Introduction
Cancer stem cells (CSCs) are a small subset of cells
within tumors that possess stem-like properties, including
self-renewal capabilities, and serve as a source for both
differentiated tumor cells and continued tumor expansion (1). Under different selection pressures,
CSCs differentiate into various functional directions, contributing
to tumor cell collective migration and heterogeneity. CSCs can
enter a quiescent state in vivo, exhibiting minimal
proliferation (2).
CSCs typically express common stem cell markers,
such as CD133, octamer-binding transcription factor 4, sex
determining region Y-box 2 (Sox2) and ATP-binding cassette
sub-family G member 2 (ABCG2), which regulate self-renewal and
differentiation (3), potentially
influencing their biological characteristics, including
interactions within the immune system (4). Additionally, CSCs often exhibit low
levels of major histocompatibility complex (MHC) class I molecules,
reducing their recognition by CD8+ T cells (5). CSCs also possess immunoregulatory
functions, modulating the activities of T cells, B cells and
natural killer (NK) cells, thereby suppressing immune responses
(6). Collectively, these features
enable CSCs to evade immune surveillance, maintaining their
presence and functionality within the body.
CSCs interact with epithelial-mesenchymal transition
(EMT), IL-4 signaling, drug efflux proteins and aldehyde
dehydrogenase (ALDH), which collectively contribute to CSC
maintenance and drug resistance, impacting tumor development and
treatment efficacy (7). The EMT is
a biological process through which tumor cells transition from
epithelial to mesenchymal states, enhancing migration, invasion and
drug resistance (7). This
transformation provides tumor cells with stem cell-like
characteristics, increasing resistance to therapy (7,8). IL-4
signaling promotes CSC proliferation and survival, reducing
sensitivity to therapeutic drugs (9). The IL-4 signaling pathway serves a
critical role in modulating the tumor microenvironment, influencing
tumor cell survival and treatment responses (9). Drug efflux proteins, including
membrane transporters, actively pump drugs out of cells, reducing
drug accumulation and efficacy, therefore contributing to tumor
cell resistance (10). The elevated
activity of ALDH is also associated with CSC drug resistance, as
ALDH enzymes facilitate the detoxification of chemotherapeutic
agents within tumor cells, thereby diminishing their cytotoxic
effects (11). Thus, these factors
collectively contribute to CSC resistance to conventional
therapies, posing significant challenges in treatment. Eliminating
CSCs is crucial for complete eradication of tumors, making them a
promising target for cancer therapy (12,13).
Targeting and eliminating lung CSCs holds
therapeutic promise. The immune system can recognize tumor-specific
peptides or neoantigen fragments, inducing cytotoxic responses
against malignant cells (14). In a
preclinical model of lung cancer, researchers at the University of
Cincinnati Cancer Centre (Cincinnati, OH, USA) isolated and
cultured lung CSCs, facilitating the development of immune-based
therapies targeting these cells (15).
Cancer immunotherapy aims to elicit or reinvigorate
cellular immune responses, particularly T cell-mediated
cytotoxicity against tumor-specific antigens and tumor-associated
antigens (TAAs), selectively targeting and destroying tumors
(16). Among immunotherapies,
immune checkpoint inhibitors (ICIs) have revolutionized the
treatment landscape for non-small cell lung cancer (NSCLC);
however, only 20% of patients with NSCLC exhibit durable responses
to ICIs, highlighting the need for alternative approaches (17).
Dendritic cell (DC) vaccines for treating tumors
have emerged as promising biological therapies (18). DCs are the most potent known
antigen-presenting cells (APCs) capable of activating naïve T
lymphocytes, which have a central role in initiating and regulating
innate and adaptive immune responses (18). As specialized APCs, DCs serve
crucial roles in initiating and regulating both cellular and
humoral immune responses, interacting with various cells of the
innate immune system, including NK cells, macrophages and mast
cells (19). Furthermore, DCs
interact with B lymphocytes to indirectly promote the proliferation
and differentiation of CD4+ T helper cells, playing a
significant role in regulating humoral immunity (20). Due to their pivotal role in
initiating immune responses, DCs are essential for antigen
presentation and vaccine strategies in cancer treatment.
Evidence has indicated that DC vaccines exhibit
efficacy against various types of cancer, such as gallbladder
(21), prostate (22), gastrointestinal (23), oral (24), pancreatic (25) and breast cancer (26), malignant glioma (27) and ovarian cancer (28), although complete tumor eradication
remains elusive, partly due to immune evasion mechanisms involving
CSCs (6). Therefore, strategies
targeting CSCs with DC vaccines to reduce immune evasion have
practical significance. Traditional DC vaccines often co-culture
DCs with autologous or allogeneic tumor cell lysates, potentially
leaving stem cells untouched, thereby posing the risk of recurrence
(21). Hence, there is an urgent
need for novel therapeutic approaches with sustained responses. The
present study explored the induction of anti-lung cancer immune
responses through A549 lung CSC lysate-sensitized DC vaccines in
vitro, aiming to advance vaccine-based therapies for lung
cancer.
Materials and methods
Cell culture
The A549 (cat. no. CL-0016) and 16HBE cell lines
(cat. no. CL-0249) were purchased from Procell Life Science &
Technology Co., Ltd. A549 lung cancer and 16HBE cells were
routinely cultured in DMEM supplemented with 10% heat-inactivated
fetal bovine serum (FBS; both Gibco; Thermo Fisher Scientific,
Inc.). 100 µg/ml streptomycin, and 100 U/ml penicillin sodium
(Beijing Solarbio Science & Technology Co., Ltd.). The cells
were cultured in an incubator at 37°C and 5% CO2.Upon
reaching confluence (100%), the culture medium was discarded, and
the cells were washed twice with PBS and then dissociated with
trypsin. The trypsin digestion was terminated by adding an equal
volume of complete culture medium. After centrifugation (10,000 × g
for 10 min at 4°C), cells were washed once with PBS, the
supernatant was discarded and cells were resuspended in 5 ml
culture medium. Gentle pipetting was used to obtain a single-cell
suspension, which was then seeded at a density of 20,000 cells/well
in low-attachment culture dishes. The culture medium (serum-free
medium) used was DMEM/F12 supplemented with B27 [Absin (Shanghai)
Biotechnology Co., Ltd.], EGF [Epidermal Growth Factor; Absin
(Shanghai) Biotechnology Co., Ltd.] and bFGF [basic Fibroblast
Growth Factor; Absin (Shanghai) Biotechnology Co., Ltd.].
Third-generation cell spheres (spherical structures formed
spontaneously in a growth factor-containing medium). were collected
and SP (Side Population) cell subpopulations were sorted using a
flow cytometer (CytoFLEX SRT; Beckman Coulter International Trading
Co., Ltd.). In the CytExpert for CytoFLEX SRT software (version
CytExpert SRT 1.0; Beckman Coulter). A single-cell suspension was
prepared at a concentration of 1×106 cells/l. Hoechst
33342 dye was added at a concentration of 5 µg/ml (Beijing Solarbio
Science & Technology Co., Ltd.). The cells were incubated for
90 min at 37°C in the dark. For the control group, verapamil was
added at a concentration of 100 mM (Beijing Solarbio Science &
Technology Co., Ltd.). A flow cytometer was used to sort SP (side
population) cell subpopulations and non-SP cell subpopulations at
an excitation wavelength of 355 nm. The sorted SP cell
subpopulation was prepared as a 1×106 cells/ml
single-cell suspension and was cultured in serum-free conditions to
form spheres (29).
Western blotting
The induced mature A549 lung CSCs were removed from
the incubator and the cell culture suspension was aspirated into a
15-ml centrifuge tube. The cell suspension was washed twice with
PBS and centrifuged at 4°C and 10,000 × g for 10 min. The
supernatant was discarded and A549 CSCs and A549 cells were lysed
with RIPA lysis buffer (Shanghai Beibo Biotechnology Co., Ltd.).
Total protein concentration was quantified using a BCA protein
assay kit (Beyotime Institute of Biotechnology). Protein samples
(20 µg/lane) underwent SDS-PAGE on a 10% gel) and the transfer of
separated proteins to nitrocellulose membranes. The membranes were
then blocked with Protein-Free Rapid Blocking Buffer (1×) (Shanghai
Yamei Biotechnology Co., Ltd.) for 20 min at 4°C, then incubated
overnight at 4°C with primary monoclonal antibodies against CD133
(1:1,000; cat. no. 48082S; CST Biological Reagents Co., Ltd.),
ABCG2 (1:1,000; cat. no. 42078T; CST Biological Reagents Co.,
Ltd.), Sox2 (1:1,000; cat. no. 5067S; CST Biological Reagents Co.,
Ltd.) and β-actin (1:3,000; cat. no. ab8226; Abcam). The membranes
were washed three times with TBS-0.1% Tween 20 (TBST) for 10 min
each and were then incubated with horseradish peroxidase-conjugated
secondary antibodies (1:10,000; cat. nos. SA00001-1 and SA00001-2;
Proteintech Group, Inc.) at room temperature for 1 h. The membranes
were then washed with TBST and incubated with ECL reagent (Beyotime
Institute of Biotechnology). The protein bands were analyzed using
Image Lab analysis software (version 4.0; Bio-Rad Laboratories,
Inc.). The experiments were performed in triplicate.
In vitro induction and culture of
human DCs
The use of all patient samples in the present study
was approved by The Ethics Committee of The Second Affiliated
Hospital of Nanchang University [Nanchang, China; approval no.
O-Medical Research Ethics Approval (2023) no. 89], and all
participants provided written consent to participate.
A total of nine healthy volunteers (age, 18 and 60
years, with an average age of 36.6. The sex ratio is 5:4 (male to
female) were recruited. Peripheral blood was collected with fresh
anticoagulant at Department of Pulmonary and Critical Care
Medicine, The Second Affiliated Hospital of Nanchang University,
and peripheral blood mononuclear cells (PBMCs) were isolated using
lymphocyte separation fluid (Cytiva). Blood collection occurred in
December 2023, January 2024 and February 2024. The specific steps
are as follows: Peripheral blood was divided equally into 50 ml
centrifuge tubes, with 15 ml blood/tube. PBS was added (15 ml, and
the contents were mixed well. The diluted blood sample was then
transferred, in a volume of 15 ml, on top of 15 ml of lymphocyte
separation fluid in four additional 50 ml centrifuge tubes. The
tubes were centrifuged at 1,200 g for 30 min at 25°C. The white
mist-like membrane from the second layer was extracted and
transferred into a 15 ml centrifuge tube. Three times the volume of
PBS was added to the tube, and the contents were mixed well before
centrifuging at 500 g for 10 min at 25°C. Supernatant was removed,
the white precipitate was collected, and it was washed twice with
PBS. The cell concentration was then adjusted to 1×10^7 cells/ml
using RPMI-1640 medium supplemented with 10% inactivated fetal
bovine serum. PBMCs were cultured in an incubator for 2 h at 37°C
and 5% CO2, non-adherent cells were removed through
aspiration and adherent cells were gently washed once with culture
medium. At this stage, adherent PBMCs were obtained. The cells were
cultured in an incubator at 37°C and 5% CO2, Each well
was supplemented with RPMI-1640 cell culture medium (Gibco; Thermo
Fisher Scientific, Inc.), containing recombinant human
granulocyte-macrophage colony-stimulating factor (GM-CSF; Gibco;
Thermo Fisher Scientific, Inc.) and recombinant human interleukin-4
(IL-4; Gibco; Thermo Fisher Scientific, Inc.). Starting from day 6,
recombinant human tumor necrosis factor-α (TNF-α; Gibco; Thermo
Fisher Scientific, Inc.) was added and cells were harvested on day
8 of DC culture at 37°C and 5% CO2). On day 8 of the DC
culture, mouse anti-human CD80 (cat. no. FHP080-025; 1:40;), CD83
(FHP083-025; 1:40; Beijing Sizhengbo Biotechnology Co., Ltd.), CD86
(FHP086-100; 1:40; Beijing Sizhengbo Biotechnology Co., Ltd.) and
HLA-DR (FHPDR-025; 1:40; all Beijing Sizhengbo Biotechnology Co.,
Ltd.) fluorescent antibodies were added separately, with mouse
anti-human IgG (410707; 1:40; BioLegend, Inc.) serving as a blank
control. After mixing antibodies with cells, the mixture was
incubated at 4°C in the dark for 30 min, followed by washing with
PBS. Cells were fixed with paraformaldehyde solution (4%) for 30
min at 4°C and analyzed using flow cytometry (FACScan; BD
Biosciences) in the Cell Quest software (version 6.0.1; Becton,
Dickinson and Company) (30).
Preparation of human T-lymphocyte
suspension (effector cells)
Peripheral blood from the nine healthy volunteers
was anticoagulated with heparin and lymphocyte separation solution
was used to obtain PBMCs. After a 2-h incubation in a cell culture
incubator (at 37°C, non-adherent cells were aspirated, T
lymphocytes were separated using nylon wool (Polysciences, Inc.)
and phytohemagglutinin (Add 100 µl to each culture flask) was
added. After 3 days of culture, IL-2 (30 U/ml; CST Biological
Reagents Co., Ltd.) was used to induce expansion, at 37°C and 5%
CO2, with medium changes every 2 days (31).
Preparation of lung CSC lysates
Lung CSCs were collected, suspended in RPMI-1640
complete culture medium, transferred to cryotubes, immersed in
liquid nitrogen for 5 min and then placed in a 37°C water bath for
5 min. This process was repeated three times. After centrifugation
(4°C; 1,000 × g for 10 min), the supernatant was collected,
transferred to a new cryotube and stored at −80°C. A549 lung cancer
cell lysates were prepared using the same method.
Effects of two different sources of
cell lysates on DCs
To verify the effects of cell lysates from two
different sources on DC maturation and antigen presentation, on day
6, 1×105 immature DCs not yet induced with TNF-α were
incubated (Incubated at 37°C and 5% CO2 for 48 h) with
sorted A549 lung CSC and A549 lung cancer cell lysates (at a 5:1
ratio). Cells were harvested 2 days later and mouse anti-human
MHC-II [abs1840769; 1:40; Absin (Shanghai) Biotechnology Co., Ltd.]
fluorescent antibodies were added. Mouse anti-human IgG (410707;
1:40; BioLegend, Inc.) was used as a negative control. After mixing
antibodies with cells, the mixture was incubated at 4°C in the dark
for 30 min, followed by washing with PBS. Cells were fixed with
paraformaldehyde solution (4%) for 30 min at 4°C and analyzed using
flow cytometry (FACScan; BD Biosciences) in the CellQuest software
(version 6.0.1; Becton, Dickinson and Company) environment
(30).
Detection of T-lymphocyte
proliferation using the Cell Counting Kit-8 (CCK-8) assay
After activating homologous T lymphocytes with the
aforementioned sensitized DCs, cells were mixed and 100 µl of each
sample was transferred into separate wells of a 96-well plate
(3,000 cells/well). Each group had three replicate wells. Cell
proliferation rates were measured using the CCK-8 assay (Dojindo
Laboratories, Inc.) at 0, 24, 48 and 72 h (incubated at 37°C for 2
h), and cell proliferation was assessed using the optical density
(OD) 450 nm) values.
Preparation of DC vaccines sensitized
with lung CSC and lung cancer cell lysates
The pre-prepared cell lysates with mature DCs
induced by TNF-α at a ratio of 5:1. The primary cells extracted
from blood in the previous groups were further divided into three
subgroups. These subgroups were sensitized with either stem cell
lysates, A549 cell lysates, or were not sensitized with any cell
lysates). GM-CSF and IL-4 cytokines were added (Incubated at 37°C
and 5% CO2) and DCs were collected the following day.
The control vaccine (DC vaccine sensitized with A549 lung cancer
cell lysates) was prepared using the same method as described for
preparing the DC vaccine sensitized with A549 lung CSC lysate
(32).
Detection of IFN-γ content in
supernatant after co-culture of A549 lung CSC lysate-sensitized DC
vaccines and T lymphocytes
A total of three types of DC vaccines (A549 lung CSC
lysate-sensitized DC vaccine, A549 lung cancer cell
lysate-sensitized DC vaccine and DC vaccine without lysate
sensitization) were mixed with prepared homologous T lymphocytes at
a 1:10 ratio. The cells were cultured in RPMI-1640 medium
containing 10% FBS, IL-12 and IL-2 for 8 days at 37°C and 5%
CO2, and the supernatants were collected daily. Human
IFN-γ ELISA KIT (CHE0017; Beijing Sizhengbo Biotechnology Co.) was
used to detect the IFN-γ content in the supernatant according to
the manufacturer's protocol (32).
In vitro induction of anti-lung cancer
immune cytotoxic effects by DC vaccines
The specific cytotoxicity of cytotoxic T lymphocytes
(CTLs) stimulated by the aforementioned three groups of DC vaccines
against A549 cells and A549 lung CSCs was assessed in vitro
using the lactate dehydrogenase release method with the Cytotox96
Non-Radioactive Cytotoxicity Assay kit (G1780; Promega
Corporation). In the experiment, the specific steps were as
follows: The target cells used were A549 lung cancer cells and A549
lung CSCs. The effector cells were CTLs stimulated by the
respective groups of DC vaccines after being cultured for 8 days at
37°C and 5% CO2. The effector cells were then mixed with
the target cells at different ratios (60:1, 30:1 and 10:1) and
added to U-bottomed 96-well culture plates. Each sample had three
replicate wells. Control groups of target cells with spontaneous
(baseline level of marker or enzyme released into the culture
medium by cells under normal conditions, without any treatment or
stimulation) and maximum release (Refers to the total amount of
marker or enzyme released into the culture medium when cells are
completely lysed or destroyed. This indicates the maximum potential
release of substances from cells under experimental conditions)
were also included. The plates were then incubated at 37°C with 5%
CO2 for 4 h. After incubation, the plates were
centrifuged at 250 × g for 4 min at 4°C. A total of 50 µl
supernatant from each well of the centrifuged plates was
transferred to a new 96-well ELISA plate. Subsequently, 50 µl
substrate mixture was added to each well and incubated at room
temperature in the dark for 30 min. The reaction was stopped with
50 µl stop solution per well in a dark room and the OD at 490 nm)
were promptly measured using a microplate reader. The cytotoxicity
percentage was calculated using the formula: Cytotoxicity (%)=[(OD
value of experimental group-OD value of spontaneous release)/(OD
value of maximum release-OD value of spontaneous release)] ×100.
Normal human airway epithelial cells (16HBE cells) were used as
negative controls for this experiment (33).
Transwell migration assay
A549 lung cancer cells were used as target cells and
CTLs stimulated with three groups of DC vaccines cultured for 8
days were used as effector cells. Effector and target cells were
mixed at a ratio of 3:1. Transwell inserts with a pore size of 8 µm
were used. Each upper chamber was loaded with 200 µl cell
suspension (The medium in the upper chamber was serum-free DMEM,
with 3×104 target cells added to each well) and the
lower chamber was filled with 500 µl complete medium containing 15%
FBS. Cells were cultured in a 5% CO2, 37°C incubator for
24 h. Cells that had migrated to the lower chamber were treated
with 3% paraformaldehyde at 25°C for 30 min, followed by staining
with crystal violet at 25°C for 15 min. The cells were then imaged
and counted using an inverted microscope (Light microscope) and
ImageJ (version 1.8.0; National Institutes of Health, USA).
Flow cytometric apoptosis assay
Effector CTLs stimulated with three groups of DC
vaccines cultured for 8 days were used as effector cells) and
target cells (A549 lung cancer cells) were mixed at a ratio of 3:1
and cultured in a 5% CO2, 37°C incubator for 24 h. Cells
were digested with trypsin solution without EDTA and adjusted to a
concentration of 1×106 cells/ml. Staining was performed
using FITC-Annexin V/PI apoptosis detection kit (Suzhou UELandy
Biotechnology Co., Ltd.), with 5 µl Annexin V-FITC and 5 µl PI,
followed by incubation in the dark at room temperature for 15 min.
Finally, flow cytometry (FACScan; BD Biosciences) was used to
detect cell apoptosis and the results were analyzed using FlowJo
(version 10; FlowJo LLC).
Statistical analysis
Data analysis was performed using GraphPad Prism
v10.2.3 software (Dotmatics). Differences between the two groups
were analyzed by unpaired Student's t-test. One-way analysis of
variance followed by Dunnett's post hoc test were used for
comparisons between multiple groups. All quantitative experiments
were repeated three times and data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Relative expression of CD133, ABCG2
and Sox2 in A549 lung CSCs
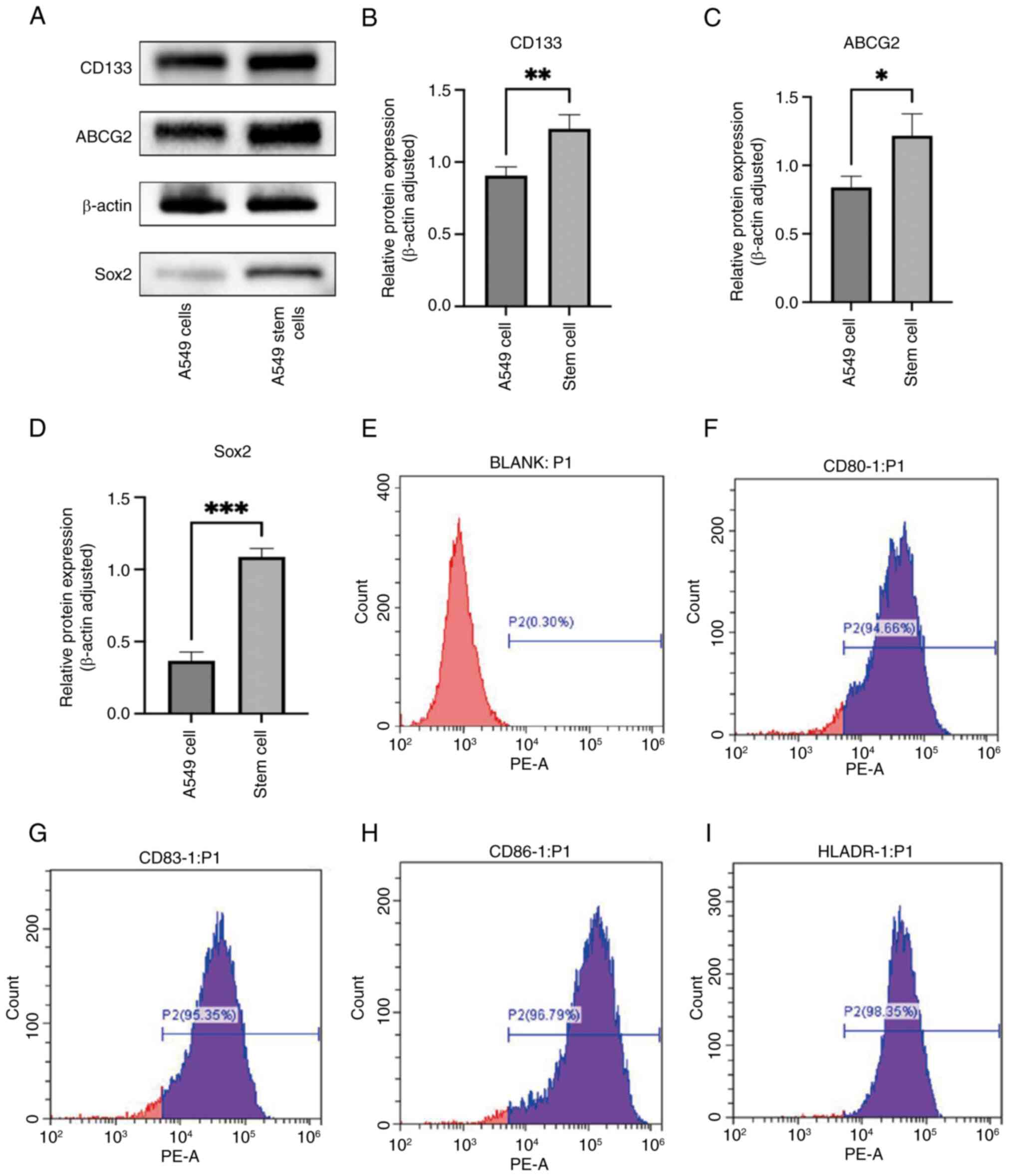
CD133, ABCG2 and Sox2 are widely recognized as
markers of lung CSCs, closely associated with the stemness and drug
resistance properties of lung CSCs (3). Therefore, CD133, ABCG2 and Sox2 were
used to validate the formation of lung cancer A549 stem cell
spheres. Western blot analysis revealed that the expression of
CD133, ABCG2 and Sox2 in lung cancer A549 stem cells was
significantly higher compared with that in A549 lung cancer cells
(Fig. 1A-D).
Flow cytometric identification of
DCs
CD80, CD83, CD86 and HLA-DR are typical markers used
to identify DCs, as they serve crucial roles in the function and
maturation status of DCs (34).
Therefore, fluorescent-labeled monoclonal antibodies against CD80,
CD83, CD86 and HLA-DR were used for flow cytometric analysis of
induced mature DCs. The expression levels of fluorescent-labeled
monoclonal antibodies on DCs were IgG, 0.3% (Fig. 1E); CD80, 94.66% (Fig. 1F); CD83, 95.35% (Fig. 1G); CD86, 96.79% (Fig. 1H); and HLA-DR, 98.35% (Fig. 1I). These findings indicated that the
purity and maturation of DCs were satisfactory and suitable for
subsequent experiments.
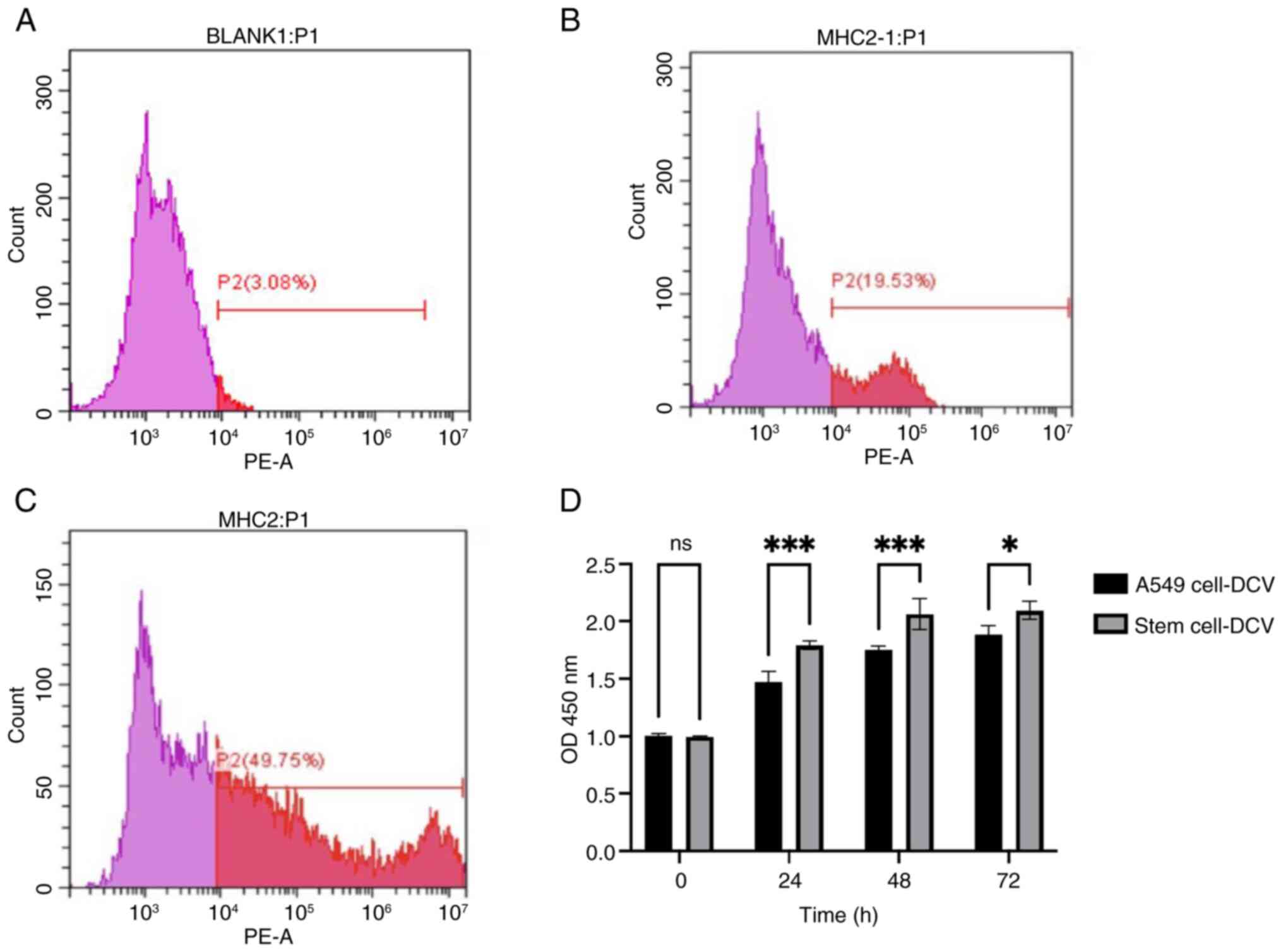
Impact of two different sources of
cell lysates on DCs
MHC-II serves as a key marker for assessing the
maturation and antigen-presenting capacity of DCs, providing direct
indications of their functional status, this is crucial for
evaluating the role of DCs in immune responses and vaccine design
(35). Therefore, MHC-II was used
to evaluate the ability of lysates from two different cell sources
to sensitize immature DCs and promote their maturation (35). The expression of fluorescent-labeled
monoclonal antibodies on DCs was 3.08% for IgG (Fig. 2A), DCs sensitized with stem cell
lysates exhibited markedly higher surface expression of MHC-II
compared with those sensitized with A549 lung cancer cell lysates
(Fig. 2B and C). These findings
indicated that stem cell lysate could more effectively activate and
mature DCs, enhancing their antigen-presenting capability and
immunogenicity.
Detection of T-lymphocyte
proliferation using the CCK-8 method
Using the CCK-8 assay, cell proliferation rates (OD
values) were measured at 0, 24, 48 and 72 h. The results showed
that the proliferation rate of T lymphocytes activated by DCs
sensitized with ALDH1+ stem cell lysates was
significantly higher than those activated by DCs sensitized with
A549 lung cancer cell lysates (Fig.
2D).
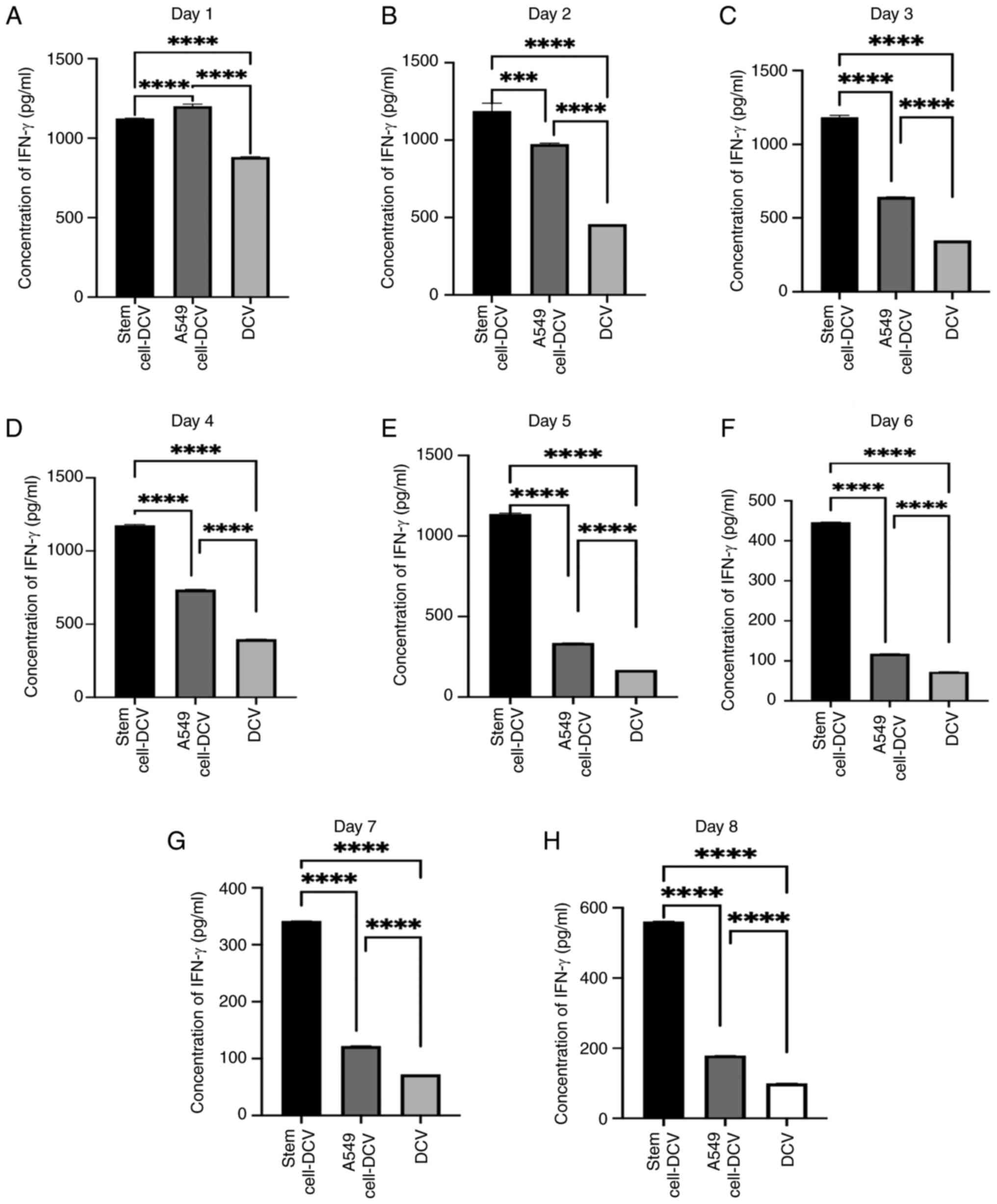
Detection of IFN-γ levels in the
supernatant after co-culture of A549 lung CSC lysate-sensitized DC
vaccine with T lymphocytes
From day 1–8, significant differences in IFN-γ
concentrations were observed in the supernatants after co-culturing
T lymphocytes with three different vaccines daily (Fig. 3A-H). IFN-γ production by T
lymphocytes stimulated with DC vaccines sensitized with A549 lung
cancer cell lysates declined starting from day 2, with the
difference from non-sensitized DC vaccines diminishing after day 2.
T lymphocytes stimulated with DC vaccines sensitized with A549 lung
CSC lysates showed no marked decline in IFN-γ production in the
first 5 days and exhibited significant differences compared with
the other two vaccine groups throughout the 8 days (Fig. 3A-H).
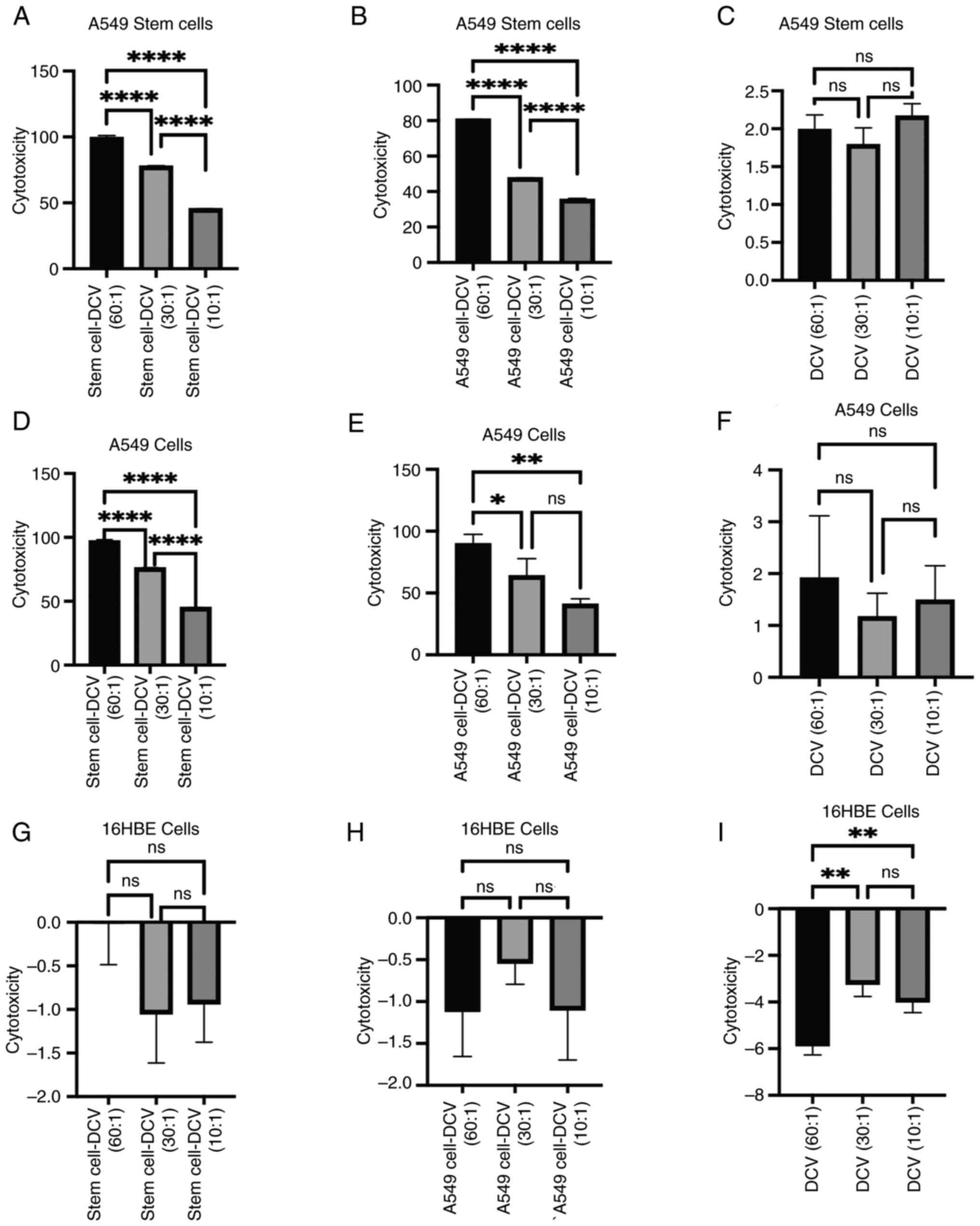
In vitro induction of anti-lung cancer
immune cytotoxic effects by DC vaccines
The results showed that DC vaccines sensitized with
stem cell lysates had the highest cytotoxicity against A549 cells
at an effector-to-target (E:T) ratio of 60:1, with cytotoxicity
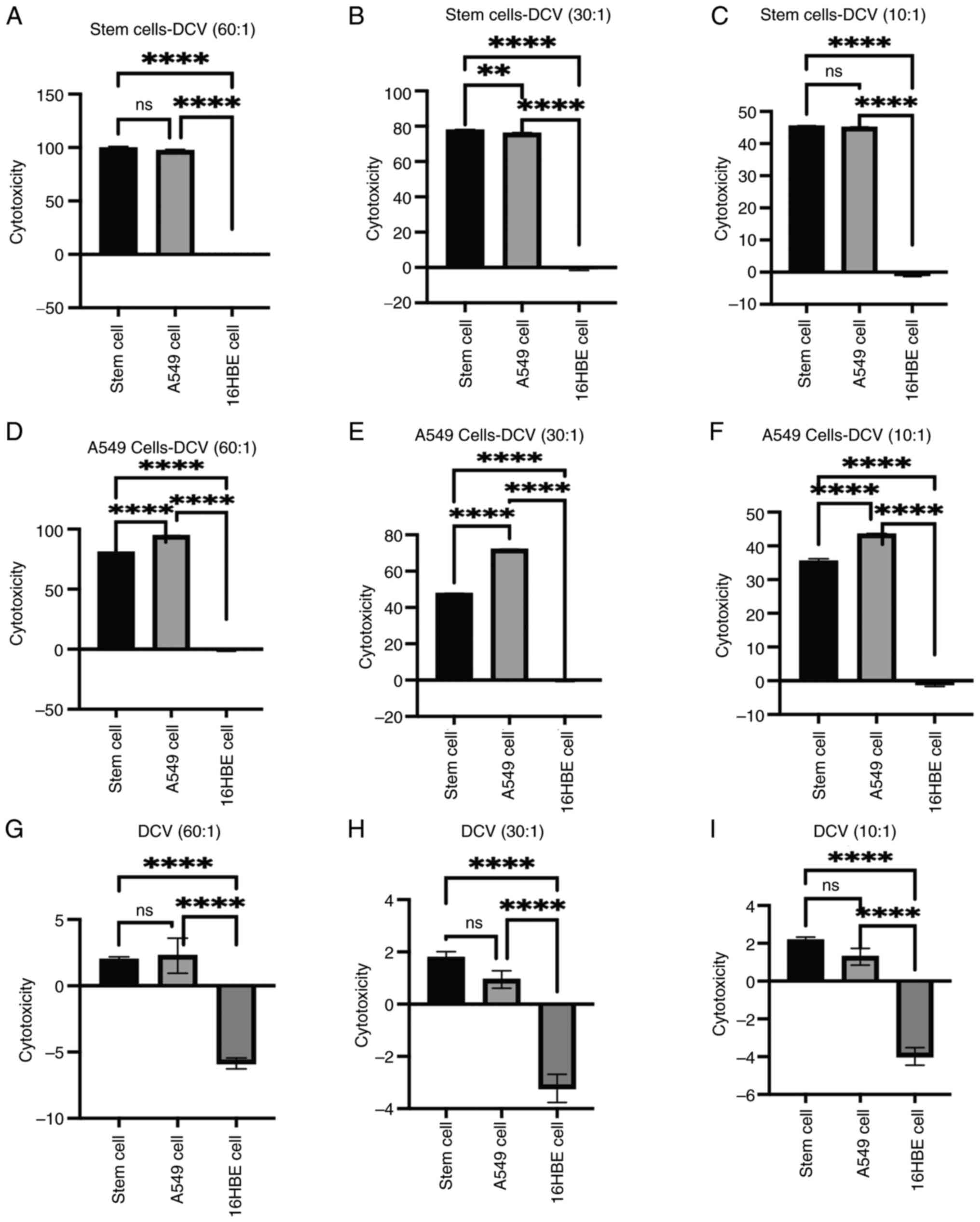
gradually decreasing as the E:T ratio decreased (Fig. 4A). Compared with DC vaccines
sensitized with stem cell lysate, the DC vaccines sensitized with
A549 cell lysates exhibited slightly lower cytotoxicity against
stem cells, with cytotoxicity also decreasing as the E:T ratio
decreased (Fig. 4B). Non-sensitized
DC vaccines at different E:T ratios showed no significant
differences in cytotoxicity against stem cells, Compared with DC
vaccines sensitized with cell lysates, non-sensitized DC vaccines
exhibited lower overall cytotoxicity (Fig. 4C).
The DC vaccines sensitized with stem cell lysates
also showed the highest cytotoxicity against A549 cells at an E:T
ratio of 60:1 (Fig. 4D). Similarly,
DC vaccines sensitized with A549 cell lysates exhibited relatively
high cytotoxicity against A549 cells at an E:T ratio of 60:1
(Fig. 4E). Non-sensitized DC
vaccines at different E:T ratios showed no significant differences
in cytotoxicity against A549 cells, Compared with the other two
groups of DC vaccines sensitized with cell lysate, the
non-sensitized DC vaccines exhibited lower overall cytotoxicity
(Fig. 4F).
All three vaccines showed no cytotoxic effects
against 16HBE cells (Fig. 4G-I).
However, non-sensitized DC vaccines at an E:T ratio of 60:1
exhibited a larger negative cytotoxicity value against 16HBE cells.
This may be due to non-specific immune responses triggered by a
large number of non-sensitized DCs in the cell culture environment,
potentially promoting cell survival or proliferation upon contact
with 16HBE cells (Fig. 4I).
The same vaccines exhibited varying cytotoxicity
against different target cells. DC vaccines sensitized with A549
stem cell lysates exhibited varying cytotoxicity against A549 stem
cells and A549 cells. At E:T ratios of 60:1 and 10:1, there was no
significant difference in cytotoxicity between A549 stem and A549
cells. However, at an E:T ratio of 30:1, the cytotoxicity against
stem cells was slightly higher than that against A549 cells
(Fig. 5A-C). DC vaccines sensitized
with A549 cell lysates demonstrated significantly higher
cytotoxicity against A549 cells compared with A549 stem cells
(Fig. 5D-F). Non-sensitized DC
vaccines showed no significant difference in cytotoxicity against
A549 stem cells and A549 cells but the results indicated negative
cytotoxicity against 16HBE cells (Fig.
5G-I). 16HBE cells may lack the expression of specific antigens
or co-stimulatory molecules, which could lead to the difficulty of
DC vaccines sensitized with lysates in effectively recognizing
these cells and activating T lymphocytes, thereby weakening the
overall immune response (36),
while 16HBE cells also exhibited natural proliferation during the
assay period.
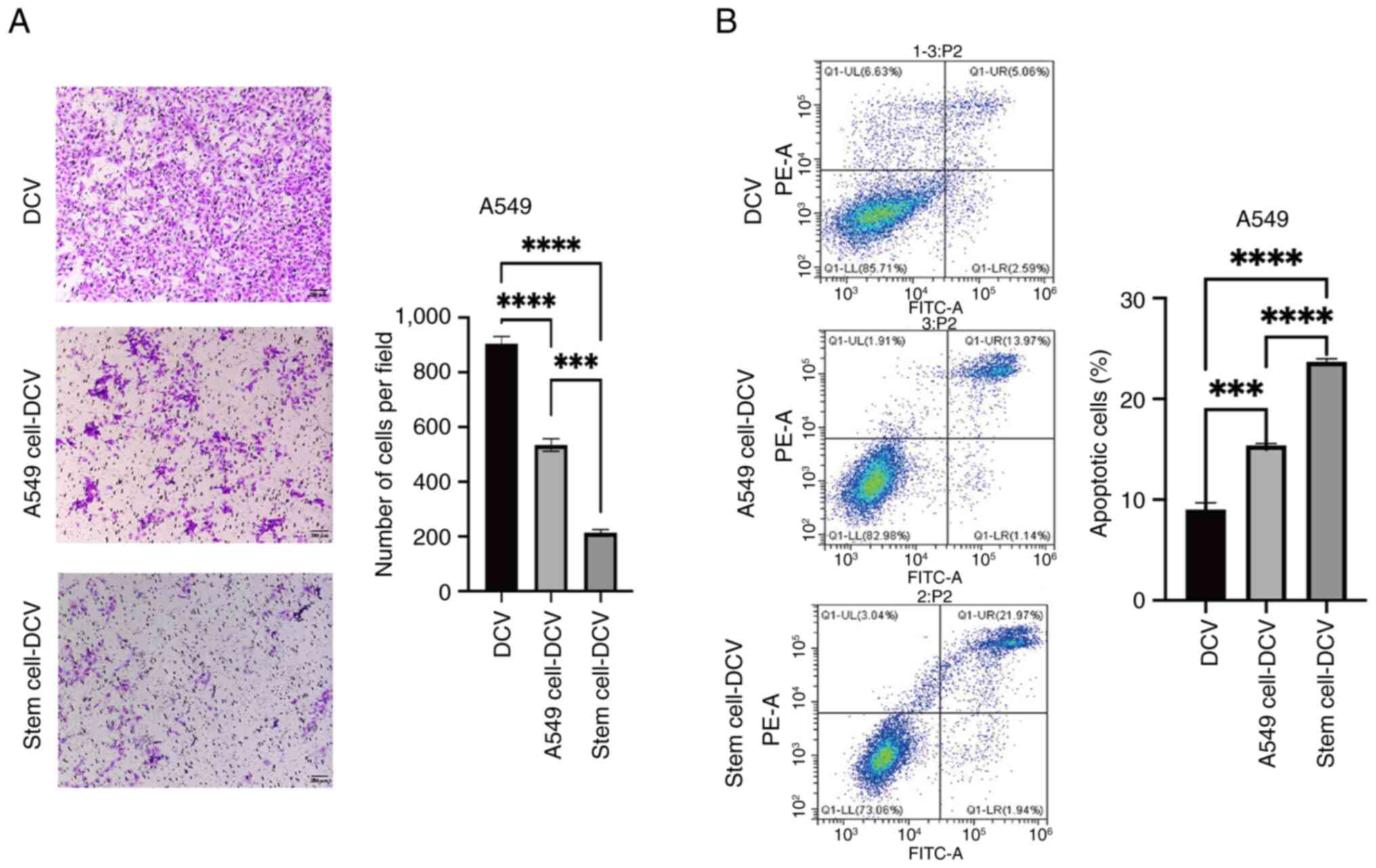
Impact of DC vaccines on A549 lung
cancer cells
DC vaccines sensitized with low doses of A549 stem
cell lysates significantly reduced the migration of A549 cells,
Compared with vaccines sensitized with stem cell lysates, the
dendritic cell vaccines sensitized with low doses of A549 cell
lysates also induced a moderate decrease in A549 cell migration,
but the effect was not as pronounced as that of the stem cell
lysate-sensitized vaccines. Compared with the other vaccine groups,
non-sensitized DC vaccines had a significantly reduced effect on
the migration of A549 cells (Fig.
6A). Flow cytometric apoptosis assays demonstrated that,
compared with other vaccines, DC vaccines sensitized with low doses
of A549 stem cell lysates notably promoted apoptosis in A549 lung
cancer cells while the effect of DC vaccines sensitized with A549
cell lysates on apoptosis in A549 lung cancer cells was less
pronounced, compared with other vaccines. Compared with the other
two vaccine groups, non-sensitized DC vaccines had a significantly
reduced effect on apoptosis in A549 cells (Fig. 6B).
Discussion
Tumor cell immune escape is a critical issue
hindering the efficacy of tumor vaccines (6). Tumor stem cells effectively evade
immune system by reducing MHC-I levels, escaping T cell-mediated
death, inhibiting T cell anti-tumor functions, and directly
interfering with T cell effector functions through their secreted
factors, that enable them to evade recognition and attack by the
immune system (6). As seen in most
tumors, the onset and maintenance of lung cancer is associated with
lung CSCs, which correspond to the malignant transformation of
respective lung stem cells (37).
Therefore, targeting and eliminating lung CSCs holds significant
therapeutic promise. The present study, to find a more effective
way to eradicate tumor stem cells, aimed to investigate the in
vitro induction of anti-lung cancer immune responses by DC
vaccines sensitized with A549 lung CSC lysates.
Firstly, A549 lung CSCs were successfully induced
and it was observed that the expression of CD133, ABCG2 and Sox2 in
A549 lung CSCs was significantly higher compared with that in A549
cells. Additionally, DCs and T lymphocytes were successfully
induced. Subsequently, using immature DCs sensitized with A549 stem
cell lysates and A549 lung cancer cell lysates, it was found that
A549 stem cell lysates were more effective in promoting DC
maturation and antigen presentation capability, and could
significantly enhance T-lymphocyte proliferation. These findings
indicated that stem cell lysates could more effectively activate
and mature DCs, enhancing their antigen presentation capability and
immunogenicity.
In subsequent experiments, three types of DC
vaccines were prepared (DC vaccines sensitized with A549 lung CSC
lysates, DC vaccines sensitized with A549 lung cancer cell lysates
and DC vaccines without sensitization) and their induction of
T-cell responses and antitumor activity were compared in
vitro. It was revealed that DC vaccines sensitized with A549
lung CSC lysate significantly stimulated the release of IFN-γ from
homologous T lymphocytes, with levels significantly higher than
those released from the other two groups.
Furthermore, in terms of cytotoxic effects on A549
cells and A549 CSCs, DC vaccines sensitized with A549 lung CSC
lysates showed efficacy in A549 stem cell cytotoxicity and
demonstrated strong cytotoxic effects on A549 cells. The cytotoxic
effect was significantly superior to the other two vaccine groups,
and all three vaccine groups did not induce immune cytotoxic
effects on 16HBE human airway epithelial cells. However, they
exhibited negative cytotoxicity on 16HBE cells. This may be due to
16HBE cells not expressing specific antigens required by DC
vaccines or related co-stimulatory molecules, rendering DC vaccines
ineffective in activating T lymphocytes to attack them. Meanwhile,
during the testing period, 16HBE cells underwent natural
proliferation and the aforementioned result may also be due to a
large number of unsensitized DCs in the in vitro cell
culture environment that triggered nonspecific immune responses
upon contact with 16HBE cells, thereby increasing cell survival
rates or even promoting cell proliferation.
Additionally, it was observed that low-dose DC
vaccines sensitized with A549 lung CSC lysates exhibited
significant anti-migratory and pro-apoptotic effects on A549 lung
cancer cells. Their effects were superior to those of DC vaccines
sensitized with A549 lung cancer cell lysates. This may be
attributed to low-dose DC vaccines not only directly killing a
small number of tumor cells but also inhibiting their activity;
however, the specific mechanisms involved require further
investigation for clarification.
Notably, neither A549 lung CSC lysate-sensitized DC
vaccines nor A549 lung cancer cell lysate-sensitized DC vaccines
achieved a 100% killing efficiency against A549 lung CSCs and A549
cells. This may be related to the use of vaccines on day 8 after
co-cultivation. The experimental results from testing the IFN-γ
levels in the supernatant after mixing three types of DC vaccines
with T lymphocytes showed that compared with the higher IFN-γ
levels observed in the first five days, the IFN-γ levels for A549
lung CSC and A549 cell-sensitized dendritic cell vaccines were
relatively lower on day 8. Additionally, this could be due to
functional defects in DCs and effector cells, such as inadequate
antigen presentation and cytokine release, which prevent them from
overcoming immune suppression that limits DC and effector cell
function (38).
Despite the potential of DC vaccines in cancer
immunotherapy, they have not demonstrated notable superiority over
traditional treatment methods, such as chemotherapy, targeted
therapy or ICIs in terms of treatment efficacy and patient survival
rates (39). DCs possess unique
biological characteristics, making the design and production of
long-lasting effective vaccines challenging (40). Ex vivo-induced differentiated
DCs exhibit tolerance to immune suppression, and have a relatively
weak and limited lifespan, thereby restricting their ability to
induce sustained immune responses (41,42).
Increasingly, studies have indicated that genetically modified DC
vaccines can significantly enhance antitumor efficacy (43,44).
Researchers have optimized DC vaccines by introducing methods such
as mRNA, small interfering RNA, viral gene transduction, and fusion
with tumor cells, the results indicate that combining these
approaches can significantly enhance the clinical effectiveness of
dendritic cell vaccines (45,46).
Previous research has demonstrated that elevated expression of
origin Recognition Complex Subunit 6) may impact DC activity,
exacerbating immune evasion by tumor cells, thereby contributing to
tumor initiation, progression and metastasis (47). Further investigation is needed to
determine whether downregulating ORC6 in DCs can enhance their
activity and the stimulation of T cells, thereby augmenting the
antitumor immune response of T cells.
In addition to modifying DCs, introducing mRNA
encoding multiple antigen epitopes can enhance the breadth and
depth of immune responses by simultaneously activating multiple
antigen-specific T-cell responses (48), mRNA can be used in conjunction with
stimuli, such as CD40L or cytokines, to enhance DC activation and
antigen epitope presentation (49).
Adjuvants are compounds that enhance the immunogenicity of vaccines
by promoting antigen uptake and processing, and activation of
immune cells, thereby boosting immune responses, and effectively
stimulating function of DCs (50).
Combination therapy aims to enhance the effectiveness of DC
vaccines, Studies have shown that combining PD-1 blockade with DC
vaccine administration can prolong the survival of treated Mice,
whereas monotherapy with any single agent does not significantly
impact the survival of animals with established tumors (51,52).
DC vaccine administration significantly increases
tumor-infiltrating lymphocytes (TILs), and the increase in PD-1
expression is associated with elevated TILs post-vaccination
(51). Chemotherapy may weaken the
immune response, so patients who have undergone chemotherapy may
have a reduced response to subsequent immunotherapy, even though
the combination of immunotherapy and chemotherapy may seem
unconventional, several clinical trials have explored this
approach, demonstrating potential synergistic effects that could
improve treatment outcomes and survival rates for patients
(53,54). Recent research has indicated that
combining DC vaccines with cytokine-induced killer (CIK) cell
therapy in patients with cancer has had a significant positive
impact on treatment (55). This
combination therapy leverages the synergistic effects of DCs and
CIK cells, as DCs effectively present tumor antigens, compensating
for the limited tumor antigen specificity of CIK cells, thus
offering promising clinical prospects for enhancing the immune
system ability to combat tumors (56). Radiotherapy can induce immunogenic
cell death of tumor cells, releasing damage-associated molecular
patterns and TAAs, thereby activating DCs and promoting their
migration to lymph nodes, which in turn induces systemic antitumor
immune responses; therefore, injecting exogenously prepared
unloaded DCs into tumors followed by radiotherapy may offer
additional benefits (57).
Notably, the production process of DC vaccines is
complex, requiring multiple technical barriers (such as
insufficient antigen presentation, migratory capacity, and cytokine
release) to be overcome to enhance the effectiveness of the
treatment (43). Compared with
other treatment modalities, such as chemotherapy and radiotherapy,
the safety of DC vaccines is predominantly reflected in their lower
rates of non-specific toxic side effects and more personalized
therapeutic approaches (58).
However, due to the current stage of research and clinical trials,
the long-term safety and efficacy of DC vaccines still require
further evaluation and confirmation through additional clinical
studies and practical experience.
In conclusion, DC vaccines sensitized with A549 lung
CSC lysates can induce more effective antitumor immune responses in
T cells. However, these experimental results have not yet been
validated in vivo. Therefore, it is necessary to establish a
mouse model of lung cancer and validate the antitumor efficacy of
DC vaccines. Additionally, the lack of testing on the effects of
different concentrations of DC vaccines on T-cell proliferation and
IFN-γ production limits the accurate determination of the optimal
dose. Hence, further validation of the optimal dose of DC vaccines
in T-cell function and immune response is needed. Moreover, the
lack of in-depth exploration into the mechanisms of interaction
between DC vaccines sensitized with stem cell lysates and tumor
stem cells highlights the need for advanced genomic sequencing
techniques, such as single-cell transcriptomics or proteomics
analysis, to be performed during co-culture, to improve
understanding of how DC vaccines influence the molecular mechanisms
of tumor stem cells and validate these biological interactions in
preclinical experiments. Furthermore, during the cultivation and
co-cultivation of DCs, the growth factors IL-2 and TNF-α were
added; therefore, after co-culturing DC vaccines with T
lymphocytes, these growth factors were not detected. These
limitations underscore the necessity for further research to
improve the understanding of how DC vaccines sensitized with A549
lung CSC lysates can effectively induce antitumor immune responses
and their potential impact in disease treatment.
With the continuous emergence of new technologies,
it is possible to reduce manufacturing and production costs
associated with DC vaccines, thereby enhancing overall
practicality. A deeper understanding of DC biology and immune
resistance mechanisms in the tumor microenvironment holds promise
for designing more optimized DC vaccines to meet the demands of
personalized therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Natural Science
Foundation of China (grant nos. 81160294 and 81960425).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Study design was performed by JX and LC. Data
collection and analysis were performed by WR and YC. LC, WR and YC
contributed to data interpretation, and to the writing and review
of the manuscript. JX and LC confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The Second Affiliated Hospital of Nanchang University
[Nanchang, China; approval no. O-Medical Research Ethics Approval
(2023) No. 89], and all participants provided written consent to
participate in this study. All experiments in this study comply
with the ethical standards of the World Medical Association
(Declaration of Helsinki).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yin W, Wang J, Jiang L and James Kang Y:
Cancer and stem cells. Exp Biol Med (Maywood). 246:1791–1801. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rowbotham SP, Goruganthu MUL, Arasada RR,
Wang WZ, Carbone DP and Kim CF: Lung cancer stem cells and their
clinical implications. Cold Spring Harb Perspect Med.
12:a0412702022.PubMed/NCBI
|
|
3
|
Walcher L, Kistenmacher AK, Suo H, Kitte
R, Dluczek S, Strauß A, Blaudszun AR, Yevsa T, Fricke S and
Kossatz-Boehlert U: Cancer stem cells-origins and biomarkers:
Perspectives for targeted personalized therapies. Front Immunol.
11:12802020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang D, Tang DG and Rycaj K: Cancer stem
cells: Regulation programs, immunological properties and
immunotherapy. Semin Cancer Biol. 52:94–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zimmermannova O, Ferreira AG, Ascic E,
Velasco Santiago M, Kurochkin I, Hansen M, Met Ö, Caiado I, Shapiro
IE, Michaux J, et al: Restoring tumor immunogenicity with dendritic
cell reprogramming. Sci Immunol. 8:eadd48172023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bayik D and Lathia JD: Cancer stem
cell-immune cell crosstalk in tumour progression. Nat Rev Cancer.
21:526–536. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doherty MR, Smigiel JM, Junk DJ and
Jackson MW: Cancer stem cell plasticity drives therapeutic
resistance. Cancers (Basel). 8:82016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Wang J, Chen J, Wu S, Zeng X, Xiong
Q, Guo Y, Sun J, Song F, Xu J, et al: Upregulation of miR-520c-3p
via hepatitis B virus drives hepatocellular migration and invasion
by the PTEN/AKT/NF-κB axis. Mol Ther Nucleic Acids. 29:47–63. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Todaro M, Alea MP, Di Stefano AB,
Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G,
Medema JP and Stassi G: Colon cancer stem cells dictate tumor
growth and resist cell death by production of interleukin-4. Cell
Stem Cell. 1:389–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dean M: ABC transporters, drug resistance,
and cancer stem cells. J Mammary Gland Biol Neoplasia. 14:3–9.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang T, Song X, Xu D, Tiek D, Goenka A,
Wu B, Sastry N, Hu B and Cheng SY: Stem cell programs in cancer
initiation, progression, and therapy resistance. Theranostics.
10:8721–8743. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shekhani MT, Jayanthy AS, Maddodi N and
Setaluri V: Cancer stem cells and tumor transdifferentiation:
Implications for novel therapeutic strategies. Am J Stem Cells.
2:52–61. 2013.PubMed/NCBI
|
|
13
|
Garvalov BK and Acker T: Cancer stem
cells: A new framework for the design of tumor therapies. J Mol Med
(Berl). 89:95–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morazán-Fernández D, Mora J and
Molina-Mora JA: In silico pipeline to identify tumor-specific
antigens for cancer immunotherapy using exome sequencing data.
Phenomics. 3:130–137. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morrison BJ, Steel JC and Morris JC:
Sphere culture of murine lung cancer cell lines are enriched with
cancer initiating cells. PLoS One. 7:e497522012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pardoll D: Does the immune system see
tumors as foreign or self? Annu Rev Immunol. 21:807–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hinterleitner C, Strähle J, Malenke E,
Hinterleitner M, Henning M, Seehawer M, Bilich T, Heitmann J, Lutz
M, Mattern S, et al: Platelet PD-L1 reflects collective
intratumoral PD-L1 expression and predicts immunotherapy response
in non-small cell lung cancer. Nat Commun. 12:70052021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gilboa E: DC-based cancer vaccines. J Clin
Invest. 117:1195–1203. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jego G, Pascual V, Palucka AK and
Banchereau J: Dendritic cells control B cell growth and
differentiation. Curr Dir Autoimmun. 8:124–139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi H, Egen JG, Huang AYC and Germain RN:
Extrafollicular activation of lymph node B cells by antigen-bearing
dendritic cells. Science. 312:1672–1676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rojas-Sepúlveda D, Tittarelli A, Gleisner
MA, Ávalos I, Pereda C, Gallegos I, González FE, López MN, Butte
JM, Roa JC, et al: Tumor lysate-based vaccines: On the road to
immunotherapy for gallbladder cancer. Cancer Immunol Immunother.
67:1897–1910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ragde H, Cavanagh WA and Tjoa BA:
Dendritic cell based vaccines: Progress in immunotherapy studies
for prostate cancer. J Urol. 172:2532–2538. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ni L: Advances in human dendritic
cell-based immunotherapy against gastrointestinal cancer. Front
Immunol. 13:8871892022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dwivedi R, Pandey R, Chandra S and
Mehrotra D: Dendritic cell-based immunotherapy: a potential player
in oral cancer therapeutics. Immunotherapy. 15:457–469. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J, Shangguan J, Eresen A, Li Y, Wang
J and Zhang Z: Dendritic cells in pancreatic cancer immunotherapy:
Vaccines and combination immunotherapies. Pathol Res Pract.
215:1526912019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qian D, Li J, Huang M, Cui Q, Liu X and
Sun K: Dendritic cell vaccines in breast cancer: Immune modulation
and immunotherapy. Biomed Pharmacother. 162:1146852023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu JH and Li G: Dendritic cell-based
immunotherapy for malignant glioma. Neurosci Bull. 24:39–44. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, He T, Li Y, Chen L, Liu H, Wu Y
and Guo H: Dendritic cell vaccines in ovarian cancer. Front
Immunol. 11:6137732021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perrigue PM, Rakoczy M, Pawlicka KP,
Belter A, Giel-Pietraszuk M, Naskręt-Barciszewska M, Barciszewski J
and Figlerowicz M: Cancer stem cell-inducing media activates
senescence reprogramming in fibroblasts. Cancers (Basel).
12:17452020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chometon TQ, Siqueira MDS, Sant Anna JC,
Almeida MR, Gandini M, Martins de Almeida Nogueira AC and Antas
PRZ: A protocol for rapid monocyte isolation and generation of
singular human monocyte-derived dendritic cells. PLoS One.
15:e02311322020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kokkinopoulos D, Perez S, Sotiriadou R,
Stinios J and Papamichail M: The use of nylon wool for the
isolation of T lymphocyte subpopulations. J Immunol Methods.
154:1–6. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo Y, Chen J, Liu M, Chen S, Su X, Su J,
Zhao C, Han Z, Shi M, Ma X and Huang H: Twist1 promotes dendritic
cell-mediated antitumor immunity. Exp Cell Res. 392:1120032020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han F, Guo S, Huang C, Cui L, Zhao Y, Ma
J, Zhu M, Chen Z, Wang M, Shen B and Zhu W: Gastric cancer
mesenchymal stem cells inhibit natural killer cell function by
up-regulating FBP1. Cent Eur J Immunol. 46:427–437. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
In H, Park JS, Shin HS, Ryu SH, Sohn M,
Choi W, Park S, Hwang S, Park J, Che L, et al: Identification of
dendritic cell precursor from the CD11c+ cells
expressing high levels of MHC class II molecules in the culture of
bone marrow with FLT3 ligand. Front Immunol. 14:11799812023.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei W, Mu S, Han Y, Chen Y, Kuang Z, Wu X,
Luo Y, Tong C, Zhang Y, Yang Y and Song Z: Gpr174 knockout
alleviates DSS-induced colitis via regulating the immune function
of dendritic cells. Front Immunol. 13:8412542022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Xiang Y, Xin VW, Wang XW, Peng XC,
Liu XQ, Wang D, Li N, Cheng JT, Lyv YN, et al: Dendritic cell
biology and its role in tumor immunotherapy. J Hematol Oncol.
13:1072020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hanna JM and Onaitis MW: Cell of origin of
lung cancer. J Carcinog. 12:62013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saxena M, Balan S, Roudko V and Bhardwaj
N: Towards superior dendritic-cell vaccines for cancer therapy. Nat
Biomed Eng. 2:341–346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bol KF, Schreibelt G, Gerritsen WR, de
Vries IJ and Figdor CG: Dendritic cell-based immunotherapy: State
of the art and beyond. Clin Cancer Res. 22:1897–1906. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kaczmarek M, Poznańska J, Fechner F,
Michalska N, Paszkowska S, Napierała A and Mackiewicz A: Cancer
vaccine therapeutics: limitations and effectiveness-a literature
review. Cells. 12:21592023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fu C, Zhou L, Mi QS and Jiang A: DC-based
vaccines for cancer immunotherapy. Vaccines (Basel). 8:7062020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hato L, Vizcay A, Eguren I, Pérez-Gracia
JL, Rodríguez J, Gállego Pérez-Larraya J, Sarobe P, Inogés S, Díaz
de Cerio AL and Santisteban M: Dendritic cells in cancer immunology
and immunotherapy. Cancers (Basel). 16:9812024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Perez CR and De Palma M: Engineering
dendritic cell vaccines to improve cancer immunotherapy. Nat
Commun. 10:54082019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mastelic-Gavillet B, Balint K, Boudousquie
C, Gannon PO and Kandalaft LE: Personalized dendritic cell
vaccines-recent breakthroughs and encouraging clinical results.
Front Immunol. 10:7662019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Abraham RS and Mitchell DA: Gene-modified
dendritic cell vaccines for cancer. Cytotherapy. 18:1446–1455.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Masoumi J, Ghorbaninezhad F, Saeedi H,
Safaei S, Khaze Shahgoli V, Ghaffari Jolfayi A, Naseri B,
Baghbanzadeh A, Baghbani E, Mokhtarzadeh A, et al: siRNA-mediated
B7H7 knockdown in gastric cancer lysate-loaded dendritic cells
amplifies expansion and cytokine secretion of autologous T cells.
Biomedicines. 11:32122023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen L, Zhang D, Chen Y, Zhu H, Liu Z, Yu
Z and Xie J: ORC6 acts as an effective prognostic predictor for
non-small cell lung cancer and is closely associated with tumor
progression. Oncol Lett. 27:962024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Campillo-Davo D, Versteven M, Roex G, De
Reu H, Heijden SV, Anguille S, Berneman ZN, Tendeloo VFIV and Lion
E: Rapid assessment of functional avidity of tumor-specific T cell
receptors using an antigen-presenting tumor cell line
electroporated with full-length tumor antigen mRNA. Cancers
(Basel). 12:2562020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Constantino J, Gomes C, Falcão A, Neves BM
and Cruz MT: Dendritic cell-based immunotherapy: A basic review and
recent advances. Immunol Res. 65:798–810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Elster JD, Krishnadas DK and Lucas KG:
Dendritic cell vaccines: A review of recent developments and their
potential pediatric application. Hum Vaccin Immunother.
12:2232–2239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Antonios JP, Soto H, Everson RG, Orpilla
J, Moughon D, Shin N, Sedighim S, Yong WH, Li G, Cloughesy TF, et
al: PD-1 blockade enhances the vaccination-induced immune response
in glioma. JCI Insight. 1:e870592016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ge Y, Xi H, Ju S and Zhang X: Blockade of
PD-1/PD-L1 immune checkpoint during DC vaccination induces potent
protective immunity against breast cancer in hu-SCID mice. Cancer
Lett. 336:253–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zitvogel L, Apetoh L, Ghiringhelli F,
André F, Tesniere A and Kroemer G: The anticancer immune response:
Indispensable for therapeutic success? J Clin Invest.
118:1991–2001. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wheeler CJ, Das A, Liu G, Yu JS and Black
KL: Clinical responsiveness of glioblastoma multiforme to
chemotherapy after vaccination. Clin Cancer Res. 10:5316–5326.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang S, Song Y, Shi Q, Qiao G, Zhao Y,
Zhou L, Zhao J, Jiang N and Huang H: Safety of dendritic cell and
cytokine-induced killer (DC-CIK) cell-based immunotherapy in
patients with solid tumor: A retrospective study in China. Am J
Cancer Res. 13:4767–4782. 2023.PubMed/NCBI
|
|
56
|
Wang S, Wang X, Zhou X, Lyerly HK, Morse
MA and Ren J: DC-CIK as a widely applicable cancer immunotherapy.
Expert Opin Biol Ther. 20:601–607. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bhattacharyya T, Purushothaman K,
Puthiyottil SS, Bhattacharjee A and Muttah G: Immunological
interactions in radiotherapy-opening a new window of opportunity.
Ann Transl Med. 4:512016.PubMed/NCBI
|
|
58
|
Ge C, Yang X, Xin J, Gong X, Wang X and
Kong L: Recent advances in antitumor dendritic cell vaccines.
Cancer Biother Radiopharm. 38:450–457. 2023.PubMed/NCBI
|