Introduction
Collecting duct carcinoma (CDC) is a rare disease
with a poor prognosis, representing ~1% of kidney tumours worldwide
(1–4). A recent study demonstrated a possible
origin for CDC in the distal convoluted tubules, which makes CDC
biologically distinct from renal cell carcinoma (RCC) and
urothelial carcinoma (5). Due to
its rarity, there is a lack of information about the treatment and
clinical management of CDC; therefore, several approaches are
adapted from urothelial carcinoma (2,3).
Furthermore, the mortality rate of advanced CDC is high; the 3-year
relative survival rate for metastatic disease is ~6% worldwide
(5,6).
The present study describes the case of a
36-year-old woman who was diagnosed with allograft CDC 18 months
after receiving a deceased-donor kidney transplant. Staging exams
revealed multiple pulmonary nodules, and left retroperitoneal and
iliac lymphadenopathy. The patient underwent nephrectomy, and
chemotherapy with gemcitabine and cisplatin. They achieved a
complete radiological response and had a 6-year disease-free
survival after completing this chemotherapy protocol.
Case report
A 36-year-old woman with end-stage renal disease
(ESRD) of unknown aetiology, undergoing haemodialysis for 2 years,
underwent a deceased-donor kidney transplant at the Clinical
Hospital of Ribeirão Preto, University of São Paulo (Ribeirão
Preto, Brazil) in July 2015, 18 months before the diagnosis of CDC.
The donor was a 53-year-old woman, with three HLA mismatches and 29
h cold ischemic time. The induction of immunosuppression was
performed with thymoglobulin [anti-thymocyte globulin (rabbit), 6
mg/kg], and was maintained with prednisone (5 mg), mycophenolate
sodium (720 mg) and tacrolimus (10 mg). An allograft biopsy was
performed due to delayed graft function and cytomegalovirus
infection, and it revealed normal renal parenchyma (17 months
before the diagnosis of CDC). The patient was treated with
ganciclovir (2.5 mg/kg q24 h) for 2 weeks, and laboratory analysis
showed creatinine levels of 1.5 mg/dl (normal range, 0.6-1.3
mg/dl), glomerular filtration rate: 46 ml/min/1.73 m2
(chronic kidney disease stage 3T) (7) and normal urinary sediment. In January
2017, the creatinine levels had increased to 3.7 mg/dl, and the
patient was admitted in the Clinical Hospital of Ribeirão Preto of
University of São Paulo for further investigation. Renal ultrasound
demonstrated a diffusely heterogeneous kidney allograft parenchyma
and a new biopsy revealed a neoplastic process in the tubules, also
with neoplastic interstitial infiltration. Immunohistochemical
evaluation of a Bouin-fixed renal biopsy (Appendix S1) showed a positive vimentin
cytoplasmic pattern; positive cytokeratin (CK) AE1/AE3; weak
positive RCC antigen; doubtful positive CD10; and negative 35BH11,
34BE12, CK7, CK20, paired box gene 8, CD117, HMB45, Melan A,
transcription factor E3, CD3, CD20, CD30, desmin, S100, CD31 and
integrase interactor 1 (SMARCB1/INI1) (data not shown). Therefore,
the result was carcinoma not otherwise specified. Kidney nuclear
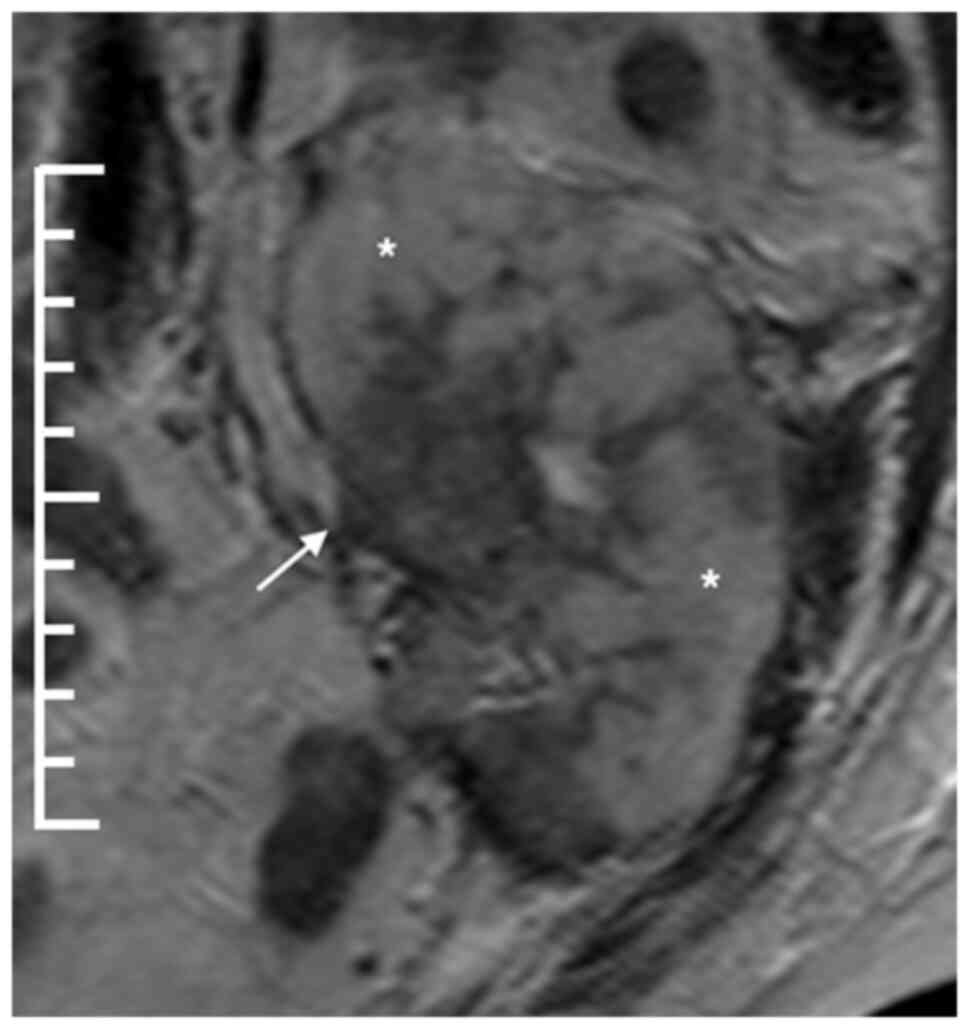
magnetic resonance imaging showed the transplanted kidney with a
diffuse, infiltrative lesion occupying the whole organ with just a
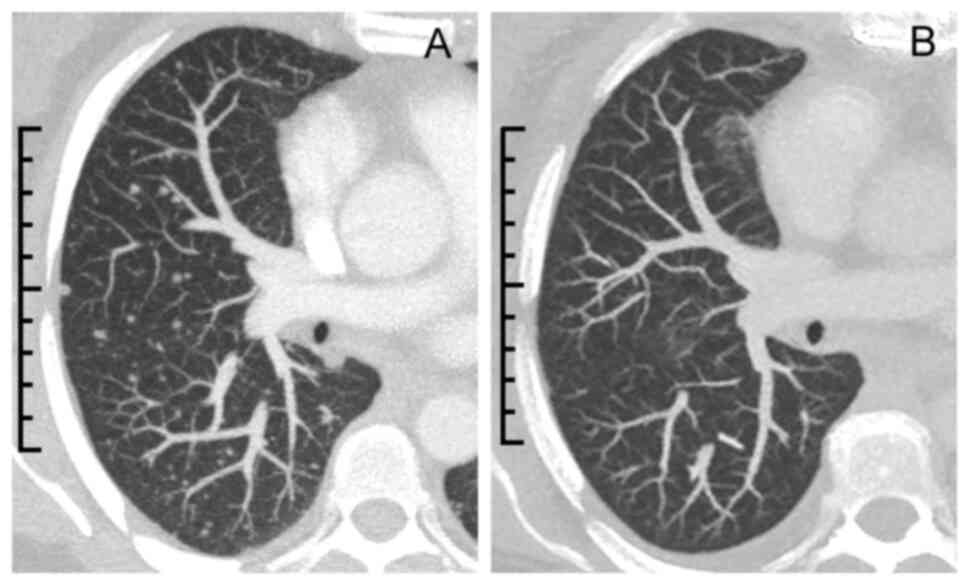
few areas of preserved renal parenchyma (Fig. 1). Staging computed tomography (CT)
scans revealed multiple pulmonary nodules, and left retroperitoneal
and iliac lymphadenopathy (Fig.
2A). The patient underwent allograft nephrectomy and left
salpingo-oophorectomy, and was restarted on chronic haemodialysis 1
month after diagnosis. Due to neoplastic involvement of the left
common and external iliac arteries, a left iliac-femoral prosthesis
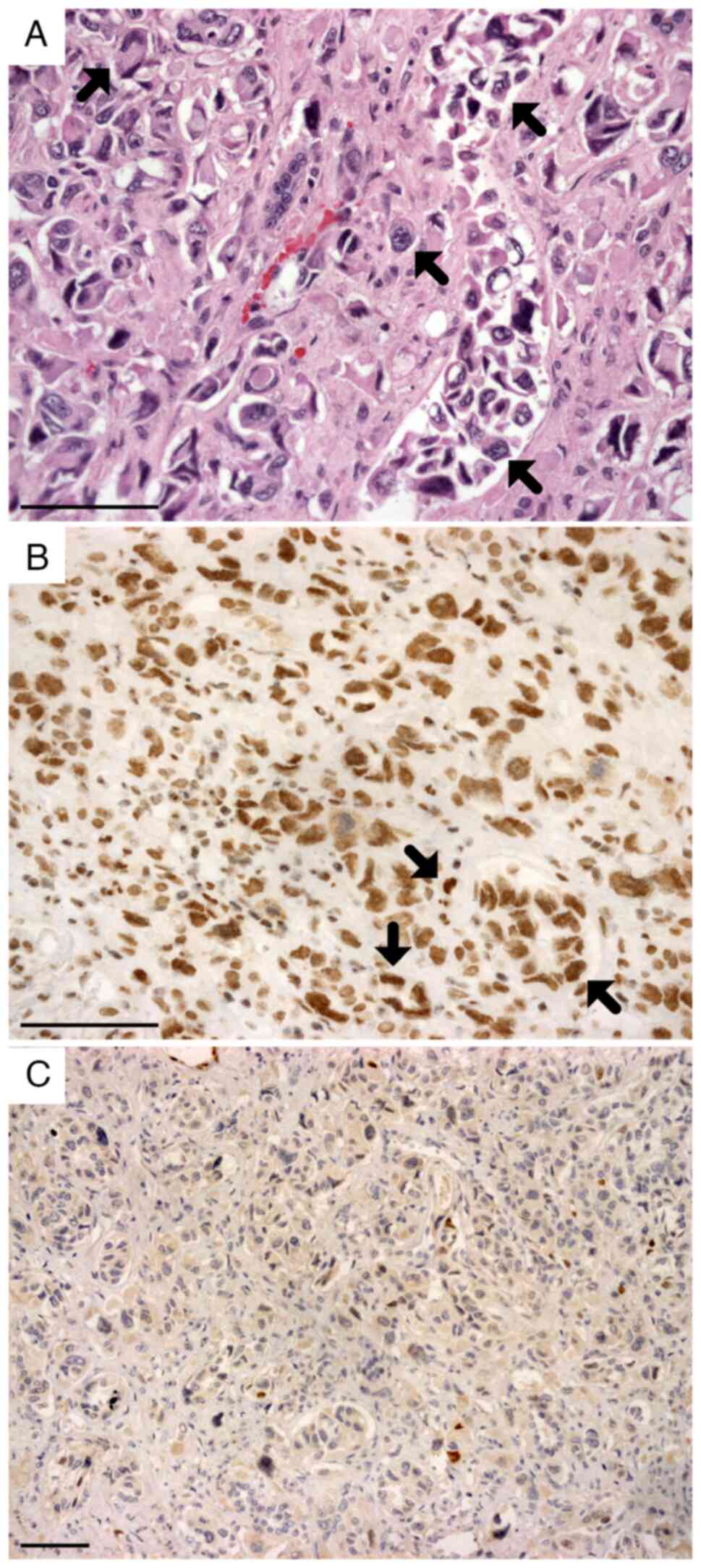
was also placed intraoperatively. A histopathological study of the
nephrectomy specimen showed malignant epithelial neoplasia with
renal cortex and medulla infiltration (Fig. 3A), extending to the perirenal
adipose tissue and the pyelocaliceal system, with venous, lymphatic
and perineural invasion. The neutral-buffered formalin-fixed
nephrectomy specimen immunohistochemical panel exhibited positivity
for CK7, CEA, CAM5.2, CK19, OCT3/4 (data not shown) and
SMARCB1/INI1 (Fig. 3B). In
addition, paired box gene 8, WT1, oestrogen receptor, CD117, CK20
(data not shown) and p63 staining was negative (Fig. 3C). Thus, a diagnosis of kidney
allograft CDC was confirmed, mainly due to SMARCB1/INI1 positivity
and p63 negativity (Table I)
(8,9). The discrepancies in
immunohistochemical findings between the renal biopsy and the
nephrectomy specimen were potentially attributed to the variance in
fixatives employed between the samples. While the renal biopsy was
fixed in Bouin's solution, the nephrectomy material was fixed in
neutral-buffered formalin. Finally, due to metastases in the left
lung, ovary, fallopian tube and iliac lymph node, the TNM staging
was pT3a pN1 pM1 (10).
 | Table I.Immunohistochemical diagnosis of renal
neoplasms. |
Table I.
Immunohistochemical diagnosis of renal
neoplasms.
| A, Positive stains
for CDC |
|---|
|
|---|
| Protein symbol | Protein name |
|---|
| SMARCB1/INI1 | Integrase interactor
1 |
| S100A1 | S100 calcium-binding
protein A1 |
|
| B, Usually
positive stains for CDC |
|
| Protein
symbol | Protein
name |
|
| CK7 | Cytokeratin 7 |
| CK19 | Cytokeratin 19 |
| 34BE12 | High molecular weight
cytokeratin 34βE12 |
| PAX8 | Paired box gene
8 |
| Vimentin | - |
|
| C, Negative stains
for CDC |
|
| Protein
symbol | Protein
name |
|
| p63 | - |
|
| D, Usually
negative stains for CDC |
|
| Protein
symbol | Protein
name |
|
| CD10 | - |
| CD117 | - |
| OCT3/4 | Octamer binding
transcription factor 3/4 |
| RCC | Renal cell carcinoma
antigen |
|
| E, Markers of
other renal neoplasms |
|
| Protein
symbol | Protein
name |
|
| AE1/AE3 | Cytokeratin
AE1/AE3 |
| CAM5.2 | Low molecular weight
cytokeratin CAM5.2 |
| CK20 | Cytokeratin 20 |
| HMB45 | Human melanoma black
45 |
| TFE3 | Transcription factor
E3 |
| WT1 | Wilms tumour protein
1 |
For treatment, a chemotherapy regimen with
gemcitabine and cisplatin was administered, with each cycle
consisting of i) Days 1 and 8: Intravenous gemcitabine 1,000
mg/m2 (full dose), followed 2 h after infusion by 3-h
haemodialysis; ii) day 2: Intravenous cisplatin 35 mg/m2
(half dose), followed 1 h after infusion by 3-h haemodialysis; and
iii) days 4–8: Subcutaneous filgrastim 300 µg/day. All
haemodialysis sessions were performed with a 300 ml/min blood flow
and a FX80 high-flow dialyzer (Fresenius Medical Care). Cisplatin
was chosen instead of carboplatin to reduce haematological
toxicity, and its dose was reduced according to creatinine
clearance. The patient was treated at the Clinical Hospital of
Ribeirão Preto, University of São Paulo, which was entirely insured
by the public health system, without access to tyrosine kinase
inhibitors or immunotherapy.
The patient underwent six cycles of the described
chemotherapy protocol, with 4-week intervals. During therapy, there
were two episodes of neutropenia-grades 2 and 3 according to the
National Cancer Institute Common Terminology Criteria for Adverse
Events (11)-without clinical
repercussions, and one episode of haemodialysis catheter-related
bloodstream infection, which was successfully treated with
vancomycin (1 g) and ceftazidime (2 g) intravenously, both three
times per week. The last chemotherapy session was performed 9
months after CDC diagnosis. The patient achieved a complete
radiological response, with remission of lung lesions on chest CT
(Fig. 2B).
Since completing the chemotherapy protocol, the
patient has had 6 years of disease-free survival. They remain under
clinical-radiological follow-up at the oncology outpatient clinic,
and continue to be treated with haemodialysis.
Discussion
CDC is a type of non-clear cell carcinoma with a
high mortality rate, with 50.3% of global cases being diagnosed as
stage IV disease (5). Furthermore,
the overall median survival time is <12 months (5). The appropriate treatment for CDC is
still unclear, and most knowledge is acquired through case reports
(4). Due to its aggressive
behaviour, treatment includes radical surgical techniques, and most
patients undergo nephrectomy plus lymphadenectomy for diagnosis and
cytoreductive therapy (5).
Conventional adjuvant chemotherapy with gemcitabine
and cisplatin was adopted in the present case following guidance
from the GETUG group phase II study (3). There are other regimens used for
urothelial carcinoma that are used for CDC, such as
paclitaxel/carboplatin;
methotrexate/vinblastine/doxorubicin/cisplatin; and
mitomycin/cisplatin (1,4). Although not well established, new and
more specific therapies have been reported, such as nivolumab,
sutinib, sorafenib, everolimus, temsirolimus and cabozantinib
(1,4,12).
However, the combination of gemcitabine and cisplatin remains as
first-line therapy, given its efficacy and better tolerability and
safety levels (3,13).
Gemcitabine is a pyrimidine nucleoside analogue.
When submitted to intracellular phosphorylation, it generates a
cytotoxic metabolite that prevents DNA synthesis and promotes cell
death (14–16). The drug is mainly excreted by the
kidneys after rapid conversion into 2′,2′-difluorodeoxyuridine
(dFdU) by tissue and plasma cytidine deaminase enzymes, 60–90 min
after infusion (12,17). dFdU is classically considered a
non-toxic metabolite; however, it has been suggested that its
elevated serum levels in chronic kidney disease (CKD) can lead to
toxicity (14–16). In a previous case report, serial
serum measurements of gemcitabine and dFdU have demonstrated
adequate removal with haemodialysis [blood flow of 200 ml/min, F7
polysulfone low-flux membrane (Fresenius Medical Care); 3.5-h
session], with dFdU clearance of 148 ml/min, a half-life of 3.9 h
and a 50% reduction in plasma levels (15). Satisfactory experiences with
800–1,000 mg/m2 gemcitabine followed by haemodialysis
after 24 h have been reported (16). In addition, other cases have used a
full dose of gemcitabine (1,000 mg/m2), but with earlier
haemodialysis, between 6 and 12 h after chemotherapy (15,17).
Cisplatin is a potent antineoplastic medication used
to treat several types of solid tumour; ~90% of the drug binds to
plasma proteins, with the free fraction being responsible for toxic
effects (12,18). Therefore, cisplatin clearance is
biphasic and consists of an initial phase of rapid urinary
excretion of the free fraction, with a half-life of 20–45 min,
followed by a long phase of excretion of the conjugated fraction,
with a half-life of 5 days (12,18).
Usually, the dose of cisplatin is reduced by 50% (35
mg/m2) in patients undergoing dialysis (19). Haemodialysis performed 1 h after
cisplatin infusion can cause its rapid clearance and reduce
undesirable myelotoxicity; this phenomenon can be explained by
early drug extraction prior to its massive protein conjugation,
that is, during the first phase of excretion (18).
A 2013 case series in Taiwan revealed successful
chemo-therapy treatment with a reduced dose of gemcitabine (600
mg/m2 biweekly) and cisplatin (30 mg/m2
weekly) in patients with CKD and urothelial, bladder, pancreatic,
and non-small cell lung cancer (14). The authors chose to reduce the
gemcitabine dose after their own negative experiences with
myelotoxicity and hepatotoxicity; however, unlike other case
reports that performed haemodialysis sooner (15,17),
these authors performed haemodialysis 24 h after chemotherapy
infusion (14). In a 2016 Japanese
study (12), which influenced the
present case report, full-dose gemcitabine (1,000 mg/m2)
and half-dose cisplatin (35 mg/m2) were prescribed for
the treatment of metastatic urothelial carcinoma. A 2-h interval
was set between the end of gemcitabine infusion and the beginning
of 3-h haemodialysis, considering that all of the drug would have
already been converted into dFdU by cytidine deaminases. Therefore,
gemcitabine pharmacokinetics would reflect what occurs in patients
with normal renal function. Furthermore, cisplatin infusion was
performed 1 h before the beginning of 3-h haemodialysis, as
reported by previous studies (12,18).
In the present case, the patient achieved a complete
radiological response. The thorax imaging findings could be due to
inflammation or infection because they were nonspecific. However,
the imaging findings were considered pulmonary metastasis (multiple
bilateral nodules with soft tissue density) due to the clinical
correlation, considering the presence of a neoplasm and the absence
of symptomatic lung infections. In addition, the appearance of
nodules during the course of the neoplasm and their progressive
reduction during chemotherapy treatment corroborates the hypothesis
of pulmonary metastases.
In conclusion, in the present case report, the
success of chemotherapy with gemcitabine and cisplatin was
demonstrated in a metastatic renal allograft CDC in a patient with
ESRD. A full dose of gemcitabine (1,000 mg/m2), followed
by haemodialysis after 2 h, and half a dose of cisplatin (35
mg/m2), followed by haemodialysis after 1 h, provided a
complete response to chemotherapy, with low side effects and no
recurrence of the disease 6 years after the end of treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was financed in part by the Coordenação de
Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)
(Finance Code 001).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TCFNA drafted the original manuscript. TCFNA, MMN,
LR and EAR contributed substantially to study conception and
design, as well as data acquisition and interpretation. BTMP and
VFM collected and analysed the anatomopathological and medical
imaging data. FNS was the oncologist who treated the patient, and
collected and analysed data from the literature to decide on the
patient's treatment. EAR, MMN and TCFNA confirm the authenticity of
all the raw data. All authors reviewed the manuscript draft and
revised it critically for intellectual content. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Ribeirão Preto Medical School (approval no.
75746623.6.0000.5440; Ribeirão Preto, Brazil). Written informed
consent was obtained from the patient and this study was performed
in accordance with The Declaration of Helsinki and according to the
Medical Ethics Committee of Ribeirão Preto Medical School.
Patient consent for publication
The patient provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mennitto A, Verzoni E, Peverelli G, Alessi
A and Procopio G: Management of metastatic collecting duct
carcinoma: An encouraging result in a patient treated with
cabozantinib. Clin Genitourin Cancer. 16:e521–e523. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Orsola A, Trias I, Raventós CX, Español I,
Cecchini L and Orsola I: Renal collecting (Bellini) duct carcinoma
displays similar characteristics to upper tract urothelial cell
carcinoma. Urology. 65:49–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oudard S, Banu E, Vieillefond A, Fournier
L, Priou F, Medioni J, Banu A, Duclos B, Rolland F, Escudier B, et
al: Prospective multicenter phase II study of gemcitabine plus
platinum salt for metastatic collecting duct carcinoma: Results of
a GETUG (Groupe d'Etudes des Tumeurs Uro-Génitales) study. J Urol.
177:1698–1702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin M, Wang W, Rosenberg J, Kaag M, Joshi
M, Holder S, Tuanquin L and Drabick JJ: Targeted therapy in
collecting duct carcinoma of the kidney: A case report and
literature review. Clin Genitourin Cancer. 14:e203–e236. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suarez C, Marmolejo D, Valdivia A,
Morales-Barrera R, Gonzalez M, Mateo J, Semidey ME, Lorente D,
Trilla E and Carles J: Update in collecting duct carcinoma: Current
aspects of the clinical and molecular characterization of an orphan
disease. Front Oncol. 12:9701992022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pepek J, Johnstone P and Jani A: Influence
of demographic factors on outcome of collecting duct carcinoma: A
surveillance, epidemiology, and end results (SEER) database
analysis. Clin Genitourin Cancer. 7:E24–E27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chapman JR: The KDIGO clinical practice
guidelines for the care of kidney transplant recipients.
Transplantation. 89:644–645. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Truong LD and Shen SS: Immunohistochemical
diagnosis of renal neoplasms. Arch Pathol Lab Med. 135:92–109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carvalho JC, Thomas DG, Mchugh JB, Shah RB
and Kunju LP: P63, CK7, PAX8 and INI-1: An optimal
immunohistochemical panel to distinguish poorly differentiated
urothelial cell carcinoma from high-grade tumours of the renal
collecting system. Histopathology. 60:597–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rini B, McKiernan J and Chang S: Kidney.
Amin M: AJCC Cancer Staging Manual. 8th edition. New York:
Springer; pp. pp7392017
|
|
11
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events (CTCAE)
Version 5.0. November 27–2017.
|
|
12
|
Rimar KJ, Meeks JJ and Kuzel TM:
Anti-programmed death receptor 1 blockade induces clinical response
in a patient with metastatic collecting duct carcinoma. Clin
Genitourin Cancer. 14:e431–e434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ezaki T, Matsumoto K, Morita S, Shinoda K,
Mizuno R, Kikuchi E and Oya M: A case of gemcitabine and cisplatin
chemotherapy in a patient with metastatic urothelial carcinoma
receiving hemodialysis. Clin Genitourin Cancer. 14:e413–e416. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang PY, Dai MS, Ho CL and Yao NS:
Administration of gemcitabine and cisplatin in cancer patients with
renal failure under hemodialysis. J BUON. 18:1058–1061.
2013.PubMed/NCBI
|
|
15
|
Kiani A, Köhne CH, Franz T, Passauer J,
Haufe T, Gross P, Ehninger G and Schleyer E: Pharmacokinetics of
gemcitabine in a patient with end-stage renal disease: Effective
clearance of its main metabolite by standard hemodialysis
treatment. Cancer Chemother Pharmacol. 51:266–270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masumori N, Kunishima Y, Hirobe M,
Takeuchi M, Takayanagi A, Tsukamoto T and Itoh T: Measurement of
plasma concentration of gemcitabine and its metabolite dFdU in
hemodialysis patients with advanced urothelial cancer. Jpn J Clin
Oncol. 38:182–185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Janus N, Thariat J, Boulanger H, Deray G
and Launay-Vacher V: Proposal for dosage adjustment and timing of
chemotherapy in hemodialyzed patients. Ann Oncol. 21:1395–403.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zahra MA, Taylor A, Mould G, Coles C,
Crawford R and Tan LT: Concurrent weekly cisplatin chemotherapy and
radiotherapy in a haemodialysis patient with locally advanced
cervix cancer. Clin Oncol. 20:6–11. 2008. View Article : Google Scholar
|
|
19
|
Horie S, Oya M, Nangaku M, Yasuda Y,
Komatsu Y, Yanagita M, Kitagawa Y, Kuwano H, Nishiyama H, Ishioka
C, et al: Guidelines for treatment of renal injury during cancer
chemotherapy 2016. Clin Exp Nephrol. 22:210–244. 2018. View Article : Google Scholar : PubMed/NCBI
|