Introduction
Tuberculosis (TB) is a communicable disease that
poses a significant global health burden and represents one of the
leading causes of mortality. An estimated 10.6 million people were
diagnosed with TB worldwide in 2021, and the TB incidence rate is
estimated to have increased by 3.6% between 2020 and 2021. Despite
ongoing efforts, the diagnosis of TB remains challenging as
existing diagnostic techniques make it difficult to quickly and
accurately differentiate TB from other diseases, with a substantial
number of cases remaining undetected according to the Global
Tuberculosis Report of 2022. Therefore, addressing this issue and
improving the TB diagnostic rates are crucial for ending the global
TB epidemic (1).
In clinical practice, patients with cancer are
occasionally misdiagnosed with pulmonary TB, causing delays in
diagnosis and inadequate treatment (2–6). In
particular, Kabashi-Muçaj et al (7) previous presented the case of a patient
who was treated for TB for ~2 years before being correctly
diagnosed with pulmonary mucinous adenocarcinoma, based on positive
sputum cytology and transthoracic biopsy results. Similarly, Shu
et al (8) analyzed 6,683
misdiagnoses of TB as lung cancer (LC), which were attributed to
similar imaging findings (51%) and positive sputum acid-fast
staining (27%).
The present case reminds doctors that a positive
pathology is the basis for a definitive diagnosis of tuberculosis.
A pathogenically negative person who has the symptoms of TB should
not immediately be diagnosed with TB in order to avoid
misdiagnosis. The patient diagnosis should be made with caution,
preferably through discussion by a panel of experts.
Case report
A 59-year-old man was referred to The Third People's
Hospital of Zhuhai (Zhuhai, China) in February 2023 with a 6-month
history of an unexplained cough and weight loss of 6 kg. The
patient had a history of close contact with his father, who had
contracted TB 20 years previously. The patient denied smoking but
had been consuming alcohol for >20 years primarily in social
settings, but without excessive drinking.
Laboratory results showed an elevated C-reactive
protein level of 4 mg/l (normal range, 0-10 mg/l) and an
erythrocyte sedimentation rate of 7 mm/h (normal range in men, 0-10
mm/h). The IFN-γ release assay (IGRA; cat. no. 20203400710; Livzon
Pharmaceutical Group, Inc.) and recombinant Mycobacterium
tuberculosis fusion protein (6-kDaA early secreted antigen
target/0-kDa culture filtrate protein) tests (EC; cat. no.
S20237004; Chongqing Zhifei Biological Products Co., Ltd.) yielded
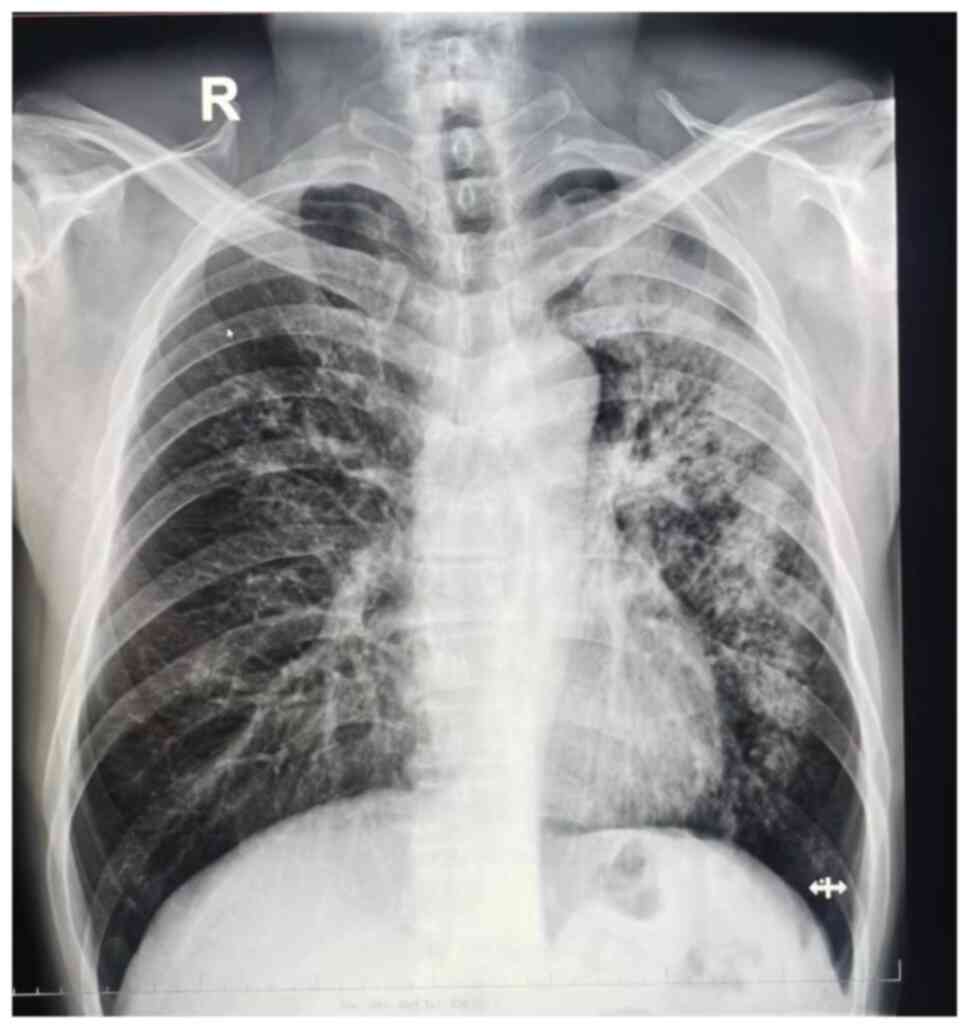
strongly positive results. Chest radiography revealed bilateral
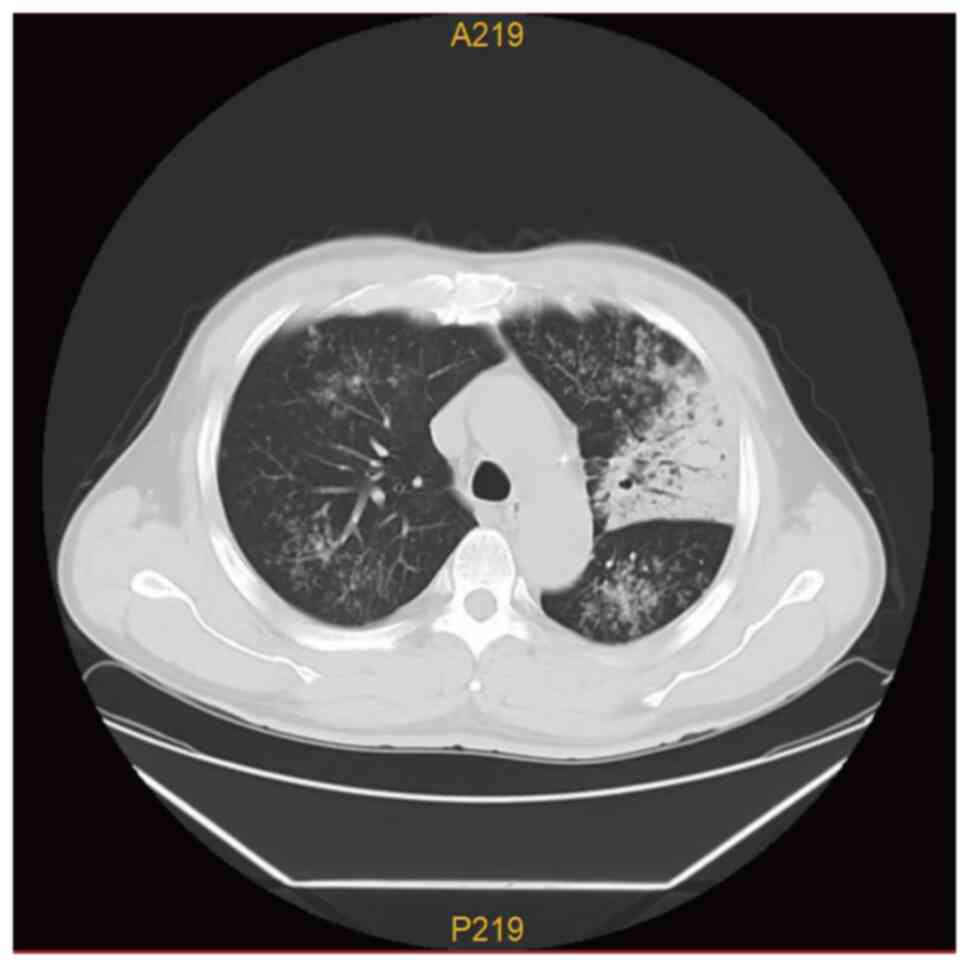
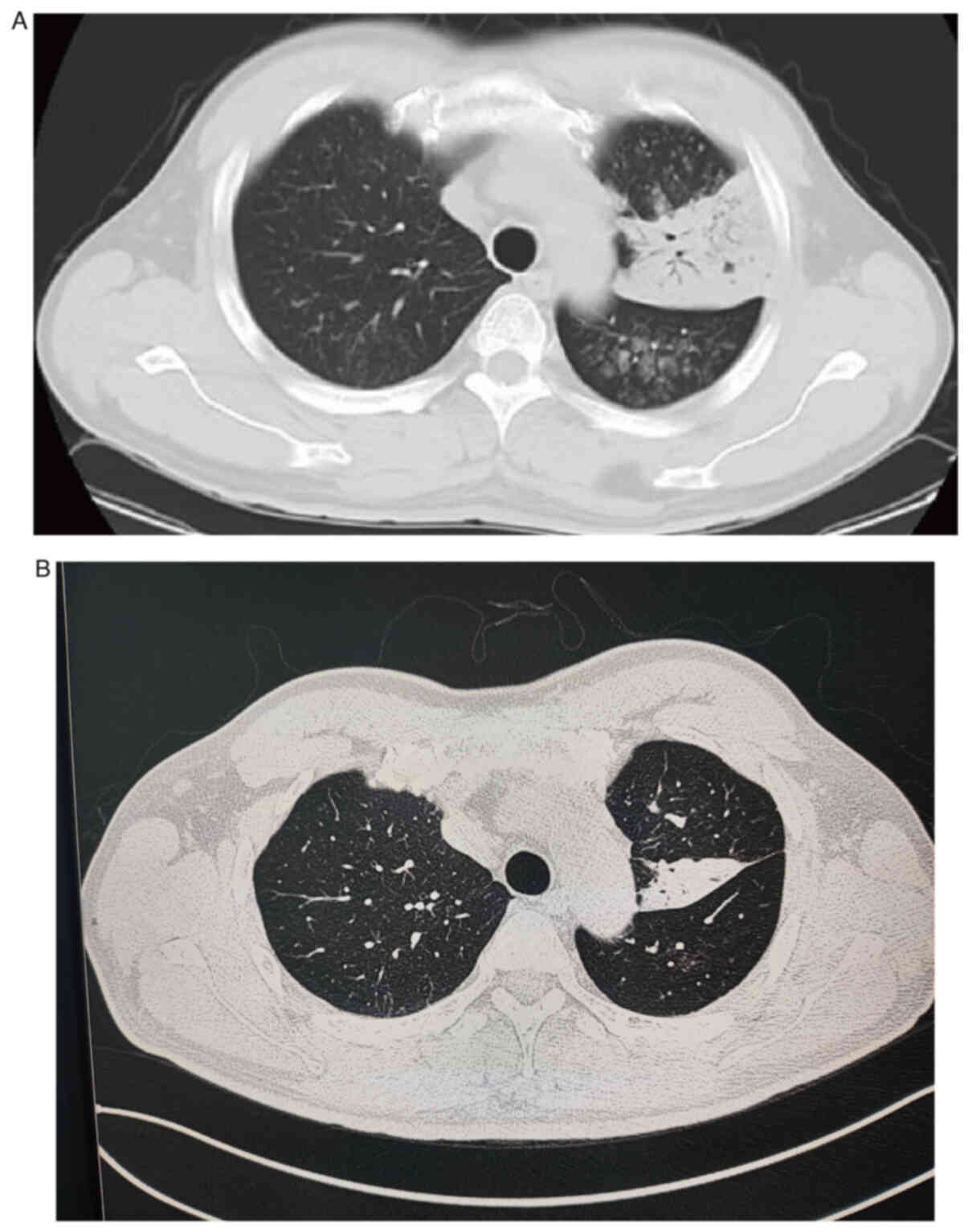
pulmonary lesions, particularly on the left lung (Fig. 1). CT revealed multiple patchy,
dendritic and small, nodular, high-density shadows in both lungs,
particularly in the left lung, which exhibited pulmonary
consolidation. Clear bronchial shadows were observed within the
lesion, with slight thickening of the pleura adjacent to the left
lung lesion (Fig. 2).
After considering the patient's symptoms of coughing
and weight loss, coupled with the history of TB contact and the
strongly positive results on IGRA and EC tests, pulmonary TB was
suspected. The patient was thereby referred to the Department of
Tuberculosis within The Third People's Hospital of Zhuhai. However,
despite repeated examinations, including a sputum acid-fast bacilli
test (cat. no. BA4091; Zhuhai Beso Cell Science and Technology Co.,
Ltd.) and TB fluorescence quantitative PCR (cat. no. 20153400357;
Daan Gene, Co., Ltd.), combined with ineffective anti-infective
therapy (days 1-10: 2 g ceftriaxone injection with 0.9% sodium
chloride injection 100-ml intravenous drip, every day; 30 mg oral
ambroxol hydrochloride dispersible tablets, three times a day; 25
mg oral compound methenamine capsules, three times a day; 3 ml
acetylcysteine solution with 3 ml 0.9% sodium chloride injection
for inhalation nebuliser, twice per day. Days 8-10: 250 ml 0.4 g
moxifloxacin hydrochloride sodium chloride by intravenous drip,
every day), a diagnosis of TB could not be established due to the
negative results. Elevated levels of the tumor markers
neuron-specific enolase (31.20 ng/ml; normal range, 0-16.3 ng/ml)
and carcinoembryonic antigen (15.04 ng/ml; normal range, 0-5 ng/ml)
were noted, where a detailed review of the chest CT scan suggested
that a neoplastic process could not be ruled out.
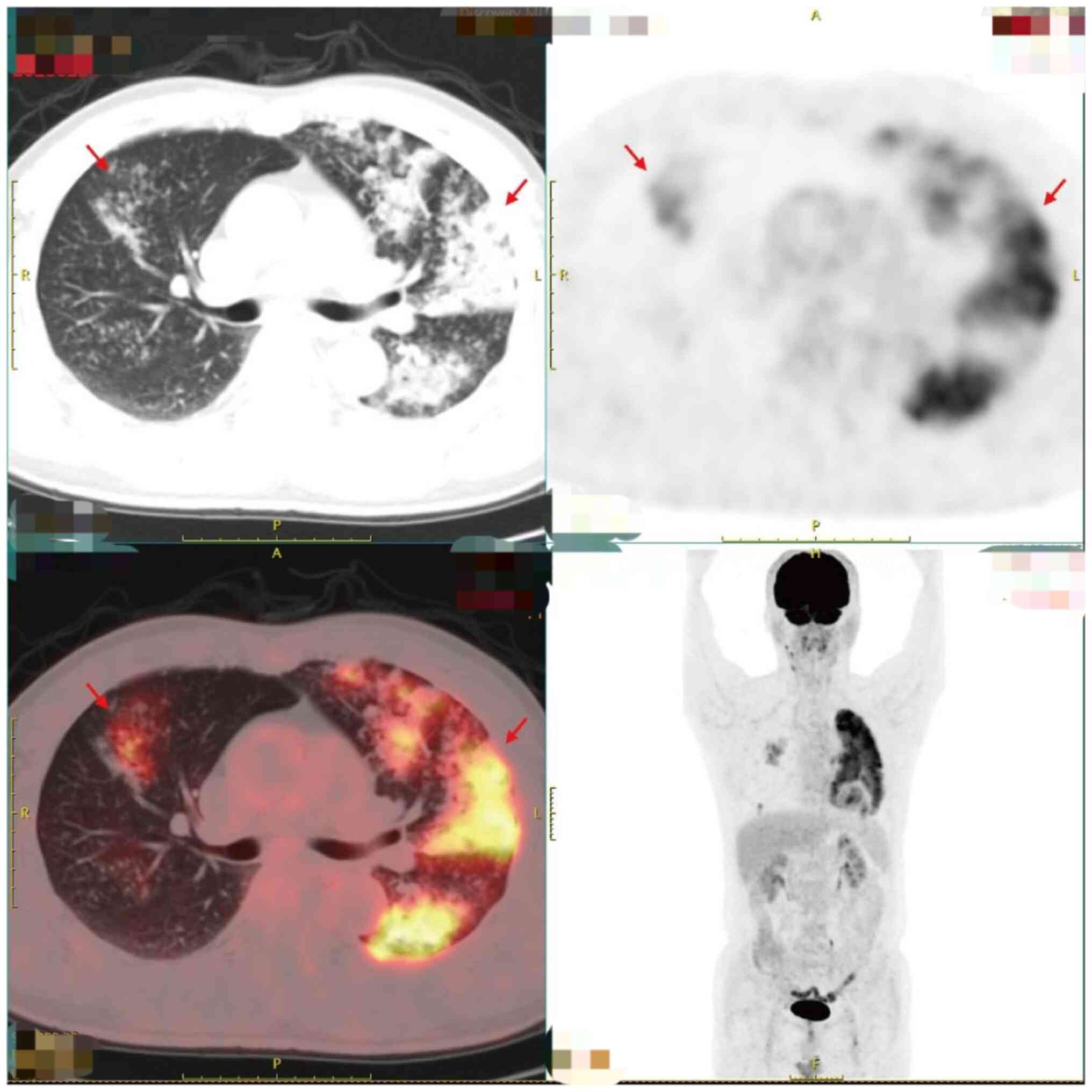
2-Deoxy2-[18F]fluoro-D-glucose PET combined with
low-dose CT (18F-FDG-PET/CT) showed multiple patchy and
nodular blurry shadows with highly elevated metabolic activity in
both lungs. The bronchial gas phase was primarily observed in the
left upper lung, accompanied by partial consolidation and
atelectasis. Contrast-enhanced scanning demonstrated enhancement,
increased FDG uptake and a maximum standardized uptake value of
~9.3. No significant swelling or radioactive concentration was
observed in the bilateral hilar or mediastinal lymph nodes. Pleural
effusion was not observed. Moderate focal FDG uptake was observed
in the myocardium (Fig. 3). The
findings indicative of myocardial involvement were suggestive of
metastatic disease. Focal metastasis is an uncommon feature in
pulmonary TB. In addition, multiple negative laboratory tests for
Mycobacterium tuberculosis provided additional support for
the exclusion of TB as a diagnostic possibility. To validate the
diagnostic findings, a bronchial section tissue sample was obtained
during bronchoscopy and forwarded for histopathological
examination. Sections measuring 3-4 µm in thickness were excised
and fixed for >10 h at room temperature using 10%
neutral-buffered formalin. The sections were then stained with
H&E for 40 min at ambient temperature and subsequently examined
under a light microscope. DNA was isolated from the tissue samples
using the QIAamp DNA FFPE Tissue kit (cat. no. 56404; Qiagen GmbH)
following the manufacturer's protocol. PCR was used for the
targeted amplification and sequencing of exons 18, 19, 20 and 21,
which are known to be frequently mutated in lung cancer, using the
Human EGFR Mutation Test Kit (cat. no. 20173404737; Wuhan Haijili
Biotechnology Co., Ltd.). The ABI-7500 PCR system (Qiagen GmbH) was
used with the following cycling conditions: Initial denaturation at
95°C for 5 min, followed by 15 cycles of 20 sec at 95°C and 30 sec
at 62°C, with another 35 cycles of 20 sec at 95°C, 40 sec at 60°C
and the fluorescence signal collection at 60°C. Data analysis of
amplification results was performed using ABI Sequencing Analysis
software version 5.4 (Applied Biosystems; Thermo Fisher Scientific,
Inc.) to detect the presence of EGFR mutations. A detailed search
was conducted for specific mutations, which found the S768 mutation
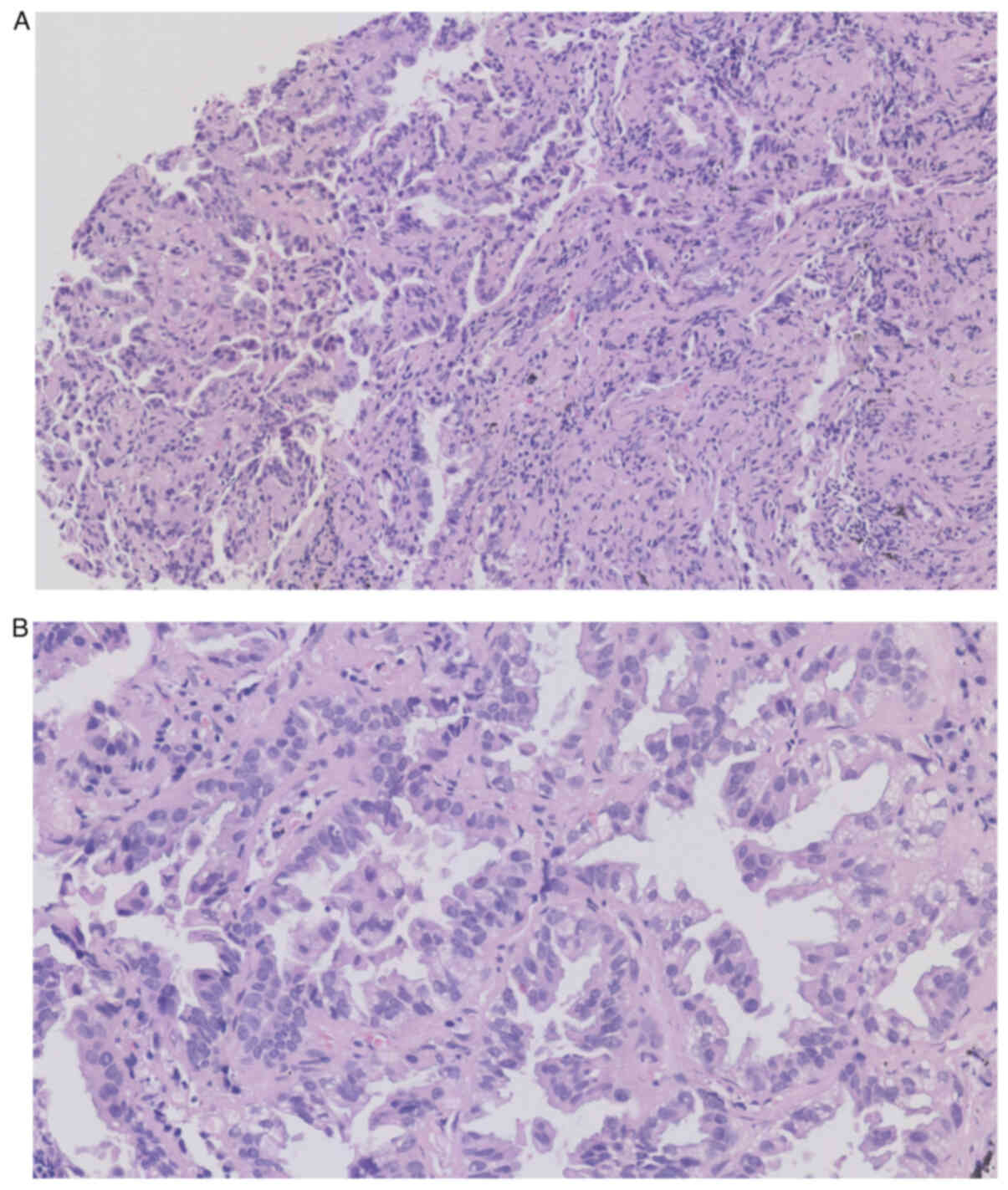
in exon 20 and the L858R mutation in exon 21. Histopathological
examination of the tissue biopsy from the bronchoscopy revealed
heterogeneously stained cells with darkly stained nuclei (Fig. 4), which ultimately led to the
diagnosis of lung adenocarcinoma.
Due to the fact that The Third People's Hospital of
Zhuhai specializes in psychiatric care rather than oncological
treatments, the patient was then referred to Zhuhai People's
Hospital (Zhuhai, China) for cancer treatment. After 2 months of
treatment with oxitinib (80 mg once daily) and bevacizumab (15
mg/kg on day 1) for 21 days per cycle, for a total of 4 cycles, a
follow-up CT scan in April 2023 showed a significant reduction in
lesion size, indicating a positive response to treatment (Fig. 5). The patient has now been
transferred to another hospital to continue treatment.
Discussion
Similarities in clinical symptoms, such as a cough,
expectoration and weight loss, couple with those in imaging
findings, accounts for the high misdiagnosis rate between TB and
certain types of LC, such as lung adenocarcinoma (6). In the present case, in the absence of
positive results on the sputum and endoscopic biopsy tests, the
initial diagnosis may have been biased by the positive IGRA and EC
test results. In addition, whilst PET/CT indicated tumor
metastasis, lung CT strongly suggested pulmonary TB. This scenario
has frequently resulted in the misdiagnosis of LC as TB, leading to
the prescription of TB medications for patients with LC in several
countries, such as Iran (9). In the
present case, the patient was diagnosed with secondary TB according
to clinical criteria and treatment with anti-infective medications
was initiated. Given the presence of multiple pulmonary foci, a
high probability of identifying a causative agent was anticipated.
However, repeated sputum smear examinations and sputum DNA tests
for TB were negative. This outcome prompted further diagnostic
evaluation. Elevated levels of the tumor markers neuron-specific
enolase and carcinoembryonic antigen were noted, where a detailed
review of the chest CT scan suggested that a neoplastic process
could not be ruled out. Following comprehensive discussions, the
patient consented to an electronic bronchoscopy with endoscopic
biopsy. The subsequent biopsy pathology results definitively
confirmed the diagnosis of lung adenocarcinoma. Histopathological
examination of the biopsied tissue provided evidence of a tumor
that could have potentially been misdiagnosed as TB.
A review of the relevant literature led to the
following key insights: i) TB is frequently found in the apical and
posterior segments of the upper lobes, in addition to the dorsal
segment of the lower lobes, whereas lung adenocarcinoma is more
frequently located in the anterior segment of the upper lobes, the
lingual lobe and the dorsal segment of the lower lobes (10). ii) Early coughing associated with
lung adenocarcinoma is typically an irritating choking type,
accompanied by small amounts of white foamy sputum. Chest pain is
typically described as a dull, pressure-like sensation that may be
perceived on the same side as the lesion or contralaterally,
presenting as a vague, poorly localized discomfort. By contrast,
the coughing in TB tends to be ‘moist’, with chest pain occurring
on the same side as the lesion, which is persistent, well localized
and progressively worsening (11).
iii) Hemoptysis in TB is commonly moderate to copious and may
resolve rapidly after treatment. Conversely, hemoptysis in lung
adenocarcinoma is characterized by small amounts of recurrent and
persistent bloody sputum. A single, non-contrast chest CT scan may
not provide essential diagnostic cues for clinicians and lacks
specificity. This is particularly true for middle-aged and older
patients with a history of TB, in which the results can easily
mislead the treating physician and result in an incorrect
diagnosis. iv) The rapid development of genetic testing technology
also provides assistance to clinicians in the differential
diagnosis of lung adenocarcinoma and TB (12). A number of studies have shown that
the current gene chip detection system can detect 17 types of
Mycobacterium tuberculosis, where the detection time is
typically 6-8 h and the success rate of Mycobacterium
tuberculosis complex identification reaching 100%. By contrast,
the success rate of non-TB Mycobacterium tuberculosis
identification can reach 95%, which is of great significance for
the rapid identification of Mycobacterium tuberculosis
(13–15). A previous study showed that EGFR is
closely associated with the occurrence and development of lung
adenocarcinoma, where its status can also affect the treatment
effect. Among patients with lung adenocarcinoma, those with a
history of TB are more likely to harbor EGFR gene mutations,
especially those on exon 21 (16).
In instances where a definitive determination cannot be
ascertained, a human EGFR mutation test may be promptly employed
for further discernment. In such cases, it is crucial to actively
pursue additional diagnostic measures, such as chest enhanced CT
and transbronchial biopsy through fiberoptic bronchoscopy to obtain
confirmatory evidence. During clinical practice, extra-pulmonary TB
is also frequently misdiagnosed as cancer in its early stages,
highlighting the diagnostic challenges in both directions (17). It should also be borne in mind that
LC and pulmonary TB differ significantly in terms of treatment and
prognosis, emphasizing the importance of making an early and
accurate distinction (18).
In this case, similarities in clinical symptoms,
such as a cough, expectoration and weight loss, and imaging
findings, between TB and lung adenocarcinoma led to the
misdiagnosis. Therefore, clinicians should consider the possibility
of LC in patients with TB-related pulmonary changes on imaging.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TL, XL and SZ made substantial contributions to the
study conception, collected clinical information and drafted the
manuscript. MH made substantial contributions to the design of the
study, and writing, reviewing and editing the manuscript. All
authors read and approved the final manuscript. TL and SZ confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
This case report was conducted in accordance with
the guidelines of the Declaration of Helsinki and was approved
(approval no. 2023061301) by the Ethics Committee of The Third
People's Hospital of Zhuhai (Zhuhai, China). Written informed
consent was obtained from the patient.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the data and the images in this case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO), . Global
tuberculosis report 2022[EB/OL]. WHO; Geneva: 2022, https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022October
27–2022
|
|
2
|
Silva DR, Valentini DF Jr, Muller AM, de
Almeida CP and Dalcin Pde T: Pulmonary tuberculosis and lung
cancer: Simultaneous and sequential occurrence. J Brasil Pneumol.
39:484–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marais BJ, Gie RP, Schaaf HS, Hesseling
AC, Obihara CC, Starke JJ, Enarson DA, Donald PR and Beyers N: The
natural history of childhood intra-thoracic tuberculosis: A
critical review of literature from the pre-chemotherapy era. Int J
Tuberc Lung Dis. 8:392–402. 2004.PubMed/NCBI
|
|
4
|
Kobashi Y, Fukuda M, Nakata M and Oka M:
Coexistence of metastatic lung cancer and pulmonary tuberculosis
diagnosed in the same cavity. Int J Clin Oncol. 10:366–370. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang HY, Li XL, Yu XS, Guan P, Yin ZH, He
QC and Zhou BS: Facts and fiction of the relationship between
preexisting tuberculosis and lung cancer risk: A systematic review.
Int J Cancer. 125:2936–2944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhatt M, Kant S and Bhaskar R: Pulmonary
tuberculosis as differential diagnosis of lung cancer. South Asian
J Cancer. 1:36–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kabashi-Muçaj S, Dedushi-Hoti K, Shatri J,
Pasha F and Dreshaj D: Pulmonary mucinous adenocarcinoma in the
presence of reactivated tuberculosis: A case report. Radiol Case
Rep. 16:3647–3651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shu CC, Chang SC, Lai YC, Chang CY, Wei YF
and Chen CY: Factors for the early revision of misdiagnosed
tuberculosis to lung cancer: A multicenter study in A
tuberculosis-prevalent area. J Clin Med. 8:7002019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keikha M and Esfahani BN: The relationship
between tuberculosis and lung cancer. Adv Biomed Res. 7:582018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suárez I, Fünger SM, Kröger S, Rademacher
J, Fätkenheuer G and Rybniker J: The diagnosis and treatment of
tuberculosis. Dtsch Arztebl Int. 116:729–735. 2019.PubMed/NCBI
|
|
11
|
Succony L, Rassl DM, Barker AP, McCaughan
FM and Rintoul RC: Adenocarcinoma spectrum lesions of the lung:
Detection, pathology and treatment strategies. Cancer Treat Rev.
99:1022372021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bloom CI, Graham CM, Berry MP, Rozakeas F,
Redford PS, Wang Y, Xu Z, Wilkinson KA, Wilkinson RJ, Kendrick Y,
et al: Transcriptional blood signatures distinguish pulmonary
tuberculosis, pulmonary sarcoidosis, pneumonias and lung cancers.
PLoS One. 8:e706302013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang H, Shangguan Y, Wang H, Ji Z, Shao J,
Zhao R, Wang S, Zheng L, Jin X, Huang S, et al: Multicenter
evaluation of the biochip assay for rapid detection of
mycobacterial isolates in smear-positive specimens. Int J Infect
Dis. 81:46–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang HY, Bang H, Kim S, Koh WJ and Lee H:
Identification of Mycobacterium species in direct respiratory
specimens using reverse blot hybridisation assay. Int J Tuberc Lung
Dis. 18:1114–1120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu L, Jiang G, Wang S, Wang C, Li Q, Yu
H, Zhou Y, Zhao B, Huang H, Xing W, et al: Biochip system for rapid
and accurate identification of mycobacterial species from isolates
and sputum. J Clin Microbiol. 48:3654–3660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma J, Fang B and Zhang CS: Targeted
treatment for advanced lung adenocarcinoma guided by mutations in
EGFR exons 19 and 21 in plasma. China Med and Pharm. 14:5–8+46.
2024.(In Chinese).
|
|
17
|
Aisenberg GM, Jacobson K, Chemaly RF,
Rolston KV, Raad II and Safdar A: Extrapulmonary tuberculosis
active infection misdiagnosed as cancer: Mycobacterium tuberculosis
disease in patients at a comprehensive cancer center (2001–2005).
Cancer. 104:2882–2887. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia H, Zhang L and Wang B: The value of
combination analysis of tumor biomarkers for early differentiating
diagnosis of lung cancer and pulmonary tuberculosis. Ann Clin Lab
Sci. 49:645–649. 2019.PubMed/NCBI
|