In 2020, breast cancer (BC) became the most
prevalent cancer, ahead of lung cancer, for the first time in
history. BC accounts for 15.5% of all cancer-related deaths in
women worldwide and therefore is a leading cause of cancer
mortality within the female population globally (1,2). The
early detection of BC with advancements in therapy effectiveness
has led to a decrease in mortality over the past two decades
(3). The 5-year relative survival
rate for early-stage BC is generally high (80–92%), but it
significantly declines to <25% for advanced BC (4).
It is necessary to understand the mechanisms
underlying the metastatic process and the complex tumor-host
interactions causing the progression of the disease, as 25% of
patients with non-metastatic BC will eventually develop distant
metastases, although initial treatment was successful (5). It was discovered that 10–50% of
patients without nodal involvement at the time of curative surgery
subsequently developed distant metastatic lesions (6–8).
Metastatic BC (MBC) is widely considered an incurable medical
condition, although the application of systemic therapies has
improved its prognosis. The median overall survival (OS) time of
MBC is ~2 years, while survival varies from a couple of months to a
few years, depending on the type of treatment used and the
molecular and patient characteristics (9). For patients with HER2+ MBC,
an OS of >5 years is now common in developed countries, whereas
individuals with triple negative BC (TNBC) have the shortest median
OS time of ~10.2 months (10).
Available data indicate that distant metastases
belong to the most significant cause of cancer mortality in
clinical practice (11,12) and are related to the aggressive
phenotype of small heterogeneous tumor cells, termed circulating
tumor cells (CTCs), which spread from the primary tumor and
circulate in the bloodstream. CTCs have a crucial role in tumor
dissemination and progression, making them a key component of the
metastatic cascade. Numerous trials have consistently demonstrated
the prognostic value of CTCs in both metastatic and primary BC
(PBC) (13–17). The role of CTCs in treatment failure
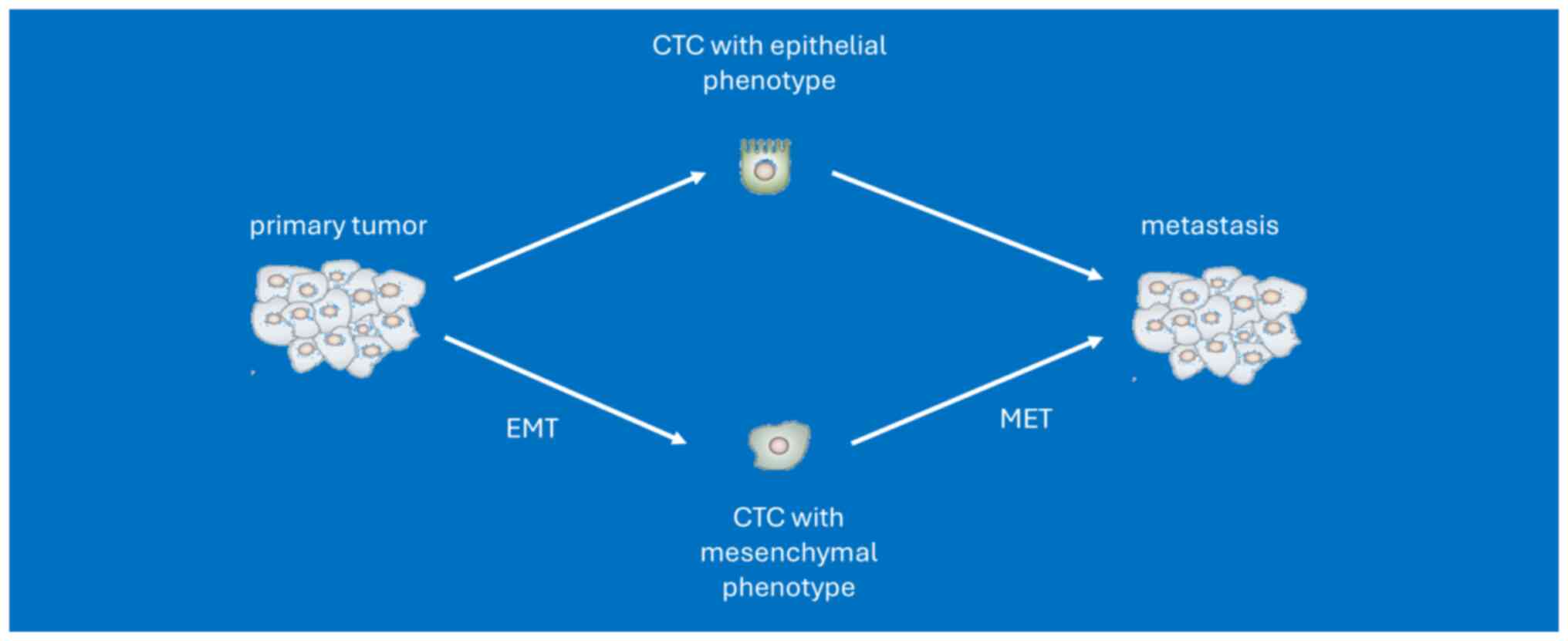
and disease progression is linked to biological processes such as
the epithelial-to-mesenchymal transition (EMT) and ‘self-seeding’,
which refers to the re-infiltration of the primary tumor or
established metastases by more aggressive CTCs (18,19).
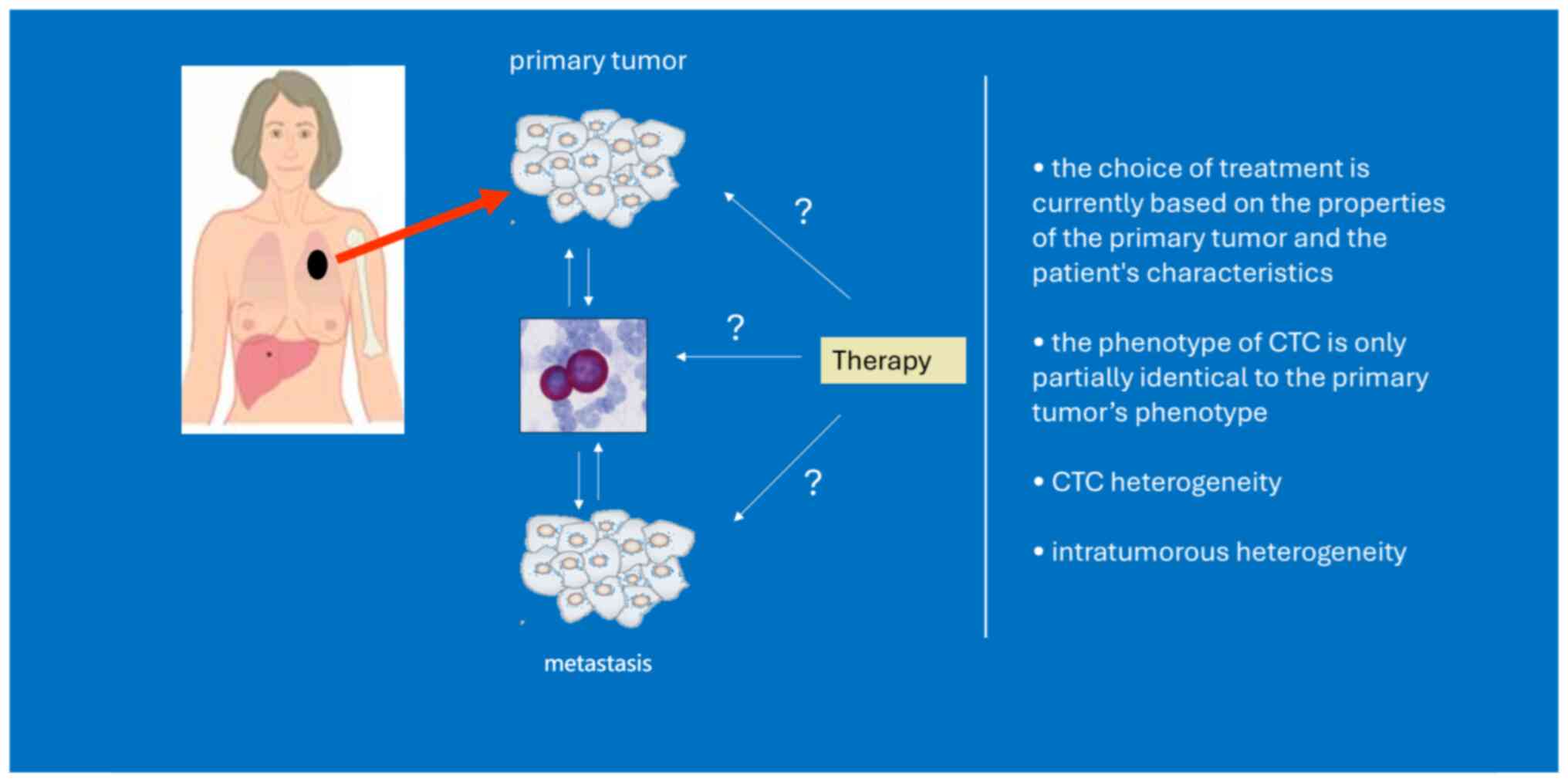
Although CTCs originate from the primary tumor, with EMT
properties, dissemination in clusters and/or exhibition of stemness
features, they differ from primary tumor cells (20). Tumor evolution represents an
important dynamic situation, illustrating modifications in tumor
heterogeneity along the temporal axis. This process can be affected
by applied therapeutic approaches (21). However, a single tumor biopsy
provides information about the specific area sampled but may not
capture the entire complexity and heterogeneity of the tumor.
Taking into consideration the ease of blood sampling, a ‘liquid
biopsy’ repeatedly during disease progression to assess the pool of
CTCs may provide evaluation of the tumor heterogeneity, prediction
of treatment response or resistance and thus provide valuable
information for novel anticancer strategy.
It took over a century to develop the appropriate
methods for CTC isolation and detection necessary for subsequent
in-depth analysis. Over the past two decades, a number of methods
for capturing CTCs have been proposed. However, the CellSearch
system is the only platform approved by the U.S. Food and Drug
Administration (FDA) and therefore it has been the most utilized
(9). The increasing number of
advanced and sensitive technologies for CTC detection has enabled
detailed investigation into the characteristics, behavior and
molecular profile of CTCs and support their clinical
implementation.
Numerous investigations in the CTC field have been
performed, mostly in BC, due to their potential role in cancer
diagnosis, prognosis and treatment monitoring, and although CTCs
have not been encompassed in clinical guidelines, their results
have projected the potential of CTCs in clinical practice (13–17,22,23).
There are numerous problems and unanswered questions
concerning the application of CTCs in clinical practice. These
issues are related to the technologies used for CTC detection,
which still primarily rely on analyzing cell count and molecular
phenotype. Additionally, there is no standardized, evidence-based
guideline for subsequent intensive or prolonged treatment based on
CTC (23). The sensitivity and
specificity of detection methods present another challenge. A
number of clinical studies lack external validation and are based
on small, single-center, case-control studies with diverse patient
characteristics (24). The
instability and uncertainty of CTC test results can influence
diagnostic and treatment decisions. The primary focus of clinical
trials has been on assessing CTC count rather than their biology
(23,25). Most notably, data on the clinical
utility of CTCs remains limited. In the present review, the
introduction of numerous CTC detection techniques is summarized to
provide a suitable framework for CTC-related technologies. Herein,
the published investigations on the role of CTCs in predicting
prognosis, their clinical importance and utility in early-stage as
well as advanced-stage BC are also reviewed. Since the CellSearch
system is one of the most widely used methods for detecting CTCs,
most of the publications included in the present review focused
almost exclusively on the CTC status assessed by this system.
CTC detection, as a non-invasive method, has the
potential to be used for prognosis and treatment monitoring;
however, the identification and isolation of CTCs among blood cells
is challenging, since they are very rare, with 1 CTC in
109 nucleated blood cells (26). CTCs also present a heterogeneous
cell population, including CTCs with partial as well as complete
EMT phenotypes with different clinical and biological properties
(27). Nonetheless, technological
development has facilitated the detection and characterization of
these cells (26).
The most accepted approach used for CTC isolation is
immune isolation. Based on the principle of immunoaffinity, CTCs
can be identified by two different methods: positive and/or
negative selection (28,29).
Positive selection is based on immunorecognition of
cancerous-related markers that are not expressed by leukocytes.
CellSearch from Veridex (USA), the only U.S. FDA-approved technique
for CTC detection, is a semi-automated immunological method based
on immunomagnetic isolation using antibodies specific for
epithelial adhesion molecule (EpCAM) and cytokeratin markers (CK8,
CK18 and CK19), with anti-CD45 antibody (anti-leukocyte) for
negative selection (30,31). Although this method is efficient and
highly reproducible, it is limited to recognizing a subset of CTCs
and may miss those with downregulated or lost markers due to EMT
(29,32–35).
Another example of a positive enrichment system is the reverse
transcription-PCR-based AdnaTest™. This technology can
identify CTCs expressing EMT-associated genes, but the kit still
includes a step to enrich EpCAM-expressing cells (36–38).
In addition, it is worth mentioning several improved alternative
immunobead technologies providing higher purity and fidelity such
as MagSweeper (39),
IsoFlux™ (40) and
CTC-µChip (41).
Negative selection uses white blood cell markers to
deplete the leukocytes from the blood sample (42). CD45 is the most prevalent antigen
used in negative selection, which is often supplemented by a
combination of additional techniques, including density gradient
centrifugation (42). A classic
example of this method is RosetteSep™, which has a
higher recovery rate than the density gradient approach (43,44).
Another bimodal selection technique is Cyttel, which has a high
detection rate (45).
Immunomagnetic methods, such as DynaBeads® and EasySep,
use magnetic beads with attached antibodies recognizing cell
surface antigens to remove unwanted cells (46).
CTCs that undergo EMT lose epithelial markers and
conventional methods may not identify CTCs with EMT
characteristics. Therefore, Mego et al (47) performed a translational study to
test an innovative approach for CTC detection, focusing on the mRNA
expression of EMT-inducing TFs in the blood of study participants
with PBC. Despite certain limitations such as a small sample size
(n=52), this was the first study to investigate a novel CTC
detection technique based on the detection of EMT-TFs,
demonstrating that EMT-CTCs may occur in the blood of patients with
PBC who have undergone neoadjuvant chemotherapy (NAC) without any
correlation between the expression levels of EMT-TFs gene and tumor
size, grade or type (47).
The alternative methodologies for recognizing CTCs
independently of surface markers are isolation platforms based on
physical features such as size, deformability, density and
electrical properties (28).
Another technique for CTC separation is density
gradient centrifugation based on the specific density of red blood
cells, leukocytes and malignant cells (54). The OncoQuick® system is
an example of a density-dependent technique, which also uses a
porous membrane to improve tumor cell enrichment (55,56).
Recent advancements in the field of CTC detection
have seen the establishment of new techniques, including those
based on microfluidics and nanotechnology elements. Microfluidic
platforms use ‘intrinsic’ vs. ‘extrinsic’ forces to separate cells
and capture target cells through different methods, such as
utilizing epithelial cell markers as antigens, the physical and
biological characteristics of malignant cells and other methods
(64,65). A powerful microfluidic platform
termed ‘CTC-chip’ captures CTCs using molecular marker-coated
micro-posts (66). Additionally, a
modified chip-based platform utilizes a chemical ligand-exchange
reaction that involves gold nanoparticles on a herringbone chip
(67). Zhang et al (68) designed an automated microfluidic
device for size-based cell isolation with high-throughput and
efficient recovery. They successfully utilized the device to sort
human BC cell lines from blood samples suggesting potential
applications in the isolation of CTCs. Wang et al (69) proposed another integrated
microfluidic platform equipped with automation capabilities for
effective CTC capture and identification within 90 min.
Furthermore, Lee and Kwak (70)
showcased a microfluidic instrument that employed differences in
magnetic field gradient and immunofluorescence to achieve on-chip
separation and simultaneous CTC characterization. This novel
microfluidic DEVICE can isolate CTCs with >99% efficiency and
can differentiate eight different subtypes of heterogenic CTCs
based on the statuses of the HER2, estrogen receptor (ER) and
progesterone receptor (PR) biomarkers guiding BC diagnosis and
prognosis. Additionally, a previous study presented the OPENchip
platform for a single CTC examination. Using this platform, Lee
et al (71) were able to
concurrently analyze both the gene activity and genetic mutations
in CTCs circulating in the blood. This was achieved using a
chip-based technology (microfluidics) combined with techniques that
analyze molecules directly in their original location (in
situ molecular profiling). Molecular analyses of single CTCs
from patients with MBC (expression of HER2 and PIK3CA mutations) or
metastatic pancreatic cancer (KRAS mutations) were demonstrated
without any off-chip procedures, proposing its possible application
of early molecular uncovering of cancer metastasis (71).
With progress in the development of nanoscale
materials and structures, nanotechnology-based techniques offer
unique advantages for real-time cancer diagnosis and detection in
terms of cost and simplicity (72).
The success of nanoparticles lies in their large surface-to-volume
ratio, which allows the adsorption of numerous targeting ligands
with the capability to bind and identify explicit cancer molecules.
Due to this property, nanomaterials offer specific benefits such as
precision of CTC-separation and highly sensitive CTC-recognition
(72). Recently, a variety of
nanomaterials, including gold nanoparticles, magnetic
nanoparticles, polymer dots, nano-fibers, nanorod arrays and
nanoparticle-coated silicon beads have been reported for CTC
detection (73). Although there
have been great expectations from the progress in the field of
nano-biotechnology, only certain nanotechnology-based techniques
have progressed to clinical trials (74). There are still a number of
challenges and limitations to be solved before their translation
into clinical applications. Namely, their reliability and
reproducibility, which can be affected by several factors such as
the interaction of nanoparticles with unintended targets, their
aggregation, unfit detection settings, possible toxicity of the
nanoparticles and the fact that most were prepared in academic
laboratories (75).
To sum up, CTC analysis has great clinical value and
the underlying basis for its subsequent application in clinical
practice is the development of reliable and reproducible
technologies for CTC detection. As aforementioned, various
CTC-related techniques have been showcased; however, further
optimization is necessary. The main goal of ongoing research is to
enhance the capabilities of these methods such as their
specificity, sensitivity and overall performance. Despite great
effort, an ideal device that can isolate a pure and viable
population of CTCs is still missing. Enhanced detection efficiency
and contaminant removal are indeed crucial for the success of CTC
detection. Several studies have highlighted this necessity
(23,25,29,34,44).
For instance, due to the low abundance of CTCs in whole peripheral
blood, the ability to distinguish CTCs from the vast majority of
non-tumor blood cells is essential for the effectiveness of CTC
capture technologies. Most of the existing methods for CTC
detection involve a two-step process of cell enrichment followed by
subsequent detection. However, the low concentration of CTCs in the
bloodstream, coupled with the heterogeneity observed among them,
renders the high-precision detection process demanding and
time-consuming. Achieving high specificity and efficiency in these
processes remains a significant challenge to ensure that CTCs can
be accurately detected and analyzed without significant
contamination. These issues necessitate the development of advanced
techniques and meticulous procedures to improve the reliability of
CTC detection (29). The current
CTC detection technologies are summarized in Table I.
Constant advancement of the methods enabling CTC
detection has expanded the application of CTCs in predicting
prognosis and outcomes in BC. In the next sections, the latest
research and the ongoing efforts to include CTC analysis as part of
regular medical care will be explored. As aforementioned,
throughout the review particular attention will be paid to studies
that almost exclusively examine CTCs detected by the CellSearch
system.
Despite these findings, the clinical utility of CTCs
in the EBC setting is constrained by their scarcity. A study that
enrolled >70 participants with carcinoma in situ showed
that 1 CTC per 22.5 ml was detected in 4.1% of cases using the
CellSearch system (82).
Non-metastatic BC typically shows <1 CTC per 10 ml of blood
(83), with rare instances of
detecting ≥5 CTCs in this volume (1–5.9%) (84). CTCs are identified in 20–25% of
individuals with non-metastatic BC at the point of initial
diagnosis using a lower threshold (≥1 CTC per 7.5 ml blood)
compared with MBC (≥5 CTCs per 7.5 ml blood) (16,84–86).
Nevertheless, extensive research on the prognostic value of CTCs
indicates that their detection in the initial diagnosis of BC is an
independent prognostic factor (84,87–97).
In 2008, during the annual American Society of
Clinical Oncology (ASCO) meeting, Rack et al (85) showcased the results of the
randomized trial, SUCCESS-A. The study, involving >2,000
patients with EBC, highlighted the significance of CTCs as an
independent prognostic marker in both pre- and post-adjuvant
chemotherapy settings. CTC positivity was associated with poorer
outcomes, disease-free survival [DFS; hazard ratio (HR)=2.28] and
OS (HR=3.95). Participants exceeding a threshold of 5 CTCs per 30
ml of blood exhibited the most unfavorable disease course and
outcome (85). Furthermore, the
ECOG-ACRIN study (E5103) along with SUCCESS-A revealed the adverse
prognostic implications of CTC persistence post neoadjuvant or
adjuvant chemotherapy (98,99). The analysis of 1,087 patients
enrolled in the SUCCESS-A trial showed that continued CTC presence
after 2 years was linked to a 2.3-fold higher recurrence risk and a
3.9-fold higher mortality risk (100). A 6-fold elevated likelihood of
recurrence was noted in the analysis of 206 patients, with
available CTC status after 5 years (99). Additionally, Sparano et al
(98) associated a ~13-fold
elevated risk of late recurrence (>5 years from the initial
diagnosis) in participants with hormone receptor+ BC in
which CTCs were detected compared with participants without CTCs,
suggesting the potential utility of CTC-positivity in the context
of long-term prognosis and risk assessment in these
individuals.
The successful attainment of a complete pathologic
response (pCR) after NAC is linked to a more favorable prognosis
(103). Nevertheless, to the best
of our knowledge, no association between the attainment of a pCR
and the presence of CTCs has been revealed so far (104).
The results of the REMAGUS02 neoadjuvant treatment
study highlight that the prechemotherapy CTC count has a
significant effect on survival outcome, whereas no significant
impact of post-chemotherapy CTC detection was observed (96). As with the phase II randomized
REMAGUS02 trial, Riethdorf et al (97) examined samples from participants
with BC treated in the GeparQuattro trial and revealed that the
incidence of CTC was 21.6% before treatment, which decreased to
10.6% after NAC, but no association between the detection of CTCs
and pCR was revealed. In addition, the presence of ≥1 CTC per 7.5
ml and ≥2 CTCs per 7.5 ml in the pre-NAC setting was significantly
related to reduced survival outcomes, specifically DFS (P=0.031)
and OS (P=0.0057), with a dose-dependent effect (≥2 CTCs per 7.5 ml
had a more notable impact). However, no such correlation was
observed in the post-NAC setting (94). The lack of correlation may be caused
by CTC viability after completing adjuvant therapy (77).
A large meta-analysis, the IMENEO study, using
individual patient data from 16 centers and 21 studies showed that
CTC detection in the pre-NAC setting had an unfavorable impact on
OS, DDFS and the locoregional relapse-free interval (LRRFI) (all
P<0.001), but was not correlated with the pCR. Participants with
varying CTC numbers (1 to ≤5 CTCs) detected in the pre-NAC setting
showed a different HR of mortality [specifically, 1.09 (95% CI,
0.65–1.69) to 6.25 (95% CI, 4.34–9.09)], illustrating a
dose-dependent impact. This finding emphasizes the quantitative
nature of CTC counts as a marker. Patients achieving pCR had
improved outcomes in terms of OS, DMFS and LRRFI. However, the
attainment of pCR did not significantly correlate with the
detection of CTCs (84).
A number of investigations have demonstrated the
prognostic value of CTCs in MBC (17,105,106). In 2005, Cristofanilli et al
(31) demonstrated for the first
time that the detection of ≥5 CTCs per 7.5 ml blood (by CellSearch)
in previously untreated participants with MBC was linked to a
significantly poorer outcome in terms of progression-free survival
(PFS) and OS. These convincing data played a key role in obtaining
FDA approval (30,31). Subsequent validation studies
(107–109), including the study by Bidard et
al (110), further reinforced
the independent prognostic effect of CTCs in MBC on the PFS (HR,
1.92; P<0.0001) and OS (HR, 2.78; P<0.0001).
A retrospective pooled analysis of >2,000
patients with MBC, categorizing participants into CTC-indolent
(<5 CTCs per 7.5 ml) and CTC-aggressive (≥5 CTCs per 7.5 ml)
groups, indicated a longer median OS time for all participants that
were Stage IV indolent compared with those that were Stage IV
aggressive (36.3 vs. 16.0 months), and likewise in the case of
participants with de novo MBC. The observed differences were
statistically significant with P<0.0001 and were consistent
across all disease subtypes (hormone receptor+,
HER2+ and TNBC). This study suggested that the CTC count
should be used as a valuable tool for staging and stratification of
MBC, emphasizing its role in prospective clinical trials (132).
In clinical settings, CTCs are applied as surrogate
biomarkers in various aggressive solid tumors such as breast, lung,
prostate, liver, gastric and pancreatic cancer (134). A summary of selected trials that
have evaluated the clinical utility of CTCs or trials that are
still on-going are shown in Tables
IV and V, respectively.
There has been significant investment in evaluating
the effectiveness of evaluating CTCs in the monitoring and
management of patients with MBC. The estimation of CTCs appears to
offer some advantages over imaging methods in the follow-up of
patients with MBC (107). A study
by Hayes et al (112)
demonstrated that elevated CTC levels, not only at the baseline but
also at any stage of treatment, serve as valuable indicators of
prognosis, specifically indicating rapid disease progression and
increased mortality in the MBC context. Determining that CTC
recognition has an unfavorable effect on clinical outcomes and that
changes in CTC levels during therapy may reflect treatment response
was an incentive to perform certain translational research
projects. The main goal of these projects was to examine the
potential utilization of monitoring and treatment decisions guided
by CTC in the context of MBC. Despite promising data, only a
limited number of studies have consistently used CTC detection for
real-time monitoring during therapy (110,112,135,136) and some of them failed to
demonstrate the clinical utility of CTC monitoring (137). The current evidence is mixed, and
its clinical utility remains to be fully established. This gap
highlights the need for more comprehensive research to validate and
standardize CTC-based monitoring protocols.
Similarly, the STIC CTC III phase study
(NCT01710605) aimed to explore the role of CTCs in first-line
treatment management of patients with hormone
receptor+HER2− MBC. Participants were
randomized into two groups: i) Physicians chose between hormone
therapy (HT) or chemotherapy according to current guidelines,
without disclosure of CTC count; and ii) treatment choice was
guided by CTC count (administration of HT for participants with
<5 CTCs per 7.5 ml of peripheral blood or chemotherapy for those
with ≥5 CTCs per 7.5 ml of peripheral blood). This trial showed
non-inferiority of the CTC-driven arm in terms of PFS, with an HR
of 0.98 (90% CI, 0.84–1.13). Analyzing the discordance between a
priori and CTC-based decision in the chosen therapy demonstrated
that switching to chemotherapy in participants with elevated
baseline CTC counts (≥5 CTC per 7.5 ml) led to improved PFS
compared with participants in the standard arm whose treatment was
clinically-driven, with statistical significance. For those
patients with a high CTC count in the clinically-driven arm treated
by HT, the median PFS time was 10.5 vs. 15.5 months in the
CTC-driven arm receiving chemotherapy. The findings of this study
are encouraging and demonstrate that CTC count could be used as a
guiding factor in choosing the first-line treatment for discrepant
cases of hormone receptor+HER2− MBC and thus
improve patient outcomes. However, this study had some limitations
such as a lack of standardized clinical criteria for chemotherapy
in the clinically-driven arm. In addition, treatment with CDK4/6
inhibitors was not used as the study was conducted after the
implementation of a new endocrine therapy (139).
In this subsection, it is emphasized that
historically BC research predominantly focused on the enumeration
of CTCs rather than their biology. For a deeper understanding of
CTC biology and more comprehensive insights into BC, moving beyond
simple counting to explore the biomolecular properties of CTCs is
of growing importance (23).
At present, available data on treatment adjusting
guided by CTC are promising but still very limited. Ongoing
prospective trials are expected to provide valuable insights.
Considering the ease of blood sampling, CTCs offer the potential
for real-time liquid biopsy, enabling repeated assessments of tumor
evolution and response to treatment. This facilitates timely and
appropriate therapy adjustments. The expression of predictive
biomarkers such as ER, PR and HER2 may evolve during the disease
course, and consequent reassessment of these markers via CTCs at
the time of disease progression could optimize treatment decisions,
making CTCs a valuable tool for personalized treatment strategies
(25,140–152). In the CirCe T-DM1 trial, which was
the first clinical trial using the phenotype of CTCs as a decision
criterion, the efficacy of trastuzumab-emtansine (T-DM1) in women
with HER2− MBC who exhibited HER2+ CTC was
assessed. However, the application of T-DM1 resulted in a partial
response in only 1 patient (140).
The DETECT study, including DETECT III, IV and V, is a
comprehensive trial investigating the effectiveness of treatment
decisions guided not only by the presence but also by the phenotype
of CTCs in women with MBC who exhibit various biological
characteristics (153). The phase
3 DETECT III study (NCT01619111), which enrolled individuals
initially diagnosed with HER2− MBC but exhibited
HER2+ CTCs, showed that application of lapatinib had a
positive impact on OS, suggesting the potential acceptance of
HER2+ CTCs as a biomarker to predict clinical benefit in
these individuals. Such findings could be clinically significant as
other HER2 drugs become accessible (141).
Reinforcing the biological role of HER2 expression
on CTCs, data from another analysis, including only participants
with HER2− MBC screened for enrollment in DETECT III and
IV with the exclusion of survival results of participants who
receiving HER2-directed treatment with lapatinib, has been
published (154). This large
multicenter analysis with nearly 2,000 patients showed that CTC
status in these patients is a strong prognostic factor and that CTC
positivity is associated with worse clinical outcomes. In this
study, ~15% of participants with HER2− MBC harbored ≥1
CTC with strong HER2 staining. The presence of CTCs with strong
HER2 staining ranged between 0.06–100% among all CTCs (mean,
15.8%). Participants with at least 1 CTC displaying strong HER2
staining had a reduced OS time compared with those with CTCs
showing only moderate HER2 staining or none at all, with OS time of
9.7 vs. 16.5 months, respectively (P=0.013). Moreover, multivariate
analysis identified hormone receptor status, CTC status, age,
Eastern Cooperative Oncology Group performance status and the
therapy line as independent predictors of OS (154).
In the DETECT V trial, the quantity or
characterization of CTCs is not included in the therapeutic
decision-making; however, one of the objectives of translational
research projects is to develop the ‘endocrine responsiveness
score’ (ERS), focusing on the expression of ER and HER2 in CTCs
(153). The findings from the
COMETI-2 study indicates that tumors expressing ER often respond
well to endocrine therapy, whereas those with upregulated HER2 are
linked to a poorer response (155). The successful establishment of the
ERS could help identify individuals who are sensitive to endocrine
therapy, thereby optimizing treatment approaches (153).
In addition, the comprehensive translational
research project, ‘DETECT-CTC’, is currently being conducted. This
project aims to utilize novel biomarkers and assays concentrated on
the molecular features of CTCs and circulating nucleic acids to
examine their potential in the clinical practice of patients with
advanced BC (153). Several
different DETECT-CTC subprojects are examining: i) The genetic and
epigenetic characteristics of CTCs, circulating free DNA and
microRNA; ii) the genomic alterations associated with DNA damage
response pathways; iii) the resistance mechanism to endocrine
therapy; iv) the expression of specific biomarkers across different
stages of cancer progression and dissemination; and v) genetic and
phenotypic changes occurring at the single-cell level within CTC
populations over time (153).
It is important to highlight the limitations
concerning current CTC test results, which include the specificity
and sensitivity of the detection methods, the lack of
standardization of biomarkers for identifying CTCs and the
biological heterogeneity CTC that could impact CTC tests
results.
The evaluation of HER2 and hormone receptors on
CTCs is critical for personalized treatment in BC. Discrepancies
between primary tumor and metastatic tissue or CTCs reported by
numerous studies are often explained as a change in the biology of
BC during the disease (142–151) (Figs.
1 and 2). However, technical
issues such as limited CTC count, variability of utilized staining
protocols as well as utilization of various CTC enrichment
techniques, contribute to this discordance (156). CTCs have potential for monitoring
disease progression and guiding therapy; however, the clinical
utility of CTC counts and molecular phenotyping has not yet been
fully validated. This hinders the ability to develop uniform
guidelines for intensive or prolonged treatment based on CTC
findings (157).
Another cause of reported receptor discrepancies is
heterogeneity within CTCs subpopulations, which can be further
complicated by processes such as EMT and mesenchymal-epithelial
transition. This limitation could lead to treatment failure. In
addition, current CTC detection methods may not capture all
subpopulations with the highest clinical validity. The lack of
established cut-off values for predictive markers on CTCs further
complicates their clinical application. Another crucial limitation
is the low level of effectiveness of CTC detection, which
significantly affects the number of CTCs detected and the
subsequent clinical decisions that can be made based on their
characterization (23,25).
Addressing these limitations, enhancing the
detection methods for capturing CTC subpopulations with the highest
clinical utility is a major future research direction.
Incorporating new platforms and standardizing biomarker expression
on CTCs are critical steps toward achieving this goal. Prospective
clinical trials are essential to validate these approaches and
determine their impact on patient outcomes. By solving these tasks,
the clinical application of CTCs as predictive markers can be
significantly improved, leading to more personalized and effective
treatment strategies (25).
Another limitation of CTC research is that a number
of clinical studies lack external validation, and the majority of
data are based on small, single-center, case-control studies with
widely varying patient characteristics. However, due to the robust
evidence provided by large-scale studies and meta-analyses such as
the study by Zhang et al (17), the clinical relevance of CTCs has
been significantly supported, leading to their incorporation into
the 2010 edition of the TNM staging manual as cM0 (i+) (158), indicating the presence of isolated
tumor cells in the blood, bone marrow or lymph nodes without
clinical signs of overt metastasis (23). The integration of CTCs into the TNM
staging system was a critical step forward underscoring their
importance in clinical oncology and marking their broader
application in oncology.
Despite the significant evidence supporting the
prognostic value of CTCs, they have not been incorporated into
clinical guidelines, such as those of the ASCO. Clinical guidelines
require high-quality evidence that a biomarker not only predicts
outcomes but also facilitates therapeutic decision-making. The aim
of future and ongoing studies is to bridge these gaps by evaluating
the impact of CTC-based interventions on patient outcomes (23). Larger, multicenter prospective
studies based on homogeneous populations are particularly needed to
determine whether CTC detection can change clinical practice and
clarify their clinical utility (17,159).
In conclusion, current therapeutic management is
based on the samples taken from primary tissues, despite the
heterogeneity that characterizes BC. Advances in the development of
detection platforms for CTCs and molecular technologies that
upgrade knowledge of the biological properties of CTCs generate the
optimism that CTCs can routinely participate in personalizing
treatment for patients with cancer. Although numerous detection
methods are available, the sensitivity and specificity of these
approaches require further enhancement and the detection of cells
escaping EpCAM selection is crucial.
It seems that characterizing CTCs is pivotal for
gaining insights into cancer prognosis as two fundamental phenomena
associated with CTCs are cancer stem cells (CSCs) and EMT. A deeper
understanding of the signaling pathways associated with EMT and the
characteristics of CSCs are critical for addressing the limitations
of CTC enumeration. The ongoing exploration of these aspects has
the potential to overcome tumor heterogeneity, drug resistance and
thus to develop targeted therapeutic strategies.
CTCs have already shown their prognostic value in a
number of clinical studies, not only in early but also in advanced
BC; however, there are still numerous challenges to be conquered
before CTC examination can be extensively utilized in clinical
practice. Although clinical utility of CTCs remains uncertain,
being easily accessible, CTCs offer an opportunity for dynamic
monitoring and thus provide valuable insights into real-time
changes in tumor characteristics and the identification of specific
mechanisms associated with resistance to treatment. Molecular
characterization of CTCs revealing the mutational profile of BC
could be beneficial to avoid ‘over/undertreatment’. Additionally,
other potential markers related to CTCs besides those already
established should be further identified.
The study of CTCs is attractive as they have
promising potential for a wide range of clinical applications. Due
to the minimally invasive nature of their sampling, CTCs offer
dynamic and real-time information, facilitating longitudinal
monitoring and may contribute to more personalized and adaptive
treatment strategies. However, the inclusion of CTC-based assays in
clinical guidelines and subsequent full integration of CTCs into
daily practice requires more clinical and molecular studies with
large cohorts of patients. Bridging the gap between CTC research
findings and clinical implementation of liquid biopsy requires
standardization, validation and collaboration between researchers
and healthcare professionals.
Not applicable.
The present research was supported by the Slovak Research and
Development Agency (grant no. APVV-16-0010).
Not applicable.
DŠR substantially contributed to the conception and
design of the manuscript; RA, PD and MJ contributed to drafting the
manuscript and the acquisition, analysis and interpretation of the
studies; MM and DP contributed to critical revisions on the
intellectual content of the manuscript. All authors read and
approved the final version of the manuscript. Data authentication
is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Dominika Šmičková Rusnáková, ORCID:
0009-0006-3638-9101; Peter Dubovan, ORCID: 0000-0002-0853-4045;
Miroslav Jurík, ORCID: 0000-0002-0290-1748; Michal Mego, ORCID:
0000-0002-6511-6827; Daniel Pinďák, ORCID: 0000-0003-0413-8907.
During the preparation of this work, artificial
intelligence tools (ChatGPT by OpenAI) were used to improve the
readability and language of the manuscript, and subsequently, the
authors revised and edited the content produced by the artificial
intelligence tool as necessary, taking full responsibility for the
ultimate content of the present manuscript.
|
1
|
Ginsburg O, Bray F, Coleman MP, Vanderpuye
V, Eniu A, Kotha SR, Sarker M, Huong TT, Allemani C, Dvaladze A, et
al: The global burden of women's cancers: A grand challenge in
global health. Lancet. 389:847–860. 2017. View Article : Google Scholar
|
|
2
|
GLOBOCAN, . 2020.New global cancer data.
https://www.uicc.org/news/globocan-2020-new-global-cancer-dataNovember
24–2021
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar
|
|
4
|
DeSantis CE, Fedewa SA, Goding Sauer A,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar
|
|
5
|
Hall CS, Valad L and Lucci A: Circulating
tumor cells in breast cancer patients. Crit Rev Oncog. 21:125–139.
2016. View Article : Google Scholar
|
|
6
|
Green M and Hortobagyi GN: Neoadjuvant
chemotherapy for operable breast cancer. Oncology (Williston Park).
16:871–884. 889–890. 892–904. 997–998. 2002.
|
|
7
|
Fisher B, Bauer M, Wickerham DL, Redmond
CK, Fisher ER, Cruz AB, Foster R, Gardner B, Lerner H, Margolese R,
et al: Relation of number of positive axillary nodes to the
prognosis of patients with primary breast cancer. An NSABP update.
Cancer. 52:1551–1557. 1983. View Article : Google Scholar
|
|
8
|
Gilbey AM, Burnett D, Coleman RE and Holen
I: The detection of circulating breast cancer cells in blood. J
Clin Pathol. 57:903–911. 2004. View Article : Google Scholar
|
|
9
|
Cueva Bañuelos JF, Rodríguez López C,
Cortegoso Mosquera A, Palacios Ozores P and Curiel García T:
Clinical relevance and therapeutic application of CTCs in advanced
breast cancer. Adv Exp Med Biol. 1220:147–164. 2020. View Article : Google Scholar
|
|
10
|
Cardoso F, Spence D, Mertz S,
Corneliussen-James D, Sabelko K, Gralow J, Cardoso MJ, Peccatori F,
Paonessa D, Benares A, et al: Global analysis of
advanced/metastatic breast cancer: Decade report (2005–2015).
Breast. 39:131–138. 2018. View Article : Google Scholar
|
|
11
|
Weiss L: Metastasis of cancer: A
conceptual history from antiquity to the 1990s. Cancer Metastasis
Rev. 19:193–383. 2000. View Article : Google Scholar
|
|
12
|
Wittekind C and Neid M: Cancer invasion
and metastasis. Oncology. 69 (Suppl 1):S14–S16. 2005. View Article : Google Scholar
|
|
13
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar
|
|
14
|
Giuliano M, Giordano A, Jackson S, De
Giorgi U, Mego M, Cohen EN, Gao H, Anfossi S, Handy BC, Ueno NT, et
al: Circulating tumor cells as early predictors of metastatic
spread in breast cancer patients with limited metastatic
dissemination. Breast Cancer Res. 16:4402014. View Article : Google Scholar
|
|
15
|
Ignatiadis M, Kallergi G, Ntoulia M,
Perraki M, Apostolaki S, Kafousi M, Chlouverakis G, Stathopoulos E,
Lianidou E, Georgoulias V and Mavroudis D: Prognostic value of the
molecular detection of circulating tumor cells using a multimarker
reverse transcription-PCR assay for cytokeratin 19, mammaglobin A,
and HER2 in early breast cancer. Clin Cancer Res. 14:2593–2600.
2008. View Article : Google Scholar
|
|
16
|
Lucci A, Hall CS, Lodhi AK, Bhattacharyya
A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM and Krishnamurthy S:
Circulating tumour cells in non-metastatic breast cancer: A
prospective study. Lancet Oncol. 13:688–695. 2012. View Article : Google Scholar
|
|
17
|
Zhang L, Riethdorf S, Wu G, Wang T, Yang
K, Peng G, Liu J and Pantel K: Meta-analysis of the prognostic
value of circulating tumor cells in breast cancer. Clin Cancer Res.
18:5701–5710. 2012. View Article : Google Scholar
|
|
18
|
Enderling H, Hlatky L and Hahnfeldt P:
Migration rules: Tumours are conglomerates of self-metastases. Br J
Cancer. 100:1917–1925. 2009. View Article : Google Scholar
|
|
19
|
Kim MY, Oskarsson T, Acharyya S, Nguyen
DX, Zhang XH, Norton L and Massagué J: Tumor self-seeding by
circulating cancer cells. Cell. 139:1315–1326. 2009. View Article : Google Scholar
|
|
20
|
Pantel K and Speicher MR: The biology of
circulating tumor cells. Oncogene. 35:1216–1224. 2016. View Article : Google Scholar
|
|
21
|
Gerlinger M, Rowan AJ, Horswell S, Math M,
Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N,
Stewart A, et al: Intratumor heterogeneity and branched evolution
revealed by multiregion sequencing. N Engl J Med. 366:883–889.
2012. View Article : Google Scholar
|
|
22
|
Bae SY, Kamalanathan KJ, Galeano-Garces C,
Konety BR, Antonarakis ES, Parthasarathy J, Hong J and Drake JM:
Dissemination of circulating tumor cells in breast and prostate
cancer: Implications for early detection. Endocrinology.
165:bqae0222024. View Article : Google Scholar
|
|
23
|
Alix-Panabières C and Pantel K: Challenges
in circulating tumour cell research. Nat Rev Cancer. 14:623–631.
2014. View Article : Google Scholar
|
|
24
|
Coumans FAW, Ligthart ST, Uhr JW and
Terstappen LWMM: Challenges in the enumeration and phenotyping of
CTC. Clin Cancer Res. 18:5711–5718. 2012. View Article : Google Scholar
|
|
25
|
Mego M and Reuben JM: Prognostic and
predictive role of circulating tumor cells in breast cancer. Curr
Breast Cancer Rep. 6:251–259. 2014. View Article : Google Scholar
|
|
26
|
Kanwar N and Done SJ: Molecular profiling
and significance of circulating tumor cell based genetic
signatures. Adv Exp Med Biol. 994:143–167. 2017. View Article : Google Scholar
|
|
27
|
Mego M, Mani SA and Cristofanilli M:
Molecular mechanisms of metastasis in breast cancer-clinical
applications. Nat Rev Clin Oncol. 7:693–701. 2010. View Article : Google Scholar
|
|
28
|
Feng Z, Wu J, Lu Y, Chan YT, Zhang C, Wang
D, Luo D, Huang Y, Feng Y and Wang N: Circulating tumor cells in
the early detection of human cancers. Int J Biol Sci. 18:3251–3265.
2022. View Article : Google Scholar
|
|
29
|
Ju S, Chen C, Zhang J, Xu L, Zhang X, Li
Z, Chen Y, Zhou J, Ji F and Wang L: Detection of circulating tumor
cells: Opportunities and challenges. Biomark Res. 10:582022.
View Article : Google Scholar
|
|
30
|
Cristofanilli M: Circulating tumor cells,
disease progression, and survival in metastatic breast cancer.
Semin Oncol. 33 (3 Suppl 9):S9–S14. 2006. View Article : Google Scholar
|
|
31
|
Cristofanilli M, Hayes DF, Budd GT, Ellis
MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC,
et al: Circulating tumor cells: A novel prognostic factor for newly
diagnosed metastatic breast cancer. J Clin oncol. 23:1420–1430.
2005. View Article : Google Scholar
|
|
32
|
Alix-Panabières C and Pantel K: Clinical
applications of circulating tumor cells and circulating tumor DNA
as liquid biopsy. Cancer Discov. 6:479–491. 2016. View Article : Google Scholar
|
|
33
|
Lianidou ES and Markou A: Circulating
tumor cells in breast cancer: Detection systems, molecular
characterization, and future challenges. Clin Chem. 57:1242–1255.
2011. View Article : Google Scholar
|
|
34
|
Andree KC, van Dalum G and Terstappen
LWMM: Challenges in circulating tumor cell detection by the
CellSearch system. Mol Oncol. 10:395–407. 2016. View Article : Google Scholar
|
|
35
|
Müller V, Riethdorf S, Rack B, Janni W,
Fasching PA, Solomayer E, Aktas B, Kasimir-Bauer S, Pantel K and
Fehm T; DETECT study group, : Prognostic impact of circulating
tumor cells assessed with the CellSearch system™ and
AdnaTest breast™ in metastatic breast cancer patients:
The DETECT study. Breast Cancer Res. 14:R1182012. View Article : Google Scholar
|
|
36
|
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig
R and Kasimir-Bauer S: Stem cell and epithelial-mesenchymal
transition markers are frequently overexpressed in circulating
tumor cells of metastatic breast cancer patients. Breast Cancer
Res. 11:R462009. View Article : Google Scholar
|
|
37
|
Danila DC, Samoila A, Patel C, Schreiber
N, Herkal A, Anand A, Bastos D, Heller G, Fleisher M and Scher HI:
Clinical validity of detecting circulating tumor cells by AdnaTest
assay compared with direct detection of tumor mrna in stabilized
whole blood, as a biomarker predicting overall survival for
metastatic castration-resistant prostate cancer patients. Cancer J.
22:315–320. 2016. View Article : Google Scholar
|
|
38
|
Gorges TM, Stein A, Quidde J, Hauch S,
Röck K, Riethdorf S, Joosse SA and Pantel K: Improved detection of
circulating tumor cells in metastatic colorectal cancer by the
combination of the CellSearch® system and the
AdnaTest®. PLoS One. 11:e01551262016. View Article : Google Scholar
|
|
39
|
Talasaz AH, Powell AA, Huber DE, Berbee
JG, Roh KH, Yu W, Xiao W, Davis MM, Pease RF, Mindrinos MN, et al:
Isolating highly enriched populations of circulating epithelial
cells and other rare cells from blood using a magnetic sweeper
device. Proc Natl Acad Sci USA. 106:3970–3975. 2009. View Article : Google Scholar
|
|
40
|
Harb W, Fan A, Tran T, Danila DC, Keys D,
Schwartz M and Ionescu-Zanetti C: Mutational analysis of
circulating tumor cells using a novel microfluidic collection
device and qPCR assay. Transl Oncol. 6:528–538. 2013. View Article : Google Scholar
|
|
41
|
Cho H, Kim J, Jeon CW and Han KH: A
disposable microfluidic device with a reusable magnetophoretic
functional substrate for isolation of circulating tumor cells. Lab
Chip. 17:4113–4123. 2017. View Article : Google Scholar
|
|
42
|
Liu Z, Fusi A, Klopocki E, Schmittel A,
Tinhofer I, Nonnenmacher A and Keilholz U: Negative enrichment by
immunomagnetic nanobeads for unbiased characterization of
circulating tumor cells from peripheral blood of cancer patients. J
Transl Med. 9:702011. View Article : Google Scholar
|
|
43
|
Naume B, Borgen E, Tøssvik S, Pavlak N,
Oates D and Nesland JM: Detection of isolated tumor cells in
peripheral blood and in BM: Evaluation of a new enrichment method.
Cytotherapy. 6:244–252. 2004. View Article : Google Scholar
|
|
44
|
Habli Z, AlChamaa W, Saab R, Kadara H and
Khraiche ML: Circulating tumor cell detection technologies and
clinical utility: Challenges and opportunities. Cancers (Basel).
12:19302020. View Article : Google Scholar
|
|
45
|
Tong B, Xu Y, Zhao J, Chen M, Xing J,
Zhong W and Wang M: Prognostic significance of circulating tumor
cells in non-small cell lung cancer patients undergoing
chemotherapy. Oncotarget. 8:86615–86624. 2017. View Article : Google Scholar
|
|
46
|
Drucker A, Teh EM, Kostyleva R, Rayson D,
Douglas S and Pinto DM: Comparative performance of different
methods for circulating tumor cell enrichment in metastatic breast
cancer patients. PLoS One. 15:e02373082020. View Article : Google Scholar
|
|
47
|
Mego M, Mani SA, Lee BN, Li C, Evans KW,
Cohen EN, Gao H, Jackson SA, Giordano A, Hortobagyi GN, et al:
Expression of epithelial-mesenchymal transition-inducing
transcription factors in primary breast cancer: The effect of
neoadjuvant therapy. Int J Cancer. 130:808–816. 2012. View Article : Google Scholar
|
|
48
|
Vona G, Sabile A, Louha M, Sitruk V,
Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M, et
al: Isolation by size of epithelial tumor cells: A new method for
the immunomorphological and molecular characterization of
circulatingtumor cells. Am J Pathol. 156:57–63. 2000. View Article : Google Scholar
|
|
49
|
Dolfus C, Piton N, Toure E and Sabourin
JC: Circulating tumor cell isolation: The assets of filtration
methods with polycarbonate track-etched filters. Chin J Cancer Res.
27:479–487. 2015.
|
|
50
|
Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo
RA, Tan DS, Lim WT, Han J, Bhagat AA and Lim CT: Isolation and
retrieval of circulating tumor cells using centrifugal forces. Sci
Rep. 3:12592013. View Article : Google Scholar
|
|
51
|
Tamminga M, Andree KC, Hiltermann TJN,
Jayat M, Schuuring E, van den Bos H, Spierings DCJ, Lansdorp PM,
Timens W, Terstappen LWMM and Groen HJM: Detection of circulating
tumor cells in the diagnostic leukapheresis product of
non-small-cell lung cancer patients comparing
CellSearch® and ISET. Cancers (Basel). 12:8962020.
View Article : Google Scholar
|
|
52
|
Miller MC, Robinson PS, Wagner C and
O'Shannessy DJ: The parsortix™ cell separation system-A
versatile liquid biopsy platform. Cytometry A. 93:1234–1239. 2018.
View Article : Google Scholar
|
|
53
|
Xu L, Mao X, Imrali A, Syed F, Mutsvangwa
K, Berney D, Cathcart P, Hines J, Shamash J and Lu YJ: Optimization
and evaluation of a novel size based circulating tumor cell
isolation system. PLoS One. 10:e01380322015. View Article : Google Scholar
|
|
54
|
Bankó P, Lee SY, Nagygyörgy V, Zrínyi M,
Chae CH, Cho DH and Telekes A: Technologies for circulating tumor
cell separation from whole blood. J Hematol Oncol. 12:482019.
View Article : Google Scholar
|
|
55
|
Rosenberg R, Gertler R, Friederichs J,
Fuehrer K, Dahm M, Phelps R, Thorban S, Nekarda H and Siewert JR:
Comparison of two density gradient centrifugation systems for the
enrichment of disseminated tumor cells in blood. Cytometry.
49:150–158. 2002. View Article : Google Scholar
|
|
56
|
Lagoudianakis EE, Kataki A, Manouras A,
Memos N, Papadima A, Derventzi A, Zografos G, Papadopoulos S,
Katergiannakis V and Konstadoulakis MM: Detection of epithelial
cells by RT-PCR targeting CEA, CK20, and TEM-8 in colorectal
carcinoma patients using OncoQuick density gradient centrifugation
system. J Surg Res. 155:183–190. 2009. View Article : Google Scholar
|
|
57
|
Gupta V, Jafferji I, Garza M, Melnikova
VO, Hasegawa DK, Pethig R and Davis DW: ApoStream(™), a
new dielectrophoretic device for antibody independent isolation and
recovery of viable cancer cells from blood. Biomicrofluidics.
6:241332012. View Article : Google Scholar
|
|
58
|
O'Shannessy DJ, Davis DW, Anderes K and
Somers EB: Isolation of circulating tumor cells from multiple
epithelial cancers with ApoStream(®) for detecting (or
monitoring) the expression of Folate receptor alpha. Biomark
Insights. 11:7–18. 2016. View Article : Google Scholar
|
|
59
|
Le Du F, Fujii T, Kida K, Davis DW, Park
M, Liu DD, Wu W, Chavez-MacGregor M, Barcenas CH, Valero V, et al:
EpCAM-independent isolation of circulating tumor cells with
epithelial-to-mesenchymal transition and cancer stem cell
phenotypes using ApoStream® in patients with breast
cancer treated with primary systemic therapy. PLoS One.
15:e02299032020. View Article : Google Scholar
|
|
60
|
Wu S, Gu L, Qin J, Zhang L, Sun F, Liu Z,
Wang Y and Shi D: Rapid label-free isolation of circulating tumor
cells from patients' peripheral blood using electrically charged
Fe3O4 nanoparticles. ACS Appl Mater
Interfaces. 12:4193–4203. 2020. View Article : Google Scholar
|
|
61
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar
|
|
62
|
Luc R, Tortorella SM, Ververis K and
Karagiannis TC: Lactate as an insidious metabolite due to the
Warburg effect. Mol Biol Rep. 42:835–840. 2015. View Article : Google Scholar
|
|
63
|
Zhu Z and Zhang YHP: In vitro metabolic
engineering of bioelectricity generation by the complete oxidation
of glucose. Metab Eng. 39:110–116. 2017. View Article : Google Scholar
|
|
64
|
Nasiri R, Shamloo A, Ahadian S, Amirifar
L, Akbari J, Goudie MJ, Lee K, Ashammakhi N, Dokmeci MR, Di Carlo D
and Khademhosseini A: Microfluidic-based approaches in targeted
cell/particle separation based on physical properties:
Fundamentalsand applications. Small. 16:e20001712020. View Article : Google Scholar
|
|
65
|
Burinaru TA, Avram M, Avram A, Mărculescu
C, Ţîncu B, Ţucureanu V, Matei A and Militaru M: Detection of
circulating tumor cells using microfluidics. ACS Comb Sci.
20:107–126. 2018. View Article : Google Scholar
|
|
66
|
Nagrath S, Sequist LV, Maheswaran S, Bell
DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky
A, et al: Isolation of rare circulating tumour cells in cancer
patients by microchip technology. Nature. 450:1235–1239. 2007.
View Article : Google Scholar
|
|
67
|
Park MH, Reátegui E, Li W, Tessier SN,
Wong KH, Jensen AE, Thapar V, Ting D, Toner M, Stott SL and Hammond
PT: Enhanced isolation and release of circulating tumor cells using
nanoparticle binding and ligand exchange in a microfluidic chip. J
Am Chem Soc. 139:2741–2749. 2017. View Article : Google Scholar
|
|
68
|
Zhang X, Zhu Z, Xiang N, Long F and Ni Z:
Automated microfluidic instrument for label-free and
high-throughput cell separation. Anal Chem. 90:4212–4220. 2018.
View Article : Google Scholar
|
|
69
|
Wang J, Li Y, Wang R, Han C, Xu S, You T,
Li Y, Xia J, Xu X, Wang D, et al: A fully automated and integrated
microfluidic system for efficient CTC detection and its application
in hepatocellular carcinoma screening and prognosis. ACS Appl Mater
Interfaces. 13:30174–30186. 2021. View Article : Google Scholar
|
|
70
|
Lee J and Kwak B: Simultaneous on-chip
isolation and characterization of circulating tumor cell
sub-populations. Biosens Bioelectron. 168:1125642020. View Article : Google Scholar
|
|
71
|
Lee AC, Svedlund J, Darai E, Lee Y, Lee D,
Lee HB, Kim SM, Kim O, Bae HJ, Choi A, et al: OPENchip: An on-chip
in situ molecular profiling platform for gene expression analysis
and oncogenic mutation detection in single circulating tumour
cells. Lab Chip. 20:912–922. 2020. View Article : Google Scholar
|
|
72
|
Zhang Y, Li M, Gao X, Chen Y and Liu T:
Nanotechnology in cancer diagnosis: Progress, challenges and
opportunities. J Hematol Oncol. 12:1372019. View Article : Google Scholar
|
|
73
|
Huang Q, Yin W, Chen X, Wang Y, Li Z, Du
S, Wang L and Shi C: Nanotechnology-based strategies for early
cancer diagnosis using circulating tumor cells as a liquid biopsy.
Nanotheranostics. 2:21–41. 2018. View Article : Google Scholar
|
|
74
|
Muthu MS and Feng SS: Theranostic
liposomes for cancer diagnosis and treatment: Current development
and pre-clinical success. Expert Opin Drug Deliv. 10:151–155. 2013.
View Article : Google Scholar
|
|
75
|
Lin D, Shen L, Luo M, Zhang K, Li J, Yang
Q, Zhu F, Zhou D, Zheng S, Chen Y and Zhou J: Circulating tumor
cells: Biology and clinical significance. Sig Transduct Target
Ther. 6:4042021. View Article : Google Scholar
|
|
76
|
Hüsemann Y, Geigl JB, Schubert F, Musiani
P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G and
Klein CA: Systemic spread is an early step in breast cancer. Cancer
Cell. 13:58–68. 2008. View Article : Google Scholar
|
|
77
|
Hosseini H, Obradović MMS, Hoffmann M,
Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C,
Maneck M, et al: Early dissemination seeds metastasis in breast
cancer. Nature. 540:552–558. 2016. View Article : Google Scholar
|
|
78
|
Rhim AD, Thege FI, Santana SM, Lannin TB,
Saha TN, Tsai S, Maggs LR, Kochman ML, Ginsberg GG, Lieb JG, et al:
Detection of circulating pancreas epithelial cells in patients with
pancreatic cystic lesions. Gastroenterology. 146:647–651. 2014.
View Article : Google Scholar
|
|
79
|
Yamaguchi J, Kokuryo T, Yokoyama Y, Ebata
T, Ochiai Y and Nagino M: Premalignant pancreatic cells seed
stealth metastasis in distant organs in mice. Oncogene.
40:2273–2284. 2021. View Article : Google Scholar
|
|
80
|
Banys M, Hahn M, Gruber I, Krawczyk N,
Wallwiener M, Hartkopf A, Taran FA, Röhm C, Kurth R, Becker S, et
al: Detection and clinical relevance of hematogenous tumor cell
dissemination in patients with ductal carcinoma in situ. Breast
Cancer Res Treat. 144:531–538. 2014. View Article : Google Scholar
|
|
81
|
Shao X, Jin X, Chen Z, Zhang Z, Chen W,
Jiang J, Wang Z, Cui Y, Fan WH, Wang K, et al: A comprehensive
comparison of circulating tumor cells and breast imaging modalities
as screening tools for breast cancer in Chinese women. Front Oncol.
12:8902482022. View Article : Google Scholar
|
|
82
|
Ignatiadis M, Rothé F, Chaboteaux C,
Durbecq V, Rouas G, Criscitiello C, Metallo J, Kheddoumi N, Singhal
SK, Michiels S, et al: HER2-positive circulating tumor cells in
breast cancer. PLoS One. 6:e156242011. View Article : Google Scholar
|
|
83
|
Thery L, Meddis A, Cabel L, Proudhon C,
Latouche A, Pierga JY and Bidard FC: Circulating tumor cells in
early breast cancer. JNCI Cancer Spectr. 3:pkz0262019. View Article : Google Scholar
|
|
84
|
Bidard FC, Michiels S, Riethdorf S,
Mueller V, Esserman LJ, Lucci A, Naume B, Horiguchi J,
Gisbert-Criado R, Sleijfer S, et al: Circulating tumor cells in
breast cancer patients treated by neoadjuvant chemotherapy: A
meta-analysis. J Natl Cancer Inst. 110:560–567. 2018. View Article : Google Scholar
|
|
85
|
Rack B, Schindlbeck C, Jückstock J,
Andergassen U, Hepp P, Zwingers T, Friedl TW, Lorenz R, Tesch H,
Fasching PA, et al: Circulating tumor cells predict survival in
early average-to-high risk breast cancer patients. J Natl Cancer
Inst. 106:dju0662014. View Article : Google Scholar
|
|
86
|
Janni WJ, Rack B, Terstappen LWMM, Pierga
JY, Taran FA, Fehm T, Hall C, de Groot MR, Bidard FC, Friedl TW, et
al: Pooled analysis of the prognostic relevance of circulating
tumor cells in primary breast cancer. Clin Cancer Res.
22:2583–2593. 2016. View Article : Google Scholar
|
|
87
|
Pierga JY, Bidard FC, Mathiot C, Brain E,
Delaloge S, Giachetti S, de Cremoux P, Salmon R, Vincent-Salomon A
and Marty M: Circulating tumor cell detection predicts early
metastatic relapse after neoadjuvant chemotherapy in large operable
and locally advanced breast cancer in a phase II randomized trial.
Clin Cancer Res. 14:7004–7010. 2008. View Article : Google Scholar
|
|
88
|
Hall C, Karhade M, Laubacher B, Anderson
A, Kuerer H, DeSynder S and Lucci A: Circulating tumor cells after
neoadjuvant chemotherapy in stage I–III triple-negative breast
cancer. Ann Surg Oncol. 22 (Suppl 3):S552–S558. 2015. View Article : Google Scholar
|
|
89
|
Hwang SB, Bae JW, Lee HY and Kim HY:
Circulating tumor cells detected by RT-PCR for CK-20 before surgery
indicate worse prognostic impact in triple-negative and HER2
subtype breast cancer. J Breast Cancer. 15:34–42. 2012. View Article : Google Scholar
|
|
90
|
Azim HA Jr, Rothé F, Aura CM, Bavington M,
Maetens M, Rouas G, Gebhart G, Gamez C, Eidtmann H, Baselga J, et
al: Circulating tumor cells and response to neoadjuvant paclitaxel
and HER2-targeted therapy: A sub-study from the NeoALTTO phase III
trial. Breast. 22:1060–1065. 2013. View Article : Google Scholar
|
|
91
|
Onstenk W, Kraan J, Mostert B, Timmermans
MM, Charehbili A, Smit VT, Kroep JR, Nortier JW, van de Ven S,
Heijns JB, et al: Improved circulating tumor cell detection by a
combined EpCAM and MCAM cellsearch enrichment approach in patients
with breast cancer undergoing neoadjuvant chemotherapy. Mol Cancer
Ther. 14:821–827. 2015. View Article : Google Scholar
|
|
92
|
Kasimir-Bauer S, Bittner AK, König L,
Reiter K, Keller T, Kimmig R and Hoffmann O: Does primary
neoadjuvant systemic therapy eradicate minimal residual disease?
Analysis of disseminated and circulating tumor cells before and
after therapy. Breast Cancer Res. 18:202016. View Article : Google Scholar
|
|
93
|
Pierga JY, Bidard FC, Autret A, Petit T,
Andre F, Dalenc F, Levy C, Ferrero JM, Romieu G, Bonneterre J, et
al: Circulating tumour cells and pathological complete response:
Independent prognostic factors in inflammatory breast cancer in a
pooled analysis of two multicentre phase II trials (BEVERLY-1 and
−2) of neoadjuvant chemotherapy combined with bevacizumab. Ann
Oncol. 28:103–109. 2017. View Article : Google Scholar
|
|
94
|
Riethdorf S, Müller V, Loibl S, Nekljudova
V, Weber K, Huober J, Fehm T, Schrader I, Hilfrich J, Holms F, et
al: Prognostic impact of circulating tumor cells for breast cancer
patients treated in the neoadjuvant ‘Geparquattro’ trial. Clin
Cancer Res. 23:5384–5393. 2017. View Article : Google Scholar
|
|
95
|
Bidard FC, Mathiot C, Delaloge S, Brain E,
Giachetti S, de Cremoux P, Marty M and Pierga JY: Single
circulating tumor cell detection and overall survival in
nonmetastatic breast cancer. Ann Oncol. 21:729–733. 2009.
View Article : Google Scholar
|
|
96
|
Bidard FC, Belin L, Delaloge S, Lerebours
F, Ngo C, Reyal F, Alran S, Giacchetti S, Marty M, Lebofsky R and
Pierga JY: Time-dependent prognostic impact of circulating tumor
cells detection in non-metastatic breast cancer: 70-Month analysis
of the REMAGUS02 study. Int J Breast Cancer. 2013:1304702013.
View Article : Google Scholar
|
|
97
|
Riethdorf S, Müller V, Zhang L, Rau T,
Loibl S, Komor M, Roller M, Huober J, Fehm T, Schrader I, et al:
Detection and HER2 expression of circulating tumor cells:
Prospective monitoring in breast cancer patients treated in the
neoadjuvant GeparQuattro trial. Clin Cancer Res. 16:2634–2645.
2010. View Article : Google Scholar
|
|
98
|
Sparano J, O'Neill A, Alpaugh K, Wolff AC,
Northfelt DW, Dang CT, Sledge GW and Miller KD: Association of
circulating tumor cells with late recurrence of estrogen
receptor-positive breast cancer: A secondary analysis of a
randomized clinical trial. JAMA Oncol. 4:1700–1706. 2018.
View Article : Google Scholar
|
|
99
|
Janni W, Rack BK, Fasching PA, Haeberle L,
Tesch H, Lorenz R, Schochter F, Tzschaschel M, De Gregorio A, Fehm
TN, et al: Persistence of circulating tumor cells in high risk
early breast cancer patients five years after adjuvant chemotherapy
and late recurrence: Results from the adjuvant SUCCESS A trial. J
Clin Oncol. 36 (Suppl):S5152018. View Article : Google Scholar
|
|
100
|
Trapp E, Janni W, Schindlbeck C, Jückstock
J, Andergassen U, de Gregorio A, Alunni-Fabbroni M, Tzschaschel M,
Polasik A, Koch JG, et al: Presence of circulating tumor cells in
high-risk early breast cancer during follow-up and prognosis. J
Natl Cancer Inst. 111:380–387. 2019. View Article : Google Scholar
|
|
101
|
Bidard FC, Fehm T, Ignatiadis M, Smerage
JB, Alix-Panabières C, Janni W, Messina C, Paoletti C, Müller V,
Hayes DF, et al: Clinical application of circulating tumor cells in
breast cancer: Overview of the current interventional trials.
Cancer Metastasis Rev. 32:179–188. 2013. View Article : Google Scholar
|
|
102
|
Hall CS, Karhade MG, Bowman Bauldry JB,
Valad LM, Kuerer HM, DeSnyder SM and Lucci A: Prognostic value of
circulating tumor cells identified before surgical resection in
nonmetastatic breast cancer patients. J Am Coll Surg. 223:20–29.
2016. View Article : Google Scholar
|
|
103
|
Cortazar P, Zhang L, Untch M, Mehta K,
Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L,
Valagussa P, et al: Pathological complete response and long-term
clinical benefit in breast cancer: The CTNeoBC pooled analysis.
Lancet. 384:164–172. 2014. View Article : Google Scholar
|
|
104
|
Walter VP, Taran FA, Wallwiener M, Hahn M,
Brucker SY and Hartkopf AD: Simultaneous detection of circulating
and disseminated tumor cells in primary breast cancer patients
following neoadjuvant chemotherapy. Arch Gynecol Obstet.
297:785–790. 2018. View Article : Google Scholar
|
|
105
|
Peeters DJE, van Dam PJ, Van den Eynden
GGM, Rutten A, Wuyts H, Pouillon L, Peeters M, Pauwels P, Van Laere
SJ, van Dam PA, et al: Detection and prognostic significance of
circulating tumour cells in patients with metastatic breast cancer
according to immunohistochemical subtypes. Br J Cancer.
110:375–383. 2014. View Article : Google Scholar
|
|
106
|
Giordano A, Giuliano M, Hsu L, Handy BC,
Ueno NT, Andreopoulou E, Alvarez RH, Valero V, Hortobagyi GN and
Cristofanilli M: Prognostic value of circulating tumor cells (CTC)
in metastatic breast cancer (MBC): Correlation with
immunohistochemically defined molecular subtypes and metastatic
disease sites. J Clin Oncol. 28 (Suppl):S10002010. View Article : Google Scholar
|
|
107
|
Budd GT, Cristofanilli M, Ellis MJ,
Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV,
Terstappen LW and Hayes DF: Circulating tumor cells versus
imaging-predicting overall survival in metastatic breast cancer.
Clin Cancer Res. 12:6403–6409. 2006. View Article : Google Scholar
|
|
108
|
Giuliano M, Giordano A, Jackson S, Hess
KR, De Giorgi U, Mego M, Handy BC, Ueno NT, Alvarez RH, De
Laurentiis M, et al: Circulating tumor cells as prognostic and
predictive markers in metastatic breast cancer patients receiving
first-line systemic treatment. Breast Cancer Res. 13:R672011.
View Article : Google Scholar
|
|
109
|
Giordano A, Giuliano M, De Laurentiis M,
Arpino G, Jackson S, Handy BC, Ueno NT, Andreopoulou E, Alvarez RH,
Valero V, et al: Circulating tumor cells in immunohistochemical
subtypes of metastatic breast cancer: Lack of prediction in
HER2-positive disease treated with targeted therapy. Ann Oncol.
23:1144–1150. 2012. View Article : Google Scholar
|
|
110
|
Bidard FC, Peeters DJ, Fehm T, Nolé F,
Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz
JA, Stebbing J, et al: Clinical validity of circulating tumour
cells in patients with metastatic breast cancer: A pooled analysis
of individual patient data. Lancet Oncol. 15:406–414. 2014.
View Article : Google Scholar
|
|
111
|
Lv Q, Gong L, Zhang T, Ye J, Chai L, Ni C
and Mao Y: Prognostic value of circulating tumor cells in
metastatic breast cancer: A systemic review and meta-analysis. Clin
Transl Oncol. 18:322–330. 2016. View Article : Google Scholar
|
|
112
|
Hayes DF, Cristofanilli M, Budd GT, Ellis
MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV and
Terstappen LW: Circulating tumor cells at each follow-up time point
during therapy of metastatic breast cancer patients predict
progression-free and overall survival. Clin Cancer Res.
12:4218–4224. 2006. View Article : Google Scholar
|
|
113
|
Cristofanilli M, Broglio KR, Guarneri V,
Jackson S, Fritsche HA, Islam R, Dawood S, Reuben JM, Kau SW, Lara
JM, et al: Circulating tumor cells in metastatic breast cancer:
Biologic staging beyond tumor burden. Clin Breast Cancer.
7:471–479. 2007. View Article : Google Scholar
|
|
114
|
Bidard FC, Vincent-Salomon A,
Sigal-Zafrani B, Diéras V, Mathiot C, Mignot L, Thiery JP,
Sastre-Garau X and Pierga JY: Prognosis of women with stage IV
breast cancer depends on detection of circulating tumor cells
rather than disseminated tumor cells. Ann Oncol. 19:496–500. 2008.
View Article : Google Scholar
|
|
115
|
Yagata H, Nakamura S, Toi M, Bando H, Ohno
S and Kataoka A: Evaluation of circulating tumor cells in patients
with breast cancer: Multi-institutional clinical trial in Japan.
Int J Clin Oncol. 13:252–256. 2008. View Article : Google Scholar
|
|
116
|
De Giorgi U, Valero V, Rohren E, Dawood S,
Ueno NT, Miller MC, Doyle GV, Jackson S, Andreopoulou E, Handy BC,
et al: Circulating tumor cells and [18F]fluorodeoxyglucose positron
emission tomography/computed tomography for outcome prediction in
metastatic breast cancer. J Clin Oncol. 27:3303–3311. 2009.
View Article : Google Scholar
|
|
117
|
Liu MC, Shields PG, Warren RD, Cohen P,
Wilkinson M, Ottaviano YL, Rao SB, Eng-Wong J,
Seillier-Moiseiwitsch F, Noone AM and Isaacs C: Circulating tumor
cells: A useful predictor of treatment efficacy in metastatic
breast cancer. J Clin Oncol. 27:5153–5159. 2009. View Article : Google Scholar
|
|
118
|
Mego M, De Giorgi U, Hsu L, Ueno NT,
Valero V, Jackson S, Andreopoulou E, Kau SW, Reuben JM and
Cristofanilli M: Circulating tumor cells in metastatic inflammatory
breast cancer. Annals Oncol. 20:1824–1828. 2009. View Article : Google Scholar
|
|
119
|
De Giorgi U, Valero V, Rohren E, Mego M,
Doyle GV, Miller MC, Ueno NT, Handy BC, Reuben JM, Macapinlac HA,
et al: Circulating tumor cells and bone metastases as detected by
FDG-PET/CT in patients with metastatic breast cancer. Annals Oncol.
21:33–39. 2010. View Article : Google Scholar
|
|
120
|
Consoli F, Grisanti S, Amoroso V, Almici
C, Verardi R, Marini M and Simoncini E: Circulating tumor cells as
predictors of prognosis in metastatic breast cancer: Clinical
application outside a clinical trial. Tumori. 97:737–742. 2011.
View Article : Google Scholar
|
|
121
|
Hartkopf AD, Wagner P, Wallwiener D, Fehm
T and Rothmund R: Changing levels of circulating tumor cells in
monitoring chemotherapy response in patients with metastatic breast
cancer. Anticancer Res. 31:979–984. 2011.
|
|
122
|
Tokudome N, Ito Y, Takahashi S, Kobayashi
K, Taira S, Tsutsumi C, Oto M, Oba M, Inoue K, Kuwayama A, et al:
Detection of circulating tumor cells in peripheral blood of heavily
treated metastatic breast cancer patients. Breast Cancer.
18:195–202. 2011. View Article : Google Scholar
|
|
123
|
De Giorgi U, Mego M, Scarpi E, Giuliano M,
Giordano A, Reuben JM, Valero V, Ueno NT, Hortobagyi GN and
Cristofanilli M: Relationship between lymphocytopenia and
circulating tumor cells as prognostic factors for overall survival
in metastatic breast cancer. Clin Breast Cancer. 12:264–269. 2012.
View Article : Google Scholar
|
|
124
|
Hayashi N, Nakamura S, Tokuda Y, Shimoda
Y, Yagata H, Yoshida A, Ota H, Hortobagyi GN, Cristofanilli M and
Ueno NT: Prognostic value of HER2-positive circulating tumor cells
in patients with metastatic breast cancer. Int J Clin Oncol.
17:96–104. 2012. View Article : Google Scholar
|
|
125
|
Pierga JY, Hajage D, Bachelot T, Delaloge
S, Brain E, Campone M, Diéras V, Rolland E, Mignot L, Mathiot C and
Bidard FC: High independent prognostic and predictive value of
circulating tumor cells compared with serum tumor markers in a
large prospective trial in first-line chemotherapy for metastatic
breast cancer patients. Annals Oncol. 23:618–624. 2012. View Article : Google Scholar
|
|
126
|
Weissenstein U, Schumann A, Reif M, Link
S, Toffol-Schmidt UD and Heusser P: Detection of circulating tumor
cells in blood of metastatic breast cancer patients using a
combination of cytokeratin and EpCAM antibodies. BMC Cancer.
12:2062012. View Article : Google Scholar
|
|
127
|
Jiang ZF, Cristofanilli M, Shao ZM, Tong
ZS, Song EW, Wang XJ, Liao N, Hu XC, Liu Y, Wang Y, et al:
Circulating tumor cells predict progression-free and overall
survival in Chinese patients with metastatic breast cancer,
HER2-positive or triple-negative (CBCSG004): A multicenter,
double-blind, prospective trial. Annals Oncol. 24:2766–2772. 2013.
View Article : Google Scholar
|
|
128
|
Liu Y, Liu Q, Wang T, Bian L, Zhang S, Hu
H, Li S, Hu Z, Wu S, Liu B and Jiang Z: Circulating tumor cells in
HER2-positive metastatic breast cancer patients: A valuable
prognostic and predictive biomarker. BMC Cancer. 13:2022013.
View Article : Google Scholar
|
|
129
|
Tarhan MO, Gonel A, Kucukzeybek Y, Erten
C, Cuhadar S, Yigit SC, Atay A, Somali I, Dirican A, Demir L and
Koseoglu M: Prognostic significance of circulating tumor cells and
serum CA15-3 levels in metastatic breast cancer, single center
experience, preliminary results. Asian Pac J Cancer Prev.
14:1725–1729. 2013. View Article : Google Scholar
|
|
130
|
Turker I, Uyeturk U, Sonmez OU, Oksuzoglu
B, Helvaci K, Arslan UY, Budakoglu B, Alkis N, Aksoy S and Zengin
N: Detection of circulating tumor cells in breast cancer patients:
Prognostic predictive role. Asian Pac J Cancer Prev. 14:1601–1607.
2013. View Article : Google Scholar
|
|
131
|
Wallwiener M, Hartkopf AD, Baccelli I,
Riethdorf S, Schott S, Pantel K, Marmé F, Sohn C, Trumpp A, Rack B,
et al: The prognostic impact of circulating tumor cells in subtypes
of metastatic breast cancer. Breast Cancer Res Treat. 137:503–510.
2013. View Article : Google Scholar
|
|
132
|
Cristofanilli M, Pierga JY, Reuben J,
Rademaker A, Davis AA, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado
R, Mavroudis D, et al: The clinical use of circulating tumor cells
(CTCs) enumeration for staging of metastatic breast cancer (MBC):
International expert consensus paper. Crit Rev Oncol Hematol.
134:39–45. 2019. View Article : Google Scholar
|
|
133
|
Magbanua MJM, Hendrix LH, Hyslop T, Barry
WT, Winer EP, Hudis C, Toppmeyer D, Carey LA, Partridge AH, Pierga
JY, et al: Serial analysis of circulating tumor cells in metastatic
breast cancer receiving first-line chemotherapy. J Natl Cancer
Inst. 113:443–452. 2021. View Article : Google Scholar
|
|
134
|
Sundling KE and Lowe AC: Circulating tumor
cells: Overview and opportunities in cytology. Adv Anat Pathol.
26:56–63. 2019. View Article : Google Scholar
|
|
135
|
Larsson AM, Jansson S, Bendahl PO, Levin
Tykjaer Jörgensen C, Loman N, Graffman C, Lundgren L, Aaltonen K
and Rydén L: Longitudinal enumeration and cluster evaluation of
circulating tumor cells improve prognostication for patients with
newly diagnosed metastatic breast cancer in a prospective
observational trial. Breast Cancer Res. 20:482018. View Article : Google Scholar
|
|
136
|
Jauch SF, Riethdorf S, Sprick MR, Schütz
F, Schönfisch B, Brucker SY, Deutsch TM, Nees J, Saini M, Becker
LM, et al: Sustained prognostic impact of circulating tumor cell
status and kinetics upon further progression of metastatic breast
cancer. Breast Cancer Res. Treat. 173:155–165. 2019.
|
|
137
|
Cabel L, Berger F, Cottu P, Loirat D,
Rampanou A, Brain E, Cyrille S, Bourgeois H, Kiavue N, Deluche E,
et al: Clinical utility of circulating tumour cell-based monitoring
of late-line chemotherapy for metastatic breast cancer: The
randomised CirCe01 trial. Br J Cancer. 124:1207–1213. 2021.
View Article : Google Scholar
|
|
138
|
Smerage JB, Barlow WE, Hortobagyi GN,
Winer EP, Leyland-Jones B, Srkalovic G, Tejwani S, Schott AF,
O'Rourke MA, Lew DL, et al: Circulating tumor cells and response to
chemotherapy in metastatic breast cancer: SWOG S0500. J Clin oncol.
32:3483–3489. 2014. View Article : Google Scholar
|
|
139
|
Bidard FC, Jacot W, Kiavue N, Dureau S,
Kadi A, Brain E, Bachelot T, Bourgeois H, Gonçalves A, Ladoire S,
et al: Efficacy of circulating tumor cell count-driven vs
clinician-driven first-line therapy choice in hormone
receptor-positive, ERBB2-negative metastatic breast cancer: The
STIC CTC randomized clinical trial. JAMA Oncol. 7:34–41. 2021.
View Article : Google Scholar
|
|
140
|
Jacot W, Cottu P, Berger F, Dubot C,
Venat-Bouvet L, Lortholary A, Bourgeois H, Bollet M, Servent V,
Luporsi E, et al: Actionability of HER2-amplified circulating tumor
cells in HER2-negative metastatic breast cancer: The CirCe T-DM1
trial. Breast Cancer Res. 21:1212019. View Article : Google Scholar
|
|
141
|
Fehm T, Müller V, Banys-Paluchowski M,
Fasching PA, Friedl TWP, Hartkopf AD, Huober J, Loehberg C, Rack B,
Riethdorf S, et al: Abstract PD3-12: Efficacy of the tyrosine
kinase inhibitor lapatinib in the treatment of patients with
HER2-negative metastatic breast cancer and HER2-positive
circulating tumor cells-results from the randomized phase III
DETECT III trial. Cancer Res. 81 (4 Suppl):PD3–12. 2021. View Article : Google Scholar
|
|
142
|
Amir E, Miller N, Geddie W, Freedman O,
Kassam F, Simmons C, Oldfield M, Dranitsaris G, Tomlinson G,
Laupacis A, et al: Prospective study evaluating the impact of
tissue confirmation of metastatic disease in patients with breast
cancer. J Clin Oncol. 30:587–592. 2012. View Article : Google Scholar
|
|
143
|
Simmons C, Miller N, Geddie W, Gianfelice
D, Oldfield M, Dranitsaris G and Clemons MJ: Does confirmatory
tumor biopsy alter the management of breast cancer patients with
distant metastases? Ann Oncol. 20:1499–1504. 2009. View Article : Google Scholar
|
|
144
|
Thompson AM, Jordan LB, Quinlan P,
Anderson E, Skene A, Dewar JA and Purdie CA; Breast Recurrence in
Tissues Study Group, : Prospective comparison of switches in
biomarker status between primary and recurrent breast cancer: The
breast recurrence in tissues study (BRITS). Breast Cancer Res.
12:R922010. View Article : Google Scholar
|
|
145
|
Curigliano G, Bagnardi V, Viale G,
Fumagalli L, Rotmensz N, Aurilio G, Locatelli M, Pruneri G, Giudici
S, Bellomi M, et al: Should liver metastases of breast cancer be
biopsied to improve treatment choice? Ann Oncol. 22:2227–2233.
2011. View Article : Google Scholar
|
|
146
|
Xiao C, Gong Y, Han EY, Gonzalez-Angulo AM
and Sneige N: Stability of HER2-positive status in breast
carcinoma: A comparison between primary and paired metastatic
tumors with regard to the possible impact of intervening
trastuzumab treatment. Ann Oncol. 22:1547–1553. 2011. View Article : Google Scholar
|
|
147
|
Sari E, Guler G, Hayran M, Gullu I,
Altundag K and Ozisik Y: Comparative study of the
immunohistochemical detection of hormone receptor status and HER-2
expression in primary and paired recurrent/metastatic lesions of
patients with breast cancer. Med Oncol. 28:57–63. 2011. View Article : Google Scholar
|
|
148
|
Amir E, Clemons M, Purdie CA, Miller N,
Quinlan P, Geddie W, Coleman RE, Freedman OC, Jordan LB and
Thompson AM: Tissue confirmation of disease recurrence in breast
cancer patients: Pooled analysis of multi-centre,
multi-disciplinary prospective studies. Cancer Treat Rev.
38:708–714. 2012. View Article : Google Scholar
|
|
149
|
Chang HJ, Han SW, Oh DY, Im SA, Jeon YK,
Park IA, Han W, Noh DY, Bang YJ and Kim TY: Discordant human
epidermal growth factor receptor 2 and hormone receptor status in
primary and metastatic breast cancer and response to trastuzumab.
Jpn J Clin Oncol. 41:593–599. 2011. View Article : Google Scholar
|
|
150
|
Dieci MV, Barbieri E, Piacentini F,
Ficarra G, Bettelli S, Dominici M, Conte PF and Guarneri V:
Discordance in receptor status between primary and recurrent breast
cancer has a prognostic impact: A single-institution analysis. Ann
Oncol. 24:101–108. 2013. View Article : Google Scholar
|
|
151
|
Lindström LS, Karlsson E, Wilking UM,
Johansson U, Hartman J, Lidbrink EK, Hatschek T, Skoog L and Bergh
J: Clinically used breast cancer markers such as estrogen receptor,
progesterone receptor, and human epidermal growth factor receptor 2
are unstable throughout tumor progression. J Clin Oncol.
30:2601–2608. 2012. View Article : Google Scholar
|
|
152
|
Alix-Panabières C and Pantel K:
Circulating tumor cells: Liquid biopsy of cancer. Clin Chem.
59:110–118. 2013. View Article : Google Scholar
|
|
153
|
Schochter F, Friedl TWP, deGregorio A,
Krause S, Huober J, Rack B and Janni W: Are circulating tumor cells
(CTCs) ready for clinical use in breast cancer? An overview of
completed and ongoing trials using CTCs for clinical treatment
decisions. Cells. 8:14122019. View Article : Google Scholar
|
|
154
|
Müller V, Banys-Paluchowski M, Friedl TWP,
Fasching PA, Schneeweiss A, Hartkopf A, Wallwiener D, Rack B,
Meier-Stiegen F, Huober J, et al: Prognostic relevance of the HER2
status of circulating tumor cells in metastatic breast cancer
patients screened for participation in the DETECT study program.
ESMO Open. 6:1002992021. View Article : Google Scholar
|
|
155
|
Paoletti C, Regan MM, Liu MC, Marcom PK,
Hart LL, Smith JW, Tedesco KL, Amir E, Krop IE, DeMichele AM, et
al: Abstract P1-01-01: Circulating tumor cell number and
CTC-endocrine therapy index predict clinical outcomes in ER
positive metastatic breast cancer patients: Results of the COMETI
phase 2 trial. Cancer Res. 77 (4 Suppl):P1-01-01. 2017. View Article : Google Scholar
|
|
156
|
Salu P and Reindl KM: Advancements in
circulating tumor cell research: Bridging biology and clinical
applications. Cancers (Basel). 16:12132024. View Article : Google Scholar
|
|
157
|
Smit DJ, Schneegans S and Pantel K:
Clinical applications of circulating tumor cells in patients with
solid tumors. Clin Exp Metastasis. 41:403–411. 2024. View Article : Google Scholar
|
|
158
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging manual (7th ed). New
York, NY: Springer; 2010
|
|
159
|
Cabel L, Proudhon C, Gortais H, Loirat D,
Coussy F, Pierga JY and Bidard FC: Circulating tumor cells:
Clinical validity and utility. Int J Clin Oncol. 22:421–430. 2017.
View Article : Google Scholar
|