Introduction
In 2020, there were an estimated 604,000 individuals
diagnosed with esophageal cancer, with >544,000 esophageal
cancer-associated deaths worldwide (1). In China, the esophageal cancer
incidence rate ranks it as the 7th most common type of cancer and
the 5th most common cause of cancer-associated death, with ~188,000
associated deaths in 2022 (2).
Squamous cell carcinoma is the most prevalent histological subtype
of esophageal cancer in Asia and Eastern Europe, especially for
cervical and upper thoracic esophageal cancer (3). Surgical resection is the primary
method of treatment for esophageal squamous cell carcinoma (ESCC)
without metastasis (4). However,
the optimal treatment plan for ESCC with supraclavicular lymph node
(SCLN) metastasis remains uncertain. Western countries typically
define SCLN metastasis as distant metastasis regardless of the
location of the ESCC (5), while in
Asian countries, only for middle and lower thoracic ESCC, SCLN
metastasis is indicative of a poor prognosis and should be defined
as a distant metastasis, whereas for upper thoracic and cervical
ESCC, it should be defined as regional lymph node metastasis
(6,7). However, in 2024, in the 12th Edition
of Staging of Esophageal Cancer released by the Japan Esophageal
Society (JES), SCLN metastasis was defined as a distant metastasis
for upper thoracic ESCC (8). Even
for cervical ESCC, SCLN metastasis is commonly indicative of a
significant deterioration in prognosis and should not be defined as
regional lymph node metastasis (9).
Previous studies regarding the prognosis of SCLN
metastasis in ESCC are based on cases undergoing surgical resection
(9–11). Although an esophagectomy can be used
to diagnose lymph node metastasis through pathological examination,
for cervical and upper thoracic ESCC, the value of esophagectomy
for prolonging overall survival rates is contested (12), Therefore, it is not accurate to
predict the prognosis of patients with SCLN metastasis based on the
survival rate of ESCC after esophagectomy. In addition,
inconsistent definitions of the supraclavicular region may also
affect the prognostic value of SCLN, as the conclusions of the
studies may not be comparable (8,13).
Chemoradiation therapy for cervical thoracic ESCC
with SCLN metastasis is widely adopted in Asian countries due to
its generally tolerable toxicity and clinical benefits (14). The aim of the present study was to
define the supraclavicular region on contrast-enhanced computed
tomography (CT) in order to investigate the prognostic value of
SCLN metastasis on cervical and upper ESCC receiving curative
chemoradiotherapy.
Patients and methods
Patients
Information on patients with ESCC diagnosed and
treated at the First Affiliated Hospital of Yangtze University
(Jingzhou, China) between December 2019 and December 2021 was
collected. The inclusion criteria were as follows: i) A
histopathological diagnosis of ESCC; ii) cases of cervical and
upper thoracic ESCC; that is, the primary tumor of the esophagus
was located between the esophageal inlet and the lower edge of the
azygos vein based on CT images; iii) patients were aged between 18
and 70 years old; iv) an Eastern Cooperative Oncology Group
performance status score of 0–1 (15); v) cases of locally advanced ESCC
that received curative radiotherapy and chemotherapy; and iv)
except for SCLN metastasis, patients showed no evidence of distant
metastasis prior to treatment. The exclusion criteria were as
follows: i) A histopathological diagnosis of adenocarcinoma or
other non-squamous cell carcinoma; ii) a hypopharyngoscopy revealed
the presence of hypopharyngeal cancer; iii) individuals receiving
palliative chemotherapy or palliative radiotherapy; and iv)
patients with a survival time of <3 months after curative
chemoradiotherapy.
The diagnostic criteria for metastatic lymph nodes
included at least one of the following: i) Lymph node with the
shortest diameter ≥0.6 cm on contrast-enhanced CT; ii)
fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT image
displayed a maximum standard uptake value >3.0; and iii) lymph
nodes confirmed as metastatic through ultrasound-guided biopsy. The
diagnosis and location of the metastatic lymph nodes were
determined by a radiologist and a radiation oncologist.
The Ethics Committee of the First Affiliated
Hospital of Yangtze University approved the present study (approval
no. 2021005), and as it was a retrospective analysis, the
requirement for informed patient consent was waived.
Radiotherapy procedure
Intensity-modulated radiation therapy or volumetric
modulation arc radiotherapy was used for all radiation treatments.
All patients included in the analysis underwent a contrast-enhanced
CT scan to obtain images of the entire cervical region, chest and
upper abdomen, with a slice thickness of 2.5 mm on a Siemens
Somatom 48 CT simulator (Siemens Healthineers). The obtained images
were transferred to the Eclipse 11 Treatment Planning System
(Varian Medical Systems, Inc.).
Gross target volume (GTV) includes gross target
volume of the primary tumor (GTV-p) and gross target
volume of the metastatic lymph nodes (GTV-n).
GTV-p includes esophageal tumors detected by CT scans,
esophageal barium examination, endoscopy, endoscopic ultrasound or
FDG-PET/CT. GTV-n is defined as the presence of
metastatic lymph nodes in the mediastinal and supraclavicular
regions. The clinical target volume of the primary tumor
(CTV-p) was positioned as a 3-cm extension along the
GTV-p and 0.5 cm on the lateral edge of the esophagus.
CTV of the lymph nodes (CTV-n) encompassed a 0.6-cm
margin around the GTV-n. The planning target volume
around the CTV (PTV-c) was established by adding a
uniform 0.5-cm margin around the CTV (CTV-p +
CTV-n). The planning target volume around the GTV
(PTV-g) was defined as a 0.3-cm margin around the GTV
(GTV-p + GTV-n).
The prescribed dosage was 50.4 Gy/28 fractions to
the PTV-c and 59.92 Gy/28 fractions to the
PTV-g. Organs at risk included the spinal cord, lungs
and heart. The treatment plan was evaluated based on the
dose-volume histogram. The treatment plan generally required that
the percentage of total lung volume treated with ≥5 Gy (V5 of the
whole lung) was ≤60%, the V20 of the whole lung was ≤28%, the V30
of the whole lung was ≤18% and the V30 of the heart was ≤30%. The
maximum spinal cord dose was <45 Gy. Cone beam CT online
validation radiotherapy was performed for 5 consecutive days in the
first week, and then once a week thereafter.
Chemotherapy and immunotherapy
The sequential, induction and consolidation
chemotherapy treatment used 5-fluorouracil + cisplatin (FP) or
docetaxel + cisplatin (TP) with the following regimen: For FP,
cisplatin (25 mg/m2/day) for 3 days and 5-fluorouracil
(750–1,000 mg/m2/day) for 5 days, repeated every 3
weeks; and for TP, docetaxel (75 mg/m2) for 1 day and
cisplatin (25 mg/m2/day) for 3 days, repeated every 3
weeks.
The concurrent chemotherapy regimen included FP, or
TP, or monotherapy with S1, with the following regimen: For FP,
cisplatin (25 mg/m2/day) for 3 days and 5-fluorouracil
(450–500 mg/m2/day) for 5 days, repeated every 4 weeks;
and for TP, docetaxel (75 mg/m2) for 1 day and cisplatin
(25 mg/m2/day) for 3 days, repeated every 4 weeks.
Alternatively, S1 was used as monotherapy, taken continuously for 2
weeks and then discontinued for 1 week, or taken only on the day of
radiotherapy (Monday-Friday), with weekend rest (40 mg twice per
day if the body surface area was <1.25 m2; 50 mg
twice per day if the body surface area was ≥1.25–1.5 m2;
or 60 mg twice per day if the body surface area was ≥1.5
m2).
Consolidation immunotherapy using either 200 mg
carolizumab or teralizumab was administered on day one, and
repeated every 3 weeks.
Definition of regional lymph
nodes
The range of lymph node regions was defined using
contrast-enhanced CT images based on the JES criteria, 12th edition
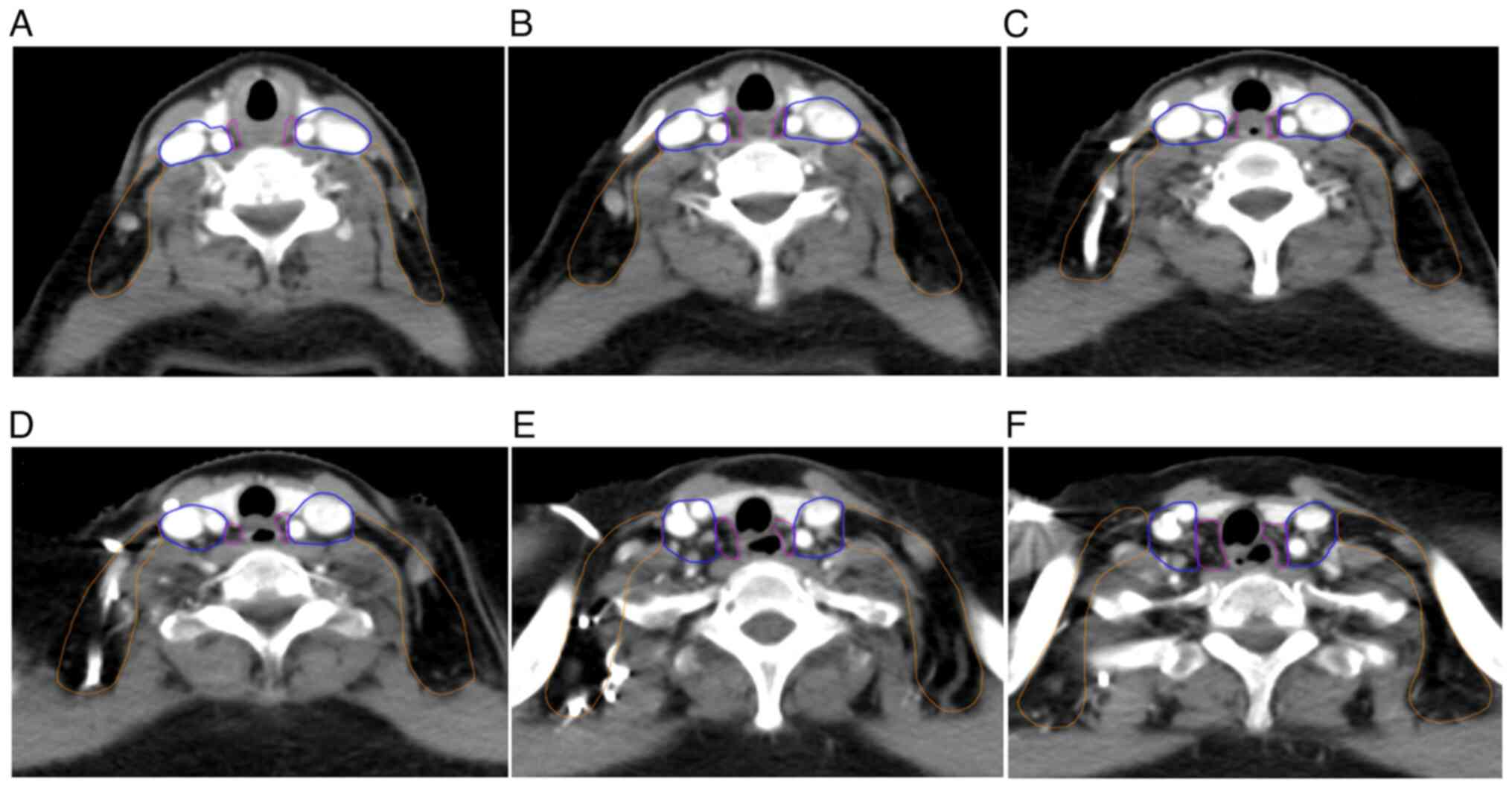
(8), with JES lymph node numbering.
Briefly, for Group I (no. 101), the cervical para-esophageal lymph
nodes, the boundaries defined on CT images were as follows: Upper
boundary, lower edge of cricoid the cartilage; lower boundary, apex
of the lung; anterior boundary, posterior edge of the thyroid;
posterior boundary, longus colli muscle; lateral boundary, medial
edge of the internal carotid artery; and inner boundary, esophageal
wall. As the probability of no. 104 lymph node metastasis is
different, lymph node region no. 104 was distinguished into Group
II and Group III. Group II was defined as the vascular sheath area
with the following boundaries: Upper boundary, lower edge of the
cricoid cartilage; lower boundary, lung apex level; anterior
boundary, anterior edge of the internal carotid artery; and
posterior boundary, posterior edge of the internal jugular vein.
Group III was defined as the vascular lateral space with the
following boundaries: Upper boundary, lower edge of the cricoid
cartilage; lower boundary, at the apex level; front boundary,
posterior margin of the internal jugular vein; and posterior
boundary, trapezius muscle. Group IV was defined as the regional
lymph nodes of the thoracic region (nos. 105-112) (8).
Follow-up and statistical
analysis
Follow-up was conducted using the hospital's
electronic case system and by telephone. The last follow-up was on
December 31, 2023, with a median follow-up time of 33 months
(range, 24–48 months). The Kaplan-Meier method was used to estimate
overall survival (OS), and a log-rank test was used to compare
survival between the Kaplan-Meier curves. Age and body mass index
(BMI) were compared using a Kruskal-Wallis test. PSM analysis
[including age, sex, BMI, tumor location, T-stage, lymph node
status, clinical stage based on the American Joint Committee on
Cancer (AJCC) staging system 8th edition (5) and treatment method] was performed
using a 1:1 nearest neighbor matching method with a caliper width
of 0.2. After balancing the clinical characteristics of the two
groups of ESCC patients, namely those with or without SCLN
metastasis, via PSM, the OS curve was plotted. The categorical
variables were evaluated using a χ2 test; when one of
the expected frequencies was ≤5, Fisher's test was used instead.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analysis was performed using GraphPad
Prism version 10 (Dotmatics).
Results
Baseline characteristics of the
patients
During the research period, data on 230 eligible
patients with ESCC was collected from the First Affiliated Hospital
of Yangtze University. A total of 208 ESCC (90.4%) were diagnosed
with lymph node metastasis by contrast-enhanced CT scans, 18 cases
(7.8%) were pathologically diagnosed with lymph node metastasis
according to ultrasound-guided biopsy and 4 cases (1.7%) were
diagnosed with lymph node metastasis by FDG-PET/CT. The number of
sequential, induction and consolidation chemotherapy cycles ranged
from 1–6, with a median of 3 cycles. The duration of consolidation
immunotherapy was 1–24 months, with a median time of 10 months. The
recruited patients were divided into two arms based on the presence
or lack of SCLN metastasis: Arm A consisted of Group I or Group IV
lymph node metastasis accompanied with no. 104 (Group II and Group
III) lymph node metastasis; and Arm B consisted of Group I (no.
101) or Group IV lymph node metastasis without no. 104 (Group II
and Group III) lymph node metastasis. As the probability of lymph
node metastasis in the supraclavicular region alone without
mediastinal lymph node metastasis was extremely low, these cases
were not calculated.
Table I shows the
detailed information of the patients with ESCC and a comparison
between the two arms. There were no significant differences in
baseline clinical characteristics between the two groups.
 | Table I.Baseline clinical characteristics of
the 230 eligible patients with esophageal squamous cell
carcinoma. |
Table I.
Baseline clinical characteristics of
the 230 eligible patients with esophageal squamous cell
carcinoma.
|
|
| Before PSM | After PSM |
|---|
|
|
|
|
|
|---|
|
Characteristics | Value | Arm Ab (n=71) | Arm Bc (n=159) | P-value | Arm Ab (n=54) | Arm Bc (n=54) | P-value |
|---|
| Median age (range),
years | 64 (45–80) | 64 | 64 | 0.861 | 64 | 64 | 0.952 |
| Sex, n (%) |
|
|
| 0.189 |
|
| 0.391 |
|
Male | 202 (87.83) | 59 | 143 |
| 45 | 49 |
|
|
Female | 28 (12.17) | 12 | 16 |
| 9 | 5 |
|
| Median BMI (range),
kg/m2 | 19.8 | 21.2 | 18.7 | 0.062 | 20.8 | 19.3 |
|
|
| (15.57–28.37) |
|
|
|
|
|
|
| Main tumor
location, n (%) |
|
|
| 0.081 |
|
| 0.202 |
|
Cervical | 63 (27.39) | 25 | 38 |
| 19 | 12 |
|
| Upper
thoracic | 167 (72.61) | 46 | 121 |
| 35 | 42 |
|
| Clinical depth of
tumor invasion |
|
|
| 0.758 |
|
| 0.999 |
|
cT1-2 | 5 (2.17) | 2 | 3 |
| 1 | 1 |
|
|
cT3 | 223 (96.96) | 68 | 155 |
| 52 | 52 |
|
|
cT4 | 2 (0.87) | 1 | 1 |
| 1 | 1 |
|
| Clinical lymph node
metastasisd, n
(%) |
|
|
| 0.236 |
|
| 0.319 |
|
cN1 | 149 (64.78) | 42 | 107 |
| 31 | 37 |
|
|
cN2 | 81 (35.22) | 29 | 52 |
| 23 | 17 |
|
| Clinical
staged, n (%) |
|
|
|
<0.0001a |
|
| - |
| II,
cT2N1M0 | 5 (2.17) | 0 | 5 |
| 0 | 0 |
|
| III,
cT3N1M0, cT1-3N2M0 | 153 (66.52) | 0 | 153 |
| 0 | 0 |
|
| IVA,
cT4N1-2M0 | 1 (0.43) | 0 | 1 |
| 0 | 0 |
|
| IVB,
cT1-4N1-2M1e | 71 (30.87) | 71 | 0 |
| 0 | 0 |
|
| Treatment status, n
(%) |
|
|
| 0.851 |
|
| 0.344 |
|
Sequential
chemoradiotherapy | 11 (4.78) | 3 | 8 |
| 3 | 3 |
|
|
Concurrent chemoradiotherapy
alone | 197 (85.65) | 63 | 134 |
| 47 | 46 |
|
|
Induction chemotherapy +
concurrent chemoradiotherapy | 7 (3.04) | 1 | 6 |
| 1 | 2 |
|
|
Concurrent chemoradiotherapy +
consolidation chemotherapy | 10 (4.35) | 3 | 7 |
| 2 | 2 |
|
|
Concurrent chemoradiotherapy +
consolidation immunotherapy | 5 (2.17) | 1 | 4 |
| 1 | 1 |
|
Distribution of metastatic lymph
nodes
All enrolled patients had lymph node metastases,
among which 51 (22.17%) cases were in Group I, 68 (29.57%) cases
were in Group II, 3 (1.30%) were in Group III and 167 (72.61%) were
in Group IV. The probability of lymph node metastasis in the
different groups is shown in Fig.
1. Due to the importance and inconsistency in the definition of
SCLNs, the range of para-esophageal and supraclavicular lymph nodes
was delineated on the CT map as shown in Fig. 2.
OS times
The median OS times of patients in Arm A and Arm B
were 17 and 30 months, respectively (P<0.001). The OS rates in
Arm A after 1, 2 and 3 years were 65, 16, and 9.7%, respectively.
The OS rates in Arm B after 1, 2 and 3 years were 80, 55, and 40%,
respectively. After PSM (54 patients in each group), the median OS
times in Arm A and Arm B were 17 and 28 months, respectively
(P<0.001). The OS rates in Arm A after 1, 2 and 3 years were 72,
18 and 11%, respectively. The OS rates in Arm B after 1, 2 and 3
years were 81, 54 and 39%, respectively (Fig. 3).
Failure patterns
During the follow-up period, there were a total of
101 ESCC cases with treatment failure in the irradiation field, 6
cases had esophageal metastasis in the non-irradiated field and 27
cases had regional lymph node metastasis in the non-irradiated
field. In addition, there were 33 cases of metastasis to the
distant lymph nodes or organ.
There was no significant difference in the local
failure rate between the groups with and without SCLN metastasis in
the irradiation field and the non-irradiation field, but the
probability of distant metastasis in the patients with SCLN
metastasis was significantly higher than that in the patients
without SCLN metastasis (P=0.025). Details of the failure patterns
are shown in Table II.
 | Table II.Failure patterns in the entire
esophageal squamous cell carcinoma cohort. |
Table II.
Failure patterns in the entire
esophageal squamous cell carcinoma cohort.
| Type of
failure | Arm Aa (n=71) | Arm Bb (n=159) | P-value |
|---|
| Irradiation field
failure, n (%) |
|
|
|
|
Esophageal recurrence | 15 (21.13) | 30 (18.87) | 0.690 |
|
Regional lymph nodes
recurrence | 17 (23.94) | 39 (24.53) | 0.924 |
| Non-irradiation
field failure, n (%) |
|
|
|
|
Esophagus failure | 1 (1.41) | 5 (3.14) | 0.669 |
|
Regional lymph nodes
failure | 8 (11.27) | 19 (11.95) | 0.999 |
| Metastasis to
distant lymph nodes or organs, n (%) | 16 (22.54) | 17 (10.69) | 0.025c |
Discussion
Due to significant differences in the pathological
types and primary tumor sites of esophageal cancer between the
Asian and Western populations, there is controversy regarding the
prognostic guiding value of SCLN metastasis. Western countries tend
to define metastases of the supraclavicular lymph nodes as distant
metastases (5), whereas Asian
countries suggest that the staging of SCLN should be determined
based on the location of the esophageal cancer (8). Additionally, the definition of the
supraclavicular region varies among different tumor types (8,13).
The description of the SCLN region in esophageal
cancer by the AJCC 8th edition (5)
and the JES 12th edition (16)
guidelines is not sufficiently detailed. For example, there is no
accurate description of the upper and lower boundaries of the
region. For surgeons, the boundary definition of the
supraclavicular region does not need to be very detailed, but this
can lead to misjudgment of SCLN metastasis, thus raising the
question of the predictive value of SCLN for prognosis. Zhong et
al (17) divided the
supraclavicular region into 6 subregions, and showed that
nasopharyngeal cancer mainly spread to the subregions of the
carotid sheath space, and vascular lateral space (VLS) I and II,
whereas ESCC tended to spread to the subregions of the
para-esophageal space, the sub-thyroid pre-trachea space, the
carotid sheath space and VLS I. Therefore, subdividing the
supraclavicular region can assist in the delineation of the CTV
during radiotherapy.
In clinical practice, it is necessary to determine
whether lymph node metastasis is located on a regional lymph node
based on contrast-enhanced CT images before treatment. The present
study provided a detailed description of the boundary lines between
the cervical para-esophageal lymph node region (no. 101) and the
SCLN region (no. 104). Based on the difference in probabilities of
lymph node metastasis in the supraclavicular region, no. 104 was
divided into two regions: Group II and Group III. Group II and
Group III are equally important for radiotherapy of head and neck
cancer (17), and breast cancer
(18), and thus both should be
included in the irradiation field. However, the present study
showed that the probability of Group III metastasis in the cervical
and upper thoracic segments of ESCC was only 1.3%. Luo et al
(19) reported that the probability
of lymph node metastasis in Group III was lower than that observed
in the present study at only 0.6%. However, the study included
esophageal cancer in the upper, middle and lower thoracic segments,
but did not include cases of cervical esophageal cancer. Yu et
al (20) analyzed the pattern
of lymph node treatment failure after surgery for thoracic ESCC and
found that lower cervical recurrence primarily occurred in the
4.3-cm area extending from both sides of the midline of the body,
which matched the Group I and Group II areas described in the
present study. Similarly, it was confirmed that prophylactic
irradiation of lymph nodes in esophageal cancer was unnecessary for
Group III (20).
Group IV and Group I are lymph node regions adjacent
to the upper thoracic and cervical esophagus, respectively. The
present results showed that the probability of metastasis in these
two regions reaches 72.61 and 22.17%, which is consistent with the
longitudinal distribution of the para-esophageal lymphatic vessels.
Group II is located in the carotid artery and venous sheath area,
and the probability of lymph node metastasis in this area reaches
29.57%. This is also a site that should be considered when elective
nodal irradiation is chosen for cervical and upper thoracic
esophageal cancer (21). However,
the gap between the posterior edge of the internal jugular vein and
the posterior edge of the sternocleidomastoid muscle has not been
separately listed, as reported by Zhong et al (17), as the probability of lymph node
metastasis at the posterior edge of the internal jugular vein was
extremely low and thus could be incorporated into Group III.
In the present study, it was found that the
probability of metastasis to supraclavicular lymph nodes (Group II
+ Group III) was 30.87%. Numata et al (9) found that the probability of SCLN
metastasis was 25.4%, but the study only included cases of cervical
ESCC that underwent radical surgery. Yu et al (20) analyzed the lymph node drainage
patterns in esophageal cancer based on postoperative lymph node
recurrence patterns. Surgery may cause blockage of some lymphatic
ducts and changes in lymph node metastasis pathways (22). In the present study, a judgment was
made based on the CT imaging at the initial diagnosis, reducing the
interference of treatment.
The results of the present study showed that the
median OS time of ESCC patients with SCLN metastasis was 17 months,
which was lower than that of patients without SCLN metastasis, but
still higher than the median OS time of 12.6 months reported in the
Keynote 590 study for locally advanced unresectable or metastatic
ESCC (23). Local treatment was
still necessary for patients with SCLN metastasis. Liu et al
(24) reported that ESCC patients
with supraclavicular lymph node metastasis could benefit from
surgery after neoadjuvant treatment (24). Moreover, a retrospective study
(6) showed that SCLN metastasis had
no significant impact on the median survival time of patients with
upper thoracic ESCC, with the median survival time of the SCLN
metastasis group and the other regional lymph node metastases group
recorded as 25.0±3.0 and 30.0±4.6 months, respectively (P=0.067).
However, Numata et al (9)
retrospectively analyzed 67 cases of cervical esophageal squamous
cell carcinoma treated with radical surgery. Compared with patients
with only para-esophageal lymph node metastasis, the 3-year
survival rate in the group with combined para-esophageal lymph node
metastasis and SCLN metastasis significantly decreased (60.1 vs.
7.8%, respectively). ESCC with SCLN metastasis was associated with
the location of the primary lesion. Okamura et al (25) reported a 5-year survival rate of
18.6–28.4% for thoracic esophageal cancer with SCLN metastasis
after curative esophagectomy. The prognosis of ESCC with SCLN
metastasis varied depending on the different segments of the
thoracic region. Wen et al (26) reported that upper thoracic
esophageal cancer with SCLC metastasis has a median survival time
of only 12 months and that surgery cannot improve the prognosis of
this group of patients. A retrospective study by Park et al
(7) analyzed 611 ESCC cases that
underwent radical esophagectomy and 3-field lymph node dissection.
The results showed that for upper ESCC, the 5-year survival rates
of patients with and without pathologically confirmed SCLN were
27.1 and 39.6%, while for middle and lower ESCC, the 5-year
survival rates of patients with and without pathologically
confirmed SCLN were 21.5 and 43.6%. The treatment method also
affected the prognosis of patients with ESCC with SCLN metastasis.
The 1-, 2- and 3-year OS rates for upper ESCC with SCLN metastasis
receiving curative radiotherapy and chemotherapy were 50, 31, and
12%, respectively, while the OS rates for the group receiving
curative surgery after 1, 2 and 3 years were 50, 0 and 0%,
respectively (26). A meta-analysis
showed that prophylactic SCLN dissection for ESCC did not prolong
the 3- and 5-year survival rates (27). Therefore, regardless of the location
of the primary lesion or the differences in treatment methods, the
occurrence of SCLN metastasis was often indicative of a very poor
prognosis. Therefore, supraclavicular lymph node metastases should
be defined as distant metastases rather than regional lymph node
metastases.
Whether accompanied by SCLN metastasis or not,
recurrence within the irradiation field was the primary cause of
treatment failure. The results of the present study showed that the
recurrence rate in the irradiation field with SCLN metastasis group
was 45.07%, while the recurrence rate in the irradiation field
without SCLN metastasis group was 43.4%. Xu et al (28) reported that the local treatment
failure rate and distant metastasis rates of locally advanced ESCC
after concurrent chemoradiotherapy were 26.6 and 16.3%, but they
did not describe the location of local failure, and there was no
indication of whether there was a difference in the probability of
distant metastasis with or without SCLN metastasis. The present
study showed that the probability of distant organ metastasis in
the group with SCLN metastasis was significantly higher than that
in the group without SCLN metastasis, at 22.54 and 10.69%,
respectively.
Autopsy anatomical data of esophageal tumor cases
indicated that the lymphatic vessel count in the longitudinal
muscle layer of the supraclavicular region was significantly lower
than that in the upper and lower mediastinum. This anatomical
feature determines that the supraclavicular region is prone to
lymph node skip metastasis, which is often indicative of a poor
prognosis (29).
The present study has several limitations. Firstly,
90.4% of the cases included in the study were defined as lymph node
metastasis based on the short diameter of lymph nodes (≥0.6 cm) on
contrast-enhanced CT, rather than being based on a pathological
diagnosis or PET-CT. This is since, in the real world, not every
patient agreed to continue with a mediastinal lymph node biopsy.
PET-CT has a higher specificity and sensitivity than CT in
diagnosing lymph node metastasis (30); however, PET-CT is expensive, and
medical insurance cannot reimburse the cost of this examination.
Most patients cannot afford the economic burden of PET-CT.
Therefore, in the real world, contrast-enhanced CT is more often
used to diagnose lymph node metastasis. Secondly, certain cases in
the present study received sequential radiotherapy and
chemotherapy, while others received chemotherapy consolidation and
immune consolidation treatment. However, the majority of patients
only received curative synchronous radiotherapy and chemotherapy,
which may have led to a certain degree of bias in the results.
Thirdly, there are slight differences between cervical and upper
thoracic ESCC. Compared with upper thoracic ESCC, cervical ESCC is
closer to the larynx, and surgeons needs to consider whether to
preserve the larynx while operating (31). However, the present study mainly
included cases of ESCC treated with radical chemoradiotherapy, so
it was not affected by surgery. In addition, the etiology,
histopathological types and lymph node metastasis patterns of
cervical ESCC are similar to those of upper thoracic ESCC. A number
of studies classified the two conditions into the same category of
upper esophageal cancer for analysis (12,28).
Therefore, when exploring the pattern of supraclavicular lymph node
metastasis and survival after curative chemoradiotherapy, the
present study included cervical and upper thoracic esophageal
cancer together, which did not affect the results.
Despite these limitations, the results of the
present study showed that for cervical and upper thoracic
esophageal cancer, SCLN metastasis was a poor prognostic factor and
should not be defined as regional lymph node metastasis.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Development Fund
Project of Yangtze University (grant no. WJ2019-22), the Hubei
Provincial Health and Family Planning Commission Joint Fund Project
(grant no. WJ2018H203) and the Jingzhou First People's Hospital
Doctoral Research Initiation Fund Project (grant no.
2022DIF01).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HZ and WZ conceived and designed the study, and
wrote and revised the manuscript. MZ and YS collected medical
record information, and collected and assessed the clinical and
pathological characteristic data of enrolled cases. SL and YX
followed up on the cases and analyzed the patterns of failure. WF
and LD conducted statistical analysis and drew the figures. MZ, YS,
SL and YX confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the First Affiliated
Hospital of Yangtze University approved the present study
(Jingzhou, China; approval no. 2021005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morgan E, Soerjomataram I, Rumgay H,
Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J and Arnold
M: The global landscape of esophageal squamous cell carcinoma and
esophageal adenocarcinoma incidence and mortality in 2020 and
projections to 2040: New estimates from GLOBOCAN 2020.
Gastroenterology. 163:649–658.e2. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng RS, Chen R, Han BF, Wang SM, Li L,
Sun KX, Zeng HM, Wei WW and He J: Cancer incidence and mortality in
China, 2022. Zhonghua Zhong Liu Za Zhi. 46:221–231. 2024.(In
Chinese). PubMed/NCBI
|
|
3
|
Verstegen MH, Harker M, van de Water C,
van Dieren J, Hugen N, Nagtegaal ID, Rosman C and van der Post RS:
Metastatic pattern in esophageal and gastric cancer: Influenced by
site and histology. World J Gastroenterol. 26:6037–6046. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Tang H, Fang Y, Tan L, Yin J, Shen
Y, Zeng Z, Zhu J, Hou Y, Du M, et al: Morbidity and mortality of
patients who underwent minimally invasive esophagectomy after
neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for
locally advanced esophageal squamous cell carcinoma: A randomized
clinical trial. JAMA Surg. 156:444–451. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rice TW, Gress DM, Patil DT, Hofstetter
WL, Kelsen DP and Blackstone EH: Cancer of the esophagus and
esophagogastric junction-major changes in the American joint
committee on cancer eighth edition cancer staging manual. CA Cancer
J Clin. 67:304–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang F, Ge X, Wang Z, Weng Y, Yin R and
You Q: Clinical significance and prognosis of supraclavicular lymph
node metastasis in patients with thoracic esophageal cancer. Ann
Transl Med. 8:902020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park SY, Lee J, Jeon YJ, Cho JH, Kim HK,
Choi YS, Zo JI and Shim YM: Clinical and pathologic supraclavicular
lymph node metastases in esophageal squamous cell carcinoma treated
by esophagectomy with three-field lymph node dissection. Ann Surg
Oncol. 31:3399–3408. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mine S, Tanaka K, Kawachi H, Shirakawa Y,
Kitagawa Y, Toh Y, Yasuda T, Watanabe M, Kamei T, Oyama T, et al:
Japanese classification of esophageal cancer, 12th edition: Part I.
Esophagus. 21:179–215. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Numata Y, Abe T, Higaki E, Hosoi T,
Fujieda H, Nagao T, Hanai N, Suzuki H, Nishikawa D, Matsuo K, et
al: Should the supraclavicular lymph nodes be considered regional
lymph nodes in cervical esophageal cancer? Ann Surg Oncol.
29:616–626. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu Y, Xu L, Chen X, Li H, Liu Q, Zhang R,
Xie H, Chen Y, Yuan L, Tan B, et al: Neoadjuvant therapy combined
with surgery is superior to chemoradiotherapy in esophageal
squamous cell cancer patients with resectable supraclavicular lymph
node metastasis: A propensity score-matched analysis. Ann Transl
Med. 10:3492022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tachimori Y, Ozawa S, Numasaki H,
Matsubara H, Shinoda M, Toh Y and Udagawa H; Registration Committee
for Esophageal Cancer of the Japan Esophageal Society, :
Supraclavicular node metastasis from thoracic esophageal carcinoma:
A surgical series from a Japanese multi-institutional nationwide
registry of esophageal cancer. J Thorac Cardiovasc Surg.
148:1224–1229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patel DC, Yang CJ, Liou DZ and Berry MF:
Treatment and outcomes of proximal esophageal squamous cell
carcinoma. Ann Surg Oncol. 30:818–827. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Zhang H, Wang L, Xie C, Yu H and
Zhong Y: Optimization of supraclavicular lymph node clinical target
volume delineation in high-risk breast cancer: A single center
experience and recommendation. BMC Cancer. 23:11682023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Zhang W, Zhang B, Qian D, Li X,
Zhang H, Wang Q, Zhao L, Pang Q and Wang P: Clinical results of
intensity-modulated radiotherapy for 250 patients with cervical and
upper thoracic esophageal carcinoma. Cancer Manag Res.
11:8285–8294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higgins MI and Master VA: Who really knows
the performance status: The physician or the patient? Cancer.
127:339–341. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Doki Y, Tanaka K, Kawachi H, Shirakawa Y,
Kitagawa Y, Toh Y, Yasuda T, Watanabe M, Kamei T, Oyama T, et al:
Japanese classification of esophageal cancer, 12th edition: Part
II. Esophagus. 21:216–269. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong Z, Wang D, Liu Y, Shao S, Chen S, He
S, Yang N, Li C, Ren J, Zhao Y, et al: Lymph drainage and cervical
fascia anatomy-oriented differential nodal CTV delineation at the
supraclavicular region for esophageal cancer and nasopharyngeal
cancer. Radiother Oncol. 177:113–120. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jing H, Tang Y, Wang ZZ, Wei R, Jin JY, Li
J, Zhao LY, Jin J, Liu YP, Song YW, et al: Individualized clinical
target volume for irradiation of the supraclavicular region in
breast cancer based on mapping of the involved ipsilateral
supraclavicular lymph nodes. Int J Radiat Oncol Biol Phys.
115:922–932. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo Y, Liu Y, Wang X, Zhang B, Yu J, Wang
C, Huang Y and Li M: Mapping patterns of nodal metastases in
esophageal carcinoma: Rethinking the clinical target volume for
supraclavicular nodal irradiation. J Thorac Dis. 8:3132–3138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu J, Ouyang W, Li C, Shen J, Xu Y, Zhang
J and Xie C: Mapping patterns of metastatic lymph nodes for
postoperative radiotherapy in thoracic esophageal squamous cell
carcinoma: A recommendation for clinical target volume definition.
BMC Cancer. 19:9272019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Zhu M, Xiang Y, Sun Y, Li S, Cai
J and Zeng H: Current and future perspectives in unresectable
locally advanced esophageal squamous cell cancer (review). Oncol
Rep. 51:652024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bassalobre M, Liebano RE, da Silva MP,
Castiglioni MLV, Sadala AY, Ferreira LM and Nahas FX: Changes in
the pattern of superficial lymphatic drainage of the abdomen after
abdominoplasty. Plast Reconstr Surg. 149:1106e–1113e. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun JM, Shen L, Shah MA, Enzinger P,
Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, et al:
Pembrolizumab plus chemotherapy versus chemotherapy alone for
first-line treatment of advanced oesophageal cancer (KEYNOTE-590):
A randomised, placebo-controlled, phase 3 study. Lancet.
398:759–771. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H,
Zhu L, Shen Y, Zhang H, Sun Y, et al: Multicenter, single-arm,
phase II trial of camrelizumab and chemotherapy as neoadjuvant
treatment for locally advanced esophageal squamous cell carcinoma.
J Immunother Cancer. 10:e0042912022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okamura A, Watanabe M, Kozuki R, Toihata
T, Yuda M, Imamura Y and Mine S: Supraclavicular and celiac
metastases in squamous cell carcinoma of the middle thoracic
esophagus. Langenbecks Arch Surg. 403:977–984. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wen J, Chen D, Zhao T, Chen J, Zhao Y, Liu
D, Wang W, Xu X, Fan M, Chen C and Chen Y: Should the clinical
significance of supraclavicular and celiac lymph node metastasis in
thoracic esophageal cancer be reevaluated? Thorac Cancer.
10:1725–1735. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsunoda S, Hoshino N, Yoshida S and Obama
K: Prophylactic supraclavicular lymph node dissection for
esophageal squamous cell carcinoma: A systematic review and
meta-analysis. Surg Today. 53:647–654. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Y, Dong B, Zhu W, Li J, Huang R, Sun Z,
Yang X, Liu L, He H, Liao Z, et al: A phase III multicenter
randomized clinical trial of 60 Gy versus 50 Gy radiation dose in
concurrent chemoradiotherapy for inoperable esophageal squamous
cell carcinoma. Clin Cancer Res. 28:1792–1799. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumakura Y, Yokobori T, Yoshida T, Hara K,
Sakai M, Sohda M, Miyazaki T, Yokoo H, Handa T, Oyama T, et al:
Elucidation of the anatomical mechanism of nodal skip metastasis in
superficial thoracic esophageal squamous cell carcinoma. Ann Surg
Oncol. 25:1221–1228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lei X, Cao Z, Wu Y, Lin J, Zhang Z, Jin J,
Ai Y, Zhang J, Du D, Tian Z, et al: Preoperative prediction of
clinical and pathological stages for patients with esophageal
cancer using PET/CT radiomics. Insights Imaging. 14:1742023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Watanabe A, Taniguchi M, Kimura Y, Kikkawa
T and Hosokawa M: Larynx-preserving hybrid surgery with endoscopic
laryngopharyngeal surgery and open surgery for cervical esophageal
cancer invading pharynx. Dis Esophagus. 33:doaa0202020. View Article : Google Scholar : PubMed/NCBI
|