Introduction
It has been reported that in addition to apoptosis,
other types of programmed cell death including ferroptosis,
necroptosis and pyroptosis are highly related to chemoresistance
(1). Inhibition of necroptosis by
application of receptor-interacting serine/threonine kinase 1
(RIP1) activity inhibitor Necrostatin-1 (Nec-1) or knockdown of
receptor-interacting serine/threonine kinase 3 (RIP3) significantly
inhibits the cisplatin-induced loss of cell viability in
apoptosis-resistant esophageal squamous cell carcinoma cells
(2), suggesting that induction of
necroptosis can increase the sensitivity of chemotherapeutic
agents.
Reactive oxygen species (ROS) are considered to be a
driving force for necroptosis (3–5). ROS
contributes to increasing necroptosis in NF-κB-deficient cells
induced by TNFα by detecting phosphorylated (p)-RIP1, RIP3 and
mixed-lineage kinase domain-like (MLKL) (6). ROS production upon TNFα/BV6 treatment
is involved in promoting the assembly of the RIP1/RIP3 necrosome in
FADD-deficient Jurkat cells by immunoprecipitation of RIP1/RIP3
(4). Sulfasalazine (SAS), a potent
cystine/glutamate transporter cystine-glutamate exchange (xCT)
inhibitor that plays an important role in maintaining glutathione
levels, impairs the ROS defense system and increases the
therapeutic efficacy of anticancer therapies (7–9). Based
on the previous results of a close correlation between necroptosis
and ROS, a hypothesis of the present study was that SAS might be
associated to the induction of necroptosis.
P62/sequestosome-1 (SQSTM1) is a multi-domain
adapter protein that is highly expressed in various tumor cells,
and it binds to multiple signaling molecules (10,11).
However, the results of previous studies highlight that p62/SQSTM1
binds to RIP1 to assemble RIP3/MLKL on the autophagosome membrane
in mouse prostate cells lacking Map3k7, suggesting that p62/SQSTM1
not only plays a role as an autophagy adaptor to degrade cellular
material, but also may induce necroptosis by recruiting RIP1
(12,13). A previous study has indicated that
deletion of the zinc finger (ZZ) domain of p62/SQSTM1 prevents the
binding of RIP1 to p62/SQSTM1, and therefore inhibits the
activation of the NF-κB signaling pathway (14). P62/SQSTM1 also exhibits pro-death
functions through interaction with caspase-8, which plays a role in
the progression of ovarian cancer (15). Notably, RIP1 activates NF-κB to
promote cell survival, and binds to caspase-8 or RIP3 to play a
role in the regulation of cell apoptosis and necroptosis (16). Thus the mechanism of necroptosis in
SKOV3/DDP cells treated with SAS through p62/SQSTM1 remains to be
elucidated.
The present study aimed to determine the effects of
SAS on cisplatin-resistant ovarian cancer SKOV3/DDP cells. Another
aim was to demonstrate that p62/SQSTM1 regulated the necroptosis of
cisplatin-resistant ovarian cancer SKOV3/DDP cells treated with
SAS, providing evidence for p62/SQSTM1 as a potential therapeutic
target to overcome drug resistance.
Materials and methods
Reagents and antibodies
SAS, necroptosis inhibitor Nec-1 and 3-(4,
5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were
purchased from Sigma-Aldrich; Merck KGaA. ViaFect™ transfection
reagent was purchased from Promega Corporation. Anti-p62/SQSTM1
[cat. no. 66184-1-Ig; 1:5,000 for western blotting (WB)], anti-RIP1
(cat. no. 17519-1-AP; 1:500 for WB) and anti-β-actin (cat. no.
60008-1-Ig; 1:5,000 for WB) were purchased from ProteinTech Group,
Inc. Anti-p-RIP1 (cat. no. 31122; 1:1,000 for WB) was purchased
from Cell Signaling Technology, Inc. Anti-RIP3 (cat. no. ab305054;
1:1,000 for WB), anti-p-RIP3 (cat. no. ab222320; 1:2,000 for WB),
anti-MLKL (cat. no. ab196436; 1:1,000 for WB) and anti-p-MLKL (cat.
no. ab279863; 1:1,000 for WB) were purchased from Abcam.
Cell lines and cell culture
Cisplatin-sensitive HOCC SKOV3 cell lines were
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences (cat. no. 1101HUM-PUMCO00027).
SKOV3/DDP were added to the medium containing 0.02 µg/ml cisplatin,
was gradually induced by increasing the concentration of cisplatin,
and kept in medium containing 0.2 µg/ml cisplatin to maintain
resistance. All cells were cultured in RPMI-1640 culture medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C
in a 5% CO2 incubator.
Cell transfection
p62/SQSTM1-small interfering (si)RNA (si-p62.1,
si-p62.2 and si-p62.3) and non-targeting siRNA (scrambled control)
were obtained from Shanghai GeneChem Co., Ltd. The target sequences
for p62-siRNA were as follows: Si-p62.1 forward,
5′-CAGATGGAGTCGGATAACT-3′, and reverse, 5′-AGTTATCCGACTCCATCTG-3′;
si-p62.2 forward, 5′-GTGACGAGGAATTGACAAT-3′, and reverse,
5′-ATTGTCAATTCCTCGTCAC-3′; si-p62.3 forward,
5′-GACATCTTCCGAATCTACA-3′, and reverse, 5′-TGTAGATTCGGAAGATGTC-3′;
and scramble control sequences were forward,
5′-TTCTCCGAACGTGTCACGT-3′, and reverse, 5′-ACGTGACACGTTCGGAGAA-3′.
The pcDNA3.1-∆ZZ-p62 plasmid (with a truncated mutation in the ZZ
domain) and empty pcDNA3.1 vector (negative control; NC) were
constructed by Sangon Biotech Co., Ltd. In total, 25×104
cells were plated into 6-well plates and cultured for 36 h.
Following culturing, cells were transfected with 2 µg siRNA or
plasmids per 6-well plate and 200 µl of total ViaFect™ transfection
reagent complex at 37°C in a 5% CO2 incubator.
Subsequent experimentation was carried out within 72 h.
Cell viability assay
In total, 8×103 cells/per well were
seeded in 96-well plates and cultured for 36 h at 37°C with 5%
CO2. Cells were treated with 0.5, 1.0, 2.0, 4.0 and 8.0
mM of SAS or 2.5, 5, 10, 25 and 50 µM of Nec-1. Cell viability was
assessed using an MTT assay which dissolved the purple formazan by
DMSO and measured at a wavelength of 570 nm using a microplate
reader (Molecular Devices, LLC).
Real-time labeled cell function
analysis (RTCA)
Cell suspensions were added to 96-well plates at a
concentration of 1.5×104 cells in 100 µl medium per
well. The 96-well plates were placed into the RTCA connection
instrument (ACEA Bioscience, Inc.; Agilent), and cells were
incubated at 37°C in 5% CO2 according to the
manufacturer's protocol. When cells reached the logarithmic growth
phase, the program was paused, the 96-well plate was removed and
the original solution was discarded. Subsequently, 0.5 mM SAS and
10 µM Nec-1 were added to SKOV3 wells, and 2.0 mM SAS and 10 µM
Nec-1 were added to SKOV3/DDP wells and the 96-well plate was
returned to the RTCA connection instrument. The real-time growth of
cells was observed and the program was completed when the
pre-planned program ended or when cell proliferation met the
requirements. Results were analyzed using the RTCA Data Analysis
Software (version, 1.0; ACEA Bioscience Inc.).
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology) supplemented
with protease and phosphatase inhibitors (2 mM AEBSF, 0.3 µM
Aprotinin, 0.13 mM Bestatin, 0.014 mM E64 and 0.01 mM Leupeptin).
Cells were centrifuged at 1,000 × g for 15 min at 4°C. The protein
determination method was BCA assay and 30 µg protein per lane was
separated using 10% SDS-PAGE. Separated proteins were subsequently
transferred onto a PVDF membrane and blocked with 5% skimmed milk
for 90 min at room temperature. Membranes were incubated with
primary antibodies at 4°C overnight. Following primary incubation,
membranes were incubated with HRP-conjugated secondary antibodies
(cat. nos. SA00001-1 and SA00001-1; 1:2,000; ProteinTech Group,
Inc.) for 2 h at room temperature. Protein bands were visualized
using ECL reagent (Thermo Fisher Scientific, Inc.) and protein
expression was quantified using a Syngene Bio Imaging system
version 4.3.17 (Synoptics).
Immunoprecipitation assay
Cells were lysed in 200 µl NP40 lysis buffer
(Beyotime Institute of Biotechnology) supplemented with protease
inhibitors (8 µM Aprotinin, 0.5 mM Bestatin, 0.15 mM E64 and 0.2 mM
Leupeptin) and were centrifuged at 12,000 × g and 4°C for 5 min.
Subsequently, 30 µl of each cell supernatant were conducted as
input. Equal amounts 1 mg of each cell supernatants were
immunoprecipitated with 2 µg of the anti-RIP3 primary antibody
overnight at 4°C. In total, 25 µl of protein A and G agarose
(Beyotime Institute of Biotechnology) was used in each sample
overnight at 4°C. The supernatants were isolated at 1,000 × g and
4°C for 5 min. Beads were washed three times with 1 ml PBS buffer,
the magnetic were centrifugated at 1,000 × g and 4°C for 1 min, and
eluted proteins were analyzed using anti-RIP1 primary antibody by
western blotting.
Statistical analysis
Data are presented as the mean ± SEM and
experimental repeats performed three times. Statistical analysis
was performed using SPSS statistical software (version, 20.0; IBM
Corp.). Comparisons between groups were conducted using one-way
ANOVA followed by a post hoc test. Dunnett's t and Dunnett's T3
tests were used for comparisons using the same control group. LSD
tests were used for comparisons between experimental, control and
SAS groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
SAS treatment activates the
necroptosis pathway in SKOV3/DDP cells
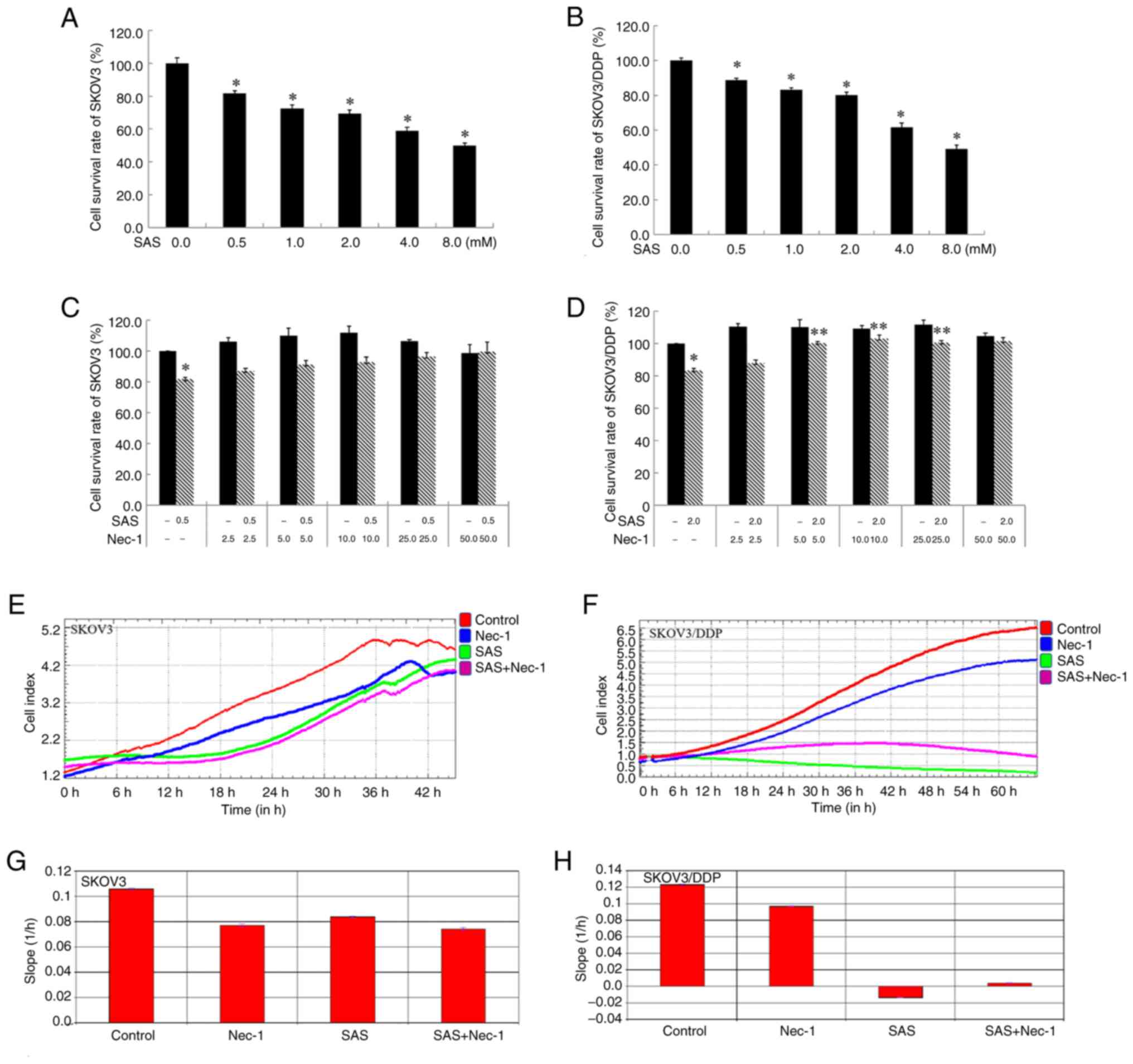
The survival rates of SKOV3 and SKOV3/DDP cells
significantly decreased in a dose-dependent manner as the
concentration of SAS increased, compared with the control group
(Fig. 1A and B). Compared with the
negative control SKOV3 cells, SKOV3/DDP cells have low sensitivity
to SAS. The survival rate for SKOV3 cells treated with 0.5 mM SAS
and 2 mM SAS were 81.9±1.4 and 69.3±2.43% respectively. The
survival rate for SKOV3/DDP treated with 0.5 mM SAS and 2 mM SAS
were 88.9±1.1 and 80.3±1.5% respectively. To observe the difference
in cell death, in subsequent experiments, 0.5 mM SAS was selected
to treat SKOV3 cells, and 2 mM SAS was selected to treat SKOV3/DDP
cells with the same cell survival rate.
Notably, it has been reported that Nec-1, a
RIP1-specific inhibitor, can block necroptosis (17). The present study tested the effect
of Nec-1 on SAS-induced death in SKOV3/DDP cells. The present study
intended to observe their cell survival rate, adhesion and
spreading and cell morphology when treated with a combination of
SAS with Nec-1. Results of the MTT assay demonstrated that,
compared with the SAS group, a combination of Nec-1 and SAS did not
increase the survival rate of SKOV3 cells, and a combination of
Nec-1 and 5, 10, 25 µM and 2 mM SAS increased the survival rate of
SKOV3/DDP cells (Fig. 1C and D).
The cell survival rate was not altered in groups treated with
different concentrations of Nec-1. The survival curve of cell index
in RTCA indicates the cell number, and the slope of cell index
indicates the adhesion and spreading, as well as cell morphology of
cells. Compared with the SAS group, the survival curve of SKOV3
cells was low in the 10 µM Nec-1 and SAS combination group
(Fig. 1E). In addition, the
survival curve of SKOV3/DDP cells in the 10 µM Nec-1 and SAS
combination group was high compared with the SAS group (Fig. 1F). Results of the RTCA demonstrated
that in SKOV3 cells, the slope of the curve was decreased in the
SAS group compared with the control. However, there was no
significant difference between the slope of the curve in the SAS
group, and that in the 10 µM Nec-1 and SAS combination group
(Fig. 1G). In SKOV3/DDP cells, the
slope of the curve was decreased in the SAS group, and showed a
negative increase compared with the control. Moreover, the slope of
the curve was increased in the 10 µM Nec-1 and SAS combination
group compared with the SAS group (Fig.
1H). By contrast, the cell index changes between the Control
and SAS groups were 4.7 and 3.7 in SKOV3 cells, respectively, and
4.0 and 0.5 in SKOV3/DDP cells respectively, indicating the
adhesion and spreading of SKOV3/DDP cells decreased following SAS
treatment in contrast to SKOV3 cells under the same cell survival
rate (~80%).
SAS treatment activates necrosome
formation in SKOV3/DDP cells
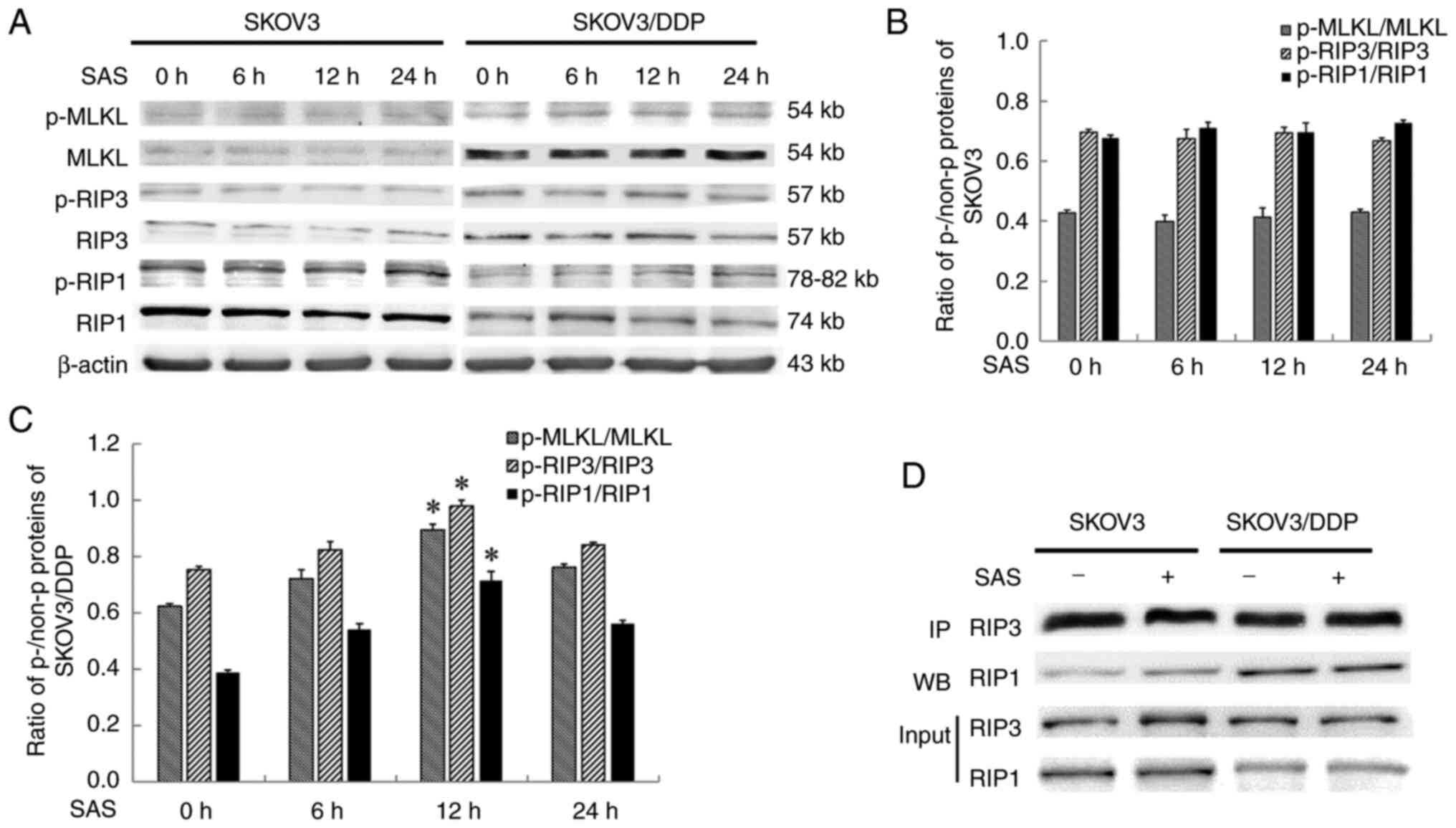
Expression levels of marker proteins associated with
the necroptosis pathway were investigated in the present study. The
expressions level of necroptosis-associated proteins; namely,
p-RIP1, RIP3 and MLKL were significantly increased in SKOV3/DDP
cells following SAS treatment for 12 h. On the other hand, in SKOV3
cells treated with SAS, the necroptosis-associated proteins p-RIP1,
RIP3 and MLKL were not significantly altered at each observed time
point (Fig. 2A-C). Notably, the
complex formed following the phosphorylation of MLKL and the
combination of RIP1 with RIP3 is the core component of necroptosis.
Therefore, the SAS-induced precipitation of RIP3 was investigated
in human ovarian epithelial carcinoma cells. Results of the
co-immunoprecipitation analysis revealed that the expression of
RIP1 and RIP3 complexes were increased in SKOV3/DDP cells compared
with SKOV3 cells (Fig. 2D).
P62/SQSTM1 adaptor plays a role in
SAS-induced necroptosis in SKOV3/DDP cells
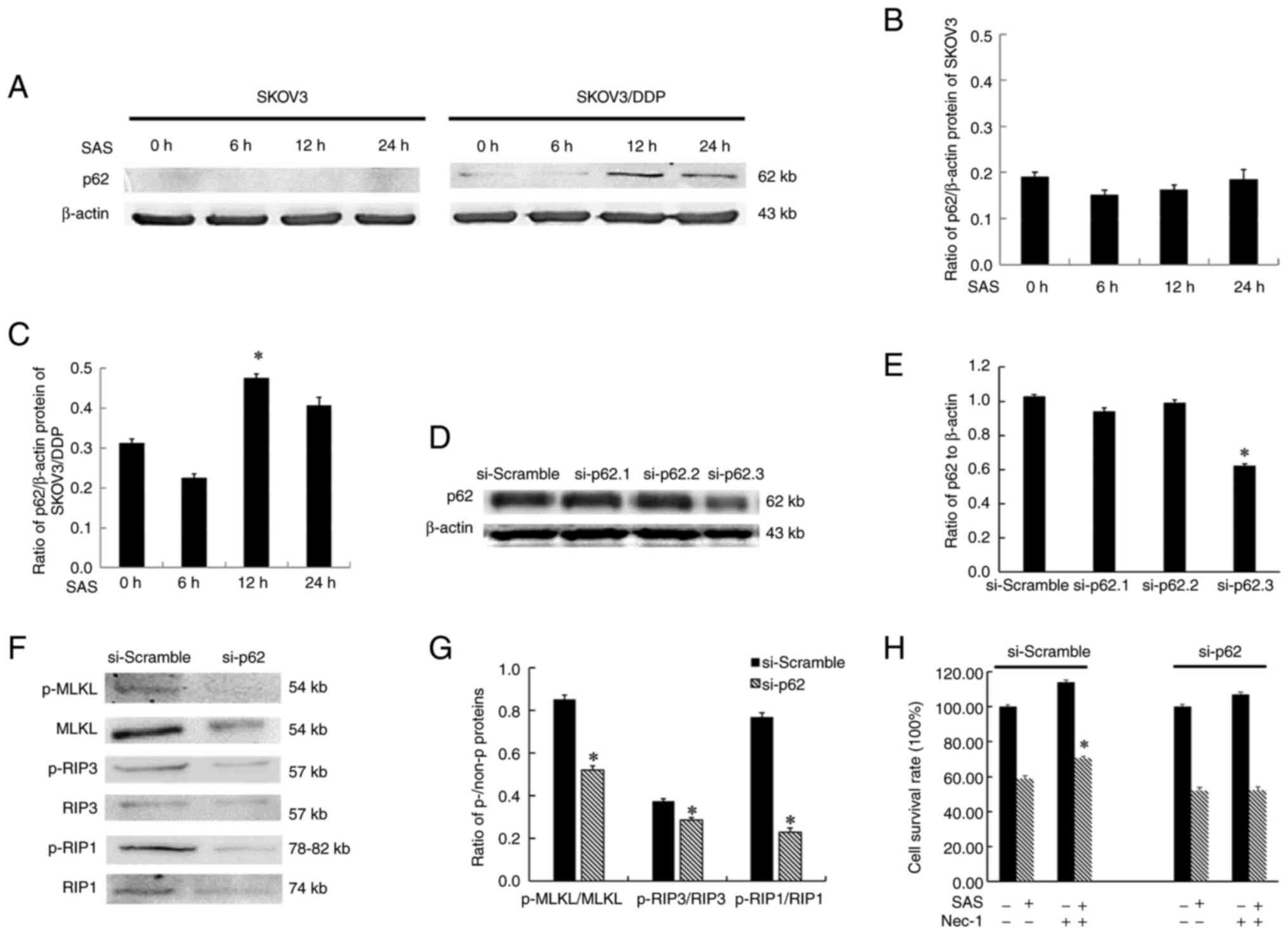
Notably, p62/SQSTM1 protein expression levels were
increased in SKOV3/DDP cells, compared with SKOV3 cells (18). During the SAS-induced death of
SKOV3/DDP cells, p62/SQSTM1 expression was significantly increased
following 12 h of SAS treatment compared with 0 h of treatment.
These results are consistent with the increased expression of
necroptosis marker proteins observed at the same time point.
Results of the present study demonstrated that p62/SQSTM1 protein
expression was minimal during the death of SKOV3 cells treated with
SAS (Fig. 3A-C). RIP1 interacts
directly with p62/SQSTM1 (19),
suggesting a mechanism that by which p62/SQSTM1 could play a role
in SAS-induced necroptosis. To test this, three si-p62 plasmids
were used in transfection experiments, and si-p62.3 was selected
for subsequent studies due to the highest level of transfection
efficiency (Fig. 3D and E). Then,
the present study immunoblotted necroptosis-related proteins after
treatment with si-p62/SQSTM1 and found the ratios of p-RIP1/RIP1,
p-RIP3/RIP3 and p-MLKL/MLKL proteins were decreased (Fig. 3F and G). Moreover, in contrast to
the si-scramble group, which increased the rate of SKOV3/DDP cell
survival after treatment with SAS and Nec-1 compared with SAS
alone, following transfection with si-p62, there was no change in
the rate of SKOV3/DDP cell survival in the 10 µM Nec-1 and SAS
combination group compared with the SAS group (Fig. 3H).
ZZ domain of p62/SQSTM1 regulates
necroptosis in SAS-induced SKOV3/DDP cells
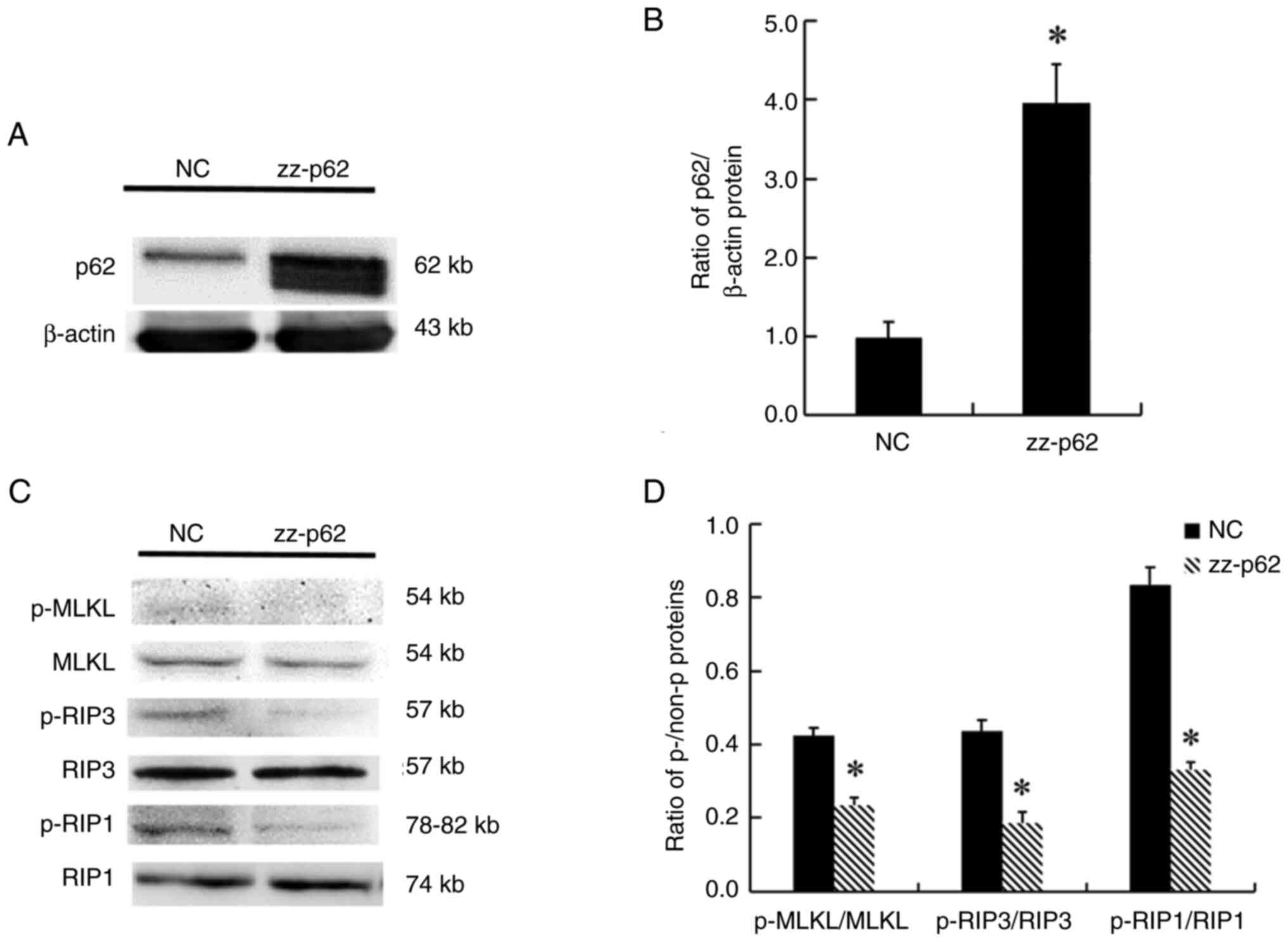
The molecular structure of p62/SQSTM1 contains a ZZ
domain that binds to RIP1. In the present study, p62/SQSTM1 protein
with a ZZ domain deletion was overexpressed in ovarian carcinoma
SKOV3 cells (Fig. 4A and B).
Following the aforementioned overexpression, the ratios of
p-RIP1/RIP1, p-RIP3/PIR3 and p-MLKL/MLKL were decreased (Fig. 4C and D).
Discussion
The modulation of necroptosis, a type of
non-apoptotic programmed cell death, seems to be a promising
approach to overcoming apoptotic drug resistance (20,21).
To improve the response to acquired resistance, trastuzumab
resistance is sensitized through the necroptosis pathway in breast
cancer, and the survival rates of patients with breast cancer are
affected (22). Results of a
previous study demonstrated that SAS induces the apoptosis of
cisplatin-tolerant small cell lung cancer cells (23), and may also induce apoptosis and
autophagy in glioma U251 cells (24). In the present study, SKOV3/DDP cells
exhibited a higher tolerance to SAS compared with SKOV3 cells.
Results of the MTT assay and RTCA demonstrated that the response to
Nec-1 also differed between SKOV3 and SKOV3/DDP cells. Notably, the
survival rate of SKOV3/DDP cells was increased following treatment
with a combination of SAS and Nec-1, while the survival rate of
SKOV3 cells was not increased following treatment with a
combination of SAS and Nec-1. In SKOV3/DDP cells, p- and
non-p-proteins, RIP1 and RIP3, formed complexes, and these were
increased following treatment with SAS. These results indicated
that the necroptosis could be induced in SKOV3/DDP cells by SAS and
increased the sensitivity of SKOV3/DDP cells to SAS.
The results of our previous study revealed that the
multifunctional protein p62/SQSTM1 is expressed at low levels in
SKOV3 cells, and expressed at high levels in SKOV3/DDP cells
(18). Results of the present study
demonstrated that the protein expression levels of p62/SQSTM1, RIP1
and RIP3 were increased in SKOV3/DDP cells following SAS treatment
for 12 h. Although autophagy directly inhibits necroptosis through
degradation of the kinase RIP1 (25), p62/SQSTM1 also co-immunoprecipitated
with RIP1 and provided a scaffold for necroptosis signaling
following treatment with tumor necrosis factor-related
apoptosis-inducing ligand (19).
The results of the present study also revealed that the expression
levels of necroptosis-associated proteins, namely, p-RIP1, p-RIP3
and p-MLKL, were decreased following SAS treatment and p62/SQSTM1
knockdown in SKOV3/DDP cells. These results suggested that the
p62/SQSTM1 protein may be required for RIP1 localization to
autophagy machinery, leading to efficient assembly and activation
of the necrosome in SAS-induced SKOV3/DDP cells. The ubiquitination
and kinase activity of RIP1 dictates whether TNF signaling leads to
activation of NF-κB; thus, protecting cells from apoptosis-mediated
death (26), and triggering
apoptosis (27) or necroptosis
(28). In addition, TNF-α promotes
necroptosis and apoptosis through various complexes involving RIP1,
and the balance of protein activity controls caspase-dependent and
-independent cell death (19). The
results of the present study demonstrated that under transfection
with si-p62, cell survival induced by Nec-1 and SAS was not
increased compared with the survival of SKOV3/DDP cells following
treatment with SAS. These results indicated that p62/SQSTM1
inhibition led to the activation of apoptosis, rather than
necroptosis. The p62/RIP1/RIP3/MLKL pathway could be involved in
necroptosis in SKOV3/DDP cells treated with SAS, and may increase
the sensitivity of cisplatin-resistant ovarian cancer cells.
Notably, the multi-domain adapter protein,
p62/SQSTM1, contains rich protein interaction sequences and
domains, and plays certain functions in tumor progression depending
on the interaction factors recruited. Mutations in the p62/SQSTM1
UBA domain increases the localization of hexokinase 2 on the
mitochondria, and increases the phosphorylated ubiquitin form of
Parkin (29). This stabilizes the
mitophagy process in ovarian cancer A2780 cells, ultimately
enabling cell survival. p62/SQSTM1 exhibits pro-death functions
through the functional domain, ubiquitin-associated domain and
LC3II-interacting region domain interacting with caspase-8
(15). In addition, the ZZ domain
of p62/SQSTM1 binds to N-terminally arginylated proteins, and these
are considered activators of the autophagic activity of p62/SQSTM1,
rather than autophagy cargos (30).
The results of a previous study demonstrated that SAS is a classic
inhibitor of NF-κB (24). Notably,
the ZZ domain of p62/SQSTM1 regulates K63-associated ubiquitination
of RIP1, and loss of the ZZ domain from p62/SQSTM1 leads to poor
proliferative capacity and high levels of apoptosis in SKOV3 cells.
This ultimately leads to higher levels of cisplatin sensitivity
(31). In the present study, the
effects of necroptosis were determined using mutant p62/SQSTM1
molecules lacking the ZZ domain required for RIP1 interaction.
Notably, the ZZ domain deletion of p62/SQSTM1 significantly
inhibited p-RIP1, p-RIP3 and p-MLKL expression levels in SKOV3
cells.
The present study exhibits limitations, for example,
there remains to be a lack of experiments on the colocalization of
p62/SQSTM1 with the necrosome after the induction of SAS treatment,
the interaction of ZZ with the necrosome and the two cells
different response when treated with the same concentration of SAS.
Moreover, in mammals, there are two autophagy receptors; namely,
neighbor of BRCA1 gene 1 and p62/SQSTM1, and these both contain a
ZZ domain. These results suggest that activation of the necrosome
was regulated by the ZZ domain of p62/SQSTM1; however, the
functional significance of the multi-specific binding ability of
the aforementioned ZZ domains is yet to be fully established.
Further studies should address these questions to fully clarify the
function of p62/SQSTM1 by serving as a scaffold for necrosome in
ovarian cancer.
Collectively, certain drugs were able to induce
necroptosis in SKOV3/DDP, while p62/RIP1/RIP3/MLKL was associated
with the induction of necroptosis and increased the sensitivity of
cisplatin-resistant ovarian cancer cells. Thus, therapeutic
targeting of necroptosis or its associated p62/RIP1/RIP3/MLKL
pathway exhibits potential for restoring cisplatin sensitivity as a
overcoming resistance therapeutic target.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The Department of
Science and Technology of Jilin Province (grant nos.
YDZJ202101ZYTS090, 20230203073SF, 20220303003SF), The National
Natural Science Foundation of China (grant no. 81541148) and The
Graduate Innovation Program from Beihua University (grant no.
2022-014, 2024-022).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NL, CY and HY contributed to design, plan and
interpret the data. SL, XZ and WT contributed to acquisition of
data. HJ, XYe and XYa carried out analysis and interpretation of
data. NL and CY confirm the authenticity of all the raw data. All
authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work (including the data)
are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MLKL
|
mixed lineage kinase domain-like
protein
|
|
SQSTM1
|
sequestosome-1
|
|
RIP1
|
receptor-interacting serine/ threonine
kinase 1
|
|
RIP3
|
receptor-interacting serine/threonine
kinase 3
|
|
SAS
|
sulfasalazine
|
|
ZZ
|
zinc finger
|
References
|
1
|
Zhang C and Liu N: Ferroptosis,
necroptosis, and pyroptosis in the occurrence and development of
ovarian cancer. Front Immunol. 13:9200592022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu Y, Lin Z, Zhao N, Zhou L, Liu F,
Cichacz Z, Zhang L, Zhan Q and Zhao X: Receptor interactive protein
kinase 3 promotes Cisplatin-triggered necrosis in
apoptosis-resistant esophageal squamous cell carcinoma cells. PLoS
One. 9:e1001272014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Su SS, Zhao S, Yang Z, Zhong CQ,
Chen X, Cai Q, Yang ZH, Huang D, Wu R and Han J: RIP1
autophosphorylation is promoted by mitochondrial ROS and is
essential for RIP3 recruitment into necrosome. Nat Commun.
8:143292017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schenk B and Fulda S: Reactive oxygen
species regulate Smac mimetic/TNFα-induced necroptotic signaling
and cell death. Oncogene. 34:5796–5806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu SK, Chang WT, Lin IL, Chen YF,
Padalwar NB, Cheng KC, Teng YN, Wang CH and Chiu CC: The role of
necroptosis in ROS-Mediated cancer therapies and its promising
applications. Cancers (Basel). 12:21852020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shindo R, Kakehashi H, Okumura K, Kumagai
Y and Nakano H: Critical contribution of oxidative stress to
TNFα-induced necroptosis downstream of RIPK1 activation. Biochem
Biophys Res Commun. 436:212–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Narang VS, Pauletti GM, Gout PW, Buckley
DJ and Buckley AR: Suppression of cystine uptake by sulfasalazine
inhibits proliferation of human mammary carcinoma cells. Anticancer
Res. 23:4571–4579. 2003.PubMed/NCBI
|
|
8
|
Ji X, Qian J, Rahman SMJ, Siska PJ, Zou Y,
Harris BK, Hoeksema MD, Trenary IA, Heidi C, Eisenberg R, et al:
xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small
cell lung cancer progression. Oncogene. 37:5007–5019. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo W, Zhao Y, Zhang Z, Tan N, Zhao F, Ge
C, Liang L, Jia D, Chen T, Yao M, et al: Disruption of xCT inhibits
cell growth via the ROS/autophagy pathway in hepatocellular
carcinoma. Cancer Lett. 312:55–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jovanović L, Nikolić A, Dragičević S,
Jović M and Janković R: Prognostic relevance of autophagy-related
markers p62, LC3, and Beclin1 in ovarian cancer. Croat Med J.
63:453–460. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adams O, Dislich B, Berezowska S, Schläfli
AM, Seiler CA, Kröll D, Tschan MP and Langer R: Prognostic
relevance of autophagy markers LC3B and p62 in esophageal
adenocarcinomas. Oncotarget. 7:39241–39255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kharaziha P, Chioureas D, Baltatzis G,
Fonseca P, Rodriguez P, Gogvadze V, Lennartsson L, Björklund AC,
Zhivotovsky B, Grandér D, et al: Sorafenib-induced defective
autophagy promotes cell death by necroptosis. Oncotarget.
6:37066–37082. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goodall ML, Cramer SD and Thorburn A:
Autophagy RIPs into cell death. Cell Cycle. 15:3014–3015. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sanz L, Sanchez P, Lallena MJ, Diaz-Meco
MT and Moscat J: The interaction of p62 with RIP links the atypical
PKCs to NF-kappaB activation. EMBO J. 18:3044–3053. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan XY, Zhong XR, Yu SH, Zhang LC, Liu YN,
Zhang Y, Sun LK and Su J: p62 aggregates mediated Caspase 8
activation is responsible for progression of ovarian cancer. J Cell
Mol Med. 23:4030–4042. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaiser WJ, Daley-Bauer LP, Thapa RJ,
Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L,
Speck SH, et al: RIP1 suppresses innate immune necrotic as well as
apoptotic cell death during mammalian parturition. Proc Natl Acad
Sci USA. 111:7753–7758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the Nomenclature Committee on cell death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu H, Su J, Xu Y, Kang J, Li H, Zhang L,
Yi H, Xiang X, Liu F and Sun L: p62/SQSTM1 involved in cisplatin
resistance in human ovarian cancer cells by clearing ubiquitinated
proteins. Eur J Cancer. 47:1585–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goodall ML, Fitzwalter BE, Zahedi S, Wu M,
Rodriguez D, Mulcahy-Levy JM, Green DR, Morgan M, Cramer SD and
Thorburn A: The Autophagy Machinery controls cell death switching
between apoptosis and necroptosis. Dev Cell. 37:337–349. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen D, Yu J and Zhang L: Necroptosis: An
alternative cell death program defending against cancer. Biochim
Biophys Acta. 1865:228–236. 2016.PubMed/NCBI
|
|
21
|
Basit F, Cristofanon S and Fulda S:
Obatoclax (GX15-070) triggers necroptosis by promoting the assembly
of the necrosome on autophagosomal membranes. Cell Death Differ.
20:1161–1173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dolapçi İB, Noyan S, Polat AY, Gürdal H
and Dedeoğlu BG: miR-216b-5p promotes late apoptosis/necroptosis in
trastuzumab-resistant SK-BR-3 cells. Turk J Biol. 47:199–207. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bagherpoor AJ, Shameem M, Luo X, Seelig D
and Kassie F: Inhibition of lung adenocarcinoma by combinations of
sulfasalazine (SAS) and disulfiram-copper (DSF-Cu) in cell line
models and mice. Carcinogenesis. 44:291–303. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su J, Liu F, Xia M, Xu Y, Li X, Kang J, Li
Y and Sun L: p62 participates in the inhibition of NF-κB signaling
and apoptosis induced by sulfasalazine in human glioma U251 cells.
Oncol Rep. 34:235–243. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bray K, Mathew R, Lau A, Kamphorst JJ, Fan
J, Chen J, Chen HY, Ghavami A, Stein M, DiPaola RS, et al:
Autophagy suppresses RIP kinase-dependent necrosis enabling
survival to mTOR inhibition. PLoS One. 7:e418312012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Kobayashi M, Blonska M, You Y and
Lin X: Ubiquitination of RIP is required for tumor necrosis factor
alpha-induced NF-kappaB activation. J Biol Chem. 281:13636–13643.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tenev T, Bianchi K, Darding M, Broemer M,
Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K
and Meier P: The Ripoptosome, a signaling platform that assembles
in response to genotoxic stress and loss of IAPs. Mol Cell.
43:432–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holler N, Zaru R, Micheau O, Thome M,
Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B and Tschopp
J: Fas triggers an alternative, caspase-8-independent cell death
pathway using the kinase RIP as effector molecule. Nat Immunol.
1:489–495. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu S, Yan X, Tian R, Xu L, Zhao Y, Sun L
and Su J: An experimentally induced mutation in the UBA Domain of
p62 changes the sensitivity of cisplatin by Up-Regulating HK2
localisation on the mitochondria and increasing mitophagy in A2780
ovarian cancer cells. Int J Mol Sci. 22:39832021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang YY, Zhang J, Liu XM, Li Y, Sui J,
Dong MQ, Ye K and Du LL: Molecular and structural mechanisms of ZZ
domain-mediated cargo selection by Nbr1. EMBO J. 40:e1074972021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan XY, Zhang Y, Zhang JJ, Zhang LC, Liu
YN, Wu Y, Xue YN, Lu SY, Su J and Sun LK: p62/SQSTM1 as an
oncotarget mediates cisplatin resistance through activating
RIP1-NF-κB pathway in human ovarian cancer cells. Cancer Sci.
108:1405–1413. 2017. View Article : Google Scholar : PubMed/NCBI
|