Introduction
In 2020, 2.3 million women worldwide were diagnosed
with breast cancer and 685,000 breast cancer-related deaths
occurred. From 2020 to January 2024, another 7.8 million women have
been diagnosed with breast cancer, making it the most prevalent
cancer worldwide (1). Generally,
cancer is characterized by abnormal tissue growth resulting from
uncontrolled cell division, which leads to a progressive increase
in the number of cells dividing autonomously (2). Notably, breast cancer is a complex
disease that involves multiple stages of initiation, progression
and metastasis; this necessitates a more comprehensive
understanding and treatment approach for breast cancer.
Treatment with chemotherapy involves the induction
of cell death to prevent the proliferation of cancer cells in the
breast; however, normal cells are also affected. This limits
chemotherapy treatment to a low-dose regimen that reduces
effectiveness. Conversely, nanotechnology allows for the design of
target-specific nanocarriers with a continuous drug release
capacity, thus contributing to a reduction in the side effects of
chemotherapeutic agents (3–5). Nanoparticles are a cornerstone of
nanotechnology, which have garnered attention from researchers due
to the development of new materials produced using noble metals,
such as silver (Ag), platinum, gold (Au) and palladium, at the
nanometer scale (6,7).
In vitro cytotoxicity testing procedures have
also received attention due to their ability to reduce the reliance
on laboratory animals (8), and have
consequently promoted the use of cultured liposomes and cells
(9). In this regard, the MCF7 and
MCF10 cell lines have been reported to serve as valuable models,
representing various critical steps in breast cancer progression
(1). The MTT assay measures
metabolic activity, such as cell viability, cytotocixity and
proliferation. This assay relies on the conversion of MTT by living
cells into formazan crystals, allowing for the determination of
mitochondrial activity. Given that total mitochondrial activity is
generally correlated with the number of viable cells within the
population, this assay is used in assessing the cytotoxic effects
of drugs on cell lines or primary patient cells in vitro
(2).
In the present study, an investigation was conducted
to evaluate the effectiveness of active nanoparticles on the MCF7
breast cancer cell line over the last decade. This assessment
encompassed an analysis of the administered dosages, the
half-maximal inhibitory concentration (IC50) values and
the proportion of cell viability (determined using the MTT test),
each with a specific focus on a 48-h incubation period. The aim of
the present study was to provide a summary of the current available
literature regarding the viability of cells in response to
different nanoparticles, and to explore concentration levels for
each nanoparticle over a 48-h period. It is anticipated that the
present findings may contribute valuable insights into the
development of novel nanoparticle-based treatments for breast
cancer.
Materials and methods
Data collection
Data selection and meta-analysis followed the
protocol outlined in the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses 2020 guidelines (10).
Criteria for database searching
A literature search was conducted within Science
Direct (https://www.sciencedirect.com/), Scopus (https://www.scopus.com/home.uri) and PubMed
(https://pubmed.ncbi.nlm.nih.gov/) to
retrieve full-text research articles published between 2013 and
2023. Key words included ‘MCF7’, ‘nanotechnology’ and ‘anticancer’.
Journals were limited to the fields of chemistry, pharmacology,
toxicology or pharmaceutical science.
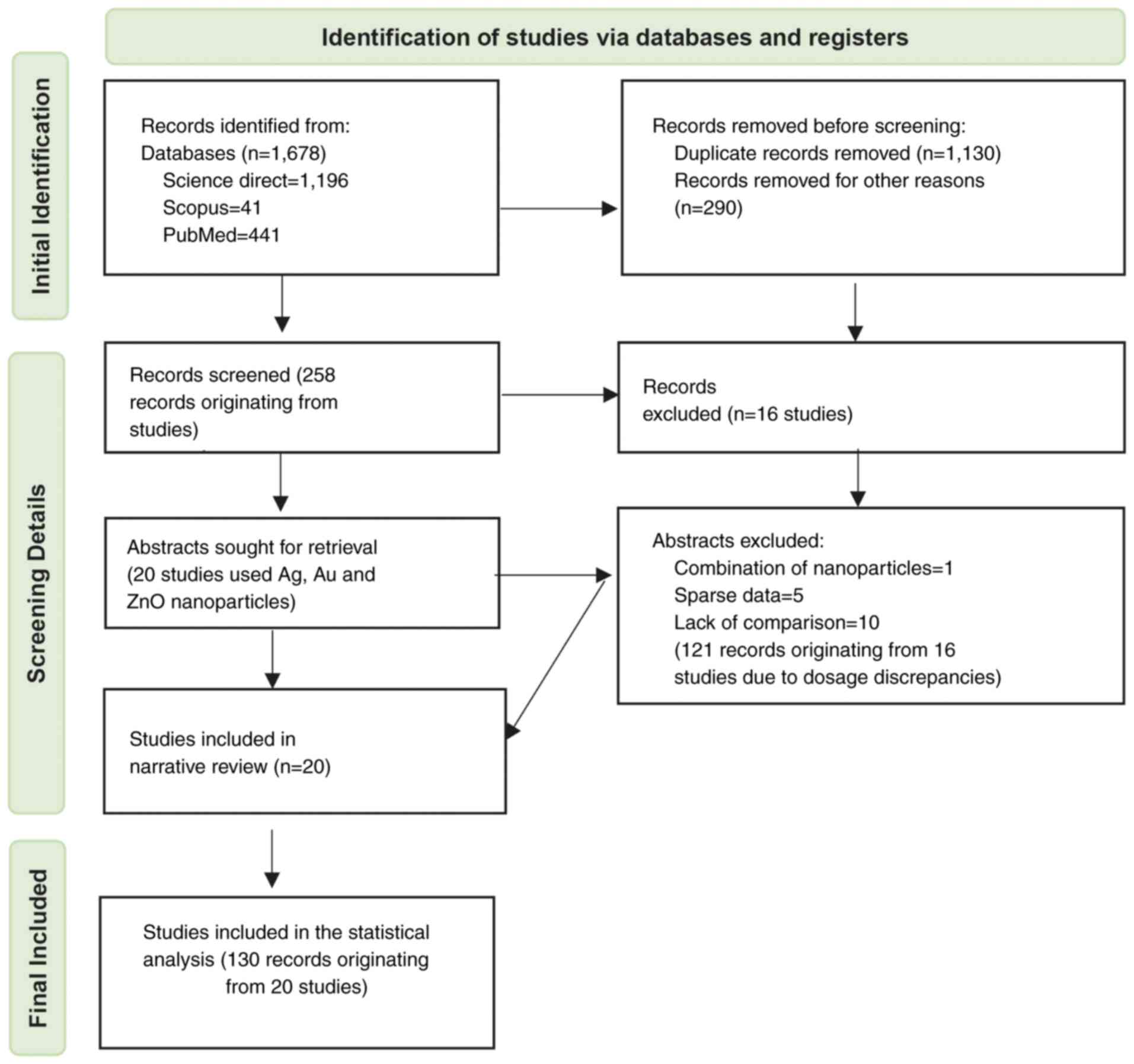
Records excluded and included
Literature screening spanned from the beginning of
2022 through to September 2023. A total of 1,678 records were
identified during the literature search (Fig. 1). Of these, 1,196 were from Science
Direct, 41 were from Scopus and the remaining 441 were from PubMed.
A total of 1,130 records were excluded as duplicates, leaving 548
to be screened. After implementing the eligibility criteria, an
additional 290 cases were disqualified for various reasons. These
included using nanoparticle combinations other than Ag, zinc oxide
(ZnO) and Au, a lack of comparisons, and reporting varied parameter
estimates unrelated to cell viability proportions. Furthermore,
full-text articles were excluded if they deviated from the specific
culture time (48 h), or if they presented unusual or specialized
conditions (e.g. light irradiation in photodynamic therapy), or had
ambiguous descriptions. This yielded 258 records that originated
from 36 studies, each employing distinct nanoparticle dosages. Only
results from studies with a 48-h culture time were included in the
meta-analysis. Consequently, 15 studies from the original 36 were
excluded from the dataset, removing 121 observations due to dosage
discrepancies. This selection process led to 20 remaining studies
and a cumulative total of 130 observations that were included in
the meta-analysis. As shown in Table
I, multiple doses were administered in each study. For example,
Al-Khedhairy and Wahab (11) tested
seven different doses ranging from 2 to 200 µg/ml Ag. Similarly,
Almalki and Khalifa (12)
experimented with five different doses of Ag ranging from 10 to 50
µg/ml. When considering these instances collectively, a total of
130 different doses of Ag, ZnO and Au were tested across 20
studies, which formed the basis for the meta-analysis.
 | Table I.Study characteristics. |
Table I.
Study characteristics.
| First author,
year | Nanoparticle
used | Number of dosages
used |
IC50 | Total cells
counted | Proportion of
viable cells, % | (Refs.) |
|---|
| Al-Khedhairy,
2022 | Ag | 7 (2–200
µg/ml) | 5.18 µg/ml | 1,000,000 | 9–100 | (11) |
| Almalki, 2020 | Ag | 5 (10–50
µg/ml) | 27–31 µg/ml | 100,000 | 15–99 | (12) |
| Balaji, 2022 | ZnO | 7 (5–60 µg/ml) | 37 µg/ml | 10,000 | 32–99 | (15) |
| Charghadchi,
2021 | Ag | 8 (1.25–160
µg/ml) | 13.4 µg/ml | 100,000 | 10–77 | (16) |
| Gomathi, 2020 | Ag | 9 (5–120
µg/ml) | 20 µg/ml | 10,000 | 15–98 | (17) |
| Hashemi, 2021 | Ag | 6 (6.5–150
µg/ml) | 21.28 µg/ml | 10,000 | 10–90 | (18) |
| Khandanlou,
2018 | Au | 5 (50–150
µg/ml) | 116.65 µg/ml | 10,000 | 3–72 | (19) |
| Khedhairy,
2022 | Ag | 7 (2–200
µg/ml) | 9.69 µg/ml | 10,000 | 21–100 | (11) |
| Krishnan, 2016 | Ag | 10 (2–200
µg/ml) | 52 µg/ml | 5,000 | 15–70 | (20) |
| Mani, 2021 | Ag | 5 (25–200
µg/ml) | 25–200 µg/ml | 5,000,000 | 13.33–51.95 | (21) |
| Nayaka, 2020 | Ag | 5 (12.5–200
µg/ml) | 72.32 µg/ml | 20,000 | 20–90 | (22) |
| Sathishkumar,
2014 | Ag | 1 (5 µg/ml) | 5 µg/ml | 1,000,000 | 50 | (23) |
| Shandiz, 2021 | ZnO | 4 (1–1,000
µg/ml) | 7.23 µg/ml | 10,000 | 0–65 | (24) |
| Shawkey, 2013 | Ag | 16 (5–50
µg/ml) | 22.4–30 µg/ml | 100,000 | 41.3–100 | (25) |
| Venugopal,
2016 | Ag | 10 (10–100
µg/ml | 52 µg/ml | 5,000 | 15–80 | (26) |
| Shewetha, 2020 | ZnO | 5 (20–100
µg/ml) | 52.64 µg/ml | 10,000 | 20–80 | (27) |
| Solairaj, 2017 | Ag | 2 (31–1,000
µg/ml) | 31 µg/ml | 500,000 | 11.27–60 | (28) |
| Ullah, 2020 | Ag | 7 (5–200
µg/ml) | 25 µg/ml | 20,000 | 10–62 | (29) |
| Venugopal,
2017 | Ag | 14 (10–100
µg/ml) | 60–70 µg/ml | 5,000 | 15–80 | (30) |
| Venugopal,
2021 | Ag | 5 (60–100
µg/ml) | 60 µg/ml | 5,000 | 15–45 | (31) |
Data items
The outcome variable for the meta-analysis was the
proportion of cell viability, derived from each nanoparticle group:
Ag, ZnO and Au. Other variables included administered dosage and
IC50 (measure of potency).
Establishing subgroups
The metal groups, along with dosages in the form of
nanoparticles, formed the basis for the subgroup analyses. Dose
applications for Ag ranged from 1.25 to 1,000 µg/ml; and the doses
for Au and ZnO were 50–150 µg/ml and 1–1,000 µg/ml, respectively.
Dosages were categorized into homogeneous subgroups, considering
the proportion of viable cells and IC50 values. In this
context, Ag doses were distributed into the following five
subgroups: <10, 10–15, 20–31, 40–50 and >60 µg/ml. Two groups
were established for Au: 0.8–8 and ≥50 µg/ml. In the case of ZnO,
four groups were formed: <10, 10–20, 30–40 and >50 µg/ml.
Statistical analysis
Primary outcomes, including data from various
nanoparticles, applied dosages and IC50 values, were
synthesized and presented using descriptive statistics in tables
and forest plots. These analyses were performed with the ‘meta’
library (package) version 6.5 (July, 2023; http://cran.r-project.org/web/packages/meta/index.html),
‘metafor’ library (package) version 4.4-0 (September 27,
2023;https://cran.r-project.org/web/packages/metafor/index.html) in
RStudio version 2023.06.0+421 (https://posit.co/download/rstudio-desktop/), as well
as the RevMan 5.4.1 software (The Cochrane Collaboration;
http://training.cochrane.org/online-learning/core-software/revman)
and SAS (version 9.4; SAS Institute). In the present study, a
robust statistical approach was employed to analyze the data,
specifically focusing on the use of the generalized linear mixed
model. More specifically, a random intercept logistic regression
model with logit transformation was used for conducting a
comprehensive meta-analysis of proportions. This statistical
technique allowed the study to effectively address the challenges
associated with synthesizing data across various studies,
especially when dealing with binary outcomes or proportions. The
random intercept logistic regression model accounts for both the
within-study and between-study variability, making it well-suited
for meta-analyses where the data comes from multiple sources with
different characteristics and study designs. The logit
transformation facilitates the modeling of proportions and the
estimation of overall effects, enabling the study to derive
meaningful insights and draw robust conclusions.
In the random-effects model, τ2 was
employed to assess the level of variation among the observed
effects in distinct studies (between-study variance). This
assessment is contingent upon the use of Cochran's homogeneity
test, which is characterized by the Q statistic. The P-value
associated with the test, which can be derived using either the
Wald test or the likelihood ratio test (LRT), follows a
χ2 distribution with degrees of freedom equal to k-1,
where k represents the number of studies included in the analysis
(13). To gauge the consistency of
results across individual studies, the I2 test was
employed. In this test, a score of <25% suggested a minimal
level of between-study heterogeneity (14). Conversely, a higher I2
score signified that the variation in effect estimates was not
merely due to chance but indicated the potential influence of a
moderator effect. Such a moderator effect can, to some extent,
influence both the direction and the magnitude of the study'
outcomes.
Notably, the random-effects model results were used
for all reported results and evaluations, regardless of the
I2 value. This approach was in strict accordance with
the Cochrane Handbook for Systematic Reviews of Interventions
guidelines (13) (https://training.cochrane.org/handbook),
ensuring consistency and rigor in the analysis and reporting of
results. Additionally, the forest plots were generated using the
‘meta’ library version 6.5 with the random-effects model applied by
default.
To evaluate the source of heterogeneity, subgroups
were created for nanoparticles, Ag, Au and ZnO; to better interpret
the results, the random-effects meta-regression approach was
employed. The Agresti-Coull interval approach that calculates
confidence intervals for proportions was used for continuity of
zero counts in any one cell. P<0.05 was considered to indicate a
statistically significant difference.
Results
Study characteristics:
Detailed information regarding the 20 studies
(11,12,15–31)
and their characteristics is provided in Table I. The number of dosages investigated
in these studies ranged from 1 to 16, resulting in a total of 130
data points included in the meta-analyses. Among these studies, 16
reported outcomes related to Ag, three to Au and three to ZnO. The
observed proportions of cell viability spanned the full spectrum
from 0 to 100%, and IC50 values ranged from 1.5 to 200
µg/ml. Further insights and specifics about the included studies
are included in Table I.
Separate meta-analyses with forest plots were
conducted for the applied dose applications of Ag, Au and ZnO;
however, due to the complexity of the findings the present analysis
focused on the most notable results. As a consequence, three
subgroup dosage comparisons were evaluated: i) Within the Ag group:
<10 µg/ml vs. 10–15 µg/ml; ii) within the Ag group the lowest
was compared to the highest dose: <10 µg/ml vs. ≥60 µg/ml; and
iii) comparisons between Ag, Au and ZnO, all at ≥60 µg/ml. To
enhance the homogeneity and interpretability of the subgroup
analysis comparing Ag, Au, and ZnO nanoparticles at ≥60 µg/ml, the
study by Sathishkumar et al (23) was excluded. This study employed a
unique nanoparticle synthesis method and reported significantly
different outcomes compared with the remaining studies.
Comparisons within the Ag group:
<10 vs. 10–15 μg/ml
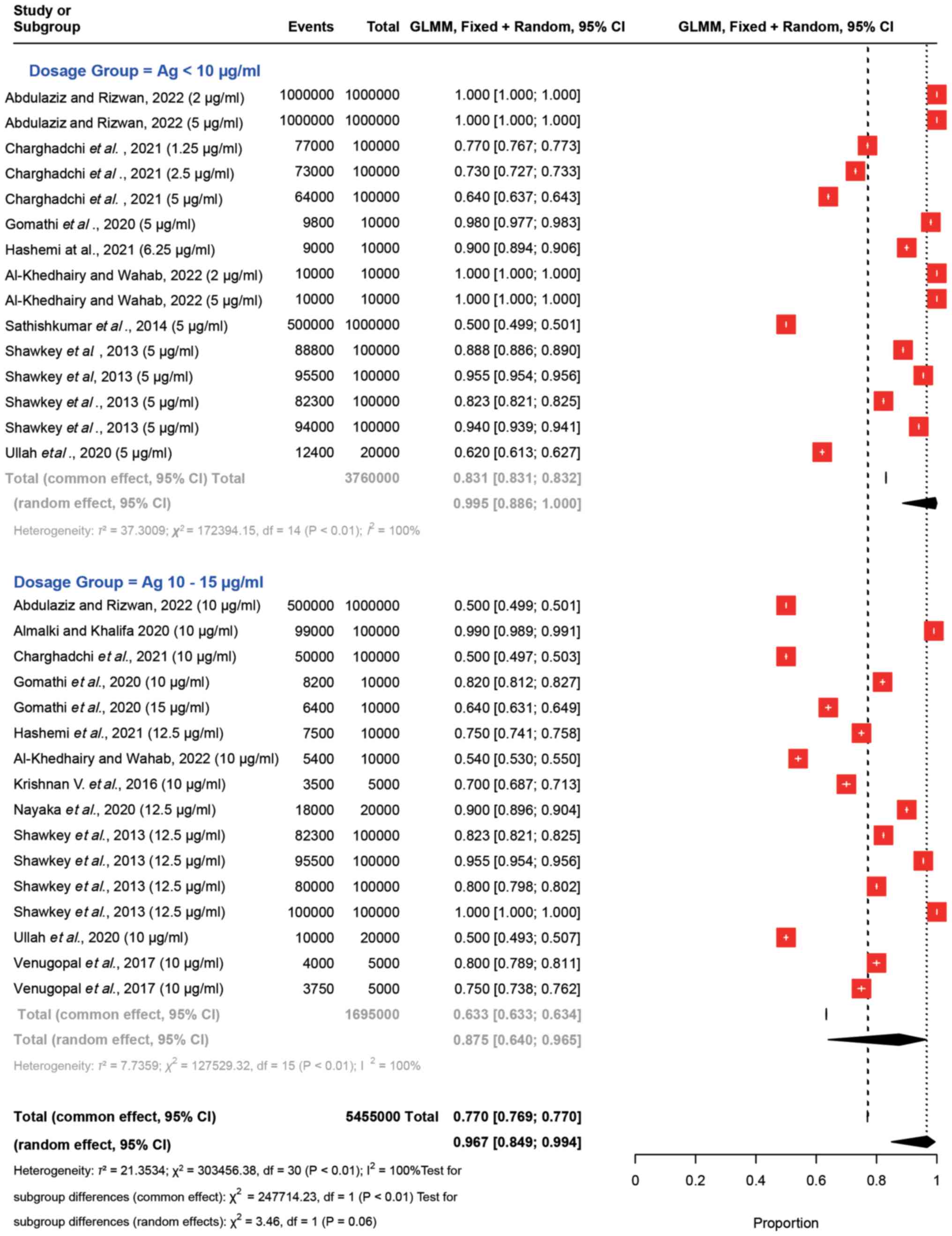
Fig. 2 presents the
forest plot comparing Ag dosages <10 µg/ml to 10–15 µg/ml. The
plot visualizes the ‘Events’ denoting the viable cell count and the
total cell count, accompanied by their respective 95% confidence
intervals (CIs). These proportions convey the impact of the
administered Ag dosages on the proportion of viable cells. For
example, Charghadchi et al (16) reported a proportion of 0.77 with a
95% CI of 0.767–0.773 for Ag dosages <10 µg/ml (Ag 1.25 µg/ml),
while a proportion of 0.50 was observed with a 95% CI of
0.497–0.503 for dosages ranging from 10 to 15 µg/ml. Notably,
similar results were observed in the study by Shawkey et al
(25) for both Ag dosages. The
summary statistics provide overall estimates of the effect. The
‘Random effects model’ estimated a proportion of cell viability of
0.967 (95% CI: 0.849–0.994) with a wider CI than fixed effect
(0.769–0.770) and an I2 value of 100%, indicating high
variability between studies. This model assumes that the true
effect may differ between studies.
In both Ag dosage groups and across all
observations, substantial heterogeneity was evident. Notably, as an
indicator of the dispersion between individual studies, the
τ2 values were elevated, reaching 37.30 for the <10
µg/ml Ag dosage group, 7.74 for the 10–15 µg/ml Ag dosage group,
and 21.35 for the overall analysis. Heterogeneity was high, as
indicated by an I2 value of 100.0%. This finding
underscores that all variations observed in the results of the
meta-analysis were attributed to genuine distinctions among the
individual studies. Both the Wald test and the LRT unequivocally
affirmed the presence of substantial heterogeneity, as denoted by a
P<0.001. This robust statistical evidence firmly supports the
notion that the effect sizes across studies are not uniform; thus,
bolstering the rationale for employing the random-effects
meta-analysis approach.
An extensive assessment was conducted to ascertain
any notable distinctions between subgroups of Ag administered at
doses <10 and 10–15 µg/ml. Both the fixed- and random-effects
models were performed; in both instances, the significance level
denoted a noteworthy contrast between the two subgroups, with a
P<0.001 for the fixed-effects model (common effect) and a
P=0.060 for the random-effects model (Fig. 2). This observation indicated the
notable variation in the impact of the dosage and the associated
trends between these two distinct groups.
Comparisons between the lowest and the
highest doses of Ag: <10 vs. ≥60 µg/ml
In order to gain further insights from the results,
a comparative analysis was conducted between the lower Ag dosages,
which were <10 µg/ml, and dosages ≥60 µg/ml. The forest plot for
this comparative analysis is depicted in Fig. 3. The results demonstrated a notable
variation in the proportions of viable cells in response to
different Ag dosages Specifically, for Ag concentrations <10
µg/ml, the observed proportions ranged from 0.50 to 1.00. By
contrast, for Ag dosages ≥60 µg/ml, the proportions of viable cells
fell within the range of 0.09 to 0.45. These results demonstrated
the impact of Ag dosages on the proportions of viable cells. For
example, Al-Khedhairy and Wahab (11) revealed that in response to Ag
concentrations <10 µg/ml, the proportion was consistently 1.00
(100%) with a narrow CI. In response to Ag dosages ≥60 µg/ml, the
proportion remained at 0.09 (9%) with narrow CIs. These findings
are similar to those across other studies and dosage groups
(26). The analysis conducted on
the forest plot provides a comprehensive assessment of
heterogeneity within the dataset. Several statistical measures were
utilized, including τ2, I2 and Higgins'
I2 adjusted for the number of studies, to gauge the
extent of heterogeneity. The results indicated significant
heterogeneity among the studies, as affirmed by both the Wald test
and the LRT, both yielding, χ2 =16.23, P<0.01
(Fig. 3).
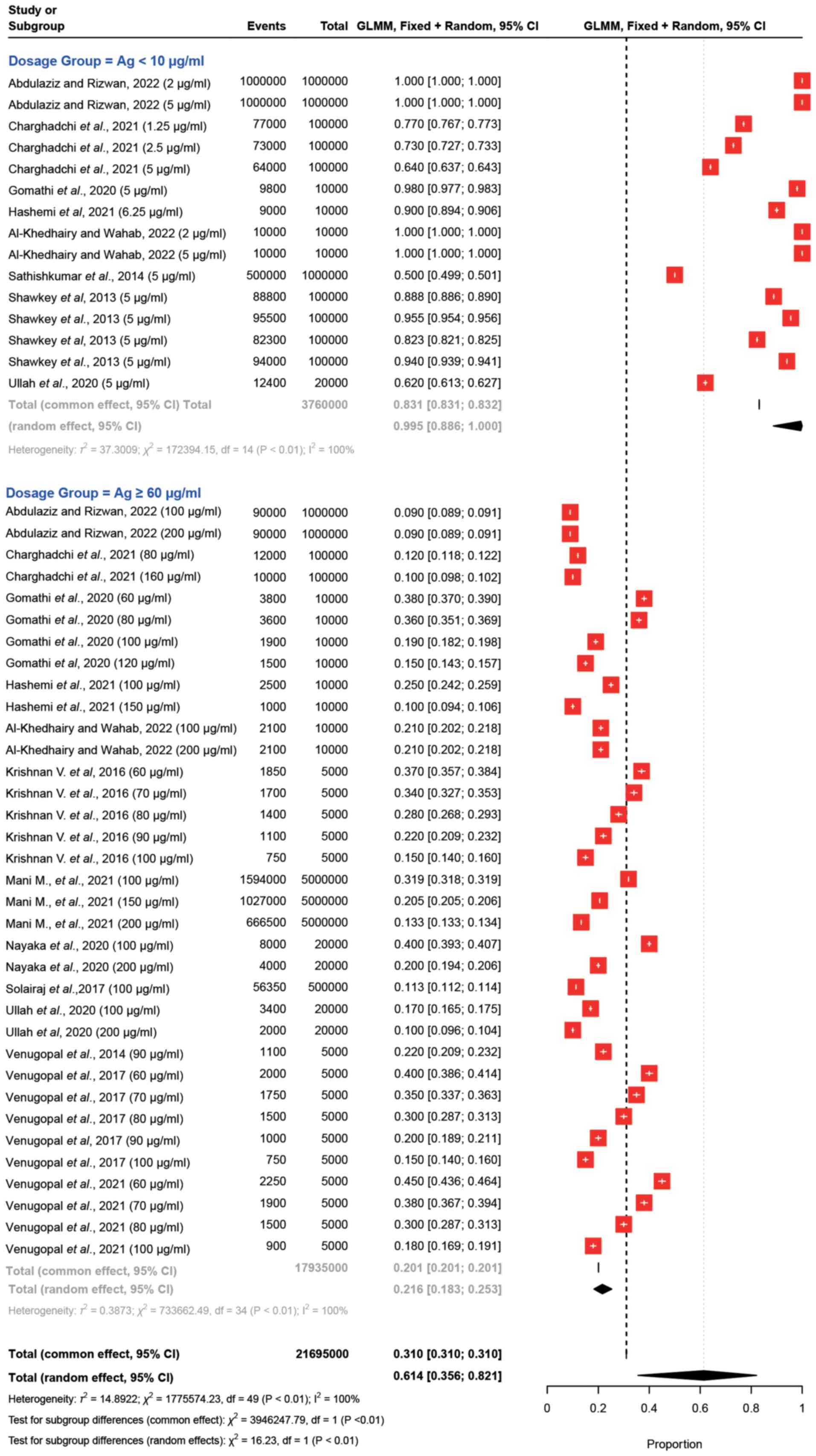 | Figure 3.Forest plots for comparing Ag
nanoparticles at dosages of <10 and ≥60 µg/ml. Each study is
represented by a red square indicating the effect estimate and its
95% CI, with the size of the square reflecting the study weight.
The plot includes individual study estimates, the total common
effect (fixed-effects model), the total random effect
(random-effects model), and heterogeneity statistics
(τ2, χ2, I2) for each group and
overall. The overall effect estimate for each group is depicted by
a diamond at the bottom of the plot, with the width of the diamond
representing the 95% CI. Ag, silver; CI, confidence interval; GLMM,
generalized linear mixed model. |
In the random-effects model, distinct patterns
emerged. Ag concentrations <10 µg/ml exhibited a viable-cell
proportion of 0.9946 (99%), coupled with a high estimated
τ2 (37.3009) value, indicating substantial heterogeneity
within this subgroup. The high τ2 suggested that there
was significant variability in effect sizes among the included
studies, beyond what would be expected by chance alone.
By contrast, Ag dosages ≥60 µg/ml demonstrated a
viable-cell proportion of 0.2161 (22%) with a relatively lower
estimated τ2 value (0.3873), suggesting less
heterogeneity within this subgroup. The lower τ2
indicates that the effect sizes across the included studies are
more consistent and similar, with less variability than would be
expected by chance. This consistency may imply that the studies in
this subgroup share more common characteristics or that the outcome
is more stable across different settings.
Comparisons between Ag, Au and ZnO,
all at ≥60 µg/ml
A comparison by group (Ag, Au and ZnO) at
concentrations ≥60 µg/ml was conducted, as a sufficient number of
studies was available for each nanoparticle type. The results for
these comparisons are shown in Fig.
4. The proportion of cell viability for both Ag and ZnO ranged
from 0.09 to 0.40; whereas Au exhibited proportions between 0.03
and 0.58. These results indicated the significant variation in the
effects of different nanoparticle dosages on cell viability when
≥60 µg/ml was applied. These findings revealed the diverse impacts
of various nanoparticle dosages on the proportion of viable cells,
emphasizing the importance of considering dosage levels in
nanoparticle studies.
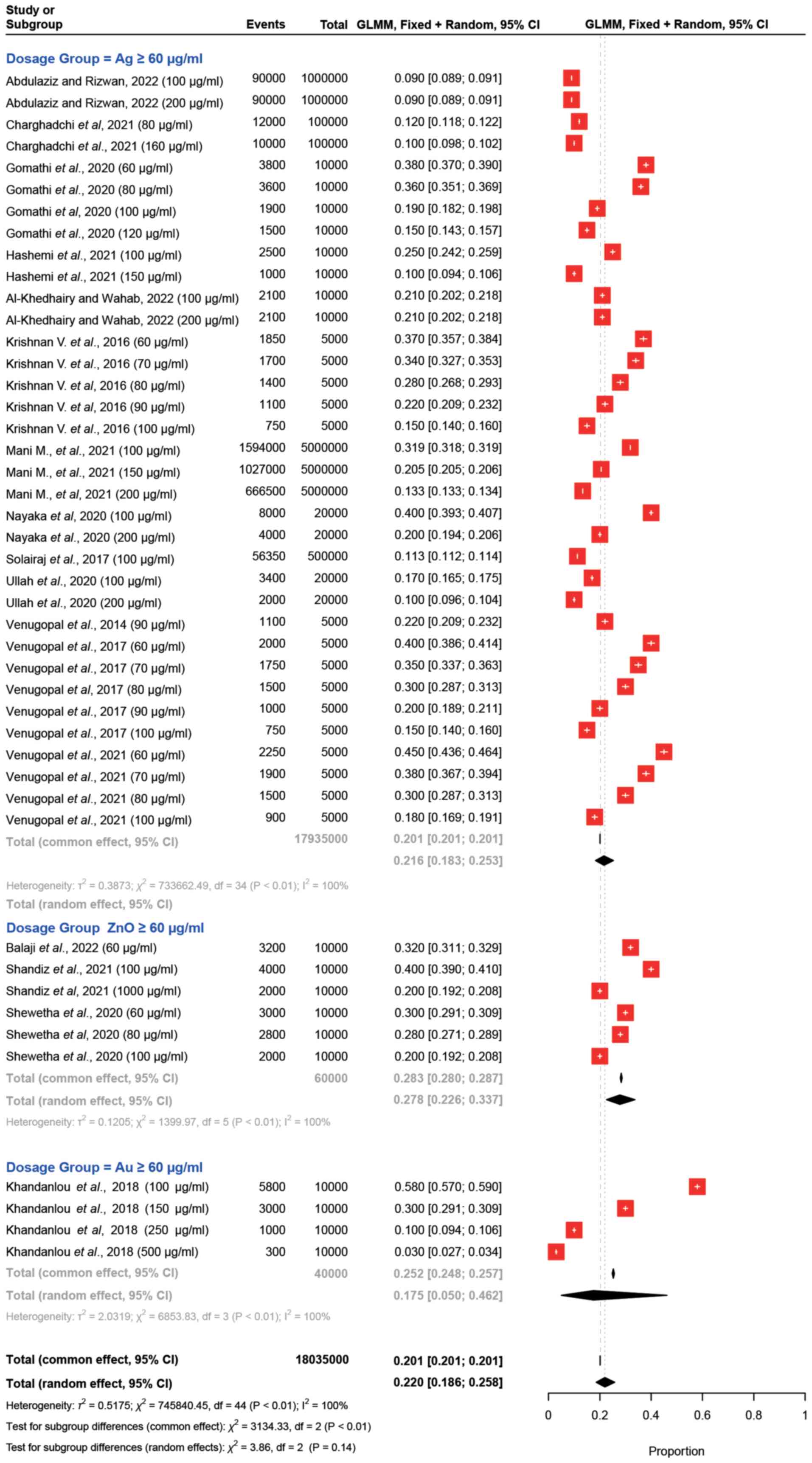 | Figure 4.Forest plots for ≥60 µg/ml dosages of
Ag, ZnO, and Au nanoparticles. The effects of Ag, ZnO and Au
nanoparticles at ≥60 µg/ml were compared on breast cancer cell
viability in vitro. Each study or subgroup is represented by
nanoparticle type, observed events (cell viability) out of the
total cells studied, and the proportion of viable cells with 95%
CIs derived from a random-effects model. The red square indicates
the effect estimate and its 95% CI, with the size of the square
reflecting the study weight. The plot includes individual study
estimates, the total common effect (fixed-effects model), the total
random effect (random-effects model) and heterogeneity statistics
(τ2, χ2, I2) for each group and
overall. The overall effect estimate for each group is depicted by
a diamond at the bottom of the plot, with the width of the diamond
representing the 95% CI. Ag, silver; Au, gold; CI, confidence
interval; GLMM, generalized linear mixed model; ZnO, zinc
oxide. |
Discussion
The present meta-analysis provides additional
insights into the impact of Ag, Au and ZnO nanoparticles on the
viability of MCF7 cells, exploring diverse concentration levels for
each nanoparticle over a 48-h period. Within the scope of MCF7
cells, previous studies (9–12) have illustrated the effects of
nanoparticles formulated with distinct active agents, examining
either the nanoparticle itself or its potential anticancer
properties. Analyzing results from studies encompassing the most
prevalent metals in nanoparticle synthesis, Ag, Au and ZnO,
revealed similar results in the proportions of viable MCF7 cells
when exposed to Ag, Au and ZnO nanoparticles at concentrations ≥60
µg/ml. These findings are consistent for data derived from live
cell rates obtained through the MTT test after a 48-h incubation
period. The present meta-analysis provided additional evidence
regarding the effect of Ag, Au and ZnO nanoparticles on the
proportion of viable MCF7 cells using different concentrations of
each nanoparticle for 48 h.
In the studies conducted by Sathishkumar et
al (23), Ullah et al
(29) and Charghadchi et al
(16), the proportion of viable
cells was determined to be 50, 62 and 64% for <10 µg/ml Ag,
respectively. Nevertheless, the outcomes of the meta-analysis
revealed that, following a 48-h incubation period with <10 µg/ml
Ag nanoparticles, the overall average proportion of cell viability
was 0.995 (95% CI, 0.888–1.000). The heterogeneity within the
studies of this group was quantified by τ2, yielding a
value of 37.3009. Significance is attributed to this heterogeneity,
as determined by the χ2 test (P<0.05). The foremost
contributors to the observed heterogeneity were considered to be
the three aforementioned studies. When comparing data within the Ag
group (i.e., <10 vs. 10–15 µg/ml), a <10 µg/ml Ag dosage was
utilized in multiple studies (11,16,18,19,23,25,29).
These studies not only synthesized Ag nanoparticles
but also incorporated diverse extracts into the experimental design
during their creation. Consequently, the observed heterogeneity
suggests that the variability in results regarding Ag nanoparticle
concentrations <10 µg/ml could be attributed to the different
active substances used alongside Ag. This indicates that the
combination of Ag with other substances may lead to differing
effects, contributing to the diversity of outcomes observed.
The present meta-analysis aimed to elucidate the
cytotoxic effects of Ag, Au and ZnO nanoparticles on MCF7 breast
cancer cells. The findings indicated that all three nanoparticles
exhibited marked cytotoxicity at higher concentrations (≥60 µg/ml).
Specifically, Ag nanoparticles reduced cell viability to a range
between 9 and 45%; ZnO nanoparticles reduced viability to between
20 and 40%; and Au nanoparticles exhibited a broader range of
viability, from 3 to 58%. This variability underscores the distinct
cytotoxic potentials of each nanoparticle type (32).
In the framework of the random-effects meta-analysis
model, the mean proportion of viable cells 48 h post-application of
Ag doses ranging from 10 to 15 µg/ml was determined to be 0.875
(95% CI, 0.640–0.965). The heterogeneity value within this specific
group was τ2=7.7359, reaching statistical significance
through the χ2 test (P<0.01). Notably, studies
conducted within this subgroup have introduced diverse active
substances into Ag nanoparticles (12,16,18,20).
The observed heterogeneity in this category may be due to the
varied use of active substances, such as Allium cepa leaf
extract, chitin and Syzygium aromaticum (clove). To allow
the homogeneity and interpretability of the subgroup analysis, the
study by Sathishkumar et al (23) was excluded from the comparison of
Ag, Au and ZnO nanoparticles at ≥60 µg/ml. This omission represents
an intriguing observation as it allows for a more focused
examination of the data within this specific concentration
range.
The results of the random-effects meta-analysis
indicated that as the applied dose concentration of Ag
nanoparticles increased, a rapid decline in the average cell
viability became evident. For example, additional analyses using
different assays, which were not depicted here, revealed that the
mean live cell ratio in response to 20 and 31 µg/ml Ag was 0.66
(95% CI, 0.52–0.77), whereas the mean live cell ratio was observed
to be 0.60 (95% CI, 0.30–0.84) for doses between 35 and 50 µg/ml
Ag, and 0.22 (95% CI, 0.18–0.25) for doses at 50 µg/ml Ag (data not
shown). Factors contributing to the cytotoxicity induced by Ag
nanoparticles may include the initiation of apoptosis through the
mitochondrial pathway in various cell lines, the production of
reactive oxygen species through lipid peroxidation in biological
membranes, and the induction of damage in structural proteins and
DNA (7,32). In a study conducted by Liu et
al (33) on MCF7 cells, it was
determined that the size and surface area of the used Ag
nanoparticles influenced cytotoxicity. Furthermore, Ag
nanoparticles induce apoptosis and necrosis at lower concentrations
(<6 µg/ml), but only lead to necrosis at higher concentrations
(>65 µg/ml) (34). A crucial
consideration for the clinical application of these findings is
whether the effective in vitro concentrations can be
replicated. The concentrations exhibiting marked cytotoxic effects
(≥60 µg/ml) must be achievable in both plasma and tumor tissues to
ensure therapeutic efficacy. For Ag, Au and ZnO nanoparticles to be
clinically viable, it is essential to evaluate their
pharmacokinetics and biodistribution in vivo. Studies have
shown that nanoparticles can accumulate in tumor tissues through
the enhanced permeability and retention effect, but achieving
therapeutic concentrations while minimizing systemic toxicity
remains a challenge (35). As
homogeneous groups, defined as groups with similar characteristics
or conditions, could not be established within the dose
applications of <60 µg/ml for Au and ZnO due to the limited
number of available studies, the present study focused on
evaluating cell viability rates 48 h after administering doses of
Ag, Au and ZnO nanoparticles at ≥60 µg/ml. This was done in
conjunction with assessing three distinct nanoparticles. The mean
proportion of cell viability in response to ZnO nanoparticles at
doses ≥60 µg/ml were recorded at 0.278 (95% CI, 0.226–0.337). By
contrast, the mean proportions of cell viability in response to Ag
and Au at doses ≥60 µg/ml were 0.22 (95% CI, 0.18–0.25) and 0.18
(95% CI, 0.05–0.46), respectively. When considering the three
nanoparticles assessed in the present study, it was evident that Au
exhibited higher heterogeneity and variability in studies involving
doses ≥60 µg/ml compared with the other two nanoparticles. The
increased use of extracts in conjunction with Ag in the evaluated
studies has led to greater heterogeneity and variability compared
with the other two particles. In the meta-analyses conducted, the
studies employed a diverse range of active substances in
combination with Ag. For example, among the 11 studies that used
Ag, only two used Ag alone, accounting for 18.2%. Similarly, out of
the three studies that used ZnO nanoparticles, only one used ZnO
alone (33.33%). On the other hand, studies involving Au conducted
experiments without using extracts. Notably, the CI obtained for
the live cell ratios of Ag nanoparticles at doses ≥60 µg/ml was
less extensive compared with that obtained for the other two
nanoparticles. While 34 results from 11 studies were reported for
Ag nanoparticles at doses ≥60 µg/ml, ZnO nanoparticles yielded six
results from three studies, and Au nanoparticles reported four
results from a single study across four different doses. The
differing results observed across studies examining Au
nanoparticles may arise from several factors, including differences
in nanoparticle size, coating materials, experimental conditions
and the biological systems used in the studies. Moreover, previous
research has indicated that Ag nanoparticles can reach therapeutic
levels in plasma and tumor tissues but require careful dosing to
avoid systemic toxicity. Au nanoparticles, noted for their
biocompatibility and potential for targeted delivery, show promise
in achieving high tumor concentrations, although precise dosage
optimization is necessary due to variable cytotoxic effects. ZnO
nanoparticles, which were associated with moderate cytotoxicity and
a higher proportion of viable cells in response to ≥60 µg/ml, may
have a wider therapeutic window but may be less effective compared
with Ag and Au nanoparticles (36,37).
The heterogeneity observed in the present study has
been suggested to be influenced by several factors, including the
application of the MTT colorimetric method to measure the
proportion of viable cells. The MTT method was applied to groups
categorized based on administered doses, synthesis methods
affecting particle size, shape and surface properties, as well as
study design and conditions. Variations in nanoparticle synthesis
methods for Ag, Au and ZnO nanoparticles may also have contributed
to this heterogeneity. This study offers valuable insights for
researchers planning future investigations by highlighting key
factors and considerations that may influence study outcomes. It
identifies potential sources of variability that involve the use of
nanoparticles in combination with anticancer agents. The findings
suggested that Ag and Au nanoparticles exhibit superior cytotoxic
effects compared with ZnO nanoparticles at similar concentrations.
This insight may guide future research on nanoparticle-based
therapies for breast cancer. Further in vivo studies are
necessary to determine the optimal dosing regimens, and to evaluate
the long-term safety and efficacy of these nanoparticles.
Additionally, the development of targeted delivery systems could
enhance the accumulation of nanoparticles in tumor tissues, thereby
increasing their therapeutic potential while minimizing systemic
side effects. Future research should also explore combination
therapies, where nanoparticles are used alongside conventional
treatments to enhance overall efficacy. In summary, the present
study highlighted the significant cytotoxic potential of Ag, Au and
ZnO nanoparticles on MCF7 breast cancer cells. Ag and Au
nanoparticles, in particular, demonstrated superior cytotoxic
effects at higher concentrations. Achieving these effective
concentrations in vivo remains a critical challenge that
must be addressed to realize the clinical potential of these
therapies. Future research should focus on optimizing nanoparticle
formulations, dosing strategies and delivery mechanisms to
translate these promising in vitro findings into viable
clinical applications.
One of the limitations of the present study is the
exclusive use of the MTT assay to assess cell viability, without
including other viability and apoptosis assays, such as
CellTiter-Blue, Caspase-Glo 3/7 and TUNEL. The inclusion of
multiple assays could have provided a more comprehensive
understanding of cell viability and apoptotic mechanisms. Each
assay has its unique sensitivity and specificity, and a combination
of these could have validated and reinforced the findings. However,
the decision to include only the MTT assay was intentional. During
the literature review, it was observed that the MTT assay was the
most consistently reported across the selected studies.
Additionally, the MTT assay, which was used in all of the studies,
is a well-established method for assessing cell metabolic activity
and viability, providing reliable and reproducible results
(2,11,34).
This consistency in reporting enabled for more direct comparisons
between the findings. Additionally, the MTT assay is a
well-established method for assessing cell metabolic activity and
viability, providing reliable and reproducible results. By focusing
on a single, widely-used assay, it was aimed to ensure that the
results were comparable to the majority of existing studies, thus
maintaining the integrity and relevance of the present findings
within the broader context of current research.
Another limitation of the present study was the
exclusive use of MCF7 cells, a specific type of breast cancer cell
line, without including other breast cancer cell lines. The
inclusion of various breast cancer cell lines may have offered a
broader perspective on the efficacy and mechanism of the treatment
across different cellular contexts. The choice to use only MCF7
cells was based on their extensive use in breast cancer research,
which allows for better comparisons with previously published data.
MCF7 cells are well-characterized, and have been widely used as a
model system to study breast cancer biology and treatment
responses. However, future studies should include a range of breast
cancer cell lines to enhance the generalizability of the
findings.
Furthermore, the present study did not include
normal non-transformed cell lines, such as MCF10A, to determine
whether the nanoparticles selectively targeted cancer cells or also
affected normal cells. Including normal cell lines could provide
insights into the selectivity and potential toxicity of the
treatment, which is crucial for assessing its safety profile. The
decision to exclude normal cell lines was made to focus on the
primary objective of evaluating the efficacy of the treatments
against breast cancer cells. Nevertheless, assessing the impact on
normal cells is essential for future studies to ensure the
therapeutic selectivity and minimize potential side effects.
In conclusion, the present study offers important
insights into the effects of treatment on breast cancer cells.
However, the limitations related to the choice of assays, the
diversity of cell lines and the absence of normal cell lines
underscore the need for further research. Future studies that
incorporate a variety of assays, different cancer cell lines and
normal cells will strengthen the reliability and relevance of the
results.
Acknowledgements
The author would like to thank Ms. Rosey Zackula
(School of Medicine, University of Kansas, Wichita, Kansas, USA)
for their guidance regarding PRISMA, support in structuring the
article and contributions to grammar. Furthermore, the author would
like to acknowledge Dr Hayrettin Okut (School of Medicine,
University of Kansas, Wichita, Kansas, USA) for their participation
in conducting the meta-analysis using RStudio.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
EYK conceived and designed the study, carried out
the data collection, performed the data analysis and interpreted
the results of the statistical analysis The author confirmed the
authenticity of all the raw data. The author read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
World Health Organization (WHO), . Breast
Cancer. WHO; Geneva: 2024, https://www.who.int/news-room/fact-sheets/detail/breast-cancerJuly
3–2024
|
|
2
|
van Meerloo J, Kaspers GJL and Cloos J:
Cell sensitivity assays: The MTT assay. Methods Mol Biol.
731:237–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen X, Fan N, Tan H, Ren B, Yuan G, Jia
Y, Li J, Xiong D, Xing X, Niu X and Hu X: Magnetic and self-healing
chitosan-alginate hydrogel encapsulated gelatin microspheres via
covalent cross-linking for drug delivery. Mater Sci Eng C Mater
Biol Appl. 101:619–629. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dalwadi C and Patel G: Thermosensitive
nanohydrogel of 5-fluorouracil for head and neck cancer:
Preparation, characterization and cytotoxicity assay. Int J
Nanomedicine. 13:31–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krutyakov YA, Kudrinskiy AA, Olenin AY and
Lisichkin GV: Synthesis and properties of silver nanoparticles:
Advances and prospects. Russ Chem Rev. 77:2332008. View Article : Google Scholar
|
|
6
|
Chaloupka K, Malam Y and Seifalian AM:
Nanosilver as a new generation of nanoproduct in biomedical
applications. Trends Biotechnol. 28:580–588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee DG, Go EB, Lee M, Pak PJ, Kim J and
Chung N: Gold nanoparticles conjugated with resveratrol induce cell
cycle arrest in MCF7 cell lines. Appl Biol Chem. 62:332019.
View Article : Google Scholar
|
|
8
|
Abraham SA, McKenzie C, Masin D, Ng R,
Harasym TO, Mayer LD and Bally MB: In vitro and in vivo
characterization of doxorubicin and vincristine coencapsulated
within liposomes through use of transition metal ion complexation
and pH gradient loading. Clin Cancer Res. 10:728–738. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Byrd JC, Lucas DM, Mone AP, Kitner JB,
Drabick JJ and Grever MR: KRN5500: A novel therapeutic agent with
in vitro activity against human B-cell chronic lymphocytic leukemia
cells mediates cytotoxicity via the intrinsic pathway of apoptosis.
Blood. 101:4547–4550. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BJM. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Khedhairy AA and Wahab R: Silver
nanoparticles: An instantaneous solution for anticancer activity
against human liver (HepG2) and breast (MCF7) cancer cells. Metals.
12:1482022. View Article : Google Scholar
|
|
12
|
Almalki MA and Khalifa AYZ: Silver
nanoparticles synthesis from Bacillus sp KFU36 and its anticancer
effect in breast cancer MCF-7 cells via induction of apoptotic
mechanism. J Photochem Photobiol B. 204:1117862020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Welch VA, Petkovic J, Jull J, Hartling L,
Klassen T, Kristjansson E, Pardo Pardo J, Petticrew M, Stott DJ,
Thomson D, et al: Chapter 16: Equity and specific populations.
Cochrane Handbook for Systematic Reviews of Interventions (2nd
ediction). Cochrane. 435–448. 2019.
|
|
14
|
West SL, Gartlehner G, Mansfield AJ, Poole
C, Tant C, Lenfestey N, Lux LJ, Amoozegar J, Morton SC, Carey TC,
et al: Comparative effectiveness review methods: Clinical
heterogeneity [Internet]. Agency for Healthcare Research and
Quality (US), Rockville, MD, 2010. https://www.ncbi.nlm.nih.gov/books/NBK53317/table/ch3.t2/
|
|
15
|
Balaji MP, Govindasamy R, Alharbi NS,
Kadaikunnan S, Thiruvengadam M, Baskar V and Rajeswari VD:
Biosynthesis of ZnONP using chamaecostus cuspidatus and their
evolution of anticancer property in MCF-7 and A549 cell lines.
Nanomaterials (Basel). 12:33842022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Charghadchi M, Gharari Z, Sadighian S,
Yazdinezhad A and Sharafi A: Green synthesized silver nanostructure
using rhus coriaria fruit extract ınhibits the growth of malignant
MCF7 Cell Line. Braz Arch Biol Technol. 64:e212100692021.
View Article : Google Scholar
|
|
17
|
Gomathi AC, Rajarathinamb SRX, Sadiqc AM
and Rajeshkumard S: Anticancer activity of silver nanoparticles
synthesized using aqueous fruit shell extract of Tamarindus indica
on MCF7 human breast cancer cell line. J Drug Deliv Sci Technol.
55:1013762020. View Article : Google Scholar
|
|
18
|
Hashemi Z, Mohammadyan M, Naderi S, Fakhar
M, Biparva P, Akhtari J and Ebrahimzadeh MA: Green synthesis of
silver nanoparticles using Ferula persica extract (Fp-NPs):
Characterization, antibacterial, antileishmanial, and in vitro
anticancer activities. Mater Today Commun. 27:1022642021.
View Article : Google Scholar
|
|
19
|
Khandanlou R, Murthy V, Saranath D and
Damani H: Synthesis and characterization of gold-conjugated
Backhousia citriodora nanoparticles and their anticancer activity
against MCF7 breast and HepG2 liver cancer cell lines. J Mater Sci.
453:3106–3118. 2018. View Article : Google Scholar
|
|
20
|
Krishnan V, Bupesh G, Manikandan E,
Thanigai Arul K, Magesh S, Kalyanaraman R and Maaza M: Green
synthesis of silver nanoparticles using piper nigrum concoction and
its anticancer activity against MCF7 and Hep-2 cell lines. J
Antimicro. 2:32016.
|
|
21
|
Mani M, Okla MK, Selvaraj S, Ram Kumar A,
Kumaresan S, Muthukumaran A, Kaviyarasu K, El-Tayeb MA, Elbadawi
YB, Almaary KS, et al: A novel biogenic Allium cepa leaf
mediated silver nanoparticles for antimicrobial, antioxidant, and
anticancer effects on MCF-7 cell line. Environ Res. 198:1111992021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nayaka S, Bhat SMP, Chakraborty B, Pallavi
SS, Airodagi D, Muthuraj R, Halaswamy HM, Dhanyakumara SB,
Shashiraj KN and Kupaneshi C: Seed extract-mediated synthesis of
silver nanoparticles from Putranjiva roxburghii Wall.,
phytochemical characterization, antibacterial activity and
anticancer activity against MCF7 cell line. Indian J Pharm Sei.
82:260–269. 2020.
|
|
23
|
Sathishkumar G, Gobinath C, Wilson A and
Sivaramakrishnan S: Dendrophthoe falcata (L.f) Ettingsh (Neem
mistletoe): A potent bioresource to fabricate silver nanoparticles
for anticancer effect against human breast cancer cells (MCF7).
Spectrochim Acta A Mol Biomol Spectrosc. 128:285–290. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shandiz SAS, Sharifian F, Behboodi S,
Ghodratpour F and Baghbani-Arani F: Evaluation of metastasis
suppressor genes expression and in vitro anti-cancer effects of
zinc oxide nanoparticles in human breast cancer cell lines MCF7 and
T47D. Avicenna J Med Biotechnol. 13:9–14. 2021.PubMed/NCBI
|
|
25
|
Shawkey AM, Rabeh MA, Abdulall AK and
Abdellatif AO: Green nanotechnology: anticancer activity of silver
nanoparticles using citrullus colocynthis aqueous extracts. Adv
Life Sci Technol. 13:60–70. 2013.
|
|
26
|
Venugopal K, Shanthi MP, Rajagopal K,
Bhaskar M, Uvarajan S and Manikandan E: Bioactivity of cancer cells
(MCF-7 and Hep-2) using plant extract enhanced surface plasmon
resonance (SPR) nanoparticles (Ag NPs). https://www.researchgate.net/publication/312164782
|
|
27
|
Shewetha UR, Latha MS, Rajith Kumar CR,
Kiran MS and Betageri VS: Facile synthesis of zinc oxide
nanoparticles using novel areca catechu leaves extract and their ın
vitro antidiabetic and anticancer studies. J Inorg Organomet Polym
Mater. 30:4876–4883. 2020. View Article : Google Scholar
|
|
28
|
Solairaj D, Rameshthangam P and
Arunachalam G: Anticancer activity of silver and copper embedded
chitin nanocomposites against human breast cancer (MCF-7) cells.
Int J Biol Macromol. 105:608–619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ullah I, Khalil AT, Ali M, Iqbal J, Ali W,
Alarifi S and Shinwari ZH: Green-synthesized silver nanoparticles
induced apoptotic cell death in MCF-7 breast cancer cells by
generating reactive oxygen species and activating caspase 3 and 9
enzyme activities. Oxid Med Cell Longev. 2020:12153952020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Venugopal K, Ahmadb H, Manikandanc E,
Thanigai Arul K, Kavithae K, Moodleyf MK, Rajagopal K, Balabhaskar
R and Bhaskar M: The impact of anticancer activity upon Beta
vulgaris extract mediated biosynthesized silver nanoparticles
(ag-NPs) against human breast (MCF-7), lung (A549) and pharynx
(Hep-2) cancer cell lines. J Photochem Photobiol B. 173:99–107.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Venugopal K, Rather HA, Rajagopal K,
Shanthi MP, Sheriff K, Illiyas M, Rather RA, Manikandan E, Uvarajan
S, Bhaskar M and Maaza M: Synthesis of silver nanoparticles (Ag
NPs) for anticancer activities (MCF 7 breast and A549 lung cell
lines) of the crude extract of Syzygium aromaticum. J
Photochem Photobiol B. 167:282–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carlson C, Hussain SM, Schrand AM,
Braydich-Stolle LK, Hess KL, Jones RL and Schlager JJ: Unique
cellular interaction of silver nanoparticles: Size-dependent
generation of reactive oxygen species. J Phys Chem B.
112:13608–13619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X and Feng R: Inhibition of epithelial
to mesenchymal transition in metastatic breast carcinoma cells by
c-Src suppression. Acta Biochim Biophys Sin (Shanghai). 42:496–501.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Çiftçi H, Türk M, Tamer U, Karahan S and
Menemen Y: Silver nanoparticles: cytotoxic, apoptotic, and necrotic
effects on MCF7 cells. Turk J Biol. 37:92013. View Article : Google Scholar
|
|
35
|
Chehelgerdi M, Chehelgerdi M, Allela OQB,
Pecho RDC, Jayasankar N, Rao DP, Thamaraikani T, Vasanthan M,
Viktor P, Lakshmaiya N, et al: Progressing nanotechnology to
improve targeted cancer treatment: overcoming hurdles in its
clinical implementation. Mol Cancer. 22:1692023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karlsson HL, Cronholm P, Gustafsson J and
Möller L: Copper oxide nanoparticles are highly toxic: A comparison
between metal oxide nanoparticles and carbon nanotubes. Chem Res
Toxicol. 21:1726–1732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dusinska M, Boland S, Saunders M,
Juillerat-Jeanneret L, Tran L, Pojana G, Marcomini A, Volkovova K,
Tulinska J, Knudsen LE, et al: Towards an alternative testing
strategy for nanomaterials used in nanomedicine: Lessons from
NanoTEST. Nanotoxicology. 9 (Suppl 1):S118–S132. 2015. View Article : Google Scholar
|