Introduction
Solitary fibrous tumors (SFTs) are mesenchymal
neoplasms of fibroblastic origin that may occur at any site of the
body (1). SFTs typically occur in
adults and were first described in 1931 in the mediastinum and
pleura (2). Superficial and deep
soft tissues, viscera or bone, including extremities, abdominal or
retroperitoneal cavity, of the head, neck and trunk may be involved
(3). SFTs rarely occur in the
central nervous system (CNS), accounting for <1% of all primary
CNS tumors (4). In the CNS SFTs
occur as intracranial extra-axial dura-based tumors, commonly
situated in the skull base, falcine or parasagittal locations
(5). The clinical presentation is
due to the mass effect or raised intracranial pressure of the
tumors and a preoperative false diagnosis of meningioma is
frequent. Histopathology with immunohistochemistry (IHC) for STAT6
forms the mainstay of diagnosis. A three-tiered grading system
based on cellularity, mitotic activity and necrosis is practiced
for these tumors according to World Health Organization (WHO)
guidelines (5th edition) (6). Gross
total resection with adjuvant radiotherapy is the preferred
treatment strategy while additional chemotherapy may be
administered for metastatic disease (4). In the present study, 3 cases of
intracranial SFTs over a period of 5 years are reported, 1 of which
had an intraventricular location, and another showed evidence of
local recurrence and distant metastasis.
Case report
Methods
All cases reported as SFT/hemangiopericytoma of the
brain between January, 2019 and December, 2023 were collected and
the clinical details were retrieved from the hospital medical
records at Rajiv Gandhi Cancer Institute and Research Centre
(RGCIRC), Delhi, India.
Histopathological examination was performed using
3-µm thick sections cut from formalin fixed (10% neutral buffered
formalin) paraffin embedded blocks (paraffin wax used at a melting
point of 56–58°C for 2-4 h). Fixation was conducted at room
temperature for 6-48 h followed by further fixation at 45–50°C
during tissue processing as per the in-house protocol. Hematoxylin
and eosin staining was performed at room temperature for ~50 min in
a Leica autostainer XL (ST5010; Leica Microsystems GmbH) according
to manufacturer's protocol. Immunostaining for STAT6 (rabbit
monoclonal antibody clone EP325; 1:100; cat. no. BSB-3809-05; Bio
SB Inc.), CD34 (rabbit monoclonal antibody clone EP88; 1:100; cat.
no. 134R-16-RUO; Cell Marque; Merck KGaA) and Ki67 (mouse
monoclonal antibody clone MIB-1; 1:100; cat. no. Z2305ML; Zeta
Corporation) at 37°C for 44 min was conducted using a Ventana
BenchMark XT 750-700 Automated IHC/ISH autostainer (Roche
Diagnostics, Ltd.) was performed as per the manufacturer's
protocol, using heat-induced antigen retrieval (100°C, 4 min) and
endogenous peroxidase blocking by 0.04% hydrogen peroxide. The
signal amplification and signal generation were accomplished using
the Ventana polymer-based OptiView system (Roche Diagnostics,
Ltd.), which includes the secondary HRP antibody multimer (cat. no.
253-4580) and is incubated at room temperature for 12 min. Finally,
the samples were stained with DAB. The hematoxylin and
eosin-stained and IHC slides were reviewed by two pathologists
under a light microscope. Ki67 assessment was conducted
manually.
A total of 3 cases were identified over the study
period with a mean age at diagnosis of 45.6 years (age range, 30-60
years). Of these patients, 2 were male and 1 was female. The
clinical presentation included vision disturbances and headache.
All 3 cases were preoperatively diagnosed as meningioma on imaging
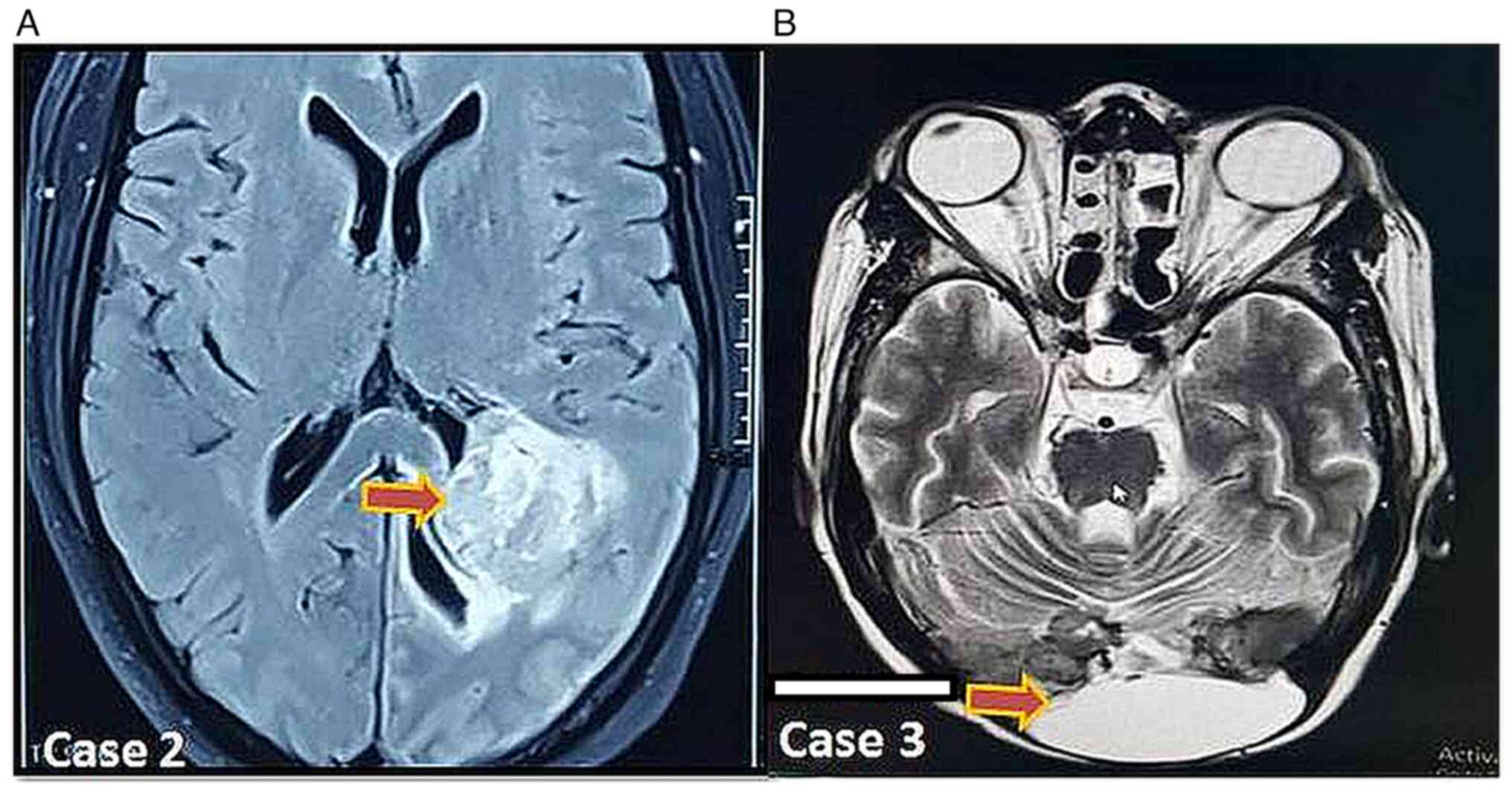
(Fig. 1). The patients underwent
decompression surgery and were referred to RGCIRC for further
treatment, evaluation and second opinion. The slides and blocks
were brought to RGCIRC for review. The clinicopathological features
of the patients are summarized in Table
I.
 | Table I.Clinicopathological parameters of the
reported cases. |
Table I.
Clinicopathological parameters of the
reported cases.
| Clinicopathological
parameters | Case 1 | Case 2 | Case 3 |
|---|
| Age, years/sex | 60/Male | 35/Male | 42/Female |
| Clinical
presentation | Blurred vision with
bifrontal headache for 1 year and amnesia for 3 months. | Severe headache 3 for
days and pain in both eyes. | Bilateral loss of
vision, memory disturbance and bilateral paresis for 6 months. |
| Imaging findings
(CEMRI) | Extra-axial mass of
60×50×40-mm in the right temporo-occipital region | Ill-defined
intraventricular mass of 46×32-mm in the left occipital lobe with
associated ventricular bleeding. | Post-op status of a
dura-based lesion in the occipital and posterior fossa. |
| Keloidal
collagen/amianthoid fibers | Present | Absent | Absent |
| Nuclear
pleomorphism | Mild to moderate | Mild | Moderate |
| Mitosis, /10 HPF | 15 | 9 | >20 |
| Necrosis | Present | Absent | Present |
|
Immunohistochemistry |
|
|
|
| CD
34 | P | N | Heterogenous P |
|
STAT6 | P | P | P |
| GFAP | N | N | N |
| CK | N | N | N |
| S100 | N | N | N |
| Ki-67 labelling
index, % | 15-20 | 8-10 | Not performed |
| Final diagnosis | SFT CNS WHO grade
3 | SFT CNS WHO grade
2 | SFT CNS WHO grade
3 |
Case 1
A 60-year-old male was referred to RGCIRC in
January, 2022 with complaints of blurred vision and bifrontal
headache for 1 year and amnesia for 3 months. Contrast enhanced
magnetic resonance imaging (CEMRI) scan findings showed an extra
axial mass in the right temporo-occipital region measuring
~6×5×4-cm. A clinical diagnosis of meningioma was considered. The
patient underwent a decompression surgery followed by radiotherapy.
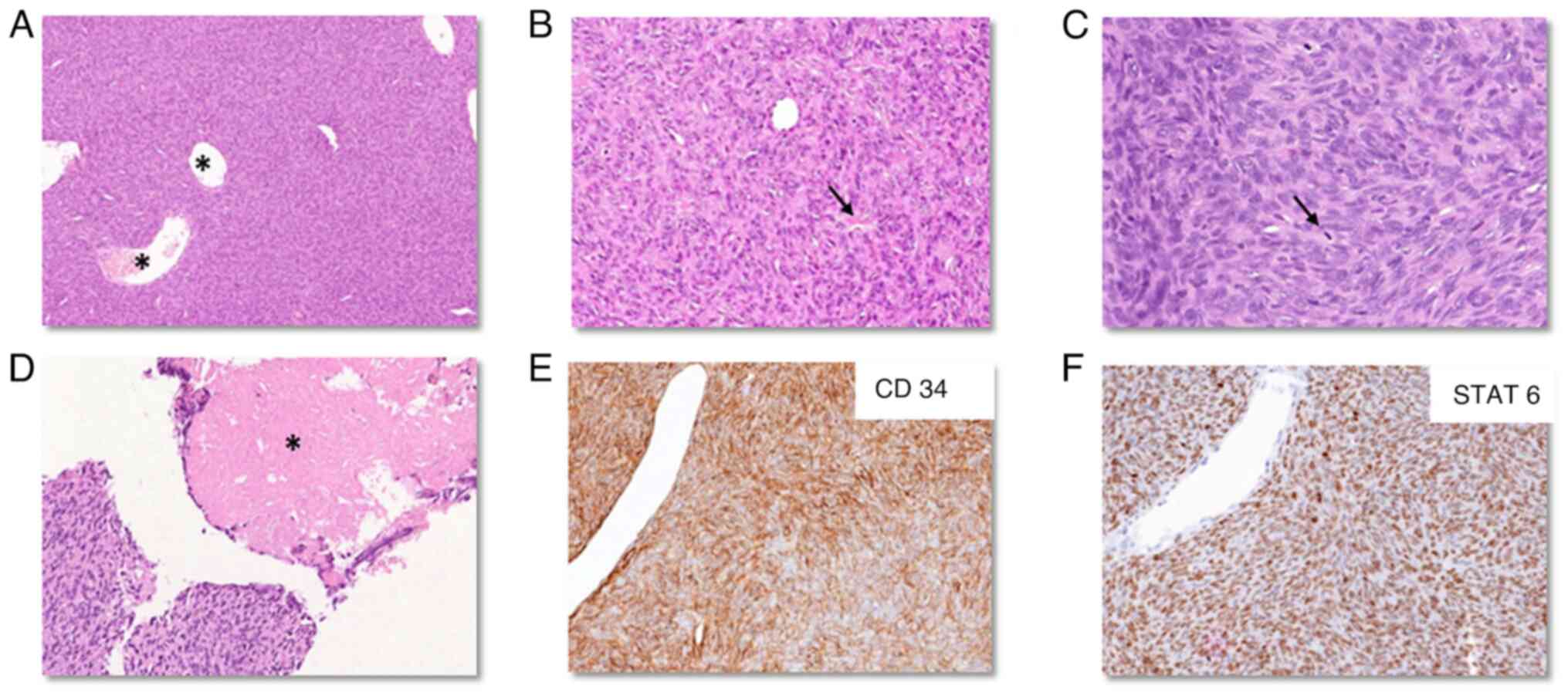
On histopathology, the tumor was composed of spindled to ovoid
monomorphic appearing cells arranged in an ill-formed fascicles and
vague storiform architecture with the presence of a dilated thin
walled (hemangiopericytomatous) vasculature and focal keloidal
collagen and amianthoid fibers (Fig. 2A
and B). The tumor cells showed mild to moderate nuclear
pleomorphism. Mitosis was 15/10 high power fields (HPF) with focal
necrosis (Fig. 2C and D). By IHC,
tumor cells were diffusely positive for CD34 (Fig. 2E) and STAT6 (Fig. 2F), but negative for CK, glial
fibrillary acidic protein (GFAP) and S100. The Ki-67 labelling
index was 15–20%. A final diagnosis of SFT CNS WHO grade 3 was
considered (6). The patient was
treated with radiotherapy at 60 Gy in 30 fractions. At present, the
patient remains disease-free after a follow-up period of 2 years
and 9 months.
Case 2
A 35-year-old diabetic male patient presented with
severe headache for 3 days associated with pain in both the eyes at
Global Rainbow Healthcare Hospital (Agra, India) in January, 2021.
Non-contrast computerized tomography of the head showed an
ill-defined intraventricular mass measuring 4.6×3.2-cm involving
the body and trigone of the left lateral ventricle, with an
associated ventricular bleed extending into the adjacent occipital
lobe. The CEMRI (Fig. 1) showed
similar findings of an intraventricular space occupying lesion
(SOL) involving the left occipital horn with intense enhancement
and hypointense signal on T2W images suggestive of an
intraventricular meningioma with subacute hemorrhage in the left
occipital lobe. The patient was also preoperatively diagnosed with
meningioma. Following surgery at Global Rainbow Healthcare Hospital
the patient was referred to RGCIRC in January, 2021 for a second
opinion and further treatment. The patient received adjuvant
radiotherapy of 60 Gy in 30 fractions at RGCIRC.
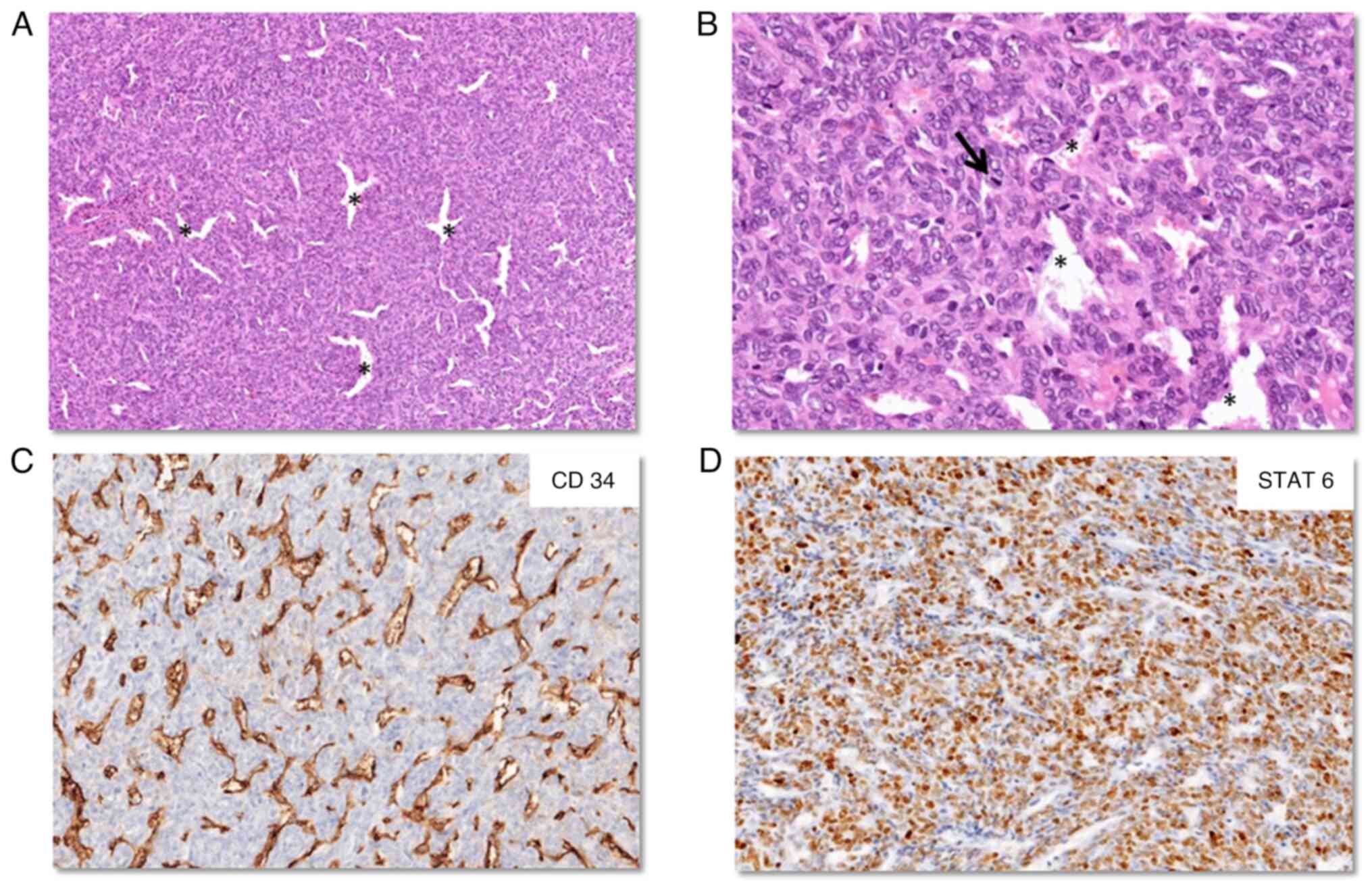
Sections from the intracranial specimen showed a
cellular tumor composed of spindle-shaped cells arranged in a
‘patternless’ architecture with numerous interspersed dilated
vessels. The cells showed indistinct cell borders with ovoid nuclei
having inconspicuous nucleoli. The mitotic rate was 9/10 HPF and no
necrosis was observed (Fig. 3A and
B). On IHC, the tumor cells diffusely expressed STAT6 (Fig. 3D), but were negative for CD34
(Fig. 3C), CK, epithelial membrane
antigen (EMA), GFAP and synaptophysin. The MIB1 labelling index was
8–10%. The integrated diagnosis given was SFT CNS WHO grade 2. The
patient remains disease free after 2 years of follow-up.
Case 3
A 42-year-old female presented with bilateral loss
of vision, memory disturbance and bilateral paresis for 6 months,
causing the patient to be bed ridden and was admitted to BHU
(Varanasi, India) in February, 2011. Surgery was performed on the
patient for a dura-based intracranial SOL, which was diagnosed as
an anaplastic meningioma CNS WHO grade 3 on histopathology. The
patient subsequently received 60 Gy of radiotherapy in 30
fractions. Surgery was again performed in 2016 and 2019 when the
patient developed recurrences while receiving radiotherapy. The
patient was admitted to RGCIRC in 2019, and following CEMRI, a
post-op status of a dura-based lesion in the occipital and
posterior fossa region with multiple bilateral cerebral nodules was
revealed. The patient underwent a PET/CT, which detected bilateral
lung metastasis in the form of sub pleural nodules, cervical
supraclavicular lymphadenopathy and a lytic lesion in the sacrum
with a soft tissue component. The slides and paraffin-embedded
blocks from the dura-based lesion were also reviewed at RGCIRC
along with a tru-cut biopsy of the lung nodule. A biopsy from the
supraclavicular lymph node showed granulomatous lymphadenitis with
no evidence of malignancy. The patient received pazopanib (tyrosine
kinase inhibitor) for 9 months at an initial dose of 400 mg twice a
day (BD) and then de-escalated to 200 mg BD. This treatment was
stopped once the patient developed a recurrence detected in 2021,
when the patient complained of headaches associated with vomiting.
On evaluation, CEMRI of the brain revealed a post-op status with a
progressive enhancing dura-based lesion in the occipital and
posterior fossa region with extensions, leptomeningeal enhancement,
subgaleal collection at the craniotomy site and persistent multiple
bilateral cerebral enhancing nodules. A re-exploration and
decompression surgery was performed followed by supportive care.
The patient developed recurrent seizures and meningitis, for which
the patient was treated with intravenous antibiotics [caspofungin
50 mg once daily, fosfomycin 4 g trice daily (TDS) and meropenem 2
g BD], antiepileptics (levetiracetam 1.5 g BD, sodium valproate 500
mg TDS and lacosamide 200 mg BD) and anti-inflammatory drugs
(paracetamol 1 g, as required) along with multivitamin syrup,
calcium gluconate and pantoprazole 40 mg iv BD. However, the
patient left the hospital against medical advice in August,
2021.
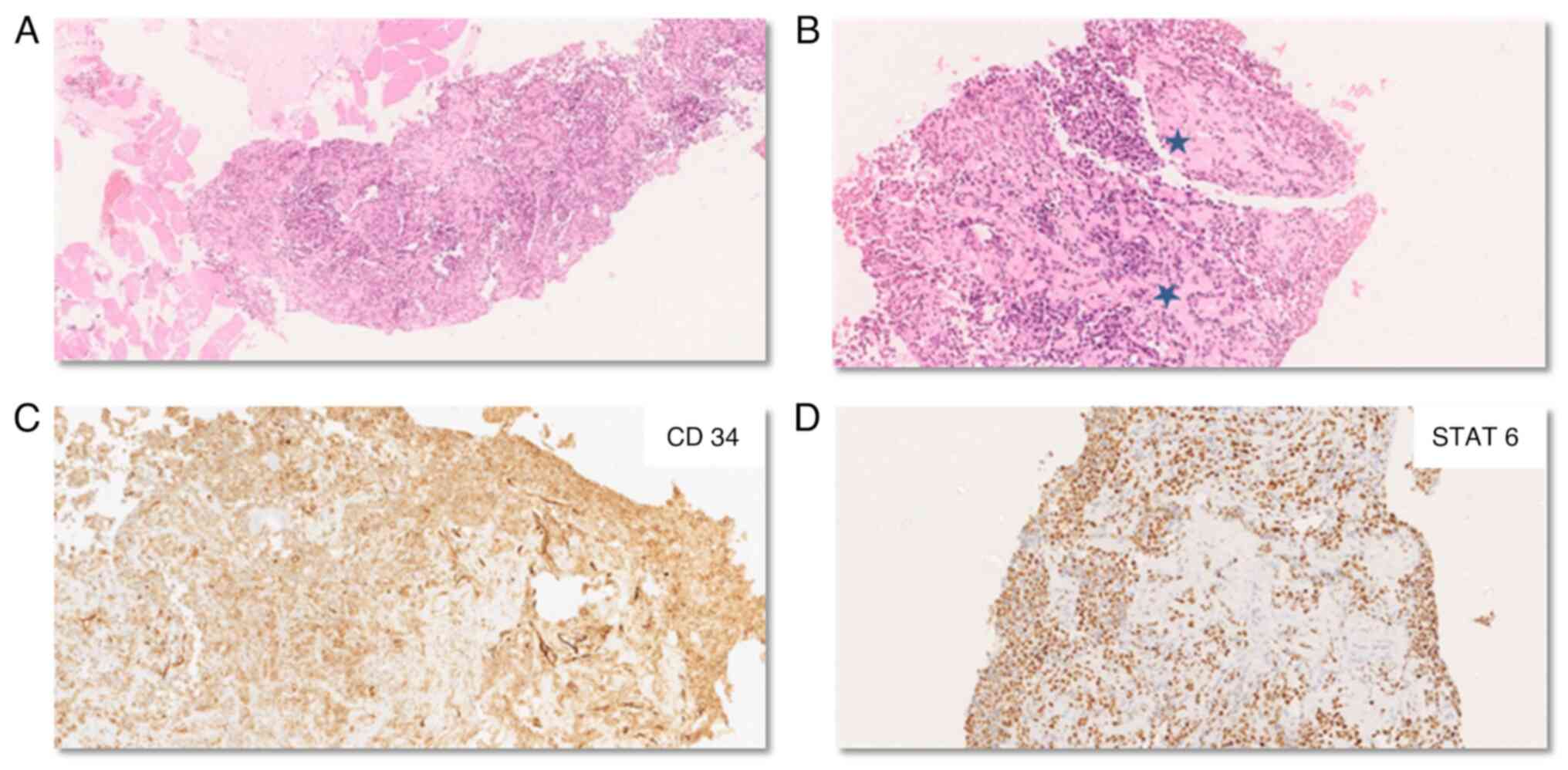
The histopathological examination of the primary
site and recurrent tumor revealed a cellular spindle cell tumor
arranged in solid sheets and a haphazard pattern with limited
intervening stroma. The tumor cells were spindle to oval-shaped
with ovoid nuclei containing vesicular chromatin and moderate
nuclear pleomorphism. A staghorn vasculature was observed
interspersed amidst the tumor (Fig. 4A
and B). Foci of hemorrhage and necrosis were also noted. The
mitosis was brisk (>20/10 HPF). On IHC, tumor cells were
observed to express STAT6, which was strong and diffuse, while CD34
showed heterogenous positivity. The tumor cells were negative for
GFAP, S100 and smooth muscle actin. An integrated diagnosis of SFT
CNS WHO grade 3 was considered (Fig. 4C
and D). Biopsy from the subpleural nodule confirmed metastasis
with similar histopathology and immunohistochemical findings. The
patient died in December, 2021.
Discussion
SFTs of the CNS are rare tumors typically occurring
as dural-based supratentorial tumors or uncommonly in the spinal
location (6). The precise cell of
origin for a CNS SFT remains uncertain and is considered to be of
fibroblastic nature, possibly arising from thick collagen bands
(7). Due to the dural-based nature
and peripheral contrast enhancement commonly known as the ‘dural
tail’, CNS SFTs are commonly misdiagnosed as meningiomas
radiologically (8). The genetic
hallmark of all SFTs is a paracentric inversion involving the long
arm of chromosome 12, leading to a fusion of the NGFI-A-binding
protein 2 and STAT6 genes that is easily demonstrable as nuclear
expression of STAT6 on IHC (9).
SFTs typically occur in older adults between the fifth and seventh
decades of life, with clinical symptoms related to increased
intracranial pressure or the mass effect caused by these tumors.
Out of the 3 cases reported in the present study, Case 1 was a
60-year-old male while Cases 2 and 3 were younger patients of 31and
40 years, respectively, who were provisionally diagnosed with
meningioma based on the imaging findings. Case 2 presented as an
intraventricular mass, which is a very rare site for SFTs. An
intraventricular location leads to differential diagnoses
encompassing intraventricular meningiomas, choroid plexus tumors,
ependymomas, gliomas or even metastasis. To date and to the best of
our knowledge, only 11 cases of intraventricular SFTs have been
reported in the literature (10–19).
Histopathological examination is the gold standard
for diagnosis of SFT. SFT is composed of uniform-appearing
spindle-shaped cells disposed in a ‘patternless’ or haphazard
arrangement with a typical thin-walled dilated branching
‘hemangiopericytomatous’ vasculature. The terms ‘solitary fibrous
tumor’ and ‘hemangiopericytoma’, which were historically considered
as two ends of a spectrum, were combined together due to the common
genetic signature. However, the latest WHO blue book series has
removed the term, hemangiopericytoma, altogether. The morphology
can vary from a hypocellular to a markedly cellular phenotype.
There can be presence of keloidal-type collagen and amianthoid
fibers focally (6). Nuclear
pseudoinclusions and calcifications observed in meningiomas are
typically not observed in SFTs. An associated adipocytic component
or dedifferentiation may be rarely observed both at the time of
primary diagnosis or at the time of recurrence (20). Mitosis (≥5/10 HPF in grade 2 and 3
tumors) and necrosis (seen only in grade 3 tumors) form the basis
of stratifying these tumors into a three-tiered grading system
(21). All 3 cases reported in the
present study showed a similar morphology: A cellular spindle cell
tumor with a hemangiopericytomatous vasculature interspersed
throughout with variable mitosis and necrosis. Cases 1 and 3 were
classified as CNS WHO grade 3 SFTs, while Case 2 was considered a
CNS WHO grade 2 SFT.
On IHC, SFTs express CD99, CD34 and bcl2, while
expression of STAT6 is considered the immunohistochemical hallmark
acting as a surrogate for the associated gene fusion. Aldehyde
dehydrogenase 1 family member A1 is a newer marker with a notable
sensitivity and specificity (6). An
IHC panel is generally employed, depending on the morphology and
location of the tumor, to rule out the differential diagnoses. A
CNS SFT needs to be differentiated from its close mimics such as
meningioma (fibrous subtype; characterized by positivity for EMA
and somatostatin receptor 2), schwannoma (S100+),
sarcomas (such as malignant peripheral nerve sheath tumors),
monophasic synovial sarcoma (EMA+, CK+ and
TLE1+) and mesenchymal chondrosarcoma (SOX9+,
NKX2.2+ and NKX3.1+) along with gliomas
(GFAP+) or even metastatic carcinomas (pancytokeratin
positive). In the present study, immunonegativity for EMA and GFAP
ruled out meningioma and glioma, respectively, while pancytokeratin
and S100 negativity was useful in negating metastasis and nerve
sheath tumors, respectively. CK and EMA negativity also ruled out a
synovial sarcoma. Strong and diffuse positivity for the highly
specific marker, STAT6, which was observed in all the 3 cases
further assisted in the unequivocal diagnosis of an SFT. In
addition, CD34 showed a variable expression, as has been described
by other researchers (22).
Meningeal SFTs show a high predilection for local
recurrence irrespective of the grade (23). This warrants a long term follow-up
and reiterates the importance of distinguishing meningeal SFTs from
their closest differential, meningioma. The risk model applied to
meningeal SFTs differs from that applied to SFTs of other locations
as patient age and tumor size have not been validated as adverse
prognostic markers in the CNS. Gross total resection is the
treatment of choice followed by adjuvant radiotherapy, which
imparts a survival benefit in higher grade tumors (grades 2 and 3)
(24). Distant metastasis was seen
in only 3% of intracranial SFTs in a large study by Lu et al
(25). The 3 cases reported in the
present series were treated by surgery followed by radiotherapy.
Case 1 remains disease free after a follow-up period of 2 years and
9 months, Case 2 remains disease free after 2 years and Case 3
suffered multiple recurrences and metastasis to the lung and sacrum
for which the patient was administered chemotherapy. The Case 3
patient died 10 years after initial presentation. Studies on the
role of chemotherapy in intracranial SFTs are scarce; however, it
may be used in metastatic cases (26).
In conclusion, intracranial SFTs are rare tumors.
The key to recognizing these tumors lies in the knowledge of their
existence with a high index of suspicion. Histomorphology and IHC,
including the surrogate marker STAT6, are imperative for a correct
and timely diagnosis and to differentiate SFTs from their close
mimics. A three-tiered grading system should be applied to risk
stratify these tumors, which show a high propensity of recurrence.
Metastasis is rare, yet a known complication of this multifaceted
tumor.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GD, PC, AS, SP and AM performed the histological
examination. ICP performed the surgical procedures and was
responsible for taking follow-up of the patients. PC, AS and GD
were major contributors in drafting the manuscript. GD and PC were
also responsible for the critical revision of the manuscript. GD
and PC confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study has been reviewed and approved by the
Institutional Review Board of Rajiv Gandhi Cancer Institute and
Research Centre (New Delhi, India; IRB no. IRB-BHR 40/2024).
Patient consent for publication
Written informed patient/next of kin consent was
obtained for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
International Agency for Research on
Cancer, . WHO Classification of Tumours Editorial Board. Soft
tissue and bone tumours (Internet). WHO classification of tumours
series; 5th edition. volume 3. Lyon, France: https://tumourclassification.iarc.who.int/chapters/33March
8–2024
|
|
2
|
Klemperer P and Rabin CB: Primary
neoplasms of the pleura. A report of five cases. Arch Pathol.
11:385–412. 1931.
|
|
3
|
Gholami S, Cassidy MR, Kirane A, Kuk D,
Zanchelli B, Antonescu CR, Singer S and Brennan M: Size and
location are the most important risk factors for malignant behavior
in resected solitary fibrous tumors. Ann Surg Oncol. 24:3865–3871.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Damodaran O, Robbins P, Knuckey N,
Bynevelt M, Wong G and Lee G: Primary intracranial
haemangiopericytoma: Comparison of survival outcomes and metastatic
potential in WHO grade II and III variants. J Clin Neurosci.
21:1310–1314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mena H, Ribas JL, Pezeshkpour GH, Cowan DN
and Parisi JE: Hemangiopericytoma of the central nervous system: A
review of 94 cases. Hum Pathol. 22:84–91. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ng Ho-Keung, Lazar AJ and Giannini C:
Mesenchymal, non meningothelial tumors involving the CNS.
Haematolymphoid Tumours WHO Classification of Tumours. 5th edition.
IARC Press; Lyon: pp. 301–305. 2021
|
|
7
|
Lin Q, Zhu J and Zhang X: Solitary fibrous
tumor of the central nervous system invading and penetrating the
skull: A case report. Oncol Lett. 25:812023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen T, Jiang B, Zheng Y, She D, Zhang H,
Xing Z and Cao D: Differentiating intracranial solitary fibrous
tumor/hemangiopericytoma from meningioma using diffusion-weighted
imaging and susceptibility-weighted imaging. Neuroradiology.
62:175–184. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schweizer L, Koelsche C, Sahm F, Piro RM,
Capper D, Reuss DE, Pusch S, Habel A, Meyer J, Göck T, et al:
Meningeal hemangiopericytoma and solitary fibrous tumors carry the
NAB2-STAT6 fusion and can be diagnosed by nuclear expression of
STAT6 protein. Acta Neuropathol. 125:651–658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nguyen A, Shan Y, Lyon K and Vance AZ:
Lateral ventricle solitary fibrous tumor: A case report and review
of the literature. Cureus. 14:e231062022.PubMed/NCBI
|
|
11
|
Li X, Tan L, Ouyang X, Jiang J, Huang S,
Huang Y, Li S and Chen D: Magnetic resonance features of meningeal
solitary fibrous tumors. Oncol Lett. 15:8825–8832. 2018.PubMed/NCBI
|
|
12
|
Bell SL, Suttner NJ and Stewart W: An
unusual intraventricular tumor. Neuropathology. 32:311–313. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vassal F, Manet R, Forest F, Camdessanche
JP, Péoc'h M and Nuti C: Solitary fibrous tumors of the central
nervous system: Report of five cases with unusual
clinicopathological and outcome patterns. Acta Neurochir (Wien).
153:377–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mekni A, Kourda J, Hammouda KB, Tangour M,
Kchir N, Zitouna M and Haouet S: Solitary fibrous tumour of the
central nervous system: Pathological study of eight cases and
review of the literature. Pathology. 41:649–654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boada M, Gómez E, Puig J and Pedraza S:
Intraventricular fibrous tumor: A case report. Radiologia.
51:512–515. 2009.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kinfe TM, Tschan CA, Stan AC and Krauss
JK: Solitary fibrous tumor of the foramen of Monro. Clin Neurol
Neurosurg. 110:404–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clarençon F, Bonneville F, Chiras J, Kujas
M and Cornu P: Cystic intraventricular solitary fibrous tumor. AJNR
Am J Neuroradiol. 28:1205–1206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Surendrababu NRS, Chacko G, Daniel RT and
Chacko AG: Solitary fibrous tumor of the lateral ventricle: CT
appearances and pathologic correlation with follow-up. AJNR Am J
Neuroradiol. 27:2135–1236. 2006.PubMed/NCBI
|
|
19
|
Tihan T, Viglione M, Rosenblum MK, Olivi A
and Burger PC: Solitary fibrous tumors in the central nervous
system. A clinicopathologic review of 18 cases and comparison to
meningeal hemangiopericytomas. Arch Pathol Lab Med. 127:432–439.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tariq MU, Din NU, Abdul-Ghafar J and Park
YK: The many faces of solitary fibrous tumor; diversity of
histological features, differential diagnosis and role of molecular
studies and surrogate markers in avoiding misdiagnosis and
predicting the behavior. Diagn Pathol. 16:322021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fritchie K, Jensch K, Moskalev EA, Caron
A, Jenkins S, Link M, Brown PD, Rodriguez FJ, Guajardo A, Brat D,
et al: The impact of histopathology and NAB2-STAT6 fusion subtype
in classification and grading of meningeal solitary fibrous
tumor/hemangiopericytoma. Acta Neuropathol. 137:307–319. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perry A, Scheithauer BW and Nascimento AG:
The immunophenotypic spectrum of meningeal hemangiopericytoma: A
comparison with fibrous meningioma and solitary fibrous tumor of
meninges. Am J Surg Pathol. 21:1354–1360. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Macagno N, Vogels R, Appay R, Colin C,
Mokhtari K; French CNS SFT/HPC Consortium; Dutch CNS SFT/HPC
Consortium, ; Küsters B, Wesseling P, Figarella-Branger D, et al:
Grading of meningeal solitary fibrous tumors/hemangiopericytomas:
Analysis of the prognostic value of the marseille grading system in
a cohort of 132 patients. Brain Pathol. 29:18–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kinslow CJ, Rae AI, Kumar P, McKhann GM,
Sisti MB, Bruce JN, Yu JB, Cheng SK and Wang TJC: Risk
stratification for management of solitary fibrous
tumor/hemangioperi-cytoma of the central nervous system. Cancers
(Basel). 15:8762023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu T, Xu H, Dong X, Jin Z and Wang Y:
Epidemiology and survival of patients with central nervous system
solitary fibrous tumors: A population-based analysis. Front Oncol.
12:9776292023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Son S, Lee SG, Jeong DH and Yoo CJ:
Malignant solitary fibrous tumor of tandem lesions in the skull and
spine. J Korean Neurosurg Soc. 54:246–249. 2013. View Article : Google Scholar : PubMed/NCBI
|