Introduction
Adenosquamous carcinoma (ASC) is defined as a
malignant tumor comprised of adenocarcinoma and components of
squamous cell carcinoma, with a low incidence rate according to the
World Health Organization (WHO) Classification of Tumors of the
Digestive System 2019 (1).
Surveillance, Epidemiology and End Results databases have reported
that the number of cases of gastrointestinal ASC in the United
States from 1973 to 2020 accounted for 20% of all ASC cases
(2). ASC can occur in various
organs, such as the lung, pancreas, gallbladder, breast, stomach,
cervix, colon and rectum. Nevertheless, primary duodenal ASC is
exceedingly rare (3–9). So far, only ~18 cases of primary
duodenal ASC have been reported, and basic and large-scale clinical
studies remain unavailable. Duodenal ASC faces several clinical
challenges. The preoperative diagnosis is difficult due to
non-specific clinical and imaging presentations, as well as the low
diagnostic accuracy of biopsies. Additionally, since only a few
cases of duodenal ASC have been reported, no standard medical
approach has been established and the prognosis of duodenal ASC
remains unclear. Previous studies (10–12)
have reported that ASC generally has a poorer prognosis and is
clinically more aggressive than its adenocarcinoma counterpart
(13).
The current report aims to present a rare case of
primary duodenal ASC and to review the literature to enhance our
clinical understanding and guide treatment strategies for the
disease.
Case report
A 55-year-old man presented to Wenzhou Central
Hospital (Wenzhou, China) with a 1-month history of jaundice and
fever in January 2023. The patient also had a history of weight
loss and anorexia, along with pruritus and clay-colored stools for
3 months. No familial/hereditary cancer syndrome was reported in
the family history. Laboratory investigations revealed the
following: Serum total bilirubin, 304.1 µmol/l (normal, 0–26
µmol/l); aspartate aminotransferase, 127 U/l (normal, 15–40 U/l);
alanine aminotransferase, 76 U/l (normal, 9–50 U/l); alkaline
phosphatase, 263 U/l (normal, 45–145 U/l); CA125, 94.4 kU/l
(normal, 0–24 kU/l) and CA19-9 (18,444 kU/l; normal, 0–25 kU/l).
AFP, 1.4 µg/l (normal, 0–7 µg/l) and CEA, 4.4 µg/l (normal, 0–5
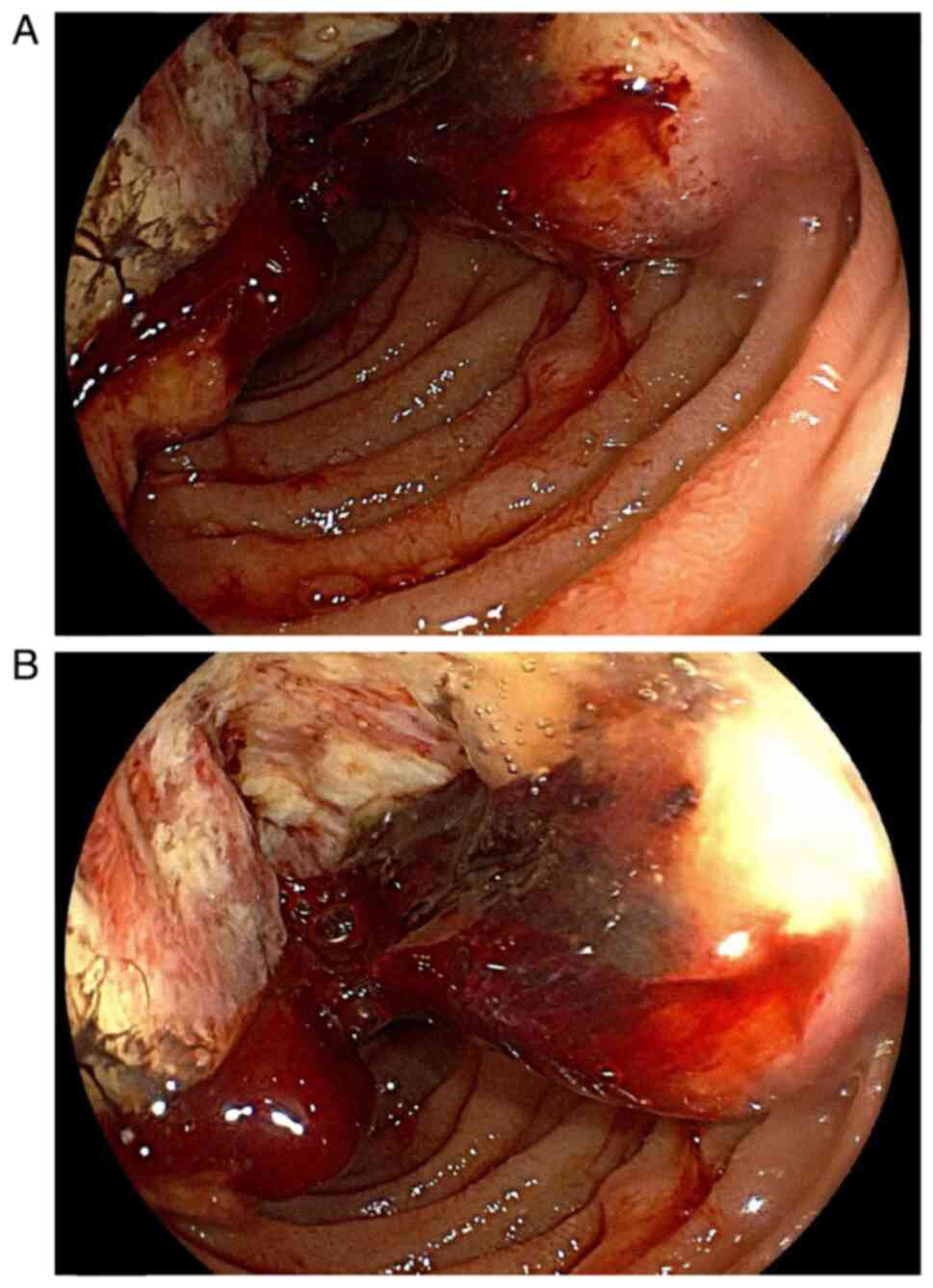
µg/l) were normal. Gastroscopy showed a hyperplastic and increased
mass in the descending portion of the duodenum, which was irregular
in shape, covered with Inflammatory necrotic tissue and blood,
presented with mucosal erosion and congestion, and encroached on
the duodenal papilla (Fig. 1A and
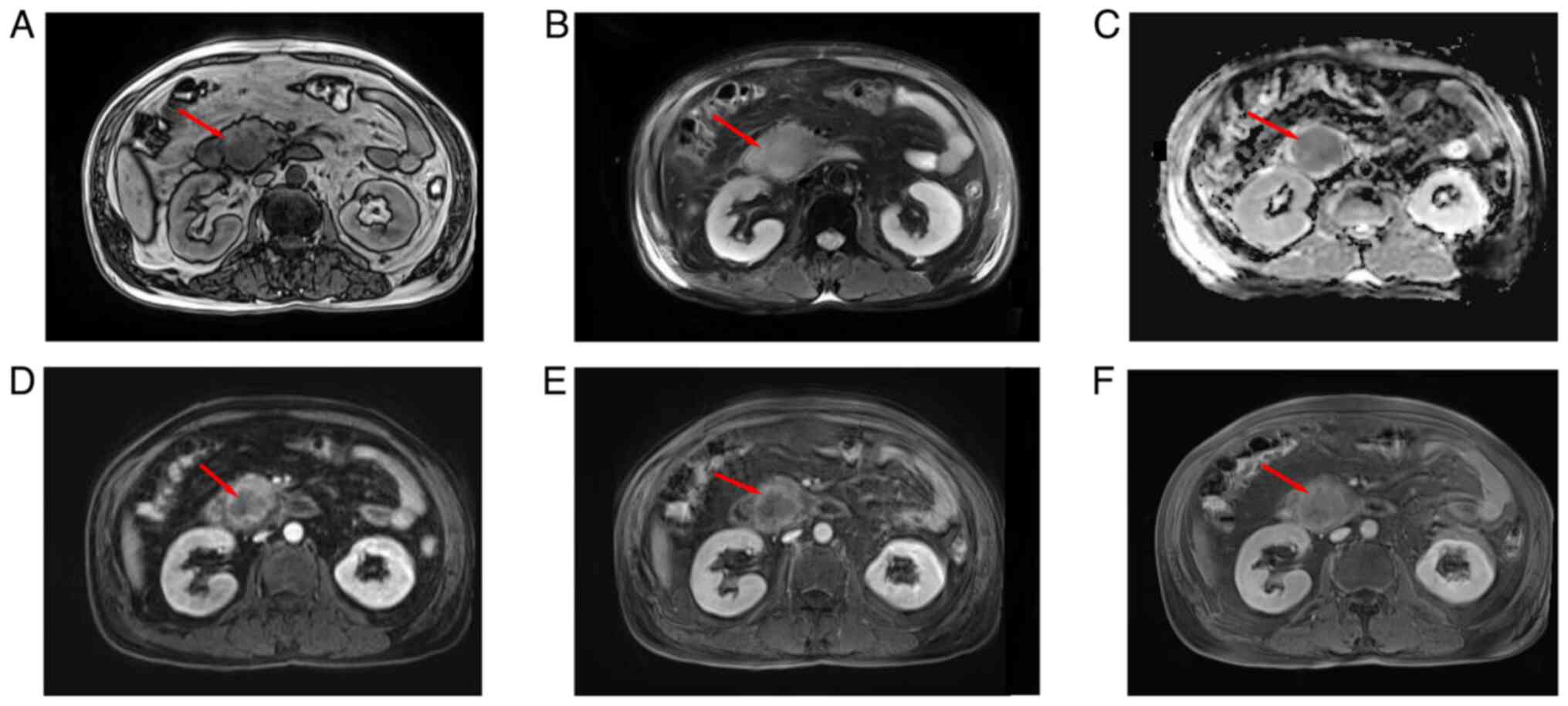
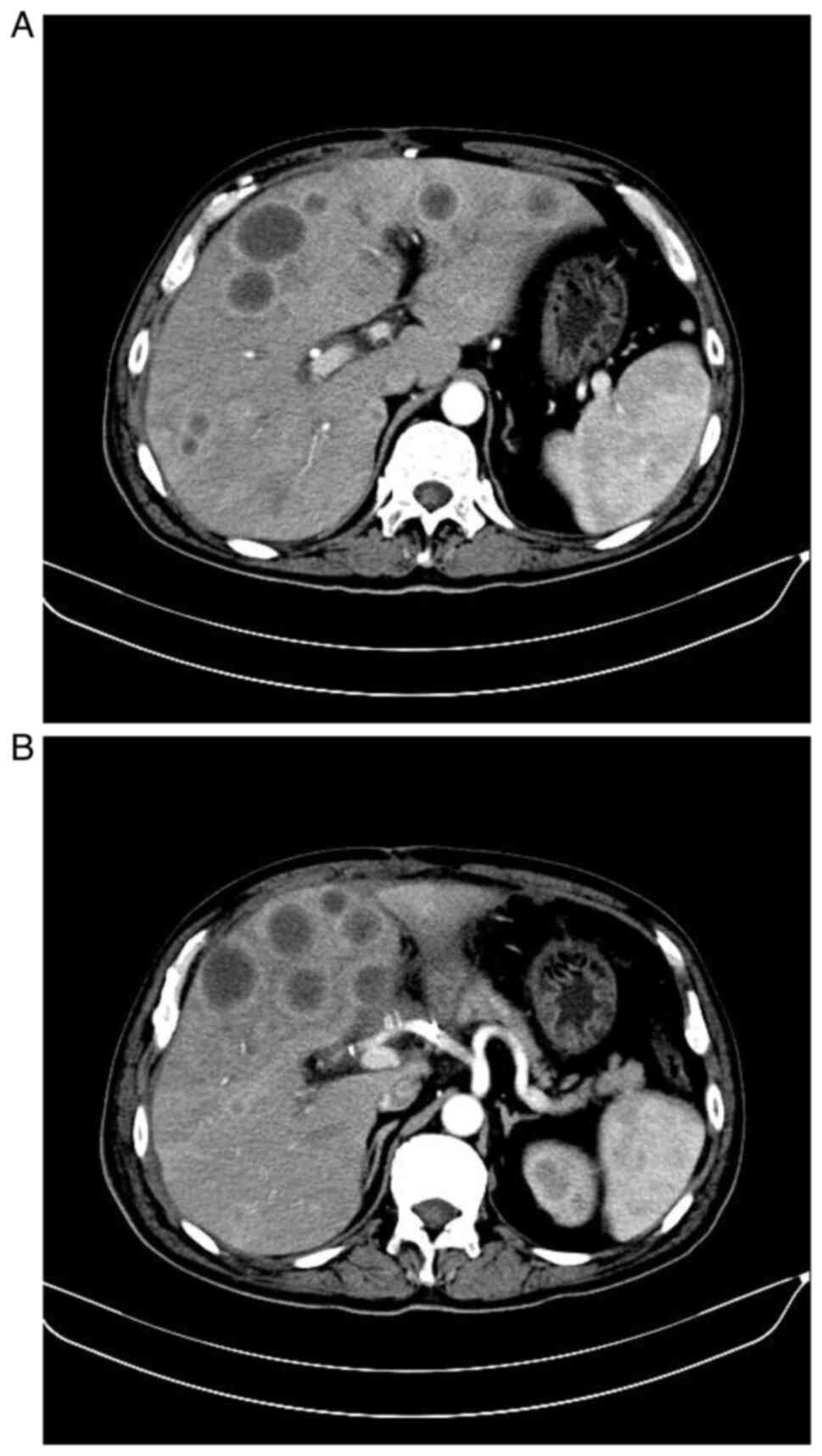
B). An enhanced magnetic resonance imaging (MRI) scan revealed
a soft-tissue mass in the descending duodenum, with dilation of the
intra- and extra-hepatic bile ducts, but without pancreatic duct
dilation. A space-occupying mass was observed in the uncinate
process of the pancreas, as well as a right intrahepatic bile duct
stone (Fig. 2A-F). There was no
evidence of lymphadenopathy or distant metastatic disease.
The patient underwent an R0 Whipple procedure.
Regional lymphatic dissection with a midline incision near the
upper abdomen was used for radical resection of the duodenal
lesion. There was no ascites, liver metastasis or peritoneal
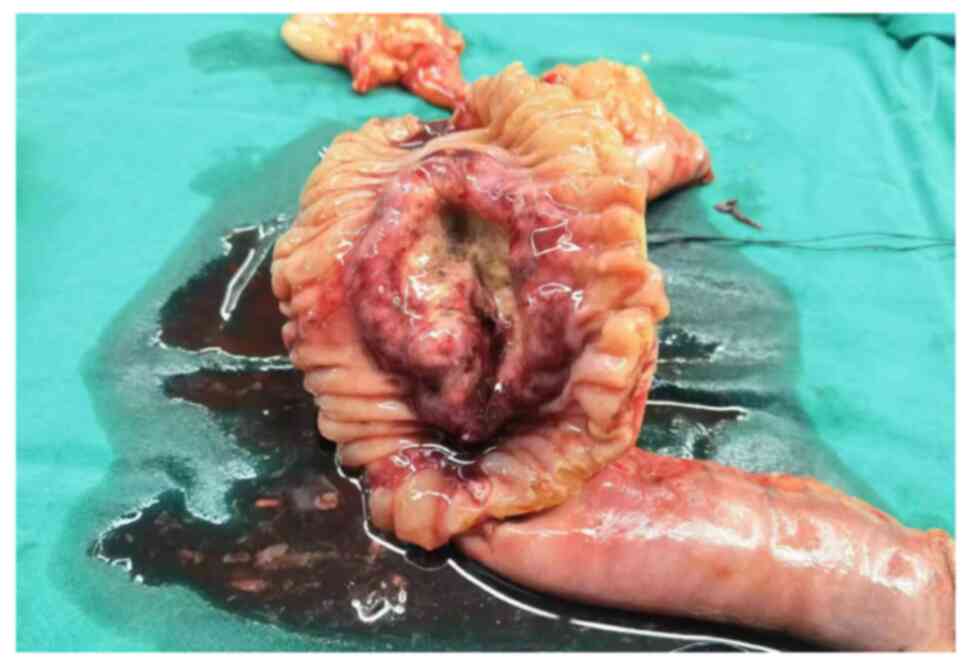
seeding. Fig. 3 shows the
surgically resected specimen. The gross tumor specimen displayed an
ulcerated mass visible at the descendant duodenum that measured
~7.0×6.8×5.5 cm, was greyish in color and had poorly defined
borders, encroaching on the head of the pancreas (Fig. 3). Tumor tissue was immersed in 10%
formalin at room temperature for 24 h for fixation, followed by
dehydration in alcohol. The tissue was then placed in xylene and
embedded in paraffin wax. Cut the wax block into 4 µm slices. The
slices are then subjected to a series of dewaxing and hydration
steps. The nucleus was subjected to hematoxylin staining for 3 min,
while the cytoplasm underwent eosin staining for 1 min at room
temperature. Finally, the prepared slides were observed under a
light microscope (magnification, ×50 and ×100). The pathological
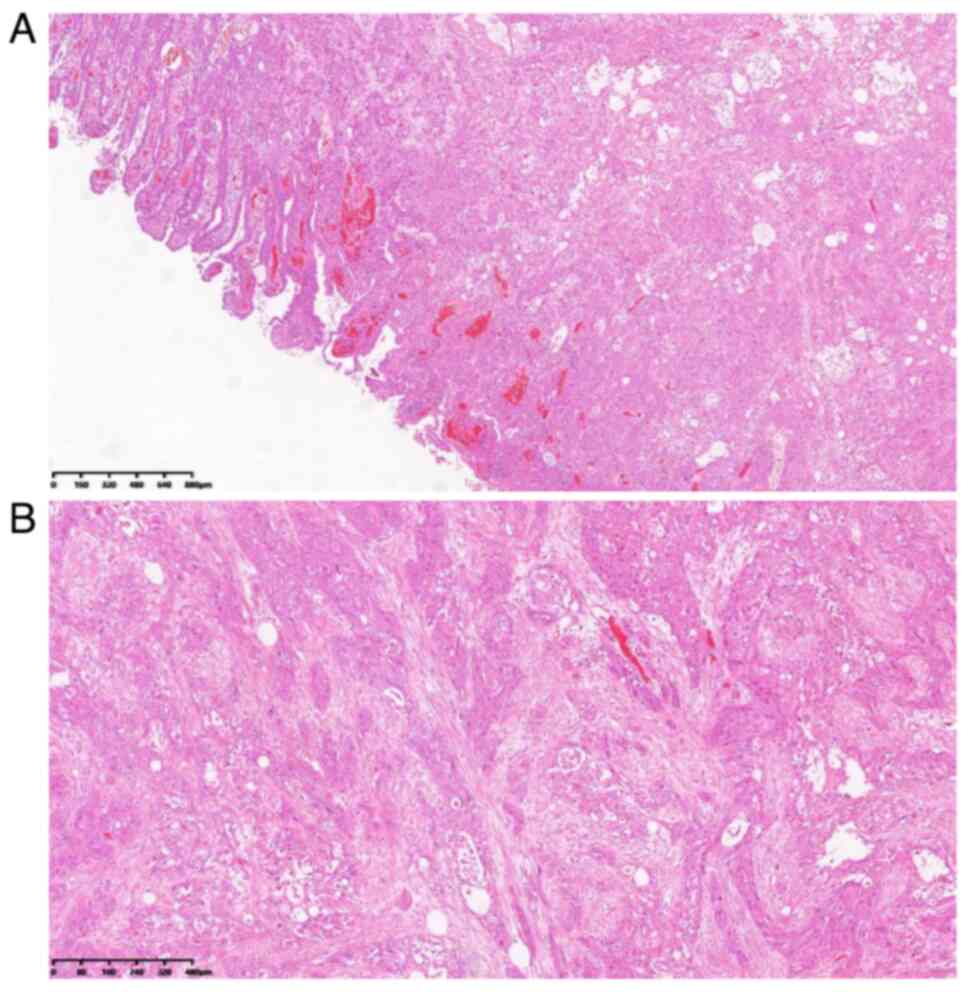
diagnosis was of a primary duodenal ASC invading the pancreas. The
tumor was comprised of two components, namely, poorly
differentiated adenocarcinoma and squamous cell carcinoma. These
two components were mixed without clear borders. The poorly
differentiated adenocarcinoma was comprised of imprinted cells with
cytoplasmic mucin, and the squamous carcinoma consisted of
keratinized polygonal cells arranged in nests (Fig. 4A and B). There were no regional
lymph node metastases or peripheral nerve and vascular infiltrative
lesions. No cancer cells were observed in the duodenal resection
margins, peripancreatic tissue resection margins, common bile duct
resection margins, pancreatic resection margins or gastric
resection margins. Tumor tissues were immersed in 10% formalin at
room temperature for 24 h for fixation, then made into paraffin
sections for immunohistochemical analysis. Tumor paraffin tissue
sections were deparaffinized, rehydrated, sealed with 3% hydrogen
peroxide, and repaired with EDTA for antigen. The slices were then
incubated with primary antibody (1:200) at 4°C overnight. They were
subsequently blocked with 5% bovine serum albumin (A1933, Sigma,
Missouri, United States) for 30 min at 37°C. Then incubated with
100 µl HRP-conjugated rabbit anti-goat IgG polymer (ZSGB-BIO,
Beijing, China, cat. no. PV-9003) at 37°C for 60 min and observed
with diaminobenzidine (DAB). The nuclei were then stained with
hematoxylin at 25°C for 3 min. Images of representative tissues are
captured using a light microscope (magnification, ×100 and ×200).
The supplier and catalog number of the primary antibody used are as
follows: CK7 (ZSGB-BIO, Beijing, China, cat. no. ZA0573), p40
(Maixin, Henan, China, cat. no. RAB0666), p63 (Maixin, Henan,
China, cat. no. MAB0694), CK5/6 (ZSGB-BIO, Beijing, China, cat. no.
ZA0683), CK19 (Maixin, Henan, China, cat. no. Kit0030), p53
(ZSGB-BIO, Beijing, China, cat. no. ZM0408), Ki-67 (ZSGB-BIO,
Beijing, China, cat. no. ZM0166). Immunohistochemical analysis
revealed that the tumor cells diffusely expressed cytokeratin 7
(CK7; Fig. 5A). The squamous
carcinoma cells were positive for tumor protein p40 (p40) (Fig. 5B) and p63 (Fig. 5C), CK5/6 (Fig. 5D), whereas other immunohistochemical
indexes were positive for CK19 (Fig.
5E) and p53 (Fig. 5F), and ~60%
positive for Ki-67 (Fig. 5G and
H).
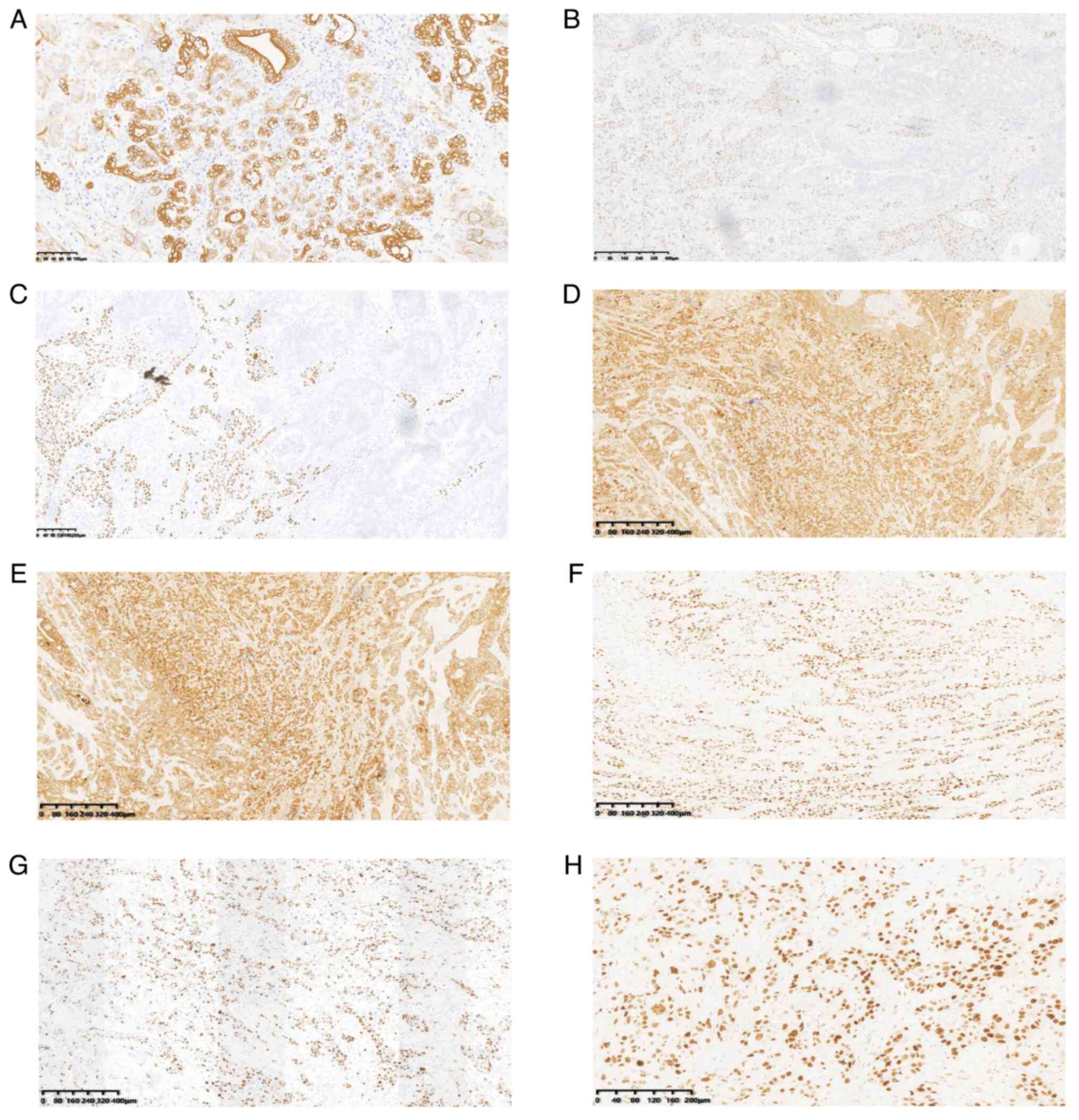 | Figure 5.Immunohistochemical analysis.
Immunohistochemical results showing that (A) the tumor cells
diffusely expressed cytokeratin 7 (scale bar, 100 µm) and that the
squamous carcinoma cells were positive for (B) p40, (C) p63, (D)
CK5/6, (E) CK19, (F) p53 and (G) Ki-67 (scale bar, 400 µm), and for
(H) Ki-67 (scale bar, 200 µm). CK, cytokeratin; p40, tumor protein
p40. |
The patient was postoperatively stable and
discharged from the hospital after 2 weeks. At the 2-month
postoperative follow-up, enhanced computed tomography (CT) scan
revealed multiple metastatic lesions in the liver (Fig. 6A and B). The pathological outcomes
of a liver puncture showed ASC cells, which were diagnosed as
duodenal ASC liver metastasis. The patient succumbed to the disease
at 6 months after surgery.
Literature review
Using ‘duodenum’ and ‘adenosquamous carcinoma’ as
the search terms, secondary adenosquamous carcinoma of duodenum and
adenosquamous carcinoma of ampulare were excluded, and primary
adenosquamous carcinoma of duodenum was excluded. Language
limitations were overcome with language translators. The search
period was limited to January 1, 1980 to January 1, 2024. A
systematic search of the PubMed (pubmed.ncbi.nlm.nih.gov), Embase
(www.embase.com), China National Knowledge
Infrastructure (https://www.cnki.net), WanFang
(http://www.wanfangdata.com.cn) and
J-STAGE databases (http://www.jstage.jst.go.jp/browse) identified 18
cases of primary duodenal ASC. Information was compiled on patient
demographics, clinical features, surgical modalities, radiotherapy
regimens, lymph node and distant metastases, and follow-up.
Table I summarizes the
demographics, clinical features, treatments and outcomes of the
reported duodenal ASC cases.
 | Table I.Clinical characteristics of cases of
primary duodenal adenosquamous carcinoma. |
Table I.
Clinical characteristics of cases of
primary duodenal adenosquamous carcinoma.
| First author,
year | Clinical feature | Age, years | Sex | Operative method | Lymph node
metastasis | Postoperative
radiation or chemotherapy | Other organ
metastasis | Follow-up time,
months; current status | (Refs.) |
|---|
| Takayoshi et
al, 2016 | Epigastric
discomfort | 76 | M | PD | + | Modified Gy FOLFOX6,
30 | Superior vena
cava | 27; dead | (17) |
| Daga and Kerkar,
2016 | Hematochezia | 78 | M | PD | - | - | Pancreas | 3 Alive | (18) |
| de la Cruz et
al, 1993 | Hematemesis,
hematochezia | 84 | F | Local tumor
resection | - | - | - | 0.13 Dead | (19) |
| Sato et al,
1980 | Anemia | 59 | M | PD, right
hemicolectomy | - | - | Colon | NM | (20) |
| Sato et al,
1980 | Jaundice,
anorexia | 49 | F | - | + | - | Liver, ovary | 2 Dead | (20) |
| Nakamura et
al, 1997 | Anemia | 77 | M | PD, right
hemicolectomy | NM | - | Pancreas, colon | 60 Alive | (21) |
| Nakamura et
al, 1997 | Abdominal mass | 75 | F | PD, right
hemicolectomy | - | - | Pancreas,
colon | 6 Dead | (21) |
| Yoneyama et
al, 2006 | Abdominal pain,
vomiting | 64 | F | PD, right
hemicolectomy, partial hepatectomy | - | - | Pancreas, liver,
colon | 6 Dead | (22) |
| Matsumoto et
al, 1990 | Abdominal pain | 45 | M | PD | + | - | - | 60 Alive | (23) |
| Ikematsu et
al, 1988 | Hematemesis,
melena | 81 | M | PD | + | - | Pancreas | NM | (24) |
| Yoshihara et
al, 1983 | Anorexia | 80 | M | - | + | - | Stomach, liver,
gallbladder, colon | 1 Dead | (25) |
| Numaga et
al, 2011 | Vomiting | 71 | M | Partial resection
of the duodenum and jejunum | - | GEM + S-1→
GEM→S-1 | Liver | 10 Dead | (26) |
| Seshimo et
al, 2007 | Hematemesis,
hematochezia | 51 | F | PD, transverse
colectomy | + | UFT | - | 27 Alive | (27) |
| Takagi et
al, 2010 | Anemia | 83 | M | Gastroduodenal bulb
resection | + | - | Liver | 0.77 Dead | (28) |
| Tanaka et
al, 2019 | None | 81 | F | PD | - | + | Liver | 10 Dead | (29) |
| Hammami et
al, 2017 | Vomiting, abdominal
distension, abdominal pain | 64 | F |
Gastrojejunostomy | + | - | Peritoneum | NM | (30) |
| Zheng et al,
2013 | Hematemesis | 25 | F | PD | + | + | Pancreas | NM | (31) |
| Present study | Jaundice, anorexia,
weight loss, clay-colored stool | 55 | M | PD | - | - | Pancreas | 5 Dead |
|
Discussion
Malignant tumors of the small intestine are
extremely rare, accounting for ~3% of all gastrointestinal
malignancies. The most prevalent histological subtypes include
adenocarcinomas, neuroendocrine tumors and gastrointestinal
mesenchymal stromal tumors, while ASC originating in the duodenum
is rare (14). To date, limited
cases have been documented in the English literature. According to
the WHO definition, ASC should be comprised of different numbers of
adenocarcinoma and squamous cell carcinoma components. The boundary
between the two components may be unclear or these two components
may be completely separated. In the present case, no clear boundary
was noted between the adenocarcinoma and squamous cell carcinoma
components. The origin of these tumors remains unclear. Several
theories have been fronted to explain the histopathological
mechanisms of squamous and glandular epithelium within the same
tumor, including i) the presence of pluripotent epithelial stem
cells that can trigger malignant transformation of both cell types,
ii) squamous metaplasia in intestinal mucosa/adenocarcinomas and
iii) collision of the two malignant tumors (15,16).
The present literature review finally identified 18
reported cases (17–31) of primary duodenal ASC (Table I). The median age of reported cases
was 73 years (range, 25–84 years), with a slight male predominance
(55.6%). The present case of a 55-year-old man fits within the
broader demographic profile. Non-specific digestive system
symptoms, as well as symptoms caused by tumor bleeding, were the
most common clinical manifestations of duodenal ASC. Available
reports indicate that duodenal ASC may present with clinical
manifestations such as fever, abdominal pain, vomiting, weight
loss, loss of appetite, hematemesis, hematochezia, jaundice,
bilirubinuria and white clay-like stools. Due to tumor invasion of
the duodenal papilla, the clinical manifestations of obstructive
jaundice and clay-colored stools in the present duodenal ASC case
are consistent with previous report (20).
From the literature review, key findings include the
challenging preoperative diagnosis and the aggressive nature of
duodenal ASC, as evidenced by its rapid metastasis and poor
prognosis. However, imaging is not specific and literature
summarizing the imaging features of duodenal ASC remains
unavailable. The most prevalent imaging presentation in the
available case reports was an ill-defined space-occupying lesion in
the duodenal lumen, with inhomogeneous enhancement of the interior
of the mass on CT or MRI-enhanced scans. Regarding the pathological
diagnosis, the WHO Classification of Tumors of the Digestive
System, 2019, does not restrict the diagnostic ratio of the two
components of ASC, and there is no consensus in the current
literature. This is due to the manual selection of the microscopic
field of view and differences in evaluating the ratio of the two
components. Although preoperative endoscopic biopsy improves the
preoperative diagnostic rate, several reports have misdiagnosed
duodenal ASC as adenocarcinoma or squamous carcinoma preoperatively
due to the inability to obtain both cellular components by puncture
biopsy (17). Thus, the diagnosis
of duodenal ASC currently relies on postoperative pathological
biopsy or autopsy. The commonly used immunohistochemical antibodies
in diagnosing ASC include p63, CK5/6, CK7 and CK8 (32–34).
The hematoxylin and eosin, and immunohistochemistry results in the
present case revealed the presence of both adenocarcinoma and
squamous carcinoma components in the tumor, which is in line with
the histological features of ASC. Meanwhile, immunohistochemical
indicators can be used to aid in diagnosing and predicting
prognosis. For instance, high levels of p63 expression in tumor
tissues have been linked to a poor prognosis in several digestive
cancer types, such as colorectal, gastric and gallbladder cancers
(35–37). Lee et al (38) and Kim et al (39) discovered that a poor prognosis in
gastric ASC may be caused by p53 upregulation and a high Ki-67
index. The immunohistochemical outcomes of the present patient
showed positive results for p63 and p53, and ~60% positivity for
Ki-67, suggesting a poor prognosis.
In terms of treatment, the ideal approach for
duodenal ASC remains unknown, and surgical resection is the
standard clinical treatment. A total of 16 (88.9%) of the 18
patients in the present literature review underwent surgical
therapy. For localized duodenal ASC, pancreaticoduodenectomy was
employed in 12 (75.0%) of the reported surgical cases. However,
guidelines recommending surgical treatment options for ASC are
unavailable. The surgical approach of the present case was
consistent with that of previous studies, using the most commonly
used pancreaticoduodenectomy for tumor removal and obtaining a
clean incisal margin.
The basic principles of surgery include negative
surgical margins, a sufficient extent of lymph node dissection and
a certain number of lymph node pathology biopsies. For advanced
duodenal ASC with distant metastases, palliative surgical
approaches were local tumor resection in 1 case (6.25%), resection
of the duodenum and jejunum in 1 case (6.25%), gastroduodenal bulb
resection in 1 case (6.25%) and gastrojejunostomy in 1 case
(6.25%). Owing to the small number of cases, it remains unclear
whether surgery can prolong patient survival. Patients with ASC are
susceptible to distal metastases and short survival times in the
early postoperative period (16,40).
Treatment interventions remain uncertain. Although surgical
resection is common, a high metastatic potential warrants further
investigation into adjuvant treatments, including radiotherapy and
chemotherapy. A total of 3 cases (16.7%) out of the 18 literature
review cases were treated with adjuvant treatment using
radiotherapy. The known chemotherapy regimens include GEM, S-1, UFT
and modified FOLFOX6 + 30 Gy adjuvant radiotherapy. Takayoshi et
al (17) described a case of
duodenal ASC in which the patient underwent 12 cycles of
chemotherapy with a modified FOLFOX6 regimen after
pancreaticoduodenectomy. The tumor developed distant metastases 5
months after chemotherapy, and resolved after 30 Gy of
radiotherapy, which relieved the systemic symptoms and suppressed
the metastatic tumor growth. Therefore, radiotherapy may be a
critical technique for adjuvant duodenal ASC treatment (17). Postoperative liver metastasis
occurred in the present case and the survival time was short.
Postoperative adjuvant radiotherapy and chemotherapy may have
improved the prognosis in this patient.
ASC is more aggressive than adenocarcinoma or
squamous cell carcinoma alone, and is more likely to develop local
and distant metastases with high malignancy resulting in a poor
prognosis (10–12,41).
Among the reviewed 18 duodenal ASC cases, lymph node metastasis
occurred in 9 (50.0%) cases and other organ metastasis in 15
(83.3%) cases, such as pancreatic invasion in 7 (46.7%) cases and
metastasis to the liver in 6 (40.0%) cases, to the colon in 5
(33.3%) cases, to the superior vena cava in 1 (6.7%) case, to the
stomach in 1 (6.7%) case, to the gallbladder in 1 (6.7%) case, to
the ovary in 1 (6.7%) case and to the peritoneum in 1 (6.7%) case.
Cases with pancreatic invasion and lymphatic vessel invasion have
worse healing (26–28). In the present case, the tumor
invaded the adjacent pancreatic tissue before surgery. Although an
R0 resection was achieved after surgery, the patient developed
liver metastasis in the second postoperative month. This also
suggests a poor postoperative prognosis. The true prognosis of
duodenal ASC should be investigated once more cases have been
reported.
Overall, duodenal ASC can occur in young,
middle-aged and elderly adults. To the best of our knowledge, no
ACS cases have been reported in children. The preoperative
diagnosis is difficult and mainly depends on the postoperative
pathological diagnosis. Duodenal ASC is locally invasive and
metastases to other organs suggest a poor prognosis. At present,
complete surgical resection with clear edges is the preferred
treatment, and a pancreaticoduodenectomy is the preferred surgical
method. However, the benefit to patients who obtain an R0 resection
is unclear. There is no uniform protocol for chemotherapy and
radiotherapy, which is still in the exploratory stage. Further
research is essential to develop standardized treatment protocols
and improve outcomes for patients with this rare malignancy.
However, the conclusions of this review are limited by the small
number of cases and the incomplete clinical information in some
reports. Therefore, there is a need for additional large-scale
multicenter prospective studies of duodenal ASC.
In conclusion, this case highlights the aggressive
nature of primary duodenal ASC and the challenges in its diagnosis
and therapy. Although surgical resection with a negative incisal
margin is the preferred method for managing duodenal ASC, the
effect of surgical treatment on patient prognosis is elusive;
radiotherapy and chemotherapy may be important treatments.
Nevertheless, additional reports are still essential to elucidate
clinical and pathological features, therapeutic strategies and the
prognosis of duodenal ASC to promote the development of
multidisciplinary joint diagnosis and treatment plans.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Wenzhou Basic Scientific
Research Project (grant no. Y20180227) and the Zhejiang Provincial
Medical and Health Technology Plan (grant nos. 2020RC113 and
2022KY1202).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HZ collected and analyzed the data and wrote the
manuscript. HS developed the treatment plan and performed the
surgery for the study case, and participated in the writing of the
manuscript. HY participated in the data collection and analysis. ZM
participated in the design of the study and revised the manuscript.
HZ and ZM confirm the authenticity of all the raw data generated
during the study. HZ and ZM confirmed the authenticity of the data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study involving humans was approved by the
Ethics Committee of Wenzhou Central Hospital (Wenzhou, China;
approval no. 2023-03-035). All procedures were performed following
the Declaration of Helsinki.
Patient consent for publication
The patient and his immediate family provided
written informed consent for the release of all data and
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board, : The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar
|
|
2
|
Benesch MGK, Ramos-Santillan VO, Rog CJ,
Nelson ED and Takabe K: Epidemiology of adenosquamous carcinomas.
World J Oncol. 15:432–453. 2024. View Article : Google Scholar
|
|
3
|
Kim SY, Kim KE, Kim Y and Chung C: A
patient with a lung adenosquamous carcinoma harboring a de novo
T790M mutation and huge nonbacterial vegetative growths
successfully treated with osimertinib: A case report. Thorac
Cancer. 14:1530–1533. 2023. View Article : Google Scholar
|
|
4
|
Bachmeyer C, Canard A, Wendum D and Amiot
X: Recent-onset diabetes mellitus and paraneoplastic hypercalcemia
revealing adenosquamous carcinoma of the pancreas. Am J Med.
136:e157–e158. 2023. View Article : Google Scholar
|
|
5
|
Fang S, Wang X, Wu X and Li H: Therapeutic
response analysis for patients with adenosquamous carcinoma of the
gallbladder: Data analysis based on the surveillance, epidemiology,
and end results (SEER) database. J Gastrointest Oncol. 14:405–419.
2023. View Article : Google Scholar
|
|
6
|
Lewis G, Fong N, Gjeorgjievski SG, Li XB,
Li Z, Wei S, Sturgis CD, Wang C, Komforti M, Zhang H, et al:
Low-grade adenosquamous carcinoma of the breast: A clinical,
morphological and immunohistochemical analysis of 25 patients.
Histopathology. 83:252–263. 2023. View Article : Google Scholar
|
|
7
|
Alsheikh C, Aljammas A, Nashar M, Alissa W
and Aljarad Z: A primary gastric adenosquamous carcinoma: Case
report. Int J Surg Case Rep. 106:1081012023. View Article : Google Scholar
|
|
8
|
Habara K, Nishikori A, Kiyama J, Nakashima
M, Koda M, Sasaki K, Sakashita T, Tanaka N and Yonehara S: A case
of coexistent poorly differentiated adenosquamous carcinoma (glassy
cell carcinoma), usual-type adenocarcinoma, and squamous cell
carcinoma in situ of the cervix. Med Mol Morphol. 56:217–224. 2023.
View Article : Google Scholar
|
|
9
|
Angerilli V, Parente P, Businello G,
Vanoli A, Paudice M, Perrone G, Munari G, Govoni I, Neri G,
Rebellato E, et al: Colorectal adenosquamous carcinoma: Genomic
profiling of a rare histotype of colorectal cancer. Virchows Arch.
482:879–885. 2023. View Article : Google Scholar
|
|
10
|
Boyd CA, Benarroch-Gampel J, Sheffield KM,
Cooksley CD and Riall TS: 415 Patients with adenosquamous carcinoma
of the pancreas: A population-based analysis of prognosis and
survival. J Surg Res. 174:12–19. 2012. View Article : Google Scholar
|
|
11
|
Masoomi H, Ziogas A, Lin BS, Barleben A,
Mills S, Stamos MJ and Zell JA: Population-based evaluation of
adenosquamous carcinoma of the colon and rectum. Dis Colon Rectum.
55:509–514. 2012. View Article : Google Scholar
|
|
12
|
Yendamuri S, Malhotra U, Hennon M, Miller
A, Groman A, Halloon A and Reid ME: Clinical characteristics of
adenosquamous esophageal carcinoma. J Gastrointest Oncol. 8:89–95.
2017. View Article : Google Scholar
|
|
13
|
Hoshimoto S, Aiura K, Shito M, Kakefuda T
and Sugiura H: Adenosquamous carcinoma of the ampulla of Vater: A
case report and literature review. World J Surg Oncol. 13:2872015.
View Article : Google Scholar
|
|
14
|
Symons R, Daly D, Gandy R, Goldstein D and
Aghmesheh M: Progress in the treatment of small intestine cancer.
Curr Treat Options Oncol. 24:241–261. 2023. View Article : Google Scholar
|
|
15
|
Kshirsagar AY, Nangare NR, Vekariya MA,
Gupta V, Pednekar AS, Wader JV and Mahna A: Primary adenosquamous
carcinoma of ampulla of Vater-a rare case report. Int J Surg Case
Rep. 5:393–395. 2014. View Article : Google Scholar
|
|
16
|
Yang SJ, Ooyang CH, Wang SY, Liu YY, Kuo
IM, Liao CH and Wu TJ: Adenosquamous carcinoma of the ampulla of
Vater-a rare disease at unusual location. World J Surg Oncol.
11:1242013. View Article : Google Scholar
|
|
17
|
Takayoshi K, Ariyama H, Tamura S, Yoda S,
Arita T, Yamaguchi T, Ozono K, Yamamoto H, Inadomi K, Kumagai H, et
al: Intraluminal superior vena cava metastasis from adenosquamous
carcinoma of the duodenum: A case report. Oncol Lett. 11:605–609.
2016. View Article : Google Scholar
|
|
18
|
Daga G and Kerkar P: Adenosquamous
carcinoma of the duodenum: A rare entity. Indian J Surg Oncol.
7:470–474. 2016. View Article : Google Scholar
|
|
19
|
de la Cruz A, de la Cruz E, Sanchez MJ,
Ortiz S, Lobato A and Merino E: Adenosquamous carcinoma of the
duodenum. An immunohistochemical study. Pathol Res Pract.
189:481–487. 1993. View Article : Google Scholar
|
|
20
|
Sato A, Hosoya Y, Muto I, Kobayashi N,
Nakadate T, Otaka H, Higuchi Y, Takahashi T, Saito Y, Fukaya H, et
al: Suprapapillary adenosquamous carcinoma of the duodenum, a case
report (author's transl). Nihon Shokakibyo Gakkai Zasshi.
77:623–628. 1980.(In Japanese).
|
|
21
|
Nakamura T, Sano Y, Ohata K, Washiyama N,
Umehara Y and Okubo T: A resected case of adenosquamous carcinoma
of the duodenum. J Jpn Surg Assoc. 30:80–83. 1997.(In
Japanese).
|
|
22
|
Yoneyama K, Toeda H and Ooyama R: A case
of adenosquamous carcinoma of the duodenum. J Jpn Surg Assoc.
67:334–337. 2006.(In Japanese). View Article : Google Scholar
|
|
23
|
Matsumoto K, Koike A, Katou K, Inamura Y,
Suzumura K, Kozima T, Kanemitsu T and Naruse T: A case of
adenosquamous carcinoma arising in the suprapapllary portion of the
duodenum. J Jpn Pract Surg Soc. 51:1275–1278. 1990.
|
|
24
|
Ikematsu Y, Tsukamoto M, Matsuo S, Tomioka
T, Eto T, Yamamoto K, Tsunoda T, Harada N, Tsuchiya R and Shima M:
An operation case of adenosquamous carcinoma of the duodenum. J Jpn
Surg Assoc. 21:885–888. 1988.(In Japanese).
|
|
25
|
Yoshihara W, Furubayashi Y, Mizumoto K,
Tsuzaki K and Kotoh K: Aspiration cytology of suprapapillary
adenosquamous carcinoma of the duodenum, a case report. J Soc Clin
Cytol. 22:80–84. 1983.(In Japanese). View Article : Google Scholar
|
|
26
|
Numaga Y, Ohya T, Takahashi N, Shimizu H,
Tago K, Matsumoto H, Iesato H, Yokomori T, Hasegawa G and Takeyoshi
I: A case of primary adenosquamous carcinoma of the small
intestine. Jpn Soc Gastroenterol Surg. 44:997–1004. 2011.(In
Japanese). View Article : Google Scholar
|
|
27
|
Seshimo I, Tomimaru Y, Ide Y, Maruyama K,
Murata K and Kinuta M: A case of adenosquamous cell carcinoma of
the duodenum with para-aortic node metastasis. J Jpn Surg Assoc.
68:2504–2507. 2007.(In Japanese). View Article : Google Scholar
|
|
28
|
Takagi T, Nakase Y, Fukumoto K, Miyagaki T
and Yanagisawa A: A case of adenosquamous carcinoma of the duodenal
bulb in an aged hemodialysis patient. J Jpn Soc Clin Surg.
71:1789–1794. 2010.(In Japanese).
|
|
29
|
Tanaka H, Miwa S, Inuma N, Kitagawa N,
Ishii K and Sato Y: A case of primary squamous cell carcinoma of
the duodenum definitively diagnosed by p16 immunostaining. J Jpn
Surg Assoc. 80:2023–2027. 2019.(In Japanese). View Article : Google Scholar
|
|
30
|
Hammami MB, Chhaparia A, Piao J, Zhou Y,
Hachem C and Lai J: Mixed adenocarcinoma and squamous cell
carcinoma of duodenum: A case report and review of the literature.
Case Rep Gastroenterol. 11:402–410. 2017. View Article : Google Scholar
|
|
31
|
Zheng H, Shi Y, Zhang L and Chen Y:
Primary adenosquamous carcinoma of duodenum: a clinicopathologic
analysis. J Diag Pathol. 20:626–628. 6332013.(In Chinese).
|
|
32
|
Dong Y, Wang J, Ma H, Zhou H, Lu G and
Zhou X: Primary adenosquamous carcinoma of the colon: Report of
five cases. Surg Today. 39:619–623. 2009. View Article : Google Scholar
|
|
33
|
He YT, Wang XJ, Gong J, Chen N and Zhou Q:
Primary adenosquamous carcinoma of the jejunum. Pathol Int.
55:590–595. 2005. View Article : Google Scholar
|
|
34
|
Ko CJ, Leffell DJ and McNiff JM:
Adenosquamous carcinoma: A report of nine cases with p63 and
cytokeratin 5/6 staining. J Cutan Pathol. 36:448–452. 2009.
View Article : Google Scholar
|
|
35
|
Albasri AM, Elkablawy MA, Ansari IA,
Alhujaily AS and Khalil AA: The prognostic significance of p63
cytoplasmic expression in colorectal cancer. An immunohistochemical
study. Saudi Med J. 40:432–439. 2019. View Article : Google Scholar
|
|
36
|
Song Y, Liu D and He G: TKTL1 and p63 are
biomarkers for the poor prognosis of gastric cancer patients.
Cancer Biomark. 15:591–597. 2015. View Article : Google Scholar
|
|
37
|
Kim K, Kim DH, Chae SW, Shin JH, Kim HJ,
Do SI, Lee HJ, Koo JH, Pyo JS and Sohn JH: Expression of cell
cycle-related proteins, p16, p53 and p63 as important prognostic
markers in gallbladder adenocarcinoma. Pathol Oncol Res.
20:409–415. 2014. View Article : Google Scholar
|
|
38
|
Lee WA, Woo DK, Kim YI and Kim WH: p53,
p16 and RB expression in adenosquamous and squamous cell carcinomas
of the stomach. Pathol Res Pract. 195:747–752. 1999. View Article : Google Scholar
|
|
39
|
Kim YS, Heo WS, Chae KH, Gang YS, Jung JH,
Kim SH, Seong JK, Lee BS, Jeong HY, Song KS, et al:
Clinicopathological features and differences of p53 and Ki-67
expression in adenosquamous and squamous cell carcinomas of the
stomach. Korean J Gastroenterol. 47:425–431. 2006.(In Korean).
|
|
40
|
Song HG, Yoo KS, Ju NR, Park JC, Jung JO,
Shin WG, Moon JH, Kim JP, Kim KO, Park CH, et al: A case of
adenosquamous carcinoma of the papilla of Vater. Korean J
Gastroenterol. 48:132–136. 2006.(In Korean).
|
|
41
|
Ge Y, Lin L, Ma X, Luo D, Shi L, Jiang M,
Fan H, He Y, Yang L and Xu Z: Adenosquamous carcinoma of the
stomach: A population-based study from the SEER database. J Cancer.
10:5705–5713. 2019. View Article : Google Scholar
|