Introduction
As cancer therapies have improved, spinal metastases
have become increasingly common in the course of oncologic disease
(1). Spinal metastases are
symptomatic in approximately 15% of patients with solid primary
tumors (2,3). Complications arising from spinal
metastases have a significant impact on patient quality of life.
The most common complications include pain (4–6) and
neurological disability (7–11). Treatment is complicated and usually
the purview of a multidisciplinary team. Numerous treatment
algorithms for metastatic spine disease are available (12). Major considerations regardless of
algorithm are spinal stability, neurologic risk of the patient, and
tumor sensitivity to radiation. The main treatment for good local
control for most tumors is high dose conformal radiation
stereotactic body radiation therapy (SBRT). Indeed, most treatment
algorithms uphold the premise that if the tumor does not respond to
low dose external beam radiation therapy (EBRT; 30 Gy in 10
fractions) than SBRT is standard.
Therefore, from a neurosurgical standpoint,
indications for a surgical intervention include: (1) the presence of radioresistant tumors
that are too close to the spinal cord to receive the full dose of
radiation, (2) neurologic risk (how
compressed the cord is), (3) spinal
instability, and (4) the need for a
tissue diagnosis. Neurological compromise from metastatic disease
to the spine is often the result of spinal cord compression from
the invading tumor; this may be the most significant indication for
a surgical intervention. Approximately 10% of patients with spinal
metastases develop spinal cord compression (3).
Bilsky et al (13) created a grading system for epidural
spinal cord compression: Grade 0 disease is confined to the bone
without any epidural spread; grade 1a indicates that there is
epidural impingement without significant deformation of the thecal
sac; 1b indicates that the thecal sac has been deformed without
abutment of the spinal cord; 1c indicates that there is spinal cord
abutment without cord compression; 2 indicates spinal cord
compression with visible cerebrospinal fluid (CSF) around the cord;
and 3 indicates that there is spinal cord compression without any
CSF visible around the spinal cord. With improved diagnostic
imaging and increased utilization of surveillance imaging in
patients with metastatic cancer, more patients with early grade
epidural disease, Bilsky grade 0 or 1, are being identified.
Unfortunately, limited data exists to guide the preferred
management of patients with low-grade epidural disease with
potential options, including local therapies such as radiation
therapy with or without surgery vs. systemic therapies. It is
unknown how the presence of grade 1 epidural disease impacts local
control following spinal stereotactic body radiation therapy
compared to spine metastasis confined to bone.
We compared outcomes and local tumor control after
SBRT between patients with disease localized to the bone (Bilsky 0)
vs. patients with mild epidural spread (Bilsky 1) to emphasize the
importance of timing of SBRT in patients with metastatic spread
prior to progression of pathology to include the central canal.
Given the mechanism of SBRT as an intervention, this minimally
invasive approach in patients without thecal sac involvement
ensures high dose delivery of radiation with maximal precision and
sparing of normal tissue, and thus improved symptom control and
reduced progression risk. Soltys et al (14) showed improved tumor control
probability with high dose regimens, but emphasized weighing the
benefits with the risks of increased toxicity; meanwhile, the
probability of toxicity is reduced the farther the pathologic locus
is from the thecal sac, further substantiating the importance of
intervening at Bilsky 0.
Materials and methods
Study design and patient
selection
We performed a retrospective analysis of a
prospectively maintained database of consecutive adult patients
with spine metastases who received spine SBRT at Michigan
Medicine-a single, large tertiary care facility in Ann Arbor,
Michigan, USA-from August 2010 to January 2021. All included
patients received SBRT based on an established treatment algorithm
(12). No patients underwent
conventional EBRT. It was determined that patients who had a
sufficiently high functional status were appropriate candidates for
SBRT therapy, and often had systemic treatment options. Patients
with poor performance status or advanced disease with limited
treatment options did not receive SBRT and were not included in
this dataset. We only included patients who had a presenting Bilsky
score of 0 or 1 (includes 1a, 1b, and 1c) and excluded patients
with a presenting Bilsky score of 2 or 3 (13). Pediatric patients (≤18 years old)
were excluded. Approval for this study was obtained from the
University of Michigan (Ann Arbor, Michigan, 48109, USA)
Institutional Review Board (ID HUM00139855); patient consent was
not required.
Clinical data
Demographic data were prospectively entered for each
patient as the patient began SBRT. Variables included age at
treatment, sex, body mass index, race (White, African American,
Asian, other, unknown), marital status (single, married, divorced,
widowed, unknown), insurance type (private, Medicare, Medicaid,
uninsured, unknown), and whether the patient had a primary care
physician. Other prospectively maintained variables included if the
patient underwent surgery, the histology of the tumor, whether
there were contiguous spinal levels of disease, if the patient had
previously undergone radiation therapy at the level of interest,
and the dose of radiation, converted to a biologically effective
doses (BED) for standardization. Tumor histology was grouped into
radiation sensitive, intermediate, and radiation resistant groups
based upon the available literature and expert opinion (JRE &
WCJ; Table I) (15–22).
Radiosensitivity, or the responsiveness to EBRT, traditionally
describes the impact that radiation therapy of this nature can have
on a specific histology compared to another. Additional variables
retrospectively gathered included the Bilsky score of the lesion at
the level of worst compression being treated, post-SBRT infield
progression of cancer, date of infield progression before death,
and survival.
 | Table I.Breakdown of cancer histologies by
radiosensitivity. |
Table I.
Breakdown of cancer histologies by
radiosensitivity.
| Radiosensitive | Intermediate | Radioresistant |
|---|
| Breast cancer | Adrenal cancer | Blood vessel
tumor |
| Prostate cancer | Bladder cancer | Colorectal
cancer |
|
| Esophageal
cancer | Melanoma |
|
| Head/neck cancer | Pancreatic
cancer |
|
| Liver cancer | Primary bone
tumor |
|
| Neuroendocrine
cancer | Renal cell
carcinoma |
|
| Non-small cell lung
carcinoma | Sarcoma |
|
| Salivary cancer |
|
|
| Thyroid cancer |
|
Clinical treatment
The goal of SBRT for all patients was to maximize
the radiation dose given to the treated tumors. While not all
treatment regiments were uniform, all obtained an appropriate dose
of radiation to treat the tumor. When sufficiently high doses of
radiation could not be administered because of proximity of the
tumor to the spinal cord, separation surgery was performed. As
previously described, separation surgery consists of transpedicular
decompression at the level of the tumor with circumferential
decompression of the thecal sac as assessed by intraoperative
ultrasound. Once sufficient decompression is achieved, pedicle
screws are placed two levels above and below the decompression
(23).
Clinical follow-up in a
multidisciplinary spinal oncology clinic
Patients who received spinal SBRT were followed in a
multidisciplinary spinal oncology clinic where their care was
coordinated between their neurosurgical team, radiation
oncologists, medical oncologists, physical therapists, and other
ancillary teams. Patients were seen in clinic at 1 and 3 months for
examination and assessment of treatment effects as well as every 3
to 6 months for a surveillance total spine MRI. The need for
additional treatment was determined in the multidisciplinary
clinic.
Statistical analysis
We examined the association of each variable against
patients presenting with Bilsky grade 0 compared to Bilsky grade 1
epidural disease using the chi-square test, Fisher exact test, or
t-test, depending on the sample size and whether the
variable was continuous or categorical. Continuous variables are
presented as mean with standard deviations. Survival analyses were
utilized to examine the associations between infield progression
and survival against patients with Bilsky 0 vs. 1 compression. An
additional stratified log rank analysis was performed for infield
progression to test between Bilsky grade while stratifying based on
levels of radiation sensitivity. Finally, a subgroup analysis was
performed by removing patients who underwent surgery, leaving
patients who only underwent SBRT. A two-sided P<.05 was
considered statistically significant. All data were analyzed using
SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
Demographics
A total of 311 spine treatment sites in 255 patients
were included. The average age of our population was 62.4±12.8
years and 34.1% were female. Other demographic data are provided in
Table II.
 | Table II.Demographics of patients presenting
with Bilsky grade 0 compared with 1 compression. |
Table II.
Demographics of patients presenting
with Bilsky grade 0 compared with 1 compression.
| Characteristic | Patients with Bilsky
grade 0 compression (n=144), no (%) | Patients with Bilsky
grade 1 compression (n=167), n (%) | P-value |
|---|
| Age, years | 63.6 (10.8) | 61.7 (13.5) | 0.19 |
| Sex |
|
| 0.19 |
|
Female | 45 (31.3%) | 64 (38.3%) |
|
| Body mass index | 28.3 (7.0) | 26.7 (5.3) | 0.05 |
| Race |
|
| 0.23 |
|
White | 125 (86.8%) | 150 (89.8%) |
|
|
Black | 9 (6.3%) | 6 (3.6%) |
|
|
Asian | 2 (1.4%) | 5 (3.0%) |
|
|
Other | 5 (3.5%) | 1 (0.6%) |
|
|
Unknown | 3 (2.1%) | 5 (3.0%) |
|
| Marital status |
|
| 0.21 |
|
Married | 80 (55.6%) | 113 (67.7%) |
|
|
Divorced | 11 (7.6%) | 7 (4.2%) |
|
|
Single | 16 (11.1%) | 16 (9.6%) |
|
|
Widowed | 7 (4.9%) | 4 (2.4%) |
|
|
Unknown | 30 (20.8%) | 27 (16.2%) |
|
| Insurance type |
|
| 0.53 |
|
Private | 81 (56.3%) | 97 (58.1%) |
|
|
Medicare | 56 (38.9%) | 57 (34.1%) |
|
|
Medicaid | 0 (0.0%) | 2 (1.2%) |
|
|
Uninsured | 5 (3.5%) | 6 (3.6%) |
|
|
Unknown | 2 (1.4%) | 5 (3.0%) |
|
| Presence of primary
care physician |
|
| 0.63 |
| No | 5 (3.5%) | 6 (3.6%) |
|
|
Yes | 137 (95.1%) | 156 (93.4%) |
|
|
Unknown | 2 (1.4%) | 5 (3.0%) |
|
| Surgical
intervention |
|
| 0.01 |
| No | 135 (93.8%) | 132 (79.0%) |
|
|
Yes | 9 (6.3%) | 35 (21.0%) |
|
Among the 311 spine treatment sites, 167 (53.7%)
exhibited Bilsky grade 1 compression while the remaining sites had
bone-only disease (Bilsky grade 0). Tumor histologies, detailed in
Table III, revealed that certain
cancers were significantly more prevalent in patients with Bilsky
grade 0 compression, such as prostate or breast cancer. Conversely,
renal cell carcinoma, non-small cell lung cancer, and sarcoma were
more commonly associated with Bilsky grade 1 compression.
 | Table III.Demographic information of tumors of
patients. |
Table III.
Demographic information of tumors of
patients.
| Characteristic | Patients with
Bilsky grade 0 compression (n=144), no (%) | Patients with
Bilsky grade 1 compression (n=167), n (%) | P-value |
|---|
| Histology
categories |
|
| <0.01 |
|
Prostate cancer | 42 (29.2%) | 19 (11.4%) |
|
| Renal
cell carcinoma | 16 (11.1%) | 32 (19.2%) |
|
|
Non-small cell lung
cancer | 9 (6.3%) | 34 (20.4%) |
|
|
Sarcoma | 8 (5.6%) | 18 (10.8%) |
|
| Breast
cancer | 19 (13.2%) | 6 (3.6%) |
|
|
Melanoma | 5 (3.5%) | 9 (5.4%) |
|
| Thyroid
cancer | 9 (6.3%) | 5 (3.0%) |
|
| Bladder
cancer | 7 (4.9%) | 5 (3.0%) |
|
| Liver
cancer | 5 (3.5%) | 2 (1.2%) |
|
|
Oropharyngeal cancer | 2 (1.4%) | 7 (4.2%) |
|
|
Colorectal cancer | 5 (3.5%) | 3 (1.8%) |
|
|
Neuroendocrine tumor | 2 (1.4%) | 6 (3.6%) |
|
|
Pancreatic cancer | 3 (2.1%) | 2 (1.2%) |
|
|
Esophageal cancer | 3 (2.1%) | 4 (2.4%) |
|
| Blood
vessel tumors | 1 (0.7%) | 1 (0.6%) |
|
|
Salivary cancer | 2 (1.4%) | 4 (2.4%) |
|
| Primary
bone tumor | 2 (1.4%) | 1 (0.6%) |
|
| Adrenal
cancer | 0 (0.0%) | 1 (0.6%) |
|
|
Other | 4 (2.8%) | 8 (4.8%) |
|
| Radiation
sensitivity of tumor |
|
| <0.01 |
|
Sensitive | 61 (42.4%) | 25 (15.0%) |
|
|
Intermediate | 42 (29.2%) | 74 (44.3%) |
|
|
Resistant | 41 (28.5%) | 68 (40.7%) |
|
| Prior radiation
therapy to site | 7 (4.9%) | 22 (13.2%) | 0.01 |
| Biologically
effective dose | 51.4 (8.7) | 53.6 (10.6) | 0.05 |
Of the 311 treatment sites, 86 (27.7%) were
radiosensitive histologies, 116 (37.3%) had intermediate
radiosensitivity, and 109 (35.0%) were radioresistant histologies.
Patients with Bilsky grade 1 compression were significantly more
likely to have tumors with intermediate (44.3% 29.2%) or resistant
(40.7% vs. 28.5%; P<.0001) radiation sensitivity compared to
those with Bilsky grade 0 compression (Table III).
Infield progression
Patients with Bilsky grade 1 were more frequently
treated with surgical intervention followed by SBRT, rather than
SBRT alone, compared to those with Bilsky grade 0 (21.0% vs. 6.3%,
P=.0002). Patients with Bilsky grade 0 and grade 1 compression
(51.4±8.7 vs. 53.6±10.6; P=.05; see Table III) received similar BEDs. Local
control rates for patients with Bilsky grade 0 compression were
92.0% at 12 months and 85.8% at 24 months, whereas for patients
with Bilsky grade 1 compression, the rates were 86.0% at 12 months
and 77.6% at 24 months. Infield progression between patients
presenting with a Bilsky grade 0 and 1 compression was not
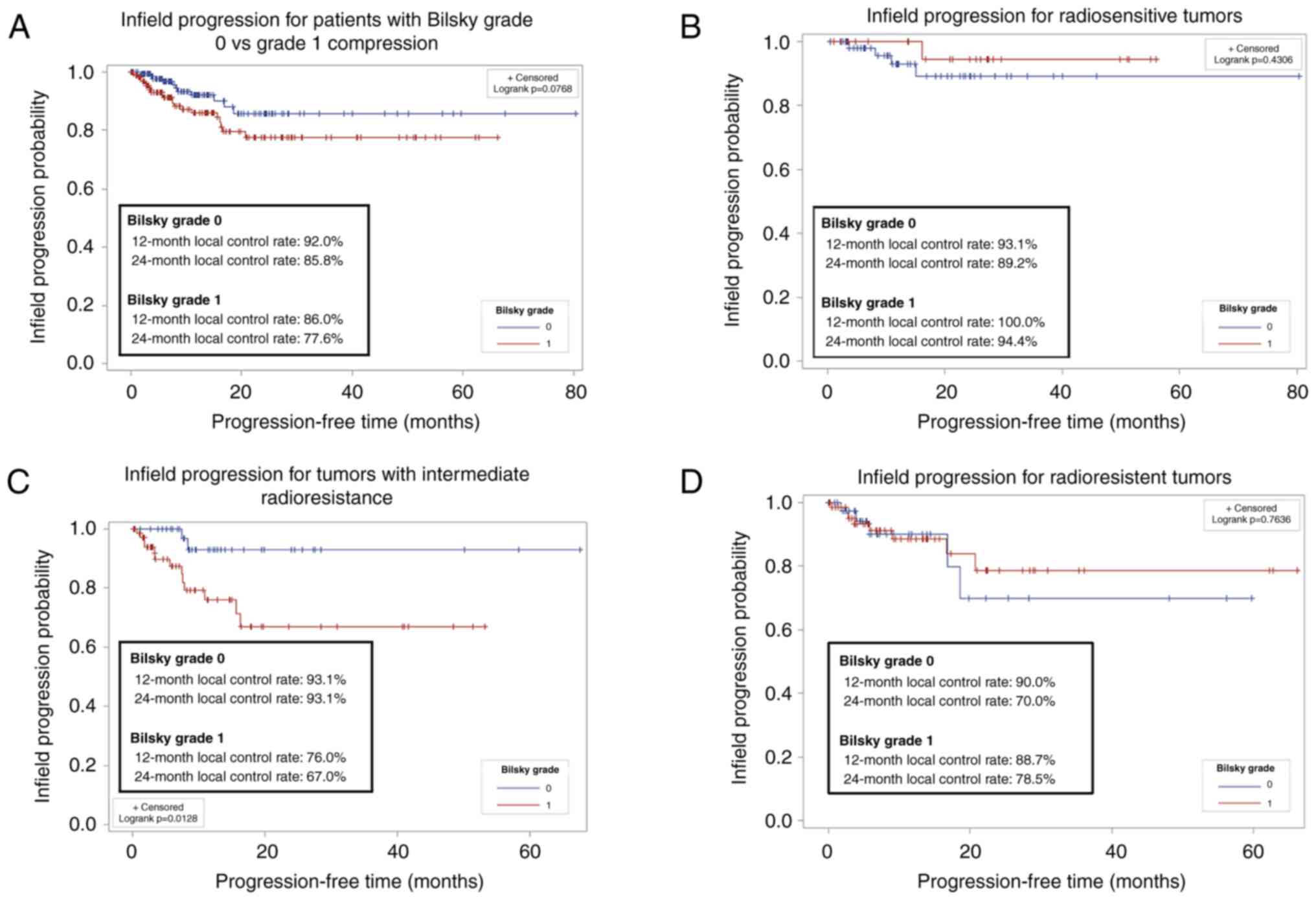
statistically different (Fig. 1A).
A stratified log rank analysis showed that no significant
difference between infield progression between Bilsky grade 0 and 1
compression when accounting for the different levels of sensitivity
to radiation (P=0.22). We performed a sensitivity analysis to
determine if radiosensitivity of the tumor impacted local control;
no significant difference between infield progression when
comparing radiosensitive and radioresistant histologies (Fig. 1B and D) resulted. However, patients
with intermediate radioresistant histologies and Bilsky grade 0
compression had significantly better local control compared to
patients with Bilsky grade 1 compression (Fig. 1C).
We performed an in-depth analysis of the specific
histologies that were within the intermediate radioresistant group
with infield progression (Table
IV) and found a trend towards more patients with non-small cell
lung cancer who had infield progression compared to no infield
progression (53.3% vs. 34.7%; P=.16). However, this was not
statistically significant.
 | Table IV.Infield progression in patients with
histologies of intermediate radioresistance. |
Table IV.
Infield progression in patients with
histologies of intermediate radioresistance.
| Histology
categories | Patients with
infield progression (n=15), n (%) | Patients without
infield progression (n=101), n (%) | P-value |
|---|
| Cancers |
|
| 0.16 |
|
Non-small cell lung | 8 (53.3%) | 35 (34.7%) |
|
|
Thyroid | 0 (0.0%) | 14 (13.9%) |
|
|
Bladder | 0 (0.0%) | 12 (11.9%) |
|
|
Liver | 0 (0.0%) | 7 (6.9%) |
|
|
Oropharyngeal | 1 (6.7%) | 8 (7.9%) |
|
|
Neuroendocrine tumor | 2 (13.3%) | 6 (5.9%) |
|
|
Esophageal | 1 (6.7%) | 6 (5.9%) |
|
|
Salivary | 2 (13.3%) | 4 (4.0%) |
|
|
Adrenal | 0 (0.0%) | 1 (1.0%) |
|
|
Other | 1 (6.7%) | 8 (7.9%) |
|
Survival
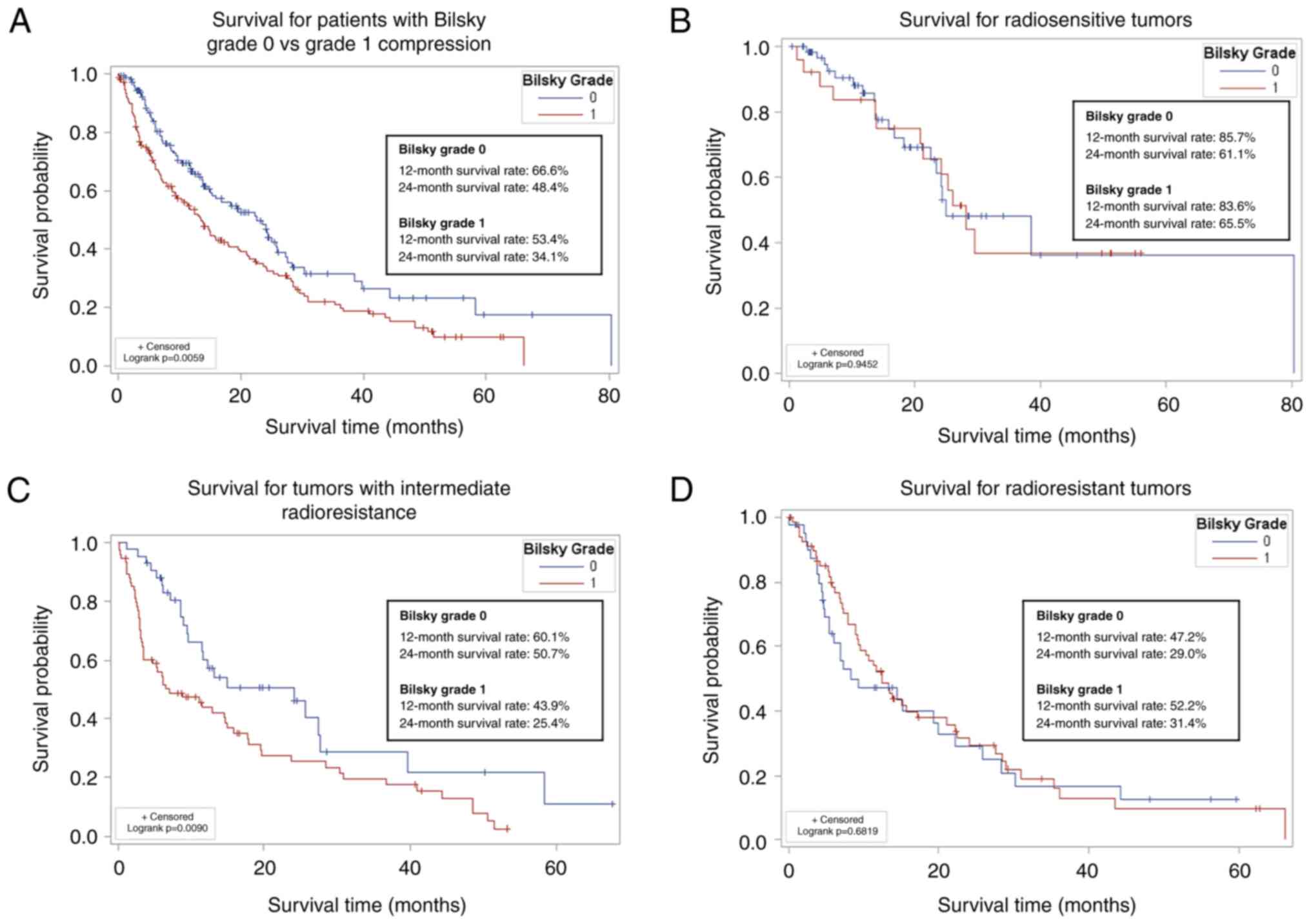
Patients with Bilsky grade 0 compression had
significantly longer survival compared to patients with Bilsky
grade 1 compression (P=.006; Fig.
2A), likely driven by the larger proportion of patients with
metastatic breast and prostate cancer in the Bilsky grade 0 group
(Table III). Patients with Bilsky
grade 0 compression had 66.6% survival at 12 months and 48.4%
survival at 24 months; patients with Bilsky grade 1 compression had
53.4% survival at 12 months and 34.1% survival at 24 months. We
performed a sensitivity analysis to determine if radiosensitivity
of the tumor impacted survival and found no significant difference
between survival in radiosensitive and radioresistant tumors
(Fig. 2B and D). However, patients
who had tumors with an intermediate radioresistance and Bilsky
grade 0 compression had significantly better survival compared to
patients with Bilsky grade 1 compression (Fig. 2C).
We performed an in-depth analysis of the specific
histologies that were within the intermediate radioresistance group
(Table V) and found more patients
with non-small cell lung cancer who had Bilsky grade 1 compression
compared to bone-only disease (45.9% vs. 21.4%; P=.04). Conversely,
thyroid, bladder, and liver cancers were more common in patients
with grade 0 compression.
 | Table V.Histologies of patients with
intermediate radioresistance. |
Table V.
Histologies of patients with
intermediate radioresistance.
| Histology
categories | Patients with
Bilsky grade 0 compression (n=42), n (%) | Patients with
Bilsky grade 1 compression (n=74), n (%) | P-value |
|---|
| Histology |
|
| 0.04 |
| Non-small cell lung
cancer | 9 (21.4%) | 34 (45.9%) |
|
| Thyroid cancer | 9 (21.4%) | 5 (6.8%) |
|
| Bladder cancer | 7 (16.7%) | 5 (6.8%) |
|
| Liver cancer | 5 (11.9%) | 2 (2.7%) |
|
| Oropharyngeal
cancer | 2 (4.8%) | 7 (9.5%) |
|
| Neuroendocrine
tumor | 2 (4.8%) | 6 (8.1%) |
|
| Esophageal
cancer | 3 (7.1%) | 4 (5.4%) |
|
| Salivary
cancer | 2 (4.8%) | 4 (5.4%) |
|
| Adrenal cancer | 0 (0.0%) | 1 (1.4%) |
|
| Other | 3 (7.1%) | 6 (8.1%) |
|
Subanalysis of patients with SBRT only
treatment
When the same analyses were performed for patients
with SBRT only (patients who underwent surgery were excluded)
results were similar compared to when surgical patients were
included. Infield progression also remained similar between
patients with Bilsky grade 0 and grade 1 (P=0.06). Additionally,
patients with Bilsky grade 0 compression had significantly longer
survival compared to patients with Bilsky grade 1 compression
(P=0.01; Fig. S1).
Discussion
As spinal metastases are becoming increasingly
common in the course of oncologic disease, physicians are
continuing to search for treatment modalities and algorithms that
both minimize patient symptoms and improve outcomes (1). Nearly 10% of patients with spinal
metastases develop spinal cord compression (3), which can cause significant pain and
neurological disability, ultimately affecting quality of life
(4–11). As diagnostic imaging has improved,
spinal metastases are detected earlier in the disease course (as
opposed to historically when most patients presented with
symptomatic high-grade spinal cord compression). With earlier
detection, data are needed to guide the preferred management for an
individual patient. Ideally it would be possible to know when to
aggressively treat spinal metastases with SBRT and possibly
separation surgery if there is not frank, symptomatic epidural
spinal cord compression.
We examined patient outcomes and local control rates
in patients treated with SBRT for either Bilsky grade 0 or 1
compression. While some variation existed in local control rates
between the two groups, the difference was not statistically
significant overall. Patients with Bilsky grade 1 compression more
often underwent separation surgery prior to SBRT. Despite the use
of different SBRT dose and fractionation schemes for treating
spinal metastases, the BED between the two groups was not
significantly different, and infield progression rates were
similar. However, patients with Bilsky grade 1 compression were
more commonly treated with separation surgery before SBRT therapy
(24–26). Separation surgery is often necessary
to achieve sufficiently high doses of SBRT when the epidural spread
does not allow a safe distance of CSF around the spinal cord
(27). While patients with Bilsky
grade 1 and 0 compression achieved statistically similar local
control, patients with Bilsky grade 1 compression, on average,
underwent more treatment with the additional surgery to achieve a
similar result.
Overall, infield progression did not differ between
Bilsky grade 0 or 1 compression; however, when examining the trend
of infield progression between Bilsky grade 0 or 1 compression of
radiosensitive, intermediate radioresistant, and radioresistant
histologies, patients with intermediate radioresistance tumors had
significantly worse infield progression if they had Bilsky grade 1
compression compared to grade 0 compression. We performed a
sensitivity analysis examining the specific histologies and found
no significant difference between infield progression between the
different histologies. It is likely that our cohort is too small to
detect more nuanced reasons for why patients with Bilsky grade 1
compared to grade 0 compression with an intermediate radioresistant
tumor would have significant differences of infield progression
when radiosensitive and radioresistant tumors did not. It is
possible that operative patterns for these tumors are different,
representing an area for future research.
We found that patients with Bilsky grade 0
compression had significantly longer survival compared to patients
with Bilsky grade 1 compression. We performed a sensitivity
analysis to examine if this difference was driven by Bilsky grade
or histology. Our data suggested that tumors that were grouped into
intermediate radioresistance had worse survival. When looking
specifically at the histologies within that group, we found that
patients with non-small cell lung cancer were more likely to have
Bilsky grade 1 compression compared to bone-only disease, which may
account for the difference in survival. Lung cancer has highly
variable responses to radiation treatments (28,29).
While not born out in the sensitivity analysis, the
possibility remains that the improved survival rate is at least
partially driven by the larger number of breast and prostate cancer
patients in the Bilsky grade 0 group and that the small numbers of
our study do not allow for statistical differences. Overall, it is
realistic that both Bilsky grade and tumor histology are jointly
critical in determining survival outcomes in patients with spinal
metastases. In fact, these variables are unlikely to be independent
in predicting survival. We did not test for correlation between
Bilsky grade and tumor histology, so this is only a logical
assumption. Meanwhile, Shah and Schwab (30) attempted to close the gap between the
ability to predict prognosis and patient-specific survival
probability. Tumor histology was a standout factor in survival
prediction (30). Bendfeldt et
al (31) found poor
survivability at the higher Bilsky scores (2–3), but
the same finding was not observed at lower grades of epidural
spinal cord compression. The combination of findings from these
studies (30,31) are consistent with expectations, but
are not granular enough to distinguish between Bilsky 0 and Bilsky
1. A larger sample size is required for granularity
Alternatively, the difference could be related to
anatomical differences between Bilsky grade 0 and 1 compression. We
postulate that this may be due to (1) later diagnosis and thus more advanced
systemic disease in patients with Bilsky grade 1 compression
compared to patients with grade 0 compression, and (2) longer periods of time off systemic
therapy for patients who underwent separation surgery before SBRT
was performed. Our findings suggest that appropriate patients may
obtain similar levels of benefit or infield progression if they are
treated when the disease is bone-only, which would minimize the
risk of needing to undergo separation surgery with associated
operative complications and possible delays in obtaining or
continuing systemic therapies.
This study is limited by the utilization of a single
center, prospectively maintained database, but many of the
variables were retrospectively obtained, potentially introducing
bias into the analysis. Since these data come from a large,
academic institution with a multidisciplinary spinal oncology
clinic, these findings may not be generalizable to all centers.
While the BED for the two patient populations was not significantly
different, multiple different SBRT dose and fraction schemes were
utilized, which introduces some minor heterogeneity into the
analysis. In addition to the BED being statistically similar
between the patient groups, all radiation doses achieved
appropriate treatment levels. Because we split the data into
specific histologies to attempt to understand infield progression
and survival patterns, the size of our data may be a limiting
factor, emphasizing the need for future, larger, multicenter
studies to obtain robust data.
In conclusion, patients with low-grade Bilsky spinal
cord compression did not have significantly different local control
rates when compared to patients with bone-only spinal metastases
following treatment with spinal SBRT. However, patients with grade
1 disease were more likely to need surgery before SBRT. In patients
with radioresistant histologies, earlier treatment before epidural
spread may eliminate the need for separation surgery and the
consequences associated with this procedure.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Mariana Grohowski
for assistance with the preparation of this manuscript. The
abstract was presented at the 39th Annual Spine Summit Meeting of
the American Association of Neurological Surgeons Mar 16–19, 2023
in Miami Beach, FL, where it received the Charles Kuntz Scholar
Award.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JRL conception and design, acquisition of data,
analysis and interpretation of data, drafting the article,
critically revising the article, statistical analysis. MJS, VGK,
PEG, LRT, JL, AT, SK, ALW, OO, MMK, and RSJ acquisition of data,
critically revising the article. SK analysis and interpretation of
data, statistical analysis. JRE and WCJ analysis and interpretation
of data, critically revising the article. NJS conception and
design, analysis and interpretation of data, critically revising
the article, reviewed submitted version of the manuscript, study
supervision. JRL and MJS confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The University of Michigan Institutional Review
Board (Ann Arbor, USA) approved this study (approval no.
HUM00139855).
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cronin KA, Scott S, Firth AU, Sung H,
Henley SJ, Sherman RL, Siegel RL, Anderson RN, Kohler BA, Benard
VB, et al: Annual report to the nation on the status of cancer,
part 1: National cancer statistics. Cancer. 128:4251–4284. 2022.
View Article : Google Scholar
|
|
2
|
Hernandez RK, Wade SW, Reich A, Pirolli M,
Liede A and Lyman GH: Incidence of bone metastases in patients with
solid tumors: Analysis of oncology electronic medical records in
the United States. BMC Cancer. 18:442018. View Article : Google Scholar
|
|
3
|
Van den Brande R, Cornips EM, Peeters M,
Ost P, Billiet C and Van de Kelft E: Epidemiology of spinal
metastases, metastatic epidural spinal cord compression and
pathologic vertebral compression fractures in patients with solid
tumors: A systematic review. J Bone Oncol. 35:1004462022.
View Article : Google Scholar
|
|
4
|
Bach F, Larsen BH, Rohde K, Borgesen SE,
Gjerris F, Boge-Rasmussen T, Agerlin N, Rasmusson B, Stjernholm P
and Sørensen PS: Metastatic spinal cord compression. Occurrence,
symptoms, clinical presentations and prognosis in 398 patients with
spinal cord compression. Acta Neurochir (Wien). 107:37–43. 1990.
View Article : Google Scholar
|
|
5
|
Posner JB: Back pain and epidural spinal
cord compression. Med Clin North Am. 71:185–205. 1987. View Article : Google Scholar
|
|
6
|
Prasad D and Schiff D: Malignant
spinal-cord compression. Lancet Oncol. 6:15–24. 2005. View Article : Google Scholar
|
|
7
|
Gilbert RW, Kim JH and Posner JB: Epidural
spinal cord compression from metastatic tumor: Diagnosis and
treatment. Ann Neurol. 3:40–51. 1978. View Article : Google Scholar
|
|
8
|
Hamilton P, Lawrence P and Eisenring CV:
Metastatic epidural spinal cord compression. J Surg Case Rep.
2020:rjaa2172020. View Article : Google Scholar
|
|
9
|
Helweg-Larsen S, Sorensen PS and Kreiner
S: Prognostic factors in metastatic spinal cord compression: A
prospective study using multivariate analysis of variables
influencing survival and gait function in 153 patients. Int J
Radiat Oncol Biol Phys. 46:1163–1169. 2000. View Article : Google Scholar
|
|
10
|
Maranzano E and Latini P: Effectiveness of
radiation therapy without surgery in metastatic spinal cord
compression: Final results from a prospective trial. Int J Radiat
Oncol Biol Phys. 32:959–967. 1995. View Article : Google Scholar
|
|
11
|
Sutcliffe P, Connock M, Shyangdan D, Court
R, Kandala NB and Clarke A: A systematic review of evidence on
malignant spinal metastases: Natural history and technologies for
identifying patients at high risk of vertebral fracture and spinal
cord compression. Health Technol Assess. 17:1–274. 2013. View Article : Google Scholar
|
|
12
|
Spratt DE, Beeler WH, de Moraes FY, Rhines
LD, Gemmete JJ, Chaudhary N, Shultz DB, Smith SR, Berlin A, Dahele
M, et al: An integrated multidisciplinary algorithm for the
management of spinal metastases: An International Spine Oncology
Consortium report. Lancet Oncol. 18:e720–e730. 2017. View Article : Google Scholar
|
|
13
|
Bilsky MH, Laufer I, Fourney DR, Groff M,
Schmidt MH, Varga PP, Vrionis FD, Yamada Y, Gerszten PC and Kuklo
TR: Reliability analysis of the epidural spinal cord compression
scale. J Neurosurg Spine. 13:324–328. 2010. View Article : Google Scholar
|
|
14
|
Soltys SG, Grimm J, Milano MT, Xue J,
Sahgal A, Yorke E, Yamada Y, Ding GX, Li XA, Lovelock DM, et al:
Stereotactic body radiation therapy for spinal metastases: Tumor
control probability analyses and recommended reporting standards.
Int J Radiat Oncol Biol Phys. 110:112–123. 2021. View Article : Google Scholar
|
|
15
|
Maranzano E, Bellavita R, Rossi R, De
Angelis V, Frattegiani A, Bagnoli R, Mignogna M, Beneventi S,
Lupattelli M, Ponticelli P, et al: Short-course versus split-course
radiotherapy in metastatic spinal cord compression: results of a
phase III, randomized, multicenter trial. J Clin Oncol.
23:3358–3365. 2005. View Article : Google Scholar
|
|
16
|
Rades D, Fehlauer F, Stalpers LJ, Wildfang
I, Zschenker O, Schild SE, Schmoll HJ, Karstens JH and Alberti W: A
prospective evaluation of two radiotherapy schedules with 10 versus
20 fractions for the treatment of metastatic spinal cord
compression: Final results of a multicenter study. Cancer.
101:2687–2692. 2004. View Article : Google Scholar
|
|
17
|
Rades D, Stalpers LJ, Hulshof MC,
Zschenker O, Alberti W and Koning CC: Effectiveness and toxicity of
single-fraction radiotherapy with 1 × 8 Gy for metastatic spinal
cord compression. Radiother Oncol. 75:70–73. 2005. View Article : Google Scholar
|
|
18
|
Rades D, Fehlauer F, Schulte R, Veninga T,
Stalpers LJ, Basic H, Bajrovic A, Hoskin PJ, Tribius S, Wildfang I,
et al: Prognostic factors for local control and survival after
radiotherapy of metastatic spinal cord compression. J Clin Oncol.
24:3388–3393. 2006. View Article : Google Scholar
|
|
19
|
Katagiri H, Takahashi M, Inagaki J,
Kobayashi H, Sugiura H, Yamamura S and Iwata H: Clinical results of
nonsurgical treatment for spinal metastases. Int J Radiat Oncol
Biol Phys. 42:1127–1132. 1998. View Article : Google Scholar
|
|
20
|
Maranzano E, Latini P, Perrucci E,
Beneventi S, Lupattelli M and Corgna E: Short-course radiotherapy
(8 Gy × 2) in metastatic spinal cord compression: An effective and
feasible treatment. Int J Radiat Oncol Biol Phys. 38:1037–1044.
1997. View Article : Google Scholar
|
|
21
|
Willcox HN and McMichael AJ: Radioactive
antigen suicide of an anti-DNP (2,4-dinitrophenyl) clone. II.
Follow-up of clones relatively resistant to radioactive antigen
suicide when initially selected. Eur J Immunol. 5:131–139. 1975.
View Article : Google Scholar
|
|
22
|
Yamada Y, Katsoulakis E, Laufer I,
Lovelock M, Barzilai O, McLaughlin LA, Zhang Z, Schmitt AM,
Higginson DS, Lis E, et al: The impact of histology and delivered
dose on local control of spinal metastases treated with
stereotactic radiosurgery. Neurosurg Focus. 42:E62017. View Article : Google Scholar
|
|
23
|
Linzey JR, Kathawate VG, Strong MJ, Roche
K, Goethe PE, Tudrick LR, Lee J, Tripathy A, Koduri S, Ward AL, et
al: Patients with progression of spinal metastases who present to
the clinic have better outcomes compared to those who present to
the emergency department. Cancer Med. 12:20177–20187. 2023.
View Article : Google Scholar
|
|
24
|
Barzilai O, Laufer I, Robin A, Xu R,
Yamada Y and Bilsky MH: Hybrid therapy for metastatic epidural
spinal cord compression: Technique for separation surgery and spine
radiosurgery. Oper Neurosurg (Hagerstown). 16:310–318. 2019.
View Article : Google Scholar
|
|
25
|
De la Garza Ramos R, Echt M, Gelfand Y,
Yanamadala V and Yassari R: Minimally invasive tubular separation
surgery for metastatic spinal cord compression: 2-dimensional
operative video. Oper Neurosurg (Hagerstown). 20:E3562021.
View Article : Google Scholar
|
|
26
|
Newman WC, Amin AG, Villavieja J, Laufer
I, Bilsky MH and Barzilai O: Short-segment cement-augmented
fixation in open separation surgery of metastatic epidural spinal
cord compression: Initial experience. Neurosurg Focus. 50:E112021.
View Article : Google Scholar
|
|
27
|
Li RF, Qiao RQ, Xu MY, Ma RX and Hu C:
Separation surgery in the treatment of spinal metastasis. Technol
Cancer Res Treat. 21:153303382211072082022. View Article : Google Scholar
|
|
28
|
Formenti SC, Rudqvist NP, Golden E, Cooper
B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari
de Andrade L, Wucherpfennig KW, et al: Radiotherapy induces
responses of lung cancer to CTLA-4 blockade. Nat Med. 24:1845–1851.
2018. View Article : Google Scholar
|
|
29
|
Willers H, Azzoli CG, Santivasi WL and Xia
F: Basic mechanisms of therapeutic resistance to radiation and
chemotherapy in lung cancer. Cancer J. 19:200–207. 2013. View Article : Google Scholar
|
|
30
|
Shah AA and Schwab JH: Predictive modeling
for spinal metastatic disease. Diagnostics (Basel). 14:9622024.
View Article : Google Scholar
|
|
31
|
Bendfeldt GA, Chanbour H, Chen JW,
Gangavarapu LS, LaBarge ME, Ahmed M, Jonzzon S, Roth SG, Chotai S,
Luo LY, et al: Does low-grade versus high-grade bilsky score
influence local recurrence and overall survival in metastatic spine
tumor surgery? Neurosurgery. 93:1319–1330. 2023. View Article : Google Scholar
|