Introduction
Gallbladder carcinoma accounts for the most common
type of biliary tract cancer. While the incidence of gallbladder
cancer (GBC) is relatively low, diagnosis often occurs at an
advanced stage. Furthermore, surgery remains the only curative
therapy for resectable diseases. For unresectable or metastatic
diseases, the prognosis is poor, curative surgery is infeasible,
and management has always been palliative because of the low
response to traditional chemotherapy (1–3).
The recent advancements in chemo-immunotherapy,
which integrates chemotherapy with immunotherapy, have improved the
treatment's efficacy in terms of survival and response rates for
metastatic cholangiocarcinoma, compared with conventional
treatments (4). Studies (i.e.,
TOPAZ-1 and KEYNOTE-966) have evaluated the effectiveness of
combining chemotherapy with immunotherapy agents (i.e., durvalumab
and pembrolizumab, respectively) in intrahepatic or extrahepatic
cholangiocarcinoma, including GBC (5,6). Both
trials demonstrated the potential benefits of adding immunotherapy
to standard chemotherapy regimens in treating biliary tract cancer
at advanced stages, showing improvements in survival rates. In the
TOPAZ-1 study, the combination therapy comprising durvalumab,
gemcitabine, and cisplatin showed an overall response rate of 26.7%
and a complete response rate of 2.7% (5). These findings underscore the
importance of continued research and clinical trials to refine and
enhance treatment protocols for improved patient outcomes. The
integration of immunotherapy represents a promising shift in the
treatment landscape, offering hope for improved long-term survival
and quality of life for patients with advanced biliary tract
cancers.
Some studies have aimed to identify the factors that
predict which patients are more likely to achieve a complete
response (7–10). These factors include molecular
profiles such as PD-L1 expression, specific genetic mutations, and
the presence of certain biomarkers. The findings suggest that a
deeper understanding of tumour biology is necessary to tailor
treatments more effectively, potentially leading to better
outcomes. As the field of precision medicine evolves, incorporating
molecular and genetic insights will be crucial in developing
personalized treatment strategies for gallbladder cancer. Future
research should also focus on identifying biomarkers that can
predict response to therapy, enabling more targeted and effective
treatment approaches. Furthermore, the role of combination
therapies in overcoming resistance to single-agent treatments
continues to be a critical area of investigation.
Case report
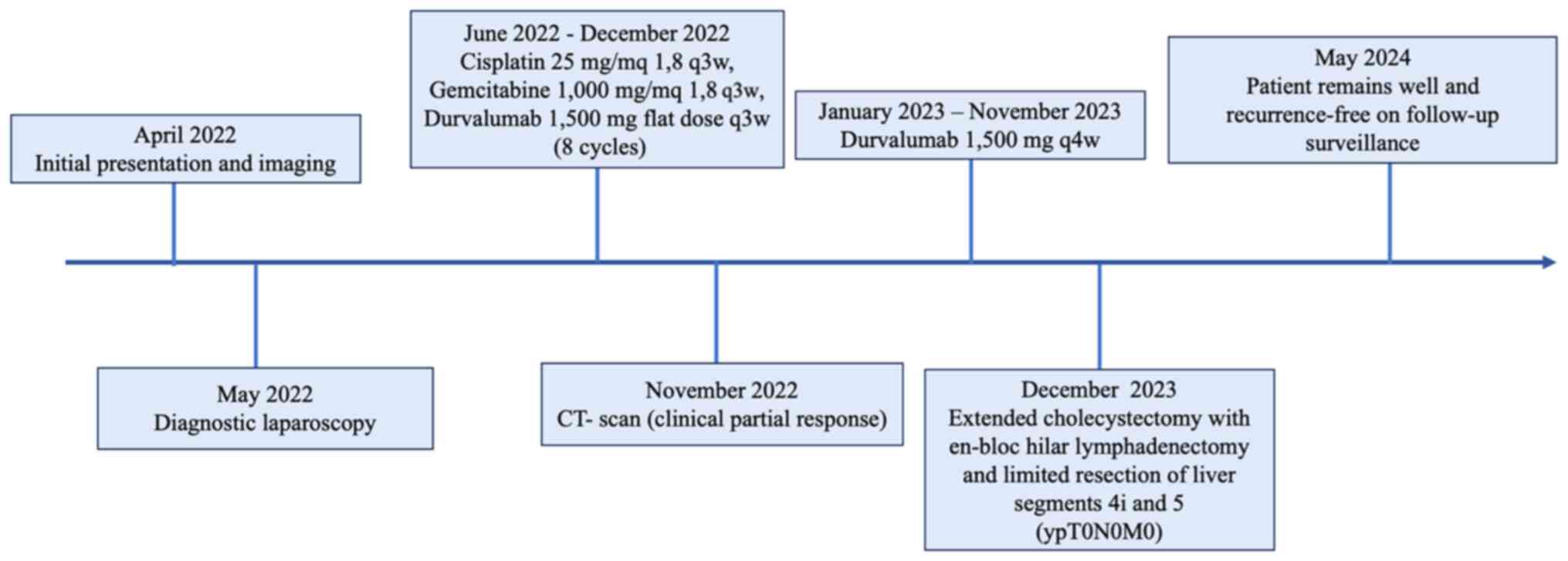
A 62-year-old male affected by GBC, unresectable at
diagnosis, received chemo-immunotherapy and then underwent surgical
resection with a pathological complete response. The events and
treatments are summarised in the timeline shown in Fig. 1.
Informed consent was obtained from the patient for
publication as a case description.
This patient had no significant medical history
(only GERD and arterial hypertension were reported).
In April 2022, for persistent abdominal pain
(particularly in the right hypochondriac region), the patient
underwent an abdominal ultrasound, which revealed a large
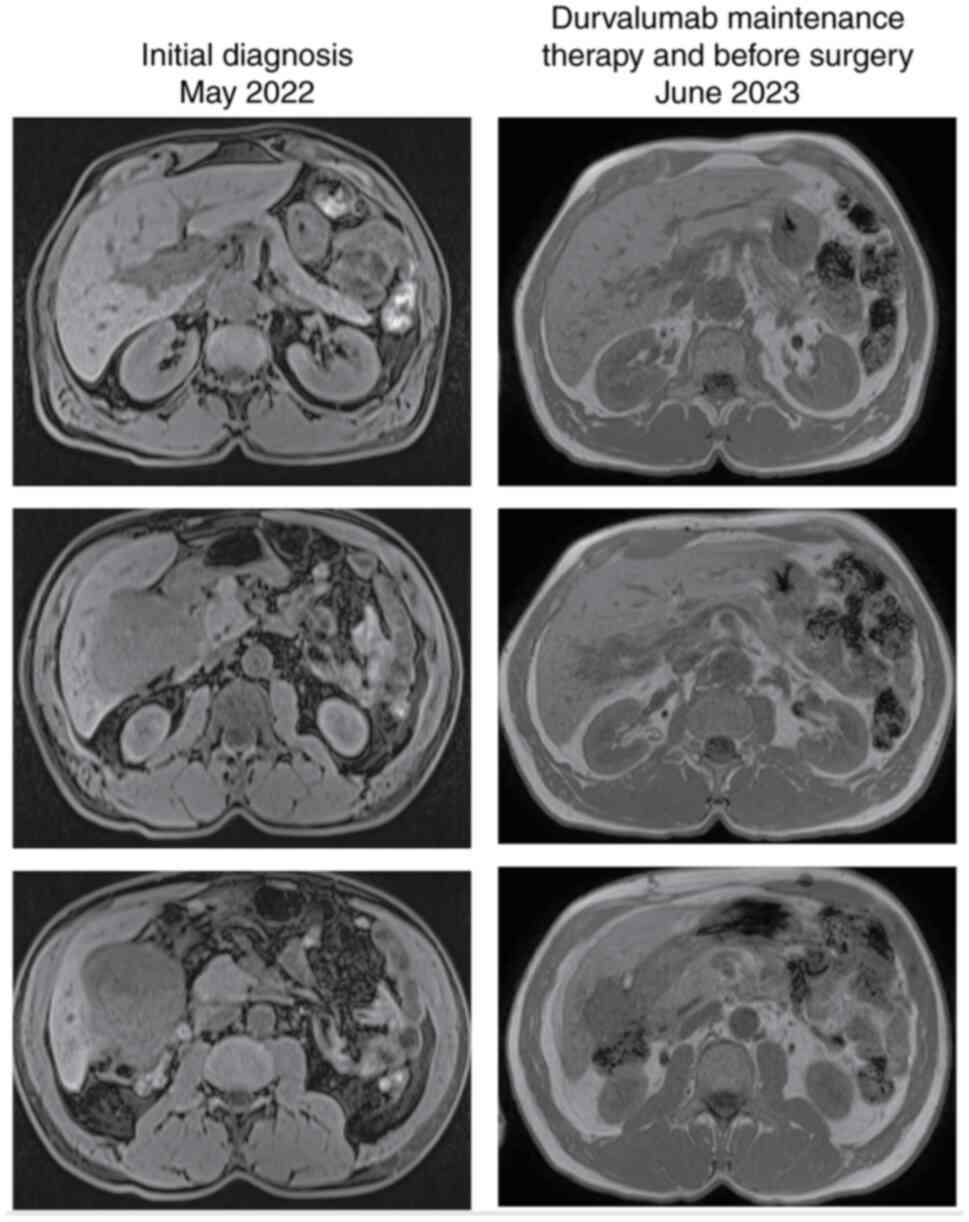
gallbladder neoplastic lesion. Computed tomography (CT) and
magnetic resonance imaging (MRI) confirmed the presence of a
gallbladder mass that extended to the liver, with suspected
duodenal infiltration (Figs. 2 and
3). An esophagogastroduodenoscopy
(EGD) was performed, and infiltration of the duodenum was
excluded.
The case was discussed among the multidisciplinary
board and an exploratory laparoscopy was performed 1 month later.
Surgeons performed multiple biopsies of the gallbladder tumour
infiltrating the hepatic pedicle and the head of the pancreas.
Histopathological findings of the core biopsy revealed poorly
differentiated squamous cell carcinoma that was negative for PD-L1
(SP263 IHC Ventana assay). Next generation sequencing was performed
using the MiSeq Illumina platform and a Myriapod NGS Cancer panel
DNA kit (Diatech), which revealed an ERBB2 R678Q mutation
(NM_004448.4:c.2033G>A) with a VAF of 29%. The melting curve was
analysed to evaluate microsatellite instability, showing that
somatic DNA from the biopsy was stable (i.e., microsatellite
stable). A subsequent CT scan was performed, showing a 97×93×120 mm
mass extending from the gallbladder to liver segments 4i and 5, the
colonic hepatic flexure, the duodenal bulb, and the head of the
pancreas. Multiple perihepatic lymphadenopathies were also
found.
Given the extent of the disease and the good
performance status of the patient, we decided to start systemic
therapy with cisplatin (25 mg/mq 1,8 q3w), plus gemcitabine (1,000
mg/mq 1,8 q3w), plus durvalumab (1,500 mg flat dose q3w), based on
the results of the TOPAZ-1 trial, within an Expanded Access
Program. At the beginning of therapy, CEA was 65.95 ng/ml and
Ca19.9 was 5,113 U/ml.
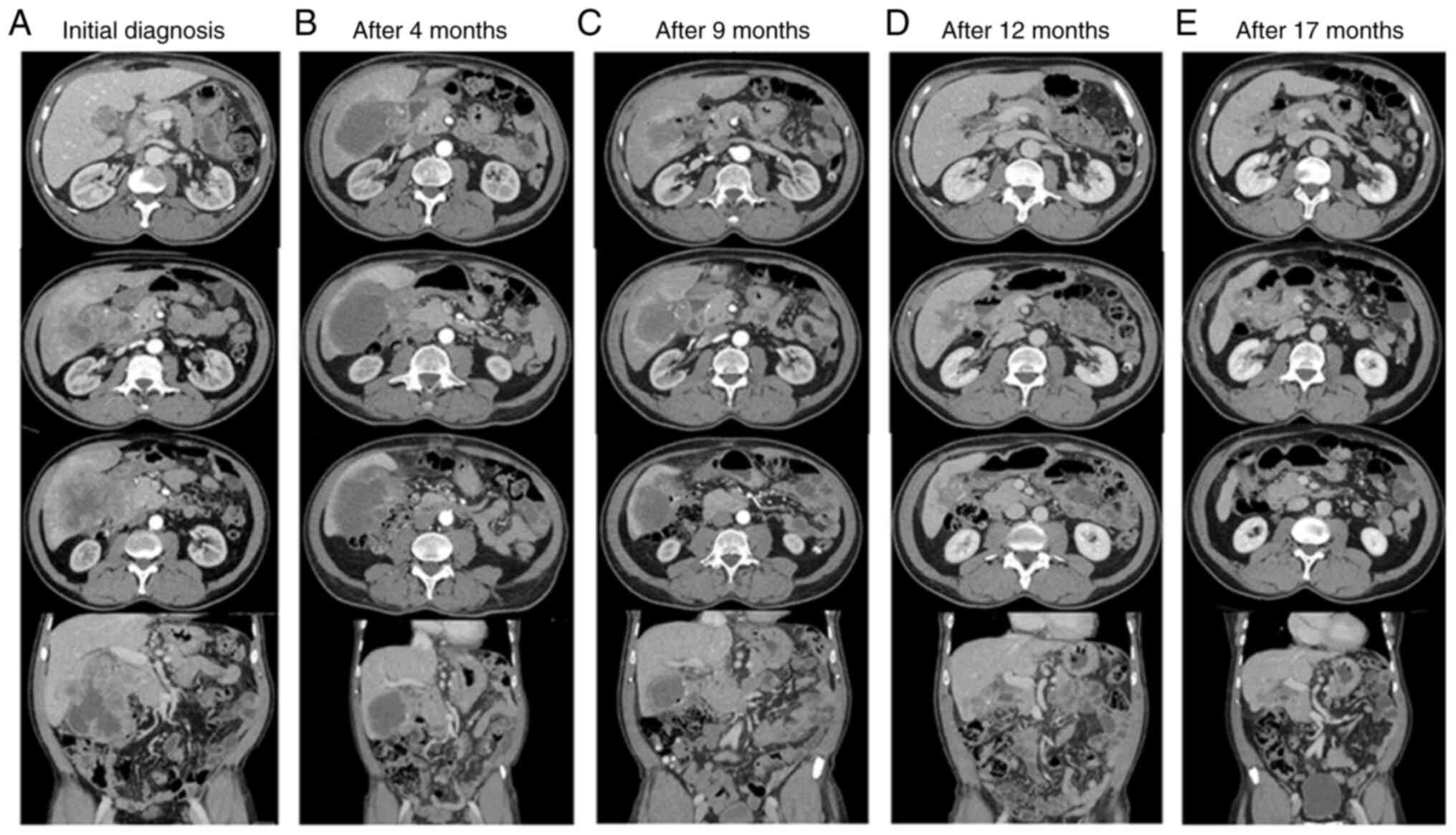
Between June and December 2022, the patient received
eight cycles of chemo-immunotherapy and exhibited excellent
clinical, biochemical, and radiological responses. In particular,
the patient reported an optimal improvement in abdominal pain, the
reduction in markers was significant, and the CT scans performed in
September and November 2022 showed significant reductions in the
tumour volume, with an increase in the necrotic component. The
therapy was maintained with durvalumab (1,500 mg q4w) starting in
January 2023. The CT scan performed 2 months later showed further
reduction in the neoplastic mass volume, and an EGD performed in
February did not show tumour cells. After 3 months, in May 2023,
the CT scan revealed further reduction in the gallbladder lesion
(45 vs. 120 mm at the time of diagnosis), and the subsequent MRI
confirmed the infiltration of the liver, with no certain cleavage
plane with the duodenum or the hepatic flexure. On June 2023, CEA
was 5.5 ng/ml and Ca19.9 was 8 U/ml. The patient maintained an
excellent performance status and received durvalumab until November
2023. The CT scan performed after 9 months of treatment with
maintaining durvalumab showed further reduction in the tumour
volume, and thus the patient underwent surgical evaluation.
In December 2023, the patient underwent a
laparotomy. A fibrotic mass extended from the gallbladder to the
right colonic flexure and the second portion of the duodenum. The
extemporaneous examination of 3 pericholecystic nodules and the
cystic duct margin revealed no tumour cells. The fibrotic mass was
detached from the colonic hepatic flexure and duodenum, revealing a
cholecystoduodenal fistula, and the duodenum was sutured. The
intraoperative ultrasound did not reveal the presence of liver
lesions, and thus an extended cholecystectomy with en-bloc hilar
lymphadenectomy and limited resection of liver segments 4i and 5
was performed. A histological exam revealed no residual tumour or
lymph node metastases. The analysed mass showed the presence of
chronic xanthogranulomatous inflammation, necrosis, and fibrosis
without the presence of tumour cells. The postoperative course was
complicated by sepsis, and a CT scan showed perihepatic fluid
collection. The patient underwent CT-guided percutaneous drainage,
and the microbial culture revealed Candida spp. Then, the patient
was treated with fluconazole to resolve the abscess.
After surgery, the patient started close
radiographic and clinical follow-up and has not shown a recurrence
of the disease.
Discussion
The majority of GBC patients present advanced stages
at diagnosis, precluding radical surgical options. Aggressive
upfront surgeries with extended resections do not benefit
higher-stage GBC owing to the greater incidence of morbidity and
mortality without oncological benefit (11). In this context, systemic therapies
have gained attention, and over the past few years, the therapeutic
landscape for unresectable GBC has evolved significantly.
Historically, gemcitabine and cisplatin (12), or oxaliplatin when cisplatin is
contraindicated (13), have been
used in standard first-line chemotherapy regimens.
The role of neoadjuvant therapy (NAT) for GBC has
not been clearly defined. The justification for using NAT is its
potential to eliminate micro-metastases, decrease the size of the
main tumour, expand the number of patients suitable for surgery,
and ultimately enhance the likelihood of survival (14). A recent study on the use of
neoadjuvant chemotherapy for extrahepatic biliary tract cancer,
including GBC, concluded that NAT was associated with better
overall survival and cancer-specific survival for patients with
advanced-stage diseases, without improving survival rates for all
surgical patients. This suggests that a tailored approach to NAT is
beneficial for certain groups of patients, underscoring the
importance of patient-specific treatment plans for enhancing
survival outcomes (15).
Pathological complete response in advanced GBC is infrequent, only
a few case reports described the use of conversion chemotherapy
(16).
However, recent advances in chemo-immunotherapy have
created new paradigms for treatment. The phase 3 TOPAZ-1 study has
been particularly pivotal, demonstrating the benefit of combining
durvalumab, an immune checkpoint inhibitor, with gemcitabine and
cisplatin (5). This combination not
only improved overall survival and progression-free survival but
also increased the rates of radiological complete response compared
with chemotherapy alone. These findings led to a shift from
traditional chemotherapy regimens to more advanced
chemo-immunotherapy protocols, where the recent chemo-immunotherapy
protocols represent the backbone of first-line treatment in
advanced cases. These advancements represent a significant leap
forward in the management of GBC, offering new hope for advanced
GBC patients with potentially curative responses, especially in
settings where only palliative care was previously feasible.
Individual cases and smaller series have reported
complete clinical responses in metastatic cholangiocarcinoma
patients treated with chemo-immunotherapy. A case utilising
pemigatinib, pembrolizumab, and chemotherapy showed a partial
response progressing to a complete metabolic response with
normalised tumour markers, highlighting the triple therapy's
potential effectiveness (7). An
approach combining dose-fractioned radiation with PD-1 inhibitors
showed significant tumour control and extended survival (8); similarly, a stage IV EBV-associated
intrahepatic cholangiocarcinoma patient achieved complete remission
with first-line anti-PD-1 immunotherapy and radiotherapy,
indicating the potential influence of high PD-L1 expression and
other genetic markers on the treatment efficacy (9). Furthermore, another patient reached
complete remission with PD-1 inhibitors and paclitaxel, maintaining
this state for over two years without relying on established
predictive biomarkers for improved efficacy, though mutations in
BRCA1, KRAS, and NTRK3 were noted (10).
Several cases of conversion surgery have been
reported in GBC (14); however,
data on chemo-immunotherapy for advanced diseases following a
surgical approach are extremely limited and predominantly consist
of case reports (17–19) (Table
I).
 | Table I.Summary of the included studies. |
Table I.
Summary of the included studies.
| First author/s,
year | Sex, age (years) | Reasons for
unresectability | CHT | Immunotherapy | PD-L1 status | Other biomarkers | Surgery (resection
assessment) | Patient
follow-up | (Refs.) |
|---|
| Satyananda et
al, 2021 | M, 59 | Stage IIIb | Gemcitabine
cisplatin | Ipilimumab
nivolumab | NA | NA | Extended right
hepatectomy with radical cholecystectomy, follow-up lymph node
dissection, Roux-Y hepaticojeju-nostomy (ypT0N0M0) | Disease-free at
10-month | (17) |
| Zhang et al,
2023 | F, 60 | Stage IVb | Gemcitabine
cisplatin | Lenvanitib
durvalumab | + | MSS | Partial
cholecistectomy and subsequent completion surgery (residual
cholecystectomy and hilar lymph node dissection) (ypT0N0M0) | Alive 1 year after
diagnosis | (18) |
| Wang et al,
2023 | F, 58 | Stage IVb | Gemcitabine
capecitabine | Carrellizumab | + | MSS | Radical resection of
gallbladder carcinoma and radiofrequency ablation of liver
lesions | Disease-free at
14-month follow-up | (19) |
| Leong et al,
2024 | M, 39 | Stage IVb | Gemcitabine | Durvalumab | NA | NA | Extended right
hepatectomy cisplatin and reconstruction with hepaticojejunostomy
(ypT1aN0M0) | Disease-free at
6-month follow-up | (20) |
In 2021, Satyananda et al (17) reported on a case that was initially
diagnosed as unresectable GBC (cT3N1M0). After
cisplatin-gemcitabine chemotherapy and trial-based
ipilimumab-nivolumab immunotherapy, a good radiological response
and reduction in tumour markers were reported. The patient
underwent right portal vein embolisation followed by an open
extended right hepatectomy, radical cholecystectomy, and roux-en-Y
hepaticojejunostomy reconstruction. Final histology showed a
complete tumour response (ypT0N0M0), and the patient was
disease-free at the 10-month follow-up.
In 2023, Zhang et al (18) reported a case of GBC with jaundice
and elevated tumour markers (i.e., CEA, CA 19.9, CA 125, and
alpha-fetoprotein). Exploratory laparoscopy with partial
cholecystectomy and biopsy revealed stage IV GBC with liver,
para-aortic, and retroperitoneal lymph node metastasis. Lenvatinib
therapy was started, but a subsequent CT scan showed an increase in
tumour mutational burden (TMB). Therapy was shifted to
cisplatin-gemcitabine chemotherapy and durvalumab immunotherapy.
After three cycles, the tumour marker levels fell to within the
normal range and radiographic assessment showed a clinical complete
response. The patient underwent residual cholecystectomy and hilar
lymph node dissection. Final histology showed a complete tumour
response (ypT0N0M0), but the CT scan performed 6 months after
surgery revealed an enlargement of the abdominal lymph nodes. SOX
regimen and durvalumab were started, and lymph node shrinkage was
observed in the subsequent CT scan. The patient was still alive 1
year after the initial diagnosis.
In 2023, Wang et al (19) reported a case of metastatic GBC in
the liver; the 58-year-old female showed a significant increase in
serum β-HCG level (6080.2 IU/l). The patient received chemotherapy
with gemcitabine and capecitabine plus immunotherapy with
carrellizumab. The CT scan after therapy revealed a drastic
reduction in the tumour size, and the serum β-HCG level decreased
to the normal range. Subsequently, radical resection of gallbladder
carcinoma and radiofrequency ablation of liver lesions were
performed. Histopathological analysis showed no tumour cells,
indicating a pathological complete response. The patient received
two more courses of postoperative chemo-immunotherapy and was
disease-free at the 14-month follow-up.
In 2024, Leong et al (20) reported a case of GBC with jaundice
and elevated CA 19.9. The preliminary diagnosis was infiltrative
GBC involving the hepatic duct confluence. The patient was a
candidate for surgery and underwent right portal vein embolisation.
The subsequent CT scan revealed disease progression, and diagnostic
laparoscopy confirmed the stage IV GBC. After cisplatin-gemcitabine
chemotherapy and durvalumab immunotherapy, the CT scan showed a
near complete response, and the patient underwent a laparoscopic
extended right hepatectomy with hepaticojejunostomy reconstruction.
Final histology confirmed the near complete pathological response
(ypT1aN0). Adjuvant gemcitabine, cisplatin, and durvalumab were
administered, and the patient was disease-free at the 6-month
follow-up.
Complete responses without pathological confirmation
are increasingly reported in the literature for cholangiocarcinoma,
and similar findings are documented for GBC, although there are
only a few cases described (21–23).
These findings, particularly those related to the characteristics
of long-term responses to immunotherapy, raise questions concerning
the role of subsequent surgery and the greater morbidity and
mortality associated with extended resections.
In the SWOG 1609 cohort, the combination of
anti-CTLA-4 and anti-PD-1 agents led to extended disease control in
advanced GBC; one patient (5% of the cohort) achieved a complete
response with a median duration of 14.8 months (21). Similarly, Tan et al (22) observed a complete response in 10% of
the patients treated with PD-1 inhibitors combined with
nab-paclitaxel, highlighting the potential of chemo-immunotherapy
to facilitate significant tumour regression. Additionally, Rao
et al (23) reported on the
complete response observed at the 11-month follow-up in a patient
who initially presented with multiple hepatic metastases from GBC
and received radical surgery treated with camrelizumab and
apatinib. The relationship between biomarkers, such as PD-L1
expression or TMB, and long-term treatment efficacy remains
underexplored in these reports, emphasising the need for targeted
research aimed at identifying reliable predictive biomarkers.
The present case harboured an ERBB2 mutation, which
is a rare activating point mutation (R678Q) in the juxtamembrane
domain (23). Aberrant expression
and signalling of the epidermal growth factor receptor family of
tyrosine kinases have been implicated in the molecular pathogenesis
of cholangiocarcinoma, thereby highlighting the potential efficacy
of agents selectively targeting these receptors; extrahepatic BTCs,
including GBC, showed a higher HER2 overexpression rate compared
with intrahepatic cholangiocarcinoma (24). The mechanisms related to the
pathological and physiological responses of GBC to combination
therapy are exceedingly complex, involving multiple factors, such
as TMB, microsatellite instability, PD-L1 expression, and
peripheral blood lymphocyte subpopulations, all contributing to the
efficacy of the therapy. Koido et al (25) reported that treatment of
cholangiocarcinoma cells with gemcitabine resulted in the
upregulation of the tumour antigen WT1, calreticulin (an eat-me
signal for cells undergoing apoptosis), and PD-L1 but did not
establish a direct link with ERBB2 mutations. The patient was PD-L1
negative, which is generally associated with a less likely response
to immune checkpoint inhibitors (26). However, a significant response was
observed, suggesting that factors beyond PD-L1 status may influence
treatment outcomes. ERBB2 mutations, including the rare R678Q
mutation found in our patient, have been implicated in various
cancers as potential drivers of oncogenesis and targets for therapy
(27), although the specific impact
of the ERBB2 R678Q mutation on the response to chemo-immunotherapy
in GBC remains unclear. The literature does not provide a
definitive correlation between this mutation and treatment
efficacy. While PD-L1 expression and TMB are commonly evaluated
biomarkers, their predictive value is not absolute, and other
factors, including specific genetic mutations, may play a role but
are not fully understood. Thus, there is justification for
combining standard chemotherapy agents with immune checkpoint
inhibitors in predominantly microsatellite stable (>97% of BTCs)
diseases to support a positive treatment response (17).
In the present study, a complete pathological
response was observed in a GBC patient with multiple distant
lymphatic metastases and local extension, exhibiting invasion of
liver segments 4i and 5, the colonic hepatic flexure, the duodenal
bulb, and the pancreatic head. After therapy with gemcitabine,
cisplatin, and durvalumab, a significant response to treatment was
observed and subsequent surgical resection resulted in a complete
pathological response.
In conclusion, first-line chemo-immunotherapy in
metastatic cholangiocarcinoma, including GBC, appears to provide
promising results, particularly for combinations using newer
immunotherapeutic agents. While complete responses are rare,
chemo-immunotherapy is associated with significant clinical
benefits and may become more common in personalised approaches
based on specific tumour characteristics. Further studies are
required to refine these therapies and better predict patients who
may benefit the most.
Acknowledgements
Not applicable.
Funding
The present study was supported by a non-profit grant from the
Associazione Malato Oncologico Piacentino.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
EO, IT, ST, AS, MAP, EA, SV, AR and MG assessed the
conception and design of the manuscript, and were involved in
acquisition, analysis and interpretation of data. MG, IT and ST
contributed to the investigation and data curation. MAP, EA and AR
drafted the article. EO, IT, ST, AS, MAP, EA, SV, AR and MG
critically revised the manuscript for important intellectual
contents. EO and MG reviewed and edited the final version of the
manuscript. EO and MG confirm the authenticity of all the raw data.
All authors have read and approved the final version of the
manuscript, and participated sufficiently in the work to take
public responsibility for appropriate portions of the content. All
authors agreed to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The case report did not require approval from a
local ethics committee; however, ethical considerations in
accordance with the Declaration of Helsinki were adhered to. The
patient provided written informed consent.
Patient consent for publication
The patient provided written informed consent for
publication of the data and images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CCA
|
cholangiocarcinoma
|
|
GBC
|
gallbladder cancer
|
|
MSS
|
micro satellite stable
|
|
NAT
|
neoadjuvant therapy
|
|
TMB
|
tumor mutational burden
|
References
|
1
|
Duffy A, Capanu M, Abou-Alfa GK, Huitzil
D, Jarnagin W, Fong Y, D'Angelica M, Dematteo RP, Blumgart LH and
O'Reilly EM: Gallbladder cancer (GBC): 10-Year experience at
memorial sloan-kettering cancer centre (MSKCC). J Surg Oncol.
98:485–489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin LX, Pitt SC, Hall BL and Pitt HA:
Aggressive surgical management of gallbladder cancer: At what cost?
Surgery. 154:266–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valle J, Kelley R, Nervi B, Oh D and Zhu
A: Biliary tract cancer. Lancet. 397:428–444. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lo JH, Agarwal R, Goff LW and Heumann TR:
Immunotherapy in biliary tract cancers: Current standard-of-care
and emerging strategies. Cancers (Basel). 15:33122023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oh DY, Lee KH, Lee DW, Kim TY and Bang YJ:
Durvalumab plus gemcitabine and cisplatin for advanced biliary
tract cancers: A phase III study (TOPAZ-1). Cancer Discov.
12:678–688. 2022.
|
|
6
|
Kelley RK, Ueno M, Yoo C, Finn RS, Furuse
J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, et al: Pembrolizumab
in combination with gemcitabine and cisplatin compared with
gemcitabine and cisplatin alone for patients with advanced biliary
tract cancer (KEYNOTE-966): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet. 401:1853–1865. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Z, Wang G, Du L, Zhao J, Pan L,
Zhang G, Wang F and Liu R: Case report: Persistent response to
combination therapy of pemigatinib, chemotherapy, and immune
checkpoint inhibitor in a patient with advanced intrahepatic
cholangiocarcinoma. Front Immunol. 14:11244822023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Zhang J, Ban X, Li Z and Fang Y: A
novel mixture of dose-fractioned radiation and PD-1 inhibitor
produces significant tumor control in a patient with advanced
intrahepatic cholangiocarcinoma: A case report. J Med Case Rep.
17:892023.
|
|
9
|
Liao W, Huang J, Liang Y, Zhou F and Pan
Z: PD-1 blockade and radiotherapy combination for advanced
Epstein-Barr virus-associated intrahepatic cholangiocarcinoma. J
Clin Oncol. 41:337–344. 2023.
|
|
10
|
He H, Zhao H, Chen L, Zhang Y and Yu H:
Long survival of immunotherapy plus paclitaxel in advanced
intrahepatic cholangiocarcinoma with high PD-L1 expression: A case
report. Cancer Biol Ther. 23:1–6. 2022.PubMed/NCBI
|
|
11
|
Balakrishnan A, Barmpounakis P, Demiris N,
Jah A, Spiers HVM, Talukder S, Martin JL, Gibbs P, Harper SJF,
Huguet EL, et al: Surgical outcomes of gallbladder cancer: The
OMEGA retrospective, multicentre, international cohort study.
EClinicalMedicine. 59:1019512023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
André T, Reyes-Vidal JM, Fartoux L, Ross
P, Leslie M, Rosmorduc O, Clemens MR, Louvet C, Perez N, Mehmud F
and Scheithauer W: Gemcitabine and oxaliplatin in advanced biliary
tract carcinoma: A phase II study. Br J Cancer. 99:862–867. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hakeem AR, Papoulas M and Menon KV: The
role of neoadjuvant chemotherapy or chemoradiotherapy for advanced
gallbladder cancer-A systematic review. Eur J Surg Oncol. 45:83–91.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Toyoda J, Sahara K, Takahashi T, Miyake K,
Yabushita Y, Sawada Y, Homma Y, Matsuyama R, Endo I and Pawlik TM:
Neoadjuvant therapy for extrahepatic biliary tract cancer: A
propensity score-matched survival analysis. J Clin Med.
12:26542023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miura Y, Ashida R, Sugiura T, Ohgi K,
Yamada M, Otsuka S, Todaka A and Uesaka K: Pathological complete
response achieved by gemcitabine plus cisplatin therapy for
initially unresectable advanced gallbladder cancer: A case report.
Surg Case Rep. 8:202022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Satyananda V, Chouliaras K, Cherkassky L
and Schwarz RE: A case from the future of HPB surgical oncology:
Resection of biliary tract cancer after immunotherapy. J Surg Case
Rep. 2021:rjab4142021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Nie J, Tai S and Zheng T: PD-L1
inhibitor plus gemcitabine and cisplatin therapy followed by
conversion surgery for initially unresectable advanced gallbladder
cancer. BMJ Case Rep. 16:e2554032023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Mu Y, Ji S, Liu Y, Lou Y, Wei S,
Dong X and Zhang B: Case report: Complete pathological remission of
human chorionic gonadotrophin-producing gallbladder carcinoma with
multiple liver metastases after treatment with chemotherapy plus an
immune checkpoint inhibitor. Front Immunol. 14:11735202023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leong EKF, Tan NCH, Pang NQ and Kow AWC:
Case report: From palliative to potentially curative-the advent of
immunotherapy providing hope to advanced gallbladder
adenocarcinoma. Front Immunol. 15:13534302024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel SP, Guadarrama E, Chae YK, Dennis
MJ, Powers BC, Liao CY, Ferri WA Jr, George TJ, Sharon E, Ryan CW,
et al: SWOG 1609 cohort 48: Anti-CTLA-4 and anti-PD-1 for advanced
gallbladder cancer. Cancer. Feb 15–2024.(Epub ahead of print).
|
|
22
|
Tan S, Yu J, Huang Q, Zhou N and Gou H:
PD-1 inhibitors plus nab-paclitaxel-containing chemotherapy for
advanced gallbladder cancer in a second-line setting: A
retrospective analysis of a case series. Front Oncol.
12:10060752022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rao J, Xia J, Yang W, Wu C, Sha B, Zheng
Q, Cheng F and Lu L: Complete response to immunotherapy combined
with an antiangiogenic agent in multiple hepatic metastases after
radical surgery for advanced gallbladder cancer: A case report. Ann
Transl Med. 8:16092020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galdy S, Lamarca A, McNamara MG, Hubner
RA, Cella CA, Fazio N and Valle JW: HER2/HER3 pathway in biliary
tract malignancies; systematic review and meta-analysis: A
potential therapeutic target? Cancer Metastasis Rev. 36:141–157.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koido S, Kan S, Yoshida K, Yoshizaki S,
Takakura K, Namiki Y, Tsukinaga S, Odahara S, Kajihara M, Okamoto
M, et al: Immunogenic modulation of cholangiocarcinoma cells by
chemoimmunotherapy. Anticancer Res. 34:6353–6361. 2014.PubMed/NCBI
|
|
26
|
Davis AA and Patel VG: The role of PD-L1
expression as a predictive biomarker: An analysis of all US food
and drug administration (FDA) approvals of immune checkpoint
inhibitors. J Immunother Cancer. 7:2782019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chmielecki J, Ross JS, Wang K, Frampton
GM, Palmer GA, Ali SM, Palma N, Morosini D, Miller VA, Yelensky R,
et al: Oncogenic alterations in ERBB2/HER2 represent potential
therapeutic targets across tumors from diverse anatomic sites of
origin. Oncologist. 20:7–12. 2015. View Article : Google Scholar : PubMed/NCBI
|