Introduction
Due to the increased use of physical examinations
and the wider application of high-resolution ultrasound and
enhanced CT, the availability of accurate early diagnostic
techniques for thyroid carcinoma has notably increased in all
malignant tumors. Papillary thyroid carcinoma (PTC) accounts for
>90% of all malignant thyroid tumors and is usually considered a
low-grade malignant tumor with good prognosis (1–3).
Recent cancer statistics, indicate that the 5-year relative
survival rate for thyroid cancer is ~99% (3). Cervical lymph node metastases are
common in patients with PTC, occurring in 30–80% of cases (4). PTC often manifests in the central
(compartments VI) and lateral neck (compartments II–V) lymph nodes,
with the central lymph node generally considered to be the first
site of PTC, with a high rate of metastasis (5). Central lymph node dissection (CLND)
does not require a longer incision and has no difference in the
probability of recurrent laryngeal nerve injury compared with those
who don't undergo CLND, therefore, CLND is routinely performed to
treat PTC (5,6). However, lateral lymph node dissection
(LLND) in patients with PTC is controversial, especially for
patients without evidence of lateral cervical metastasis (cN1b-).
Furthermore, the 2015 American Thyroid Association (ATA) guidelines
state that prophylactic lateral neck dissection is not recommended
in patients with PTC without clinically involved lateral
compartment lymph nodes (cN1b+) (7). However, even after appropriate
treatment, 10–20% of patients that are cN1b- experience lateral
lymph node recurrence (LLNR) and require re-operation during
follow-up (8). Furthermore, there
are certain patients with suspicious ultrasound images including
loss of the fatty hilum, a rounded rather than oval shape, cystic
change, calcifications and peripheral vascularity, of the lateral
lymph node metastasis (LLNM) but without preoperative fine-needle
aspiration (FNA) cytological results or negative FNA results;
whether to perform LLND in these cases is considered to be
controversial (5).
In the present retrospective study, the association
between central lymph node metastasis (CLNM) and LLNM in patients
with sonographically suspicious lateral lymph nodes, as well as the
incidence of LLNR in postoperative patients that were classed as
cN1b- were investigated. The aim of the present study was to
provide a basis for the selection of clinical treatment strategies
in these patients.
Materials and methods
Study patients
The present study reviewed patients with PTC who
underwent surgery from June 2019 to June 2021 at the Thyroid
Department of The First Affiliated Hospital of Anhui Medical
University (Hefei, China). The exclusion criteria included: i)
Inadequate medical records; ii) distant metastases; iii) a history
of treatments for other types of cancer; and iv) follow-up data of
<2 years. A total of 1,061 patients were included in the present
study and 128 of these patients had preoperative ultrasonic imaging
examination results that were indicative of LLNM (9). Patient characteristics and cervical
lymph node clinicopathologic features were carefully reviewed. The
numbers of metastatic and central lymph node yield, the central
lymph node ratio (CLNR; the number of positive central lymph nodes
divided by the number of central lymph node yield) and CLNM were
identified using postoperative pathological reports. All patients
were pathologically confirmed to have PTC through pathologic
examination. The present study was approved by the Ethics Committee
of the First Affiliated Hospital of Anhui Medical University
(Hefei, China).
Treatments and follow-up
All patients included in the study were examined by
preoperative high-resolution ultrasonography at the ultrasound
department of The First Affiliated Hospital of Anhui Medical
University (Hefei, China), according to the European Thyroid
Association guidelines definition of ultrasonography-detectable
LLNM (9).
Hemi-thyroidectomy, isthmus and ipsilateral CLND was
routinely performed for unilateral PTC, and total thyroidectomy and
bilateral CLND were performed for bilateral PTC, regardless of
central lymph node involvement (as per the Chinese Thyroid
Association guidelines) (10). The
central lymph node is bordered superiorly by the hyoid bone,
laterally by the carotid sheaths and inferiorly by the innominate
(brachiocephalic) artery and is closely associated with the
prelaryngeal lymph nodes, pretracheal lymph nodes and the
paratracheal lymph nodes. Patients that were suspected to have LLNM
underwent primary thyroid tumor resection, and central region VI
and lateral cervical selective lymph node dissection (region II–V)
(11,12).
All 1,061 patients were treated with thyroid
stimulating hormone suppressive therapy after surgery (12). Postoperative radioiodine treatment
was administered to patients who had intermediate or high-risk
stratification for recurrence according to intraoperative and
pathologic findings (7).
Patients were followed up every 3 months during the
first year and then every 6 months thereafter. Clinical
examinations, thyroid function tests, thyroid and cervical lymph
node ultrasonography and chest radiography were used to assess the
condition of the patients. LLNR was defined as the first occurrence
of positive lymph nodes in the lateral cervical region during the
follow-up period after the initial standard operation and was
diagnosed using ultrasound-guided FNA cytology.
Statistical analysis. χ2 tests and
Fisher's exact tests were used as appropriate to identify single
risk factors for LLNM. Logistic regression analysis was used to
calculate the odds ratios (ORs) of certain parameters. Results were
presented as ORs with 95% confidence intervals (CI) and P-values.
Receiver operating characteristic (ROC) curve analysis was
performed to determine the cut-off number of positive central lymph
nodes. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
SPSS (version 26; IBM Corp.).
Results
Patient characteristics
A total of 1,061 patients were included in the
present study (Table I). Of these
patients, 128 were individuals with sonographically suspicious LLNM
that received both central and LLND. Among these, 94 (73.4%)
patients were diagnosed with CLNM and 106 (82%) with lymph node
metastasis in both the central and lateral compartments using
postoperative pathology examination. The mean number of metastatic
lymph nodes in the central compartment was 3.68±3.05, the mean
number of central lymph node yield was 6.25±3.37 and the CLNR was
0.57±0.37. A total of 933 patients without LLNM (cN1b-) were
included in the present study, and underwent CLND directly after
the discovery of the lesion. There were 503 (53.9%) cases of
pathologic central lymph node metastases. The mean number of
metastatic lymph nodes in the central neck was 1.11±1.44, the mean
number of central lymph node yield was 5.14±2.59 and the CLNR was
0.16±0.23.
 | Table I.Characteristics of included patients
with PTC (n=1,061). |
Table I.
Characteristics of included patients
with PTC (n=1,061).
| Characteristic | CLND + LLND | CLND | LLN recurrence |
|---|
| No. of patients | 128 | 933 | 31 |
| Patient sex, n
(%) |
|
|
|
| Male | 36 (28.1) | 260 (27.9) | 12 (38.7) |
|
Female | 92 (71.9) | 673 (72.1) | 19 (61.3) |
| Age, n (%) |
|
|
|
| <55
years | 81 (63.3) | 654 (70.1) | 26 (83.9) |
| ≥55
years | 47 (36.7) | 279 (29.9) | 5 (26.1) |
| CLNM, n (%) | 94 (73.4) | 503 (53.9) | 25 (80.6) |
| LLNM, n (%) | 106 (82.0) | - | - |
| LLN recurrence, n
(%) | - | 31 (3.4) | - |
| No. of metastatic
lymph nodes in central neck, mean (SD) | 3.68 (3.05) | 1.11 (1.44) | 3.48 (2.94) |
| Central lymph node
yield, mean (SD) | 6.25 (3.37) | 5.14 (2.59) | 6.58 (3.68) |
| Lymph node ratio in
central neck, mean (SD) | 0.57 (0.37) | 0.16 (0.23) | 0.52 (0.37) |
| Follow-up time in
months, median (range) | 32 (25–50) | 32 (25–50) | 25 (18–36) |
During a median follow-up period of 32 months
(range, 25–50 months), 31 of the patients were diagnosed with
lateral cervical lymph node recurrence and underwent modified
radical LLND. A total of 12 male patients and 19 female patients
had lateral neck recurrence, and among these, 26 (83.9%) were
<55 years of age. The pathological data relating to the primary
surgery in these recurrent patients were reviewed, and the overall
rate of CLNM was 25/31 patients (80.6%) in the first surgery. The
mean number of metastatic lymph nodes in the central compartment
was 3.48±2.94, the mean number of central lymph node yield was
6.58±3.68 and the CLNR was 0.52±0.37.
Association between CLNM and lateral
neck lymph node metastasis
The association between CLNM and lateral neck lymph
node metastasis was investigated and the difference was
statistically significant (P<0.001). Patients with suspicious
LLNM had a significantly higher incidence of positive central lymph
nodes [84 (79.2%) vs. 10 (45.5%), P=0.001; Table II].
 | Table II.Relationship between CLN ratio and
LLNM in patients with PTC (n=128). |
Table II.
Relationship between CLN ratio and
LLNM in patients with PTC (n=128).
| CLN status | LLNM-positive,
(n=106) | LLNM-negative,
(n=22) | P-value |
|---|
| Positive, n (%) | 84 (79.2) | 10 (45.5) | 0.001 |
| Negative, n
(%) | 22 (21.8) | 12 (54.5) |
|
Univariate analysis showed that the CLNR and the
number of positive central lymph nodes was significantly associated
with LLNM (P<0.05). Multivariate analysis showed that the CLNR
(OR=8.538, P=0.016) and the number of positive central lymph nodes
(OR=2.234, P=0.023) were significant independent risk factors for
LLNM (Table III).
 | Table III.Univariate and multivariate analysis
of CLNR, number of positive central lymph node and lateral lymph
node metastasis. |
Table III.
Univariate and multivariate analysis
of CLNR, number of positive central lymph node and lateral lymph
node metastasis.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Characteristic | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| CLNR | 13.035
(3.018–66.053) | <0.001 | 8.538
(1.547–53.411) | 0.016 |
| No. of positive
central lymph nodes | 2.554
(1.188–18.031) | 0.001 | 2.234
(0.732–9.138) | 0.023 |
The association between LLNM and the number of
positive central lymph nodes was further analyzed (Table IV). The 128 patients were divided
into groups according to the number of positive central lymph
nodes. The rate of LLNM notably increased with the number of
positive central lymph nodes as follows: 9.1% in patients with one
positive central lymph node, 63.6% in patients with two positive
central lymph nodes and 80–100% in patients with ≥3 positive
central lymph nodes.
 | Table IV.Patients with LLNM grouped according
to the number of positive central lymph nodes. |
Table IV.
Patients with LLNM grouped according
to the number of positive central lymph nodes.
|
| No. of positive
central lymph nodes |
|---|
|
|
|
|---|
| Parameter | 0 | 1 | 2 | 3 | 4 | 5 | 6 | ≥7 |
|---|
| No. of patients,
(n=128) | 34 | 11 | 11 | 20 | 16 | 15 | 5 | 16 |
| No. of patients
with LLNM, (n=106) | 22 | 9 | 7 | 18 | 16 | 14 | 4 | 16 |
| Incidence of
patients with LLNM, % | 64.7 | 9.1 | 63.6 | 90.0 | 100.0 | 93.3 | 80.0 | 100.0 |
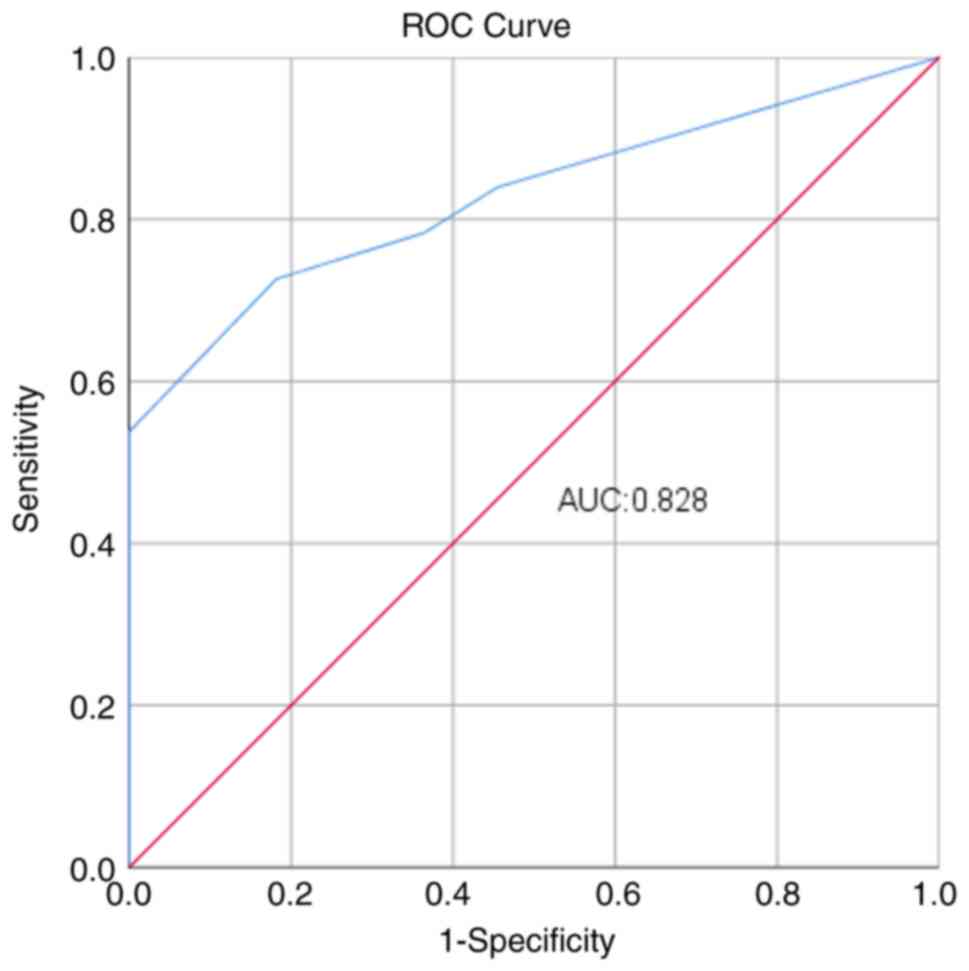
The optimal cut-off number of positive central lymph
nodes to differentiate patients with or without LLNM was 3, which
was used to divide the patients into three groups (Fig. 1). The groups included: i) Group A,
no positive central lymph nodes; ii) group B, 1–2 positive central
lymph nodes; and iii) group C, ≥3 positive central lymph nodes
(Table V). Comparisons between the
three groups were examined using the χ2 test, and the
incidence of LLNM was shown to be significantly increased in group
C compared with that of groups A and B (P<0.05). There was no
statistically significant difference identified between group A and
group B (P=0.530).
 | Table V.Relationship between the number of
positive CLNs and LLNM. |
Table V.
Relationship between the number of
positive CLNs and LLNM.
| Parameter | Group A | Group B | Group C | P-value |
|---|
| No. of
patients | 34 | 22 | 72 |
|
| LLNM, n (%) |
|
|
| 0.530a |
|
Negative | 12 (35.3) | 6 (27.3) | 4 (5.66) |
<0.001b |
|
Positive | 22 (64.7) | 16 (72.7) | 68 (94.4) | 0.004c |
The association between LLNM and CLNR was further
examined by division of the lateral neck lymph nodes into
compartments II–V, according to 2015 ATA guidelines (7). The logistic regression analysis
results showed that with the increase in CLNR, the risk of lymph
node metastasis involving levels III, IV and V increased (Table VI).
 | Table VI.Univariate analysis of CLNR and
different regional lateral lymph node metastases. |
Table VI.
Univariate analysis of CLNR and
different regional lateral lymph node metastases.
|
| Univariate |
|---|
| Lateral neck lymph
node compartments |
|
|---|
| OR (95% CI) | P-value |
|---|
| Level II | 0.833
(0.312–2.943) | 0.719 |
| Level III | 3.217
(1.304–8.956) | 0.030 |
| Level IV | 7.124
(2.733–24.439) | <0.001 |
| Level V | 9.636
(1.258–67.759) | 0.037 |
Association between CLNM and lateral neck lymph node
recurrence. In the 933 patients that were cN1b-, the CLNR was 53.5%
(398/744). A total of 31 of these patients developed secondary
lateral cervical lymph node metastasis. Univariate analyses
indicated that there was no association between CLNR and lateral
cervical lymph node recurrence. However, the number of positive
central lymph nodes was significantly associated with lateral neck
recurrence. Specifically, when the number of CLNM was ≥3, the risk
of lateral neck lymph node recurrence was significantly increased
(P<0.05; Table VII).
 | Table VII.Univariate and multivariate analysis
of CLNR, the number of positive central lymph nodes and recurrence
of lateral neck lymph node. |
Table VII.
Univariate and multivariate analysis
of CLNR, the number of positive central lymph nodes and recurrence
of lateral neck lymph node.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Characteristic | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| CLNR | 1.018
(0.968–1.030) | 0.231 | 0.938
(0.917–1.011) | 0.412 |
| No. of positive
central lymph nodes | 3.521
(1.604–10.531) | 0.024 | 2.544
(0.664–8.183) | 0.043 |
Discussion
CLNM is the most common metastasis in patients with
PTC and often follows a stepwise spread pattern, initially
occurring in the central lymph node (level VI) and spreads to the
LLN (level II–V) (13). Due to
this, the association between CLNM and lateral neck lymph node
metastasis has been investigated in numerous studies. Previous
reports have shown that CLNM is an important independent predictive
factor for LLNM (13–15).
The extent of cervical lymph node dissection is a
subject of debate; specifically, whether CLND alone or LLND
combined directly affects follow-up treatment, and whether it is
related to the prognosis (16,17).
According to the guidelines of 2015 ATA, therapeutic lateral neck
compartmental lymph node dissection should be performed for
patients with biopsy-proven metastatic lateral cervical
lymphadenopathy (7). However, not
all patients are suitable for FNA, including those with cystic
lymph nodes, whose cyst fluid tends to cause cancer cells to
spread. Furthermore, in certain patients with PTC, the puncture
result is negative, but imaging results are indicative of LLNM.
The present study differed from previous studies as
it included patients with PTC that had the lymph nodes in the
lateral cervical region that were identified as suspicious
metastases through cervical ultrasounds. These patients underwent
CLND and LLND, regardless of the FNA results. Postoperative
pathological examination confirmed that the rate of CLNM and LLNM
was 73.4 and 82%, respectively. Previous studies have reported that
ultrasonography and enhanced CT are specific and sensitive for the
recognition of cervical lymph node metastasis (18,19).
The present study aimed to find alternate methods to improve the
diagnostic accuracy of lateral CLNM, besides preoperative
ultrasound and CT evaluation. Therefore, the association between
CCLM and LLNM was analyzed and it was found that patients with
suspected LLNM had an increased incidence of positive central lymph
nodes. Using univariate and multivariate analyses, it was shown
that the number of positive central lymph nodes and the rate of
central lymph nodes were independent risk factors for LLNM in
patients with PTC. Therefore, further assessment of these factors
was carried out.
Firstly, the number and incidence of LLNM were
assessed in each group according to the number of positive central
lymph nodes, and it was found that the incidence of LLNM increased
with the number of positive central lymph nodes. Similar results
were found in previous studies (20,21)
including that by Wang et al (22) who found that differences in LLNM
incidences were significant among the 0, 1–2 and ≥3 CLNM groups
(16.53% vs. 41.61% vs. 64.58%; P<0.001). In the present study,
when the number of positive central lymph nodes was ≥3, the
accuracy of the diagnosis of LLNM reached 94.4% when in combination
with the suspicious results of preoperative imaging. Notably, the
model incorporating preoperative suspicious ultrasound results and
the number of positive lymph nodes during surgery demonstrated
increased accuracy (94.4% vs. 64.58%) in predicting CLNM, when
compared with the study by Wang et al (22). Furthermore, the present study
demonstrated that three positive central lymph nodes was the
optimal cut-off number for differentiating patients with PTCs with
or without LLNM using the ROC curve. Hence, the patients with PTC
were divided into three groups depending on whether the number of
positive central lymph nodes diagnosed. It was found that the
incidence of LLNM in patients with PTC with ≥3 positive central
lymph nodes was significantly increased compared with those with 1
and 2 positive central lymph nodes or with no positive central
lymph nodes.
Secondly, published ATA guidelines have queried
whether LLNM should include level IIb, III and IV lymph nodes in
patients with clinical lateral metastases (7). The results of the present study
suggested that with the increase of CLNR, the risk of lymph node
metastasis in levels III, IV and V increases. Therefore, it is not
recommended for patients with PTC with increased CLNR to undergo
selective lymph node dissection in level IIb, III and IV, which
could potentially lead to the omission of lymph nodes in other
regions. It could be suggested that due to the low metastasis rate
in area Va and the difficulty of exposure (22–24),
the extent of LLND should include at least level IIa, IIb, III, IV
and Vb lymph nodes.
Ipsilateral lateral neck recurrence is a common mode
of recurrence, which usually involves lymph node metastases
(25–27). In the present study, LLNR was
detected in 2.6% (31/933) of patients, which was in accordance with
a previous report (28). Recent
studies focus particularly on the association between CLNM and
recurrence of lateral neck lymph node. Xu et al (29) performed a study that included ~2,500
patients who underwent thyroidectomy and unilateral central
compartment dissection. They found that an increased risk of LNR
was associated with >6 CLNMs and the 5-year lateral neck
recurrent-free survival rate was significantly worse for patients
with >3 CLNMs compared with that in patients with £3 CLNMs. Gui
et al (30) found that
recurrence was significantly increased in patients with >5
positive lymph nodes.
Kang et al (31) identified that LNR values ≥0.29 were
an independent prognostic factor for recurrence in a retrospective
study. They found that patients with an LNR value ≥0.29 had an
increased risk of recurrence compared with those with a lower LNR
(31). In the present study, there
was no statistically significant association identified between LNR
and lateral neck lymph node recurrence. This was in accordance with
a study by Lee et al (32)
which also failed to find a significant difference between LNR and
recurrence-free survival in 136 patients with PTC with cN1b who
underwent thyroidectomy and therapeutic central and lateral neck
dissection. However, the present study found that when the number
of positive lymph nodes in the central region was >3, the risk
of lateral neck lymph node recurrence increased 3.5-fold.
Therefore, patients with ≥3 CLNMs should be closely followed up,
and early intervention should be carried out if there are imaging
abnormalities of lateral neck lymph nodes.
The present study, however, had several limitations.
Firstly, as a retrospective study, it was susceptible to selection
bias. Secondly, the present study was conducted as a single-center
retrospective analysis with certain inherent limitations. Thirdly,
all the patients included in the present study were of Chinese
ethnicity. Therefore, this may limit the application of the
conclusions of the present study with respect to patients of other
ethnicities with PTC. Future studies should include a multi-center
prospective study with a larger sample size that will obtain more
accurate and objective conclusions.
In conclusion, CLNM was more likely to occur in
patients with PTC, and the rate and number of CLNMs was associated
with LLNM. During the surgical treatment of these patients with PTC
presenting with suspicious lateral lymph nodes but lack cytological
results from lymph node (FNA) or have negative FNA results at the
time of diagnosis, the CLNR level and number of positive central
lymph nodes could be assessed through intraoperative frozen section
examination, if the number of positive central lymph nodes is ≥3,
it is advisable to extend the incision length in real time to
search for suspicious lateral lymph nodes and assess the presence
of LLNM. After confirming CLNM ≥3 in the postoperative pathological
results, it is recommended to intensify postoperative observation,
conduct regular follow-ups, and repeat FNA in suspicious lateral
lymph nodes for patients with PTC.
Acknowledgements
Not applicable.
Funding
This work was supported by the Clinical Research Project of the
First Affiliated Hospital of Anhui Medical University (grant no.
LCYJ2021YB014) and the University Research Project of Anhui
Province (grant no. 2022AH040161).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YX and CZ designed the research scheme. YX collected
data. YX and CZ wrote and revised the manuscript. YX performed the
statistical analysis. CZ and YX read and approved the final
manuscript. YX and CZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Anhui Medical University (Hefei,
China; approval no. PJ2023-04-44) and informed patient consent was
waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li M, Dal Maso L and Vaccarella S: Global
trends in thyroid cancer incidence and the impact of overdiagnosis.
Lancet Diabetes Endocrinol. 8:468–470. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sancho J, Lennard T, Paunovic I, Triponez
F and Sitges-Serra A: Prophylactic central neck disection in
papillary thyroid cancer: A consensus report of the european
society of endocrine surgeons (ESES). Langenbecks Arch Surg.
399:155–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aygun N, Kostek M, Isgor A and Uludag M:
Role and extent of neck dissection for neck lymph node metastases
in differentiated thyroid cancers. Sisli Etfal Hastan Tip Bul.
55:438–449. 2021.PubMed/NCBI
|
|
6
|
Eskander A, Merdad M, Freeman JL and
Witterick IJ: Pattern of spread to the lateral neck in metastatic
well-differentiated thyroid cancer: A systematic review and
meta-analysis. Thyroid. 23:583–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim Y, Roh JL, Gong G, Cho KJ, Choi SH,
Nam SY and Kim SY: Risk factors for lateral neck recurrence of
N0/N1a papillary thyroid cancer. Ann Surg Oncol. 24:3609–3616.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leenhardt L, Erdogan MF, Hegedus L, Mandel
SJ, Paschke R, Rago T and Russ G: 2013 European thyroid association
guidelines for cervical ultrasound scan and ultrasound-guided
techniques in the postoperative management of patients with thyroid
cancer. Eur Thyroid. 2:147–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao M, Ge M, Ji Q, Cheng R, Lu H, Guan H,
Gao L, Guo Z, Huang T, Huang X, et al: 2016 Chinese expert
consensus and guidelines for the diagnosis and treatment of
papillary thyroid microcarcinoma. Cancer Biol Med. 14:203–211.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guidelines Working Committee of Chinese
Society of Clinical Oncology, . Guidelines of Chinese society of
clinical oncology (CSCO) differentiated thyroid cancer. J Cancer
Control Treat. 34:1164–1201. 2021.
|
|
12
|
Stack BC Jr, Ferris RL, Goldenberg D,
Haymart M, Shaha A, Sheth S, Sosa JA, Tufano RP and American
Thyroid Association Surgical Affairs Committee: American thyroid
association consensus review and statement regarding the anatomy,
terminology, and rationale for lateral neck dissection in
differentiated thyroid cancer. Thyroid. 22:501–508. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agrawal N, Evasovich MR, Kandil E,
Noureldine SI, Felger EA, Tufano RP, Kraus DH, Orloff LA, Grogan R,
Angelos P, et al: Indications and extent of central neck dissection
for papillary thyroid cancer: An American head and neck society
consensus statement. Head Neck. 39:1269–1279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang H, Xu S, Ni S, Wang X and Liu S: A
nomogram for predicting lateral lymph node metastasis in cN0
unifocal papillary thyroid microcarcinoma. BMC Cancer. 23:7182023.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong J, Zhu B, Liu W, Shi C, Xia C, Zeng L
and Lv Y: Risk factors for lymph node metastasis in papillary
thyroid carcinoma: A retrospective study. Horm Metab Res.
55:315–322. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng J, Yang X, Wu B, Sun D, Jiang Y and
Qu Z: Predictive factors for central lymph node and lateral
cervical lymph node metastases in papillary thyroid carcinoma. Clin
Transl Oncol. 21:1482–1491. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deligiorgi M, Panayiotidis M and Trafalis
D: Prophylactic lymph node dissection in clinically no
differentiated thyroid carcinoma: Example of personalized
treatment. Per Med. 17:317–338. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong Y, Li J, Huang Y, Zhou J, Liu T, Guo
Y, Yu J, Zhou S, Wang Y and Chang C: Ultrasound-Based Radiomic
Nomogram for Predicting Lateral Cervical Lymph Node Metastasis in
Papillary Thyroid Carcinoma. Acad Radiol. 28:1675–1684. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Wu X, Mao N, Zheng G, Zhang H, Mou
Y, Jia C, Mi J and Song X: Computed tomography-based radiomics
model to predict central cervical lymph node metastases in
papillary thyroid carcinoma: A multicenter study. Front Endocrinol
(Lausanne). 12:7416982021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng J, Qin A, Ye J, Pan H, Jiang Y and Qu
Z: Predictive factors for lateral lymph node metastasis and skip
metastasis in papillary thyroid carcinoma. Endocr Pathol. 31:67–76.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang Y, Yin Y and Zhou W: Risk Factors
for central and lateral lymph node metastases in patients with
papillary thyroid micro-carcinoma: Retrospective analysis on 484
cases. Front Endocrinol (Lausanne). 12:6405652021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Deng C, Shu X, Yu P, Wang H, Su X
and Tan J: Risk factors and a prediction model of lateral lymph
node metastasis in CN0 papillary thyroid carcinoma patients with
1–2 central lymph node metastases. Front Endocrinol (Lausanne).
12:7167282021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang W, Zhang Z, Zhao Y, Xue W, Xia F and
Li X: Management of lateral multiple-level metastasis in N1b
papillary thyroid microcarcinoma. Front Oncol. 10:15862020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song K, Jin Y, Kim M, Moon S, Heo DB, Won
HR, Chang JW and Koo BS: Patterns of occult metastasis to level Va
and Vb in clinically lateral node-positive papillary thyroid
carcinoma. Ann Surg Oncol. 29:2550–2556. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asimakopoulos P, Shaha AR, Nixon IJ, Shah
JP, Randolph GW, Angelos P, Zafereo ME, Kowalski LP, Hartl DM,
Olsen KD, et al: Management of the neck in well-differentiated
thyroid cancer. Curr Oncol Rep. 23:12020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun W, Lan X, Zhang H, Dong W, Wang Z, He
L, Zhang T and Liu S: Risk factors for central lymph node
metastasis in CN0 papillary thyroid carcinoma: A systematic review
and meta-analysis. PLoS One. 10:e01390212015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang LH, Yin KX, Wen QL, Chen C, Ge MH
and Tan Z: Predictive risk-scoring model for central lymph node
metastasis and predictors of recurrence in papillary thyroid
carcinoma. Sci Rep. 10:7102020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu SY, Ren ZF, Liu J, Huang H, Zhang ZM,
Liu SY, Wang XL and Xu ZG: Establishment of model to predict
lateral neck recurrence of central lymph node metastasis in
papillary thyroid carcinoma. Zhonghua Zhong Liu Za Zhi. 43:775–780.
2021.(In Chinese). PubMed/NCBI
|
|
29
|
Xu S, Huang H, Huang Y, Wang X, Xu Z, Liu
S and Liu J: Risk stratification of lateral neck recurrence for
patients with pN1a papillary thyroid cancer. BMC Cancer.
22:12462022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gui Y, Huang D, Hou Y, Wei X, Zhang J and
Wang J: Predictive factors for recurrence of papillary thyroid
carcinoma in children and adolescents. Front Oncol. 12:8337752022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang IK, Park J, Bae JS, Kim JS and Kim K:
Lymph node ratio predicts recurrence in patients with papillary
thyroid carcinoma with low lymph node yield. Cancers (Basel).
15:29472023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee CW, Roh JL, Gong G, Cho KJ, Choi SH,
Nam SY and Kim SY: Risk factors for recurrence of papillary thyroid
carcinoma with clinically node-positive lateral neck. Ann Surg
Oncol. 22:117–124. 2015. View Article : Google Scholar : PubMed/NCBI
|