Introduction
According to the latest global cancer data, there
are expected to be 2.3 million new cases of breast cancer worldwide
by 2022, accounting for 11.6% of all cancer cases. In 157
countries, breast cancer is the most common cancer among women
(1). The incidence of bone
metastasis (BM) in patients with breast cancer is ~8%, but can
reach 30–85% in cases of advanced breast cancer (2). Breast cancer remains the leading cause
of cancer-associated deaths among women. It is estimated that
666,000 women succumbed to breast cancer in 2022 worldwide, with
metastatic disease being the main cause of death rather than the
primary cancer (1,3). BM can disrupt bone metabolism, leading
to bone-related events such as bone pain, pathological fractures,
spinal cord compression, and hypercalcemia, which markedly affect
the quality of life of patients and can even be life-threatening
(4). A previous study showed that
BM is a crucial factor affecting the prognosis of patients with
breast cancer. The 5-year survival rate for patients with
early-stage breast cancer without metastasis is as high as 90%, but
once BM occurs, the 5-year survival rate drops to 10% (5). There is currently no cure for patients
with breast cancer and BM; however, appropriate treatment can
prolong survival and improve the quality of life of the patient.
Therefore, it is important to accurately assess whether patients
with breast cancer have BM (6).
X-ray imaging is routinely used to screen for bone
disease, but is not effective for early BM detection because it
only identifies lesions after a 30–50% loss of calcium (7). Bone scans are imaging techniques with
high sensitivity but low specificity for the detection of bone
lesions (8). Therefore, more
sensitive and accurate methods are necessary to detect the BM
associated with breast cancer earlier so that intervention can be
initiated sooner, and thereby improve the survival time of the
patient. Positron emission tomography/computed tomography (PET/CT)
is an advanced diagnostic imaging technology that provides both
metabolic information and precise anatomical localization. It has
broad applications in the diagnosis, staging, location and
treatment evaluation of various malignant tumors (9,10).
Fluorine-18 fluorodeoxyglucose (18F-FDG) is a PET/CT
tracer that is widely used for the diagnosis, staging and follow-up
of patients with breast cancer due to its high diagnostic
performance for lesions (11). A
bone-specific radiotracer, 18F-sodium fluoride
(18F-NaF), is effective in revealing changes in bone
activity and has been widely used for the clinical detection of
bone lesions (12,13). In patients with breast cancer and
BM, metastases are predominantly osteolytic, but are osteogenic in
15–20% of cases (14–16). It has been shown that
18F-FDG is most sensitive in the detection of osteolytic
metastases (17). Therefore, the
present meta-analysis reviewed studies on the detection of BM in
patients with breast cancer using PET/CT. The aim was to
quantitatively evaluate and compare the diagnostic performance of
18F-FDG and 18F-NaF as PET/CT tracers in the
detection of BM associated with breast cancer.
Patients and methods
Literature search to identify relevant
studies
The present study was conducted in accordance with
the Cochrane Collaboration's Systematic Review guidelines and
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
requirements (18). The English
literature on 18F-FDG PET/CT or 18F-NaF
PET/CT in the detection of BM in breast cancer was retrieved from
the PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Embase
(https://www.embase.com/) databases. A systematic
search was performed used multiple keywords: (‘PET/CT’ OR ‘PET-CT’
OR ‘positron emission tomography/computed tomography’ OR ‘positron
emission tomography-computed tomography’) AND (‘breast cancer’ OR
‘breast carcinoma’ OR ‘mammary cancer’ OR ‘breast tumor;) AND
(‘bone metastasis’ OR ‘skeletal metastases’ OR ‘osseous
metastasis’) AND (‘18F-fluorodeoxyglucose’ OR
‘18F-FDG’ OR ‘18F-NaF’ OR
‘18F-fluoride’). The publication period was limited from
January 1, 2000 to January 31, 2022. The final list of articles was
supplemented by cross-checking the reference lists of all retrieved
articles.
Study selection and quality
assessment
Two reviewers independently screened all titles and
read abstracts. The full text of the selected articles was reviewed
to determine eligibility. Data extraction and evaluation were
performed independently by two authors, with disputes resolved by a
third reviewer. Studies included in the meta-analysis met all of
the following criteria: i) Patients of any age with breast cancer
at any stage of disease, regardless of treatment status; ii)
18F-FDG PET/CT or 18F-NaF PET/CT used in the
imaging and characterization of BM in patients with breast cancer;
iii) histopathological findings or CT, magnetic resonance imaging
(MRI) or clinical follow-up over 6 months included as reference
standards; iv) a 2×2 contingency table could be constructed using
directly extracted data or by the calculation of true positive
(TP), false positive (FP), false negative (FN) and true negative
(TN) values based on the sensitivity, specificity, and positive and
negative prediction values provided in the article. Exclusion
criteria were: i) Studies with <10 patients with breast cancer;
ii) studies where the PET/CT tracer was not 18F-FDG or
18F-NaF; iii) studies with multiple published data or
subsets of data; iv) case reports, letters, editorials, reviews,
animal studies, in vitro studies and studies without
original data; v) studies presenting results from different imaging
modalities jointly, or those in which it was not possible to
distinguish between the test performance assessments of individual
imaging modalities.
The QUADAS-2 tool was used for the quality
assessment of diagnostic accuracy, covering four key areas: Patient
selection, index tests, reference standards and the flow and timing
of patients through the study (19).
Data extraction
Data extraction was performed independently by two
investigators. For each relevant study, the following data were
collected: i) Basic information such as the first author,
publication year, country and sample size; ii) patient age, patient
selection (continuous or non-continuous) and clinical background;
iii) study design information; iv) examination results, including
the numbers of TP, FP, TN and FN cases; v) parameters of the CT
techniques used for 18F-FDG PET or 18F-NaF
PET/CT. If there was a dispute between the reviewers, a third
researcher evaluated all discordant items until a consensus was
reached.
Statistical analysis
Stata software version 14.0 (StataCorp LP) was used
to perform the statistical analysis. The diagnostic performance of
18F-FDG PET/CT and 18F-NaF PET/CT in the
detection of BM in breast cancer was evaluated using specificity,
sensitivity, positive likelihood ratio (PLR), negative likelihood
ratio (NLR), diagnostic odds ratio (DOR) and summary receiver
operating characteristic (SROC) curves based on TP, FP, FN, and TN
values extracted from the included studies. The area under the
curve (AUC) and 95% confidence intervals (CIs) were calculated.
Analyses were performed using the DerSimonan-Laird method, a
random-effects model, to calculate weighted mean pooled
sensitivity, specificity, PLR, NLR and DOR and their corresponding
95% CIs. Variability was assessed graphically by plotting metrics
with 95% CIs for each study separately in a forest plot. Values of
pooled PLR >10 and DOR >100 indicate that a positive test
result helps to confirm the presence of BM, while pooled NLR values
<0.1 indicate that a negative test result helps to exclude BM
(20). Hierarchical logistic
regression models were used to estimate the sensitivity and
specificity of the included studies. For each study included in a
forest plot, the corresponding 95% CIs were shown to graphically
represent the index being measured. Heterogeneity among the studies
was assessed using Cochran's Q test and Higgins I2 test
(20). In Cochran's Q test,
P<0.05 indicated the presence of heterogeneity. The degree of
heterogeneity was assessed using the following criteria: An
inconsistency index (I2) <50% indicated low
heterogeneity; an I2 of 50–80% indicated moderate
heterogeneity; and an I2 >80% indicated high
heterogeneity. Subgroup analyses for 18F-FDG PET/CT were
performed based on study sample size, mean patient age, study
design type, attenuation correction, minimum scan slice thickness,
imaging system supplier and whether the study was patient- or
lesion-based. Publication bias was assessed using a funnel plot and
Deek's asymmetry test for both 18F-FDG PET/CT and
18F-NaF PET/CT (21).
The potential publication bias was estimated using Egger's
quantitative test. A two-sample Z-test was used to evaluate the
difference in diagnostic performance between the two methods for
the detection of BM in breast cancer, with P<0.05 considered to
indicate a statistically significant result.
Results
Eligible studies and quality
assessment
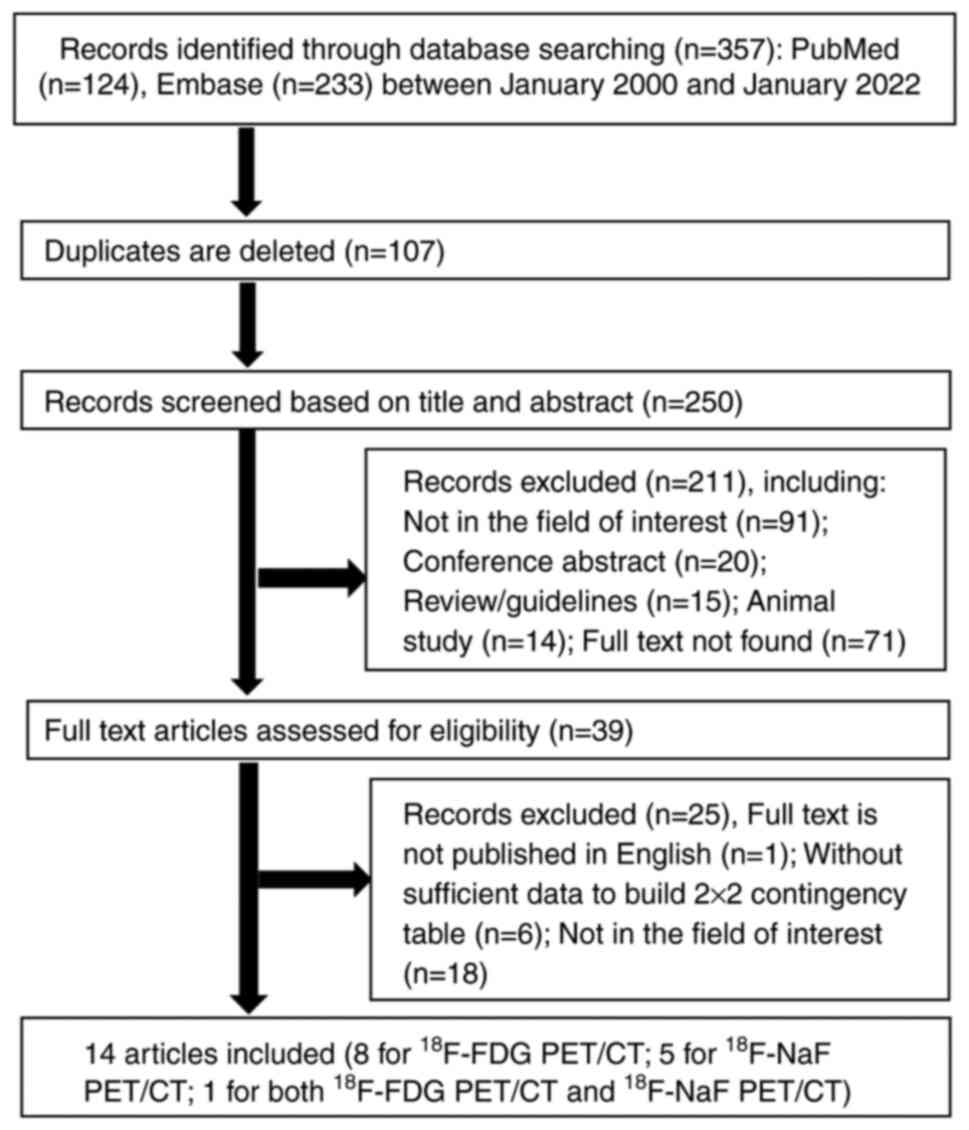
A literature search identified357 potentially
relevant articles. After the exclusion of 107 duplicates, the
screening of titles and abstracts led to the exclusion of a further
211 articles for being reviews or guidelines (n=15), conference
papers (n=20), animal studies (n=14) or on irrelevant topics
(n=91), or due to the full text not being available (n=71). After
reading the full texts of the remaining 39 articles, 25 articles
were excluded due to not being published in English (n=1), lacking
the data to construct a 2×2 contingency table (n=6), or not being
relevant to the area of interest (n=18). Finally, 14 articles on
the diagnostic performance of 18F-FDG or
18F-NaF PET/CT in breast cancer BM met the criteria for
inclusion in the present meta-analysis. The identification and
selection process for the studies is shown in Fig. 1.
A total of 14 articles (15,22–34)
were included in the study. These comprised 8 studies on
18F-FDG PET/CT, 5 studies on 18F-NaF PET/CT,
and 1 study on both, including a total of 919 patients and 2,054
lesions. The sample sizes in the studies ranged from 20 to 150
patients, with mean ages ranging from 43.8 to 64 years. All 14
articles were published between 2010 and 2019, and comprised 8
prospective studies and 6 retrospective studies. Among these, 3
studies included patients with breast cancer who had previously
received treatment, 3 studies included patients newly diagnosed
with breast cancer who were clinically suspected of having BM, and
8 studies included both treated and newly diagnosed patients. The
baseline characteristics of each study are presented in Table I, and the PET/CT parameters used in
each study are presented in Table
SI. The quality of each study was assessed using the QUADAS-2
tool. This assessment revealed that all studies met at least 5 of
the 7 reference criteria, which included 4 items associated with
the risk of bias, namely patient selection, index test, reference
standard, and flow and timing, and 3 items associated with
application concerns, namely patient selection, index test and
reference standard; therefore, they were considered satisfactory
(35). With regard to patient
selection, 5 studies (22,24,25,29,32)
were considered high-risk for reference standards, as only imaging
and follow-up results were used as the reference standards.
Additionally, one study had only a 2-month follow-up period
(24), which was also considered
high-risk. The risk of bias for flow and timing was unclear in all
studies because the time interval between the index test and the
reference standard was not reported. The results of the QUADAS-2
assessment are shown in Table
SII.
 | Table I.Clinical characteristics and
diagnostic results of the detection of bone metastases reported in
each eligible study. |
Table I.
Clinical characteristics and
diagnostic results of the detection of bone metastases reported in
each eligible study.
| A,
18F-FDG PET/CT |
|---|
|
|---|
|
|
|
|
| Study design |
|
| Patient-based
analysis | Lesion-based
analysis |
|
|
|---|
| First author,
year | Country | No. of
patients | No. of lesions |
| Clinical
setting | Mean age (range),
years |
|
| Reference
standard | (Refs.) |
|---|
| Prospective | Multicenter | Consecutive | TP | FP | FN | TN | TP | FP | FN | TN |
|---|
| Koizumi et
al, 2019 | Japan | 120 | - | No | No | Yes | New + | - | 34 | 8 | 1 | 77 | - | - | - | - | HP follow-up >6
months | (30) |
|
|
|
|
|
|
|
| treated |
|
|
|
|
|
|
|
|
|
|
|
| Caglar et
al, 2016 | Turkey | 150 | - | No | No | Yes | New + treated | 52 (27–85) | 84 | 1 | 1 | 64 | - | - | - | - | HP; follow-up
>10 months | (26) |
| Hahn et al,
2011 | Germany | 28 | 129 | No | No | Yes | New + treated | 57.5 (35–78) | 7 | 0 | 1 | 20 | 67 | 5 | 3 | 54 | MRI follow-up | (22) |
| Damle et al,
2013 | India | 72 | - | Yes | No | Yes | Treated | 52 (30–77) | 25 | 1 | 9 | 37 | - | - | - | - | HP; consensus from
MRI/thin-slice CECT/skeletal radiograph findings | (15) |
| Al-Muqbel,
2017 | Jordan | 35 | - | No | No | Yes | Treated | 48.1 | 25 | 0 | 9 | 1 | - | - | - | - | Staging or
follow-up 18F-FDG-PET/CT. | (25) |
| Botsikas et
al, 2019 | Switzerland | 80 | 175 | Yes | No | Yes | New + treated | 48 | 6 | 0 | 3 | 71 | 18 | 0 | 8 | 149 | HP; follow-up
>12 months | (34) |
| Rager et al,
2018 | Switzerland | 25 | 109 | No | Yes | Yes | New | 55 (38–82) | 10 | 0 | 2 | 13 | 43 | 0 | 48 | 18 | Follow-up >21
months | (24) |
| Heusner et
al, 2010 | Germany | 20 | - | Yes | No | Yes | New + treated | 54.5
(25.4–78.2) | 7 | 0 | 0 | 13 | - | - | - | - | Consensus from MRI
and bone scan | (28) |
| Teke et al,
2015 | Turkey | - | 496 | No | No | - | New | 44.5 (28–81) | - | - | - | - | 141 | 2 | 10 | 343 | Follow-up >6
months | (32) |
|
| B,
18F-NaF PET/CT |
|
|
|
|
|
| Study
design |
|
| Patient-based
analysis | Lesion-based
analysis |
|
|
| First author,
year | Country | No. of
patients | No. of
lesions |
| Clinical
setting | Mean age
(range), years |
|
| Reference
standard | (Refs.) |
|
Prospective |
Multicenter |
Consecutive | TP | FP | FN | TN | TP | FP | FN | TN |
|
| Yoon et al,
2013 | Korea | - | 119 | Yes | No | - | New + treated | 55.6 | - | - | - | - | 49 | 36 | 3 | 31 | HP; follow-up
>12 months | (33) |
| Broos et al,
2018 | Netherlands | 118 | - | Yes | No | - | New + | 64 | 50 | 6 | 2 | 60 | - | - | - | - | Follow-up >6
months | (23) |
|
|
|
|
|
|
|
| treated |
|
|
|
|
|
|
|
|
|
|
|
| Damle et al,
2013 | India | 72 | - | Yes | No | Yes | Treated | 52 (30–77) | 34 | 11 | 0 | 27 | - | - | - | - | HP; consensus from
MRI/thin-slice CECT/skeletal radiograph findings | (15) |
| Passah et
al, 2017 | India | - | 199 | Yes | No | - | New | 43.8 | - | - | - | - | 178 | 0 | 0 | 21 |
99mTc-MDP skeletal
scintigraphy | (29) |
| Abikhzer et
al, 2016 | UK | 41 | 284 | Yes | No | - | New + treated | 58 (30–75) | 21 | 3 | 0 | 17 | 73 | 6 | 7 | 198 | HP; follow-up
>33 months | (31) |
| Piccardo et
al, 2012 | Italy | 39 | 662 | Yes | - | - | Treated | 60 | 27 | 0 | 0 | 12 | 491 | 11 | 51 | 109 | Follow-up >12
months | (27) |
Diagnostic accuracy
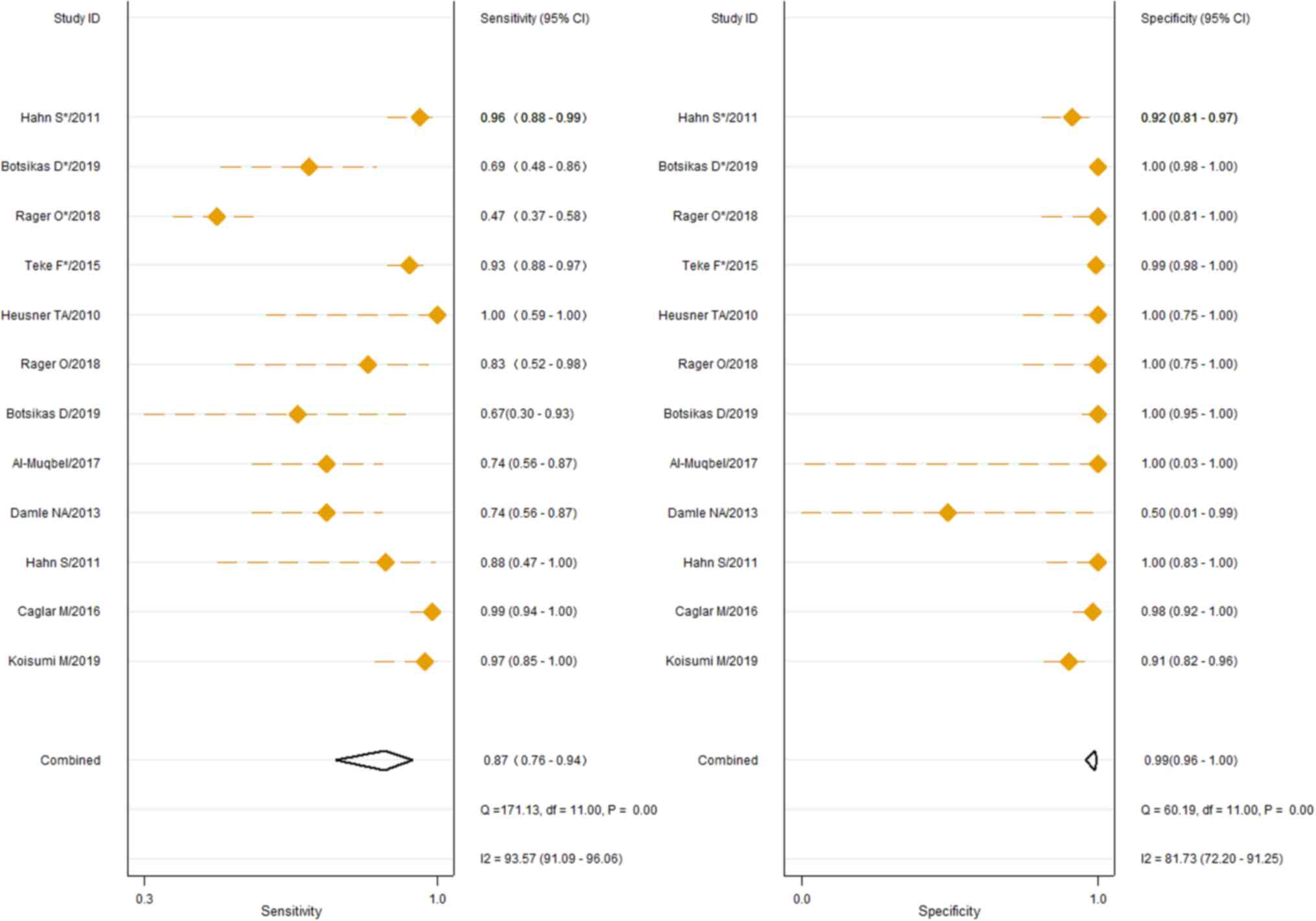
The 9 studies using the 18F-FDG PET/CT
method had sensitivities ranging from 0.47 (95% CI, 0.37–0.58) to
1.0 (95% CI, 0.59–1.00) for the identification of breast cancer BM,
and specificities ranging from 0.91 (95% CI, 0.82–0.96) to 1.0 (95%
CI, 0.98–1.00). The pooled sensitivity and specificity of
18F-FDG PET/CT for the identification of BM derived from
breast cancer were 0.88 (95% CI, 0.76–0.94) and 0.99 (95% CI,
0.97–1.00), respectively, as shown in Fig. 2. In addition, Cochran's Q test and
Higgins I2 test indicated high heterogeneity in
sensitivity (Q, 168.81, P≤0.01; I2, 93.48) and moderate
heterogeneity in specificity (Q, 44.38, P≤0.01; I2,
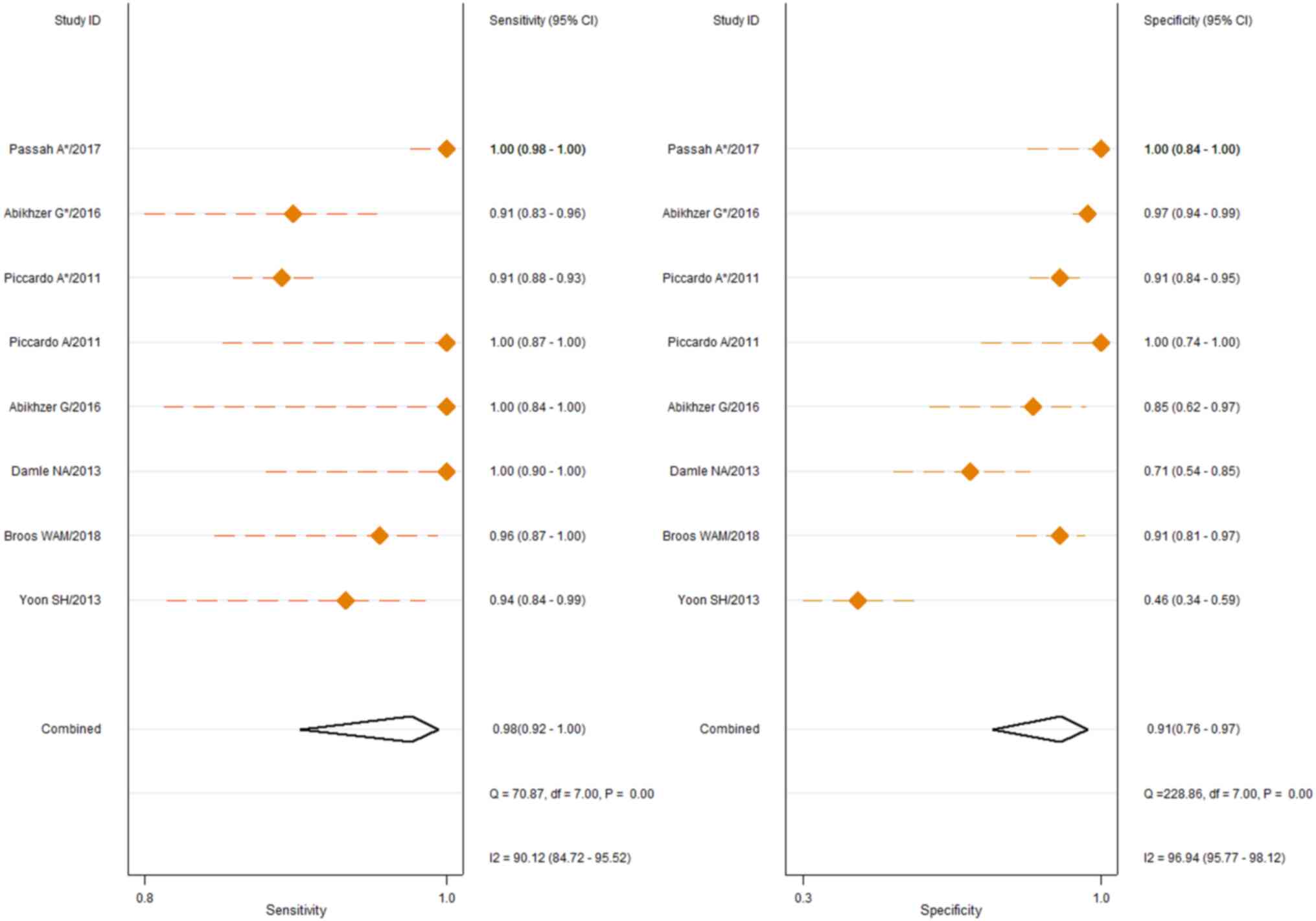
75.21). The 6 studies describing the use of 18F-NaF
PET/CT in the detection of breast cancer BM had sensitivities
ranging from 0.91 (95% CI, 0.83–0.96) to 1.00 (95% CI, 0.84–1.00)
and specificities ranging from 0.46 (95% CI, 0.34–0.59) to 1.00
(95% CI, 0.74–1.00). The pooled sensitivity and specificity were
0.98 (95% CI, 0.92–1.00) and 0.91 [95% CI, 0.76–0.97),
respectively, as shown in Fig. 3.
Cochran's Q test and Higgins I2 test also showed high
heterogeneity in sensitivity (Q, 70.87, P≤0.01; I2,
90.12) and specificity (Q, 228.86, P≤0.01; I2, 96.94)
among the studies. The pooled PLR and NLR for 18F-FDG
PET/CT were 129.2 (95% CI, 27.1–616.4) and 0.13 (95% CI,
0.06–0.25), respectively. For 18F-NaF PET/CT, the pooled
PLR and NLR were 10.9 (95% CI, 3.8–31.5) and 0.02 (95% CI,
0.01–0.1), respectively. The pooled DOR for 18F-FDG
PET/CT in the diagnosis of breast cancer BM was 1,028 (95% CI,
244–4,330), while for 18F-NaF PET/CT, the pooled DOR was
489 (95% CI, 65–3,654), as shown in Table II. These data suggest that a
positive result from FDG testing helps confirm the presence of BM,
while a negative result from NaF testing helps to rule out BM. No
significant difference in the DOR between 18F-FDG PET/CT
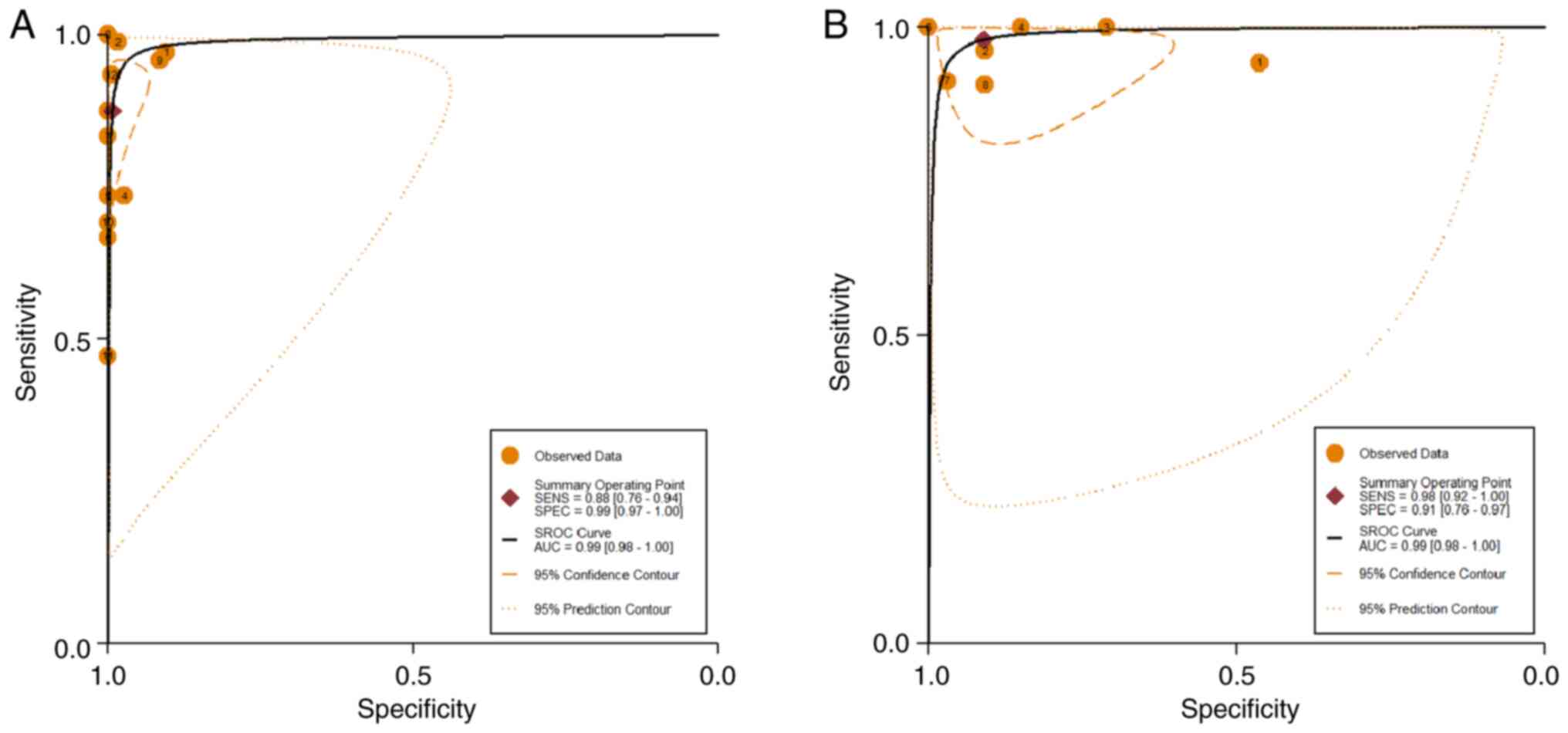
and 18F-NaF PET/CT was detected. The area under the SROC
curves for 18F-FDG PET/CT and 18F-NaF PET/CT
were both 0.99 (95% CI, 0.98–1.00), as shown in Fig. 4.
 | Table II.Summary of the diagnostic performance
characteristics of 18F-FDG and 18F-NaF PET/CT
in breast cancer bone metastases. |
Table II.
Summary of the diagnostic performance
characteristics of 18F-FDG and 18F-NaF PET/CT
in breast cancer bone metastases.
|
| 18F-FDG
PET/CT | 18F-NaF
PET/CT |
|---|
|
|
|
|
|---|
| Parameter | Estimate | 95% CI | Estimate | 95% CI |
|---|
| Sensitivity | 0.88 | 0.76, 0.94 | 0.98 | 0.92, 1.00 |
| Specificity | 0.99 | 0.97, 1.00 | 0.91 | 0.76, 0.97 |
| Positive likelihood
ratio | 129.2 | 27.1, 616.4 | 10.9 | 3.8, 31.5 |
| Negative likelihood
ratio | 0.13 | 0.06, 0.25 | 0.02 | 0.01, 0.1 |
| Diagnostic odds
ratio | 1,028 | 244, 4,330 | 489 | 65, 3,654 |
| AUC | 0.99 | 0.98, 1.00 | 0.99 | 0.98, 1.00 |
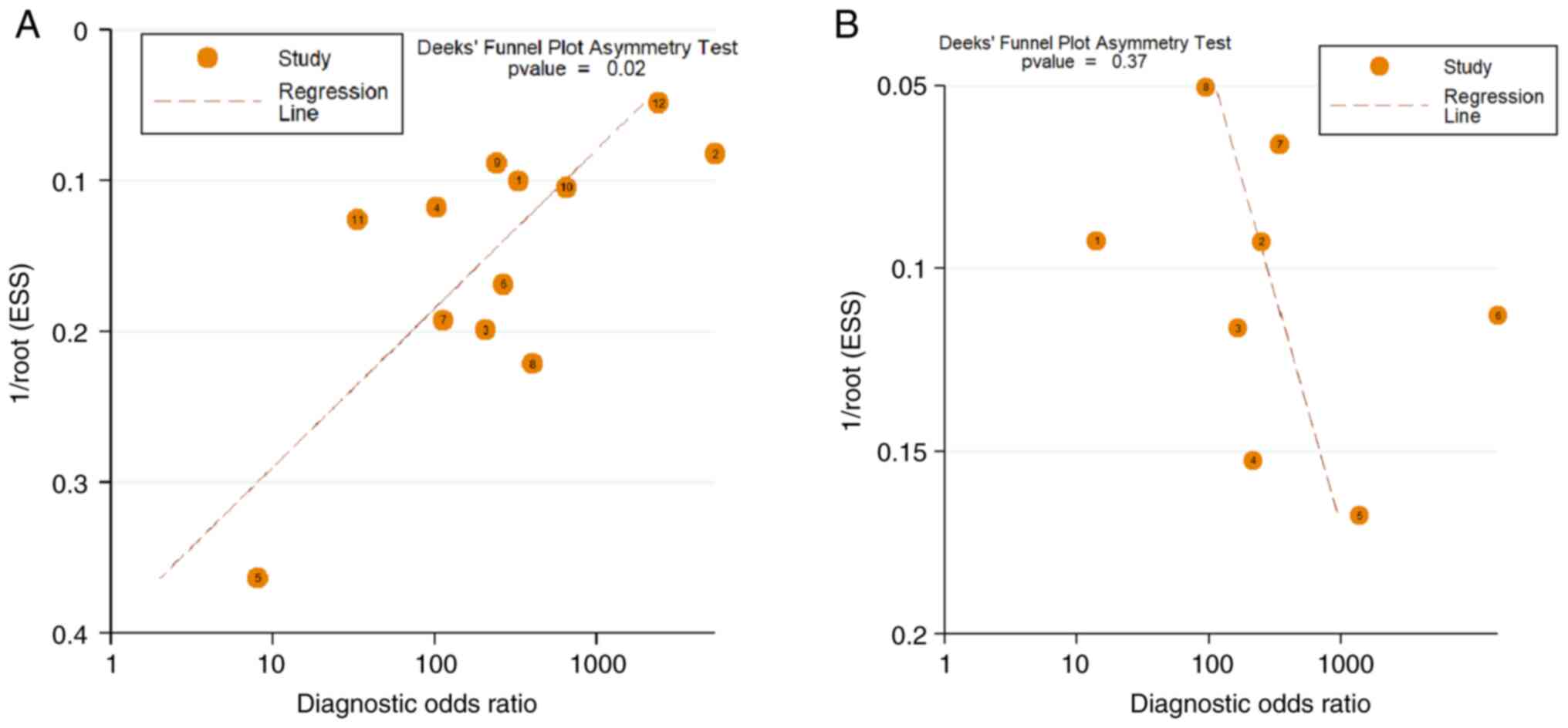
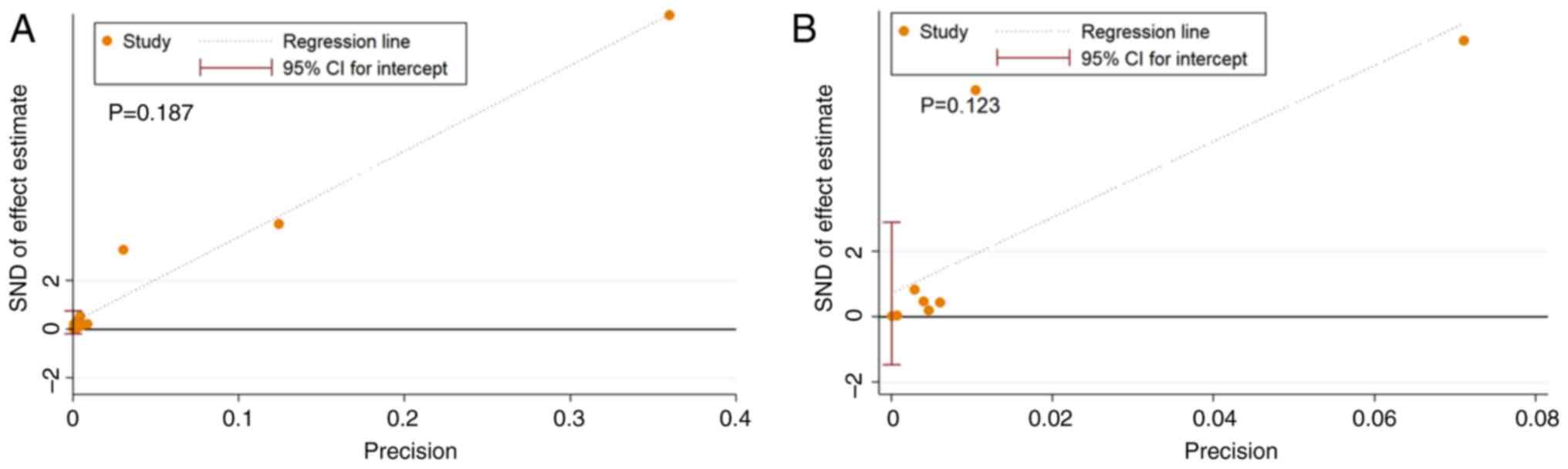
Publication bias
Deek's funnel plots for publication bias in the
studies on 18F-FDG PET/CT and 18F-NaF PET/CT
are shown in Fig. 5. The
statistical significance of the slope coefficient for
18F-FDG PET/CT (P=0.02) is suggestive of publication
bias. However, the slope coefficient for 18F-NaF PET/CT
lacked significance (P=0.37), indicating a low possibility of
publication bias. When analyzed using Egger's test, both
18F-FDG PET/CT and 18F-NaF PET/CT exhibited
no evidence of publication bias (P=0.187 and P=0.123, respectively;
Fig. 6).
Exploration of heterogeneity
The results of the meta-regression analysis are
shown in Table III. Eight studies
reported patient-based results for the performance of
18F-FDG PET/CT in the diagnosis of breast cancer BM,
with a sensitivity of 0.89 (95% CI, 0.80–0.99) and a specificity of
0.99 (95% CI, 0.98–1.00). Four studies, including 909 lesions, were
lesion-based, with a sensitivity of 0.84 (95% CI, 0.67–1.00) and a
specificity of 1.00 (95% CI, 0.98–1.00). The 7 studies in which the
mean age of the patients was ≥50 years had a sensitivity of 0.88
(95% CI, 0.78–0.99) and specificity of 0.98 (95% CI, 0.96–1.00),
while the 4 studies in which the mean age of the patients was
<50 years had a sensitivity of 0.80 (95% CI, 0.60–0.99) and
specificity of 1.00 (95% CI, 0.99–1.00). Patient-based analysis,
mean patient age, slice thickness and imaging system supplier were
not found to be responsible for the between-study heterogeneity
(P>0.05). However, study design, sample size, attenuation
correction value and the different imaging system supplier were
identified as sources of heterogeneity in the diagnostic
performance of 18F-FDG PET/CT in breast cancer BM
(P<0.05). Due to the small number of studies on
18F-NaF PET/CT, it was not possible to perform a further
subgroup analysis to identify the causes of heterogeneity.
 | Table III.Meta-regression analysis results for
fluorine-18 fluorodeoxyglucose positon emission tomography/computed
tomography in the detection of bone metastases in patients with
breast cancer. |
Table III.
Meta-regression analysis results for
fluorine-18 fluorodeoxyglucose positon emission tomography/computed
tomography in the detection of bone metastases in patients with
breast cancer.
| Parameter | No. of studies | Sensitivity (95%
CI) | P-value | Specificity | P-value |
|---|
| Basis |
|
| 0.88 |
| 0.21 |
|
Patient | 8 | 0.89
(0.80–0.99) |
| 0.99
(0.98–1.00) |
|
|
Lesion | 4 | 0.84
(0.67–1.00) |
| 1.00
(0.98–1.00) |
|
| Design |
|
| 0.13 |
| <0.01 |
|
Prospective | 4 | 0.78
(0.55–1.00) |
| 1.00
(0.99–1.00) |
|
|
Retrospective | 8 | 0.91
(0.83–0.98) |
| 0.99
(0.97–1.00) |
|
| Mean age,
years |
|
| 0.73 |
| 0.38 |
|
≥50 | 7 | 0.88
(0.78–0.99) |
| 0.98
(0.96–1.00) |
|
|
<50 | 4 | 0.80
(0.60–0.99) |
| 1.00
(0.99–1.00) |
|
| Sample no. |
|
| 0.65 |
| <0.01 |
|
>50 | 4 | 0.91
(0.81–1.00) |
| 0.98
(0.95–1.00) |
|
|
≤50 | 4 | 0.87
(0.71–1.00) |
| 1.00
(1.00–1.00) |
|
| Vendor |
|
| 0.82 |
| 0.07 |
| GE
Healthcare | 4 | 0.92
(0.81–1.00) |
| 1.00
(0.99–1.00) |
|
| Siemens
Healthineers | 6 | 0.89
(0.77–1.00) |
| 1.00
(0.99–1.00) |
|
| AC |
|
| 0.85 |
| 0.02 |
|
Yes | 7 | 0.89
(0.78–1.00) |
| 1.00
(0.98–1.00) |
|
| No | 5 | 0.86
(0.72–1.00) |
| 0.99
(0.98–1.00) |
|
| Slice thickness,
mm |
|
| 0.55 |
| 0.08 |
| ≥4 | 4 | 0.90
(0.76–1.00) |
| 0.99
(0.95–1.00) |
|
|
<4 | 4 | 0.83
(0.64–1.00) |
| 0.99
(0.95–1.00) |
|
Discussion
In the present meta-analysis, covering 919 patients
and 2,054 lesions from 14 studies, the diagnostic performance of
18F-NaF PET/CT and 18F-FDG PET/CT was
compared in the detection of breast cancer BM. The results indicate
that 18F-NaF PET/CT is more sensitive than
18F-FDG PET/CT for the detection of BM in patients with
breast cancer (98 vs. 88%), while 18F-FDG PET/CT is more
specific than 18F-NaF PET/CT for this purpose (99 vs.
91%). However, these differences are not statistically significant,
suggesting that both tracers have a good diagnostic performance,
with both having an AUC of 0.99 (95% CI, 0.98–1.00) when used in
PET/CT imaging for the detection of BM associated with breast
cancer.
18F-FDG PET/CT is a sensitive molecular
imaging method that is able to diagnose BM by detecting the
increased uptake of FDG in metastatic cancer cells (36). Previous meta-analyses have shown
that 18F-FDG PET/CT has high diagnostic performance in
the identification of lymph node metastasis, staging, evaluation of
treatment efficacy and assessment of the prognosis of patients with
breast cancer after chemotherapy (37–41).
The present meta-analysis, which included 9 studies on
18F-FDG PET/CT with 530 patients and 909 lesions, showed
that 18F-FDG PET/CT has a good diagnostic performance.
As an osteophytic tracer, 18F-NaF offers the
advantageous features of high and rapid bone uptake accompanied by
very rapid blood clearance. This results in a high
bone-to-background ratio in a short time and allows areas of
altered skeletal activity to be displayed, which makes it an
increasingly favored agent for use in the detection of bone lesions
(42). Previous studies have shown
that 18F-NaF PET/CT can accurately detect BM in
malignant tumors such as non-small cell lung cancer, breast cancer
and prostate cancer. In particular, it is useful for assessing the
extent of BM and aiding in treatment decisions, making it a good
tool for the early and accurate detection of BM (15,43).
The present meta-analysis, which included 6 studies on
18F-NaF PET/CT, showed that 18F-NaF PET/CT is
more sensitive but less specific than 18F-FDG PET/CT in
the detection of breast cancer BM. This lower specificity may be
due to benign diseases also being able to cause new bone formation
and increase NaF uptake, which can create false positives (26). Moreover, it is notable that in
addition to showing high accuracy in the detection of BM,
18F-FDG is also highly accurate in the identification of
distant organ tissue metastasis and lymph node metastasis (38,39).
The results of the present meta-analysis indicate that
18F-FDG and 18F-NaF have comparable accuracy
in the detection of BM in patients with breast cancer. Therefore,
it is suggested that 18F-FDG should be considered first
in clinical practice, and additional 18F-NaF
examinations may not be necessary. Previous studies revealed that
18F-FDG PET/CT is more useful than bone imaging for the
detection of osteolytic BM, and that it more accurately detects
pure bone marrow metastases, particularly fast-growing lesions
(41,44), while it is not recommended for
detecting blastic BM (45). For BM
with low 18F-FDG intake, 18F-NaF PET/CT has
been shown to be a better choice due to greater sensitivity
(46). Although the current study
indicates that 18F-FDG and 18F-NaF have
similar diagnostic value, the choice of imaging agent may differ
according to the clinical situation.
The current meta-analysis revealed heterogeneity in
pooled sensitivity and specificity for the studies on both
18F-FDG PET/CT and 18F-NaF PET/CT. Subgroup
analysis showed that study design, sample size and the use of
attenuation correction were factors contributing to heterogeneity
among the studies. Specifically, the specificity of retrospective
studies was lower than that of prospective studies, possibly due to
inherent bias in patient selection. In addition, studies with a
sample size of <50 patients showed higher specificity, which may
be due to the fact that a small sample size means that the
diversity of the sample may be reduced. The specificity of studies
using attenuation correction was higher than that of those without,
likely due to improved image quality and clearer visualization of
the lesions after attenuation correction (44). The meta-regression results indicated
that lesion-based analysis was more specific than patient-based
analysis, and that the specificity of studies with a mean patient
age <50 years was greater than that of studies with a higher
mean patient age, but these differences were not statistically
significant. Slice thickness was not found to contribute to the
heterogeneity between studies observed in the present
meta-analysis. Meta-regression analysis of 18F-NaF
PET/CT was not possible because only 6 studies met the inclusion
criteria, and some data were not available.
The main limitation of the present meta-analysis is
the limited number of eligible studies, particularly those on
18F-NaF PET/CT. During data extraction, it was found
that two articles had inconsistencies in the reported TP, FP, FN
and TN values, and their sensitivity and specificity; therefore,
these studies were excluded (45,46).
Additionally, heterogeneity in the assessment of diagnostic
accuracy among the studies on 18F-FDG PET/CT and
18F-NaF PET/CT limits the quality of the meta-analysis.
Histopathological validation was not available for BM in all
patients; instead, imaging-based reference standards such as CT and
MRI were used, which may increase clinical heterogeneity. However,
as it is impractical and unethical to obtain histological evidence
for all skeletal lesions, non-invasive imaging results that are not
rigorously validated by histological examination are considered
acceptable. Although the present study compared the diagnostic
performance of 18F-NaF PET/CT and 18F-FDG
PET/CT in the detection of breast cancer BM, limited data may
affect the estimates of diagnostic efficacy. However, the
diagnostic performance of these imaging techniques provides a
reference for clinical practice and helps to avoid the subjective
interpretation of results.
In conclusion, the current study shows that
18F-NaF PET/CT and 18F-FDG PET/CT are
accurate methods for the detection of BM in patients with breast
cancer, and are comparable in diagnostic accuracy. Moreover, it
contributes to a more comprehensive understanding of the use of
18F-FDG and 18F-NaF as PET/CT imaging agents
for the detection of BM in patients diagnosed with breast cancer,
and may serve as a point of reference for patient care.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural Science Foundation
of the People's Republic of China, NSFC (grant no. 82260353).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JC, XH and HH conceived the study and designed the
structure of the manuscript. XH and JC were involved in the
methodology, data validation, writing, reviewing and editing the
study, and project supervision/CMS validated the data, carried out
investigation, wrote, reviewed and edited the manuscript, and
completed project supervision. JC, ZL, WY, DL and SL were involved
in the conceptualization of the study, data visualization,
supervision and project administration. XH and JC confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
18F
|
fluorine-18
|
|
BM
|
bone metastasis
|
|
FDG
|
fluorodeoxyglucose
|
|
NaF
|
sodium fluoride
|
|
PET/CT
|
positron emission tomography/computed
tomography
|
|
TP
|
true positive
|
|
FP
|
false positive
|
|
FN
|
false negative
|
|
TN
|
true negative
|
|
PLR
|
positive likelihood ratio
|
|
NLR
|
negative likelihood ratio
|
|
DOR
|
diagnostic odds ratio
|
|
SROC
|
summary receiver operating
characteristic
|
|
AUC
|
area under the curve
|
|
CI
|
confidence interval
|
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cook GJ, Houston S, Rubens R, Maisey MN
and Fogelman I: Detection of bone metastases in breast cancer by
18FDG PET: differing metabolic activity in osteoblastic and
osteolytic lesions. J Clin Oncol. 16:3375–3379. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zengel B, Kilic M, Tasli F, Simsek C,
Karatas M, Ozdemir O, Cavdar D, Durusoy R, Bas KK and Uslu A:
Breast cancer patients with isolated bone metastases and
oligometastatic bone disease show different survival outcomes. Sci
Rep. 11:201752021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pantel K and Hayes DF: Disseminated breast
tumour cells: Biological and clinical meaning. Nat Rev Clin Oncol.
15:129–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Makhoul I, Montgomery CO, Gaddy D and Suva
LJ: The best of both worlds-managing the cancer, saving the bone.
Nat Rev Endocrinol. 12:29–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brook N, Brook E, Dharmarajan A, Dass CR
and Chan A: Breast cancer bone metastases: Pathogenesis and
therapeutic targets. Int J Biochem Cell Biol. 96:63–78. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu T, Cheng T, Xu W, Yan WL, Liu J and
Yang HL: A meta-analysis of 18FDG-PET, MRI and bone scintigraphy
for diagnosis of bone metastases in patients with breast cancer.
Skeletal Radiol. 40:523–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cook GJ, Azad GK and Goh V: Imaging bone
metastases in breast cancer: Staging and response assessment. J
Nucl Med. 57 (Suppl 1):27S–33S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hansen JA, Naghavi-Behzad M, Gerke O, Baun
C, Falch K, Duvnjak S, Alavi A, Høilund-Carlsen PF and Hildebrandt
MG: Diagnosis of bone metastases in breast cancer: Lesion-based
sensitivity of dual-time-point FDG-PET/CT compared to low-dose CT
and bone scintigraphy. PLoS One. 16:e02600662021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garami Z, Hascsi Z, Varga J, Dinya T,
Tanyi M, Garai I, Damjanovich L and Galuska L: The value of 18-FDG
PET/CT in early-stage breast cancer compared to traditional
diagnostic modalities with an emphasis on changes in disease stage
designation and treatment plan. Eur J Surg Oncol. 38:31–37. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bastawrous S, Bhargava P, Behnia F, Djang
DS and Haseley DR: Newer PET application with an old tracer: Role
of 18F-NaF skeletal PET/CT in oncologic practice. Radiographics.
34:1295–1316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiong Z, Deng G, Huang X, Li X, Xie X,
Wang J, Shuang Z and Wang X: Bone metastasis pattern in initial
metastatic breast cancer: A population-based study. Cancer Manag
Res. 10:287–295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsuzuki S, Park SH, Eber MR, Peters CM and
Shiozawa Y: Skeletal complications in cancer patients with bone
metastases. Int J Urol. 23:825–832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Damle NA, Bal C, Bandopadhyaya GP, Kumar
L, Kumar P, Malhotra A and Lata S: The role of 18F-fluoride PET-CT
in the detection of bone metastases in patients with breast, lung
and prostate carcinoma: A comparison with FDG PET/CT and 99mTc-MDP
bone scan. Jpn J Radiol. 31:262–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Muqbel KM, Yaghan RJ, Al-Omari MH,
Rousan LA, Dagher NM and Al Bashir S: Clinical relevance of
18F-FDG-negative osteoblastic metastatic bone lesions noted on
PET/CT in breast cancer patients. Nucl Med Commun. 37:593–601.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gaeta CM, Vercher-Conejero JL, Sher AC,
Kohan A, Rubbert C and Avril N: Recurrent and metastatic breast
cancer PET, PET/CT, PET/MRI: FDG and new biomarkers. Q J Nucl Med
Mol Imaging. 57:352–366. 2013.PubMed/NCBI
|
|
18
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ. 339:b27002009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Whiting PF, Rutjes AW, Westwood ME,
Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA and Bossuyt
PM: QUADAS-2: A revised tool for the quality assessment of
diagnostic accuracy studies. Ann Intern Med. 155:529–536. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deeks JJ: Systematic reviews in health
care: Systematic reviews of evaluations of diagnostic and screening
tests. BMJ. 323:157–162. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deeks JJ, Macaskill P and Irwig L: The
performance of tests of publication bias and other sample size
effects in systematic reviews of diagnostic test accuracy was
assessed. J Clin Epidemiol. 58:882–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hahn S, Heusner T, Kümmel S, Köninger A,
Nagarajah J, Müller S, Boy C, Forsting M, Bockisch A, Antoch G and
Stahl A: Comparison of FDG-PET/CT and bone scintigraphy for
detection of bone metastases in breast cancer. Acta Radiol.
52:1009–1014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Broos W, van der Zant FM, Wondergem M and
Knol R: Accuracy of 18F-NaF PET/CT in bone metastasis detection and
its effect on patient management in patients with breast carcinoma.
Nucl Med Commun. 39:325–333. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rager O, Lee-Felker SA, Tabouret-Viaud C,
Felker ER, Poncet A, Amzalag G, Garibotto V, Zaidi H and Walter MA:
Accuracy of whole-body HDP SPECT/CT, FDG PET/CT, and their
combination for detecting bone metastases in breast cancer: An
intra-personal comparison. Am J Nucl Med Mol Imaging. 8:159–168.
2018.PubMed/NCBI
|
|
25
|
Al-Muqbel KM: Bone marrow metastasis is an
early stage of bone metastasis in breast cancer detected clinically
by F18-FDG-PET/CT imaging. Biomed Res Int. 2017:98526322017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Caglar M, Kupik O, Karabulut E and
Høilund-Carlsen PF: Detection of bone metastases in breast cancer
patients in the PET/CT era: Do we still need the bone scan. Rev Esp
Med Nucl Imagen Mol. 35:3–11. 2016.PubMed/NCBI
|
|
27
|
Piccardo A, Altrinetti V, Bacigalupo L,
Puntoni M, Biscaldi E, Gozza A, Cabria M, Iacozzi M, Pasa A,
Morbelli S, et al: Detection of metastatic bone lesions in breast
cancer patients: fused (18)F-Fluoride-PET/MDCT has higher accuracy
than MDCT. Preliminary experience. Eur J Radiol. 81:2632–2638.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heusner TA, Kuemmel S, Koeninger A, Hamami
ME, Hahn S, Quinsten A, Bockisch A, Forsting M, Lauenstein T,
Antoch G and Stahl A: Diagnostic value of diffusion-weighted
magnetic resonance imaging (DWI) compared to FDG PET/CT for
whole-body breast cancer staging. Eur J Nucl Med Mol Imaging.
37:1077–1086. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Passah A, Tripathi M, Ballal S, Yadav MP,
Kumar R, Roesch F, Meckel M, Sarathi Chakraborty P and Bal C:
Evaluation of bone-seeking novel radiotracer
68Ga-NO2AP-Bisphosphonate for the detection of skeletal
metastases in carcinoma breast. Eur J Nucl Med Mol Imaging.
44:41–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koizumi M, Motegi K and Umeda T: A novel
biomarker, active whole skeletal total lesion glycolysis (WS-TLG),
as a quantitative method to measure bone metastatic activity in
breast cancer patients. Ann Nucl Med. 33:502–511. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abikhzer G, Srour S, Fried G, Drumea K,
Kozlener E, Frenkel A, Israel O, Fogelman I and Kagna O:
Prospective comparison of whole-body bone SPECT and sodium
18F-fluoride PET in the detection of bone metastases from breast
cancer. Nucl Med Commun. 37:1160–1168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Teke F, Teke M, Inal A, Kaplan MA,
Kucukoner M, Aksu R, Urakci Z, Tasdemir B and Isikdogan A:
Significance of hormone receptor status in comparison of
18F-FDG-PET/CT and 99mTc-MDP bone scintigraphy for evaluating bone
metastases in patients with breast cancer: Single center
experience. Asian Pac J Cancer Prev. 16:387–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoon SH, Kim KS, Kang SY, Song HS, Jo KS,
Choi BH, Lee SJ, Yoon JK and An YS: Usefulness of (18)F-fluoride
PET/CT in breast cancer patients with osteosclerotic bone
metastases. Nucl Med Mol Imaging. 47:27–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Botsikas D, Bagetakos I, Picarra M, Da
Cunha Afonso Barisits AC, Boudabbous S, Montet X, Lam GT, Mainta I,
Kalovidouri A and Becker M: What is the diagnostic performance of
18-FDG-PET/MR compared to PET/CT for the N- and M-staging of breast
cancer. Eur Radiol. 29:1787–1798. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu X, Li D, Liang Z, Liao Y, Yang L, Wang
R, Wang P and Cai J: Indirect comparison of the diagnostic
performance of 18F-FDG PET/CT and MRI in differentiating benign and
malignant ovarian or adnexal tumors: A systematic review and
meta-analysis. BMC Cancer. 21:10802021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Evangelista L, Panunzio A, Polverosi R,
Ferretti A, Chondrogiannis S, Pomerri F, Rubello D and Muzzio PC:
Early bone marrow metastasis detection: The additional value of
FDG-PET/CT vs. CT imaging. Biomed Pharmacother. 66:448–453. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han S and Choi JY: Impact of 18F-FDG PET,
PET/CT, and PET/MRI on staging and management as an initial staging
modality in breast cancer: A systematic review and meta-analysis.
Clin Nucl Med. 46:271–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Liu Y, Luo H and Zhang J: PET/CT
and MRI for identifying axillary lymph node metastases in breast
cancer patients: Systematic review and meta-Analysis. J Magn Reson
Imaging. 52:1840–1851. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han S and Choi JY: Prognostic value of
18F-FDG PET and PET/CT for assessment of treatment response to
neoadjuvant chemotherapy in breast cancer: A systematic review and
meta-analysis. Breast Cancer Res. 22:1192020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Diao W, Tian F and Jia Z: The prognostic
value of SUVmax measuring on primary lesion and ALN by 18F-FDG PET
or PET/CT in patients with breast cancer. Eur J Radiol. 105:1–7.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Krüger S, Buck AK, Mottaghy FM, Hasenkamp
E, Pauls S, Schumann C, Wibmer T, Merk T, Hombach V and Reske SN:
Detection of bone metastases in patients with lung cancer:
99mTc-MDP planar bone scintigraphy, 18F-fluoride PET or 18F-FDG
PET/CT. Eur J Nucl Med Mol Imaging. 36:1807–1812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grant FD, Fahey FH, Packard AB, Davis RT,
Alavi A and Treves ST: Skeletal PET with 18F-fluoride: Applying new
technology to an old tracer. J Nucl Med. 49:68–78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schirrmeister H, Guhlmann A, Kotzerke J,
Santjohanser C, Kühn T, Kreienberg R, Messer P, Nüssle K, Elsner K,
Glatting G, et al: Early detection and accurate description of
extent of metastatic bone disease in breast cancer with fluoride
ion and positron emission tomography. J Clin Oncol. 17:2381–2389.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bebbington NA, Zacho HD and Holdgaard PC:
Lesion detection in 18F-sodium fluoride bone imaging: A comparison
of attenuation-corrected versus nonattenuation-corrected PET
reconstructions from modern PET-CT systems. Nucl Med Commun.
43:78–85. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Capitanio S, Bongioanni F, Piccardo A,
Campus C, Gonella R, Tixi L, Naseri M, Pennone M, Altrinetti V,
Buschiazzo A, et al: Comparisons between glucose analogue
2-deoxy-2-((18)F)fluoro-D-glucose and (18)F-sodium fluoride
positron emission tomography/computed tomography in breast cancer
patients with bone lesions. World J Radiol. 8:200–209. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Balci TA, Koc ZP and Komek H: Bone scan or
(18)f-fluorodeoxyglucose positron emission tomography/computed
tomography; which modality better shows bone metastases of breast
cancer. Breast Care (Basel). 7:389–393. 2012. View Article : Google Scholar : PubMed/NCBI
|