Introduction
Immune checkpoint inhibitor (ICI) therapy directed
at programmed cell death protein 1 (PD-1) has been shown to improve
survival outcomes in patients diagnosed with non-small cell lung
cancer (NSCLC) (1). Anti-PD-1
antibodies, including pembrolizumab and nivolumab, are considered
to exert their anticancer effects by blocking the interaction of
PD-1 with programmed death-ligand 1 (PD-L1), as this interaction
has an inhibitory effect on T-cell activity. However, the function
of PD-L1 as a physiological regulator of the activation of CD8 T
cells also prevents the development of chronic autoimmune
inflammation (2). As a consequence,
immune-related adverse events (irAEs) are the predominant toxicity
associated with ICI therapy (3).
IrAEs can impact any organ and result from the effects of immune
dysregulation on normal tissues. Individuals with pre-existing
autoimmune diseases are frequently excluded from clinical trials
involving ICIs (4,5) due to concerns about the potential
exacerbation of underlying autoimmune diseases. This limits the
treatment options for these patients. Bullous pemphigoid (BP) is an
autoimmune skin blistering disease characterized by the deposition
of autoantibodies in the epithelial basement membrane zone (BMZ).
Predominantly affecting older adults, the condition typically
manifests with generalized pruritic urticarial plaques and
subepithelial tension blisters (6).
A recent case report has demonstrated the recurrence of BP in a
patient undergoing treatment for lung cancer with pembrolizumab
(7). Although retrospective studies
have explored the safety of using ICIs in patients with autoimmune
diseases (8), the evaluation of
these drugs in various clinically relevant scenarios has been
limited. The safe delivery of immunotherapy to this distinctive
population without worsening their autoimmune condition presents a
substantial clinical challenge as well as an unmet medical
need.
The present study reports a case involving a patient
diagnosed with NSCLC complicated by BP, who underwent treatment
with pembrolizumab. Throughout the treatment course, the potential
exacerbation of known autoimmune diseases was monitored and the
antitumor effect was evaluated.
Case report
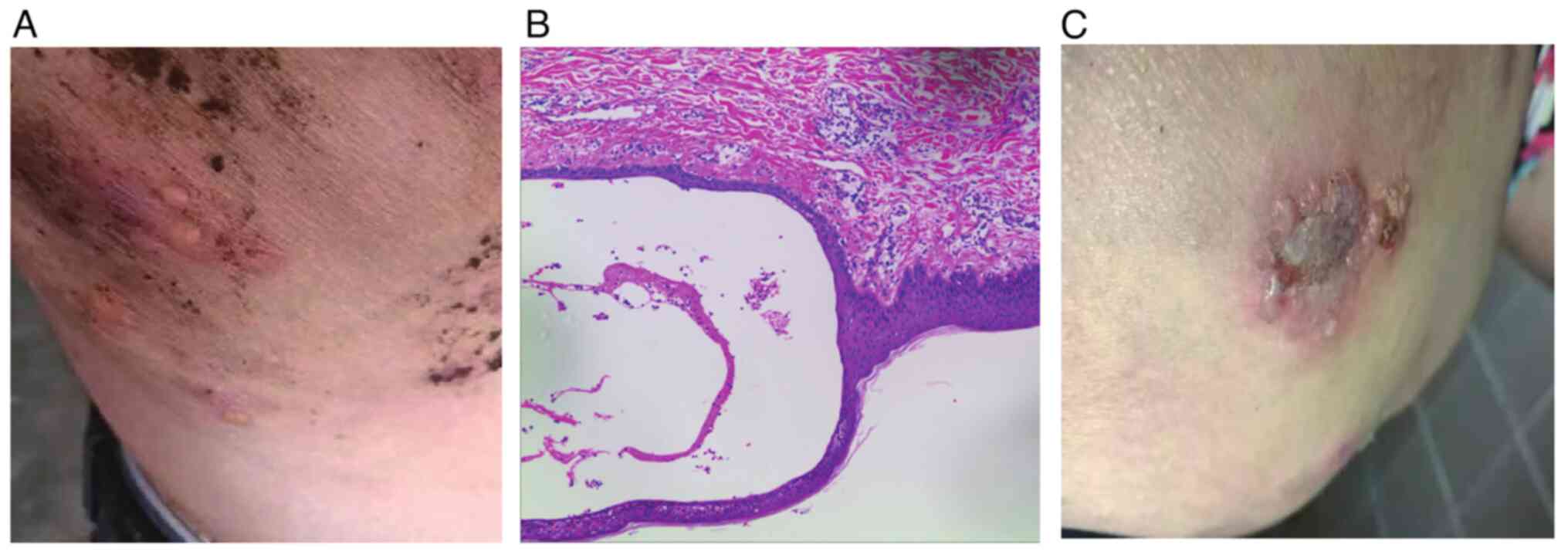
In November 2020, a 52-year-old man consulted with a
dermatologist at the Affiliated Hospital of Guangdong Medical
University (Zhanjiang, China) due to the presence of multiple
blisters on his back, which were characterized by thick walls and a
hemispheric morphology (Fig. 1A).
The histopathological examination of the skin biopsy reported the
presence of subepidermal blisters, visible on HE staining,
accompanied by inflammatory cell infiltration at the base of the
blisters and the absence of significant eosinophils (Fig. 1B). Immunofluorescence demonstrated
the presence of linear deposits of IgG, C3 and IgA within the
basement membrane, which is consistent with the diagnosis of BP
(data not shown). The patient initially received treatment with
oral prednisone at a dose of 10 mg once daily. After 3 years of
treatment, the skin symptoms improved but were not completely cured
(Fig. 1C). Due to the prolonged
duration of treatment, the patient neglected to monitor the
progression of the disease and discontinued treatment in May
2023.
The history of NSCLC began in March 2023, when the
patient experienced pain in the right chest and back. A subsequent
positron emission tomography and computed tomography (PET-CT) scan
revealed a maximum standardized uptake value (SUV) of 17.58,
indicating a high likelihood of lung cancer. The scan also revealed
multiple lymph node metastases in the bilateral clavicle area, the
inner or underside surfaces of the right pectoralis major and minor
muscles, as well as in the right hilum and mediastinum (Fig. 2A and C). Pathological examination
following biopsy of the mass in the upper right lung indicated
infiltrating adenocarcinoma, a type of NSCLC, with PD-L1 expression
at 3% (Fig. S1). No genetic
mutations were detected (Fig.
S2).
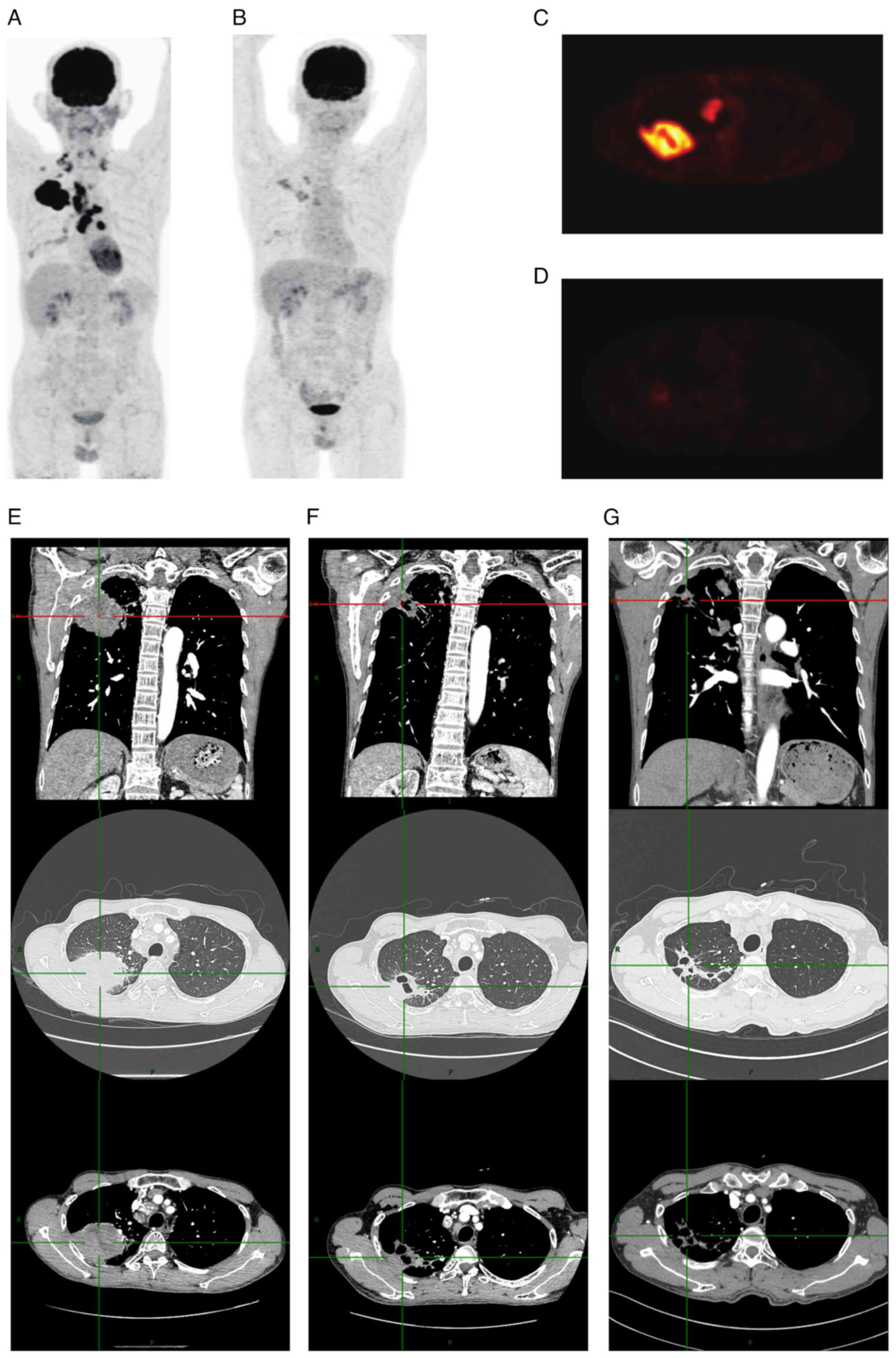 | Figure 2.PET-CT and CT images of the patient at
different time points. PET-CT images of the body (A) at the time of
diagnosis of lung cancer and (B) after comprehensive treatment.
PET-CT images of the lung (C) at the time of diagnosis and (D)
after comprehensive treatment. Enhanced CT images at different
angles and window levels (E) before treatment, (F) after two cycles
of treatment and (G) after four cycles of treatment. Each set of
images comprises three distinct panels: a upper panel, a middle
panel, and a lower panel. The top panel displays coronal images of
the CT mediastinal window, while the middle and bottom panels
present axial images of the same slice in the lung window and
mediastinal window, respectively. The images in Group E illustrate
the presence of a large tumor prior to treatment, which has
resulted in compression of the surrounding tissues. The images in
Group F demonstrate a notable reduction in tumor volume following
two cycles of treatment, accompanied by partial recovery of the
lung parenchyma. The images in Group G exhibit further tumor
shrinkage after four cycles of treatment, with enhanced recovery of
the surrounding lung tissue and structure. PET, positron emission
tomography; CT, computed tomography. |
In July 2023, the patient underwent further
treatment at the Affiliated Hospital of Guangdong Medical
University. CT re-examination revealed the presence of an irregular
round soft tissue mass in the upper lobe of the right lung,
measuring ~58×59×52 mm (Fig. 2E).
Considering the previous examination results, the patient was
diagnosed with cT3N3M0 stage IIIC according to the Cancer Staging
Manual of the American Joint Committee on Cancer, 8th version
(9). Following evaluation by a
multidisciplinary team (MDT) and a comprehensive assessment of the
risks and benefits associated with treatment, a combined treatment
approach of pembrolizumab (200 mg every 3 weeks) and chemotherapy
(pemetrexed 940 mg every 3 weeks + carboplatin 500 mg every 3
weeks) was initiated. A CT scan conducted in August 2023 indicated
a noticeable reduction in tumor size (Fig. 2F). Following the completion of four
cycles of combined therapy, CT (Fig.
2G) and PET-CT (Fig. 2B and D)
scans were performed for re-examination. The PET-CT scan revealed a
maximum SUV of 3.0, indicating a significant reduction in the size
of the lung cancer lesions in the upper lobe of the right lung
compared with that at the previous scan. Furthermore, the multiple
lymph node metastases in the bilateral supraclavicular fossa,
mediastinum and right hilar area were markedly reduced in number
and smaller than before. The tumor response was evaluated as a
partial response according to the Response Evaluation Criteria in
Solid Tumors criteria, version 1.1 (10). The patient exhibited no adverse
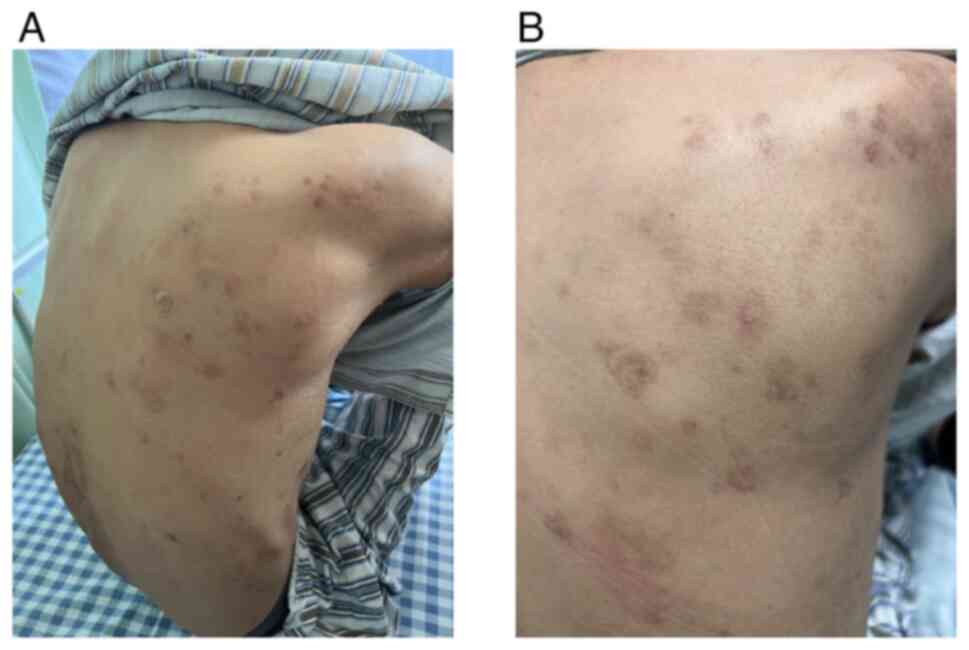
reactions during the treatment, and the BP showed no signs of
exacerbation; instead improvements in this skin condition were
observed (Fig. 3A). After four
treatment cycles, the superficial BP lesions of the patient had
resolved (Fig. 3B). Following
immunotherapy combined with chemotherapy, the patient was diagnosed
with ypT2aN0M0 stage IB. At this time, the combination therapy was
completed, with no evidence of disease progression and no
occurrence of any other adverse reactions. Consequently, the
immunomodulatory maintenance therapy was continued. The patient's
cancer indicators are now normal, with no significant side effects,
and he is currently undergoing maintenance therapy with pablizumab
monotherapy (200 mg every three weeks), with pre-treatment blood
sampling for cancer markers and follow-up chest CT scans conducted
every three months.
Discussion
In the present case, effectively managing lung
cancer while not exacerbating the autoimmune disease was of the
utmost importance. Symptoms stemming from tumor-secreted hormones,
peptides, cytokines or immune cross-reactions between malignant and
normal tissues in various organ systems are collectively referred
to as paraneoplastic syndromes (11). According to a meta-analysis
published in 2018, the incidence of malignant tumors in patients
with BP is 11% (12). The
occurrence of BP is also associated with lung cancer, mantle cell
lymphoma and cutaneous squamous cell carcinoma as part of a
paraneoplastic syndrome (13–15).
In response to this phenomenon, various pathogenic mechanisms have
been proposed. One suggestion is that, in instances where
malignancy precedes BP, antibodies directed against tumor-specific
antigens may cross-react with basement membrane antigens, such as
BP antigens, ultimately resulting in skin blistering (16). Such an antigen may be laminin-332, a
protein crucial for keratinocyte-basal cell membrane adhesion that
is also produced by various solid malignancies, including breast,
pancreatic, colon and lung cancer. It has also been postulated that
the tumor produces anti-BMZ cross-reactive antigens or other
molecules that disrupt the basement membrane, and that this
disruption leads to the production of anti-BMZ antibodies (17). As of yet, no specific
histopathological factors have been identified that are able to
differentiate tumor-associated pemphigoid from conventional
pemphigoid. Due to the concurrent discovery of BP and NSCLC in the
present patient, guided by Curth's postulates (Table I) and recent studies (11–17),
it was proposed that the pemphigoid may have been induced by NSCLC.
Consequently, the effective treatment of the primary tumor was
crucial for controlling the associated skin disease.
 | Table I.Curth's postulates for the diagnosis
of cutaneous paraneoplastic syndromes. |
Table I.
Curth's postulates for the diagnosis
of cutaneous paraneoplastic syndromes.
| No. | Criterion |
|---|
| 1 | Neoplasia and
cutaneous condition began concurrently |
| 2 | Development followed
a parallel coursea |
| 3 | Cutaneous condition
is not associated with a genetic syndrome |
| 4 | A specific type of
neoplasia occurs with a characteristic cutaneous condition |
| 5 | The cutaneous
condition is rare in the general population |
| 6 | High frequency of
association between both conditions |
Recent research findings have indicated that
neoadjuvant nivolumab in combination with chemotherapy
significantly extends event-free survival compared with
chemotherapy alone in patients with resectable NSCLC, with a higher
proportion of patients achieving a pathological complete response
(18). Although PD-1/PD-L1
inhibitors may induce autoimmune diseases, their efficacy in
patients with pre-existing autoimmune diseases is generally
comparable with that in other patients, and most irAEs are mild and
manageable (19). A meta-analysis
demonstrated that the addition of chemotherapy to ICIs improves
their treatment efficacy in the first-line treatment of advanced
NSCLC. Specifically, the combination of chemotherapy with
pembrolizumab or atezolizumab exhibits consistently higher efficacy
than chemotherapy alone, any other ICI-based combination or
monotherapy, particularly in patients with non-squamous histology
(20). In addition, in another
study, pembrolizumab plus chemotherapy exhibited the optimum
overall survival in patients with PD-L1 expression ≥1% In addition,
pembrolizumab was associated with fewer grade ≥3 adverse events
compared with other immunotherapies combined with chemotherapy
(21). In the present case,
following approval in the MDT meeting, a regimen involving a
combination of pembrolizumab and chemotherapy was devised. The
survival and response rates of cisplatin plus paclitaxel are
comparable with those of carboplatin plus paclitaxel. However, as
carboplatin plus paclitaxel has been shown to be associated with
lower toxicity (22), carboplatin
was selected for use in the present case. Due to its good efficacy
and tolerability, pemetrexed remains a key agent in the treatment
of patients with advanced non-squamous NSCLC (23), and pemetrexed was therefore chosen
as the chemotherapy regimen for the present case. Following four
cycles of treatment, the patient experienced a marked reduction in
lung tumors, and the skin lesions completely disappeared. To date,
the treatment has not yet concluded. Given that irAEs frequently
manifest during the maintenance phase, it is imperative that the
condition of the patient is monitored during the follow-up
treatment period.
In conclusion, in the present case, the use of
pembrolizumab combined with chemotherapy successfully reduced the
size of the tumor, and the BP symptoms gradually improved. A
potential paraneoplastic syndrome was suspected. However, to the
best of our knowledge, no studies have examined the combination of
immunosuppressive agents with chemotherapy for the treatment of
autoimmune diseases. This may be due to the mechanism of action of
chemotherapy not being strongly associated with the pathogenesis of
autoimmune diseases. Furthermore, recent case reports have
suggested that pemphigoid may recur after pembrolizumab treatment
(7). Therefore, the treatment plan
used in the current study holds certain importance as a clinical
reference; however, its safety requires verification through use in
more cases. Further exploration of the mechanism by which
pemphigoid and malignant tumors are associated is also necessary.
In addition, the relationship between steroid and ICI therapies is
complex. It has been proposed that the use of baseline
corticosteroids at a dosage of ≥10 mg prednisone equivalent is
associated with a poorer outcome in patients with NSCLC who are
treated with PD-1/PD-L1 inhibitors (24). Consequently, it is suggested that
this combination therapy should be used with caution and its
feasibility should be further explored in future studies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AL and FL were responsible for conceptualization.
ZM, YP, BD and ZH were responsible for the collection and collation
of clinical data and background information. AL and FL wrote the
original draft of the manuscript. ZL and JC contributed to the data
acquisition and analysis, and reviewed it critically for important
intellectual content. AL and FL confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the anonymized data and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Doroshow DB, Sanmamed MF, Hastings K,
Politi K, Rimm DL, Chen L, Melero I, Schalper KA and Herbst RS:
Immunotherapy in non-small cell lung cancer: Facts and hopes. Clin
Cancer Res. 25:4592–4602. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boutros C, Tarhini A, Routier E, Lambotte
O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S,
Berdelou A, et al: Safety profiles of anti-CTLA-4 and anti-PD-1
antibodies alone and in combination. Nat Rev Clin Oncol.
13:473–486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reckamp KL, Redman MW, Dragnev KH,
Minichiello K, Villaruz LC, Faller B, Al Baghdadi T, Hines S,
Everhart L, Highleyman L, et al: Phase II randomized study of
ramucirumab and pembrolizumab versus standard of care in advanced
non-small-cell lung cancer previously treated with
immunotherapy-lung-MAP S1800A. J Clin Oncol. 40:2295–2306. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xing P, Wang M, Zhao J, Zhong W, Chi Y, Xu
Z and Li J: Study protocol: A single-arm, multicenter, phase II
trial of camrelizumab plus apatinib for advanced nonsquamous NSCLC
previously treated with first-line immunotherapy. Thorac Cancer.
12:2825–2828. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hammers CM and Stanley JR: Mechanisms of
disease: Pemphigus and bullous pemphigoid. Annu Rev Pathol.
11:175–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaul S, Wang A, Grushchak S and Albrecht
J: Pembrolizumab-induced reactivation of bullous pemphigoid. Int J
Dermatol. 60:757–758. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tison A, Quéré G, Misery L, Funck-Brentano
E, Danlos FX, Routier E, Robert C, Loriot Y, Lambotte O, Bonniaud
B, et al: Safety and efficacy of immune checkpoint inhibitors in
patients with cancer and preexisting autoimmune disease: A
nationwide, multicenter cohort study. Arthritis Rheumatol.
71:2100–2111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rami-Porta R, Asamura H, Travis WD and
Rusch VW: Lung cancer-major changes in the American joint committee
on cancer eighth edition cancer staging manual. CA Cancer J Clin.
67:138–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thiers BH, Sahn RE and Callen JP:
Cutaneous manifestations of internal malignancy. CA Cancer J Clin.
59:73–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lucariello RJ, Villablanca SE, Mascaró JM
Jr and Reichel M: Association between bullous pemphigoid and
malignancy: A meta-analysis. Australas J Dermatol. 59:253–260.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Das A, Das S, Das SK and Basuthakur S: A
case of paraneoplastic bullous pemphigoid in association with
squamous cell carcinoma of lung. J Postgrad Med. 61:197–199. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Md Radzi AB and Kasim SS: A case report of
paraneoplastic bullous pemphigoid associated with mantle cell
lymphoma: A rare presentation. Medicine (Baltimore).
102:e328222023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Albadri Z, Thorslund K, Häbel H, Seifert O
and Grönhagen C: Increased risk of squamous cell carcinoma of the
skin and lymphoma among 5,739 patients with bullous pemphigoid: A
Swedish nationwide cohort study. Acta Derm Venereol.
100:adv002892020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balestri R, Magnano M, La Placa M, Patrizi
A, Angileri L, Tengattini V and Bardazzi F: Malignancies in bullous
pemphigoid: A controversial association. J Dermatol. 43:125–133.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moro F, Fania L, Sinagra JLM, Salemme A
and Di Zenzo G: Bullous pemphigoid: Trigger and predisposing
factors. Biomolecules. 10:14322020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant Nivolumab plus chemotherapy in resectable
lung cancer. N Engl J Med. 386:1973–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leonardi GC, Gainor JF, Altan M, Kravets
S, Dahlberg SE, Gedmintas L, Azimi R, Rizvi H, Riess JW, Hellmann
MD and Awad MM: Safety of programmed death-1 pathway inhibitors
among patients with non-small-cell lung cancer and preexisting
autoimmune disorders. J Clin Oncol. 36:1905–1912. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dafni U, Tsourti Z, Vervita K and Peters
S: Immune checkpoint inhibitors, alone or in combination with
chemotherapy, as first-line treatment for advanced non-small cell
lung cancer. A systematic review and network meta-analysis. Lung
Cancer. 134:127–140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu L, Bai H, Wang C, Seery S, Wang Z,
Duan J, Li S, Xue P, Wang G, Sun Y, et al: Efficacy and safety of
first-line immunotherapy combinations for advanced NSCLC: A
systematic review and network meta-analysis. J Thorac Oncol.
16:1099–1117. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomasini P, Barlesi F, Mascaux C and
Greillier L: Pemetrexed for advanced stage nonsquamous non-small
cell lung cancer: Latest evidence about its extended use and
outcomes. Ther Adv Med Oncol. 8:198–208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arbour KC, Mezquita L, Long N, Rizvi H,
Auclin E, Ni A, Martínez-Bernal G, Ferrara R, Lai WV, Hendriks LEL,
et al: Impact of baseline steroids on efficacy of programmed cell
death-1 and programmed death-ligand 1 blockade in patients with
non-small-cell lung cancer. J Clin Oncol. 36:2872–2878. 2018.
View Article : Google Scholar : PubMed/NCBI
|