Introduction
Mucinous tubular and spindle cell carcinoma (MTSCC)
represents a rare subtype of renal cell carcinoma characterized by
unique biological features. In the 2004 WHO classification of renal
tumors, it was first recognized as a distinct entity (1). However, in the 2016 edition of the
classification standards, the description of it as malignant and
low-grade tumor was removed (2). It
has a wide age distribution (13–82 years), with a mean age of onset
of 53 years, and is more common in women (3). Due to the lack of significant clinical
manifestations and imaging characteristics, the diagnosis of MTSCC
primarily relies on pathological histology and immunohistochemical
examination (4). Pathologically,
MTSCC is characterized by tightly packed, elongated tubules
transitioning to spindle cell areas and mucinous stroma (5). Although surgery is the primary
treatment modality and most patients have a favorable prognosis,
the risk of recurrence and distant metastasis should not be
overlooked (6). Ged et al
(7) conducted a study of 25 cases
at their institution, reporting a 3-year survival rate of 84.0%,
with a median follow-up time of 3.9 years (range, 1 month to 10.3
years). The present article reports the treatment of a patient with
MTSCC at Zhuji People's Hospital (Zhuji, China) in October 2020,
who experienced renal recurrence and retroperitoneal metastasis
shortly after surgery, with rapid deterioration of the condition.
Additionally, it summarizes literature-reported metastatic cases,
aiming to provide clinical insights by reviewing their clinical
manifestations, treatment strategies and prognoses.
Case report
A 31-year-old male patient was admitted at Zhuji
Fourth People's Hospital (Zhuji, China) in October 2020, with a
half-month history of painless gross hematuria, which was
continuous, light red without clots, and worsened after consuming
spicy food. An initial urinary system ultrasound performed
externally suggested the presence of a mass in the right kidney.
The patient then sought treatment at Zhuji People's Hospital
(Zhuji, China) in October 2020. A physical examination demonstrated
no significant positive findings, including negative rebound
tenderness in the abdomen, no tenderness upon percussion in the
renal area and no palpable abdominal mass. Laboratory tests
revealed the following: Urine white blood cell count, 233 U/µl
(reference values, <25.0 U/µl); urine red blood cell count,
10,204/µl (reference values: 0.0–20.0 U/µl); uric acid level, 571
µmol/l (reference values: Male 202–416 µmol/l); lactate
dehydrogenase, 296 U/l (reference values: 120–250 U/l); and BCA,
creatinine, urea nitrogen and tumor markers (CEA, AFP, CA 19-9, CA
72-4 and CA 125) were all negative. Further upper abdominal CT
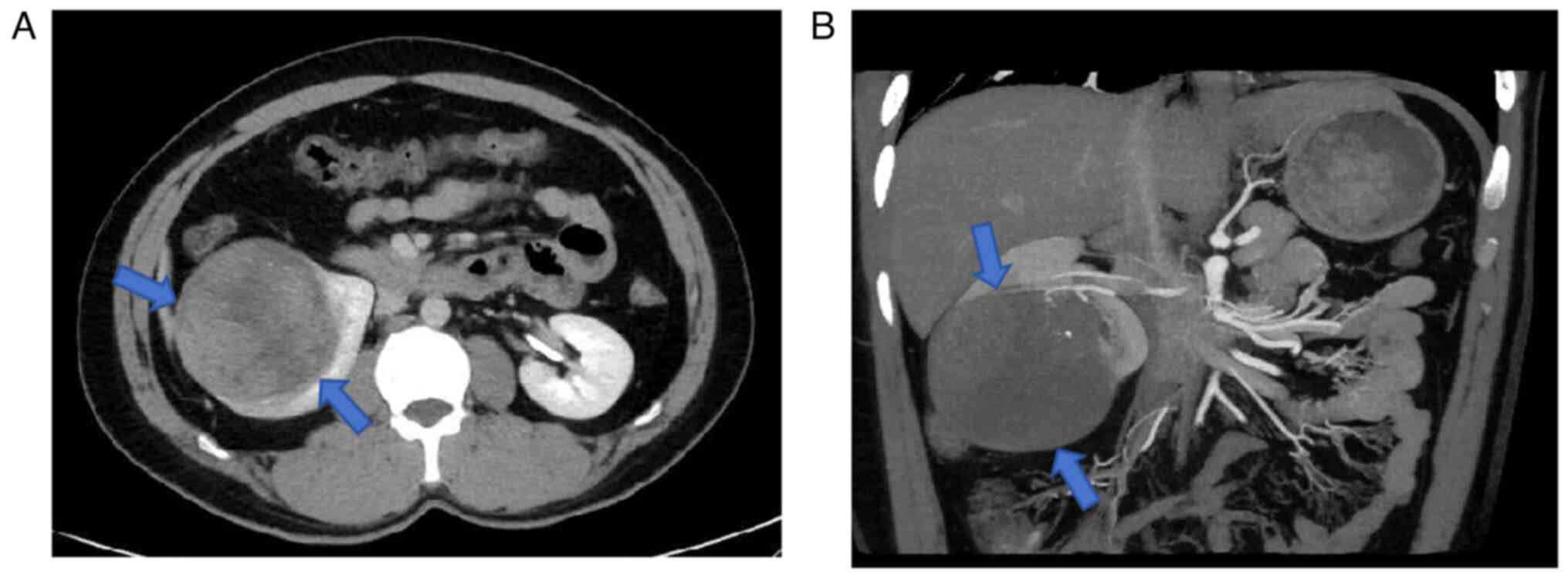
scans with and without contrast revealed a roughly circular mass at
the lower pole of the right kidney with clear boundaries, smooth
edges and uneven internal density, including patchy hemorrhage,
necrosis and fine calcifications, measuring ~7.6×6.7 cm (Fig. 1A). Contrast-enhanced CT showed mild,
uneven enhancement in the lesion area, no significant
lymphadenopathy and thrombosis. Renal artery CT angiography
indicated that the tumor was primarily supplied by the renal artery
with sparse internal vasculature (Fig.
1B). A chest CT showed no metastatic lesions. Based on these
findings, a preoperative diagnosis of a malignant tumor with
insufficient right kidney blood supply was made.
Under general anesthesia, the patient underwent
laparoscopic right radical nephrectomy, which lasted 3.5 h with an
estimated blood loss of ~200 ml. The resected kidney specimen
(measuring ~8×7×7 cm) partially protruded from the kidney surface,
with an intact capsule, and the cut surface revealed a soft tumor
containing necrotic tissue without noticeable mass in the renal
pelvis. A biopsy specimen was first fixed using Zhejiang Jinhua
Tonghe Biotechnology Co., Ltd. Biological Tissue Fixative (10%
neutral buffered formalin fixative; ready-to-use) at 25°C for 12 h,
after which samples were cut to 3 µm-thick. The sections were
stained with HE stain at 25°C for 45 min and imaged using a Zeiss
Axio-Lab-A1 microscope. Microscopically, the tumor tissue showed
infiltrative growth, invading the renal parenchyma but not the
perirenal fat tissue (Fig. 2A).
Tumor cells were partly plump and spindle-shaped, with eosinophilic
cytoplasm, arranged in bundles. Certain cells had unclear
boundaries and transparent cytoplasm, with oval nuclei and atypical
mitoses (Fig. 2B). The stroma
showed focal mucinous changes, vascular proliferation and extensive
necrosis (Fig. 2C). No nerve,
vascular invasion or intravascular tumor thrombus was seen. For
immunohistochemistry, tissue sections (3-µm thick) were fixed in
Zhejiang Jinhua Tonghe Biotechnology Co., Ltd., Biological Tissue
Fixative (10% neutral buffered formalin) at 25°C for 12 h, and
embedded in paraffin. Staining was performed using the DAKO
Autostainer Link 48 system (Agilent Technologies, Inc.). The
following primary antibodies (prediluted by the manufacturer) from
Guangzhou Anbiping Medical Laboratory Co., Ltd., were used: Ki67
(cat. no. IM098), CD10 (cat. no. IM025), EMA (cat. no. IR074),
PAX-8 (cat. no. IR191), Vimentin (cat. no. IM142), CK7 (cat. no.
IM061), CK (cat. no. IM067), CD34 (cat. no. IM034), P504s (cat. no.
IR127), CK20 (cat. no. IR385), SMA (cat. no. IHC-M005), P53 (cat.
no. IM123) and CD30 (cat. no. IM032). All primary antibodies were
incubated with the samples at 25°C for 30 min. The secondary
antibodies EnVision FLEX+, Mouse, High pH (Link; prediluted by the
manufacturer; cat. no. K8002; Agilent Technologies, Inc.; EnVision
FLEX+) were used at 25°C for 20 min. Blocking was performed with 3%
peroxidase blocking reagent (cat. no. DAKO SM801; Agilent
Technologies, Inc.) at 25°C for 5 min, followed by incubation with
EnVision FLEX/HRP (cat. no. DAKO SM802; Agilent Technologies, Inc.)
at 25°C for 20 min. DAB incubation was carried out at 25°C for 5
min (cat. no. DAKO DM827). The microscope used for examination was
an Olympus BX-51 microscope with a camera adaptor (Olympus
U-TV0.5XC-3; Olympus Corporation) for obtaining images.
Immunohistochemistry results revealed Ki-67 labeling index (~40%;
Fig. 2D), CD10 (−; Fig. 2E), epithelial membrane antigen [EMA
(++); Fig. 2F], paired box gene 8
[PAX8 (−); Fig. 2G],Vimentin (+++;
Fig. 2H), CK7 (−; Fig. 2I), CK (++; Fig. 2J), α-methylacyl-CoA racemase [P504S
(−) Fig. 2K], CD34 vessels (+;
Fig. 2L),CK20 (−; data not shown),
SMA (−; data not shown), P53 (−; data not shown), CD30 (−; data not
shown), placental alkaline phosphatase (++; data not shown) and
Desmin (−; data not shown). Based on morphology and
immunohistochemistry, a final diagnosis of high-grade MTSCC (tumor
size ~8×4×3.5 cm) was made. No tumor invasion was seen in the
ipsilateral ureter or vascular margins. The tumor was classified as
T2N0M0 (8), with extensive necrosis
and atypical mitoses, World Health Organization (WHO)/International
Society of Urological Pathology grade 3 (2), but without sarcomatoid
transformation.
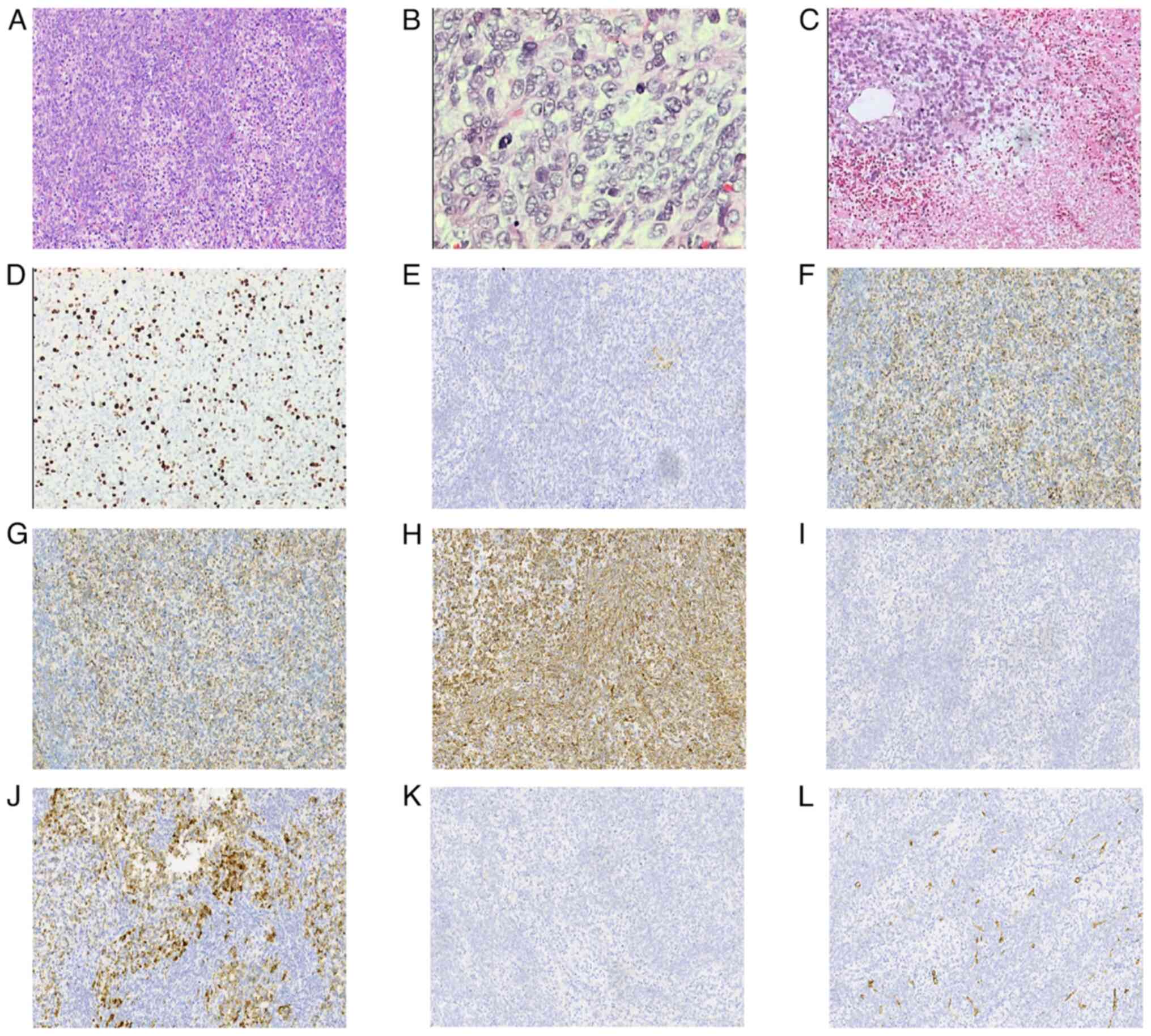 | Figure 2.Histological and immunohistochemical
findings. (A) Tumor cells are partly plump and spindle-shaped, with
eosinophilic cytoplasm, arranged in bundles (magnification, ×200).
(B) Certain cells had unclear boundaries and transparent cytoplasm,
with oval nuclei and atypical mitoses (magnification, ×400). (C)
The stroma revealed focal mucinous changes, vascular proliferation
and extensive necrosis (magnification, ×200). (D) Positive
cytoplasmic staining of Ki-67 (magnification, ×200). (E) Negative
cytoplasmic staining of CD10 (magnification, ×200). (F) Positive
cytoplasmic staining of epithelial membrane antigen (magnification,
×200). (G) Negative cytoplasmic staining of paired box gene 8
(magnification, ×200). (H) Positive cytoplasmic staining of
Vimentin (magnification, ×200). (I) Negative cytoplasmic staining
of CK7 (magnification, ×200). (J) Positive cytoplasmic staining of
CK (magnification, ×200). (K) Negative cytoplasmic staining of
P504S (magnification, ×200). (L) Positive cytoplasmic staining of
CD34 vessels (magnification, ×200). |
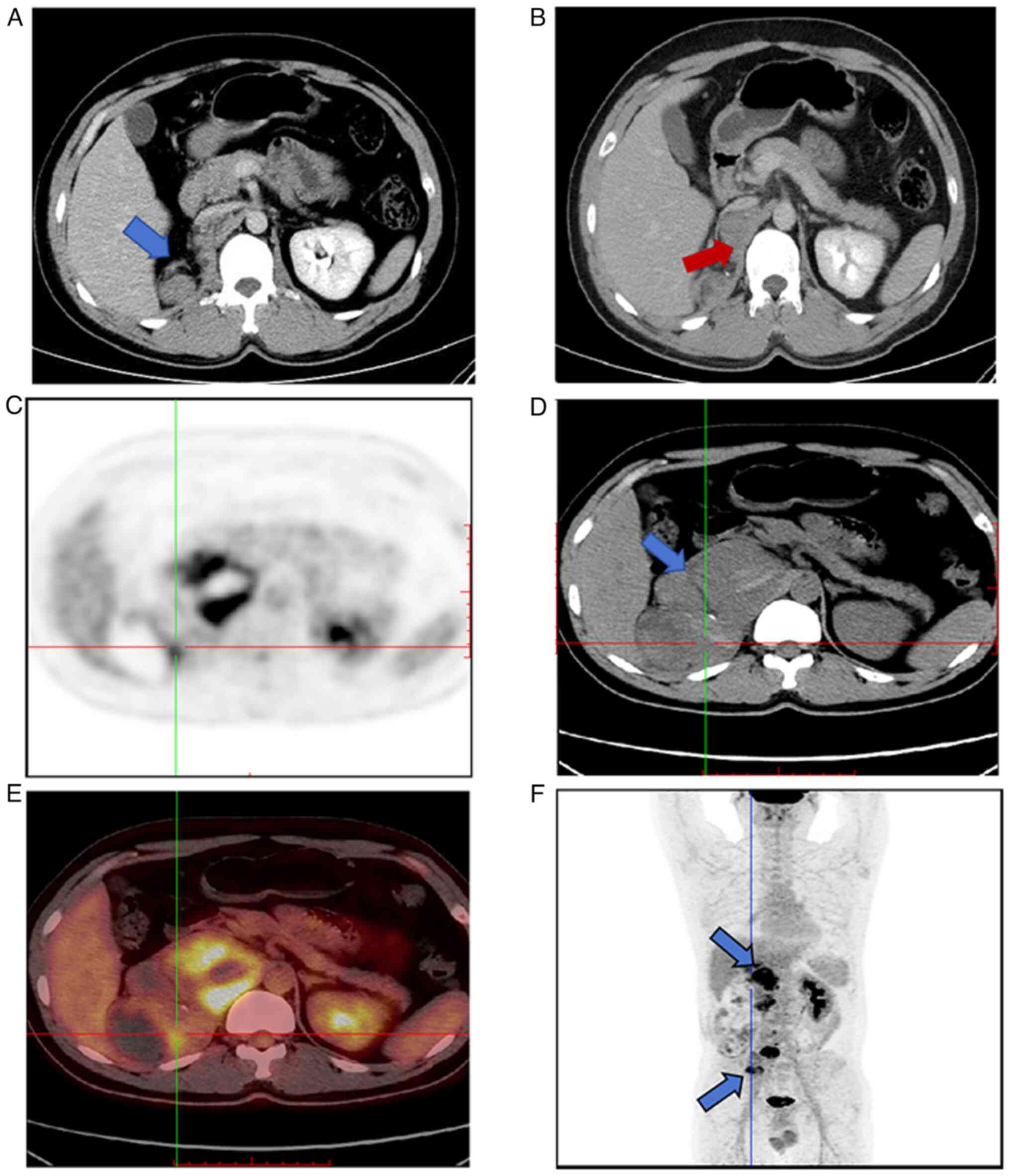
A total of 3 months post-operation, abdominal
enhanced CT with contrast revealed scattered soft tissue nodules
with clear boundaries next to the right renal area, one of which
was associated with hemorrhage (Fig. 3A
and B). CT values ranged from 23–56 Hu and the largest nodule
measured ~1.9×1.3 cm, showing mild uneven enhancement. The patient
sought further treatment at Zhejiang Cancer Hospital (Hangzhou,
China) in March 2021, where a PET-CT scan indicated multiple
recurrences or metastases around the right kidney surgical area
(Fig. 3C-E), inferior vena cava,
right psoas muscle (Fig. 3F), right
mesentery, peritoneum and serosal surface of the right hemicolon,
with possible hemorrhage within the masses. The right liver,
diaphragm, right psoas muscle, right iliopsoas muscle and right
abdominal wall muscles were involved, with potential emboli
formation in the inferior vena cava and left common iliac artery.
The treatment with 200 mg tislelizumab (intravenously once every 3
weeks) and 800 mg pazopanib (orally once a day) was initiated for
one cycle, but the response was poor.
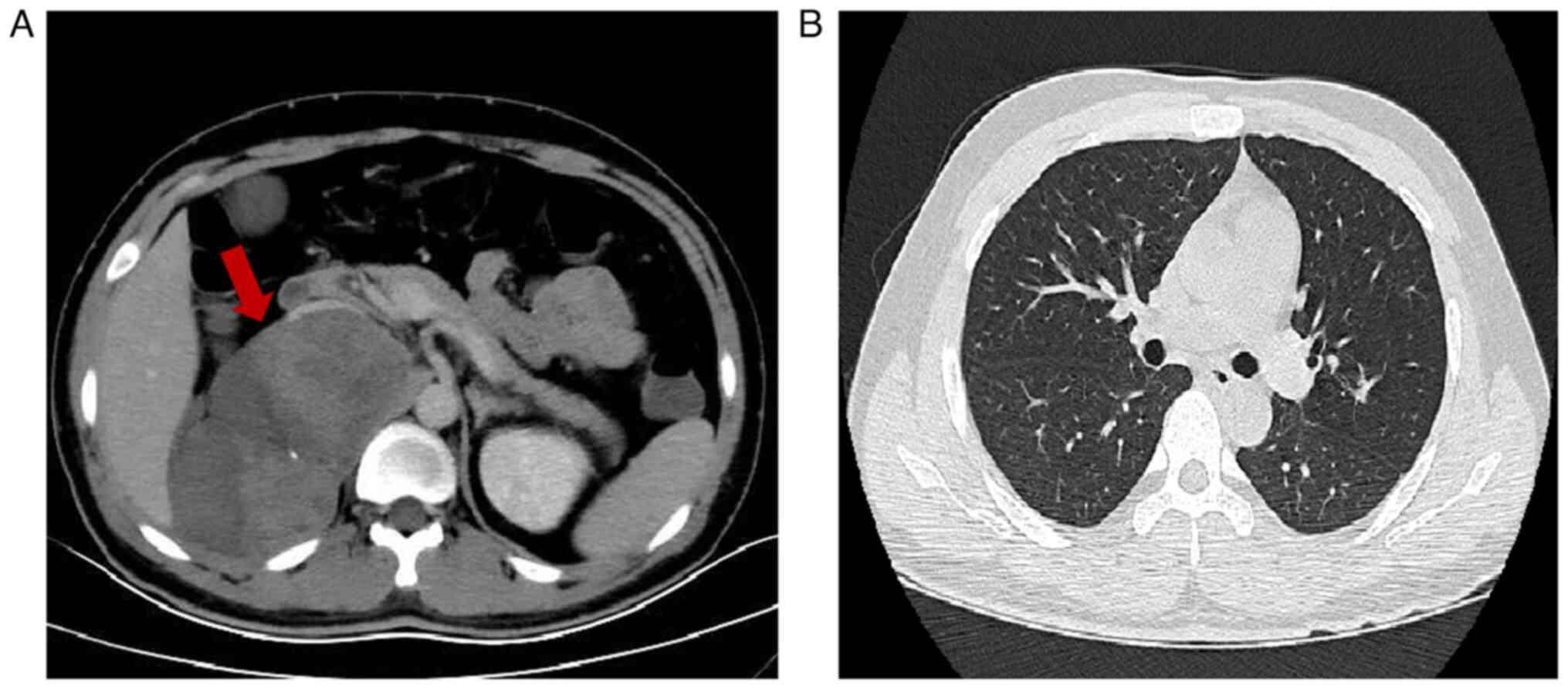
A total of 5 months post-operation, the patient was
readmitted at Zhuji People's Hospital (Zhuji, China) in March 2021
due to a fever, and a full abdominal enhanced CT identified
multiple soft tissue nodules and mass shadows in the right renal
area, retroperitoneum, paraspinal muscles and surrounding the
ascending colon and inguinal area (Fig.
4A). Certain nodules fused into masses with internal bleeding
and necrosis, the largest mass measured ~12.3×7.2 cm, with areas
showing mild uneven enhancement. Moreover, chest CT revealed
inflammation in the lower lobe of the right lung, without obvious
metastatic lesions (Fig. 4B). The
patient was treated with intravenous linezolid glucose injection
(every 12 h), combined with 0.3 g biapenem (every 8 h) and 10 ml
oral ibuprofen suspension for fever reduction. Despite these
interventions, the high fever persisted, leading to progressive
severe anemia and oliguria. The patient was administered
transfusions of packed red blood cells (1.5 U) to improve
oxygen-carrying capacity, antipyretics (ibuprofen suspension 10
ml/6 h) and linezolid glucose injection (0.6 g IV infusion once
every 12 h) combined with biapenem (0.3 g IV infusion once every 8
h) for anti-infection treatment. However, no significant
improvement was recorded. Subsequently, the patient developed
Gram-positive bacterial sepsis and acute renal failure, and was
discharged in April 2021, at the request of the patient. Follow-up
via phone later that month confirmed the patient had died.
Discussion
MTSCC is a rare subtype of renal cancer that
typically manifests as a low-grade and indolent tumor, and accounts
for <1% of all renal cancer incidences (9). However, the exact incidence of MTSCC
is unclear. In the study by Xu et al (10), it is reported that the incidence of
MTSCC at their institution was 0.52% of all cases of renal cell
cancer (22/4,197 cases). MTSCC is distinguished by its unique
morphology of renal tubules and spindle cells, along with a
mucinous extracellular matrix, classified by the WHO in 2004 as a
low-grade polymorphic renal epithelial tumor (11). Although MTSCC affects a wide age
range, with a male-to-female ratio of ~1:4, most cases are
incidentally discovered during physical examinations, with a
minority presenting with flank pain or painless hematuria (12). The patient in the present case was
male and presented with painless hematuria, which is consistent
with reported cases (6,7,12).
Imaging findings for MTSCC tumors typically reveal a solitary solid
lesion located within the kidney, sometimes protruding from the
renal parenchyma (13). These
lesions have relatively uniform density, with occasional punctate
or patchy calcifications, small areas of hemorrhage or cystic
necrosis (14). Enhanced CT shows
that tumor enhancement during the cortical phase is usually lower
than that of the renal parenchyma, whilst in the renal parenchymal
and excretory phases, it exhibits gradual and persistent mild
enhancement (15). The tumor
characteristics of the patient described in the present report
study are consistent with those reported in the literature,
presenting as a solitary roundish mass within the renal parenchyma,
accompanied by scattered patchy hemorrhage, necrosis and fine
punctate calcifications, with persistent mild enhancement.
From a pathological perspective, typical MTSCC
features include spindle cells and tubular structures interspersed
with mucinous stroma, with varying proportions of different
components within the tumor (16).
Although the vast majority of MTSCCs are low-grade, with
inconspicuous nucleoli and minimal atypia, literature reports have
also described high-grade MTSCCs, characterized by larger nuclei,
prominent nucleoli, coagulative necrosis and areas of sarcomatoid
transformation, indicating the malignant potential of MTSCC
(17). This present case showed
prominent nucleoli, atypical mitoses and extensive necrosis, with
no invasion into perirenal fat, vessels or nerves, diagnosed as
pT2a stage. Although no sarcomatoid transformation was observed, it
indicates the malignant potential of MTSCC.
Immunohistochemically, MTSCC typically expresses
distal renal tubular markers such as cytokeratin, vimentin and EMA,
whilst proximal tubular markers such as CD10 and villin are often
negative (18). The literature
reports that vimentin, PAX8, EMA, P504S and low molecular weight
cytokeratins (such as, CK7, CK8/18 and 34βE12) are usually
positively expressed, whilst CD10 is partially positive. Markers
such as carbonic anhydrase IX, CK20 and GATA binding protein 3 are
typically negative, and the Ki-67 index may be elevated in
high-grade tumors (19). The
present case demonstrated a positive expression of EMA and
vimentin, and a negative expression of PAX8, CK7 and CD10. Combined
with its morphological appearance and immunohistochemistry, the
diagnosis was MTSCC. Furthermore, the Ki-67 index of ~40% suggested
a high-grade tumor.
Despite being generally regarded as low-grade and
indolent tumors, reports of lymph node and distant metastases of
MTSCC have gradually increased in recent years, highlighting its
potential malignancy (6,12,15–19). A
review of the literature and a summary of metastatic cases,
including this case, totaled 22 cases (6,12,15–29),
in which there were 16 male and 6 female patients, with a
male-to-female ratio of 2.6:1, and the median age was 64 years
(range, 31–82 years). Although MTSCC can occur at any age and is
more common in females, the summarized cases demonstrated that
middle-aged and older males may be more at risk of metastasis in
MTSCC, consistent with the report by Huang et al (24). However, a larger number of cases is
needed to verify this. Furthermore, the average size of the tumor
was ~7.1 cm (range, 1–18 cm). A total of two cases were located
within the renal pelvis and 14 cases were in the renal parenchyma
near the medullary region, with larger lesions protruding beyond
the renal outline externally and inward toward the renal sinus.
Imaging of 12 cases (54%) showed heterogeneous density with
necrosis, hemorrhage or cystic changes. Preoperative imaging and
postoperative pathology indicated that 12 cases (60%) had invasion
into the perirenal fat, lymphatic vessels or veins, with one case
developing an inferior vena cava thrombus. This indicates that when
tumors are large, located deep within the kidney (near the
medullary sinus area) and accompanied by infiltration of
surrounding tissues, lymphatic vessels or veins, as well as the
presence of necrosis or hemorrhage, the aggressiveness of the tumor
increases, consistent with reports by Ursani et al (6) and Uchida et al (12) (Table
I). Furthermore, of the summarized cases, 6 showed sarcomatoid
transformation, 10 had necrosis, and 8 exhibited atypical mitoses
of grade ≥3. A total of 5 cases (22%) suggested low-grade tumors,
and among these, 3 had metastasis preoperatively, 1 showed inferior
vena cava thrombus and 2 died during follow-up. Although most
metastatic behaviors are observed in high-grade MTSCC, the
possibility of high malignancy and metastasis in low-grade tumors
cannot be entirely excluded (6,20,23,25,26),
emphasizing the importance of regular postoperative follow-ups.
Moreover, of the 22 summarized cases, 7 had distant metastasis
preoperatively and 9 had lymph node metastasis. Except for one
patient who received sunitinib treatment without surgery, the
remaining 19 patients underwent partial or radical nephrectomy,
with 8 receiving adjuvant therapy (such as tyrosine kinase
inhibitors, monoclonal antibodies and radiotherapy). Following
aggressive treatment, 11 patients were still alive during
follow-up, with survival times ranging from 3 weeks to 130 months.
A total of four cases achieved tumor-free status, whilst 7 cases
exhibited coexistence with the disease. Prior to surgery, seven
patients were diagnosed with a clinical stage of IV; however,
following active treatment, five patients remained alive. Even if
MTSCC has metastasized distantly, aggressive surgical treatment,
metastasectomy and molecular targeted or immunosuppressive therapy
remain recommended treatment methods, helping to control disease
progression (17,24,25).
Furthermore, out of the summarized cases, during the follow-up
period, the shortest time to first detection of metastasis after
surgery was 3 weeks in one case (27), and the longest was 91 months
(25), indicating a wide time span.
This highlights the importance of developing personalized follow-up
strategies for early detection of disease recurrence or
metastasis.
 | Table I.Summary of the characteristics of
cases of mucinous tubular and spindle cell carcinoma in the
literature. |
Table I.
Summary of the characteristics of
cases of mucinous tubular and spindle cell carcinoma in the
literature.
| First author/s,
year | Case | Sex | Age, years | Location | Size, cm | pTNM stage | Invasion of
surrounding tissues | Pre-operative
metastasis | Nephrectomy | Necrosis | Nuclear
gradea | Sarcomat-oid
transformation | Adjuvant therapy | Postoperative
metastasis sites | Prognosis | (Refs.) |
|---|
| Present case | 1 | Male | 31 | Right | 8 | T2aN0M0 | No | No | RN | Yes | High | No | Tislelizumab | Retroperito- | DOD | - |
|
|
|
|
|
|
|
|
|
|
|
| (grade 3) |
| and pazopanib | neum, muscle | (5 weeks) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| and mesentery |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (3 months) |
|
|
| Ursani et
al, | 2 | Female | 64 | Left | 18 | T3aN1M1 | Perirenal fat | Liver and | RN | No | Low | No | No | Liver and LN | AWD | (6) |
| 2010 |
|
|
|
|
|
|
| LN |
|
|
|
|
|
| (16 months) |
|
| Uchida et
al, | 3 | Male | 71 | Right | 3 | T3aN0M0 | Blood vessel | No | PN | No | High | No | Sunitinib, | Lung, bone, | DOD | (12) |
| 2017 |
|
|
|
|
|
|
|
|
|
| (grade 3) |
| temsirolimus | liver and
pleura) | (24 months) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| and axitinib | (1 months |
|
|
|
| 4 | Male | 64 | Right | 8 | T3aN0M0 | Blood vessel | No | RN | No | High | No | No | Lung (6
months) | DOD |
|
|
|
|
|
|
|
|
|
|
|
|
| (grade 3) |
|
|
| (9 months) |
|
| Gong et
al, | 5 | Male | 70 | Left | 4.4 | T3aN0M1 | No | Bladder | RN | Yes | Low | No | Sunitinib, | None | NED | (15) |
| 2020 |
|
|
|
|
|
|
|
|
|
|
|
| gemcitabine |
| (36 months) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| and cisplatin |
|
|
|
|
| 6 | Male | 61 | Left | 10.7 | T3aN1M0 | Blood vessel | No | RN | Yes | Low | No | No | None | NED |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (80 months) |
|
| Sakatani et
al, | 7 | Male | 82 | Right | 2.1 | T3aN1M0 | Lymphatic | LN | RN | No | High | No | No | LN, liver and | DOD | (16) |
| 2017 |
|
|
|
|
|
| vessel |
|
|
| (grade 3) |
|
| brain (5
months) | (5 months) |
|
| Ivey et
al, | 8 | Male | 39 | Left | 14 | T2bN1M0 | No | LN | RN | Yes | Low | No | No | None | NED | (17) |
| 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (12 months) |
|
| Kubota et
al, | 9 | Male | 72 | Right | 4.6 | T3aN2M1 | Perirenal fat | LN | RN | Yes | High | Yes | No | Lung, Bone | DOD | (18) |
| 2018 |
|
|
|
|
|
|
|
|
|
| (grade 3) |
|
| and pleura | (66 months) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (12 months) |
|
|
|
| 10 | Male | 64 | Right | 11 | T3bN0M0 | Perirenal fat | No | RN | Yes | High | Yes | No | Mesentery | AWD |
|
|
|
|
|
|
|
|
|
|
|
|
| (grade 4) |
|
| (24 months) | (130 months) |
|
| Shen et al, | 11 | Female | 77 | Left | 2 | T1aN0M0 | No | No | RN | Yes | Low | No | Unknown | Lung (6
months) | DOD | (19) |
| 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (15 months) |
|
|
| 12 | Male | 69 | Left | 5.5 | T3aN0M0 | Perirenal fat | No | RN | No | High | No | No | Lung (6
months) | DOD |
|
|
|
|
|
|
|
|
|
|
|
|
| (grade 3) |
|
|
| (6 months) |
|
| Isono et
al, | 13 | Male | 43 | Left | 5 | T3aN1M0 | Lymphatic | LN | RN | No | Low | No | Sunitinib, | Peritoneum | DOD | (20) |
| 2020 |
|
|
|
|
|
| vessel |
|
|
|
|
| axitinib and | (4 months) | (12 months) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| nivolumab |
|
|
|
| Miura et
al, | 14 | Female | 77 | Left | 1 | T3aN1M0 | Vein | LN | RN | No | High | Yes | Pazopanib | Lung, LN and | AWD | (21) |
| 2020 |
|
|
|
|
|
|
|
|
|
| (grade 4) |
| and axitinib | bone (15
months) | (25 months) |
|
| Takahashi et
al, | 15 | Male | 68 | Right | 6.4 | T3aN1M0 | Perirenal fat | LN | RN | No | Low | No | Axitinib and | Lung and bone | AWD | (22) |
| 2019 |
|
|
|
|
|
|
|
|
|
|
|
| nivolumab | (7 months) | (12 months) |
|
| Kobayashi et
al, | 16 | Female | 75 | Left | 3.5 | T3aN0M0 | Renal sinus | No | RN | No | Low | No | No | Lung, LN and | DOD | (23) |
| 2019 |
|
|
|
|
|
| fat |
|
|
|
|
|
| bone (1
months) | (4 months) |
|
| Huang et
al, | 17 | Male | 60 | Left | 3.7 | T1aN0M1 | Renal | Bone | RN | No | Low | No | No | Bone | AWD | (24) |
| 2018 |
|
|
|
|
|
| capsule |
|
|
|
|
|
|
|
|
|
| Mikami et
al, | 18 | Male | 87 | Right | 6.5 | T1bN0M0 | No | No | RN | No | Low | No | No | LN (91 months) | AWD | (25) |
| 2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (115 months) |
|
| Larkin et
al, | 19 | Female | 61 | Right | 3.5 | T1aN1M1 | No | LN, bone | No | No | Low | No | Sunitinib | LN, bone and | AWD | (26) |
| 2010 |
|
|
|
|
|
|
| and AdG |
|
|
|
|
| AdG | (7 months) |
|
| Simon et
al, | 20 | Female | 64 | Left | 15 | T3aN0M1 | Perirenal fat | Bone and | RN | Yes | Low | Yes | Radiotherapy | Liver and bone | DOD | (27) |
| 2008 |
|
|
|
|
|
|
| AdG |
|
|
|
|
| (3W) | (3 weeks) |
|
| Kobari et
al, | 21 | Male | 53 | Left | 11.5 | T3aN0M0 | Perirenal fat | AdG, LN | RN | Yes | Low | Yes | Radiotherapy, | AdG, LN and | DOD | (28) |
| 2023 |
|
|
|
|
|
|
| and liver |
|
|
|
| pazopanib and | liver (2
months) | (24 months) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| axitinib |
|
|
|
| Fuchizawa et
al, | 22 | Male | 69 | Left | 9 | T2aN0M1 | No | Bone | CN | Yes | High | Yes | Ipilimumab | None | NED | (29) |
| 2021 |
|
|
|
|
|
|
|
|
|
| (grade 3) |
| and nivolumab |
| (21 months) |
|
Due to the rarity of metastatic MTSCC, there is
currently no recommended systemic treatment. In case 3 (12), partial nephrectomy was followed by
treatment with sunitinib, temsirolimus and axitinib, along with
palliative radiation to the left ribs to control pain. However, the
patient showed no significant response and died of respiratory
failure 2 years later. In case 5 (15), preoperative bladder metastasis was
treated with radical nephroureterectomy and bladder resection,
followed by 1.2 g gemcitabine and 60 mg cisplatin chemotherapy,
with no metastasis observed during a 36-month follow-up. This
suggests that a combination of sunitinib and gemcitabine +
cisplatin chemotherapy may be effective. In case 13 (20), peritoneal metastasis was treated
with sunitinib, resulting in a reduction of disseminated tumors and
stable disease for 3 months. However, 9 months post-operation, the
patient developed ascites and was treated with axitinib and
nivolumab, but with poor outcomes, dying 12 months post-operation.
In case 14 (21), preoperative
chemotherapy (gemcitabine + cisplatin for 1 month, and
methotrexate, vinblastine, doxorubicin and cisplatin for 4 cycles)
failed to control the tumor, leading to a left nephrectomy and
regional lymphadenectomy. Postoperatively, the patient received 600
mg/day pazopanib and later switched to 10 mg/day axitinib. However,
15 months later, CT revealed lung and supraclavicular lymph node
metastases. Despite continued treatment, the patient remained alive
but continued to experience tumor metastasis and recurrence. In
case 15 (22), postoperative lung,
vertebral and iliac metastases were treated with axitinib and later
nivolumab as second-line therapy, resulting in complete remission
of metastatic sites. In case 19 (26), preoperative multiple metastases were
treated with sunitinib for 7 months with a positive response. In
case 20 (27), preoperative
vertebral and ipsilateral adrenal metastases were treated with
radiation, tumor embolization and radical nephrectomy, but the
patient died 3 weeks post-operation due to further metastases. In
case 21 (28), preoperative
multiple distant and lymph node metastases were treated with a
radical nephrectomy and metastasectomy, followed by continuous
systemic treatment with tyrosine kinase inhibitors, pazopanib and
axitinib, with radiotherapy to all metastatic sites. However, the
outcomes were poor and the patient died 2 years later. In case 22
(29), multiple preoperative bone
metastases were treated with cytoreductive nephrectomy, followed by
ipilimumab and nivolumab combined therapy, resulting in controlled
disease, with the patient remaining disease-free. Therefore, immune
checkpoint inhibitors appear effective against metastatic MTSCC. In
summary, the primary treatment strategy for MTSCC involves radical
or partial nephrectomy and for metastatic cases, aggressive
surgical treatment, metastasectomy and molecular targeted or
immunosuppressive therapy may be effective treatment methods.
In conclusion, based on the literature that is
currently available, treatment strategies for MTSCC need to follow
a detailed protocol. Before surgical treatment, a thorough imaging
assessment is essential to determine the size and location of the
tumor, and its invasion of adjacent tissues, and to evaluate the
presence of lymph node spread or distant metastasis. After surgery,
comprehensive pathological and immunohistochemical analysis of the
resected tumor is crucial to identify the tumor grade, especially
the presence of high-grade features such as sarcomatoid changes,
mitotic activity and areas of necrosis. To develop and optimize
comprehensive treatment plans for MTSCC, including selecting the
appropriate surgical technique, determining adjuvant treatment
options and formulating follow-up strategies, there is an urgent
need to expand the scope of research and increase the sample size
of cases. Additionally, more genetic and molecular biology studies
are necessary to fully understand the pathogenesis and therapeutic
targets of MTSCC. Such expanded research will provide deeper
insights, making the management of MTSCC more precise and
effective.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XM, JX and JF designed the study and participated in
the literature search. XM obtained medical images, contributed to
the literature review, and prepared the draft manuscript. XM and JF
critically revised the manuscript for important intellectual
content and provided general supervision. XM, XY, FY, and ZW were
instrumental in revising the manuscript, participating in data
analysis and providing treatment recommendations for the patient.
XM and JF confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Zhuji People's Hospital [Zhuji, China; approval no.
(2024) MedEthics no. (0115)].
Patient consent for publication
Written consent for publication was obtained from
the family of the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lopez-Beltran A, Scarpelli M, Montironi R
and Kirkali Z: 2004 who classification of the renal tumors of the
adults. Eur Urol. 49:798–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ling C, Tan R, Li J and Feng J: Mucinous
tubular and spindle cell carcinoma of the kidney: A report of seven
cases. BMC Cancer. 23:8152023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang H, Xu W, Chang S, Yuan J, Bai X,
Zhang J, Guo H, Ye H and Wang H: Mucinous tubular and spindle cell
carcinomas of the kidney (MTSCC-Ks): CT and MR imaging
characteristics. Jpn J Radiol. 40:1175–1185. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zou ZG, Wang YH, Zhou JX, Zhan SH, Zheng
YS, Liu WS, Yuan X and Guo LC: Renal mucinous tubular and spindle
cell carcinoma: Clinicopathological and whole exome sequencing
analyses. Zhonghua Bing Li Xue Za Zhi. 50:762–767. 2021.(In
Chinese). PubMed/NCBI
|
|
6
|
Ursani NA, Robertson AR, Schieman SM,
Bainbridge T and Srigley JR: Mucinous tubular and spindle cell
carcinoma of kidney without sarcomatoid change showing metastases
to liver and retroperitoneal lymph node. Hum Pathol. 42:444–448.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ged Y, Chen YB, Knezevic A, Donoghue MTA,
Carlo MI, Lee CH, Feldman DR, Patil S, Hakimi AA, Russo P, et al:
Mucinous tubular and spindle-cell carcinoma of the kidney: Clinical
features, genomic profiles, and treatment outcomes. Clin Genitourin
Cancer. 17:268–274.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guinan P, Sobin LH, Algaba F, Badellino F,
Kameyama S, MacLennan G and Novick A: TNM staging of renal cell
carcinoma: Workgroup No. 3. Union international Contre le cancer
(UICC) and the American joint committee on cancer (AJCC). Cancer.
80:992–993. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adamane SA, Menon S, Prakash G, Bakshi G,
Joshi A, Popat P and Desai SB: Mucinous tubular and spindle cell
carcinoma of the kidney: A case series with a brief review of the
literature. Indian J Cancer. 57:267–281. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu X, Zhong J, Zhou X, Wei Z, Xia Q, Huang
P, Shi C, Da J, Tang C, Cheng W and Ge J: Mucinous tubular and
spindle cell carcinoma of the kidney: A study of clinical, imaging
features and treatment outcomes. Front Oncol. 12:8652632022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kai K, Tobu S, Kido S, Mikami S, Takeuchi
K, Dobashi A, Togashi Y, Noguchi M and Aishima S: ALK
rearrangement-associated renal cell carcinoma morphologically
mimicking mucinous tubular and spindle cell carcinoma: A case
report. Diagn Pathol. 17:522022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uchida S, Suzuki K, Uno M, Nozaki F, Li
CP, Abe E, Yamauchi T, Horiuchi S, Kamo M, Hattori K and Nagashima
Y: Mucin-poor and aggressive mucinous tubular and spindle cell
carcinoma of the kidney: Two case reports. Mol Clin Oncol.
7:777–782. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu D, Yuan W, Zhu Q, Ye J, Zhu W and Chen
W: Comparative study of CT and MRI appearances in mucinous tubular
and spindle cell carcinoma and papillary renal cell carcinoma. Br J
Radiol. 94:202105482021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cornelis F, Ambrosetti D, Rocher L, Derchi
LE, Renard B, Puech P, Claudon M, Rouvière O, Ferlicot S, Roy C, et
al: CT and MR imaging features of mucinous tubular and spindle cell
carcinoma of the kidneys. A multi-institutional review. Eur Radiol.
27:1087–1095. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong P, Zhuang Q, Wang X, Xu R, Ding T,
Yin S and He X: Mucinous tubular and spindle cell carcinoma of the
kidney: Five case reports and review of the literature. Oncol Lett.
20:3372020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakatani T, Okumura Y, Kuroda N,
Magaribuchi T, Nakano Y, Shirahase T, Watanabe J, Taki Y, Okigaki
M, Ikehara S and Adachi Y: Mucinous tubular and spindle cell
carcinoma with a high nuclear grade and micropapillary pattern: A
case report. Mol Clin Oncol. 7:976–980. 2017.PubMed/NCBI
|
|
17
|
Ivey JA III, Cortese C, Baird BA, Thiel DD
and Lyon TD: Mucinous tubular and spindle cell carcinoma of the
kidney with nodal metastasis managed with surgical resection. Eur
Urol Open Sci. 29:10–14. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kubota M, Yamasaki T, Teramoto Y, Ito K,
Takada H, Magaribuchi T, Sawada A, Akamatsu S, Negoro H, Saito R,
et al: Two cases of metastatic and recurrent non-clear cell renal
cell carcinoma re-diagnosed as renal mucinous tubular and spindle
cell carcinoma during long-term follow-up. Hinyokika Kiyo.
64:111–115. 2018.(In Japanese). PubMed/NCBI
|
|
19
|
Shen Q, Liu YX and He Q: Mucinous tubular
and spindle cell carcinoma of kidney: Clinicopathology and
prognosis. Beijing Da Xue Xue Bao Yi Xue Ban. 55:276–282. 2023.(In
Chinese). PubMed/NCBI
|
|
20
|
Isono M, Seguchi K, Yamanaka M, Miyai K,
Okubo K and Ito K: Rapid progression of mucinous tubular and
spindle cell carcinoma of the kidney without sarcomatoid changes: A
case report. Urol Case Rep. 31:1011622020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miura K, Adachi Y, Shirahase T, Nagashima
Y, Suemune K, Sakaida N, Nakano Y, Sakai Y, Shimizu S and Ikehara
S: A case of high-grade mucinous tubular and spindle cell
carcinoma. J Surg Case Rep. 2020:rjaa0142020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi Y, Numakura K, Aoyama Y, Okada
S, Saito T, Muto Y, Koizumi A, Nara T, Chiba S, Kanda S, et al:
METASTATIC mucinous tubular and spindle cell carcinoma treated with
nivolumab successfully : A case report. Hinyokika Kiyo. 65:363–367.
2019.(In Japanese). PubMed/NCBI
|
|
23
|
Kobayashi Y, Arai H, Honda M, Terada H and
Yasuhara Y: A case of rapidly advancing renal mucinous tubular and
spindle cell carcinoma. Nihon Hinyokika Gakkai Zasshi. 110:249–254.
2019.(In Japanese). PubMed/NCBI
|
|
24
|
Huang ZX, Zhang XP, Dong S, Liu SJ, Yang
RL, Zhou YS and Ma WG: Renal mucinous tubular and spindle cell
carcinoma combined with multiple bone metastasis: A case report and
literature review. Beijing Da Xue Xue Bao Yi Xue Ban. 50:732–736.
2018.(In Chinese). PubMed/NCBI
|
|
25
|
Mikami H, Endo Y, Yanagi M, Nemoto K,
Hamasaki T, Kimura G, Suzuki Y and Kondo Y: Laparoscopic
lymphadenectomy for postoperative lymph-node metastasis of renal
mucinous tubular and spindle cell carcinoma: a case report. Nihon
Hinyokika Gakkai Zasshi. 108:30–34. 2017.(In Japanese). PubMed/NCBI
|
|
26
|
Larkin J, Fisher R, Pickering L, Thway K,
Livni N, Fisher C and Gore M: Metastatic mucinous tubular and
spindle cell carcinoma of the kidney responding to sunitinib. J
Clin Oncol. 28:e539–e540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simon RA, di Sant'agnese PA, Palapattu GS,
Singer EA, Candelario GD, Huang J and Yao JL: Mucinous tubular and
spindle cell carcinoma of the kidney with sarcomatoid
differentiation. Int J Clin Exp Pathol. 1:180–184. 2008.PubMed/NCBI
|
|
28
|
Kobari Y, Yoshida K, Minoda R, Fukuda H,
Hata K, Unagami K, Iizuka J, Ishida H, Nagashima Y and Takagi T:
Long-time survival of a renal transplant recipient with metastatic
mucinous tubular and spindle cell carcinoma: A case report. In
Vivo. 37:1394–1398. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fuchizawa H, Kijima T, Takada-Owada A,
Nagashima Y, Okazaki A, Yokoyama M, Nishihara D, Ishida K and Kamai
T: Metastatic mucinous tubular and spindle cell carcinoma of the
kidney responding to nivolumab plus ipilimumab. IJU Case Rep.
4:333–337. 2021. View Article : Google Scholar : PubMed/NCBI
|