dependent on CD8+
T cells, were found to be significantly upregulated in preclinical
investigations conducted on primary tumor cells and animal models
(71). Additionally, major
histocompatibility complex class I and natural killer group 2,
member D (NKG2D) ligand are more highly expressed in heated tumor
cells, rendering them more vulnerable to lysis by CD8 and natural
killer T cells (72).
The idea of heat generation and selective
distribution of magnetic particles was initially proposed by
Gilchrist et al (73) in the
late 1950s. Stea et al (74)
presented the first clinical trial describing the use of MH for
primary malignant brain tumors in 1990 and subsequently conducted a
prospective phase I/II experiment in 1994 (28) assessing the efficacy of
brachytherapy (BCT) and interstitial thermotherapy in treating
primary and recurrent high-grade glioma (HGG) following surgical
resection. In this study, a noteworthy correlation between survival
and hyperthermia [hazard ratio (HR), 0.53; 95% confidence interval
(CI), 0.29–0.94) in the case of primary tumors was discovered.
However, a relevant proportion of complications were also
documented in the study, which included hydrocephalus, intracranial
hemorrhage, episodic seizures and elevated edema. In a separate
phase I investigation, 6 patients with recurrent GBM were found to
exhibit a substantial edematous response to superparamagnetic iron
oxide nanoparticles. Of these patients, 4 required high-dose
corticosteroid therapy, and another craniotomy was necessary to
remove the particles (70).
Direct stereotactic injection of nanoparticles into
the tumor is another proposed form of delivery. The results of a
prospective non-randomized study involving 59 patients with
recurrent GBM who received repeated sessions of stereotactic RT and
thermotherapy were presented by Maier-Hauff et al (29). When patients receiving MH were
compared with historical controls from previous investigations, the
authors found a significant survival advantage (OS from recurrence,
13.2 vs. 6.2 months). However, the study also reported significant
side effects, including worsening of motor deficits, focal
convulsions and grade 1–3 thermal stress.
The optimal treatment strategy for MH remains
inadequately evaluated. Although preliminary research indicates
that it may be useful in treating HGGs, more validation in
randomized controlled trials is essential. However, MH also
deserves careful examination due to the relationship with multiple
potential complications, which may limit its application in routine
clinical practice.
TTF
TTF represents a divisive and debated therapeutic
approach to GBM management. TTF constitutes an antimitotic therapy
that uses transducer arrays applied to the shaved scalp to deliver
low-intensity, intermediate-frequency (200 kHz) alternating
electric fields. These fields exert anti-mitotic effects by
disrupting microtubule assembly during cell division, resulting in
tumor cell death (75–77). The TTF device for recurrent GBM
received US Food and Drug Administration (FDA) approval in 2011
based on a phase III clinical trial (EF-11). This trial compared
the efficacy of TTF with the best choice of chemotherapy (as
determined by the physician) in patients with recurrent GBM,
demonstrating comparable efficacy between the two treatments.
However, patients receiving TTF experienced an improved quality of
life and less toxicity (30). In
2015, TTF therapy received FDA approval for treating patients with
newly diagnosed GBM. This approval was based on the EF-14 clinical
study (NCT00916409), which demonstrated a significant extension of
PFS and OS time when TTF therapy was combined with maintenance TMZ
(31). The National Comprehensive
Cancer Network also incorporated the TTF device as a viable option
for treating newly diagnosed GBM. However, Despite FDA endorsement,
doubts persist surrounding this therapy (75).
After gaining approval in the US, subsequent
regulatory approvals in Europe and Asia in recent years have
heightened awareness of TTF therapy among a wider range of patient
populations and treatment centers. In a recent meta-analysis, Ballo
et al (77) analyzed 1,430
patients with GBM in a pooled analysis for OS time. The
meta-analysis of comparative studies indicated a significant
improvement in OS time for patients receiving TTF plus standard of
care (SOC) vs. SOC alone (P<0.001). Specifically, the OS time
was 22.6 months for patients treated with TTF plus SOC, compared
with 17.4 months for those receiving SOC alone.
Although a number of studies have assessed the
safety and effectiveness of TTF, questions remain regarding
research design, quality of life and therapeutic costs. These
problems require further investigation, and ongoing trials are
intended to yield more information regarding treatment outcomes and
interactions in combination regimens.
Local treatment approaches and delivery
systems for malignant gliomas
Given the notable drawbacks of systemic drug
delivery options, local drug delivery techniques are seen as viable
substitutes. The drawbacks of systemic methods include long
delivery routes, which increase the chance of medication absorption
by unwanted organs or clearance during blood circulation.
Furthermore, the potential for systemic toxicity remains a notable
concern. By contrast, local drug delivery techniques provide a
focused and efficient approach, delivering drugs directly into the
brain bypassing the limits of the BBB (9,78–82).
Local drug delivery approaches encompass a growing number of
research topics, including the continuous discovery of therapeutic
nanomaterials and the continued development of pharmaceutical
molecular design (9,10,78–82).
Numerous intracranial implant-based delivery techniques are now
under investigation (83–94).
Convention-enhanced delivery
(CED)
Since the 1994 study by Bobo et al (79), CED for gliomas has primarily
remained under investigation in preclinical models (10). In CED, catheters extend past the
cannula tip into the targeted tumor location and are proximally
coupled to a syringe pump that contains the treatments components.
Chemotherapy CED has demonstrated success in laboratory settings,
but its application in clinical environments has encountered
several challenges (80–82). These challenges highlight the
necessity of addressing specific issues to enhance the
implementation of CED in patient care. Failures of CED in glioma
treatment can be attributed to several factors: i) Achieving
uniform distribution within the tumor; ii) limitations in the
design and placement of the probe; and iii) tracking the delivery
of the infusate. Since GBMs are highly infiltrative, effective
drugs must discriminate between malignant and normal tissue while
penetrating the tissue deeply to reduce the possibility of severe
side effects (80–82). Several ongoing trials are
investigating the CED of tumor-targeting compounds, encompassing
cytokines, viruses, gene therapies and antibodies. Further details
on these approaches will be discussed below.
Implanted delivery
Various preclinical models are currently under
investigation to explore hydrogels, nanofibers and spray devices
(80). Furthermore, nanoparticles
can be used as carriers for the transport of pharmacological
compounds and genetic material across the BBB. If properly
engineered, nanoparticles can interact specifically with tumor
cells, limiting damage to healthy cells (56). In this regard, biomaterial implants
are becoming increasingly flexible therapeutic platforms capable of
providing novel approaches in GBM treatments (83). A number of non-biodegradable
ethylene-vinyl acetate copolymers (EVAcs) and biodegradable
materials [such as polyanhydride
poly(bis(p-carboxy-phenoxy)propane-sebacic acid) copolymer, fatty
acid dimer-sebacic acid copolymer, poly(lactic-co-glycolic acid)
copolymer (PLGA) and poly-ε-caprolactone] have been explored for
the local delivery of therapeutic agents including chemotherapy
[such as paclitaxel, doxorubicin and bis-chloroethylnitrosourea
(BCNU)] and anti-angiogenic (such as minocycline and endostatin
fragment) drugs (84–86). These implantable biomaterials can be
in different forms including wafers, discs, films, rods or meshes
and can be fabricated using different methods such as
electrospinning, solvent casting, extrusion or compression molding.
The effectiveness of implantable drug delivery systems in treating
recurrent cancer is potentially heterogenous. Gliosis may hinder
the delivery of drugs into the brain parenchyma and hamper access
to tumor cells at tumor recurrence (56). The local administration of the
anti-angiogenesis drug, minocycline, either in combination with
systemic BCNU or alone, resulted in notable improvements in the
median survival time in a rat brain tumor model without the need
for excision (84).
Implanting a device containing EVAc and BCNU proved
effective in reducing systemic toxicity while increasing local drug
concentration (87). The device,
created by dissolving the drug and polymer in methylene chloride,
demonstrated potential antitumor effects in rat intracranial glioma
models when delivering chemotherapy drugs, such as amsacrine and
mitoxantrone, using EVAc. Additionally, PLGA, an FDA-approved
copolymer, has been explored in studies. Xie and Wang (88) developed paclitaxel-loaded PLGA
ultrafine-fiber implants for brain glioma treatment via
electrospinning, achieving sustained drug release for >60 days.
Another group utilized PLGA and paclitaxel in treating glioma, with
the constructed electrospun paclitaxel-loaded PLGA fibrous meshes
supporting drug release for 80 days (89). These implants resulted in much
smaller tumors in a mouse subcutaneous C6 glioma in vivo
model compared with the placebo and Taxol® injected
control groups (89).
The topic of theranostic applications for the brain
has seen a recent increase in interest, with an emphasis on the use
of electronic devices such sensors and actuators. This represents a
recent proposal that adds a new dimension to the ongoing
exploration of these technologies in the context of brain-related
applications. The goal of this multidisciplinary strategy is to
combine macroscopic and microscopic tactics to potentially
revolutionize drug delivery options (78). For instance, in clinical practice, a
solid implant for intracranial drug delivery in GBM is the
biodegradable CWs. The US FDA approved the CW implantation strategy
for the treatment of recurrent GBM and newly diagnosed HGG in 1996
and 2003, respectively, establishing a therapeutic bridge between
adjuvant therapy and surgical resection (83). Despite the early promising results,
the implantation of CWs in HGG has gradually been abandoned in
typical clinical practice for a number of reasons (33–38,90).
In a recent collaborative review by the Society for
Neuro-Oncology-European Society of Neuro-Oncology, the authors
stated that CWs offer a limited survival advantage of ~2 months,
suggesting a limited use, primarily due to concerns related to
safety and tolerability risks (91,92).
Furthermore, the exclusion of CW-treated patients in later clinical
trials, to prevent potential confounding effects brought by CWs,
discourages its broad implementation (91,92).
Nevertheless, recent long-term follow-up studies
have shown survival benefits in newly diagnosed patients with GBM
treated with CW implantation, prompting a reevaluation of the
therapeutic efficacy of CWs in selected cases, such as young
patients with small lesions without ventricle opening during
surgery. According to a recent study by Iuchi et al
(93), implantation of CWs
significantly extends OS (median OS time, 27.4 months; 2-year OS
rate, 46%) in younger patients with an extent of resection >95%.
The aforementioned study supports the critique directed towards the
efficacy of CWs highlighted in a study by Champeaux and Weller
(94), a 9-year nationwide
retrospective study in which it was demonstrated that clinical and
surgical factors (such as age, tumor volume, tumor side and extent
of resection) may influence the survival benefit in patients
receiving CWs, underling its potential efficacy in specific cases.
In addition, although implantable CWs have been criticized in
recent research, their efficacy and safety may be increased by
adjusting the chemicals, dosage and implantation methods (95,96).
Human recombinant bone morphogenetic protein 4
(hrBMP4) has shown promise in preclinical studies for its ability
to induce differentiation and reduce the self-renewal capacity of
GBM stem-like cells (GSCs), which are often implicated in the
aggressive nature and treatment resistance of GBM. By targeting
these GSCs, BMP4 could potentially reduce tumor recurrence and
improve patient outcomes (95). In
a recent human phase I dose escalation trial, the efficacy and
safety of hrBMP4 administered via CED was investigated in 15
patients with recurrent GBM. The results demonstrated that hrBMP4
was well-tolerated with no serious adverse events directly
attributed to the treatment (39).
The findings of these investigations suggest that local hrBMP4
delivery can inhibit tumor growth in areas exposed to the protein,
highlighting the potential for hrBMP4 as a therapeutic approach for
GBM.
Overall, CED bypasses the BBB to directly administer
targeted therapies into malignant glioma tissue and surrounding
areas. Despite being invasive, wafer and reservoir delivery systems
offer the potential for prolonged compound delivery during disease
progression. Although CED shows significant potential for advancing
the treatment of GBM, continuous research and refinement are
necessary to fully harness its capabilities and address current
challenges. Enhancing the design of the catheter to minimize
backflow and improving the materials of the catheter to prevent
scarring may increase the effectiveness of CED delivery. Catheter
design, number of catheters, catheter location, infusion rate,
start-up infusion protocol, infusion duration, type of drug
infused, possible drug encapsulation and methods of evaluation for
drug distribution are critical factors that need to be considered
in the future. At present, CED is still a potent and promising
treatment modality for GBM.
Local RT
RT is currently the primary method for controlling
the growth of GBM. Since publication of the study by Stupp et
al (1) in 2005, the dose of 60
Gy in 30 sessions has been delivered along with chemotherapy in the
postoperative setting to the residual disease (when present) and
the surgical bed with an adequate clinical margin (97). Unfortunately, even after RT, disease
recurrence is inevitable, and in 70–80% of cases, it occurs within
the treatment field (98). Several
clinical trials have attempted to increase the dose of ionizing
radiation in combination with TMZ using external beams RT (99). Nevertheless, the advantages in terms
of survival have been marginal, while the adverse effects induced
by radiation have significantly exacerbated.
To augment the dose directed at the tumor bed and
mitigate harm to adjacent tissue, diverse BCT approaches have been
explored, yielding differing levels of success (100). BCT and intraoperative RT (IORT)
are the two main types of localized RT. BCT consists of placing a
source of ionizing radiation into the surgical cavity, and IORRT
treats the surgical bed immediately after surgery using a dedicated
linear accelerator. Both upfront and recurrent settings have been
assessed in clinical trials. Nevertheless, to date, there are a
limited number of published clinical studies, and most studies are
retrospective and single-centered in design. In 2007, Chen et
al (43) reported the results
of a phase I study on 18 patients with GBM treated with BCT after
the first surgical intervention (all patients had undergone radical
resection). The median dose of ionizing radiation delivered through
the placement of permanent iodine-125 (I-125) seeds at a depth of
0.5 cm was 400 Gy, followed by postoperative external beam RT.
Despite PFS and OS results consistent with those reported by the
aforementioned Stupp study, 11 patients underwent surgery for the
development of radionecrosis (without evidence of disease
progression). As a result of the high rate of toxicity, the trial
was prematurely stopped. Similar results in terms of OS following
BCT treatment at first diagnosis were also reported by Welsh et
al (44). In addition, Fabrini
et al (46) evaluated the
viability and effectiveness of perioperative high-dose-rate BCT in
2009, administering an 18 Gy radiation at a 5 mm depth to 21
patients with recurrent GBM. The median OS time was 21.7 and 8
months from diagnosis and tumor recurrence, respectively. Chan
et al (40) reported similar
survival outcomes for 24 patients who received BCT at the time of
the second surgical intervention, which likely indicates selection
bias by the neurosurgeon affected the data. This was due to patient
selection (ideal patients eligible for a second surgery were
selected), a prolonged time since the end of RT and a tumor
recurrence that is easily resectable and preferably localized
within the RT treatment fields. The study reported a median
survival time of 23.3 months from diagnosis and 9.1 months from the
date of recurrence.
In a study by Chino et al (45), the survival time of 26 patients
treated with BCT after the second surgical intervention was 7.1
months. Gabayan et al (42)
observed that patients who received BCT at the time of recurrence
had a median survival time of 36.3 weeks from the date of BCT,
which was consistent with earlier studies. In 2014, Kickingereder
et al (101) reported a
retrospective case series of patients with inoperable GBM treated
with BCT at diagnosis and recurrence (103 patients treated at
diagnosis and 98 at disease recurrence) between 1990 and 2012. A
median dose of 60 Gy was administered through low-dose-rate
stereotactic I-125 BCT. The treatment-related mortality was 0% and
toxicities occurred in <7.5% of patients. It was found that
patients treated with BCT at diagnosis and at recurrence had the
same length of disease control (6.2 vs. 5.9 months, respectively;
P=0.11). This result was likely affected by the patient selection
(102,103). In 2015, Schwartz et al
(48) published the results of a
retrospective study on 68 patients with recurrent GBM who had been
treated with I-125 BCT (the reference dose was 50.0 Gy, calculated
to the boundary of the tumor). The median survival time was 41.8
months (95% CI, 29.2–55.9) and the perioperative morbidity was
2.9%. However, this study exhibited notable bias in patient
selection, rendering the evaluation of OS data challenging.
Based on the available published studies, it can be
concluded that BCT is likely safe, meriting further investigation
in dedicated prospective clinical trials. However, the
retrospective nature of currently published BCT studies, the
variability in patient selection criteria, the prolonged enrollment
periods resulting in heterogeneity according to various WHO
classifications and the differing techniques and doses utilized
present significant challenges to definitively determining the
impact of this technique on patient survival. Future prospective
studies are anticipated to provide critical insights.
Clinical experiences assessing the application of
IORT in patients with GBM have revealed comparable limitations. In
2005, Schueller et al (41)
reported the results of a retrospective study conducted on 71
patients with glioma treated with IORT. IORT demonstrated
feasibility, with perioperative complication rates remaining
unchanged. However, the survival outcomes generally did not exhibit
improvement when compared with a historical control group. Disease
recurrence exhibited similar survival rates as primary tumors, and
GBM displayed a slightly elevated survival, suggesting potential
indications for the use of IORT. Investigations by Usychkin et
al (47) and Sarria et
al (49) described findings
indicating less favorable outcomes in terms of safety. The two
studies assessed the viability of IORT in new and recurrent GBM..
Radionecrosis occurred in 35 and 25.5% of patients in the
respective studies. In addition, Usychkin et al (47) evaluated the impact of IORT in terms
of survival and toxicity in a retrospective single-center study of
17 patients with GBM treated between 1992 and 2002. Each patient
received high-dose IORT (20 Gy), followed by post-operative
external beam RT. For the whole group, the median OS time was 13
months, consisting of 10.4 months for recurrent cases and 14 months
for primary cases. Of the complications recorded, 3 patients
presented with radionecrosis, 1 with osteomyelitis at the
craniotomy bone flap, 1 with intracerebral hemorrhage and 1 with
pulmonary embolism. In addition, 2 patients had a fatal outcome.
Sarria et al (49) evaluated
51 patients with GBM. IORT was performed in a single session
immediately after surgery (10–40 Gy prescribed at the applicator
surface using low-energy photon) and was followed by standard
radiochemotherapy treatment. Although no grade 4 radionecrosis was
recorded, G1-G3 radionecrosis occurred in 25.4% of patients. At
present, there is a lack of data supporting the use of IORT or BCT
for diffuse glioma. Furthermore, the main clinical randomized trial
investigating the efficacy of I-125 has failed to demonstrate a
survival benefit (104).
The FDA clearance of GammaTile, incorporating Cs-131
titanium seeds within a resorbable collagen-based tile, has renewed
the interest in BCT and introduced the concept of surgically
targeted RT. Early clinical reports suggest a reasonable safety
profile, but potential delayed seed settling during collagen
absorption raises uncertainty about efficacy. Ongoing clinical
trials are actively exploring the safety and efficacy of GammaTile
for CNS tumors (105,106).
Local immunotherapy
Immunotherapy stands out as one of the extensively
explored novel approaches for GBM treatment. The low immunogenicity
of the tumor, along with an immunosuppressive TME, allows it to
evade an immune response. For a number of reasons, including its
low tumor mutational burden, low number of tumor-infiltrating T
cells and low programmed cell death protein 1 (PD-1)/programmed
death-ligand 1 expression, GBM is recognized as an immunologically
inert tumor, particularly when compared with other cancer types
that have responded favorably to immunotherapy. The high
heterogeneity of GBM further facilitates immune evasion (7). In addition, although steroids are
often essential for managing peritumoral edema, they can compromise
the effectiveness of immunotherapies (7,107,108). Until now, several phase III
clinical trials that focused on immune therapy for GBM have
encountered difficulties, mainly related to individual components
of the antitumor immune response. Learning from these setbacks, the
potential success of immunotherapy for GBM appears most optimistic
when utilizing a combination of immunotherapies to address the
significant immunosuppression disease-related. It is thus crucial
to detect reliable biomarkers both for appropriate patient
selection and tumor evolution monitoring (9,10).
In this section, current findings and continuing
clinical research in the field of immunotherapy for GBM will be
discussed, which includes immune checkpoint inhibitors (ICIs),
vaccines, chimeric antigen receptor T cell (CAR-T) treatment and
viral therapy. In total, two different approaches of immunotherapy
are described in literature: Passive immunotherapy (using
antibodies and immune checkpoint modulators) and active
immunotherapy (using tumor vaccination with viral vectors or
dendritic cells and CAR-T treatments). A complete list of trials
with clinical relevance using different immunotherapy strategies in
the treatment of glioma are reported in Table III.
Viral therapy
Oncolytic viruses (OVs) selectively replicate in
cancer cells and stimulate antitumor immunity, inducing immunogenic
cell death (109). Studies have
confirmed the efficacy and safety of OVs for the treatment of
glioma; however, it is required to determine which OVs provide the
most efficient treatment (adenovirus and herpes virus) (53,54,110).
VB-11 is an adenovirus that directly disrupts the angiogenic
vessels and induces a direct tumor immune response. Phase III
trials have tested the efficacy of VB-11 with controversial
results. A study by Brenner et al (110) demonstrated a significantly longer
OS time (HR, 0.48; P=0.043) in patients primed with VB-111 in
combination with bevacizumab. However, another phase III trial
failed to demonstrate a benefit with this combination (OS time, 6.8
vs. 7.9 months; HR, 1.2; P=0.19) (53).
Oncolytic herpes simplex virus (HSV) is an
attractive class of anticancer therapy due to a highly stable
genome, potent cytolytic capability and effective drugs to treat
adverse events. Westphal et al (54) demonstrated an improvement in median
time to death (or re-intervention) with the perilesional injection
of HSV-thymidine kinase followed by ganciclovir plus SOC. The
survival time of the indicated treatment vs. SOC alone after tumor
resection in newly diagnosed GBM was 308 vs. 268 days (HR, 1.53;
P=0.006).
CAR-T cells
CARs are immunoglobulin T cell receptors that can
activate T cells recognizing specific antigens, which have
generated a recent particular interest due to favorable activity in
hematologic malignancies (111).
The most notable targets of these cells include EGFR, IL-13R2 and
HER2; however, more recently discovered targets include mucin 1,
CD147, GD2 and Eph receptor A2 (112). These cells appear to eliminate
tumor cells with precision and demonstrate an increase in the
immunogenicity of the GBM microenvironment (7). Encouraging data are also emerging in
diffuse intrinsic pontine glioma. A study by Vitanza et al
(113) demonstrated satisfactory
tolerability to intraventricular infusion of B7-H3 CAR-T and 1 of
the 3 patients with diffuse intrinsic pontine glioma enrolled had
radiographic and clinical improvement through 12 months of the
study. Further scientific details on this topic are discussed in
greater depth in Chapter 8.
ICIs
ICIs target PD-1 or cytotoxic T-lymphocyte
associate protein 4 (CTLA-4) to induce the immune response of
T-cells. Although ICIs now represent the SOC in a number of cancer
types with encouraging preclinical data (including in GBM), in a
clinical context, ICIs do not improve the survival of patients with
GBM (114,115). Different factors may limit immune
cell trafficking, reducing the efficacy of suppressive TME.
Although some encouraging data are emerging from the neoadjuvant
phase investigations, these trials are still too immature and
require a comparison arm. (116).
Regarding intratumor activity, certain phase I/II clinical trials
administering a combination of ICIs and CAR-T or OV make possible
the application of ICIs in patients with glioma (52). Nassiri et al (52) in a multicenter phase 1/2 study,
investigated the combination of intratumoral delivery of the OV,
DNX-2401, followed by intravenous administration of anti-PD-1
antibody (pembrolizumab) in recurrent GBM. The authors demonstrated
that the combination of DNX-2401 and pembrolizumab was safe and
showed a notable survival benefit in selected patients. The OS rate
at 12 months (52.7%; 95% CI, 40.1–69.2%), was significantly higher
than the prespecified control rate of 20%. The median OS time was
12.5 months (10.7–13.5 months). Patients who achieved objective
responses had a longer survival (HR, 0.20; 95% CI, 0.05–0.87).
Additionally, 56.2% (95% CI, 41.1–70.5%) of patients experienced
clinical benefits, defined as stable disease or better. Notably, 3
patients had durable responses and were alive at 45, 48 and 60
months.
Advances in CAR-T cell technology and beyond
for HGG
Despite efforts in the genomic, transcriptomic,
epigenetic and proteomic characterization of GBM specimens, little
progress has been made in the survival of patients with GBM. The
failure of treatments can be attributed to a number of GBM gene
alterations, including mutations in KRAS, c-MET, PI3KCA, BRAF,
telomerase reverse transcriptase, TP53 and PTEN, as well as the
mutational status of the EGFR and platelet derived growth factor
receptor α genes (7). Furthermore,
the inability of tyrosine kinase inhibitors to cross the BBB
reduces the efficacy of chemotherapy (116). Consequently, accurate tumor
antigen targeting and effective intracranial drug delivery are
essential for the success of GBM treatments (117). Comprehensive single-cell
RNA-sequencing analysis of cancer stem cells, cells that remain in
recurrent and resistant GBM inside the TME, may provide valuable
data for future research on effective targeting strategies for this
fatal illness (118). At present,
several clinical trials are being conducted to treat GBM. These
trials include vaccine therapy, immunotherapy and CAR-T cell
therapy (119).
CAR-T cell technology envisages the production of T
lymphocytes redirected to express a single-chain variable fragment
(scFv) of an antibody to target and eliminate tumor cells that
overexpress a specific tumor-associated antigen (TA) (120). CAR antigens that have been
primarily targeted in patients with GBM include EGFR variant III
(vIII), HER2, IL-13Rα2, B7-H3 and NKG2D (51,121–123). EGFRvIII CAR-T has been explored in
several trials but has shown little OS benefit. Since CAR-T cell
abundance in the blood is correlated with tumor regression,
Suryadevara et al (124)
have demonstrated that greater lymphodepletion induced by TMZ-dose
intensified (TMZ-DI) is required to stimulate the proliferation and
persistence of EGFRvIII CAR-T cells in a murine model. In light of
this, a phase I clinical trial was established that involved
individuals diagnosed with newly onset GBM, utilizing TMZ-DI as a
preconditioning regimen preceding CAR-T cell immunotherapy
(NCT02664363). According to Brown et al (51), intracranial infusion of IL-13Rα2
CAR-T cells initially demonstrated GBM regression but ultimately
resulted in recurrence. In line with this, an ongoing phase I trial
has been established to study the side effects and mechanism of
IL-13Rα2 CAR-T cells when administered alone or in combination with
the ICIs, nivolumab (anti-PD-1 mAb) and ipilimumab (anti-CTLA-4
mAb), in treating recurrent GBM cases (NCT04003649). As a result,
CAR-T cell therapy for GBM will develop; however, several issues
must be addressed, including TA heterogeneity, T-cell exhaustion,
T-cell infiltration and the tumor immunosuppressive
microenvironment.
Specifically, as demonstrated in the aforementioned
studies, EGFRvIII CAR-T cell therapy led to a reduction in the
number of EGFRvIII-expressing cells in patients with GBM. However,
examination of post-infusion surgical resections demonstrated that,
although EGFRvIII CAR-T cells effectively penetrated brain tumors,
a high level of wild-type EGFR (wt EGFR) expression persisted in
the residual tumor (51,123,124). To overcome this antigen
heterogeneity, Choi et al (125) developed a sophisticated modified
EGFRvIII CAR-T cells to produce a bispecific T-cell engager (BiTE)
with the aim of targeting the residual EGFRvIII− GBM
cells in mice. BiTE was conceived to incorporate two scFvs, one
directed against wt EGFR and the other to engage and activate T
cells by binding CD3. BiTEs secreted by EGFRvIII CAR-T cells were
also able to recruit local bystander T-cell effector activity. This
platform may also apply to other tumor types that have demonstrated
heterogenous EGFRvIII expression, including medulloblastomas and
breast and ovarian carcinoma.
To overcome the limited efficacy of CAR-T cells,
multitargeting CAR-T cells were also tested in preclinical GBM
models. Novel CAR-T cells were developed to simultaneously target
IL-13Rα2 and EphA2 or HER2 and IL-13Rα2 or EGFRvIII and IL-13Rα2
(126–128) and have shown additive T cell
activation and antitumor activity. Trivalent CAR-T (IL-13Rα2, HER2
and EphA2) products were also developed. These CAR-T cells
demonstrated a notably improved tumor clearance of autologous
orthotopic glioma [patient-derived xenografts (PDXs)] compared with
univalent and bivalent T-cell products (129). However, to the best of our
knowledge, no first-in-human clinical trials of the latter proposed
innovation have been listed on clinicaltrials.gov at present.
One of the cutting-edge therapeutic approaches to
stimulate the immune system against solid tumors is the use of
T-cell bispecific antibodies (TCB). TCBs are engineered to
incorporate binding sites for CD3ε of the T-cell receptor and a TA.
For instance, EGFRvIII-TCB was developed against EGFRvIII, which is
upregulated solely in GBM and is absent in healthy tissues
(130). Peripheral infusion of
EGFRvIII-TCB induced a strong antitumor activity in orthotopic
humanized and PDX GBM models (130). The favorable preclinical data
supported the use of this molecule in a first-in-human clinical
trial (NCT05187624) in patients with newly diagnosed or recurrent
GBM.
There are also currently ongoing clinical trials
using the immune checkpoint inhibitor, lymphocyte activating 3
(LAG-3) mAb, alone or in combination regimens (NCT02658981 and
NCT03493932). Unfortunately, immunotherapy using ICIs for the
treatment of GBM still has drawbacks, such as high toxicity and
poor efficacy (131).
The shortcomings in clinical outcomes underscore
the imperative to pinpoint targets that can enhance the antitumor
activity of CD8+ T-cells, suggesting that T-cell
dysfunction may impact the GBM microenvironment. Ye et al
(132) reported that the adoptive
transfer of CD8+ T cells with protein disulfide
isomerase family A member 3 (PDIA3), α-1,6-mannosylglycoprotein
6-β-N-acetylglucosaminyltransferase, epithelial membrane
protein 1 or LAG-3 gene editing enhances the survival of
GBM-bearing mice. In summary, it was shown that mutant human
CD8+ T cells PDIA3−/−EGFRvIII
CAR-T cells compared with wt EGFRvIII CAR-T cells enhanced killing
of the GBM cell line, U87-EGFRvIII.
Although myeloid cell infiltration in the
colorectal carcinoma (CRC) TME does not appear to be
immunosuppressive, but instead is associated with a favorable
clinical course of the disease (133,134), the presence of myeloid cells in
the TME of GBM and most epithelial tumors, including renal cell
carcinoma, is immunosuppressive (135,136).
Marked T cell dysfunction in GBM has also been
demonstrated in two studies, which indicates why there is little
antitumor immunity in GBM. Gangoso et al (136) demonstrated that GBM cells develop
myeloid-like transcriptional and epigenetic programs in response to
the immune microenvironment, which serves as a means of immune
evasion. Another notable study by Ravi et al (137), explained the relationship between
the release of the immunosuppressive cytokine, IL-10, from
CD163+HMOX1+ myeloid cells in the GBM
microenvironment and effector T-cell exhaustion and dysfunction.
Ravi et al (137)
discovered that chemically depleted myeloid cells caused T cells to
produce more Granzyme B and less IL-10 and, based on these
findings, the authors treated a patient with recurrent GBM with
ruxolitinib, an inhibitor of the Janus kinase/STAT pathway, in a
neoadjuvant setting in an effort to partially rescue the
immunosuppressive environment. These studies provide insights into
potential future therapeutic strategies that enhance T cell
activation by decreasing immunosuppressive programs, beyond the use
of CAR-T cell technology.
Conclusions
Multiple factors contribute to the poor prognosis
of GBM and its resistance to current treatments, which include: i)
The heterogeneity of GBM, which limits the options and efficacy of
targeted therapies; ii) the pro-tumorigenic role of the TME, which
activates resistance to radiation and chemotherapy in GBM; and iii)
the low immunogenicity of GBM, which impedes a robust immunological
response. In addition, the treatment for GBM incurs significant
costs without providing an effective cure. Consequently, there is a
pressing medical need for more effective treatments. To effectively
address this lethal disease, future treatment approaches are needed
that involve a combination of targeted local and systemic
therapies, rather than relying on a single strategy.
Recurrences of GBM most typically occur within or
near the resection cavity. Focusing therapeutic interventions
directly on the tumor cavity has the potential of enhancing
treatment effectiveness. Although LT for GBM has garnered
attention, numerous innovative strategies are still in development.
A combination of different approaches, such as dual targeting,
should be considered. The exploration of post-operative implants
holds particular appeal when compared with conventional
chemotherapies for four reasons: i) LT allows the start of bridging
therapy between surgery and conventional standard treatments; ii)
LT implants offer the advantage of circumventing the BBB, allowing
consideration of various chemotherapeutic agents and establishing a
reservoir of active molecules in close proximity to the pathology;
iii) the localized administration of active molecules results in
limited systemic toxicity; and iii) given the complexity of GBM and
the potential for multi-targeting, employing ligands specific to
various components of the TME could yield a synergistic effect in
treatments. This innovative approach could revolutionize GBM
treatment, offering new hope to patients and medical professionals
alike.
Before the WHO 2021 classification, all
investigations regarding LT and GBM were partially representative,
adding bias and reducing the generalizability of results in the
context of subsequent molecular discoveries. Prior classifications
did not include extensive molecular and genetic profiling that
currently separates a number of GBM subtypes. This oversight could
mean that the patients or data selected for downstream analysis did
not fairly represent the range of GBM cases, potentially distorting
study findings. To ensure more accurate and inclusive research, it
is beneficial and essential to reinterpret historical data in light
of the new classification and define future prospective studies in
this challenging clinical setting.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
Conceptualization was conducted by TI, TS, FP, MCaf
and GL; methodology was conducted by TI, JB and FV; formal analysis
and data interpretation were conducted by TI, TS, FP, JB, FV, MCac,
EC, CC, GP, GS, MZ, CG, MCaf and GL; literature analysis was
conducted by TI, TS, FP, JB, FV, MCac, EC, CC, GS, MZ, CG, MCaf and
GL; writing of the original draft was conducted by TI, TS, FP, JB,
FV, MCac, EC, CC, GP, GS, MZ, CG, MCaf and GL; reviewing and
editing of the manuscript was conducted by TI, TS, FP, CC, MZ, MCaf
and GL; visualization was conducted by TI, TS, FP, JB, FV, MCac,
EC, CC, GP, GS, MZ, CG, MCaf and GL; supervision was conducted by
TI, TS, FP, CC, MZ, MCaf and GL; project administration was
conducted by TI, GP and CC. All authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weller M, van den Bent M, Preusser M, Le
Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven
L, et al: EANO guidelines on the diagnosis and treatment of diffuse
gliomas of adulthood. Nat Rev Clin Oncol. 18:170–186. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ius T, Sabatino G, Panciani PP, Fontanella
MM, Rudà R, Castellano A, Barbagallo GMV, Belotti F, Boccaletti R,
Catapano G, et al: Surgical management of Glioma Grade 4: technical
update from the neuro-oncology section of the Italian Society of
Neurosurgery (SINch®): A systematic review. J
Neurooncol. 162:267–293. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu P, Du XL, Lu G and Zhu JJ: Survival
benefit of glioblastoma patients after FDA approval of temozolomide
concomitant with radiation and bevacizumab: A population-based
study. Oncotarget. 8:44015–44031. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pombo Antunes AR, Scheyltjens I, Duerinck
J, Neyns B, Movahedi K and Van Ginderachter JA: Understanding the
glioblastoma immune microenvironment as basis for the development
of new immunotherapeutic strategies. Elife. 9:e521762020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Menna G, Manini I, Cesselli D, Skrap M,
Olivi A, Ius T and Della Pepa GM: Immunoregulatory effects of
glioma-associated stem cells on the glioblastoma peritumoral
microenvironment: A differential PD-L1 expression from core to
periphery? Neurosurg Focus. 52:E42022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agosti E, Panciani PP, Zeppieri M, De
Maria L, Pasqualetti F, Tel A, Zanin L, Fontanella MM and Ius T:
Tumor microenvironment and glioblastoma cell interplay as promoters
of therapeutic resistance. Biology (Basel). 12:7362023.PubMed/NCBI
|
|
8
|
Brandes AA, Tosoni A, Franceschi E, Sotti
G, Frezza G, Amistà P, Morandi L, Spagnolli F and Ermani M:
Recurrence pattern after temozolomide concomitant with and adjuvant
to radiotherapy in newly diagnosed patients with glioblastoma:
Correlation With MGMT promoter methylation status. J Clin Oncol.
27:1275–1279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Solinge TS, Nieland L, Chiocca EA and
Broekman MLD: Advances in local therapy for glioblastoma-taking the
fight to the tumour. Nat Rev Neurol. 18:221–236. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Young JS, Morshed RA, Hervey-Jumper SL and
Berger MS: The surgical management of diffuse gliomas: Current
state of neurosurgical management and future directions. Neuro
Oncol. 25:2117–2133. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reulen HJ, Suero Molina E, Zeidler R,
Gildehaus FJ, Böning G, Gosewisch A and Stummer W: Intracavitary
radioimmunotherapy of high-grade gliomas: Present status and future
developments. Acta Neurochir (Wien). 161:1109–1124. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO Classification of Tumors of the
Central Nervous System: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mohammadi AM, Hawasli AH, Rodriguez A,
Schroeder JL, Laxton AW, Elson P, Tatter SB, Barnett GH and
Leuthardt EC: The role of laser interstitial thermal therapy in
enhancing progression-free survival of difficult-to-access
high-grade gliomas: A multicenter study. Cancer Med. 3:971–979.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomas JG, Rao G, Kew Y and Prabhu SS:
Laser interstitial thermal therapy for newly diagnosed and
recurrent glioblastoma. Neurosurg Focus. 41:E122016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beaumont TL, Mohammadi AM, Kim AH, Barnett
GH and Leuthardt EC: magnetic resonance imaging-guided laser
interstitial thermal therapy for glioblastoma of the corpus
callosum. Neurosurgery. 83:556–565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamath AA, Friedman DD, Akbari SHA, Kim
AH, Tao Y, Luo J and Leuthardt EC: Glioblastoma treated with
magnetic resonance imaging-guided laser interstitial thermal
therapy: Safety, efficacy, and outcomes. Neurosurgery. 84:836–843.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Connor KP, Palejwala AH, Milton CK, Lu
VM, Glenn CA, Sughrue ME and Conner AK: Laser interstitial thermal
therapy case series: Choosing the correct number of fibers
depending on lesion size. Oper Neurosurg (Hagerstown). 20:18–23.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Traylor JI, Patel R, Muir M, de Almeida
Bastos DC, Ravikumar V, Kamiya-Matsuoka C, Rao G, Thomas JG, Kew Y
and Prabhu SS: Laser interstitial thermal therapy for glioblastoma:
A single-center experience. World Neurosurg. 149:e244–e252. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Groot JF, Kim AH, Prabhu S, Rao G,
Laxton AW, Fecci PE, O'Brien BJ, Sloan A, Chiang V, Tatter SB, et
al: Efficacy of laser interstitial thermal therapy (LITT) for newly
diagnosed and recurrent IDH wild-type glioblastoma. Neurooncol Adv.
4:vdac0402022.PubMed/NCBI
|
|
22
|
Johnson GW, Han RH, Smyth MD, Leuthardt EC
and Kim AH: Laser interstitial thermal therapy in grade 2/3 IDH1/2
Mutant Gliomas: A preliminary report and literature review. Curr
Oncol. 29:2550–2563. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muir M, Patel R, Traylor JI, de Almeida
Bastos DC, Kamiya C, Li J, Rao G and Prabhu SS: Laser interstitial
thermal therapy for newly diagnosed glioblastoma. Lasers Med Sci.
37:1811–1820. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaisman-Elbaz T, Xiao T, Grabowski MM,
Barnett GH and Mohammadi AM: The impact of extent of ablation on
survival of patients with newly diagnosed glioblastoma treated with
laser interstitial thermal therapy: A large single-institutional
cohort. Neurosurgery. 93:427–435. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jubran JH, Scherschinski L, Dholaria N,
Shaftel KA, Farhadi DS, Oladokun FC, Hendricks BK and Smith KA:
Magnetic resonance-guided laser interstitial thermal therapy for
recurrent glioblastoma and radiation necrosis: A single-surgeon
case series. World Neurosurg. 182:e453–e462. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guthkelch AN, Carter LP, Cassady JR,
Hynynen KH, Iacono RP, Johnson PC, Obbens EA, Roemer RB, Seeger JF,
Shimm DS, et al: Treatment of malignant brain tumors with focused
ultrasound hyperthermia and radiation: Results of a phase I trial.
J Neurooncol. 10:271–284. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carpentier A, Canney M, Vignot A, Reina V,
Beccaria K, Horodyckid C, Karachi C, Leclercq D, Lafon C, Chapelon
JY, et al: Clinical trial of blood-brain barrier disruption by
pulsed ultrasound. Sci Transl Med. 8:343re22016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stea B, Rossman K, Kittelson J, Shetter A,
Hamilton A and Cassady JR: Interstitial irradiation versus
interstitial thermos radiotherapy for supratentorial malignant
gliomas: A comparative survival analysis. Int J Radiat Oncol Biol
Phys. 30:591–600. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maier-Hauff K, Ulrich F, Nestler D,
Niehoff H, Wust P, Thiesen B, Orawa H, Budach V and Jordan A:
Efficacy and safety of intratumoral thermotherapy using magnetic
iron-oxide nanoparticles combined with external beam radiotherapy
on patients with recurrent glioblastoma multiforme. J Neurooncol.
103:317–324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stupp R, Wong ET, Kanner AA, Steinberg D,
Engelhard H, Heidecke V, Kirson ED, Taillibert S, Liebermann F,
Dbalý V, et al: NovoTTF-100A versus physician's choice chemotherapy
in recurrent glioblastoma: A randomised phase III trial of a novel
treatment modality. Eur J Cancer. 48:2192–2202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stupp R, Taillibert S, Kanner AA, Kesari
S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink
KL, et al: Maintenance therapy with tumor-treating fields plus
temozolomide vs temozolomide alone for glioblastoma: A Randomized
clinical trial. JAMA. 314:2535–2543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vinjamuri M, Adumala RR, Altaha R, Hobbs
GR and Crowell EB Jr: Comparative analysis of temozolomide (TMZ)
versus 1,3-bis (2-chloroethyl)-1 nitrosourea (BCNU) in newly
diagnosed glioblastoma multiforme (GBM) patients. J Neurooncol.
91:221–225. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Bonis P, Anile C, Pompucci A,
Fiorentino A, Balducci M, Chiesa S, Maira G and Mangiola A: Safety
and efficacy of Gliadel wafers for newly diagnosed and recurrent
glioblastoma. Acta Neurochir (Wien). 154:1371–1378. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Catalán-Uribarrena G, Bilbao-Barandica G,
Pomposo-Gaztelu I, Undabeitia-Huertas J, Ruiz de Gopegui-Ruiz E,
Galbarriatu-Gutiérrez L, Canales-Llantada M, Aurrecoechea-Obieta J,
Igartua-Azkune A and Carbayo-Lozano G: Prognostic factors and
survival in a prospective cohort of patients with high-grade glioma
treated with carmustine wafers or temozolomide on an
intention-to-treat basis. Acta Neurochir (Wien). 154:211–222;
discussion 222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Noël G, Schott R, Froelich S, Gaub MP,
Boyer P, Fischer-Lokou D, Dufour P, Kehrli P and Maitrot D:
Retrospective comparison of chemoradiotherapy followed by adjuvant
chemotherapy, with or without prior gliadel implantation
(carmustine) after initial surgery in patients with newly diagnosed
high-grade gliomas. Int J Radiat Oncol Biol Phys. 82:749–755. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pallud J, Audureau E, Noel G, Corns R,
Lechapt-Zalcman E, Duntze J, Pavlov V, Guyotat J, Hieu PD, Le Reste
PJ, et al: Long-term results of carmustine wafer implantation for
newly diagnosed glioblastomas: A controlled propensity-matched
analysis of a French multicenter cohort. Neuro Oncol. 17:1609–1619.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roux A, Peeters S, Zanello M, Bou Nassif
R, Abi Lahoud G, Dezamis E, Parraga E, Lechapt-Zalcmann E, Dhermain
F, Dumont S, et al: Extent of resection and Carmustine wafer
implantation safely improve survival in patients with a newly
diagnosed glioblastoma: A single center experience of the current
practice. J Neurooncol. 135:83–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Akiyama Y, Kimura Y, Enatsu R, Mikami T,
Wanibuchi M and Mikuni N: Advantages and disadvantages of combined
chemotherapy with carmustine wafer and bevacizumab in patients with
newly diagnosed glioblastoma: A single-institutional experience.
World Neurosurg. 113:e508–e514. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bos EM, Binda E, Verploegh ISC, Wembacher
E, Hoefnagel D, Balvers RK, Korporaal AL, Conidi A, Warnert EAH,
Trivieri N, et al: Local delivery of hrBMP4 as an anticancer
therapy in patients with recurrent glioblastoma: A first-in-human
phase 1 dose escalation trial. Mol Cancer. 22:1292023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chan TA, Weingart JD, Parisi M, Hughes MA,
Olivi A, Borzillary S, Alahakone D, Detorie NA, Wharam MD and
Kleinberg L: Treatment of recurrent glioblastoma multiforme with
GliaSite brachytherapy. Int J Radiat Oncol Biol Phys. 62:1133–1139.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schueller P, Micke O, Palkovic S,
Schroeder J, Moustakis C, Bruns F, Schuck A, Wassmann H and Willich
N: 12 years' experience with intraoperative radiotherapy (IORT) of
malignant gliomas. Strahlenther Onkol. 181:500–506. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gabayan AJ, Green SB, Sanan A, Jenrette J,
Schultz C, Papagikos M, Tatter SP, Patel A, Amin P, Lustig R, et
al: GliaSite brachytherapy for treatment of recurrent malignant
gliomas: A retrospective multi-institutional analysis.
Neurosurgery. 58:701–9; discussion 701–709. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen AM, Chang S, Pouliot J, Sneed PK,
Prados MD, Lamborn KR, Malec MK, McDermott MW, Berger MS and Larson
DA: Phase I trial of gross total resection, permanent iodine-125
brachytherapy, and hyperfractionated radiotherapy for newly
diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys.
69:825–830. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Welsh J, Sanan A, Gabayan AJ, Green SB,
Lustig R, Burri S, Kwong E and Stea B: GliaSite brachytherapy boost
as part of initial treatment of glioblastoma multiforme: A
retrospective multi-institutional pilot study. Int J Radiat Oncol
Biol Phys. 68:159–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chino K, Silvain D, Grace A, Stubbs J and
Stea B: Feasibility and safety of outpatient brachytherapy in 37
patients with brain tumors using the GliaSite Radiation Therapy
System. Med Phys. 35:3383–3388. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fabrini MG, Perrone F, De Franco L,
Pasqualetti F, Grespi S, Vannozzi R and Cionini L: Perioperative
high-dose-rate brachytherapy in the treatment of recurrent
malignant gliomas. Strahlenther Onkol. 185:524–529. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Usychkin S, Calvo F, dos Santos MA,
Samblás J, de Urbina DO, Bustos JC, Diaz JA, Sallabanda K, Sanz A,
Yélamos C, et al: Intra-operative electron beam radiotherapy for
newly diagnosed and recurrent malignant gliomas: Feasibility and
long-term outcomes. Clin Transl Oncol. 15:33–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schwartz C, Romagna A, Thon N, Niyazi M,
Watson J, Belka C, Tonn JC, Kreth FW and Nachbichler SB: Outcome
and toxicity profile of salvage low-dose-rate iodine-125
stereotactic brachytherapy in recurrent high-grade gliomas. Acta
Neurochir (Wien). 157:1757–1764; discussion 1764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sarria GR, Sperk E, Han X, Sarria GJ, Wenz
F, Brehmer S, Fu B, Min S, Zhang H, Qin S, et al: Intraoperative
radiotherapy for glioblastoma: An international pooled analysis.
Radiother Oncol. 142:162–167. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Keu KV, Witney TH, Yaghoubi S, Rosenberg
J, Kurien A, Magnusson R, Williams J, Habte F, Wagner JR, Forman S,
et al: Reporter gene imaging of targeted T cell immunotherapy in
recurrent glioma. Sci Transl Med. 9:eaag21962017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Brown CE, Alizadeh D, Starr R, Weng L,
Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J,
Simpson J, et al: Regression of glioblastoma after chimeric antigen
receptor T-cell therapy. N Engl J Med. 375:2561–2569. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nassiri F, Patil V, Yefet LS, Singh O, Liu
J, Dang RMA, Yamaguchi TN, Daras M, Cloughesy TF, Colman H, et al:
Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent
glioblastoma: A phase 1/2 trial. Nat Med. 29:1370–1378. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cloughesy TF, Brenner A, de Groot JF,
Butowski NA, Zach L, Campian JL, Ellingson BM, Freedman LS, Cohen
YC, Lowenton-Spier N, et al: A randomized controlled phase III
study of VB-111 combined with bevacizumab vs bevacizumab
monotherapy in patients with recurrent glioblastoma [GLOBE]. Neuro
Oncol. 22:705–717. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Westphal M, Ylä-Herttuala S, Martin J,
Warnke P, Menei P, Eckland D, Kinley J, Kay R and Ram Z; ASPECT
Study Group, : Adenovirus-mediated gene therapy with sitimagene
ceradenovec followed by intravenous ganciclovir for patients with
operable high-grade glioma [ASPECT]: A randomised, open-label,
phase 3 trial. Lancet Oncol. 14:823–833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Desjardins A, Gromeier M, Herndon JE II,
Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F,
Muscat AM, Nair S, et al: Recurrent glioblastoma treated with
recombinant poliovirus. N Engl J Med. 379:150–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Caffo M, Caruso G, Curcio A, Laera R,
Crisafulli C, Passalacqua M and Germanò A: The Role of
Nanotechnology in Brain Tumors. Human Brain and Spinal Cord Tumors:
From Bench to Bedside. Vol 1. Springer International Publishing;
pp. 193–207. 2022
|
|
57
|
Langen UH, Ayloo S and Gu C: Development
and cell biology of the blood-brain barrier. Annu Rev Cell Dev
Biol. 35:591–613. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Patel B, Yang PH and Kim AH: The effect of
thermal therapy on the blood-brain barrier and blood-tumor barrier.
Int J Hyperthermia. 37:35–43. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Holste KG and Orringer DA: Laser
interstitial thermal therapy. Neurooncol Adv.
2:vdz0352019.PubMed/NCBI
|
|
60
|
Mohammadi AM, Sharma M, Beaumont TL,
Juarez KO, Kemeny H, Dechant C, Seas A, Sarmey N, Lee BS, Jia X, et
al: Upfront magnetic resonance imaging-guided stereotactic
laser-ablation in newly diagnosed glioblastoma: A multicenter
review of survival outcomes compared to a matched cohort of
biopsy-only patients. Neurosurgery. 85:762–772. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fadel HA, Haider S, Pawloski JA, Zakaria
HM, Macki M, Bartlett S, Schultz L, Robin AM, Kalkanis SN and Lee
IY: Laser Interstitial thermal therapy for first-line treatment of
surgically accessible recurrent glioblastoma: Outcomes compared
with a surgical cohort. Neurosurgery. 91:701–709. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Quadri SA, Waqas M, Khan I, Khan MA,
Suriya SS, Farooqui M and Fiani B: High-intensity focused
ultrasound: past, present, and future in neurosurgery. Neurosurg
Focus. 44:E162018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mauri G, Nicosia L, Xu Z, Di Pietro S,
Monfardini L, Bonomo G, Varano GM, Prada F, Della Vigna P and Orsi
F: Focused ultrasound: Tumour ablation and its potential to enhance
immunological therapy to cancer. Br J Radiol. 91:201706412018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hu S, Zhang X, Unger M, Patties I, Melzer
A and Landgraf L: Focused ultrasound-induced cavitation sensitizes
cancer cells to radiation therapy and hyperthermia. Cells.
9:25952020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hersh AM, Bhimreddy M, Weber-Levine C,
Jiang K, Alomari S, Theodore N, Manbachi A and Tyler BM:
Applications of focused ultrasound for the treatment of
glioblastoma: A new frontier. Cancers (Basel). 14:49202022.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Elhelf IAS, Albahar H, Shah U, Oto A,
Cressman E and Almekkawy M: High intensity focused ultrasound: The
fundamentals, clinical applications and research trends. Diagn
Interv Imaging. 99:349–359. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fomenko A and Lozano AM: Neuromodulation
and ablation with focused ultrasound-toward the future of
noninvasive brain therapy. Neural Regen Res. 14:1509–1510. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cohen-Inbar O, Xu Z and Sheehan JP:
Focused ultrasound-aided immunomodulation in glioblastoma
multiforme: A therapeutic concept. J Ther Ultrasound. 4:22016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cazares-Cortes E, Cabana S, Boitard C,
Nehlig E, Griffete N, Fresnais J, Wilhelm C, Abou-Hassan A and
Ménager C: Recent insights in magnetic hyperthermia: From the
‘hot-spot’ effect for local delivery to combined
magneto-photo-thermia using magneto-plasmonic hybrids. Adv Drug
Deliv Rev. 138:233–246. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Grauer O, Jaber M, Hess K, Weckesser M,
Schwindt W, Maring S, Wölfer J and Stummer W: Combined
intracavitary thermotherapy with iron oxide nanoparticles and
radiotherapy as local treatment modality in recurrent glioblastoma
patients. J Neurooncol. 141:83–94. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Toraya-Brown S, Sheen MR, Zhang P, Chen L,
Baird JR, Demidenko E, Turk MJ, Hoopes PJ, Conejo-Garcia JR and
Fiering S: Local hyperthermia treatment of tumors induces CD8(+) T
cell-mediated resistance against distal and secondary tumors.
Nanomedicine. 10:1273–1285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ito A, Tanaka K, Kondo K, Shinkai M, Honda
H, Matsumoto K, Saida T and Kobayashi T: Tumor regression by
combined immunotherapy and hyperthermia using magnetic
nanoparticles in an experimental subcutaneous murine melanoma.
Cancer Sci. 94:308–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gilchrist RK, Medal R, Shorey WD,
Hanselman RC, Parrott JC and Taylor CB: Selective inductive heating
of lymph nodes. Ann Surg. 146:596–606. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Stea B, Cetas TC, Cassady JR, Guthkelch
AN, Iacono R, Lulu B, Lutz W, Obbens E, Rossman K, Seeger J, et al:
Interstitial thermoradiotherapy of brain tumors: Preliminary
results of a phase I clinical trial. Int J Radiat Oncol Biol Phys.
19:1463–1471. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fabian D, Guillermo Prieto Eibl MDP,
Alnahhas I, Sebastian N, Giglio P, Puduvalli V, Gonzalez J and
Palmer JD: Treatment of Glioblastoma (GBM) with the Addition of
Tumor-Treating Fields (TTF): A Review. Cancers (Basel). 11:1742019.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shah PP, White T, Khalafallah AM, Romo CG,
Price C and Mukherjee D: A systematic review of tumor treating
fields therapy for high-grade gliomas. J Neurooncol. 148:433–443.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ballo MT, Conlon P, Lavy-Shahaf G, Kinzel
A, Vymazal J and Rulseh AM: Association of Tumor Treating Fields
(TTFields) therapy with survival in newly diagnosed glioblastoma: A
systematic review and meta-analysis. J Neurooncol. 164:1–9. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Joo H, Lee Y, Kim J, Yoo JS, Yoo S, Kim S,
Arya AK, Kim S, Choi SH, Lu N, et al: Soft implantable drug
delivery device integrated wirelessly with wearable devices to
treat fatal seizures. Sci Adv. 7:eabd46392021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bobo RH, Laske DW, Akbasak A, Morrison PF,
Dedrick RL and Oldfield EH: Convection-enhanced delivery of
macromolecules in the brain. Proc Natl Acad Sci USA. 91:2076–2080.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cha GD, Kang T, Baik S, Kim D, Choi SH,
Hyeon T and Kim DH: Advances in drug delivery technology for the
treatment of glioblastoma multiforme. J Control Release.
328:350–367. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Haar PJ, Chen ZJ, Fatouros PP, Gillies GT,
Corwin FD and Broaddus WC: Modelling convection-enhanced delivery
in normal and oedematous brain. J Med Eng Technol. 38:76–84. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
White E, Bienemann A, Malone J, Megraw L,
Bunnun C, Wyatt M and Gill S: An evaluation of the relationships
between catheter design and tissue mechanics in achieving high-flow
convection-enhanced delivery. J Neurosci Methods. 199:87–97. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chew SA and Danti S: Biomaterial-Based
implantable devices for cancer therapy. Adv Healthc Mater.
6:16007662017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bow H, Hwang LS, Schildhaus N, Xing J,
Murray L, Salditch Q, Ye X, Zhang Y, Weingart J, Brem H and Tyler
B: Local delivery of angiogenesis-inhibitor minocycline combined
with radiotherapy and oral temozolomide chemotherapy in 9L glioma.
J Neurosurg. 120:662–669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bersini S, Jeon JS, Dubini G, Arrigoni C,
Chung S, Charest JL, Moretti M and Kamm RD: A microfluidic 3D in
vitro model for specificity of breast cancer metastasis to bone.
Biomaterials. 35:2454–2461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liao J, Guo X, Grande-Allen KJ, Kasper FK
and Mikos AG: Bioactive polymer/extracellular matrix scaffolds
fabricated with a flow perfusion bioreactor for cartilage tissue
engineering. Biomaterials. 31:8911–8920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yang Y, Hu X, Liu Y, Ouyang B, Zhang J,
Jin H, Yu Z, Liu R, Li Z, Jiang L, et al: An implantable
ultrasound-powered device for the treatment of brain cancer using
electromagnetic fields. Sci Adv. 8:eabm50232022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Xie J and Wang CH: Electrospun micro- and
nanofibers for sustained delivery of paclitaxel to treat C6 glioma
in vitro. Pharm Res. 23:1817–1826. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Benoit MA, Ribet C, Distexhe J, Hermand D,
Letesson JJ, Vandenhaute J and Gillard J: Studies on the potential
of microparticles entrapping pDNA-poly(aminoacids) complexes as
vaccine delivery systems. J Drug Target. 9:253–266. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ius T, Cesselli D, Isola M, Toniato G,
Pauletto G, Sciacca G, Fabbro S, Pegolo E, Rizzato S, Beltrami AP,
et al: Combining clinical and molecular data to predict the
benefits of carmustine wafers in newly diagnosed high-grade
gliomas. Curr Treat Options Neurol. 20:32018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ricciardi L, Manini I, Cesselli D, Trungu
S, Piazza A, Mangraviti A, Miscusi M, Raco A and Ius T: Carmustine
wafers implantation in patients with newly diagnosed high grade
glioma: Is It still an option? Front Neurol. 13:8841582022.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wen PY, Weller M, Lee EQ, Alexander BM,
Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM,
Chiocca EA, et al: Glioblastoma in adults: A Society for
Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO)
consensus review on current management and future directions. Neuro
Oncol. 22:1073–1113. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Iuchi T, Inoue A, Hirose Y, Morioka M,
Horiguchi K, Natsume A, Arakawa Y, Iwasaki K, Fujiki M, Kumabe T
and Sakata Y: Long-term effectiveness of Gliadel implant for
malignant glioma and prognostic factors for survival: 3-year
results of a postmarketing surveillance in Japan. Neurooncol Adv.
4:vdab1892022.PubMed/NCBI
|
|
94
|
Champeaux C and Weller J: Implantation of
carmustine wafers (Gliadel®) for high-grade glioma
treatment. A 9-year nationwide retrospective study. J Neurooncol.
147:159–169. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wu Q and Yao J: BMP4, a new prognostic
factor for glioma. World J Surg Oncol. 11:2642013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yang DY, Bu XY, Zhou ZL, Yan ZY, Ma CX, Qu
MQ, Zhao YW, Kong LF, Wang YW and Luo JC: Enhanced antitumor
effects of radiotherapy combined local nimustine delivery
rendezvousing with oral temozolomide chemotherapy in glioblastoma
patients. J Cancer Res Ther. 14:78–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Niyazi M, Andratschke N, Bendszus M,
Chalmers AJ, Erridge SC, Galldiks N, Lagerwaard FJ, Navarria P,
Munck Af Rosenschöld P, Ricardi U, et al: ESTRO-EANO guideline on
target delineation and radiotherapy details for glioblastoma.
Radiother Oncol. 184:1096632023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Tu Z, Xiong H, Qiu Y, Li G, Wang L and
Peng S: Limited recurrence distance of glioblastoma under modern
radiotherapy era. BMC Cancer. 21:7202021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Singh R, Lehrer EJ, Wang M, Perlow HK,
Zaorsky NG, Trifiletti DM, Bovi J, Navarria P, Scoccianti S, Gondi
V, et al: Dose Escalated Radiation Therapy for Glioblastoma
Multiforme: An International Systematic Review and Meta-Analysis of
22 Prospective Trials. Int J Radiat Oncol Biol Phys. 111:371–384.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Barbarite E, Sick JT, Berchmans E, Bregy
A, Shah AH, Elsayyad N and Komotar RJ: The role of brachytherapy in
the treatment of glioblastoma multiforme. Neurosurg Rev.
40:195–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kickingereder P, Hamisch C, Suchorska B,
Galldiks N, Visser-Vandewalle V, Goldbrunner R, Kocher M, Treuer H,
Voges J and Ruge MI: Low-dose rate stereotactic iodine-125
brachytherapy for the treatment of inoperable primary and recurrent
glioblastoma: Single-center experience with 201 cases. J
Neurooncol. 120:615–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Pasqualetti F, Barberis A, Zanotti S,
Montemurro N, De Salvo GL, Soffietti R, Mazzanti CM, Ius T, Caffo
M, Paiar F, et al: The impact of survivorship bias in glioblastoma
research. Crit Rev Oncol Hematol. 188:1040652023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Pasqualetti F, Montemurro N, Desideri I,
Loi M, Giannini N, Gadducci G, Malfatti G, Cantarella M, Gonnelli
A, Montrone S, et al: Impact of recurrence pattern in patients
undergoing a second surgery for recurrent glioblastoma. Acta Neurol
Belg. 122:441–446. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Laperriere NJ, Leung PM, McKenzie S,
Milosevic M, Wong S, Glen J, Pintilie M and Bernstein M: Randomized
study of brachytherapy in the initial management of patients with
malignant astrocytoma. Int J Radiat Oncol Biol Phys. 41:1005–1011.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Odia Y, Gutierrez AN and Kotecha R:
Surgically targeted radiation therapy (STaRT) trials for brain
neoplasms: A comprehensive review. Neuro Oncol. 24 (Suppl
6):S16–S24. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gessler DJ, Neil EC, Shah R, Levine J,
Shanks J, Wilke C, Reynolds M, Zhang S, Özütemiz C, Gencturk M, et
al: GammaTile® brachytherapy in the treatment of
recurrent glioblastomas. Neurooncol Adv. 4:vdab1852021.PubMed/NCBI
|
|
107
|
Cristescu R, Mogg R, Ayers M, Albright A,
Murphy E, Yearley J, Sher X, Liu XQ, Lu H, Nebozhyn M, et al:
Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based
immunotherapy. Science. 362:eaar35932018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Thorsson V, Gibbs DL, Brown SD, Wolf D,
Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy
JA, et al: The Immune Landscape of Cancer. Immunity.
48:812–830.e14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Alayo QA, Ito H, Passaro C, Zdioruk M,
Mahmoud AB, Grauwet K, Zhang X, Lawler SE, Reardon DA, Goins WF, et
al: Glioblastoma infiltration of both tumor- and virus-antigen
specific cytotoxic T cells correlates with experimental virotherapy
responses. Sci Rep. 10:50952020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Brenner AJ, Peters KB, Vredenburgh J,
Bokstein F, Blumenthal DT, Yust-Katz S, Peretz I, Oberman B,
Freedman LS, Ellingson BM, et al: Safety and efficacy of VB-111, an
anticancer gene therapy, in patients with recurrent glioblastoma
Results of a phase I/II study. Neuro Oncol. 22:694–704. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Agosti E, Zeppieri M, De Maria L, Tedeschi
C, Fontanella MM, Panciani PP and Ius T: Glioblastoma
Immunotherapy: A Systematic Review of the Present Strategies and
Prospects for Advancements. Int J Mol Sci. 24:150372023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Luksik AS, Yazigi E, Shah P and Jackson
CM: CAR T Cell Therapy in Glioblastoma: Overcoming Challenges
Related to Antigen Expression. Cancers (Basel). 15:14142023.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Vitanza NA, Wilson AL, Huang W, Seidel K,
Brown C, Gustafson JA, Yokoyama JK, Johnson AJ, Baxter BA, Koning
RW, et al: Intraventricular B7-H3 CAR T Cells for Diffuse Intrinsic
Pontine Glioma: Preliminary First-in-Human Bioactivity and Safety.
Cancer Discov. 13:114–131. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Omuro A, Brandes AA, Carpentier AF, Idbaih
A, Reardon DA, Cloughesy T, Sumrall A, Baehring J, van den Bent M,
Bähr O, et al: Radiotherapy combined with nivolumab or temozolomide
for newly diagnosed Glioblastoma with unmethylated MGMT promoter:
An international randomized phase III trial. Neuro Oncol.
25:123–134. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Reardon DA, Brandes AA, Omuro A,
Mulholland P, Lim M, Wick A, Baehring J, Ahluwalia MS, Roth P, Bähr
O, et al: Effect of nivolumab vs bevacizumab in patients with
recurrent glioblastoma: The checkmate 143 phase 3 Randomized
clinical trial. JAMA Oncol. 6:1003–1010. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Schalper KA, Rodriguez-Ruiz ME, Diez-Valle
R, López-Janeiro A, Porciuncula A, Idoate MA, Inogés S, de Andrea
C, López-Diaz de Cerio A, Tejada S, et al: Neoadjuvant nivolumab
modifies the tumor immune microenvironment in resectable
glioblastoma. Nat Med. 25:470–476. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Liu X, Chen X, Shi L, Shan Q, Cao Q, Yue
C, Li H, Li S, Wang J, Gao S, et al: The third-generation EGFR
inhibitor AZD9291 overcomes primary resistance by continuously
blocking ERK signaling in glioblastoma. J Exp Clin Cancer Res.
38:2192019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Rong L, Li N and Zhang Z: Emerging
therapies for glioblastoma: Current state and future directions. J
Exp Clin Cancer Res. 41:1422022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Marei HE, Althani A, Caceci T, Arriga R,
Sconocchia T, Ottaviani A, Lanzilli G, Roselli M, Caratelli S,
Cenciarelli C and Sconocchia G: Recent perspective on CAR and
Fcγ-CR T cell immunotherapy for cancers: Preclinical evidence
versus clinical outcomes. Biochem Pharmacol. 166:335–346. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Karachi A, Dastmalchi F, Nazarian S, Huang
J, Sayour EJ, Jin L, Yang C, Mitchell DA and Rahman M: Optimizing T
cell-based therapy for glioblastoma. Front Immunol. 12:7055802021.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
O'Rourke DM, Nasrallah MP, Desai A,
Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem
S, Maloney E, Shen A, et al: A single dose of peripherally infused
EGFRvIII-directed CAR T cells mediates antigen loss and induces
adaptive resistance in patients with recurrent glioblastoma. Sci
Transl Med. 9:eaaa09842017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ahmed N, Brawley V, Hegde M, Bielamowicz
K, Kalra M, Landi D, Robertson C, Gray TL, Diouf O, Wakefield A, et
al: HER2-Specific chimeric antigen receptor-modified virus-specific
T cells for progressive glioblastoma: A phase 1 dose-escalation
trial. JAMA Oncol. 3:1094–1101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Marei HE, Althani A, Afifi N, Hasan A,
Caceci T, Pozzoli G and Cenciarelli C: Current progress in chimeric
antigen receptor T cell therapy for glioblastoma multiforme. Cancer
Med. 10:5019–5030. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Suryadevara CM, Desai R, Abel ML, Riccione
KA, Batich KA, Shen SH, Chongsathidkiet P, Gedeon PC, Elsamadicy
AA, Snyder DJ, et al: Temozolomide lymphodepletion enhances CAR
abundance and correlates with antitumor efficacy against
established glioblastoma. Oncoimmunology. 7:e14344642018.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Choi BD, Yu X, Castano AP, Bouffard AA,
Schmidts A, Larson RC, Bailey SR, Boroughs AC, Frigault MJ, Leick
MB, et al: CAR-T cells secreting BiTEs circumvent antigen escape
without detectable toxicity. Nat Biotechnol. 37:1049–1058. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Muhammad N, Wang R, Li W, Zhang Z, Chang
Y, Hu Y, Zhao J, Zheng X, Mao Q and Xia H: A novel TanCAR targeting
IL13Rα2 and EphA2 for enhanced glioblastoma therapy. Mol Ther
Oncolytics. 24:729–741. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hegde M, Mukherjee M, Grada Z, Pignata A,
Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KK,
et al: Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate
tumor antigen escape. J Clin Invest. 131:e1524772021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Schmidts A, Srivastava AA, Ramapriyan R,
Bailey SR, Bouffard AA, Cahill DP, Carter BS, Curry WT, Dunn GP,
Frigault MJ, et al: Tandem chimeric antigen receptor (CAR) T cells
targeting EGFRvIII and IL-13Rα2 are effective against heterogeneous
glioblastoma. Neurooncol Adv. 5:vdac1852022.PubMed/NCBI
|
|
129
|
Bielamowicz K, Fousek K, Byrd TT, Samaha
H, Mukherjee M, Aware N, Wu MF, Orange JS, Sumazin P, Man TK, et
al: Trivalent CAR T cells overcome interpatient antigenic
variability in glioblastoma. Neuro Oncol. 20:506–518. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Iurlaro R, Waldhauer I, Planas-Rigol E,
Bonfill-Teixidor E, Arias A, Nicolini V, Freimoser-Grundschober A,
Cuartas I, Martínez-Moreno A, Martínez-Ricarte F, et al: A Novel
EGFRvIII T-Cell bispecific antibody for the treatment of
glioblastoma. Mol Cancer Ther. 21:1499–1509. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Marei HE, Hasan A, Pozzoli G and
Cenciarelli C: Cancer immunotherapy with immune checkpoint
inhibitors (ICIs): Potential, mechanisms of resistance, and
strategies for reinvigorating T cell responsiveness when resistance
is acquired. Cancer Cell Int. 23:642023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ye L, Park JJ, Dong MB, Yang Q, Chow RD,
Peng L, Du Y, Guo J, Dai X, Wang G, et al: In vivo CRISPR screening
in CD8 T cells with AAV-Sleeping Beauty hybrid vectors identifies
membrane targets for improving immunotherapy for glioblastoma. Nat
Biotechnol. 37:1302–1313. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Sconocchia G, Zlobec I, Lugli A, Calabrese
D, Iezzi G, Karamitopoulou E, Patsouris ES, Peros G, Horcic M,
Tornillo L, et al: Tumor infiltration by FcγRIII (CD16)+ myeloid
cells is associated with improved survival in patients with
colorectal carcinoma. Int J Cancer. 128:2663–2672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Sconocchia G, Eppenberger-Castori S,
Zlobec I, Karamitopoulou E, Arriga R, Coppola A, Caratelli S,
Spagnoli GC, Lauro D, Lugli A, et al: HLA class II antigen
expression in colorectal carcinoma tumors as a favorable prognostic
marker. Neoplasia. 16:31–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Sconocchia G, Spagnoli GC, Del Principe D,
Ferrone S, Anselmi M, Wongsena W, Cervelli V, Schultz-Thater E,
Wyler S, Carafa V, et al: Defective infiltration of natural killer
cells in MICA/B-positive renal cell carcinoma involves
beta(2)-integrin-mediated interaction. Neoplasia. 11:662–671. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Gangoso E, Southgate B, Bradley L, Rus S,
Galvez-Cancino F, McGivern N, Güç E, Kapourani CA, Byron A,
Ferguson KM, et al: Glioblastomas acquire myeloid-affiliated
transcriptional programs via epigenetic immunoediting to elicit
immune evasion. Cell. 184:2454–2470.e26. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ravi VM, Neidert N, Will P, Joseph K,
Maier JP, Kückelhaus J, Vollmer L, Goeldner JM, Behringer SP,
Scherer F, et al: T-cell dysfunction in the glioblastoma
microenvironment is mediated by myeloid cells releasing
interleukin-10. Nat Commun. 13:9252022. View Article : Google Scholar : PubMed/NCBI
|