Introduction
Rhabdomyosarcoma (RMS) is a malignant tumour that
develops from mesenchymal cells and affects the head and neck,
followed by the urogenital system, extremities, and rarely, the
perianal area (1,2). Perianal RMS accounts for only 2% of
RMS cases, is associated with high risks of mortality and low cure
rates, and has a poor prognosis (3). RMS can be divided into four types
based on histological and genetic characteristics: Embryonal RMS
(ERMS), mainly composed of rhabdomyoblasts and small round cells;
alveolar RMS, mainly composed of large round cells and
rhabdomyoblasts; adult pleomorphic RMS, composed mainly of
pleomorphic rhabdomyoblasts; and spindle cell/sclerosing RMS,
composed mainly of spindle-shaped rhabdomyoblasts (4). ERMS is most common in children <10
years of age and rarely occurs in adults (5). Therefore, perianal ERMS in adults is
extremely rare. Perianal EMRS in adults sometimes presents as
perianal pain and an increased skin temperature, which can easily
be misdiagnosed as a perianal abscess. The present report describes
a case of perianal ERMS in an adult male that was misdiagnosed as a
perianal abscess but later confirmed pathologically. This case is
presented to improve our understanding of ERMS and reduce its
future misdiagnosis.
Case report
Case presentation
A 30-year-old man was referred to the Emergency
Department of Xiaoshan Affiliated Hospital of Wenzhou Medical
University (Hangzhou, China) in November 2014, complaining of
severe left perianal pain for 1 day, without chills, fever or other
discomfort. Perianal examination showed that the skin temperature
of the left perianal region was relatively high, and a mass was
palpable at the anal edge at 5 o'clock in the lithotomy position,
measuring ~4.0×3.0 cm in size, with pain, fluctuating sensation and
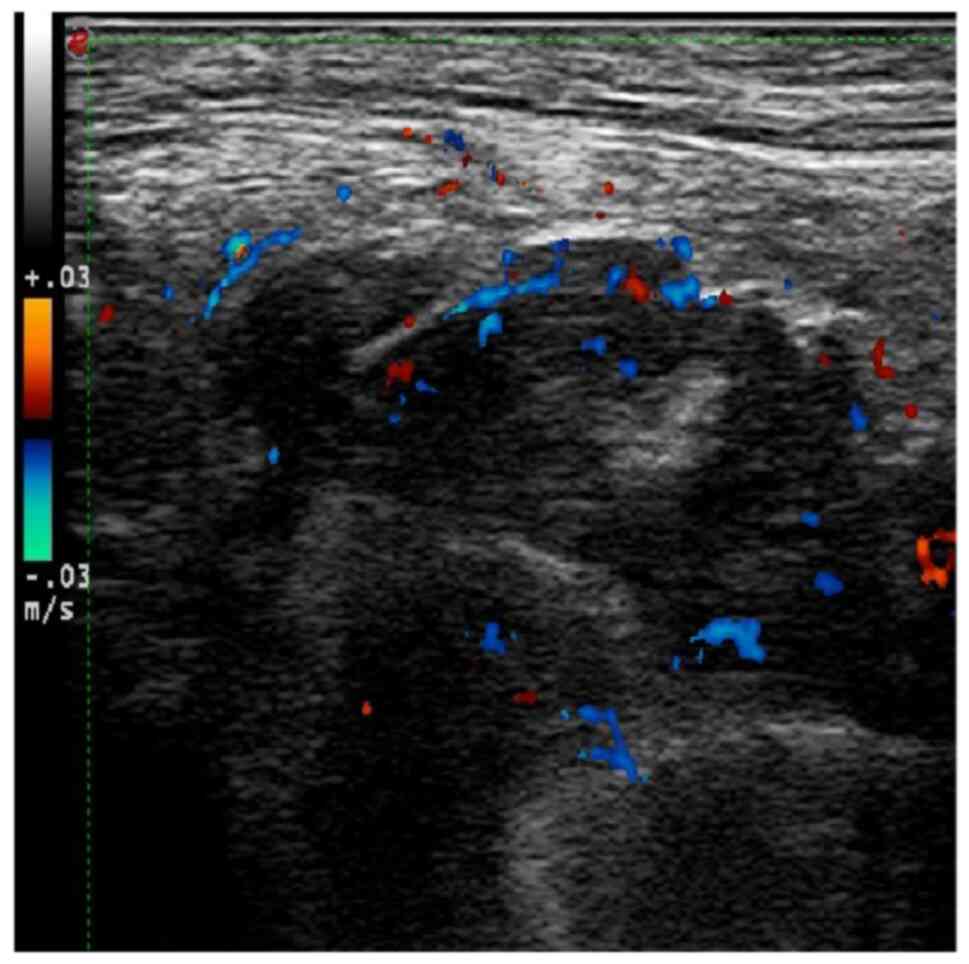
no ulceration or pus. Perianal B-ultrasound showed a 3.6×2.2×4.2-cm
hypoechoic dark area under the left perianal skin. The internal
fluid was thick, and obvious blood flow signals could be seen,
suggesting that it might be a left perianal subcutaneous
multilocular abscess (Fig. 1).
Based on the B-ultrasound results and the patient's symptoms, a
perianal abscess was diagnosed, and perianal abscess incision and
drainage were performed under general anaesthesia on the same day.
During the operation, the mass was incised, and no obvious purulent
liquid was found. The mass was tough in texture, and necrotic
tissue and dark red jelly-like objects were seen. Three pieces of
tissue were taken for pathological examination during the
operation. The anti-infective drug ceftriaxone (2 g per day) was
administered for five days after surgery; however, the patient's
perianal pain was not significantly relieved.
Postoperative pathological microscopic observations
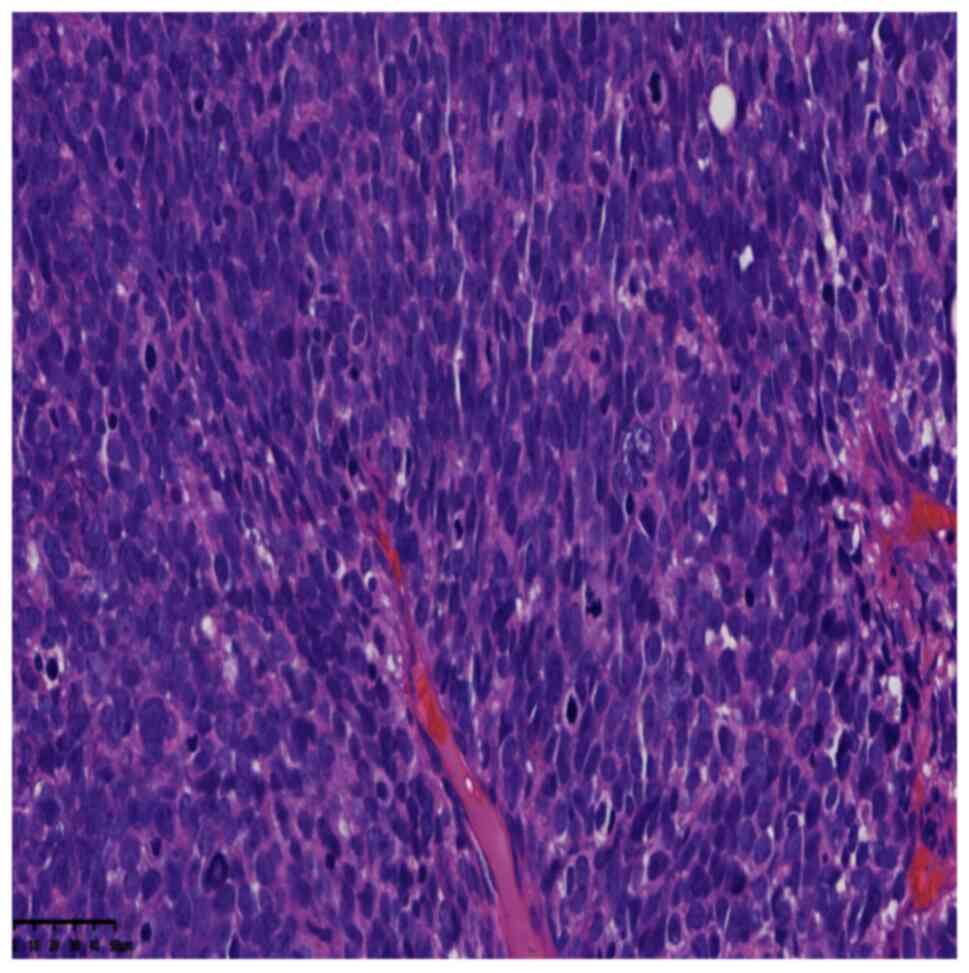
revealed that the tumour was comprised of round cells that grew
diffusely and infiltrated the adipose tissue (Fig. 2). The round cells were of medium
size, with round or oval nuclei, a high nuclear-to-cytoplasmic
ratio, frequent mitoses, obvious atypia, darkly stained chromatin,
small nucleoli and regional tumour necrosis (Fig. 3). Immunohistochemical (IHC) staining
and specific staining indicated the following results: Desmin(+)
(Fig. 4), myoblast determination
protein 1 (MyoD1)(+) (Fig. 5),
Ki-67(+; 75%), Melan-A(−), S-100(−), human melanoma black 45
(HMB45) (−), leukocyte common antigen(−), prostate-specific
antigen(−), chromogranin A(−), synaptophysin(−), CDX-2(−), creatine
kinase (CK)(−) and epithelial membrane antigen(−). The pathological
diagnosis was of a perianal ERMS. We recommended that the patient
undergoes postoperative radiotherapy and chemotherapy, but the
patient did not accept it and requested to be discharged from the
hospital 10 days after the surgery. The patient then visited a
higher-level hospital for radiotherapy. However, during the
follow-up period, the patient died of multiple metastases and
multiorgan failure at 6 months post-surgery.
Staining methods
Postoperative pathology
The tissue was fixed with 4% neutral formalin (24 h
at 25°C) and embedded in paraffin, and 4-µm serial sections were
prepared and subjected to staining with H&E (Beijing Jinqiao
Zhongshan Biological Co. Ltd.; OriGene Technologies, Inc.) for 8 h
at 25°C. Observation was performed using a Leica DM2000 light
microscope (Leica Microsystems GmbH).
IHC staining
The undyed tissue sections (4 µm) were placed in an
oven at 60°C for 120 min and then dewaxed in xylene (500 ml) three
times at 25°C for 10 min each. The sections were rehydrated by
washing in an ethanol gradient series (100 and 95% for 3 min, and
85 and 75% for 1 min) and then rinsed with distilled water. The
sections were placed at 100°C in EDTA (pH 9.0±0.2) buffer (1:50;
cat. no. ZLI9069; Beijing Zhongshan Jinqiao Biological Co. Ltd.;
OriGene Technologies, Inc.) and the repair solution was used for
antigen retrieval for 20 min (hot repair at 100°C in EDTA 1:50,
2,500 ml liquid for 20 min). Subsequently, sections were washed
with distilled water, treated with 3% H2O2
(blocking reagent) solution at 25°C for 10 min to inhibit
endogenous peroxidase activity and washed with PBS. Tissue sections
were then incubated at room temperature for 40 min with primary
antibodies. Following primary incubation, sections were washed
three times with PBS for 5 min each time and incubated with sheep
anti-rat/rabbit IgG polymer labeled with HRP (ready-to-use type;
cat. no. PV-8000D; Origene Technologies, Inc.) at 25°C for 15 min.
Sections were washed three times with PBS for 5 min each time.
Tissues were incubated with 3,3′-diaminobenzidine color development
solution (1:50 dilution; cat. no. PV-8000D; Beijing Zhongshan
Jinqiao Biological Co. Ltd.) at 25°C for 5-10 min, and then washed
with distilled water. Hematoxylin was applied at 25°C for 1 min and
samples were washed in tap water and then blued in PBS. Afterwards,
the slide was washed with 75, 85, 95 and 100% ethanol (500 ml each)
for 1 min each to remove excess water and facilitate observation
under the microscope. Finally, tissue sections were placed in
xylene (500 ml) three times for 1 min each and a drop of neutral
gum was added to seal. IHC sections were observed under a light
microscope (Leica DM2000; Leica Microsystems GmbH) without software
analysis. IHC was performed using an EnVision IHC kit (polymer
method; cat. no. KIT-0014; Beijing Zhongshan Jinqiao Biotechnology
Co., Ltd.; Origene Technologies, Inc.) using primary antibodies
obtained from Beijing Zhongshan Jinqiao Biological Co., Ltd. and
Fuzhou Maixin Biotechnology development Co., Ltd. to target the
following proteins (pre-diluted working solutions unless otherwise
indicated): Desmin (working solution; cat. no. 20092713), MyoD1
(working solution; cat. no. 20121719), Ki-67 (1:200 dilution; cat.
no. 21030436), Melan-A (working solution; cat. no. 19122684), S-100
(working solution; cat. no. 2012240585C8), HMB45 (working solution;
cat. no. 21065615), leukocyte common antigen (working solution;
cat. no. 201140037a), prostate-specific antigen (working solution;
cat. no. 2012160146f), chromogranin A (working solution; cat. no.
20090705), synaptophysin (working solution; cat. no. 2101060742a),
CDX-2 (working solution; cat. no. 2105190631CEPR2764Y), CK (1:200
dilution; cat. no. 21061509) and epithelial membrane antigen (EMA;
1:100 dilution; cat. no. 21020730).
Discussion
ERMS originates from myogenic precursor cells and is
more common in children and adolescents (6). The disease occurs in various
locations, with recent reports focusing on the head and neck,
urogenital tract, trunk and extremities (7,8). ERMS
arising in the perianal region is rare (9). Perianal ERMS often presents as
extensive diffuse lesions around the anus, with unclear edges,
normal or grey skin colour, no obvious tenderness or fluctuation,
and a hard, fixed texture (2). When
complicated by an infection, there may be symptoms such as redness,
swelling, heat and pain that need to be differentiated from the
indications of a perianal abscess. Clinically, there have been a
number of cases of misdiagnosis as a perianal abscess (10,11).
The present case was initially misdiagnosed as a perianal abscess;
however, it was later pathologically confirmed as an ERMS.
The clinical manifestations of ERMS are diverse,
characterised by the poor differentiation of tumour cells, rapid
tumour growth, strong invasiveness, high rate of metastasis and
mortality, and can only be diagnosed pathologically (12). The morphological appearance under
ERMS microscopy is mainly similar to the muscles during the
7-10-week embryonic development, but it can also resemble the
morphology of muscle cells at various stages of development. The
tumour cells are round or oval with a very small amount of
eosinophilic cytoplasm, deeply stained nuclei and
well-differentiated smooth muscle blasts, with large round or
bizarre nuclei (13).
Immunohistochemical labelling is an essential method for diagnosing
ERMS. Most ERMS tumours are positive for desmin, Myogenin and
MyoD1. Among these, desmin and MyoD1 are sensitive markers for
identifying RMS and require nuclear staining to be considered
positive (13,14).
Perianal RMS treatment includes surgery,
radiotherapy and chemotherapy. A combined abdominoperineal radical
resection can be performed for localised perianal tumours. In
larger cases, radiotherapy can be administered, and surgery is
performed after the tumour shrinks (15). In addition, when there are no
suitable treatment options, multi-target inhibitors and supportive
care may be good alternatives for patients with EMRS (5,16).
These tumours are now routinely tested for molecular
characterization, particularly TP53 mutation status. However, since
perianal ERMS is a rare disease, the hospital had little experience
of such cases in the present study and not much was known about the
molecular characteristics at that time. The patient received brief
treatment in the Xiaoshan Affiliated Hospital of Wenzhou Medical
University and was discharged to a higher-level hospital for
treatment after the pathological results were obtained. Therefore,
no molecular characterization tests were performed on the tumour.
The patient did not receive any treatment and was discharged to
another hospital for consultation. During the follow-up period, the
patient died of multiple metastases and multi-organ failure at 6
months after the operation.
In summary, the present study reports a case of
perianal ERMS in an adult that was initially misdiagnosed as a
perianal abscess. The rarity of perianal ERMS in adults poses a
challenge with regard to its aetiology and diagnosis; therefore, a
detailed evaluation is required before surgery. The prognosis of
perianal ERMS in adults is poor and requires active postoperative
treatment and close follow-up. In addition, the analysis of
clinical signs and immunohistochemistry deepens our understanding
of perianal ERMS and provides a diagnostic reference for clinicians
encountering these conditions in the future.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the Hangzhou Medical and
Health Technology Project (grant no. B20220132).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YJ and JL performed case data collection, drafting
of the manuscript and conception of the study. BH and GL obtained
medical images and analyzed patient data. JL and YJ confirm the
authenticity of all the raw data. YJ revised the manuscript and
interpreted the data. In addition, all authors agreed on the
journal to which the article has been submitted and agreed to be
accountable for all aspects of the work. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaseb H, Kuhn J, Gasalberti DP and Babiker
HM: Rhabdomyosarcoma. StatPearls Publishing; Treasure Island, FL:
2024
|
|
2
|
Yang N, Kong D, Wang X and Liu Y: Perianal
rhabdomyosarcoma in an adult: A case report and review of the
literature. Med (Baltimore). 102:e362762023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Casey DL, Wexler LH, LaQuaglia MP, Meyers
PA and Wolden SL: Patterns of failure for rhabdomyosarcoma of the
perineal and perianal region. Int J Radiat Oncol Biol Phys.
89:82–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agaram NP: Evolving classification of
rhabdomyosarcoma. Histopathology. 80:98–108. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu JJ, Chen MB, Gao XJ, Zhang Y, Liu YY,
Yong Y and Li P: Gross perianal embryonal rhabdomyosarcoma with
severe multiple bone metastases throughout the body: A case report.
J Int Med Res. 50:30006052210870502022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eftekhari K, Chambers CB, Goldstein SM,
Katowitz WR and Katowitz JA: Alveolar rhabdomyosarcoma masquerading
as embryonal subtype: The value of modern molecular diagnostic
testing. Ophthalmic Plast Reconstr Surg. 31:e43–e45. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guenther M, Richter M, Doenst T, Rachow T,
Lang S and Sandhaus T: Mediastinal rhabdomyosarcoma feeding off the
left anterior Descending Artery. Thorac Cardiovasc Surg Rep.
11:e17–e19. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu W, Jiang L, Jin Y, Yang B and Lai TY:
Alveolar rhabdomyosarcoma of the sphenoid sinus mimicking optic
neuritis presenting with intermittent visual loss in an adult. Onco
Targets Ther. 9:6333–6336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hafiz Yusuf F, Shaikh I, Hussain M, Arif
A, Rahim D, Hafeez Siddiqui A, Farrukh S, Saleem Tebha S and Huma
ZE: Laryngeal embryonal rhabdomyosarcoma: A rare adult neoplasm.
Ear Nose Throat J. 1455613221083795. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodrigues BD, Alves MC, da Silva AL and
Reis IG: Perianal endometriosis mimicking recurrent perianal
abscess: Case report and literature review. Int J Colorectal Dis.
31:1385–1386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu YN, Zhu Y, Tan JJ, Shen GS, Huang SL,
Zhou CG, Huangfu SH, Zhang R, Huang XB, Wang L, et al: Extranodal
natural killer/T-cell lymphoma (nasal type) presenting as a
perianal abscess: A case report. World J Clin Cases. 7:992–1000.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Linea C, Sinagra E, Gioia F and Rimi C:
Perianal embryonal rhabdomyosarcoma diagnosed by endoscopic
ultrasound-guided fine needle aspiration. Endoscopy. 44 (Suppl
2):UCTN. E342–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh AP, Mangal K, Tanger R, Gupta AK,
Ansari M and Shukla AK: Perianal and perineal spindle cell variant
of embryonal rhabdomyosarcoma in an infant. J Indian Assoc Pediatr
Surg. 24:219–221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmad Z, Din NU, Ahmad A, Imran S, Pervez
S, Ahmed R and Kayani N: Rhabdomyosarcoma-An epidemiological and
histopathologic study of 277 cases from a major tertiary care
center in Karachi, Pakistan. Asian Pac J Cancer Prev. 16:757–760.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leaphart C and Rodeberg D: Pediatric
surgical oncology: management of rhabdomyosarcoma. Surg Oncol.
16:173–185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hua Z, Song G, Minzhi Y, Min S, Chao X,
Jianmin Z, Jing W, Siqi H, Chenjie X, Jing Ma, et al: Analysis of
infantile fibrosarcoma reveals extensive T-cell responses within
tumors: Implications for immunotherapy. Pediatr Blood Cancer.
65:2018.PubMed/NCBI
|