Introduction
Lung cancer is one of the most common causes of
cancer-related deaths worldwide, and was estimated to account for
21% of cancer-related deaths in the United States in 2023 (1). Patients with metastatic lung cancer
who are eligible for targeted therapy survive longer than those who
are ineligible (2,3). Most patients with advanced non-small
cell lung cancer (NSCLC) with an oncogenic mutation of epidermal
growth factor receptor (EGFR) benefit significantly from EGFR
tyrosine kinase inhibitors (TKIs); however, patients typically
progress after 9–13 months of treatment with first- or
second-generation EGFR-TKIs (4–8). Among
these patients, the resistant EGFR T790M mutation (p.-Thr790Met) is
found in 50–60% of tumors (9–12). The
third-generation EGFR-TKI osimertinib is effective for treating the
T790M mutation, but disease progression occurs after a median time
of 10.1 months (13,14).
When osimertinib is used as a first-line therapy or
treatment for NSCLC with the resistant T790M mutation in EGFR, the
acquired resistance mechanisms are complex, including EGFR-mediated
T790M C797S mutation, MET amplification, HER2 amplification and
histological transformation; however, the resistance mechanisms in
approximately half of cases remain unclear (15–17).
The treatment options are limited, and platinum-based chemotherapy
is the main treatment option for these patients.
Immunotherapy-based combination therapies are the
standard treatment for EGFR/ALK-negative advanced NSCLC. However,
clinical trials have indicated that patients with EGFR-mutant NSCLC
have a poor response to anti-programmed cell death-1
(PD-1)/programmed cell death ligand-1 (PD-L1) single-agent therapy
(18–23), and immunotherapy-based combinations
may be a potentially effective strategy. Therefore, the present
study evaluated the efficacy of immune checkpoint inhibitors (ICIs)
combined with chemotherapy with or without bevacizumab in patients
with advanced EGFR-mutant NSCLC after TKI failure to inform
clinical practice regarding treatment strategies for these
patients.
Materials and methods
Patients
The medical records of all patients with lung cancer
at Fujian Cancer Hospital (Fuzhou, China) from March 1, 2019 to
July 15, 2023 were reviewed. The eligible patients had EGFR-mutant
advanced lung adenocarcinoma, with an Eastern Cooperative Oncology
Group (ECOG) performance-status score (24) of 0–2 and at least one measurable
tumor. Only patients who: i) experienced treatment failure with
first-/second-generation EGFR-TKIs who were T790M mutation negative
or who experienced treatment failure with a third-generation
EGFR-TKI, ii) received ICIs plus chemotherapy with or without
bevacizumab therapy, and iii) were stages IVA or IVB according to
the 8th TNM classification (25).
were included in the study. The study was approved by the Ethics
Committee of Fujian Cancer Hospital (approval no.
SQ2021-176-01).
Molecular diagnostics
Analysis of EGFR mutations in biopsy specimens or
circulating tumor DNA (ctDNA) from all patients was performed by
amplification-refractory mutation system (ARMS) PCR using an
ADx-ARMS EGFR kit (Amoy Diagnostics Co., Ltd.) or by
next-generation sequencing (NGS) at diagnosis. The EGFR T790M
mutation was detected in biopsy specimens or ctDNA using the
ADx-ARMS EGFR kit or NGS, or by droplet digital PCR (ddPCR) using
an EGFR T790M (S-ddPCR) kit (CB240008; Shanghai Yuanqi Biomedical
Technology Co., Ltd.) when patients failed first-/second-generation
EGFR-TKI treatment. The primer sequences used for ARMS-PCR were as
follows: EGFR 19E746_A750del-S,
5′-GTTAAAATTCCCGTCGCTATCAAGACATCT-3′; EGFR 19E746_S752>A-S,
5′-AGAAAGTTAAAATTCCCGTCGCTATCAAGGCTCC-3′; EGFR-L747_S752del-S,
5′-AATTCCCGTCGCTATCAAGGAACC-3′; EGFR-L747_E749del-S,
5′-GTTAAAATTCCCGTCGCTATCAAGGAAGC-3′; EGFR-19-R,
5′-CACAGCAAAGCAGAAACTCACAT-3′; EGFR-21L858R-S,
5′-GCAGCATGTCAAGATCACAGATTTTGGGCG-3′; EGFR-21L861Q-S,
5′-GATCACAGATTTTGGGCTGGCCAAACA-3′; EGFR-21-R,
5′-GTCAGGAAAATGCTGGCTGACCTAAAG-3′; EGFR 20T790M-S,
5′-CCTCACCTCCACCGTGCARCTCATCAT-3′; EGFR-20T790M-R,
5′-GAGCCAATATTGTCTTTGTGTTCCCG-3′; EGFR-18G719A-FR,
5′-TATACACCGTGCCGAACGCACCGGAGG-3′; EGFR-18G719C-FR,
5′-CCGTGCCGAACGCACCGGAGCA-3′; and EGFR-18-FF,
5′-GGAGCCTCTTACACCCAGTGGAGA-3′. ARMS-PCR was carried out using the
following thermocycling conditions: Incubation at 95°C for 10 min,
followed by 15 cycles of 95°C for 40 sec, 64°C for 40 sec and 72°C
for 30 sec, and then 28 cycles of 93°C for 40 sec, 60°C for 45 sec
and 72°C for 30 sec. The primer sequences used for ddPCR were:
T790M-F, 5′-GCCGCCTGCTGGCAT-3′ and T790M-R,
5′-TGTGTTCCCGGACATAGTCCAG-3′; reference gene primer-F,
5′-ACTACTTGGAGGAGGACCGTCGC-3′ and reference gene primer-R,
5′-TTCTGCATGGTATTCTTTCTC-3′. ddPCR was carried out using the
following thermocycling conditions: Incubation at 95°C for 10 min,
followed by 40 cycles of 94°C for 15 sec, 58°C for 60 sec and 98°C
for 10 min, then a 4°C hold. A total of 18 specimens underwent NGS
performed by Xiamen Spacegen Co., Ltd., including 7 specimens at
diagnosis and 11 specimens after the development of
first-/second-generation EGFR-TKI resistance. The PD-L1 tumor
proportion score (TPS) was measured by immunohistochemistry
(Dako28-8; Agilent Technologies, Inc.) in 17 patients after
progression on EGFR-TKIs. The immunohistochemistry of PD-L1
expression was carried out using the following procedure: 5-µm
sections were cut from each biopsy specimen. Tissue sections were
incubated at 60°C overnight, and incubated 40°C for 1 h, followed
by separation with xylene and ethanol. Tissue sections were treated
with PBS at 37°C for 12 h and subjected to IHC staining. Antigen
repair was performed by water bath method at 97°C for 20 min, and
the repair solution was EnVision Flex TRS(pH 6.1); the antibody of
PD-L1 (28–8) was diluted at 1:40 to 1:20, used at
room temperature for 20 min. EnVision Flex+ was applied for 20 min,
with CuSO4 enhanced DAB color development. A Dako
AutoStainer Link 48 platform (Agilent Technologies, Inc.) was used
for detection.
Treatment regimens and response
evaluation
Enrolled patients had received PD-1 inhibitors every
3 weeks, including 200 mg camrelizumab (Jiangsu Hengrui Medicine
Co., Ltd.), 200 mg tislelizumab (BeiGene, Ltd.) and 200 or 240 mg
toripalimab (Shanghai Junshi Biosciences Co., Ltd.) plus
chemotherapy with or without bevacizumab. RECIST version 1.1 was
used to evaluate the treatment responses of the patients (26). Progression-free survival (PFS)
represented the length of survival from treatment with PD-1
inhibitor and chemotherapy/bevacizumab to progression, and overall
survival (OS) represented the survival from treatment with PD-1
inhibitor to death. The response to PD-1 inhibitor-based therapy
was defined as a complete response (CR), partial response (PR),
stable disease (SD) or progressive disease (PD) during the course
of therapy. The overall response rate (ORR) was defined as the
percentage of patients with a CR or PR: ORR (%)=(CR + PR)/total
number of patients ×100. The disease control rate (DCR) was defined
as the percentage of patients with a CR, PR or SD: DCR (%)=(CR + PR
+ SD)/total number of patients ×100. Adverse events (AEs) were
graded according to the National Cancer Institute Common
Terminology Criteria for Adverse Events (NCICTC-AE) v5.0 (27).
Statistical analysis
Kaplan-Meier analysis and the log-rank test were
used to compare differences in survival. The ORR and DCR of
different subgroups were compared using Fisher's exact tests. In
the tests, two-sided P<0.05 was considered statistically
significant. SPSS version 24.0 (IBM Corp.) statistical software was
used to perform all the statistical analyses.
Results
Patient population and
characteristics
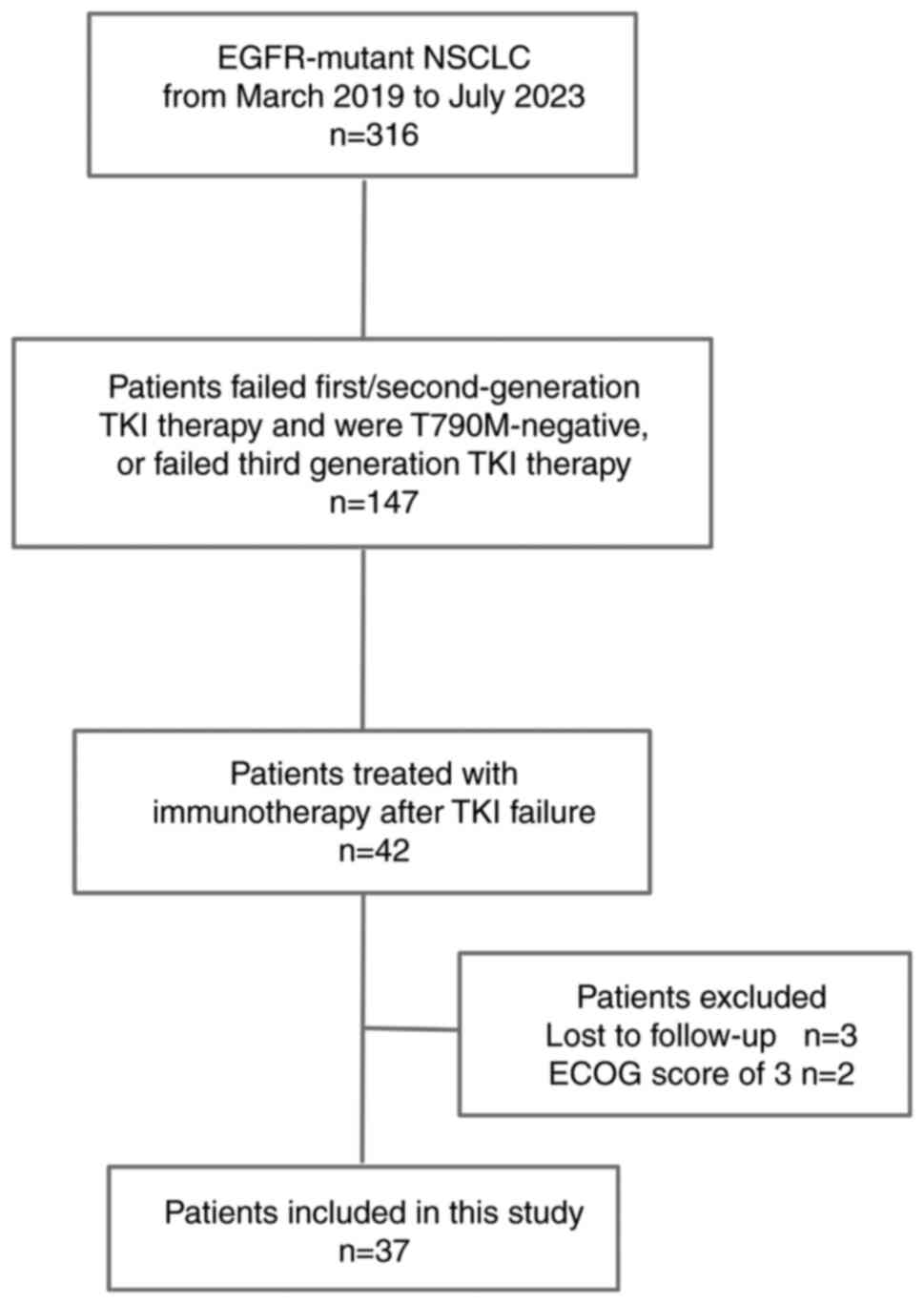
There were 316 patients with EGFR-mutant advanced
NSCLC who were treated with EGFR-TKIs from March 1, 2019 to July
15, 2023, of whom 147 had experienced failure when previously
treated with TKIs. These included 42 patients who were treated with
PD-1 inhibitors after TKI failure. However, 2 patients were lost to
follow-up and 3 patients had a ECOG score of 3. Finally, a total of
37 patients with EGFR-mutant advanced NSCLC were included in the
study (Fig. 1). The baseline
clinicopathological characteristics of these patients are
summarized in Table I. The median
age was 56 years (range, 32–72 years). A total of 19 patients were
female. Most (n=29) patients had an ECOG score of 0 or 1. The EGFR
mutation subtypes were EGFR exon 19 deletion mutation (n=18), EGFR
exon 21 L858R mutation (n=15) and rare double EGFR rare mutations
G719X/L861Q (n=2), G719A/S861I (n=1) and G719X/S861I (n=1). A total
of 25 patients received first-generation EGFR-TKIs as first-line
treatment, 14 patients acquired the T790M mutation (Table SI) when the disease progressed and
were treated with osimertinib, and 12 patients received osimertinib
as first-line treatment. The total duration of previous TKI
treatment was ≤12 months for 20 patients and >12 months in the
remaining 17 patients.
 | Table I.Characteristics of all patients and
clinical response to immunotherapy. |
Table I.
Characteristics of all patients and
clinical response to immunotherapy.
|
|
| ORR | DCR | mPFS |
|---|
|
|
|
|
|
|
|---|
|
Characteristics | N (%) | n/N (%) | P-value | n/N (%) | P-value | Months | 95% CI | P-value |
|---|
| Sex |
|
| 0.170 |
| 1.000 |
|
| 0.929 |
|
Male | 18 (48.6) | 8/18 (44.4) |
| 15/18 (83.3) |
| 5.2 | 2.665–7.735 |
|
|
Female | 19 (51.4) | 4/19 (21.1) |
| 15/19 (78.9) |
| 5.2 | 3.984–6.416 |
|
| Age, years |
|
| 0.274 |
| 1.000 |
|
| 0.315 |
|
>60 | 13 (35.1) | 6/13 (46.2) |
| 11/13 (84.6) |
| 4.5 | 3.080–5.720 |
|
|
≤60 | 24 (64.9) | 6/24 (25.0) |
| 19/24 (79.2) |
| 5.4 | 3.245–8.102 |
|
| ECOG-PS |
|
| 1.000 |
| 0.156 |
|
| 0.006 |
|
0-1 | 29 (78.4) | 10/29 (34.5) |
| 25/29 (86.2) |
| 5.4 | 2.735–8.065 |
|
| 2 | 8 (21.6) | 2/8 (25.0) |
| 5/8 (62.5) |
| 2.4 | 0.321–4.479 |
|
| EGFR mutations |
|
| 0.818 |
| 0.223 |
|
| 0.461 |
|
19del | 18 (48.7) | 6/18 (33.3) |
| 15/18 (83.3) |
| 4.6 | 2.937–6.2637 |
|
|
21L858R | 15 (40.5) | 5/15 (33.3) |
| 11/15 (73.3) |
| 4.5 | 1.452–7.548 |
|
|
Others | 4 (10.8) | 1/4 (25.0) |
| 4/4 (100) |
| 5.2 | 0 |
|
| TNM stage |
|
| 0.306 |
| 0.007 |
|
| 0.083 |
|
IVA | 14 (37.8) | 3/14 (21.4) |
| 8/14 (57.1) |
| 3.5 | 2.100–4.300 |
|
|
IVB | 23 (62.2) | 9/23 (39.1) |
| 22/23 (95.7) |
| 5.4 | 2.894–8.506 |
|
| Brain
metastases |
|
| 1.000 |
| 0.308 |
|
| 0.734 |
|
Present | 8 (21.6) | 2/8 (25.0) |
| 8/8 (100) |
| 5.2 | 3.404–6.996 |
|
|
Absent | 29 (78.4) | 10/29 (34.5) |
| 22/29 (75.9) |
| 5.2 | 2.817–7.583 |
|
| T790M status
(post-TKIs) |
|
| 0.306 |
| 0.390 |
|
| 0.041 |
|
Negative | 23 (62.2) | 9/23 (39.1) |
| 20/23 (87.0) |
| 6.2 | 3.265–9.135 |
|
|
Positive | 14 (37.8) | 3/14 (21.4) |
| 10/14 (71.4) |
| 4.4 | 2.923–5.877 |
|
| Prior
EGFR-TKIs |
|
| 0.239 |
| 0.670 |
|
|
|
|
First-generation | 11 (29.7) | 3/11 (27.3) |
| 10/11 (90.9) |
| 7.1 | 3.863–10.337 | 0.068 |
|
First/third-generation | 14 (37.8) | 3/14 (21.4) |
| 10/14 (71.4) |
| 4.4 | 2.923–5.877 |
|
|
Third-generation | 12 (32.5) | 6/12 (50.0) |
| 10/12 (83.3) |
| 5.2 | 2.314–8.086 |
|
| Total duration of
previous |
|
| 1.000 |
| 1.000 |
|
| 0.069 |
| TKIs, months |
|
|
|
|
|
|
|
|
|
≤12 | 20 (54.1) | 6/20 (30.0) |
| 16/20 (80.0) |
| 4.4 | 2.209–6.591 |
|
|
>12 | 17 (45.9) | 6/17 (35.3) |
| 14/17 (82.4) |
| 5.4 | 1.197–9.603 |
|
| Line of ICI |
|
| 0.007 |
| 0.016 |
|
| <0.001 |
|
Front-line | 26 (70.3) | 12/26 (46.2) |
| 24/26 (92.3) |
| 6.2 | 2.655–9.745 |
|
|
Late-line | 11 (29.7) | 0/11 (0) |
| 6/11 (54.5) |
| 2.4 | 0.309–4.491 |
|
| Combination
treatment strategy |
|
| 0.036 |
| 0.028 |
|
| 0.681 |
| ICI +
C | 22 (59.5) | 4/22 (18.2) |
| 15/22 (68.1) |
| 4.4 | 1.826–6.974 |
|
| ICI + C
+ A | 15 (40.5) | 8/15 (53.3) |
| 15/15 (100) |
| 6.2 | 3.279–9.121 |
|
Treatment characteristics
There were 26 patients who immediately received PD-1
inhibitors after TKI failure, which was defined as front-line
therapy, and 11 patients who received late-line PD-1 inhibitor
therapy because they had received other systemic treatments between
EGFR-TKIs and ICI therapy. Regarding the combination treatment
strategy, 22 patients were treated with PD-1 inhibitors plus
chemotherapy, and the remaining 15 patients were treated with PD-1
inhibitors plus chemotherapy and bevacizumab (Table I).
Overall clinical outcomes
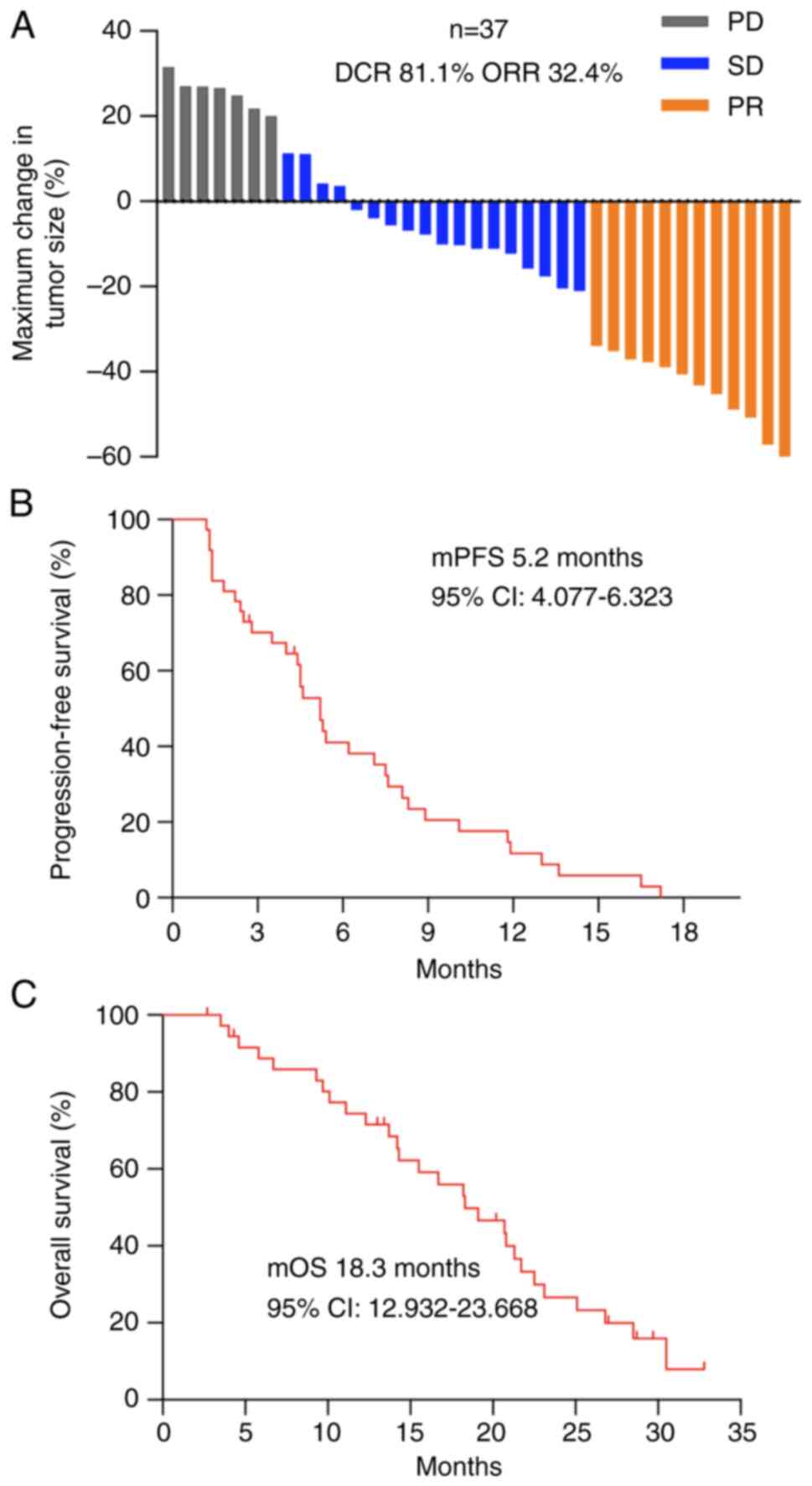
At the last follow-up on January 15, 2024, the
median follow-up time was 13.4 months (range, 2.7–32.8 months). The
median PFS (mPFS) of all patients was 5.2 months (95% CI,
4.077–6.323 months; Fig. 2B), and
the median OS (mOS) was 18.3 months (95% CI, 12.932–23.668 months;
Fig. 2C). Disease progression
occurred in 94.6% (35/37) of patients, and 75.7% (28/37) of the
patients died. Overall, 32.4% (12/37), 48.6% (18/37) and 18.9%
(7/37) of the patients exhibited a PR, SD or PD respectively, with
a DCR of 81.1% and an ORR of 32.4% (Fig. 2A).
Survival outcomes in selected patient
subgroups
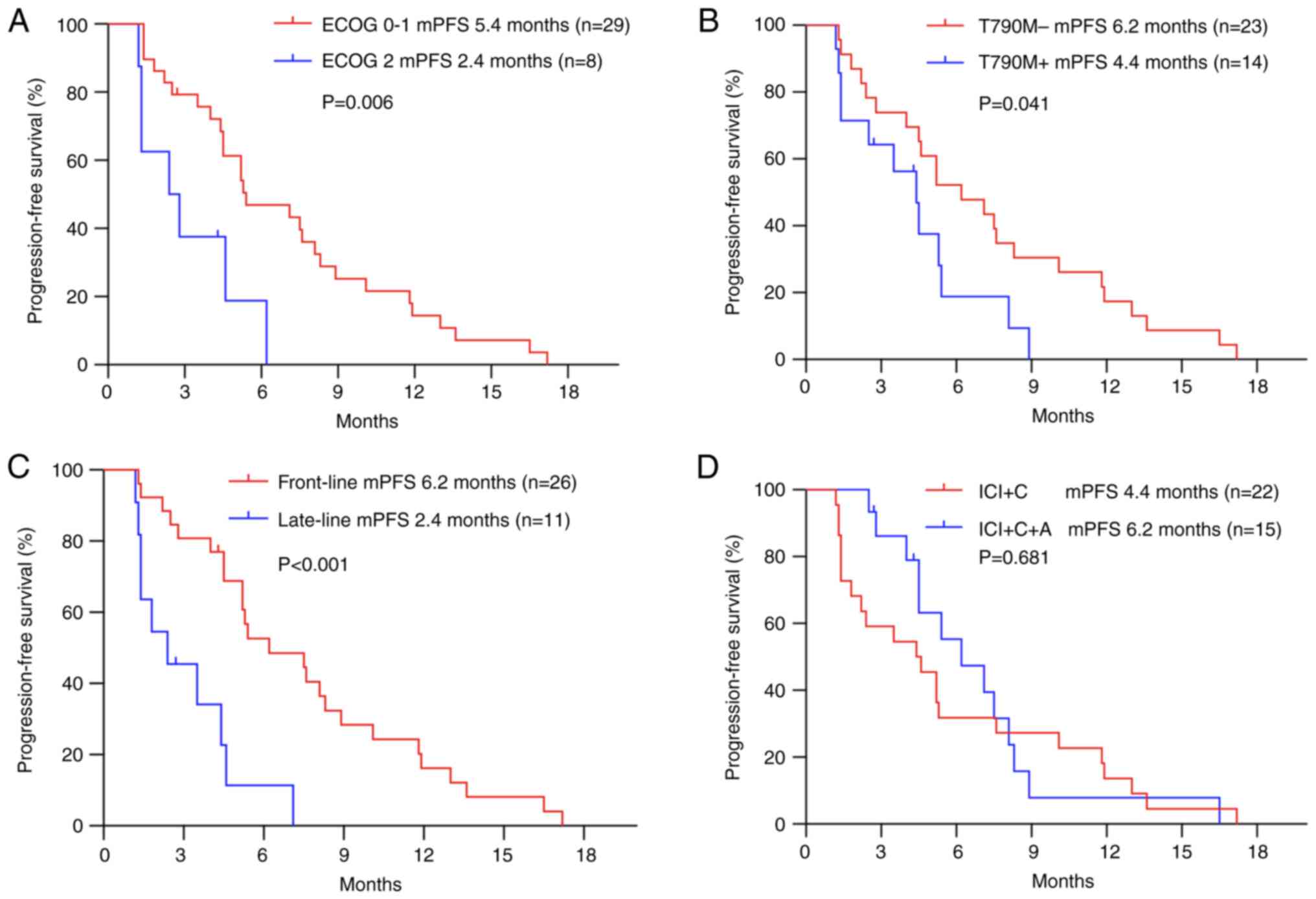
Subgroup analyses based on all 37 patients revealed
that patients with an ECOG-PS score of 0 or 1 had a similar ORR but
longer PFS than those with an ECOG-PS score of 2 (ORR, 34.5 vs.
25.0%, P=1.000; mPFS, 5.4 vs. 2.4 months, P=0.006; Table I, Fig.
3A). The analysis revealed a PFS improvement in EGFR
T790M-negative patients, with a median PFS of 6.2 months (95% CI,
3.265–9.135 months), which was longer than that in EGFR
T790M-positive patients (4.4 months; 95% CI, 2.923–5.877 months)
(P=0.041; Table I; Fig. 3B). The patients treated with
ICI-based therapy as front-line therapy showed a higher ORR and
longer PFS than those treated with ICI-based therapy as late-line
therapy (ORR, 46.2 vs. 0%, P=0.007; mPFS, 6.2 vs. 2.4 months,
P<0.001; Table I; Fig. 3C). In the subgroups based on
different types of EGFR mutations, TNM stage, the presence or
absence of brain metastases, the total duration of previous TKI
treatment and the type of ICI-based therapy (with or without
bevacizumab), no significant differences in PFS were observed
(Table I; Fig. 3D). However, the ORR and DCR of
patients treated with ICIs plus chemotherapy and bevacizumab were
higher than those of patients treated with ICIs plus chemotherapy
(ORR, 53.3 vs. 18.2%, P=0.036; DCR, 100 vs. 68.1%, P=0.028;
Table I). Cox multivariate
regression analysis revealed that the ECOG-PS score, EGFR T790M
status post-EGFR-TKIs and timing of immunotherapy were independent
predictors of PFS in patients treated with immunotherapy-based
combinations (P<0.05; Table
II).
 | Table II.Univariate and multivariable analyses
of covariables associated with progression-free survival in
patients treated with immunotherapy. |
Table II.
Univariate and multivariable analyses
of covariables associated with progression-free survival in
patients treated with immunotherapy.
|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
|
Characteristics | N | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| ECOG-PS score |
|
|
|
|
|
|
0-1 | 29 | Ref. | 0.010 | Ref. | 0.017 |
| 2 | 8 | 3.338
(1.335–8.347) |
| 3.328
(1.245–8.896) |
|
| T790M status
post-TKIs |
|
|
|
|
|
|
Negative | 24 | Ref. | 0.048 | Ref. | 0.021 |
|
Positive | 13 | 1.987
(0.918–4.298) |
| 2.166
(1.0064.662) |
|
| Line of
immunotherapy |
|
|
|
|
|
|
Front-line | 26 | Ref. | 0.001 | Ref. | 0.004 |
|
Late-line | 11 | 4.465
(1.876–0.628) |
| 2.113
(1.370–3.260) |
|
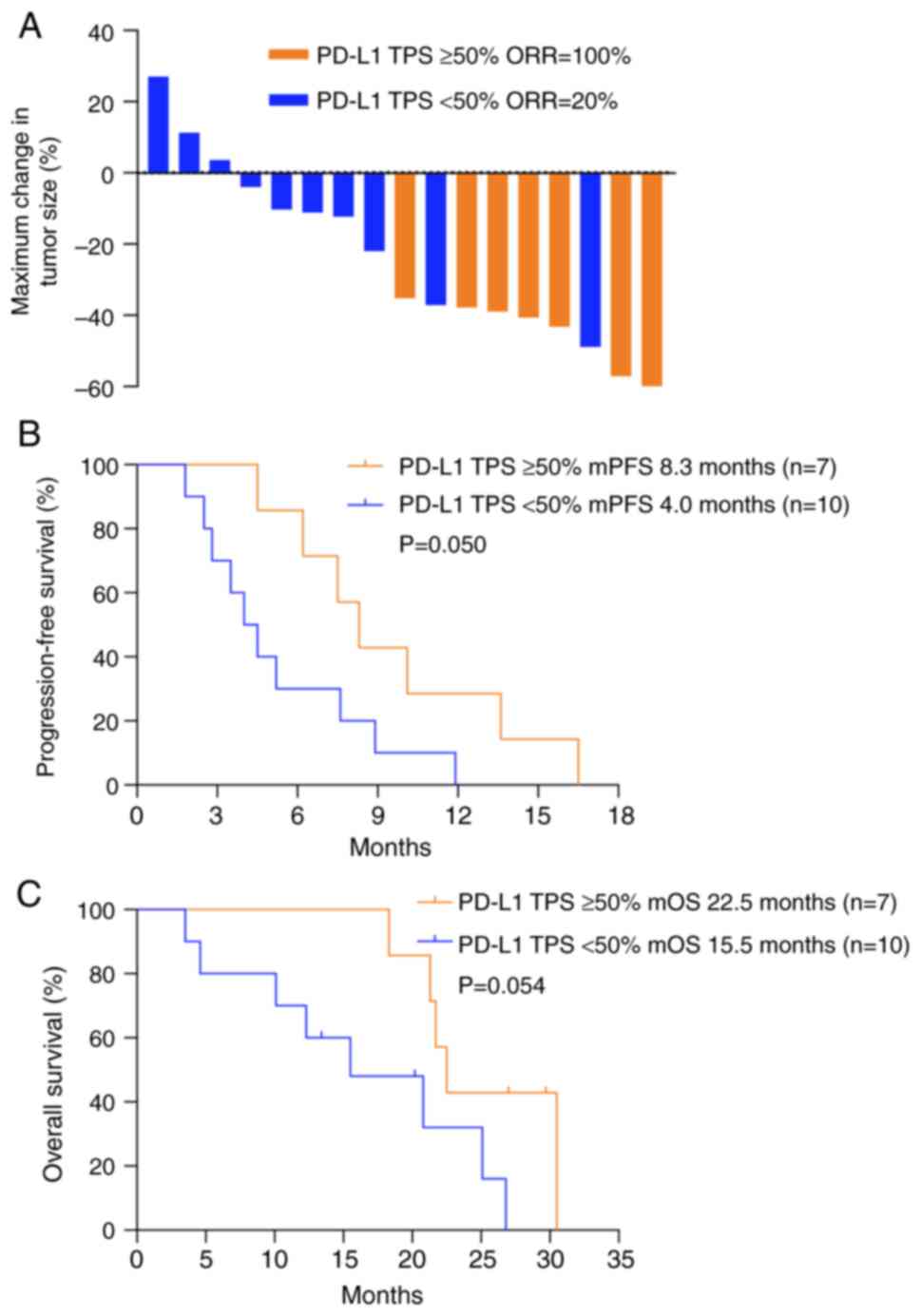
Efficacy according to PD-L1 TPS
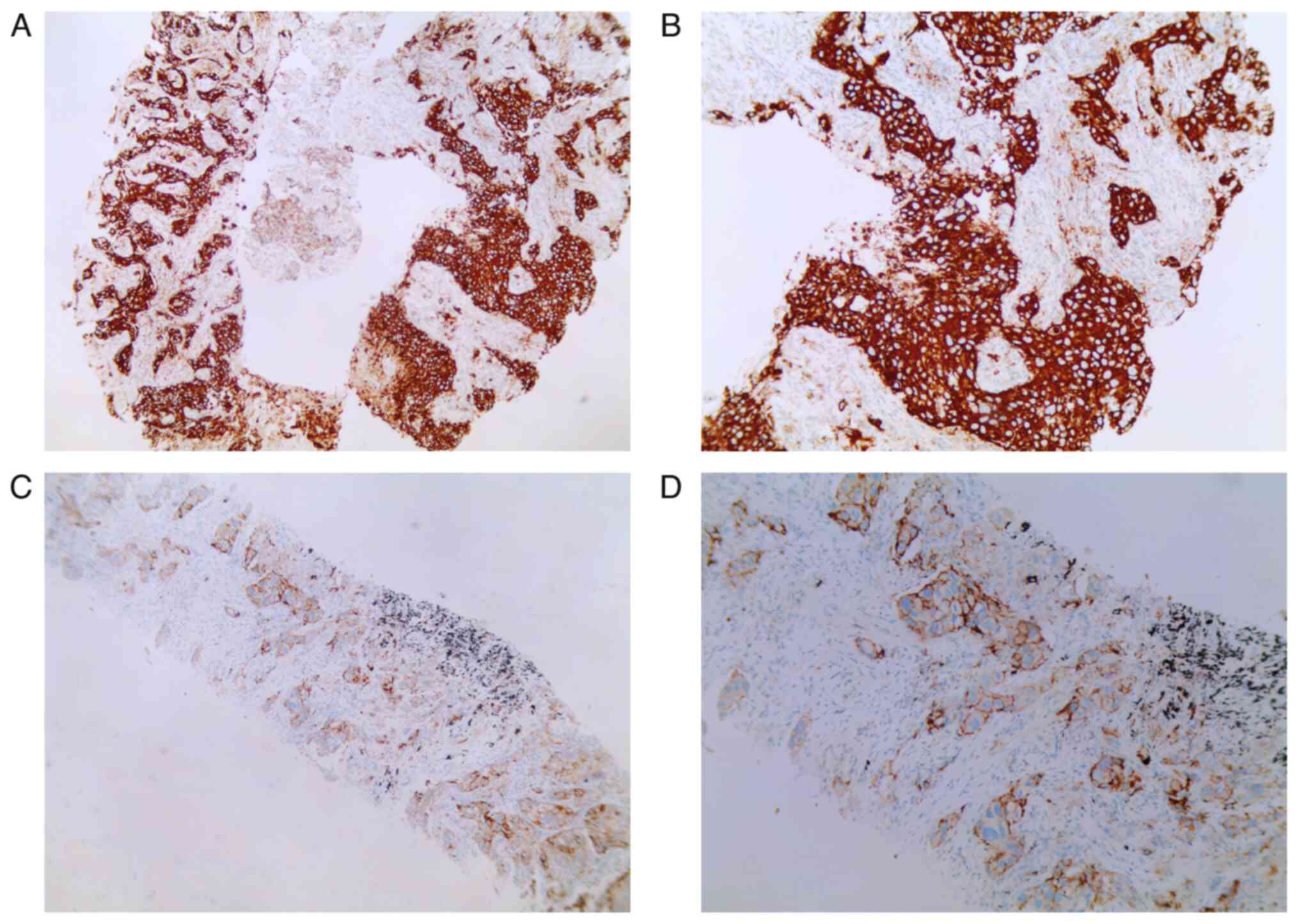
The PD-L1 TPS was measured in 45.9% (17/37) of
patients with re-biopsy specimens post-EGFR-TKI treatment (Table SII). Four patients were negative
for the PD-L1 TPS and 7 patients had a PD-L1 TPS ≥50% (Fig. 4). In these 17 patients, the optimal
efficacy was achieved in patients with a PD-L1 TPS ≥50%, with an
ORR of 100%, while patients with a PD-L1 TPS <50% had an ORR of
only 20% (Fig. 5A). The mPFS was
8.3 months (95% CI, 6.247–10.353 months) for patients with a PD-L1
TPS ≥50%, which was longer than that for patients with a PD-L1 TPS
<50% (median PFS, 4.0 months; 95% CI, 2.450–5.550 months)
(P=0.050; Fig. 5B). In addition,
the mOS was 22.5 months for patients with a PD-L1 TPS ≥50%, which
tended to be prolonged compared with that of patients with a PD-L1
TPS <50% (P=0.054; Fig. 5C).
Safety
The median number of PD-1 inhibitor cycles was 6
(range, 1–35). AEs associated with any component of treatment
occurred in 28/37 (75.7%) patients. However, no mortalities
associated with the treatment occurred. The grade 3 or 4 AEs
associated with the treatment were leukopenia in 4/37 (10.8%)
patients, as well as fatigue, rash and pneumonitis, each of which
occurred in 1/37 (2.7%) of patients (Table III). One patient discontinued
immunotherapy due to grade 3 fatigue, and 4 patients discontinued
immunotherapy due to grade 2/3 pneumonitis.
 | Table III.Treatment-related adverse events in
the 37 patients. |
Table III.
Treatment-related adverse events in
the 37 patients.
|
| Patients, n
(%) |
|---|
|
|
|
|---|
| Event | All grades | Grade ≥3 |
|---|
| Leukopenia | 13 (35.1) | 4 (10.8) |
| Fatigue | 7 (18.9) | 1 (2.7) |
| Rash | 6 (17.1) | 1 (2.7) |
| Nausea | 3 (11.5) | - |
| ALT elevation | 5 (13.5) | - |
| AST elevation | 5 (13.5) | - |
| Pneumonitis | 4 (10.8) | 1 (2.7) |
| Capillary
proliferation | 1 (2.7) | - |
| Hypertension | 1 (2.7) | - |
| Proteinuria | 1 (2.7) | - |
Discussion
PD-1/PD-L1 inhibitors have become a standard
treatment option for EGFR/ALK-negative advanced NSCLC. The
potential of immunotherapy in patients with EGFR mutations, who
account for ~50% of Asian patients with NSCLC (28), requires further exploration. In the
present study, the effect and safety of PD-1 inhibitors combined
with chemotherapy with or without bevacizumab were evaluated. The
results showed that the mPFS of patients receiving PD-1
inhibitor-based combination therapy was 5.2 months, which is
similar to that of patients receiving platinum-based double drug
chemotherapy as a first-line treatment in advanced NSCLC but longer
than that of immune monotherapy reported in previous studies
(21,23,29).
Data from a multicenter phase II trial of the PD-1 inhibitor
toripalimab plus chemotherapy showed an mPFS of 7.0 months when
used as a second-line treatment in patients with EGFR-mutant
advanced NSCLC after the failure of prior EGFR-TKIs (30). However, in real-world settings, the
mPFS was found to be ~5 months for patients treated with these
immunotherapy-based combinations (31–35).
Unfortunately, the outcome of patients with EGFR-mutant tumors in
the IMpower130, CheckMate-722 and KEYNOTE-789 clinical trials also
did not suggest an advantage for immunotherapy combined with
chemotherapy in TKI-refractory EGFR-mutant NSCLC (36–38).
Therefore, the interplay between the tumor immune microenvironment
(TME), PD-L1 expression in tumors, tumor mutation burden (TMB) and
vascular endothelial growth factor (VEGF) receptor inhibitors may
be affecting the efficacy of treatment.
The precise mechanisms underlying the unsatisfactory
response to immunotherapy in patients with EGFR-mutant NSCLC remain
unclear. The generation of tumor neoantigens, antigen presentation
and identification, and activation of T cells have been suggested
to impact the effect of immunotherapy (39). The low TMB in patients with EGFR
mutations who do non-smoke has been suggested as a potential reason
for the poor effect of immunotherapy (40). In addition, low PD-L1 expression may
impact the efficacy of immunotherapy in patients with EGFR
mutations (19,21). Although chemotherapy can kill tumor
cells, increase tumor neoantigen levels and improve the efficacy of
immunotherapy (41,42), no survival benefit was observed in
patients treated with immunotherapy combined with chemotherapy in
previous studies (36,38). Based on the approach of combining
immunotherapy with other treatments, the final exploratory analyses
of the IMpower 150 trial showed a survival benefit in patient
subgroups with EGFR mutations when treated with a combination of
atezolizumab, bevacizumab and chemotherapy, even in those patients
who had previously been treated with TKIs (43,44).
In addition, the ORIENT-31 trial reported the successful use of a
PD-1 inhibitor with bevacizumab biosimilar plus chemotherapy
(45). However, the use of a PD-1
inhibitor combined with chemotherapy and bevacizumab did not show a
clear advantage on mPFS compared with the use of a PD-1 inhibitor
combined with chemotherapy alone in the present study, although the
ORR and DCR were improved. VEGFs can regulate the TME and stimulate
regulatory T cells, thereby improving the efficacy of immunotherapy
(46). Therefore, the combination
of chemotherapy and immunotherapy with antiangiogenic agents may be
a promising treatment strategy for EGFR-mutant advanced NSCLC.
However, further clinical studies are necessary to confirm
this.
In the present study, a subgroup analysis was
performed to evaluate the patients who were more likely to benefit
from immunotherapy-based combinations. Patients with an ECOG-PS
score of 0 or 1 were found to have an improved response to PD-1
inhibitor-based combination therapy (mPFS, 5.4 months) compared
with those with an ECOG-PS score of 2, and EOCG-PS was identified
as an independent predictor of PFS in patients treated with
immunotherapy-based combinations (P=0.017). T790M mutation status
was identified as another independent predictor of the efficacy of
immunotherapy-based combinations (P=0.021) in the present study.
The T790M-negative patients had an mPFS of 6.2 months, which was
longer than the 4.4 months of T790M-positive patients (P=0.041).
One possible explanation for this is that T790M-negative tumors are
characterized by high PD-L1 expression and a high TMB.
Unfortunately, only some of the patients in our study were suitable
for PD-L1 testing, and none of the patients underwent TMB testing
because of insufficient specimens or the expense of testing after
EGFR-TKI failure. A study by Haratani et al (47) indicated that patients with
T790M-negative tumors are more likely than those with
T790M-positive tumors to benefit from nivolumab after EGFR-TKIs,
and suggested that this may be due to high PD-L1 expression in
T790M-negative tumors. Similar results were also reported in a
IMMUNOTARGET registry study (48).
However, prospective clinical trials are required to verify these
findings.
The TME contains immune cells and immune factors,
and is a key factor affecting the efficacy of immunotherapy. PD-L1
expression and the TME dynamically change with tumor treatment
(49). Regrettably, information on
the TME was lacking in the present study. However, in a previous
study of a lung cancer model with EGFR mutations, it was observed
that as EGFR-TKI resistance developed, immune effector cells
gradually disappeared, and myeloid-derived suppressor cells
continued to proliferate with subsequent increases in IL-10 and
chemokine (C-C motif) ligand 2 levels (50). Therefore, the timing of
immunotherapy may impact its efficacy. In the present study,
front-line PD-1 inhibitor-based combination therapy was associated
with a longer PFS than late-line therapy following TKI failure
(mPFS, 6.2 vs. 2.4 months; P<0.001). As NSCLC progresses, the
TME becomes more complex and less conducive to immunotherapy.
Consequently, front-line immunotherapy-based combinations could be
recommended for clinical use after TKI failure.
Currently, biomarkers for the efficacy of
immunotherapy in EGFR-mutant NSCLC have not been clearly
identified. However, PD-L1 is the most important predictor of the
efficacy of immunotherapy in patients with NSCLC (51,52).
Preclinical evidence suggests that EGFR mutations can upregulate
PD-L1 expression (53,54), and EGFR-TKIs may even increase PD-L1
expression in EGFR-mutant tumors (55,56).
However, some studies have reported opposite findings (57,58).
In the present study, PD-L1 expression was evaluated in 17
patients. Of these, the 7 patients with a PD-L1 TPS ≥50% had an ORR
of 100% and a median PFS of 8.3 months, which were improved
compared with those of patients with a PD-L1 TPS <50%. The
Keynote-010 study revealed the preliminary efficacy of the PD-1
inhibitor pembrolizumab in patients with EGFR-mutant,
PD-L1-positive NSCLC (20). In
addition, in the ATLANTIC study, durvalumab exhibited greater
clinical activity in patients with EGFR-mutant and heavily
pretreated NSCLC with ≥25% PD-L1 expression than in those with
<25% PD-L1 expression (59).
Similarly, the ATTLAS trial showed that patients with EGFR-mutant
NSCLC who were PD-L1 positive could benefit from immunotherapy plus
chemotherapy and bevacizumab, and that patients with high PD-L1
expression had a longer PFS (60).
Further investigations are required to verify the utility of PD-L1
expression as a predictive biomarker of treatment in patients with
EGFR mutations.
In conclusion, the treatment options for EGFR-mutant
advanced NSCLC after TKI failure are limited. Immunotherapy-based
combinations may be a potentially effective strategy, and treatment
outcomes are influenced by the TME, the TMB, PD-L1 expression in
tumors and prior TKI treatment. In the present study,
immunotherapy-based combination therapy was the recommended
treatment option for patients with EGFR-mutant advanced NSCLC after
TKI failure. In addition, ECOG-PS scores of 0 or 1,
T790M-negativity or high PD-L1 expression indicated an improved
prognosis for patients with EGFR-mutant NSCLC who experienced tumor
progression following EGFR-TKI treatment. Immunotherapy and
chemotherapy in combination with antiangiogenic agents appears to
be a promising combination therapy for these patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Fujian Province (grant no. 2022J011047), High-level
Talent Development Program (grant no. 2024YNG11) and the Innovation
of Science and Technology, Fujian Province (grant no.
2021Y0056).
Availability of data and materials
The original NGS data generated in the present study
may be found in the SRA under accession number PRJNA1092050. All
other data generated in the present study may be requested from the
corresponding author.
Authors' contributions
ML and ZH designed the study. CL, JL, SC and LW
contributed to data collection and investigation. ML and CL wrote
the original draft of the manuscript. ML and ZH confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Fujian Cancer Hospital (approval no.
SQ2021-176-01). Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References Uncategorized References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall Survival with Osimertinib in
Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin JJ, Cardarella S, Lydon CA, Dahlberg
SE, Jackman DM, Jänne PA and Johnson BE: Five-Year Survival in
EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs.
J Thorac Oncol. 11:556–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi Y, Zhang L, Liu X, Zhou C, Zhang L,
Zhang S, Wang D, Li Q, Qin S, Hu C, et al: Icotinib versus
gefitinib in previously treated advanced non-small-cell lung cancer
(ICOGEN): A randomised, double-blind phase 3 non-inferiority trial.
Lancet Oncol. 14:953–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park K, Tan EH, O'Byrne K, Zhang L, Boyer
M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, et al: Afatinib versus
gefitinib as first-line treatment of patients with EGFR
mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase
2B, open-label, randomised controlled trial. Lancet Oncol.
17:577–589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kobayashi S, Boggon TJ, Dayaram T, Jänne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yun CH, Mengwasser KE, Toms AV, Woo MS,
Greulich H, Wong KK, Meyerson M and Eck MJ: The T790M mutation in
EGFR kinase causes drug resistance by increasing the affinity for
ATP. Proc Natl Acad Sci USA. 105:2070–2075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gainor JF and Shaw AT: Emerging paradigms
in the development of resistance to tyrosine kinase inhibitors in
lung cancer. J Clin Oncol. 31:3987–3996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu SG and Shih JY: Management of acquired
resistance to EGFR TKI-targeted therapy in advanced non-small cell
lung cancer. Mol Cancer. 17:382018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Janne PA, Yang JC, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung
Cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oxnard GR, Hu Y, Mileham KF, Husain H,
Costa DB, Tracy P, Feeney N, Sholl LM, Dahlberg SE, Redig AJ, et
al: Assessment of resistance mechanisms and clinical implications
in patients WithEGFRT790M-Positive lung cancer and acquired
resistance to osimertinib. JAMA Oncol. 4:1527–1534. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Tsui ST, Liu C, Song Y and Liu D:
EGFR C797S mutation mediates resistance to third-generation
inhibitors in T790M-positive non-small cell lung cancer. J Hematol
Oncol. 9:592016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leonetti A, Sharma S, Minari R, Perego P,
Giovannetti E and Tiseo M: Resistance mechanisms to osimertinib in
EGFR-mutated non-small cell lung cancer. Br J Cancer. 121:725–737.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lisberg A, Cummings A, Goldman JW,
Bornazyan K, Reese N, Wang T, Coluzzi P, Ledezma B, Mendenhall M,
Hunt J, et al: A Phase II Study of Pembrolizumab in EGFR-Mutant,
PD-L1+, Tyrosine Kinase Inhibitor Naive Patients With Advanced
NSCLC. J Thorac Oncol. 13:1138–1145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell
Lung Cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): a phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee CK, Man J, Lord S, Cooper W, Links M,
Gebski V, Herbst RS, Gralla RJ, Mok T and Yang JC: Clinical and
molecular characteristics associated with survival among patients
treated with checkpoint inhibitors for advanced non-small cell lung
carcinoma: A systematic review and meta-analysis. JAMA Oncol.
4:210–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gettinger S, Rizvi NA, Chow LQ, Borghaei
H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman
JW, et al: Nivolumab monotherapy for first-line treatment of
advanced non-small-cell lung cancer. J Clin Oncol. 34:2980–2987.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Azam F, Latif MF, Farooq A, Tirmazy SH,
AlShahrani S, Bashir S and Bukhari N: Performance Status Assessment
by Using ECOG (Eastern Cooperative Oncology Group) score for cancer
patients by oncology healthcare professionals. Case Rep Oncol.
12:728–736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lim W, Ridge CA, Nicholson AG and
Mirsadraee S: The 8(th) lung cancer TNM classification and clinical
staging system: Review of the changes and clinical implications.
Quant Imaging Med Surg. 8:709–718. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-Version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi Y, Li J, Zhang S, Wang M, Yang S, Li
N, Wu G, Liu W, Liao G, Cai K, et al: Molecular epidemiology of
EGFR mutations in asian patients with advanced non-small-cell lung
cancer of adenocarcinoma Histology-Mainland China subset analysis
of the PIONEER study. PLoS One. 10:e01435152015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gandhi L, Rodriguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang T, Wang P, Zhang J, Zhao Y, Zhou J,
Fan Y, Shu Y, Liu X, Zhang H, He J, et al: Toripalimab plus
chemotherapy as second-line treatment in previously EGFR-TKI
treated patients with EGFR-mutant-advanced NSCLC: a multicenter
phase-II trial. Signal Transduct Target Ther. 6:3552021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu R, Zhao Z, Shi Y, Shi M, Xia G, Yu S
and Feng J: Immune checkpoint inhibitors combined with
chemotherapy/bevacizumab therapy for patients with advanced lung
cancer and heavily treated with EGFR mutation: A retrospective
analysis. J Thorac Dis. 13:2959–2967. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang L, Hao X, Hu X, Wang Z, Yang K, Mi Y,
Yang Y, Xu H, Yang G and Wang Y: Superior efficacy of
immunotherapy-based combinations over monotherapy for EGFR-mutant
non-small cell lung cancer acquired resistance to EGFR-TKIs. Thorac
Cancer. 11:3501–3509. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lam TC, Tsang KC, Choi HC, Lee VH, Lam KO,
Chiang CL, So TH, Chan WW, Nyaw SF, Lim F, et al: Combination
atezolizumab, bevacizumab, pemetrexed and carboplatin for
metastatic EGFR mutated NSCLC after TKI failure. Lung Cancer.
159:18–26. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen CI, Chao HS, Shiao TH, Chiang CL,
Huang HC, Luo YH, Chiu CH and Chen YM: Comparison of the outcome
between immunotherapy alone or in combination with chemotherapy in
EGFR-mutant non-small cell lung cancer. Sci Rep. 11:161222021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tian T, Yu M, Li J, Jiang M, Ma D, Tang S,
Lin Z, Chen L, Gong Y, Zhu J, et al: Front-Line ICI-Based
combination therapy Post-TKI resistance may improve survival in
NSCLC patients with EGFR mutation. Front Oncol. 11:7390902021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mok T, Nakagawa K, Park K, Ohe Y, Girard
N, Kim HR, Wu YL, Gainor J, Lee SH, Chiu CH, et al: Nivolumab plus
chemotherapy in epidermal growth factor receptor-mutated metastatic
non-small-cell lung cancer after disease progression on epidermal
growth factor receptor tyrosine kinase inhibitors: Final results of
checkmate 722. J Clin Oncol. 42:1252–1264. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang JC, Lee DH and Lee JS: Pemetrexed and
platinum with or without pembrolizumab for tyrosine kinase
inhibitor (TKI)-resistant, EGFR-mutant, metastatic nonsquamous
NSCLC: Phase 3 KEYNOTE-789 study. J Clin Oncol. Jun 7–2023.(Epub
ahead of print). doi: doi.org/10.1200/JCO.2023.41.17_suppl.LBA90.
View Article : Google Scholar
|
|
38
|
West H, McCleod M, Hussein M, Morabito A,
Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, et
al: Atezolizumab in combination with carboplatin plus
nab-paclitaxel chemotherapy compared with chemotherapy alone as
first-line treatment for metastatic non-squamous non-small-cell
lung cancer (IMpower130): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:924–937. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Galluzzi L, Zitvogel L and Kroemer G:
Immunological mechanisms underneath the efficacy of cancer therapy.
Cancer Immunol Res. 4:895–902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zappasodi R, Merghoub T and Wolchok JD:
Emerging concepts for immune checkpoint blockade-based combination
therapies. Cancer Cell. 33:581–598. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Reck M, Mok TSK, Nishio M, Jotte RM,
Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu
D, Moro-Sibilot D, et al: Atezolizumab plus bevacizumab and
chemotherapy in non-small-cell lung cancer (IMpower150): Key
subgroup analyses of patients with EGFR mutations or baseline liver
metastases in a randomised, open-label phase 3 trial. Lancet Respir
Med. 7:387–401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Socinski MA, Nishio M, Jotte RM, Cappuzzo
F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D,
Moro-Sibilot D, Thomas CA, et al: IMpower150 final overall survival
analyses for atezolizumab plus bevacizumab and chemotherapy in
first-line metastatic nonsquamous NSCLC. J Thorac Oncol.
16:1909–1924. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu S, Wu L, Jian H, Cheng Y, Wang Q, Fang
J, Wang Z, Hu Y, Han L, Sun M, et al: Sintilimab plus chemotherapy
for patients with EGFR-mutated non-squamous non-small-cell lung
cancer with disease progression after EGFR tyrosine-kinase
inhibitor therapy (ORIENT-31): second interim analysis from a
double-blind, randomised, placebo-controlled, phase 3 trial. Lancet
Respir Med. 11:624–636. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao Y, Guo S, Deng J, Shen J, Du F, Wu X,
Chen Y, Li M, Chen M, Li X, et al: VEGF/VEGFR-Targeted therapy and
immunotherapy in non-small cell lung cancer: targeting the tumor
microenvironment. Int J Biol Sci. 18:3845–3858. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Haratani K, Hayashi H, Tanaka T, Kaneda H,
Togashi Y, Sakai K, Hayashi K, Tomida S, Chiba Y, Yonesaka K, et
al: Tumor immune microenvironment and nivolumab efficacy in EGFR
mutation-positive non-small-cell lung cancer based on T790M status
after disease progression during EGFR-TKI treatment. Ann Oncol.
28:1532–1539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mazieres J, Drilon A, Lusque A, Mhanna L,
Cortot AB, Mezquita L, Thai AA, Mascaux C, Couraud S, Veillon R, et
al: Immune checkpoint inhibitors for patients with advanced lung
cancer and oncogenic driver alterations: results from the
IMMUNOTARGET registry. Ann Oncol. 30:1321–1328. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Isomoto K, Haratani K, Hayashi H, Shimizu
S, Tomida S, Niwa T, Yokoyama T, Fukuda Y, Chiba Y, Kato R, et al:
Impact of EGFR-TKI treatment on the tumor immune microenvironment
in EGFR mutation-positive non-small cell lung cancer. Clin Cancer
Res. 26:2037–2046. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jia Y, Li X, Jiang T, Zhao S, Zhao C,
Zhang L, Liu X, Shi J, Qiao M, Luo J, et al: EGFR-targeted therapy
alters the tumor microenvironment in EGFR-driven lung tumors:
Implications for combination therapies. Int J Cancer.
145:1432–1444. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus Chemotherapy for PD-L1-Positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen N, Fang W, Zhan J, Hong S, Tang Y,
Kang S, Zhang Y, He X, Zhou T, Qin T, et al: Upregulation of PD-L1
by EGFR activation mediates the immune escape in EGFR-Driven NSCLC:
Implication for optional immune targeted therapy for NSCLC Patients
with EGFR Mutation. J Thorac Oncol. 10:910–923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hsu PC, Jablons DM, Yang CT and You L:
Epidermal growth factor receptor (EGFR) Pathway, Yes-Associated
Protein (YAP) and the regulation of programmed Death-Ligand 1
(PD-L1) in non-small cell lung cancer (NSCLC). Int J Mol Sci.
20:38212019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Suda K, Rozeboom L, Furugaki K, Yu H,
Melnick MAC, Ellison K, Rivard CJ, Politi K, Mitsudomi T and Hirsch
FR: Increased EGFR phosphorylation correlates with higher
programmed death ligand-1 expression: Analysis of TKI-Resistant
lung cancer cell lines. Biomed Res Int. 2017:76942022017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Omori S, Kenmotsu H, Abe M, Watanabe R,
Sugino T, Kobayashi H, Nakashima K, Wakuda K, Ono A, Taira T, et
al: Changes in programmed death ligand 1 expression in non-small
cell lung cancer patients who received anticancer treatments. Int J
Clin Oncol. 23:1052–1059. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dong ZY, Zhang JT, Liu SY, Su J, Zhang C,
Xie Z, Zhou Q, Tu HY, Xu CR, Yan LX, et al: EGFR mutation
correlates with uninflamed phenotype and weak immunogenicity,
causing impaired response to PD-1 blockade in non-small cell lung
cancer. Oncoimmunology. 6:e13561452017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Soo RA, Lim SM, Syn NL, Teng R, Soong R,
Mok TSK and Cho BC: Immune checkpoint inhibitors in epidermal
growth factor receptor mutant non-small cell lung cancer: Current
controversies and future directions. Lung Cancer. 115:12–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Garassino MC, Cho BC, Kim JH, Mazières J,
Vansteenkiste J, Lena H, Corral Jaime J, Gray JE, Powderly J,
Chouaid C, et al: Durvalumab as third-line or later treatment for
advanced non-small-cell lung cancer (ATLANTIC): An open-label,
single-arm, phase 2 study. Lancet Oncol. 19:521–536. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Park S, Kim TM, Han JY, Lee GW, Shim BY,
Lee YG, Kim SW, Kim IH, Lee S, Kim YJ, et al: A phase III,
randomized study of atezolizumab plus bevacizumab and chemotherapy
in patients with EGFR or ALK mutated in non-small cell lung cancer
(ATTLAS, KCSG-LU19-04). J Clin Oncol. 42:1241–1251. 2024.
View Article : Google Scholar : PubMed/NCBI
|