Introduction
Microsatellites are short tandem repeats, formed of
a set of 1–6 nucleotides, which repeat 3–60 times on DNA sequences
(1). DNA slippage, repetitive
elements prone to mutations during DNA replication (2), often occurs at short tandem repeats
during replication, which leads to sequence insertion or deletion;
this phenomenon is termed microsatellite instability (MSI)
(3–5). The human DNA mismatch repair (MMR)
system functions to repair replication errors. Specifically, the
mutS homolog (MSH) 2-MSH6 heterodimer recognizes a DNA mismatch and
the mutL homolog (MLH) 1-postmeiotic segregation increased 2 (PMS2)
heterodimer repairs the DNA mismatch using proficient MMR (pMMR)
systems (6). Deficient MMR (dMMR)
leads to increased tumor mutational burden, with a heightened risk
of neoplasia (7,8). Certain types of cancer are
characterized by MSI-high/hypermutated (MSI-H), including 30% of
primary endometrial cancer (EC) cases and 25% of colorectal cancer
(CRC) cases (9–14).
Treatment options for patients with cancer typically
include surgery, chemotherapy and immunotherapy, and the
appropriate treatment is chosen based on tumor stage and
characteristics (15,16). In the CheckMate 142 study including
patients with metastatic CRC (mCRC) treated with nivolumab and
low-dose ipilimumab, an overall survival rate of 65% and a disease
control rate of 81% at 12 months were observed. In MSI-H or dMMR
advanced EC, the response rates for the PD-1 inhibitors,
dostarlimab and pembrolizumab, were 49 and 57%, respectively, while
the PD-L1 inhibitors, avelumab and durvalumab, demonstrated
response rates of 27 and 43%, respectively (17,18).
In 2017, the U.S. Food and Drug Administration (FDA) approved
pembrolizumab for the treatment of MSI-H or dMMR tumors, regardless
of the location in the body the cancer originated. In July 2018,
the FDA approved the combination of nivolumab and low-dose
ipilimumab in the treatment of patients with MSI-H/dMMR mCRC who
have been previously treated with standard chemotherapy drugs
(19). In March 2022, the FDA
approved pembrolizumab as a single agent for patients with advanced
endometrial carcinoma, that is patients with MSI-H or dMMR who have
disease progression following prior systemic therapy in any setting
and who are not candidates for curative surgery or radiation
(20). Immunotherapy is an
appropriate treatment option for patients with MSI-H/dMMR tumors,
which are characterised by an increasing number of mutations and a
higher number of neoantigens. CD8+ T cells recognize
these neoantigens, resulting in immune cell infiltration into
tumors higher than microsatellite-stable (MSS) or pMMR tumors
(21). However, patients with
MSI-H/dMMR CRC tumors are reported to have a favorable prognosis
and no benefit using 5-fluorouracil (5-FU), a first-line
chemotherapy treatment for CRC (22). In tumor cells with pMMR, the
MSH2-MSH6 heterodimers recognize 5-FU-induced DNA breaks and the
excessive accumulation of DNA breaks induces cell apoptosis
(23,24). In dMMR cells, the MSH2-MSH6
heterodimer loses the ability to recognize DNA errors, which leads
to the evasion of cell apoptosis (25).
Personalized treatments for patients with dMMR and
MSI-H should be considered. Traditionally, surgery remains a
crucial component of treatment, especially for localized disease.
Unresectable locally advanced or mCRC is treated with 5-FU-based
chemotherapy. However, although immunotherapy strategies have been
established, there remains a need to explore the choice between
single-agent or dual checkpoint inhibitor treatment, as well as
primary or secondary resistance.
The European Society for Medical Oncology (ESMO) and
the National Comprehensive Cancer Network's Clinical Practice
Guidelines in Oncology recommended MMR and MSI detection using
immunohistochemistry (IHC) staining or PCR tests, prior to the
treatment of patients with CRC or EC (26,27).
IHC for MMR and MSI testing detects the level of four MMR proteins:
MLH1, MLH2, PMS2 and MSH6, to evaluate the pMMR or dMMR status in
the tumor (28). Although there is
a high degree of concordance between MSI-PCR and MMR-IHC tests,
discordances of 3–5% between the two assays can occur via a number
of mechanisms (29–31). MMR protein dysfunction causes an
increase in missense mutations. However, in certain cases the
epitope of a monoclonal antibody may be maintained, which can lead
to intact MMR protein expression and false negative IHC results.
Consequently, these MSI-PCR assays may yield MSI-H results. Next
generation sequencing (NGS) analysis of an IHC and MSI-PCR
inconclusive case revealed missense and frameshift mutations in the
PMS2 and MSH6 genes, respectively (32–34).
Therefore, the College of American Pathologists Guideline
recommended using both IHC and PCR-based MSI tests for the
detection of MSI status (35). The
Bethesda panel is a set of markers used for MSI testing in patients
with cancer. It was initially recommended by the National Cancer
Institute in the United States. The panel includes two
mononucleotide repeat markers (BAT-25 and BAT-26) and three
dinucleotide repeat markers (D2S123, D5S346 and D17S250) (36). Dinucleotide repeats are reported to
have a lower sensitivity in the detection of MSI status compared
with mononucleotide repeats and therefore short mononucleotide
repeats (SMRs) of 21–27 bp are often used to detect MSI status in
patients with colorectal cancer (37). MSI status is determined using the
number of positive markers: MSI-H refers to detection of ≥30%
unstable microsatellite loci or ≥2 positive markers, low frequency
of MSI (MSI-L) refers to detection of a singular positive marker
and MSS refers to a lack of detected positive markers (38). However, the use of SMRs is currently
insufficient for MSI analysis as equivocal (cases with ambiguous
results or small shifts) were detected, especially in EC (39).
In a previous study, a shift of 1 nucleotide was
observed in multiple markers in 76% of MSI-H EC cases, whereas only
12% of MSI-H CRC cases displayed a 1 nucleotide shift in one of
five markers (40). The equivocal
results were subtle (MSI-PCR with one or two nucleotides shifting)
particularly in endometrial cancer. In such cases, patients may not
receive an appropriate treatment regimen. A concurrent limitation
is that DNA extracted from formalin-fixed paraffin-embedded (FFPE)
tissue samples from patients is of low quality and is easily
fragmented, although a number of companies are refining their
products, such as the MSI Analysis System (Promega Corporation), to
overcome this limitation (41–43).
The present study suggested that long mononucleotide repeats (LMRs)
of >40 bp have longer shift sizes, which could improve PCR-based
MSI detection. LMR markers produce more pronounced fragment length
changes, and therefore could improve the detection of MSI that may
have otherwise gone undetected with the use of SMR markers alone.
The present study aimed to investigate the LMR markers to improve
PCR-based MSI test for patients with CRC and EC.
Materials and methods
Sample preparation
Data from 100 patients with either CRC or EC, who
received surgical treatment or adjuvant chemotherapy between
September 2017 and July 2022 at the National Cheng Kung University
Hospital (NCKUH; Tainan, Taiwan) were retrospectively analyzed in
the present study. All histopathological biopsies were
independently graded by two pathologists, using the American Joint
Committee on Cancer classification (44). Matched tumor (with at least 20%
tumor content) and adjacent normal tissue samples were selected,
which included tissue samples from 50 patients with CRC and 50
patients with EC. The protocols in the present study were approved
by the NCKUH Institutional Review Board (approval nos. B-ER-109-15
and A-ER-108-311; Tainan, Taiwan) and all identifiable patient data
were anonymized prior to analysis.
IHC
IHC was used to detect MMR protein expression to
categorize tissue samples into either the dMMR or pMMR group. The
thickness of the unstained sections was 5-µm per sample and the
sections were prepared from FFPE tissue blocks. Deparaffinization
and rehydration were performed using xylene and ethanol,
respectively. The endogenous peroxidase was blocked using 3%
hydrogen peroxide. Antigen retrieval was conducted using the Ph 9.0
Target Retrieval Solution (Dako; Agilent Technologies, Inc.). The
sections were then incubated with primary antibodies for MLH1
(clone, M1; cat. no. 760-5091; diluted 1:1; Roche Tissue
Diagnostics), PMS2 (clone, A16-4; cat. no. 760-5094; diluted 1:1;
Roche Tissue Diagnostics), MSH2 (clone, G219-1129; cat. no.
760-5093; diluted 1:1; Roche Tissue Diagnostics) and MSH6 (clone,
SP93; cat. no. 760-5092; diluted 1:1; Roche Tissue Diagnostics) at
4°C overnight. Secondary antibodies were prepared for immediate use
and were applied for 30 min at room temperature using EnVision+
System-HRP Labelled Polymer anti-rabbit (cat. no. K4003) and
anti-mouse (cat. no. K4001) (Dako; Agilent Technologies, Inc.).
Visualization of these proteins was achieved using the Liquid DAB+
Substrate Chromogen System (Dako; Agilent Technologies, Inc.).
Sections were counterstained with hematoxylin for 5 min at room
temperature. Stained tissue slides were visualized by light
microscopy (OLYMPUS BX53) and analyzed using cellSens software
(OLYMPUS cellSens Entry 3.1; http://www.olympus-lifescience.com/en/software/cellsens/).
The results were evaluated by a pathologist who determined that the
absence of nuclear staining in tumor cells and the presence of
positive staining in surrounding stromal cells indicated a loss of
signal. This was defined as a loss of MMR proteins, which is
characteristic of dMMR tumors. Conversely, tumors that exhibited no
loss of MMR proteins were classified as pMMR. Partial patient data
(CRC nos. 47 and 50) were reviewed in our previous study (45) (Fig.
S1).
DNA extraction
To extract DNA from tissue samples, 3–5 sections of
FFPE tissue samples from a single patient were placed into a 1.5 ml
tube and the QIAamp DNA FFPE Tissue Kit (cat. no. 56404; Qiagen
GmbH) was used according to the manufacturer's instructions. DNA
concentration was quantified using a NanoDrop® 2000
(NanoDrop Technologies; Thermo Fisher Scientific, Inc.). Samples
containing ≥30 ng of DNA were used in subsequent experiments.
MSI detection by SMR-MSI and LMR-MSI
tests
In accordance with ESMO recommendations, five
poly(A) SMRs (BAT-25, BAT-26, NR-21, NR-24 and NR-27) were selected
for the SMR-MSI test based on data from our previous study
(32,45). The LMR test was performed using
patent protected markers for 40-A, 40-B, 40-C, 40-D and 44. The
patent protected markers were used in the detection kit for MSI in
biological samples. To select the LMR markers, the human reference
genome (GRCh38; accession no. GCF_000001405.26) and the genome
annotation from the National Centre for Biotechnology Information
(NCBI) RefSeq database (https://www.ncbi.nlm.nih.gov/refseq/) was obtained
(46). The reference genome was
parsed to identify ≥40 consecutive bases of adenines (or thymines)
that represented LMRs. For each LMR, the closest flanking genes
(based on the RefSeq annotation) were identified. The present study
selected five LMRs that had flanking genes (KIT, KIT
proto-oncogene receptor tyrosine kinase; RAC1, Rac family
small GTPase 1; KLF4, krüppel-like factor 4; INFAR1,
IFN-α and β receptor 1; and FHIT, fragile histidine triad
diadenosine triphosphatase) involved in CRC and EC. The selected
LMR sequences, together with the flanking sequences (80 bp), were
used to design primers via the NCBI Primer-Basic Local Alignment
Search Tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi) (47) and Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/)
with the default parameters (Table
SI).
The DNA extracted from the patient tissue samples
were amplified using the Hot Start PCR Master Mix (Thermo Fisher
Scientific, Inc.) and 10 pmol/µl forward and reverse primers. The
following thermocycling conditions were used: Initial denaturation
at 95°C for 5 min; 40 cycles of 95°C for 30 sec, 54°C for 45 sec
and 72°C for 1 min; and a final extension at 72°C for 10 min, using
a T100 Thermal Cycler (Bio-Rad Laboratories, Inc.). Capillary
electrophoresis analysis using the PCR products was performed using
a QIAxcel DNA Screening Kit (Qiagen GmbH) according to the
manufacturer's instructions, using a QIAxcel Advanced Instrument
(Qiagen GmbH).
Tissue samples that presented MSI markers with
either band shifts or smears were considered to be positive for
MSI. For the SMR-MSI and LMR-MSI tests, classifications of MSI-H,
MSI-L and MSS described the detection of ≥2, 1 and 0 positive
markers, respectively.
Cell culture
The HCT116 human colon carcinoma cell line was
cultured in McCoy's 5A (Gibco; Thermo Fisher Scientific, Inc.; cat.
no. 16600) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.; cat. no. 26140079) and 1% streptomycin
(Gibco; Thermo Fisher Scientific, Inc.; cat. no. 15140-122) and
cultured at 37°C in a humidified atmosphere of 5%
CO2.
Limit of detection (LoD) of LMR
assay
The genomic DNA from MSI-H HCT116 cells, a CRC cell
line harboring a heterozygous MLH1 c.755C>A mutation, was mixed
with that of normal FFPE DNA to contain a total of 30 ng of DNA in
each reaction. Genomic DNA was extracted from cells and FFPE using
the QIAamp DNA Micro Kit (cat. no. 56304; Qiagen GmbH) and the
QIAamp DNA FFPE Tissue Kit (cat. no. 56404; Qiagen GmbH),
respectively. The samples contained final concentrations of 100.0,
50.0, 20.0, 10.0, 7.5, 5.0 and 2.5% HCT116 DNA for the LoD
assay.
Concordance between SMR-MSI and
LMR-MSI analyses
The concordance rate was calculated as the number of
dMMR cases classed as MSI-H and pMMR cases classed as either MSS or
MSI-L cases using the SMR-MSI or LMR-MSI tests, and the resulting
number of cases was divided by the number of total cases.
Sensitivity, or positive percentage agreement, was calculated as
the number of MSI-H cases detected using the SMR or LMR-MSI tests
divided by the number of dMMR cases detected using MMR-IHC.
Specificity, or negative percentage agreement, was calculated as
the number of MSI-L and MSS cases detected using the SMR or LMR-MSI
tests divided by the number of pMMR cases detected using IHC.
Target NGS of MMR pathway genes
The Human Colorectal Cancer Focus Panel (cat. no.
PHS-103Z; Qiagen GmbH) was used to perform NGS (Table SII) of CRC case nos. 48 and 49,
which were classed as pMMR using the MMR-IHC assay but were classed
as MSI-L using the SMR-MSI test and as MSI-H using the LMR-MSI
test. The Human Breast Cancer Focus Panel (cat. no. PHS-102Z;
Qiagen GmbH) was also performed on the EC case nos. 13 and 14,
which were classed as MSI-H using the SMR and LMR-MSI tests, but
classed as pMMR using the dMMR-IHC assay. The panel included the
following MMR pathway related genes: MLH1, MLH3, MSH2, MSH3,
PMS2 and MSH6 (Table
SII).
QIAamp DNA FFPE Kits (cat. no. 56404; Qiagen GmbH)
were used for the purification of FFPE DNA for NGS sequencing. A
total of 250 ng FFPE DNA was used for library construction.
Amplicons were dual barcoded for sample identification.
Construction of the DNA libraries was performed according to the
manufacturer's instructions. Library QC was performed with QIAxcel
DNA High Resolution Kit (cat. no. 929002; Qiagen GmbH) to check the
correct size distribution of the library. The library was
quantified using QIAseq Library Quant Assay Kit (cat. no. 333314;
Qiagen GmbH). The loading concentration of the final library was 10
pM, measured using a Qubit fluorometer. The sequencing run was
performed using the MiSeq Reagent Kit v2. (cat. no. MS-102-2002).
Paired ends libraries (2X 150 bp) were sequenced using the Illumina
MiSeq sequencer (Illumina, Inc.). Mean sequence depths of at least
800X were achieved for the tumor tissue.
Data analysis and interpretation was performed using
the GeneGlobe Data Analysis Center (https://geneglobe.qiagen.com/us/analyze). Data
analysis was focused on the MMR pathway and homologous
recombination (HR)-related genes (the MMR pathway genes: MLH1,
MLH3, MSH2, MSH3, PMS2 and MSH6; the HR genes: BRCA1,
BRCA2, RAD51B, RAD51C, RAD51D and RAD54L) using the
QIAGEN Clinical Insights Interpret software (version
9.1.0.20230224; Qiagen GmbH) as it offered flexible and automatable
interpretation workflows. The variants were classified as either
pathogenic or likely pathogenic using the ClinVar database
(https://www.ncbi.nlm.nih.gov/clinvar/)
Statistical analysis
The sample size estimate was calculated using an
online calculator (https://turkjemergmed.com/calculator) for statistical
power calculations (48). The
calculations were performed at 95% CI and the minimum sample size
was calculated to be 97 cases for the present study. A two-tailed
unpaired Student's t-test and Fisher's exact were performed using
SPSS (version 17.0; IBM Corp.) to evaluate the shift sizes between
the PCR-based MSI analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Study population and samples
To evaluate the LMR test for patients with CRC or
EC, tissue sections were collected and independently validated by
two board-certified pathologists. Patients with CRC had an age
range of 36–89 years and a mean age of 64 years (Table I). Patients with EC had an age range
of 29–89 years and a mean age of 57 years. Of the patients with
CRC, 64% were male (32/50) and 36% were female (18/50). The mean
percentage of tumor cells in CRC and EC tissue samples were 52.7%
(range, 20–80%) and 54.4% (range, 10–90%), respectively.
 | Table I.Clinicopathologic features of CRC and
EC cases in the present study. |
Table I.
Clinicopathologic features of CRC and
EC cases in the present study.
| Clinicopathologic
features | CRC (total,
n=50) | EC (total,
n=50) |
|---|
| Mean age at
diagnosis, years ± SD | 63.6±13.2 | 56.6±12.9 |
| Sex, n (%) |
|
|
|
Male | 32 (64) | 0 (0) |
|
Female | 18 (36) | 50 (100) |
| Histologic type, n
(%) |
|
|
|
Adenocarcinoma | 44 (88) | 45 (90) |
|
Mucinous adenocarcinoma | 6 (12) | 5 (10) |
| Histological
features |
|
|
| Well
differentiated | 7 (14) | 11 (22) |
|
Moderately differentiated | 35 (70) | 20 (40) |
| Poorly
differentiated | 8 (16) | 19 (38) |
| TNM stage, n
(%) |
|
|
|
0-I | 3 (6) | 10 (20) |
| II | 15 (30) | 17 (34) |
|
III | 28 (56) | 19 (38) |
| IV | 4 (8) | 4 (8) |
| T stage, n (%) |
|
|
| 1 | 1 (2) | 18 (36) |
| 2 | 2 (4) | 14 (28) |
| 3 | 33 (66) | 18 (36) |
| 4 | 14 (28) | 0 (0) |
| N stage, n (%) |
|
|
| 0 | 18 (36) | 25 (50) |
| 1 | 17 (34) | 21 (42) |
| 2 | 15 (30) | 4 (8) |
| M stage, n (%) |
|
|
| 0 | 31 (62) | 28 (56) |
| 1 | 19 (38) | 22 (44) |
Comparison of MMR-IHC, SMR-MSI and
LMR-MSI assays
To examine LMR marker availability in patients with
CRC or EC, DNA extracted from FFPE tissues were analyzed using
MMR-IHC, SMR-MSI and LMR-MSI, the latter of which used LMR markers
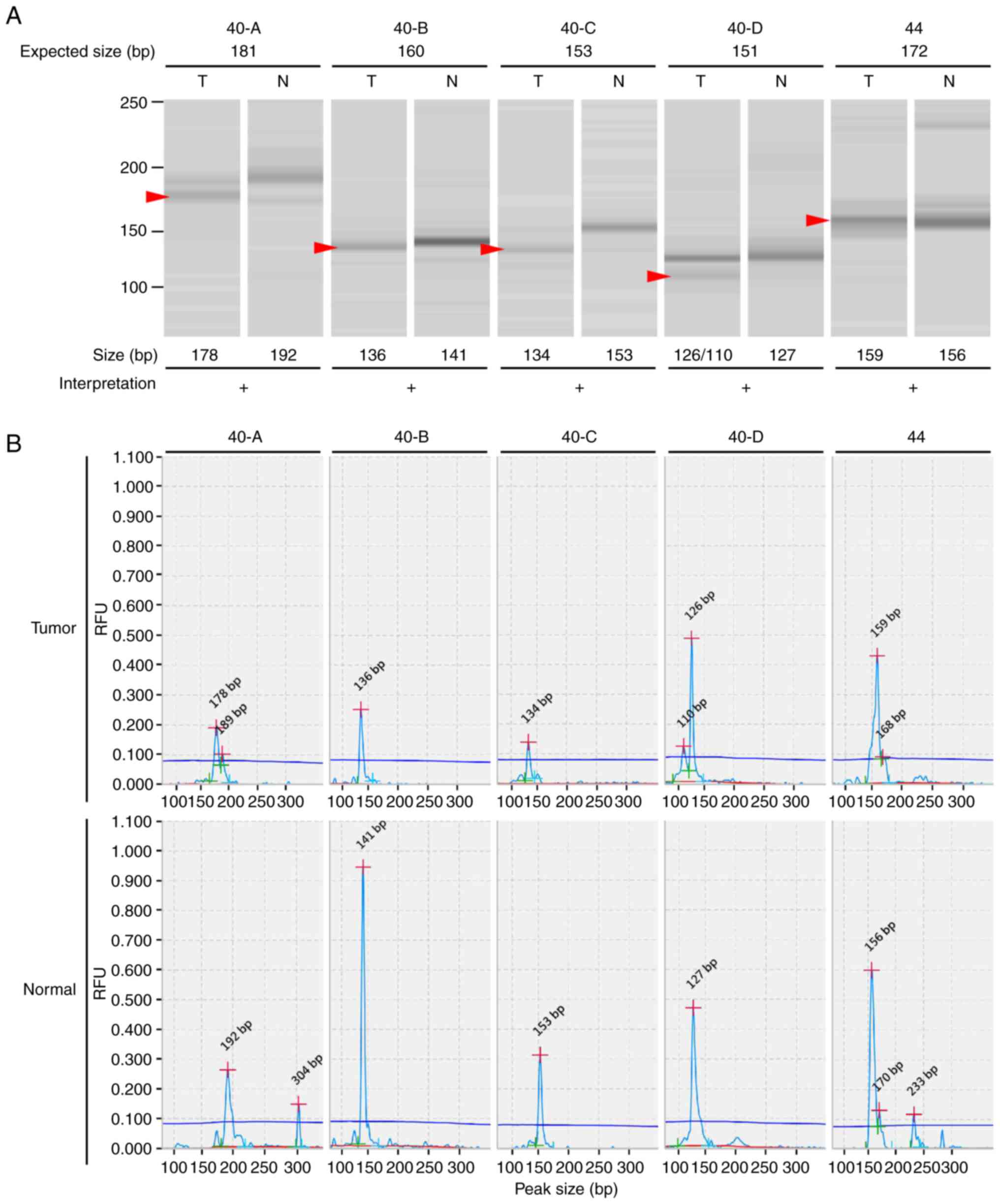
40-A, 40-B, 40-C, 40-D and 44. Differences between tumor and normal
allelic size of ≥3 bp were considered to represent a band shift;
the presence of a number of newly generated bands in tumor tissues
in comparison to normal tissues was considered to be indicative of
band smears (Figs. 1 and S2). The LMR-MSI test LoD was evaluated by
the detection of increasing ratios of MSI-positive DNA from HCT116
cells with patient DNA. The LoD was 2.5% for LMR markers 40-A,
40-B, 40-C, 40-D and 44 when using the QIAxcel high-resolution
screening gel (Fig. S3).
In CRC cases, the concordance rates of the SMR and
LMR-MSI assays were both 96.0% when compared with MMR-IHC (Table II). A large proportion of CRC cases
showed high concordance across the MMR-IHC, SMR and LMR-MSI tests.
Case nos. 1–26 were classed as dMMR with MSI-H using both SMR and
LMR-MSI test results, while case nos. 27–46 were classed as pMMR
with MSS using both SMR and LMR-MSI test results (Table III). Out of the present 50 CRC
cases, only 4 cases had discrepancies across the MSI tests. This
indicated that there was no overt tendency of false negative or
positive results using the novel LMR-MSI test. Case nos. 47 and 50
were classed as dMMR with MSS and MSI-L using the SMR-MSI test,
which represented potential false negatives, as the LMR-MSI test
indicated the cases were MSI-H. Case nos. 48 and 49 were classed as
pMMR using the MMR-IHC, MSI-L using the SMR-MSI test, but MSI-H
using the LMR-MSI test, which represented a potential false
positive using the LMR-MSI test. Tissue samples from the last two
CRC cases, nos. 48 and 49, were used for NGS analysis.
 | Table II.Concordance rate of SMR-MSI and
LMR-MSI assays compared with MMR-IHC in CRC and EC cases. |
Table II.
Concordance rate of SMR-MSI and
LMR-MSI assays compared with MMR-IHC in CRC and EC cases.
|
| CRC | EC |
|---|
|
|
|
|
|---|
| Variable | SMR | LMR | SMR | LMR |
|---|
| Concordance rate,
% | 96.0 | 96.0 | 87.8 | 95.1 |
 | Table III.MMR and MSI status using MMR-ICH,
SMR-MSI and LMR-MSI assays in 50 cases of patients with CRC. |
Table III.
MMR and MSI status using MMR-ICH,
SMR-MSI and LMR-MSI assays in 50 cases of patients with CRC.
|
|
| MMR-IHC
markers |
| SMR-MSI
markers |
| LMR-MSI
markers |
|---|
| CRC case no. | MMR-IHC status |
| SMR-MSI status |
| LMR-MSI status |
|
|---|
| MLH1 | MSH2 | PMS2 | MSH6 | BAT-25 | BAT-26 | NR-21 | NR-24 | NR-27 | 40-A | 40-B | 40-C | 40-D | 44 |
|---|
| 1 | dMMR | + | - | + | - | MSI-H | + | + | + | + | + | MSI-H | + | + | + | + | + |
| 2 | dMMR | + | - | + | - | MSI-H | + | + | + | + | + | MSI-H | + | - | + | + | + |
| 3 | dMMR | + | - | + | - | MSI-H | + | + | + | + | + | MSI-H | + | - | + | - | + |
| 4 | dMMR | + | - | + | - | MSI-H | + | + | + | + | + | MSI-H | - | + | + | - | + |
| 5 | dMMR | - | - | - | - | MSI-H | + | + | + | + | + | MSI-H | + | + | + | - | - |
| 6 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | + | + | + | + |
| 7 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | + | + | + | + |
| 8 | dMMR | - | + | - | + | MSI-H | +a | + | + | + | + | MSI-H | + | + | + | + | + |
| 9 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | + | + | + | + |
| 10 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | + | + | - | + |
| 11 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | - | + | + | + | + |
| 12 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | - | + | + | + | + |
| 13 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | - | + | + | + | + |
| 14 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | - | + | + | - | + |
| 15 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | - | + | + | - | + |
| 16 | dMMR | - | + | - | + | MSI-H | + | + | - | + | + | MSI-H | - | - | + | - | + |
| 17 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | - | + | - | + |
| 18 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | - | + | - | + |
| 19 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | + | + | + | + |
| 20 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | + | + | - | - |
| 21 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | + | - | - | + |
| 22 | dMMR | + | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | + | + | + | + |
| 23 | dMMR | + | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | + | + | + | + |
| 24 | dMMR | + | + | - | + | MSI-H | + | + | + | - | + | MSI-H | + | + | + | - | + |
| 25 | dMMR | + | + | - | + | MSI-H | +a | +a | +a | +a | +a | MSI-H | + | - | + | - | + |
| 26 | dMMR | + | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | - | + | - | - |
| 27 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 28 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 29 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 30 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 31 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 32 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 33 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 34 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 35 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 36 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 37 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 38 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 39 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 40 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 41 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 42 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 43 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 44 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 45 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 46 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 47 | dMMR | + | + | - | + |
MSS | - | - | - | - | - | MSI-H | - | - | - | + | + |
| 48 | pMMR | + | + | + | + |
MSI-L | - | - | + | - | - | MSI-H | - | - | - | + | + |
| 49 | pMMR | + | + | + | + |
MSI-L | - | - | - | + | - | MSI-H | + | - | - | + | + |
| 50 | dMMR | + | + | + | - |
MSI-L | - | - | - | + | - | MSI-H | + | - | + | - | - |
In the EC cases, 7 cases were excluded as MMR-IHC
results were unavailable. The concordance rates of the SMR-MSI and
LMR-MSI tests were 87.8 and 95.1% respectively, compared with
MMR-IHC (Table II). For the EC
cases with MMR-IHC results, a large proportion of cases
demonstrated a high concordance across MMR-IHC, SMR-MSI and LMR-MSI
tests. Case nos. 1–10 were classed as dMMR with MSI-H using both
SMR-MSI and LMR-MSI tests, while case nos. 15–40 were classified as
pMMR with MSS using both SMR-MSI and LMR-MSI tests (Table IV). In 41 EC cases with IHC
results, 5 cases showed discrepancies between the three analyses.
Case nos. 48–50 were classed as dMMR with MSS or MSI-L using the
SMR-MSI test which represented a potential false negative, while
the novel LMR-MSI tests indicated MSI-H for these cases. Case nos.
13 and 14 were classed as pMMR with MSI-H using both SMR-MSI and
LMR-MSI tests. MMR proteins may be dysfunctional but retained
structures recognized by antibodies, resulting in pMMR
classification when using MMR-IHC detection (49). Tissue samples from EC case nos. 13
and 14 were used for NGS analysis.
 | Table IV.MMR and MSI status using different
MMR-ICH, SMR-MSI and LMR-MSI assays in 50 cases of patients with
EC. |
Table IV.
MMR and MSI status using different
MMR-ICH, SMR-MSI and LMR-MSI assays in 50 cases of patients with
EC.
|
|
| MMR-IHC
markers |
| SMR-MSI
markers |
| LMR-MSI
markers |
|---|
| EC case no. | MMR-IHC status |
| SMR-MSI status |
| LMR-MSI status |
|
|---|
| MLH1 | MSH2 | PMS2 | MSH6 | BAT-25 | BAT-26 | NR-21 | NR-24 | NR-27 | 40-A | 40-B | 40-C | 40-D | 44 |
|---|
| 1 | dMMR | - | + | - | + | MSI-H | - | +a | - | +a | +a | MSI-H | + | + | + | - | + |
| 2 | dMMR | - | + | - | + | MSI-H | + | + | + | - | - | MSI-H | + | + | + | - | + |
| 3 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | - | + | - | + |
| 4 | dMMR | - | + | - | + | MSI-H | +a | +a | +a | +a | +a | MSI-H | + | - | + | + | + |
| 5 | dMMR | - | + | - | + | MSI-H | +a | - | +a | +a | - | MSI-H | - | - | + | - | + |
| 6 | dMMR | - | + | - | - | MSI-H | + | + | + | - | + | MSI-H | + | - | + | - | + |
| 7 | dMMR | - | - | + | + | MSI-H | + | + | - | + | + | MSI-H | + | - | + | - | + |
| 8 | dMMR | + | - | + | - | MSI-H | + | + | + | + | + | MSI-H | + | - | + | - | + |
| 9 | dMMR | + | - | + | - | MSI-H | + | + | - | + | + | MSI-H | + | - | + | - | + |
| 10 | dMMR | + | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | + | + | - | + |
| 11 | dMMR | - | + | - | + | MSI-H | + | - | + | + | + | MSI-H | + | - | + | - | - |
| 12 | dMMR | - | + | - | + | MSI-H | + | + | + | + | + | MSI-H | + | - | - | - | + |
| 13 | pMMR | + | + | + | + | MSI-H | +a | +a | +a | +a | +a | MSI-H | + | + | + | - | - |
| 14 | pMMR | + | + | + | + | MSI-H | + | + | + | + | + | MSI-H | - | - | + | + | + |
| 15 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 16 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 17 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 18 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 19 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 20 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 21 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 22 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 23 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 24 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 25 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 26 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 27 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 28 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 29 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 30 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 31 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 32 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 33 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 34 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 35 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 36 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 37 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 38 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 39 | pMMR | + | + | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 40 | pMMR | NA | NA | + | + |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 41 | NA | NA | NA | NA | NA |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 42 | NA | NA | NA | NA | NA |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 43 | NA | NA | NA | NA | NA |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 44 | NA | NA | NA | NA | NA |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 45 | NA | NA | NA | NA | NA |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 46 | NA | NA | NA | NA | NA |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 47 | NA | NA | NA | NA | NA |
MSS | - | - | - | - | - |
MSS | - | - | - | - | - |
| 48 | dMMR | + | + | + | - |
MSS | - | - | - | - | - | MSI-H | + | + | - | - | + |
| 49 | dMMR | + | + | + | - |
MSS | - | - | - | - | - | MSI-H | + | + | - | + | - |
| 50 | dMMR | + | + | + | - |
MSI-L | - | - | - | + | - | MSI-H | + | + | + | + | + |
LMR tests demonstrated larger shift
sizes
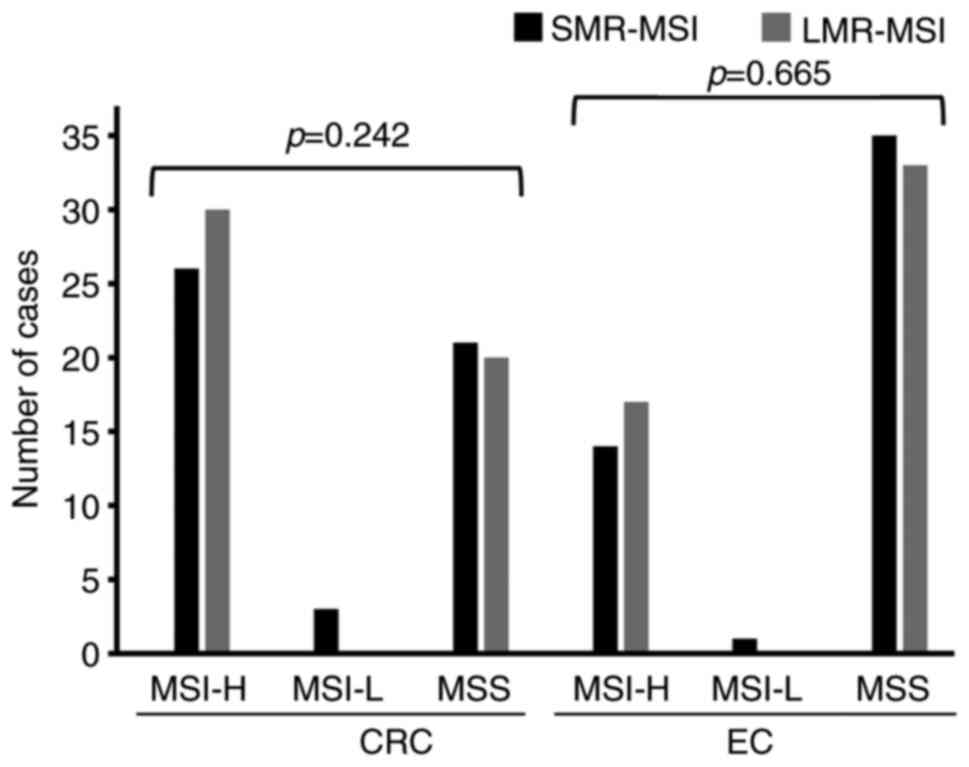
The SMR-MSI test detected a small number of MSI-L
cases, 3 CRC cases and 1 EC case (Fig.
2). The LMR-MSI test did not detect any MSI-L cases in the
present patient cohorts. The cases detected as MSI-L by the SMR-MSI
test were changed to an MSI-H classification following application
of the LMR-MSI test (Tables III
and IV). Of the 4 cases, 2 were
classified as dMMR, while the other 2 cases were classified as
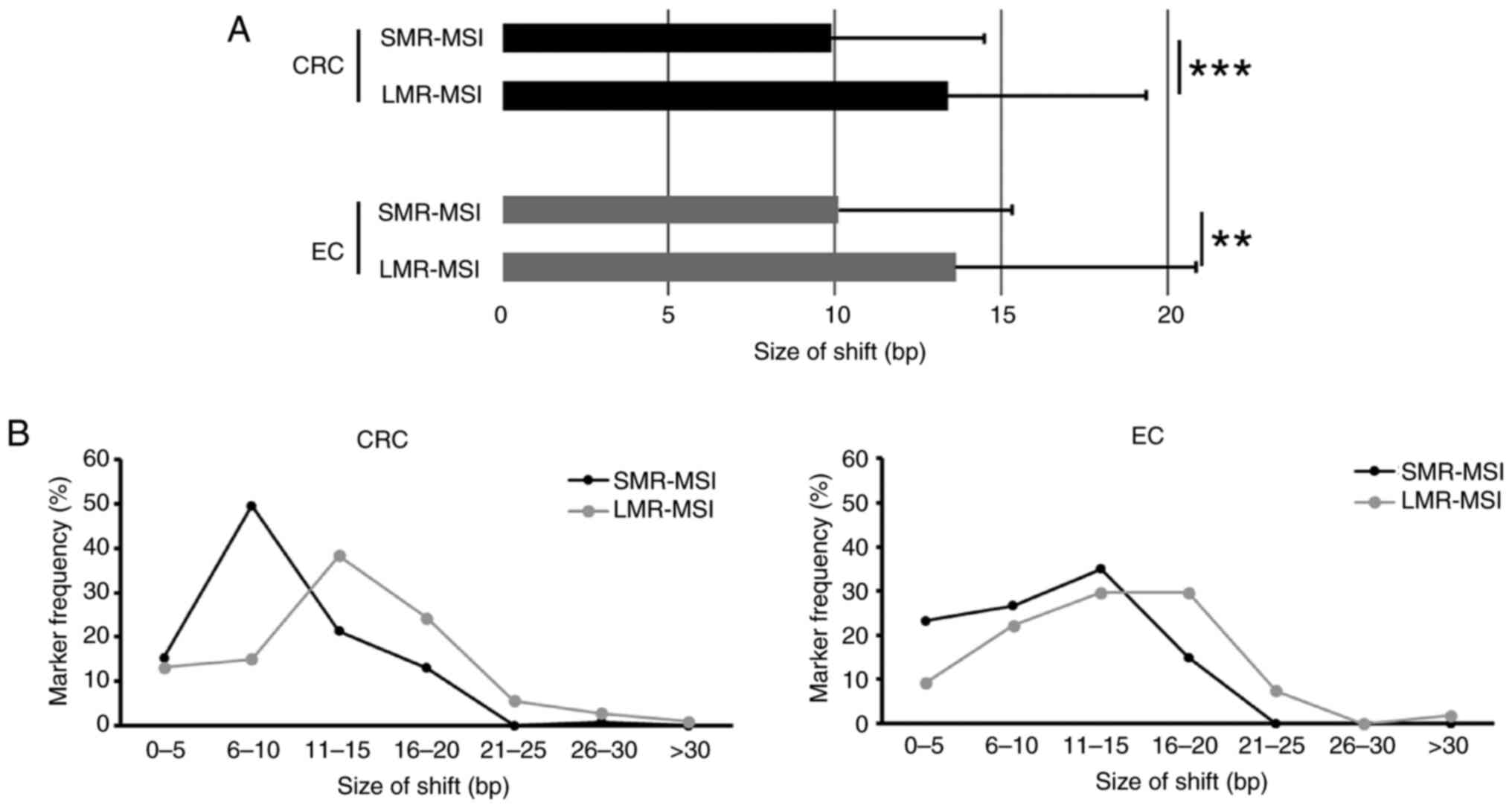
pMMR. The LMR markers exhibited significantly larger shift sizes
and a significantly higher mean shift size (13 bp) in MSI-H tumors
compared with the SMR-MSI test (10 bp). The average shift sizes of
LMR markers were 1.3-fold higher in both CRC (P<0.0001) and EC
(P=0.003) (Fig. 3).
NGS
The 4 discrepant cases (CRC, case nos. 48 and 49;
EC, case nos. 13 and 14) that were initially classed as pMMR but
LMR-MSI tests indicated were MSI-H, were submitted for NGS. Of
these, 3 of the cases did not demonstrate any pathological
variations using the NGS panel. Only 1 EC case, no. 13, presented
as MSI-H but with no loss of protein in the MMR-IHC, which
represented pMMR. The NGS results demonstrated that the tumor
sample of EC case no. 13 harbored one major somatic mutation: 1.25%
alleles with a MSH3 c.4091-2A>T mutation (accession no.
NM_001040108.2), which occurs in the splice acceptor region,
clinical significance was according to the ClinVar database
(50). It was proposed that the
MSH3 c.4091-2A>T mutation could lead to premature
translation termination and result in aberrant protein production
of MSH3. This could potentially disrupt the normal function
of the MMR and lead to MSI-H.
Discussion
The present study used LMR markers, including 40-A,
40-B, 40-C, 40-D and 44, to detect MSI status in patients with CRC
and EC, using PCR amplification and capillary electrophoresis
analysis. The LMR-MSI test showed high concordance rates compared
with MMR-IHC analysis and traditional SMR-MSI tests. The present
pilot study involved 100 patient cases, 50 of which were cases of
patients with CRC and 50 were patients with EC. In total, 7 cases
were excluded as MMR-IHC results were unavailable. Of the remaining
93 cases that had all three test results available, 82 were
consistent across all three platforms. All 38 cases classed as
dMMR/MSI-H cases using MMR-IHC/SMR-MSI assays were detected to be
MSI-H cases using the LMR-MSI test. All 46 cases classed as
pMMR/MSS cases using MMR-IHC/SMR-MSI assays, were detected to be
MSS cases using the LMR-MSI test. The remaining 9 cases were
categorized into two groups: i) MSI-L cases detected using SMR-MSI
tests; and ii) cases with discrepancies between MMR-IHC and SMR-MSI
tests.
LMR-MSI tests reduced the number of equivocal case
generated compared with SMR-MSI tests. Cases classed as MSI-L using
SMR-MSI tests were detected as MSI-H in 3 CRC cases and in 1 EC
case, with use of the LMR-MSI assay. MSI-L is often misclassified
as MSS and in such cases, the patients may potentially not receive
the appropriate medication recommendations (51). In this study, 1 CRC case (no. 50)
and 1 EC case (no. 50) were classed as MSI-L using SMR-MSI testing
but were classed as dMMR using MMR-IHC and as MSI-H using the
LMR-MSI test. Using the MMR-IHC and LMR-MSI results, these
aforementioned cases were most likely dMMR cases. At present, the
patients with tumors classed as dMMR cases used chemotherapy as the
first-line therapy, despite the potential for long-term survival.
In these cases, immune-checkpoint inhibitor (ICI) therapy could be
considered as second-line therapy. In the case of CRC case no. 50,
the patient commenced chemotherapy in November 2017, with the best
treatment response recorded as ‘stable disease’ following 6 months
of treatment. At the time-of-writing (May, 2024) the patient
continues to attend regular follow-up appointments. In the case of
EC case no. 50, the patient received chemotherapy in July 2018,
with the best treatment response recorded as ‘complete remission’
following 18 months of treatment. The two aforementioned cases were
treated with first-line chemotherapy, which resulted in long-term
survival and a positive response to treatment. Jaffrelot et
al (52) reviewed the
occurrence of MMR-IHC-only loss of PMS2 or MSH6, which was present
in 15% of dMMR tumors examined for all MMR IHC markers. The
subgroups were found to be highly associated with MSI-H (81%), with
1 case detected to be MSI-L, similar to CRC case no. 50 and EC case
no. 50 reported in the present study. The previous study further
analyzed the NGS panel and found that the tumor mutational burden
(TMB) of 20 cases (38%) was reported to be TMB-high or
TMB-intermediate cases. Of the cases examined, 2 cases showed a
complete response after immunotherapy.
The 2 MSI-L cases of the present study, CRC case
nos. 48 and no. 49 were classed as pMMR using MMR-IHC but classed
as MSI-H using the LMR-MSI assay. NGS of the 2 aforementioned tumor
samples did not reveal any pathological mutations. The results
suggested potential false positive results of the LMR-MSI test in
the aforementioned CRC cases. However, although the NGS panel used
was primarily designed for CRC samples and included several
MMR-related genes, it may be far from fully comprehensive. Further
investigation is required to determine the nature of the 2
aforementioned cases with pMMR, such as the methylation status of
MLH1, MSH2, PMS2 and MSH6 promoter regions. Unfortunately, the 2
samples were unavailable for further investigation.
The 2 pMMR cases detected using MMR-IHC in EC cases
of the present study were classed as MSI-H by both SMR and LMR-MSI
tests. PCR-based MSI tests detect microsatellites at the DNA level
and IHC analyzes MMR systems at the protein level using antibody
specificity (4). Both methods have
limitations, such as a low level of tumor tissue percentage (for
example <10%), which can affect the tumor DNA concentration in
the PCR-based MSI test, and the MMR-IHC test is limited in the
detection of tumors containing functionally deleterious mutations
but unaffected MMR protein expression levels (53). The two methods may be used in
combination to provide patients with more comprehensive MSI test
results. Of the discordant cases reported in the present study, EC
case no. 13 exhibited pMMR using MMR-IHC, but was demonstrated to
have a missense mutation in the MSH3 gene using NGS.
Sole MSH6 deficiency may cause discrepancies between
MMR-IHC and traditional SMR-MSI tests. MSH6 forms a heterodimer
with MSH2 and is involved in DNA repair in MMR systems (7). Loss of MSH6 protein expression levels
detected through IHC alone may not confirm MSI-H in patients. When
the MSH6 protein is mutated the MSH2/MSH3 heterodimer still
operates and DNA mismatch errors are partially corrected. A number
of patients with CRC no longer exhibit MSH6 expression following
neoadjuvant therapy (54,55). Therefore, it would be necessary to
use further methods, in addition to MMR-IHC, in such cases. In the
present study, 4 cases exhibited MSH6 loss (3 EC cases and 1 CRC
case), all without prior neoadjuvant therapy. The LMR-MSI test
detected MSI-H in all 4 cases, while the SMR-MSI test indicated
either MSS or MSI-L. Therefore, LMR-MSI test may have the potential
to verify MSI status in cases with sole MSH6 loss without
neoadjuvant therapy, particularly in patients with EC. CRC case no.
47 was classed as dMMR (PMS2 deficient alone) using the MMR-IHC
testing but was classed as MSS using the SMR-MSI test. The LMR-MSI
test indicated MSI-H in this case, which was consistent with
MMR-IHC test results. The SMR-MSI test was repeated and
demonstrated the same result of MSS status in the aforementioned
CRC case. The reason that MSS was detected in the aforementioned
case using the SMR-MSI test is currently unclear (56). However, a possible limitation is
that the Bethesda panel (dinucleotides repeat markers) is less
sensitive and specific than the LMR-MSI.
FFPE is beneficial for long-term tissue storage;
however, FFPE-extracted DNA is occasionally fragmented and of low
quality (41). The DNA extracted
from FFPE tissue was sufficient for the LMR-MSI assay in the
present study. The results of the present study have implications
for the improvement of personalized treatment in certain types of
cancer. Certain assay detection equipment, such as the MSI Analysis
System (Promega Corporation), uses fluorescent signals for MSI
analysis (57,58). A non-fluorescent targeted LMR-MSI
test was used in the present study. The advantages of
non-fluorescent systems are the low costs and no risk of
ineffective fluorescent reagents due to improper storage or
photobleaching (59). Idylla
(Biocartis Group NV) have launched a non-fluorescent system that
uses fully automated real-time PCR testing for MSI detection in
FFPE tissues (60,61). NGS is also considered an MSI
detection method; however, it is time-consuming with an operation
time of 2 weeks and is relatively expensive compared with
alternative MSI tests available (53). Each type of tumor requires screening
in a number of databases during NGS data analysis (49,53).
The present study sought to evaluate the performance
of the LMR-MSI assay by analyzing LMR markers in tumor and normal
tissues using QIAxcel, a non-fluorescent system. The results
demonstrated that the sensitivity of the LMR-MSI test was higher
than that of the SMR-MSI test in both types of cancer. Notably, the
LMR-MSI assay did not detect any cases of MSI-L in either the CRC
or EC tissue samples. The present study demonstrated a possible
benefit in the use of LMR-MSI testing compared with SMR-MSI tests
for MSI analysis in patients with EC. The method presented a
time-saving and non-fluorescent method for detecting MSI in FFPE
tissues, as it mitigated the effects of using only SMRs in the
detection of MSI status.
However, the present study had several limitations,
such as a small sample size. Additionally, the patient cohorts with
CRC or EC in the present study were selected based on 100 cases
with MMR status, determined by MMR-IHC and SMR-MSI test results.
Therefore, the present pilot study required a larger patient cohort
size to confirm findings. An advantage in performance of the
LMR-MSI assay is that microsatellites with longer homopolymer
regions increases the size shift for the detection of dMMR/MSI-H
cases in CRC and EC. The larger allele size shifts of the LMR
markers compared with SMR markers provide increased confidence in
shift interpretation. The discrepancies between the three tests are
unclear and further evidence is needed to clarify these
discrepancies.
The results of the present study were obtained by
comparison of tumor tissues with normal tissues. In the future, the
LMR-MSI test may be applied to a greater number of cases and other
types of cancer, in particular stomach adenocarcinoma, which has a
22% detection rate of MSI-H according to The Cancer Genome Atlas
database (62). The present study
demonstrated a potential cost-effective and non-fluorescent method
for the detection of MSI using LMR markers, which markedly reduced
the number of equivocal MSI-L cases in patients with CRC and
EC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Yu-Min Yeh
(Department of Oncology, National Cheng Kung University, Tainan,
Taiwan, R.O.C.) for providing the HCT116 cell line.
Funding
This study was supported by intramural grants from the NCKUH
(grant no. NCKUH-11308004) and the National Science and Technology
Council (grant nos. NSTC 111-2320-B-006-025 and NSTC
112-2320-B-006-044).
Availability of data and materials
The data generated in the present study may be found
in the National Centre for Biotechnology Information (NCBI)
database under at the following URLs: CRC case no. 48, https://trace.ncbi.nlm.nih.gov/Traces/index.html?view=run_browser&acc=SRR23096330&display=metadata;
CRC case no. 49, https://trace.ncbi.nlm.nih.gov/Traces/index.html?view=run_browser&acc=SRR23096329&display=metadata;
EC case no. 13, https://trace.ncbi.nlm.nih.gov/Traces/index.html?view=run_browser&acc=SRR23096328&display=metadata;
and EC case no.14, https://trace.ncbi.nlm.nih.gov/Traces/index.html?view=run_browser&acc=SRR23096327&display=metadata.
Authors' contributions
YiC, CH, WH and TL conceived and designed the
experiments. YiC and WC confirm the authenticity of all the raw
data. YiC, PC and WC performed the experiments. YiC, PC, TL and YaC
analyzed the data. YiC, CH, TL, YaC and PC wrote the manuscript. CL
and NC interpreted MMR-IHC data. CL interpreted the PCR-based MSI
tests. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the National Cheng Kung University Hospital
(Tainan, Taiwan). The study design involved the retrospective
collection of data from patients with either CRC or EC diagnosed at
the National Cheng Kung University Hospital (Tainan, Taiwan)
between September 2017 and July 2022. The MMR status was analyzed
following an approved protocol (approval nos. B-ER-109-152 and
A-ER-108-311; Tainan, Taiwan). Written informed consent was
obtained from the participating patients with discrepant cases
between pMMR-IHC and MSI-PCR assays. Written informed consent was
obtained from the relevant patients for use of their data for NGS
analysis. Written informed consent was waived for analyses that
would not influence treatment decisions. All patient tissues and
data were anonymized prior to analysis.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Richard GF, Kerrest A and Dujon B:
Comparative genomics and molecular dynamics of DNA repeats in
eukaryotes. Microbiol Mol Biol Rev. 72:686–727. 2008. View Article : Google Scholar
|
|
2
|
Lynch HT, Shaw MW, Magnuson CW, Larsen AL
and Krush AJ: Hereditary factors in cancer. Study of two large
midwestern kindreds. Arch Intern Med. 117:206–212. 1966. View Article : Google Scholar
|
|
3
|
Dean DA, Wadl PA, Hadziabdic D, Wang X and
Trigiano RN: Analyzing microsatellites using the QIAxcel system.
Methods Mol Biol. 1006:223–243. 2013. View Article : Google Scholar
|
|
4
|
Li K, Luo H, Huang L, Luo H and Zhu X:
Microsatellite instability: A review of what the oncologist should
know. Cancer Cell Int. 20:162020. View Article : Google Scholar
|
|
5
|
Fan H and Chu JY: A brief review of short
tandem repeat mutation. Genomics Proteomics Bioinformatics. 5:7–14.
2007. View Article : Google Scholar
|
|
6
|
Roudko V, Cimen Bozkus C, Greenbaum B,
Lucas A, Samstein R and Bhardwaj N: Lynch Syndrome and MSI-H
Cancers: From Mechanisms to ‘Off-The-Shelf’ Cancer Vaccines. Front
Immunol. 12:7578042021. View Article : Google Scholar
|
|
7
|
Reyes GX, Schmidt TT, Kolodner RD and
Hombauer H: New insights into the mechanism of DNA mismatch repair.
Chromosoma. 124:443–462. 2015. View Article : Google Scholar
|
|
8
|
Tamura K, Kaneda M, Futagawa M, Takeshita
M, Kim S, Nakama M, Kawashita N and Tatsumi-Miyajima J: Genetic and
genomic basis of the mismatch repair system involved in Lynch
syndrome. Int J Clin Oncol. 24:999–1011. 2019. View Article : Google Scholar
|
|
9
|
Hause RJ, Pritchard CC, Shendure J and
Salipante SJ: Classification and characterization of microsatellite
instability across 18 cancer types. Nat Med. 22:1342–1350. 2016.
View Article : Google Scholar
|
|
10
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar
|
|
11
|
McMeekin DS, Tritchler DL, Cohn DE, Mutch
DG, Lankes HA, Geller MA, Powell MA, Backes FJ, Landrum LM, Zaino
R, et al: Clinicopathologic significance of mismatch repair defects
in endometrial cancer: An NRG Oncology/Gynecologic oncology group
study. J Clin Oncol. 34:3062–3068. 2016. View Article : Google Scholar
|
|
12
|
Bonneville R, Krook MA, Kautto EA, Miya J,
Wing MR, Chen HZ, Reeser JW, Yu L and Roychowdhury S: Landscape of
microsatellite instability across 39 cancer types. JCO Precis
Oncol. 2017.PO.17.00073, 2017. View Article : Google Scholar
|
|
13
|
Green AK, Feinberg J and Makker V: A
Review of immune checkpoint blockade therapy in endometrial cancer.
Am Soc Clin Oncol Educ Book. 40:1–7. 2020.
|
|
14
|
Boland CR and Goel A: Microsatellite
instability in colorectal cancer. Gastroenterology.
138:2073–2087.e3. 2010. View Article : Google Scholar
|
|
15
|
Petrelli F, Ghidini M, Cabiddu M, Pezzica
E, Corti D, Turati L, Costanzo A, Varricchio A, Ghidini A, Barni S,
et al: Microsatellite instability and survival in stage ii
colorectal cancer: A systematic review and meta-analysis.
Anticancer Res. 39:6431–6441. 2019. View Article : Google Scholar
|
|
16
|
Lin A, Zhang J and Luo P: Crosstalk
Between the MSI status and tumor microenvironment in colorectal
cancer. Front Immunol. 11:20392020. View Article : Google Scholar
|
|
17
|
Makker V, Colombo N, Casado Herraez A,
Santin AD, Colomba E, Miller DS, Fujiwara K, Pignata S, Baron-Hay
S, Ray-Coquard I, et al: Lenvatinib plus Pembrolizumab for advanced
endometrial cancer. N Engl J Med. 386:437–448. 2022. View Article : Google Scholar
|
|
18
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar
|
|
19
|
Lenz HJ, Van Cutsem E, Luisa Limon M, Wong
KYM, Hendlisz A, Aglietta M, Garcia-Alfonso P, Neyns B, Luppi G,
Cardin DB, et al: First-Line nivolumab plus Low-Dose ipilimumab for
microsatellite Instability-High/Mismatch repair-deficient
metastatic colorectal cancer: The phase II CheckMate 142 study. J
Clin Oncol. 40:161–170. 2022. View Article : Google Scholar
|
|
20
|
Green AK, Feinberg J and Makker V: A
review of immune checkpoint blockade therapy in endometrial cancer.
Am Soc Clin Oncol Educ Book. 40:1–7. 2020.
|
|
21
|
Narayanan S, Kawaguchi T, Peng X, Qi Q,
Liu S, Yan L and Takabe K: Tumor infiltrating lymphocytes and
macrophages improve survival in microsatellite unstable colorectal
cancer. Sci Rep. 9:134552019. View Article : Google Scholar
|
|
22
|
Diao Z, Han Y, Chen Y, Zhang R and Li J:
The clinical utility of microsatellite instability in colorectal
cancer. Crit Rev Oncol Hematol. 157:1031712021. View Article : Google Scholar
|
|
23
|
Pal A, Greenblatt HM and Levy Y:
Prerecognition diffusion mechanism of human DNA mismatch repair
proteins along DNA: Msh2-Msh3 versus Msh2-Msh6. Biochemistry.
59:4822–4832. 2020. View Article : Google Scholar
|
|
24
|
Marsischky GT, Filosi N, Kane MF and
Kolodner R: Redundancy of saccharomyces cerevisiae MSH3 and MSH6 in
MSH2-dependent mismatch repair. Genes Dev. 10:407–420. 1996.
View Article : Google Scholar
|
|
25
|
Hewish M, Lord CJ, Martin SA, Cunningham D
and Ashworth A: Mismatch repair deficient colorectal cancer in the
era of personalized treatment. Nat Rev Clin Oncol. 7:197–208. 2010.
View Article : Google Scholar
|
|
26
|
Luchini C, Bibeau F, Ligtenberg MJL, Singh
N, Nottegar A, Bosse T, Miller R, Riaz N, Douillard JY, Andre F, et
al: ESMO recommendations on microsatellite instability testing for
immunotherapy in cancer, and its relationship with PD-1/PD-L1
expression and tumour mutational burden: A systematic review-based
approach. Ann Oncol. 30:1232–1243. 2019. View Article : Google Scholar
|
|
27
|
Benson AB, Venook AP, Al-Hawary MM, Arain
MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, et
al: Colon cancer, version 2.2021, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 19:329–359. 2021. View Article : Google Scholar
|
|
28
|
Funkhouser WK Jr, Lubin IM, Monzon FA,
Zehnbauer BA, Evans JP, Ogino S and Nowak JA: Relevance,
pathogenesis, and testing algorithm for mismatch repair-defective
colorectal carcinomas: A report of the association for molecular
pathology. J Mol Diagn. 14:91–103. 2012. View Article : Google Scholar
|
|
29
|
Cicek MS, Lindor NM, Gallinger S, Bapat B,
Hopper JL, Jenkins MA, Young J, Buchanan D, Walsh MD, Le Marchand
L, et al: Quality assessment and correlation of microsatellite
instability and immunohistochemical markers among population- and
clinic-based colorectal tumors results from the colon cancer family
registry. J Mol Diagn. 13:271–281. 2011. View Article : Google Scholar
|
|
30
|
Hechtman JF, Rana S, Middha S, Stadler ZK,
Latham A, Benayed R, Soslow R, Ladanyi M, Yaeger R, Zehir A, et al:
Retained mismatch repair protein expression occurs in approximately
6% of microsatellite instability-high cancers and is associated
with missense mutations in mismatch repair genes. Mod Pathol.
33:871–879. 2020. View Article : Google Scholar
|
|
31
|
Cohen R, Hain E, Buhard O, Guilloux A,
Bardier A, Kaci R, Bertheau P, Renaud F, Bibeau F, Flejou JF, et
al: Association of primary resistance to immune checkpoint
inhibitors in metastatic colorectal cancer with misdiagnosis of
microsatellite instability or mismatch repair deficiency status.
JAMA Oncol. 5:551–555. 2019. View Article : Google Scholar
|
|
32
|
Huang W, Ho CL, Lee CT, Chen WL, Yang SC,
Chow NH and Chen YL: High concordance rate of capillary
electrophoresis workflow for microsatellite instability analysis
and mismatch repair (MMR) immunostaining in colorectal carcinoma.
PLoS One. 18:e02842272023. View Article : Google Scholar
|
|
33
|
Murphy KM, Zhang S, Geiger T, Hafez MJ,
Bacher J, Berg KD and Eshleman JR: Comparison of the microsatellite
instability analysis system and the Bethesda panel for the
determination of microsatellite instability in colorectal cancers.
J Mol Diagn. 8:305–311. 2006. View Article : Google Scholar
|
|
34
|
Vikas P, Messersmith H, Compton C, Sholl
L, Broaddus RR, Davis A, Estevez-Diz M, Garje R, Konstantinopoulos
PA, Leiser A, et al: Mismatch repair and microsatellite instability
testing for immune checkpoint inhibitor therapy: ASCO endorsement
of college of american pathologists guideline. J Clin Oncol.
41:1943–1948. 2023. View Article : Google Scholar
|
|
35
|
Taieb J, Svrcek M, Cohen R, Basile D,
Tougeron D and Phelip JM: Deficient mismatch repair/microsatellite
unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur
J Cancer. 175:136–157. 2022. View Article : Google Scholar
|
|
36
|
Rodriguez-Bigas MA, Boland CR, Hamilton
SR, Henson DE, Jass JR, Khan PM, Lynch H, Perucho M, Smyrk T, Sobin
L, et al: A National cancer institute workshop on hereditary
nonpolyposis colorectal cancer syndrome: Meeting highlights and
Bethesda guidelines. J Natl Cancer Inst. 89:1758–1762. 1997.
View Article : Google Scholar
|
|
37
|
Umar A, Boland CR, Terdiman JP, Syngal S,
de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ,
Hamelin R, et al: Revised Bethesda Guidelines for hereditary
nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite
instability. J Natl Cancer Inst. 96:261–268. 2004. View Article : Google Scholar
|
|
38
|
Bacher JW, Flanagan LA, Smalley RL, Nassif
NA, Burgart LJ, Halberg RB, Megid WM and Thibodeau SN: Development
of a fluorescent multiplex assay for detection of MSI-High tumors.
Dis Markers. 20:237–250. 2004. View Article : Google Scholar
|
|
39
|
Goel A, Nagasaka T, Hamelin R and Boland
CR: An optimized pentaplex PCR for detecting DNA mismatch
repair-deficient colorectal cancers. PLoS One. 5:e93932010.
View Article : Google Scholar
|
|
40
|
Wang Y, Shi C, Eisenberg R and
Vnencak-Jones CL: Differences in microsatellite instability
profiles between endometrioid and colorectal cancers: A potential
cause for False-Negative results? J Mol Diagn. 19:57–64. 2017.
View Article : Google Scholar
|
|
41
|
Do H and Dobrovic A: Sequence artifacts in
DNA from formalin-fixed tissues: Causes and strategies for
minimization. Clin Chem. 61:64–71. 2015. View Article : Google Scholar
|
|
42
|
McDonough SJ, Bhagwate A, Sun Z, Wang C,
Zschunke M, Gorman JA, Kopp KJ and Cunningham JM: Use of
FFPE-derived DNA in next generation sequencing: DNA extraction
methods. PLoS One. 14:e02114002019. View Article : Google Scholar
|
|
43
|
Wu S, Liu X, Wang J, Zhou W, Guan M, Liu
Y, Pang J, Lu T, Zhou L, Shi X, et al: DNA Mismatch repair
deficiency detection in colorectal cancer by a new microsatellite
instability analysis system. Interdiscip Sci. 12:145–154. 2020.
View Article : Google Scholar
|
|
44
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar
|
|
45
|
Lee CT, Chow NH, Chen YL, Ho CL, Yeh YM,
Lin SC, Lin PC, Lin BW, Chu CA, Tsai HW, et al: Clinicopathological
features of mismatch repair protein expression patterns in
colorectal cancer. Pathol Res Pract. 217:1532882021. View Article : Google Scholar
|
|
46
|
O'Leary NA, Wright MW, Brister JR, Ciufo
S, Haddad D, McVeigh R, Rajput B, Robbertse B, Smith-White B,
Ako-Adjei D, et al: Reference sequence (RefSeq) database at NCBI:
Current status, taxonomic expansion, and functional annotation.
Nucleic Acids Res. 44:D733–D745. 2016. View Article : Google Scholar
|
|
47
|
Ye J, Coulouris G, Zaretskaya I,
Cutcutache I, Rozen S and Madden TL: Primer-BLAST: A tool to design
target-specific primers for polymerase chain reaction. BMC
Bioinformatics. 13:1342012. View Article : Google Scholar
|
|
48
|
Akoglu H: User's guide to sample size
estimation in diagnostic accuracy studies. Turk J Emerg Med.
22:177–185. 2022. View Article : Google Scholar
|
|
49
|
Yu F, Makrigiorgos A, Leong KW and
Makrigiorgos GM: Sensitive detection of microsatellite instability
in tissues and liquid biopsies: Recent developments and updates.
Comput Struct Biotechnol J. 19:4931–4940. 2021. View Article : Google Scholar
|
|
50
|
Liu HX, Zhou XL, Liu T, Werelius B,
Lindmark G, Dahl N and Lindblom A: The role of hMLH3 in familial
colorectal cancer. Cancer Res. 63:1894–1899. 2003.
|
|
51
|
Kawakami H, Zaanan A and Sinicrope FA:
Microsatellite instability testing and its role in the management
of colorectal cancer. Curr Treat Options Oncol. 16:302015.
View Article : Google Scholar
|
|
52
|
Jaffrelot M, Farés N, Brunac AC, Laurenty
AP, Danjoux M, Grand D, Icher S, Meilleroux J, Mery E, Buscail E,
et al: An unusual phenotype occurs in 15% of mismatch
repair-deficient tumors and is associated with non-colorectal
cancers and genetic syndromes. Mod Pathol. 35:427–437. 2022.
View Article : Google Scholar
|
|
53
|
Wang C, Zhang L, Vakiani E and Shia J:
Detecting mismatch repair deficiency in solid neoplasms:
Immunohistochemistry, microsatellite instability, or both? Mod
Pathol. 35:1515–1528. 2022. View Article : Google Scholar
|
|
54
|
Bao F, Panarelli NC, Rennert H, Sherr DL
and Yantiss RK: Neoadjuvant therapy induces loss of MSH6 expression
in colorectal carcinoma. Am J Surg Pathol. 34:1798–1804. 2010.
View Article : Google Scholar
|
|
55
|
Kuan SF, Ren B, Brand R, Dudley B and Pai
RK: Neoadjuvant therapy in microsatellite-stable colorectal
carcinoma induces concomitant loss of MSH6 and Ki-67 expression.
Hum Pathol. 63:33–39. 2017. View Article : Google Scholar
|
|
56
|
Guyot D'Asnières De Salins A, Tachon G,
Cohen R, Karayan-Tapon L, Junca A, Frouin E, Godet J, Evrard C,
Randrian V, Duval A, et al: Discordance between immunochemistry of
mismatch repair proteins and molecular testing of microsatellite
instability in colorectal cancer. ESMO Open. 6:1001202021.
View Article : Google Scholar
|
|
57
|
Bacher JW, Sievers CK, Albrecht DM, Grimes
IC, Weiss JM, Matkowskyj KA, Agni RM, Vyazunova I, Clipson L,
Storts DR, et al: Improved detection of microsatellite instability
in early colorectal lesions. PLoS One. 10:e01327272015. View Article : Google Scholar
|
|
58
|
Lin JH, Chen S, Pallavajjala A, Guedes LB,
Lotan TL, Bacher JW and Eshleman JR: Validation of long
mononucleotide repeat markers for detection of microsatellite
instability. J Mol Diagn. 24:144–157. 2022. View Article : Google Scholar
|
|
59
|
Turner EH, Dickerson JA, Ramsay LM,
Swearingen KE, Wojcik R and Dovichi NJ: Reaction of fluorogenic
reagents with proteins III. Spectroscopic and electrophoretic
behavior of proteins labeled with Chromeo P503. J Chromatogr A.
1194:253–256. 2008. View Article : Google Scholar
|
|
60
|
Zwaenepoel K, Holmgaard Duelund J, De
Winne K, Maes V, Weyn C, Lambin S, Dendooven R, Broeckx G,
Steiniche T and Pauwels P: Clinical performance of the idylla MSI
test for a rapid assessment of the DNA microsatellite status in
human colorectal cancer. J Mol Diagn. 22:386–395. 2020. View Article : Google Scholar
|
|
61
|
Siemanowski J, Schomig-Markiefka B, Buhl
T, Haak A, Siebolts U, Dietmaier W, Arens N, Pauly N, Ataseven B,
Buttner R, et al: Managing difficulties of microsatellite
instability testing in endometrial Cancer-Limitations and
advantages of four different PCR-Based approaches. Cancers (Basel).
13:12682021. View Article : Google Scholar
|
|
62
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar
|