Introduction
Thyroid cancer, a malignancy ranking ninth worldwide
in terms of incidence, can occur in people of any sex and any age
(1). Papillary thyroid cancer (PTC)
is the predominant type of thyroid cancer, making up ~80% of cases
(2). Despite a more favorable
overall prognosis compared with other forms of thyroid cancer
(3), patients with PTC are more
likely to have central lymph node metastasis (CLNM), and the
incidence of CLNM is 30–80% (4,5), which
is considered to be the most important risk factor of regional
recurrence and poor survival (6).
Therefore, to achieve the goal of radical tumor resection, surgeons
will often perform therapeutic central lymph node dissection (CLND)
in patients with PTC (7). However,
it is still controversial as to whether CLN dissection should be
performed in patients with PTC with clinically negative (cN0) CLNM.
Certain cN0 patients have potential CLNM, therefore prophylactic
CLND can lower the rate of postoperative regional recurrence rate
and avoid a second surgery (8,9).
However, prophylactic CLND is not particularly cost-effective, and
the risk of recurrent laryngeal nerve injury, permanent
hypoparathyroidism and other associated complications is greatly
increased (10). Furthermore,
ultrasound (US)-guided ablation is a safe, effective and minimally
invasive substitute for surgical resection for patients with
low-risk PTC without CLNM (11).
Therefore, it is important to evaluate the central lymph nodes
accurately and comprehensively before operation to avoid
overtreatment and undertreatment, and to provide a more reasonable
surgical plan for patients.
Given its non-invasiveness, non-radiation and high
resolution, US is the preferred preoperative imaging technique for
assessing thyroid nodules and cervical lymph nodes. It can clearly
show the tumor size, location, shape, margin, composition,
echogenicity, microcalcification and blood flow signal (12,13).
However, owing to the influence of the anatomical structures of the
central neck, the effect of US in detecting CLNM is not ideal
(4,14). Meanwhile, US is also limited by the
reliance on operator skills and the incapacity to visualize deep
structures (15). Therefore, the
American Thyroid Association guidelines recommend contrast-enhanced
computed tomography (CT) as an adjunct to US to improve the
accuracy of preoperative diagnosis (7). Contrast-enhanced CT effectively avoids
the shortcomings of US. First, contrast-enhanced CT can provide
comprehensive cross-sectional images of the thyroid gland and
neighboring structures including the trachea, esophagus, blood
vessels and lymph nodes (16,17).
Second, due to the absence of gas and bone restrictions,
contrast-enhanced CT may better visualize lymph node metastasis,
capsule invasion and extrathyroidal extension (18–20).Therefore, the combination of US and
contrast-enhanced CT diagnosis would be complementary, to make up
for the deficiency of the single application of contrast-enhanced
CT or US for diagnosing thyroid nodules, and improve the diagnostic
specificity and sensitivity.
Currently, most studies predicting CLNM in patients
with PTC have focused on clinical and US features, and the results
are consistent (21–23). Furthermore, to the best of our
knowledge, few studies have investigated the association between
contrast-enhanced CT features and CLNM in patients with PTC.
Therefore, the present study used contrast-enhanced CT features to
determine the risk factors for CLNM in patients with cN0 PTC,
aiming to identify key predictors and establish a new nomogram for
predicting the risk of CLNM in patients with PTC to facilitate
preoperative decision making for prophylactic CLND.
Materials and methods
Patients selection
Owing to the retrospective study design, approval
from the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong
University School of Medicine (Shanghai, China) was obtained and
the requirement for informed consent was waived.
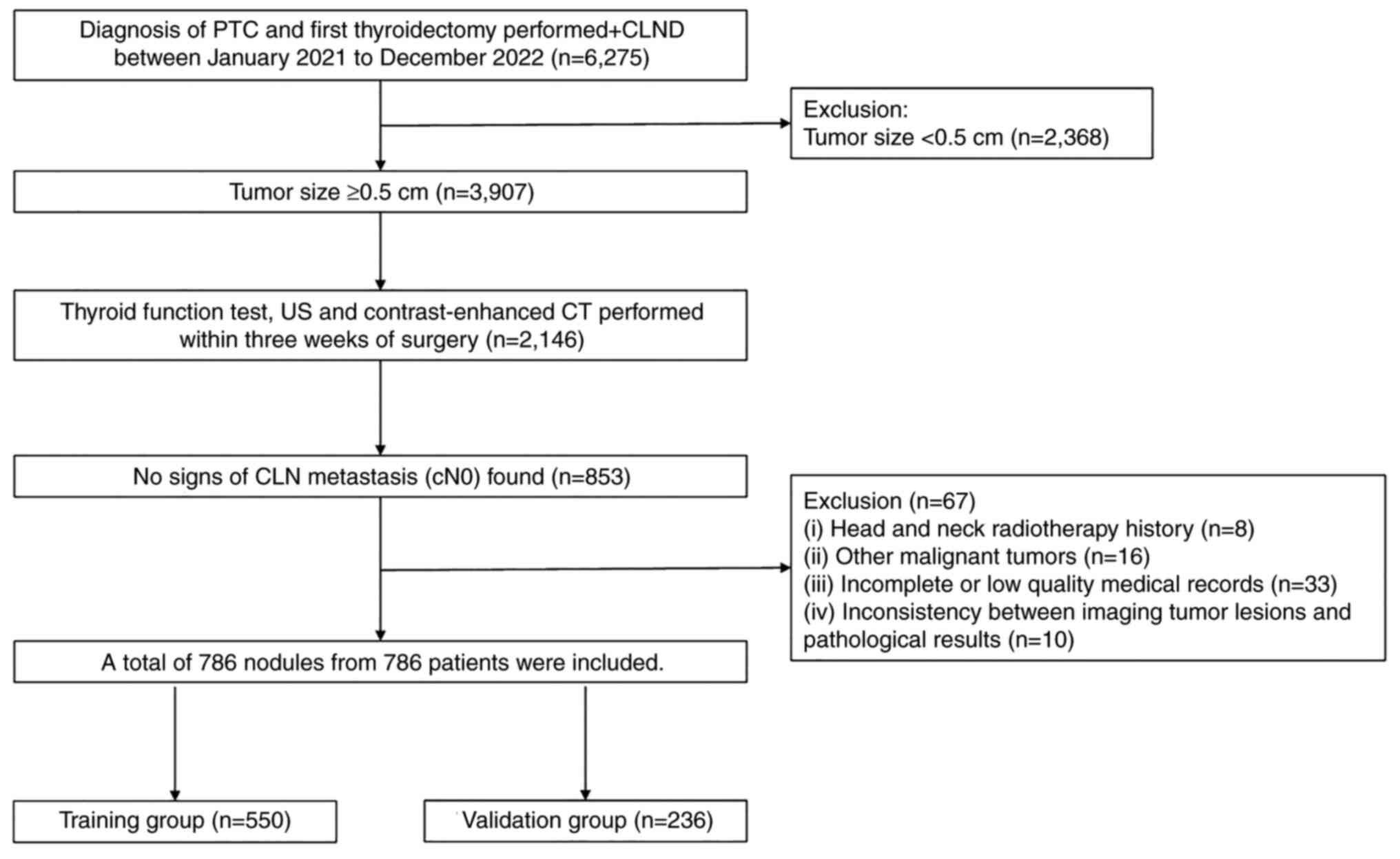
A total of 6,275 patients who underwent
thyroidectomy along with CLND and were histopathologically
confirmed to have PTC at Ruijin Hospital from January 2021 to
December 2022 were enrolled in the present study. The inclusion
criteria were as follows: i) Treatment with primary thyroid surgery
and CLND, and a BRAF V600E mutation test; ii) histopathologically
confirmed PTC; iii) no signs of lymph node metastasis (cN0),
conventional US and contrast-enhanced CT performed, and a medical
history collection within 3 weeks before surgery; iv) preoperative
thyroid function tests performed and no prior history of thyroxine
treatment [including thyroid stimulating hormone (TSH),
thyroglobulin (TG), TG antibody (TGAb) and thyroid peroxidase
antibody (TPOAb)]; and v) only the largest nodule was included for
patients with multiple nodules (with at least two of which
confirmed as PTC). Exclusion criteria were as follows: i) Tumor
size of <0.5 cm; ii) treatment of head and neck radiotherapy
therapy; iii) presence of other malignant tumors, such as
nasopharyngeal carcinoma and breast cancer; iv) incomplete or low
quality medical records; and v) inconsistent imaging tumor lesions
with the pathological results. Based on the aforementioned
inclusion and exclusion criteria, the data from 786 patients with
cN0 PTC were analyzed in the present study. The flowchart depicting
the selection process is presented in Fig. 1.
US assessment
All participants were evaluated using US equipment
(MyLab™ 9, Esaote S.p.A; DC-8, Shenzhen Mindray Bio-Medical
Electronics Co., Ltd.; and iU22, Philips Medical Systems B.V.) with
5–13 MHz linear probe. The patient was positioned in the supine
position with the neck fully exposed. A total of two radiologists
possessing 15 years of experience in thyroid US imaging evaluated
the following sonographic features in consensus: Tumor location,
size, orientation, margin, internal composition, echogenicity,
microcalcification and blood flow signal. Representative US
features are presented in Fig. 2.
Any disagreements between the two radiologists were resolved by a
third radiologist with 25 years of experience in thyroid
sonography.
BRAF V600E mutation testing
BRAF V600E mutation testing was performed and the
results were reviewed by experienced technicians in the clinical
laboratory of Ruijin Hospital. Genomic DNA was extracted from the
thyroid tissue samples using QIAamp® DNA Micro Kit
(Qiagen, Inc.; cat. no. 56304) according to the instructions. The
extracted DNA was subjected to PCR amplification (reagents: Ampli
Taq Gold™ 360 Premix of Applied Biosystems; Thermo Fisher
Scientific, Inc.; thermocycling conditions: 95°C for 3 min, 95°C 15
sec, 58°C 30 sec, 72°C 1 min, 72°C 7 min, 35 cycles in total) and
Sanger sequencing, and the sequencing data were interpreted using
the low-frequency mutation analysis software Minor Variant Finder
of Applied Biosystems (version 1.1; Thermo Fisher Scientific,
Inc.). The sequencing primers used for BRAF V600E mutation testing
are provided in Fig. S1.
Sequencing traces for Sanger sequencing are shown in Fig. S2.
Contrast-enhanced CT assessment
All patients underwent scanning using multidetector
CT scanners (GE Discovery CT750 HD 64 Slice CT Scanner, Cytiva; uCT
760; Shanghai United Imaging Healthcare Co., Ltd; and Philips
Brilliance iCT 256, Philips Medical Systems B.V.) to collect CT
data. All patients provided written informed consent and underwent
iodine allergy testing before the examination. The slice thickness
was 3.0 or 2.5 mm. Contrast-enhanced scans were performed at 45–65
sec after intravenous injection of non-ionic iodine contrast agent
(2.5 m/s). The scanning range was scanned from C7 up to the base of
the posterior fossa. The CT findings of the following nodules were
evaluated by two radiologists with extensive experience in thyroid
CT imaging: i) Mean CT values of the lesions in the plain phase
(UCT) and the venous phase (VCT). A circular region of interest was
drawn at the maximum diameter of the lesion, excluding
calcification, cystic components and artifacts, with the goal of
covering >80% of the whole lesion area. ΔCT=VCT-UCT was used to
evaluate the absolute enhanced CT value. The average value of two
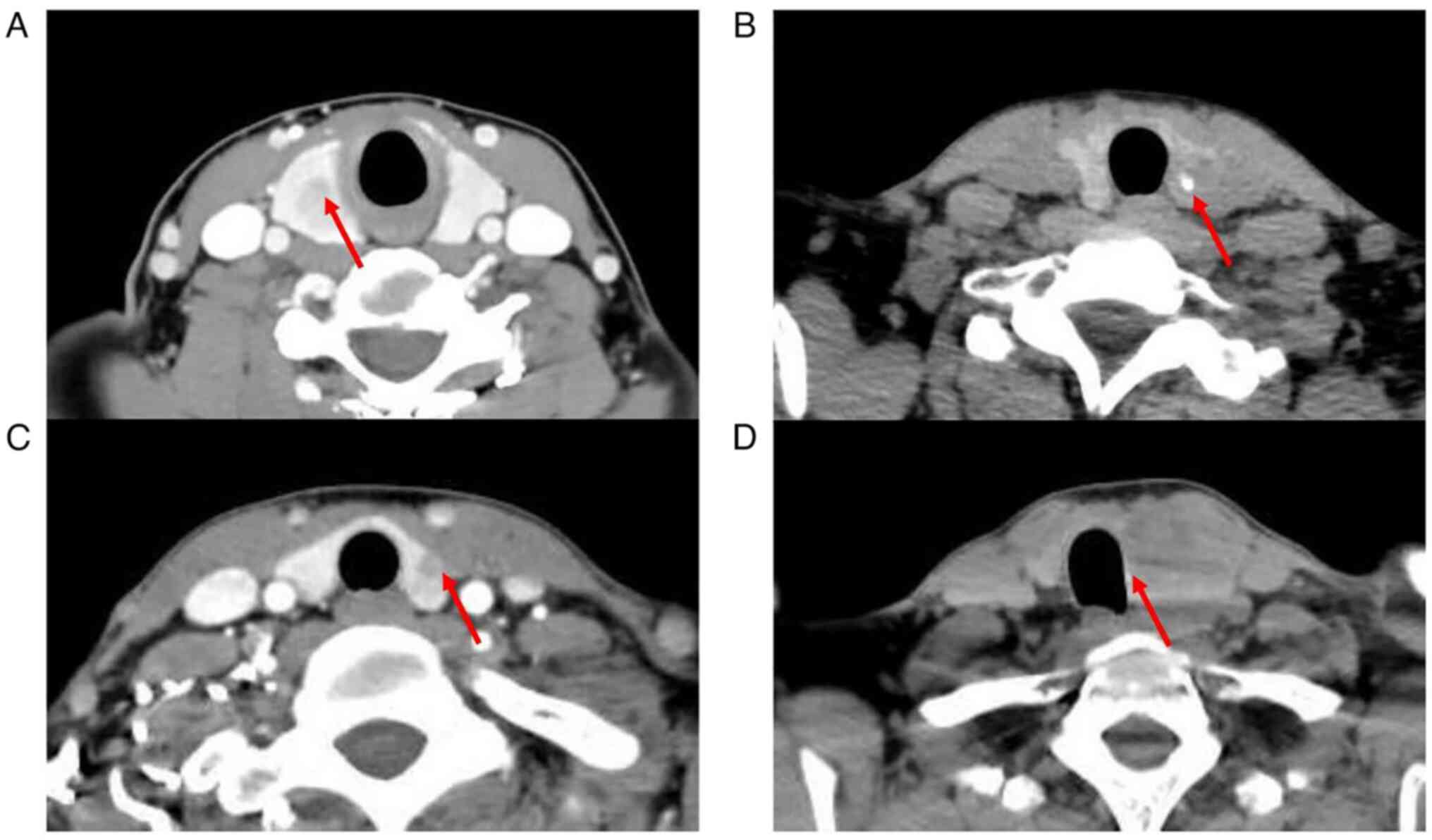
radiologists was used for further analysis; ii) homogeneity of
enhancement was divided into homogeneity and inhomogeneity
(Fig. 3A); iii) calcification
(Fig. 3B); iv) capsule invasion
(Fig. 3C); and v) tracheal
deviation (Fig. 3D).
Variable definition and
evaluation
Data for the following characteristics were
collected to construct a retrospective database: i) Basic features:
Age (45 years old as the cut point in accordance with the 7th Union
for International Cancer Control/American Joint Committee on Cancer
tumor-node-metastasis staging system) (24), sex (male/female), body mass index
[BMI; 20.92 kg/m2 as the cut point according to receiver
operating characteristic (ROC) curve analysis], Hashimoto
thyroiditis (HT; yes/no), BRAF V600E mutation (yes/no), TSH
(reference, 0.35–4.94 µIU/ml), TG (reference, 3.5–77 ng/ml), TGAb
(reference, <4.11) IU/ml) and TPOAb (reference, <5.61 IU/ml);
ii) conventional US features: Tumor location (isthmus/non-isthmus),
tumor number (unifocal/multifocal), tumor size (papillary thyroid
microcarcinoma ≤1.0 cm and PTC >1.0 cm), tumor orientation
(taller-than-wide/wider-than-tall), tumor margin
(regular/irregular), internal composition (solid/non-solid),
echogenicity (markedly hypoechoic/hypoechoic/isoechoic), capsule
contact (yes/no; defined as thyroid nodule touching the thyroid
boundary with or without capsule uplift), microcalcification
(yes/no) and blood flow signal (poor/rich)' and iii)
contrast-enhanced CT features: UCT, VCT, ΔCT, homogeneity of
enhancement (homogeneity/inhomogeneity; defined as the degree of
homogeneity of enhancement within the thyroid nodule),
calcification (yes/no), capsule invasion (yes/no; defined as the
maximum diameter of the nodule was located at the junction of the
nodule and thyroid gland or at the lateral side of the thyroid
gland, known as ‘cookie bite sign’) and tracheal deviation
(yes/no). ROC curve analysis revealed that the UCT value was 65.35
Hu, the VCT value was 183.90 Hu, and ΔCT was 111.50 Hu as the
cut-off point of CLNM in the population of the present study (data
not presented).
Statistical analysis
Continuous data were transformed into categorical
data using cut-off values established through ROC curve analysis
for enhanced clinical comprehension. Data are presented as the
frequency or mean ± standard deviation. UCT, VCT and ΔCT were
analyzed using an independent samples t-test, and TSH and
echogenicity were analyzed using Fisher's exact test. All other
variables were analyzed using the χ2 test. Multivariate
logistic regression analysis was used to determine independent
factors. Based on the results of multivariate logistic regression
analysis, a nomogram for predicting CLNM was developed and
evaluated using ROC curves, calibration curves and decision curve
analysis (DCA) curves. All statistical analyses were performed
using SPSS version 27.0 (IBM Corp.) and R version 4.3.2 (The R
Foundation) software. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of patients
Patients were divided into the training group
(n=550) and validation group (n=236). CLNM occurred in 39.1%
(215/550) of the patients in the training group and 38.6% (91/236)
of the patients in the validation group. In total, 38.9% of
patients (306/786) had an CLNM(−) status before surgery, but had
confirmed CLNM using postoperative pathology. As demonstrated in
Table I, there was no significant
difference between the two groups (P>0.05), which indicated
their rationality as training and validation groups.
 | Table I.Characteristics of all patients in
the training and validation group. |
Table I.
Characteristics of all patients in
the training and validation group.
| A, Clinical
characteristics |
|---|
|
|---|
| Characteristic | Training group
(n=550) | Validation group
(n=236) | P-value |
|---|
| Age (%) |
|
| 0.620 |
| ≤45
years | 401 (72.91) | 168 (71.19) |
|
| >45
years | 149 (27.09) | 68 (28.81) |
|
| Sex (%) |
|
| 0.399 |
|
Male | 138 (25.09) | 66 (27.97) |
|
|
Female | 412 (74.91) | 170 (72.03) |
|
| BMI,
kg/m2 | 23.74±3.57 | 23.59±3.59 | 0.596 |
| With HT (%) |
|
| 0.150 |
|
Yes | 100 (18.18) | 33 (13.98) |
|
| No | 450 (81.82) | 203 (86.02) |
|
| BRAF V600E mutation
(%) |
|
| 0.863 |
|
Yes | 448 (81.45) | 191 (80.93) |
|
| No | 102 (18.55) | 45 (19.07) |
|
| TSH (%) |
|
| 0.191 |
|
Low | 9 (1.64) | 3 (1.27) |
|
|
Normal | 536 (97.45) | 227 (96.19) |
|
|
High | 5 (0.91) | 6 (2.54) |
|
| TG (%) |
|
| 0.105 |
|
Low | 95 (17.27) | 29 (12.29) |
|
|
Normal | 425 (77.27) | 188 (79.66) |
|
|
High | 30 (5.45) | 19 (8.05) |
|
| TGAb (%) |
|
| 0.125 |
|
Negative | 351 (63.82) | 164 (69.49) |
|
|
Positive | 199 (36.18) | 72 (30.51) |
|
| TPOAb (%) |
|
| 0.658 |
|
Negative | 428 (77.82) | 187 (79.24) |
|
|
Positive | 122 (22.18) | 49 (20.76) |
|
|
| B, US
characteristics |
|
|
Characteristic | Training group
(n=550) | Validation group
(n=236) | P-value |
|
| Tumor location
(%) |
|
| 0.682 |
|
Isthmus | 22 (4.00) | 8 (3.39) |
|
|
Non-isthmus | 528 (96.00) | 228 (96.61) |
|
| Tumor number
(%) |
|
| 0.472 |
|
Unifocal | 412 (74.91) | 171 (72.46) |
|
|
Multifocal | 138 (25.09) | 65 (27.54) |
|
| Tumor
size (%) |
|
| 0.589 |
| ≤1.0
mm | 395 (71.82) | 165 (69.92) |
|
| >1.0
mm | 155 (28.18) | 71 (30.08) |
|
| Tumor shape
(%) |
|
| 0.768 |
|
Taller-than-wide | 278 (50.55) | 122 (51.69) |
|
|
Wider-than-tall | 272 (49.45) | 114 (48.31) |
|
| Margin (%) |
|
| 0.500 |
|
Irregular | 511 (92.91) | 216 (91.53) |
|
|
Regular | 39 (7.09) | 20 (8.47) |
|
| Composition
(%) |
|
| 0.943 |
|
Solid | 525 (95.45) | 225 (95.34) |
|
|
Non-solid | 25 (4.55) | 11 (4.66) |
|
| Echogenicity
(%) |
|
| 0.140 |
|
Markedly hypoechoic | 19 (3.45) | 8 (3.39) |
|
|
Hypoechoic | 527 (95.82) | 222 (94.07) |
|
|
Isoechoic | 4 (0.73) | 6 (2.54) |
|
| Capsule contact
(%) |
|
| 0.557 |
|
Yes | 405 (73.64) | 169 (71.61) |
|
| No | 145 (26.36) | 67 (28.39) |
|
| Microcalcification
(%) |
|
| 0.238 |
|
Yes | 407 (74.00) | 184 (77.97) |
|
| No | 143 (26.00) | 52 (22.03) |
|
| Blood flow signal
(%) |
|
| 0.159 |
|
Rich | 89 (16.18) | 48 (20.34) |
|
|
poor | 461 (83.82) | 188 (79.66) |
|
|
| C, CT
characteristics |
|
|
Characteristic | Training group
(n=550) | Validation group
(n=236) | P-value |
|
| UCT, Hu | 61.11±17.51 | 62.92±16.84 | 0.174 |
| VCT, Hu | 130.69±35.10 | 131.64±34.84 | 0.726 |
| ΔCT, Hu | 69.58±28.95 | 67.72±29.71 | 0.419 |
| Homogeneity of
enhancement (%) |
|
| 0.219 |
|
Inhomogeneity | 488 (88.73) | 202 (85.59) |
|
|
Homogeneity | 62 (11.27) | 34 (14.41) |
|
| Calcification
(%) |
|
| 0.091 |
|
Yes | 91 (16.55) | 51 (21.61) |
|
| No | 459 (83.45) | 185 (78.39) |
|
| Capsule invasion
(%) |
|
| 0.382 |
|
Yes | 254 (46.18) | 117(49.58) |
|
| No | 296 (53.82) | 119 (50.42) |
|
| Tracheal deviation
(%) |
|
| 0.852 |
|
Yes | 15 (2.73) | 7 (2.97) |
|
| No | 535 (97.27) | 229 (97.03) |
|
Univariate analysis of CLNM
In the univariate analysis, CLNM was significantly
associated with a younger age (≤45 years; P<0.001), the male sex
(P<0.001), no HT (P=0.001), negative TGAb (P=0.004) and negative
TPOAb (P=0.002). However, there were no significant differences for
BMI, presence of BRAF V600E mutation, level of TSH or level of
TG.
Among the US features, tumor location (isthmus;
P<0.001), tumor size (>1.0 cm; P<0.001), presence of
microcalcification (P<0.001) and capsule contact (yes;
P<0.001) were significantly different between CLNM and non-CLNM
groups. However, there was no significant difference for tumor
number, tumor shape, tumor margin, internal composition,
echogenicity or blood flow signal.
In terms of contrast-enhanced CT characteristics,
there were significant differences for an inhomogeneous enhancement
(P=0.002), presence of calcification (P=0.007) and capsule invasion
(P<0.001), but there were no significant differences for UCT,
VCT, ΔCT or tracheal deviation between the two groups (Table II).
 | Table II.Univariate analysis of
characteristics in the training group. |
Table II.
Univariate analysis of
characteristics in the training group.
| A, Clinical
characteristics |
|---|
|
|---|
| Characteristic | CLNM(+) group
(n=215) | CLNM(−) group
(n=335) | P-value |
|---|
| Age (%) |
|
|
<0.001a |
| ≤45
years | 174 (80.93) | 227 (67.76) |
|
| >45
years | 41 (19.07) | 108 (32.24) |
|
| Sex (%) |
|
|
<0.001a |
|
Male | 77 (35.81) | 61 (18.21) |
|
|
Female | 138 (64.19) | 274 (81.79) |
|
| BMI (%) |
|
| 0.168 |
| ≤20.92
kg/m2 | 43 (20.00) | 84 (25.07) |
|
|
>20.92
kg/m2 | 172 (80.00) | 251(74.93) |
|
| With HT (%) |
|
| 0.001a |
|
Yes | 25 (11.63) | 75 (22.39) |
|
| No | 190 (88.37) | 260 (77.61) |
|
| BRAF V600E mutation
(%) |
|
| 0.187 |
|
Yes | 181 (84.19) | 267 (79.70) |
|
| No | 34 (15.81) | 68 (20.30) |
|
| TSH (%) |
|
| 0.757 |
|
Low | 3 (1.40) | 6 (1.79) |
|
|
Normal | 211 (98.14) | 325 (97.01) |
|
|
High | 1 (0.47) | 4 (1.19) |
|
| TG (%) |
|
| 0.107 |
|
Low | 28 (13.02) | 67 (20.00) |
|
|
Normal | 175 (81.40) | 250 (74.63) |
|
|
High | 12 (5.58) | 18 (5.37) |
|
| TGAb (%) |
|
| 0.004a |
|
Negative | 153 (71.16) | 198 (59.10) |
|
|
Positive | 62 (28.84) | 137 (40.90) |
|
| TPOAb (%) |
|
| 0.002a |
|
Negative | 182 (84.65) | 246 (73.43) |
|
|
Positive | 33 (15.35) | 89 (26.57) |
|
|
| B, US
characteristics |
|
|
Characteristic | CLNM(+) group
(n=215) | CLNM(−) group
(n=335) | P-value |
|
| Tumor location
(%) |
|
|
<0.001a |
|
Isthmus | 16 (7.44) | 6 (1.79) |
|
|
Non-isthmus | 199 (92.56) | 329 (98.21) |
|
| Tumor number
(%) |
|
| 0.538 |
|
Unifocal | 158 (73.49) | 254 (75.82) |
|
|
Multifocal | 57 (26.51) | 81 (24.18) |
|
| Tumor size (%) |
|
|
<0.001a |
| ≤1.0
mm | 128 (59.53) | 267 (79.70) |
|
| >1.0
mm | 87 (40.47) | 68 (20.30) |
|
| Tumor shape
(%) |
|
| 0.414 |
|
Taller-than-wide | 104 (48.37) | 174 (51.94) |
|
|
Wider-than-tall | 111 (51.63) | 161 (48.06) |
|
| Margin (%) |
|
| 0.148 |
|
Irregular | 204 (94.88) | 307 (91.64) |
|
|
Regular | 11 (5.12) | 28 (8.36) |
|
| Composition
(%) |
|
| 0.457 |
|
Solid | 207 (96.28) | 318 (94.93) |
|
|
Non-solid | 8 (3.72) | 17 (5.07) |
|
| Echogenicity
(%) |
|
| 0.263 |
|
Markedly hypoechoic | 4 (1.86) | 15 (4.48) |
|
|
Hypoechoic | 209 (97.21) | 318 (94.93) |
|
|
Isoechoic | 2 (0.93) | 2 (0.60) |
|
| Capsule contact
(%) |
|
|
<0.001a |
|
Yes | 179 (83.26) | 226 (67.46) |
|
| No | 36 (16.74) | 109 (32.54) |
|
| Microcalcification
(%) |
|
|
<0.001a |
|
Yes | 178 (82.79) | 229 (68.36) |
|
| No | 37 (17.21) | 106 (31.64) |
|
| Blood flow signal
(%) |
|
| 0.508 |
|
Rich | 32 (14.88) | 57 (17.01) |
|
|
Poor | 183 (85.12) | 278 (82.99) |
|
|
| C, CT
characteristics |
|
|
Characteristics | CLNM(+) group
(n=215) | CLNM(−) group
(n=335) | P-value |
|
| UCT (%) |
|
| 0.155 |
| ≤65.35
Hu | 135 (62.79) | 230 (68.66) |
|
|
>65.35 Hu | 80 (37.21) | 105 (31.34) |
|
| VCT (%) |
|
| 0.208 |
| ≤183.90
Hu | 200 (93.02) | 320 (95.52) |
|
|
>183.90 Hu | 15 (6.98) | 15 (4.48) |
|
| ΔCT (%) |
|
| 0.173 |
| ≤111.50
Hu | 194 (90.23) | 313 (93.43) |
|
|
>111.50 Hu | 21 (9.77) | 22 (6.57) |
|
| Homogeneity of
enhancement (%) |
|
| 0.002a |
|
Inhomogeneity | 202 (93.95) | 286 (85.37) |
|
|
Homogeneity | 13 (6.05) | 49 (14.63) |
|
| Calcification
(%) |
|
| 0.007a |
|
Yes | 47 (21.86) | 44 (13.13) |
|
| No | 168 (78.14) | 291 (86.87) |
|
| Capsule invasion
(%) |
|
|
<0.001a |
|
Yes | 155 (72.09) | 99 (29.55) |
|
| No | 60 (27.91) | 236 (70.45) |
|
| Tracheal deviation
(%) |
|
| 0.643 |
|
Yes | 5 (2.33) | 10 (2.99) |
|
| No | 210 (97.67) | 325 (97.01) |
|
Multivariate logistic regression
analysis of CLNM
The characteristics with statistical significance
identified in the univariate analysis were further analyzed using
multivariate logistic regression analysis. The results demonstrated
that the following predictors were significantly independently
associated with promoting CLNM in patients with PTC: Age of ≤45
years old [odds ratio (OR)=0.964; 95% confidence interval (CI),
0.945–0.982; P<0.001], male sex (OR=2.147; 95% CI, 1.332–3.459;
P=0.002), no HT (OR=2.515; 95% CI, 1.208–5.239; P=0.014), isthmic
tumor (OR=0.211; 95% CI, 0.067–0.669; P=0.008), presence of
microcalcification (OR=0.589; 95% CI, 0.355–0.979; P=0.041),
inhomogeneous enhancement (OR=2.711; 95% CI, 0.355–0.979; P=0.041).
95%CI 1.268–5.798, P=0.010) and capsule invasion (OR=6.463; 95% CI,
4.103–10.181; P<0.001; Table
III).
 | Table III.Multivariate analysis of
characteristics in the training group. |
Table III.
Multivariate analysis of
characteristics in the training group.
| Variable | B coefficient | OR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
| ≤45
years | −0.037 | 0.964 | 0.945–0.982 |
<0.001a |
| >45
years |
|
|
|
|
| Sex |
|
|
|
|
|
Male | 0.764 | 2.147 | 1.332–3.459 | 0.002a |
|
Female |
|
|
|
|
| With HT |
|
|
|
|
|
Yes | 0.922 | 2.515 | 1.208–5.239 | 0.014a |
| No |
|
|
|
|
| TGAb |
|
|
|
|
|
Negative | 0.196 | 1.216 | 0.681–2.173 | 0.509 |
|
Positive |
|
|
|
|
| TPOAb |
|
|
|
|
|
Negative | −0.629 | 0.533 | 0.277–1.025 | 0.059 |
|
Positive |
|
|
|
|
| US-tumor
location |
|
|
|
|
|
Isthmus | −1.554 | 0.211 | 0.067–0.669 | 0.008a |
|
Non-isthmus |
|
|
|
|
| US-tumor size |
|
|
|
|
| ≤1.0
mm | −0.264 | 0.768 | 0.466–1.265 | 0.300 |
| >1.0
mm |
|
|
|
|
| US-capsule
contact |
|
|
|
|
|
Yes | −0.418 | 0.659 | 0.399–1.088 | 0.103 |
| No |
|
|
|
|
|
US-microcalcification |
|
|
|
|
|
Yes | −0.529 | 0.589 | 0.355–0.979 | 0.041a |
| No |
|
|
|
|
| CT-homogeneity of
enhancement |
|
|
|
|
|
Inhomogeneity | 0.997 | 2.711 | 1.268–5.798 | 0.010a |
|
Homogeneity |
|
|
|
|
| CT-capsule
invasion |
|
|
|
|
|
Yes | 1.866 | 6.463 | 4.103–10.181 |
<0.001a |
| No |
|
|
|
|
|
CT-calcification |
|
|
|
|
|
Yes | −0.450 | 0.638 | 0.368–1.106 | 0.110 |
| No |
|
|
|
|
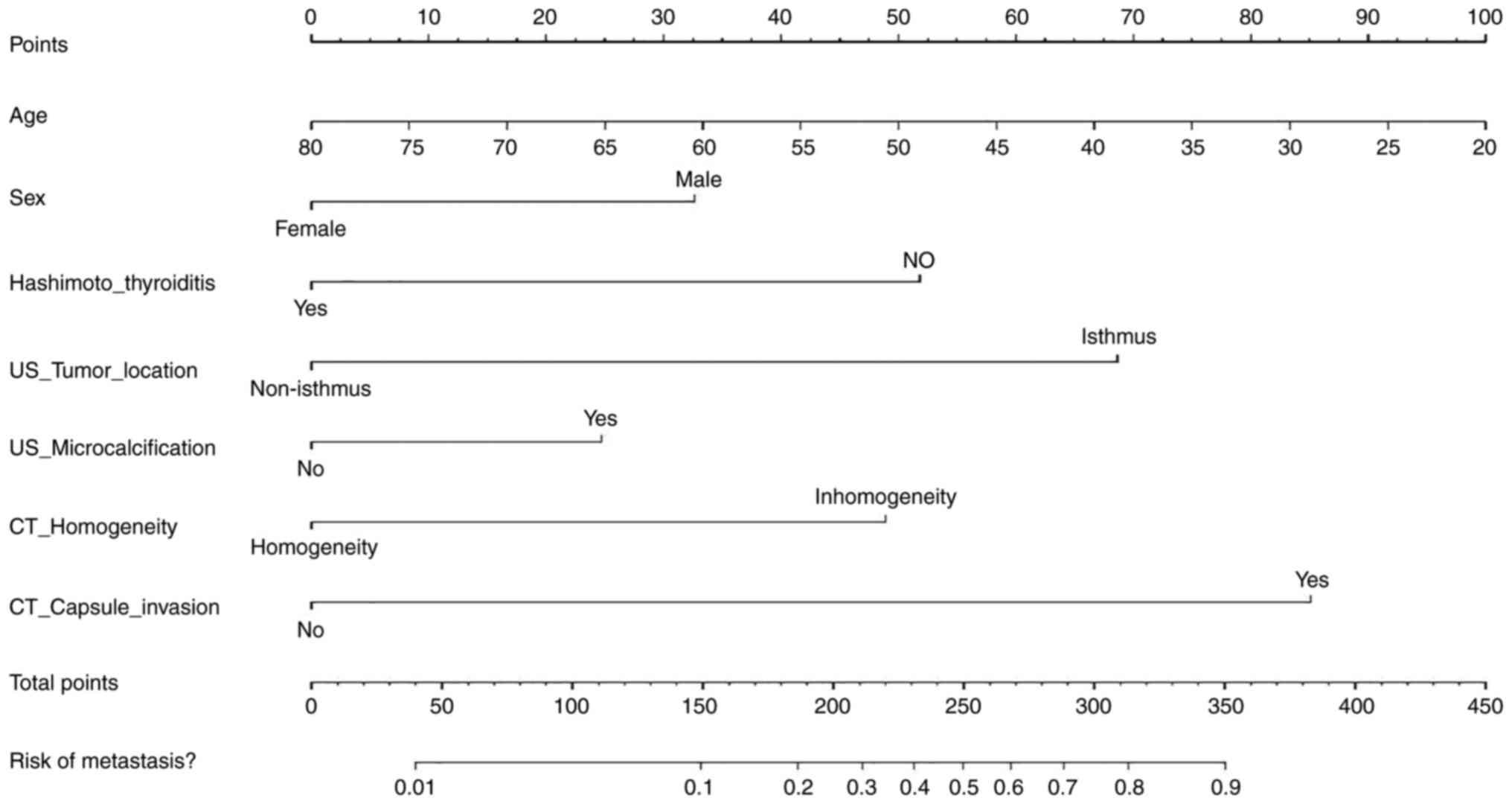
Development and validation of the
individualized prediction nomogram
According to the results of multivariate logistic
regression analysis, 7 variables including age, sex, presence of
HT, tumor location, microcalcification, homogeneity of enhancement
and capsule invasion were used in the development of a personalized
prediction nomogram for predicting CLNM in patients with PTC
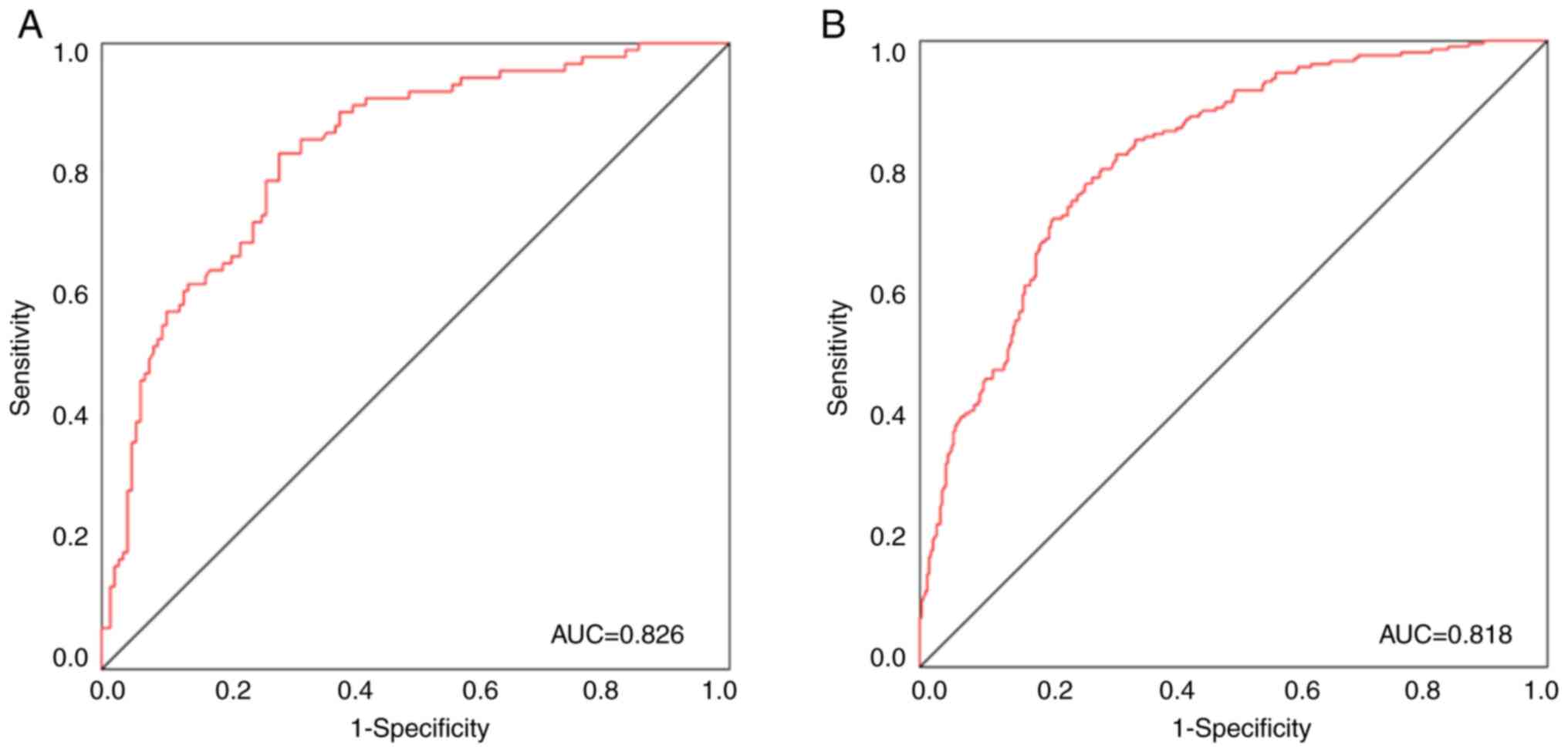
(Fig. 4). According to the ROC
curve, the area under the curve (AUC) was 0.826, the sensitivity
was 0.824 and the specificity was 0.717 for the training group,
whilst the AUC was 0.818, the sensitivity was 0.725 and the
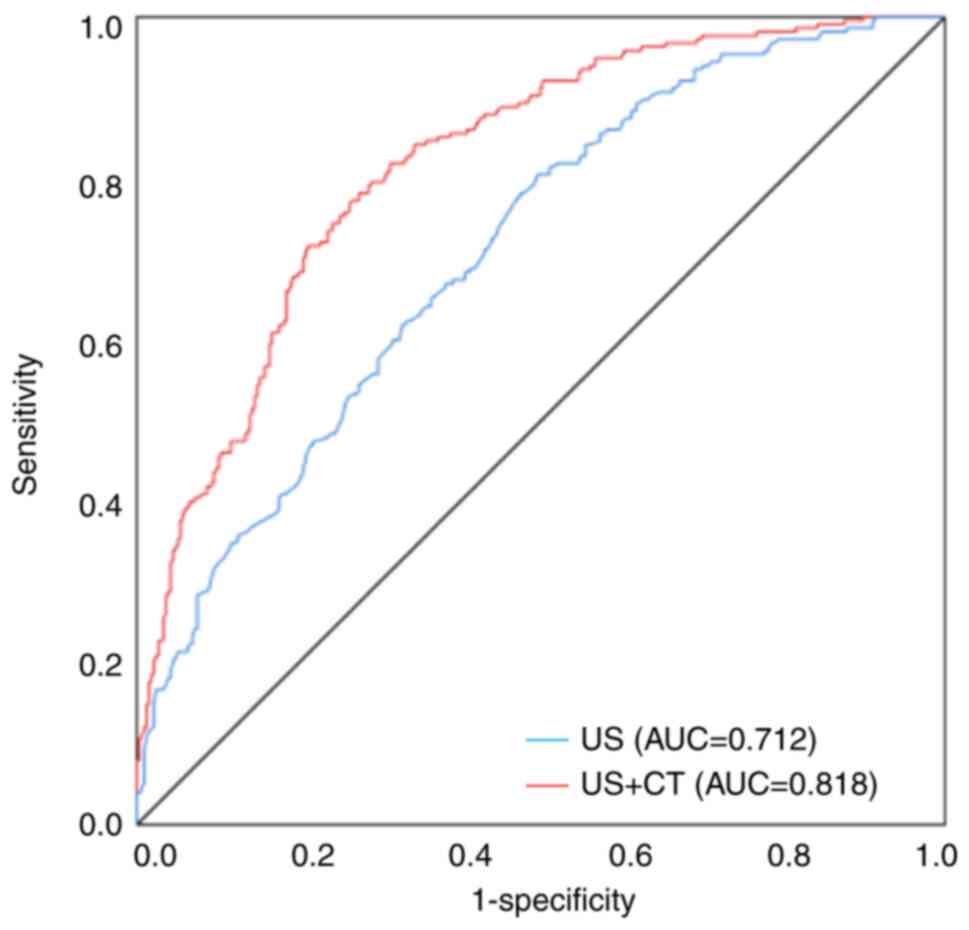
specificity was 0.781 for the validation group (Fig. 5A and B). In addition, the AUC for
predicting CLNM without combined contrast-enhanced CT was 0.712,
and the AUC of predicting CLNM increased to 0.818 when clinical and
conventional US and contrast-enhanced CT features were combined
(Fig. 6). This further demonstrates
the advantage of the US combined CT model.
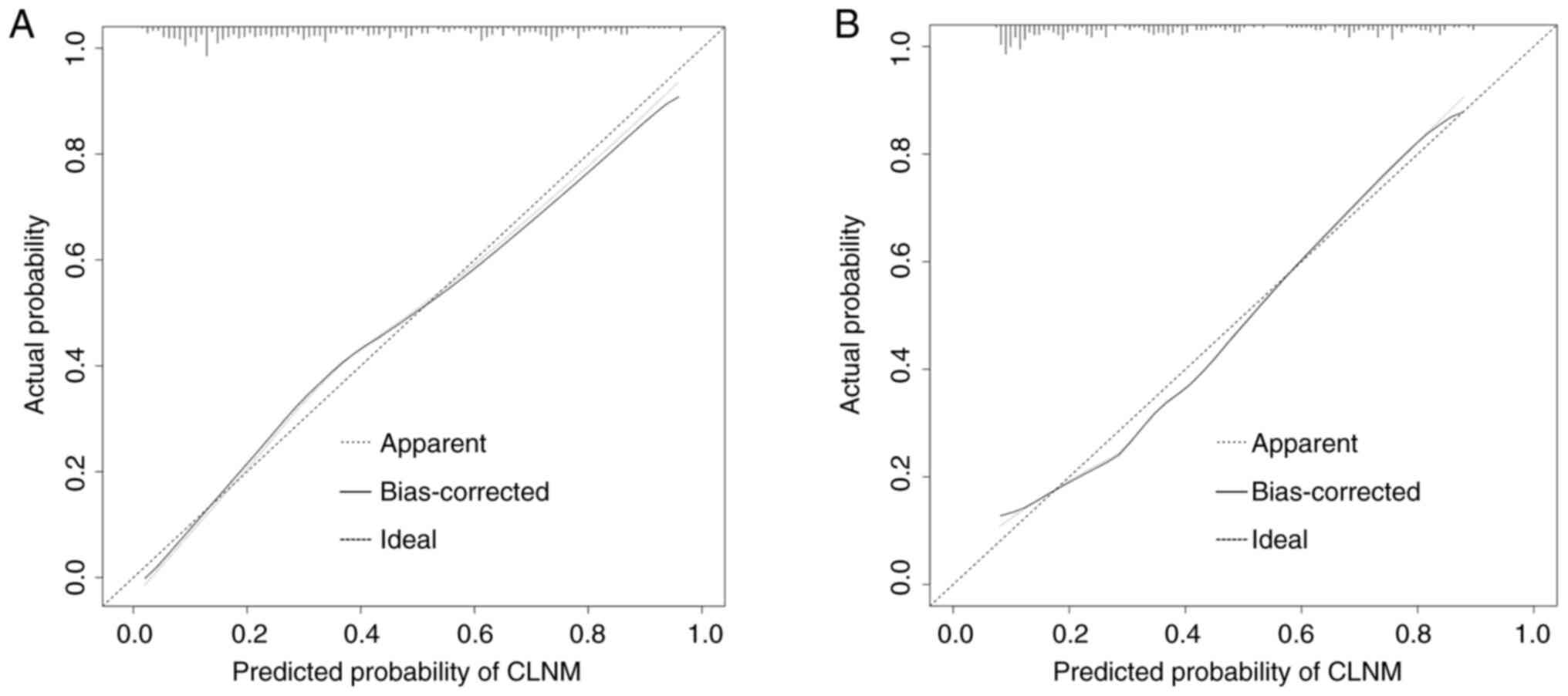
Furthermore, calibration curves depicting the CLNM
risk nomogram in patients with PTC were generated to assess the
effectiveness of the nomogram. The curves indicated a satisfactory
agreement in both the training and validation groups, with mean
absolute errors of 0.021 (Fig. 7A)
and 0.023 (Fig. 7B),
respectively.
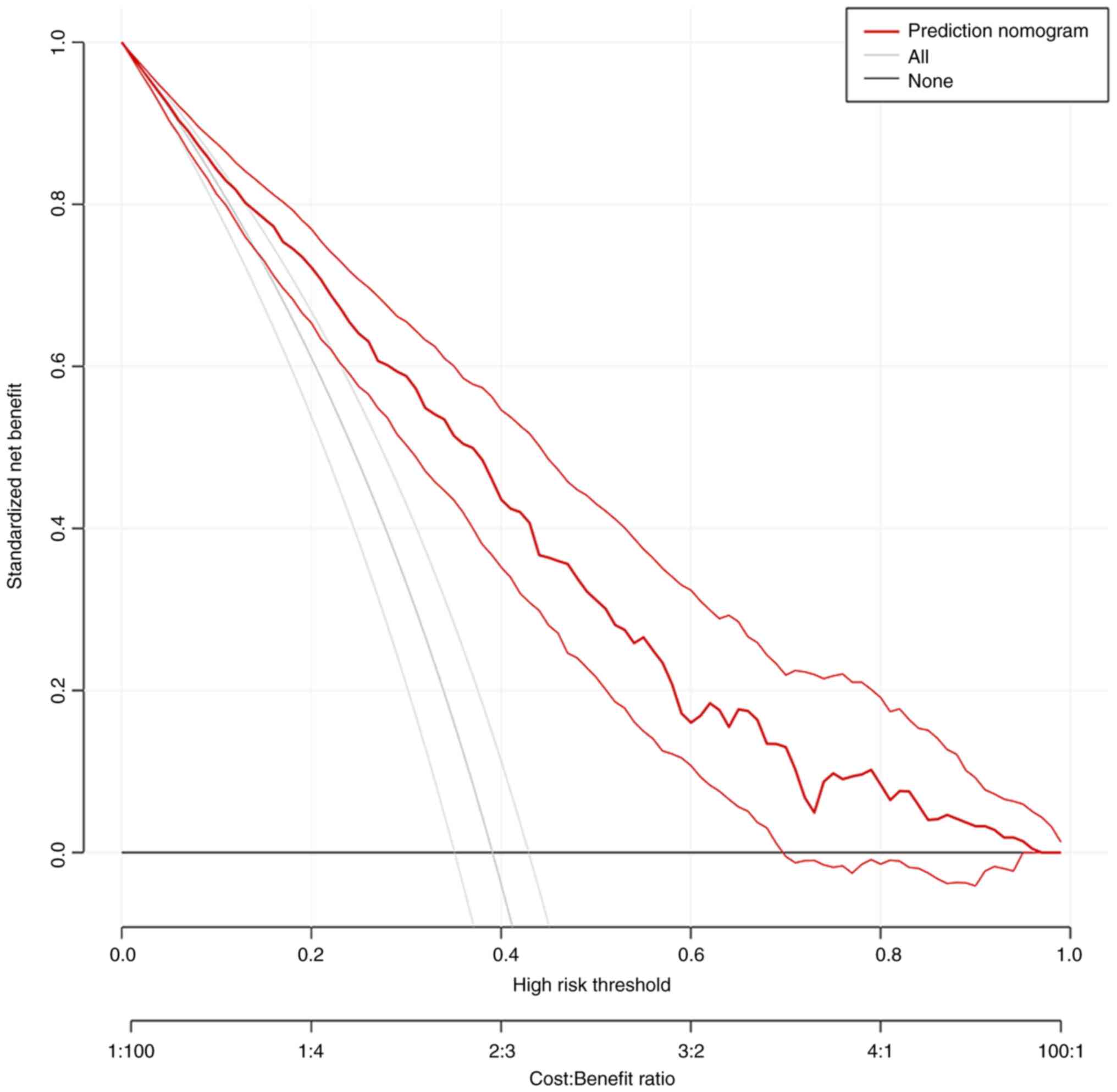
Clinical application
Finally, DCA was performed to evaluate the
performance of the model in detecting CLNM for patients with PTC
(Fig. 8). The DCA curve
demonstrated that it would be beneficial to predict CLNM with the
nomogram when the threshold probability ranges from 0.1 to 1.0.
Discussion
Most PTCs show a slow and indolent growth pattern,
and the overall prognosis is favorable, with a current 5-year
survival rate of >90% (25).
CLNM is occurs in 12–64% of patients with PTC, and exhibits a
strong association with increased recurrence and poor overall
survival (26). Therefore, precise
preoperative prediction of CLNM can be advantageous for patients
with PTC (cN0), and creating an effective prediction model would
serve as a viable solution. Previous studies have reported that US
features of PTC can help predict CLNM in patients, but few of them
mentioned the role of CT in this (27–29).
In the present study, the US and CT features of patients with PTC
were reviewed and the value of US combined with contrast-enhanced
CT for predicting CLNM was evaluated.
Many studies have reported that sex and age are
independent risk factors for CLNM in PTC, among which the male sex
and a younger age (≤45 years) have a greater risk of CLNM (30–32).
This is consistent with the findings obtained in the present study.
However, the multifocality and tumor size characteristics did not
differ significantly between the two groups in the present study,
which is not consistent with previous studies (22,31,33).
The potential reasons contributing to this variation may be the
different sample sizes and evaluation criteria used for
characteristics.
HT is the most common autoimmune thyroid disease and
10–58% of patients with PTC have it (34). In most studies, HT has been regarded
as a protective factor for CLNM in PTC (35,36),
and Jara et al (37) also
noted that HT correlated with less aggressive disease and a reduced
incidence of lymph node metastasis. Moreover, the present study
also demonstrated that patients with PTC but without coexistent HT
were more prone to CLNM. However, Mao et al (38) and Liu et al (39) suggested that HT had no significant
effect on the incidence of lymph node metastasis. Therefore, the
effect of HT on CLNM in PTC is uncertain, and more clinical trials
emphasizing the influence of HT on the progression of PTC are worth
performing.
To the best of our knowledge, the relationship
between PTC tumor location and lymph node metastasis is
controversial. Certain studies reported that there is no
significant association between tumor location and CLNM (22,40).
However, Li et al (41) and
Lyu et al (42) suggested
that isthmic tumors are more prone to CLNM compared with lateral
lobe tumors. The present study also demonstrated that tumor
location in the isthmus was significantly associated with CLNM. The
thyroid isthmus is typically situated anteriorly to the
cartilaginous ring of the second to fourth trachea, where the gland
thins to a thickness of only ~2 mm. Due to the specific location of
the tumor, the isthmus tumor is adjacent to the trachea and thyroid
capsule, so the incidence of extrathyroidal extension, CLNM and
multifocality is higher than that of thyroid lobe tumor (43). In addition, the lymphatic drainage
pattern of the thyroid isthmus differs from that of the thyroid
lobe (41). The presence of the
aforementioned features will increase the risk of CLNM in in
isthmic tumors (42). Notably,
although patients with isthmic tumors were demonstrated to have a
higher risk of CLNM, the incidence of this feature was low,
accounting for only 4.0% (22/550) of the total cases in the present
study.
US is known to provide a better soft tissue
resolution than CT, and microcalcifications seen by US are not
necessarily shown on CT (44,45).
Therefore, in the present study, microcalcifications were evaluated
by US, whilst calcifications evaluated by CT were generally
macrocalcifications. The present study confirmed that the presence
of microcalcification was an independent predictor of CLNM in cN0
PTC and that macrocalcification was not significantly associated
with CLNM. Previous studies have also reported an association
between microcalcification and CLNM in PTC (46–48).
Microcalcifications are characterized as punctate bright echoes
with or without accompanying acoustic shadowing, mainly small
psammoma bodies of 10–100 µm, arranged in concentric layers
(49). Therefore, as
microcalcification may be a predictive marker for CLNM (50), when microcalcification is found in
thyroid nodules by preoperative examination, a more meticulous
evaluation of the central cervical lymph nodes is warranted.
Angiogenesis is known to be associated with
aggressive tumor growth and metastasis (51). Contrast-enhanced CT provides
improved visualization of the tumor microvascular distribution
(33). There were a large number of
neovascularization in the thyroid tumor tissue, which appeared to
be enhanced after enhancement. However, at the same time, this
malignant growth will destroy a lot of tissue structures and blood
vessels, so the degree of enhancement is lower than that of normal
thyroid (52). Furthermore,
heterogeneous vascular distribution can lead to inhomogeneous
enhancement shown on contrast-enhanced CT images. The present study
demonstrated that although the incidence of inhomogeneous
enhancement was relatively high, it was also significantly
associated with predicting CLNM.
Capsule invasion is generally regarded as being
associated with CLNM. However, whether US or contrast-enhanced CT
is superior in predicting capsule invasion is still controversial
(19). Yang et al (23) reported that observation of the
anterior thyroid capsule by US is influenced by US near-field
artifacts, whilst observation of the lateral and posterior thyroid
capsules is hindered by the presence of blood vessels and the
trachea, which may not be distinctly depicted (8). In addition, considering the strong
dependence of US on the operator (15), the present study included capsule
contact on US images and capsule invasion on CT images, which were
associated but not consistent. The present study demonstrated that
capsule invasion assessed by CT is an independent risk factor for
CLNM, and its mechanism may be linked to the abundant thyroid
lymphatic network. If the tumor breaks through the capsule, it has
the potential to readily induce lymph node metastasis in the
central region (8,53).
Few studies have investigated the relationship
between contrast-enhanced CT features and CLNM. For example, Peng
et al (54) and Mou et
al (55) collected data from
preoperative CT images to predict CLNM in patients with cN0 PTC,
but the studies only had small sample sizes. Moreover, Zhao et
al (33) used a simple
risk-scoring system to predict CLNM. To the best of our knowledge,
the present study was the first with an adequate sample size to
construct a nomogram combining US with contrast-enhanced CT for
predicting CLNM.
Nonetheless, there were several limitations of the
present study that should be acknowledged. First, the study design
was retrospective, making it susceptible to inherent bias in
patient recruitment and data collection. Second, the retrospective
nature of the present study may limit the analysis of additional
potential variables. Analyses were performed only on the basis of
the characteristics of the primary tumor. Furthermore, the present
study lacks external validation. Therefore, it is imperative to
prioritize additional external validation cohorts from prospective
studies to comprehensively assess the viability of the nomogram in
the present study. In addition, for multifocal tumors, analysis was
performed only on the largest tumor, and the features of the
remaining tumors were unknown. Notably, the present study did not
assess patients with PTCs that were <0.5 cm.
Despite the limitations, the present study presents
certain highlights. Based on the aforementioned clinical, US and
contrast-enhanced CT characteristics, the present study developed
and validated a novel nomogram, which has an improved diagnostic
performance in predicting CLNM than no combination of
contrast-enhanced CT. Moreover, the nomogram serves as a
user-friendly diagnostic tool for predicting CLNM. By adding the
specific scores of each predictor, the corresponding CLNM
probability for thyroid nodules can be obtained. Overall, this
prediction model could make it possible to personalize the CLNM
prediction of most patients with PTC and help surgeons make
decisions on surgical options to maximize the benefits of
patients.
In conclusion, the findings of the present study
suggest that a young age, the male sex, no presence of HT, isthmic
tumor, microcalcification, inhomogeneous enhancement and capsule
invasion are significantly associated with CLNM in patients with
cN0 PTC. Furthermore, the constructed nomogram has the potential to
be used for preoperative risk assessment of CLNM, which can help
surgeons better develop appropriate surgical plans, providing a
novel approach to managing patients with cN0 PTC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QZ, SX, JY and WZ conceived and designed the study.
QZ, SX, QS and YM contributed to data collection and data analyses.
QZ and SX, YH and YM performed the data interpretation. QZ, SX, YH
and YM contributed to the statistical analysis. QZ, SX, YH and YM
drafted the manuscript. QS, JY and WZ revised the manuscript
critically for important intellectual content. QS, JY and WZ
confirm the authenticity of all the raw data. QZ, SX, QS, YM, YH,
JY and WZ discussed the results and contributed to the revision of
the final manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Ruijin Hospital, Shanghai Jiao Tong University School
of Medicine (Shanghai, China; approval no. 2023-129). The
requirement for informed consent to participate was waived by the
Ethics Committee as the present study is retrospective. All methods
were performed in accordance with the Helsinki Declaration and
local legislation and institutional requirements.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PTC
|
papillary thyroid cancer
|
|
CLNM
|
central lymph node metastasis
|
|
CLND
|
central lymph node dissection
|
|
US
|
ultrasound
|
|
CT
|
computed tomography
|
|
ROC
|
receiver operating characteristic
|
|
DCA
|
decision curve analysis
|
|
AUC
|
area under the curve
|
|
TSH
|
thyroid stimulating hormone
|
|
TG
|
thyroglobulin
|
|
TGAb
|
TG antibody
|
|
TPOAb
|
thyroid peroxidase antibody
|
|
OR
|
odd ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen DW, Lang BHH, McLeod DSA, Newbold K
and Haymart MR: Thyroid cancer. Lancet. 401:1531–1544. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papaleontiou M, Evron JM, Esfandiari NH,
Reyes-Gastelum D, Ward KC, Hamilton AS, Worden F and Haymart MR:
Patient report of recurrent and persistent thyroid cancer. Thyroid.
30:1297–1305. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai Q, Liu D, Tao Y, Ding C, Li S, Zhao C,
Wang Z, Tao Y, Tian J and Leng X: Nomograms based on preoperative
multimodal ultrasound of papillary thyroid carcinoma for predicting
central lymph node metastasis. Eur Radiol. 32:4596–4608. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al Afif A, Williams BA, Rigby MH, Bullock
MJ, Taylor SM, Trites J and Hart RD: Multifocal papillary thyroid
cancer increases the risk of central lymph node metastasis.
Thyroid. 25:1008–1012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heng Y, Yang Z, Lin J, Liu Q, Cai W and
Tao L: Risks of central lymph node metastasis in papillary thyroid
carcinoma with or without multifocality in at least one lobe: A
multi-center analysis. Oral Oncol. 134:1061852022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guang Y, He W, Zhang W, Zhang H, Zhang Y
and Wan F: Clinical study of ultrasonographic risk factors for
central lymph node metastasis of papillary thyroid carcinoma. Front
Endocrinol (Lausanne). 12:7919702021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mazzaferri EL, Doherty GM and Steward DL:
The pros and cons of prophylactic central compartment lymph node
dissection for papillary thyroid carcinoma. Thyroid. 19:683–689.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaha AR: Central lymph node metastasis in
papillary thyroid carcinoma. World J Surg. 42:630–631. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xue J, Teng D and Wang H: Efficacy and
safety of ultrasound-guided radiofrequency ablation for papillary
thyroid microcarcinoma: A systematic review and meta-analysis. Int
J Hyperthermia. 39:1300–1309. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo
B, Li J, Qian L, Cui L, Chen W, et al: 2020 Chinese guidelines for
ultrasound malignancy risk stratification of thyroid nodules: The
C-TIRADS. Endocrine. 70:256–279. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alexander EK and Cibas ES: Diagnosis of
thyroid nodules. Lancet Diabetes Endocrinol. 10:533–539. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roh JL, Park JY, Kim JM and Song CJ: Use
of preoperative ultrasonography as guidance for neck dissection in
patients with papillary thyroid carcinoma. J Surg Oncol. 99:28–31.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang HS and Orloff LA: Efficacy of
preoperative neck ultrasound in the detection of cervical lymph
node metastasis from thyroid cancer. Laryngoscope. 121:487–491.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim E, Park JS, Son KR, Kim JH, Jeon SJ
and Na DG: Preoperative diagnosis of cervical metastatic lymph
nodes in papillary thyroid carcinoma: Comparison of ultrasound,
computed tomography, and combined ultrasound with computed
tomography. Thyroid. 18:411–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suh CH, Baek JH, Choi YJ and Lee JH:
Performance of CT in the preoperative diagnosis of cervical lymph
node metastasis in patients with papillary thyroid cancer: A
systematic review and meta-analysis. AJNR Am J Neuroradiol.
38:154–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Traylor KS: Computed tomography and MR
imaging of thyroid disease. Radiol Clin North Am. 58:1059–1070.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoo RE, Kim JH, Hwang I, Kang KM, Yun TJ,
Choi SH, Sohn CH and Park SW: Added value of computed tomography to
ultrasonography for assessing LN metastasis in preoperative
patients with thyroid cancer: Node-by-node correlation. Cancers
(Basel). 12:11902020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeon YH, Lee JY, Yoo RE, Rhim JH, Lee KH,
Choi KS, Hwang I, Kang KM and Kim JH: Validation of ultrasound and
computed tomography-based risk stratification system and biopsy
criteria for cervical lymph nodes in preoperative patients with
thyroid cancer. Korean J Radiol. 24:912–923. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng Y, Min Y, Chen H, Xiang K, Wang X and
Yin G: Construction and validation of a nomogram for predicting
cervical lymph node metastasis in classic papillary thyroid
carcinoma. J Endocrinol Invest. 44:2203–2211. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Sun P, Huang T, Li L, He S, Ai X,
Xiao H and Xue G: Preoperative prediction of central lymph node
metastasis in cN0T1/T2 papillary thyroid carcinoma: A nomogram
based on clinical and ultrasound characteristics. Eur J Surg Oncol.
48:1272–1279. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Z, Heng Y, Lin J, Lu C, Yu D, Tao L
and Cai W: Nomogram for predicting central lymph node metastasis in
papillary thyroid cancer: A retrospective cohort study of two
clinical centers. Cancer Res Treat. 52:1010–1018. 2020.PubMed/NCBI
|
|
24
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer staging handbook: from the AJCC
cancer staging manual. 7th edition. Springer-Verlag; New York, NY:
2010
|
|
25
|
Coca-Pelaz A, Shah JP, Hernandez-Prera JC,
Ghossein RA, Rodrigo JP, Hartl DM, Olsen KD, Shaha AR, Zafereo M,
Suarez C, et al: Papillary thyroid cancer-aggressive variants and
impact on management: A narrative review. Adv Ther. 37:3112–3128.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YM, Sung TY, Kim WB, Chung KW, Yoon JH
and Hong SJ: Risk factors for recurrence in patients with papillary
thyroid carcinoma undergoing modified radical neck dissection. Br J
Surg. 103:1020–1025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng JW, Hong LZ, Wang F, Wu WX, Hu J, Liu
SY, Jiang Y and Ye J: A nomogram based on clinical and ultrasound
characteristics to predict central lymph node metastasis of
papillary thyroid carcinoma. Front Endocrinol (Lausanne).
12:6663152021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao X, Luo W, He L, Cheng J and Yang L:
Predictors and a prediction model for central cervical lymph node
metastasis in papillary thyroid carcinoma (cN0). Front Endocrinol
(Lausanne). 12:7893102021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo QW, Gao S, Lv X, Li SJ, Wang BF, Han
QQ, Wang YP, Guan QL and Gong T: A novel tool for predicting the
risk of central lymph node metastasis in patients with papillary
thyroid microcarcinoma: A retrospective cohort study. BMC Cancer.
22:6062022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gui CY, Qiu SL, Peng ZH and Wang M:
Clinical and pathologic predictors of central lymph node metastasis
in papillary thyroid microcarcinoma: A retrospective cohort study.
J Endocrinol Invest. 41:403–409. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Zhang H, Zhou Y and Cheng R: Risk
factors for central lymph node metastasis in the cervical region in
papillary thyroid carcinoma: A retrospective study. World J Surg
Oncol. 19:1382021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Chang Q, Zhang H, Du G, Li S, Liu
Y, Sun H and Yin D: A clinical predictive model of central lymph
node metastases in papillary thyroid carcinoma. Front Endocrinol
(Lausanne). 13:8562782022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao S, Yue W, Wang H, Yao J, Peng C, Liu
X and Xu D: Combined conventional ultrasound and contrast-enhanced
computed tomography for cervical lymph node metastasis prediction
in papillary thyroid carcinoma. J Ultrasound Med. 42:385–398. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JH, Kim Y, Choi JW and Kim YS: The
association between papillary thyroid carcinoma and histologically
proven Hashimoto's thyroiditis: A meta-analysis. Eur J Endocrinol.
168:343–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Zheng J, Hu X, Chang Q, Qiao Y,
Yao X and Zhou X: A retrospective study of papillary thyroid
carcinoma: Hashimoto's thyroiditis as a protective biomarker for
lymph node metastasis. Eur J Surg Oncol. 49:560–567. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Issa PP, Omar M, Buti Y, Issa CP, Chabot
B, Carnabatu CJ, Munshi R, Hussein M, Aboueisha M, Shama M, et al:
Hashimoto's thyroiditis minimizes lymph node metastasis in BRAF
mutant papillary thyroid carcinomas. Biomedicines. 10:20512022.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jara SM, Carson KA, Pai SI, Agrawal N,
Richmon JD, Prescott JD, Dackiw A, Zeiger MA, Bishop JA and Tufano
RP: The relationship between chronic lymphocytic thyroiditis and
central neck lymph node metastasis in North American patients with
papillary thyroid carcinoma. Surgery. 154:1272–1280. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mao J, Zhang Q, Zhang H, Zheng K, Wang R
and Wang G: Risk factors for lymph node metastasis in papillary
thyroid carcinoma: A systematic review and meta-analysis. Front
Endocrinol (Lausanne). 11:2652020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Y, Lv H, Zhang S, Shi B and Sun Y: The
impact of coexistent Hashimoto's thyroiditis on central compartment
lymph node metastasis in papillary thyroid carcinoma. Front
Endocrinol (Lausanne). 12:7720712021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun J, Jiang Q and Wang X, Liu W and Wang
X: Nomogram for preoperative estimation of cervical lymph node
metastasis risk in papillary thyroid microcarcinoma. Front
Endocrinol (Lausanne). 12:6139742021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, Gao X, Guo T and Liu J: Development
and validation of nomograms for predicting the risk of central
lymph node metastasis of solitary papillary thyroid carcinoma of
the isthmus. J Cancer Res Clin Oncol. 149:14853–14868. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lyu YS, Pyo JS, Cho WJ, Kim SY and Kim JH:
Clinicopathological significance of papillary thyroid carcinoma
located in the isthmus: A meta-analysis. World J Surg.
45:2759–2768. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu F, Li F, Xie X, Wu Y and Wang W:
Investigating the impact of tumor location and size on the risk of
recurrence for papillary thyroid carcinoma in the isthmus. Cancer
Med. 12:13290–13299. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schmitz G and Dencks S: Ultrasound
imaging. Recent Results Cancer Res. 216:135–154. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bin Saeedan M, Aljohani IM, Khushaim AO,
Bukhari SQ and Elnaas ST: Thyroid computed tomography imaging:
Pictorial review of variable pathologies. Insights Imaging.
7:601–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tian X, Song Q, Xie F, Ren L, Zhang Y,
Tang J, Zhang Y, Jin Z, Zhu Y, Zhang M and Luo Y: Papillary thyroid
carcinoma: An ultrasound-based nomogram improves the prediction of
lymph node metastases in the central compartment. Eur Radiol.
30:5881–5893. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pyo JS, Kang G, Kim DH, Park C, Kim JH and
Sohn JH: The prognostic relevance of psammoma bodies and
ultrasonographic intratumoral calcifications in papillary thyroid
carcinoma. World J Surg. 37:2330–2335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ferreira LB, Lima RT, Bastos ACSDF, Silva
AM, Tavares C, Pestana A, Rios E, Eloy C, Sobrinho-Simões M, Gimba
ERP and Soares P: OPNa overexpression is associated with matrix
calcification in thyroid cancer cell lines. Int J Mol Sci.
19:29902018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li X, Zhou W and Zhan W: Clinical and
ultrasonographic features of medullary thyroid microcarcinomas
compared with papillary thyroid microcarcinomas: A retrospective
analysis. BMC Med Imaging. 20:492020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu J, Jia X, Gu Y, Chen X, Guan L, Yan J,
Zhai H, Zhou N, Dong Y, Zhan W, et al: Thyroid parenchyma
microcalcifications on ultrasound for predicting lymph node
metastasis in papillary thyroid carcinoma: A prospective
multicenter study in China. Front Oncol. 11:6090752021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang F, Qiao Y and Zhang H: Value of CT
features in the diagnosis of papillary thyroid tumors in incidental
thyroid nodules. Int J Endocrinol. 2020:93423172020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Park JP, Roh JL, Lee JH, Baek JH, Gong G,
Cho KJ, Choi SH, Nam SY and Kim SY: Risk factors for central neck
lymph node metastasis of clinically noninvasive, node-negative
papillary thyroid microcarcinoma. Am J Surg. 208:412–418. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Peng Y, Zhang ZT, Wang TT, Wang Y, Li CH,
Zuo MJ, Lin HS and Gong LG: Prediction of central lymph node
metastasis in cN0 papillary thyroid carcinoma by CT radiomics. Acad
Radiol. 30:1400–1407. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mou Y, Han X, Li J, Yu P, Wang C, Song Z,
Wang X, Zhang M, Zhang H, Mao N and Song X: Development and
validation of a computed tomography-based radiomics nomogram for
the preoperative prediction of central lymph node metastasis in
papillary thyroid microcarcinoma. Acad Radiol. 31:1805–1817. 2023.
View Article : Google Scholar : PubMed/NCBI
|