Introduction
Renal carcinoma and bladder cancer are the most
common cancer types among the urinary system tumors, and their
incidence and mortality rates account for a considerable proportion
of the global population with cancer. Statistics on 36 types of
cancer in 185 countries and regions around the world in 2022 showed
that bladder cancer and kidney cancer ranked 9 and 14th
respectively, with incidence rates of 3.1 and 2.2%, respectively
(1). The main subtypes of renal
carcinoma include clear cell carcinoma, chromophobe cell carcinoma
and papillary carcinoma, with clear cell renal cell carcinoma
(ccRCC) accounting for 87.7% of cases in a multicenter study in
Europe and the United States in 2005 (2). However, the latest 2024 National
Comprehensive Cancer Network (NCCN) guidelines indicate that ccRCC
accounts for 70% of renal tumors (3). In the 5th edition of the
classification of urinary and male reproductive organ tumors
released by the World Health Organization in 2022, the
subclassification of type 1/2 papillary RCC was cancelled, and
‘clear cell papillary RCC’ was named ‘clear cell renal cell tumor’
(4).
Initially diagnosed bladder cancer can be roughly
divided into three stages, of which non-muscle invasive bladder
cancer (NMIBC) accounts for 70–75%, MIBC accounts for 20–25%, and
locally advanced or metastatic bladder cancer accounts for 5%
(5,6). In total, ~90% of patients with bladder
cancer are pathologically diagnosed with urothelial carcinoma (UC),
whereas the remaining 10% are diagnosed with sarcoma, squamous cell
carcinoma, adenocarcinoma, neuroendocrine carcinoma or small cell
carcinoma (5,6). UC, also known as transitional cell
carcinoma (TCC), is divided into upper tract UC (UTUC; originating
from the renal pelvis and ureter), bladder UC (bUC; originating
from the bladder and accounting for the largest proportion) and
urethral UC (originating from the urethra) (7).
To the best of our knowledge, although both ccRCC
and bUC are common diseases, their co-occurrence in the same
patient is rare, and the current evidence on this disease is
limited to case reports or small series. The total number of
patients with ccRCC and bUC co-occurrence published in the
worldwide literature is 380 [328 cases in the Surveillance,
Epidemiology and End Results (SEER) database (8) + 17 cases in small series (9) + 35 cases in case reports]. The present
report describes a rare case of concurrent ccRCC and bUC. The
clinical features, treatments and outcomes of this rare disease
have also been summarized based on previously published literature.
To the best of our knowledge, the present study conducted the first
literature review of the multiple primary malignancies associated
with ccRCC and bUC.
Case report
In July 2021, a 65-year-old man was admitted to the
Huanghe Sanmenxia Hospital Affiliated to Henan University of
Science and Technology (Sanmenxia, China) with complaints of
intermittent hematuria for 8 months, accompanied by frequent
urination, urgency, dysuria, and incomplete and interrupted
urination. The patient had no other abnormalities such as
hypertension or heart disease. In 2004, the patient underwent a
cholecystectomy for cholecystitis. The patient had type 2 diabetes
for 6 years, and their blood sugar level was under satisfactory
control using metformin [500 mg once a day (qd)] + glimepiride
tablets (2.5 mg qd). The patient had smoked 10 cigarettes per day
for 40 years. Color ultrasonography of the urinary system revealed
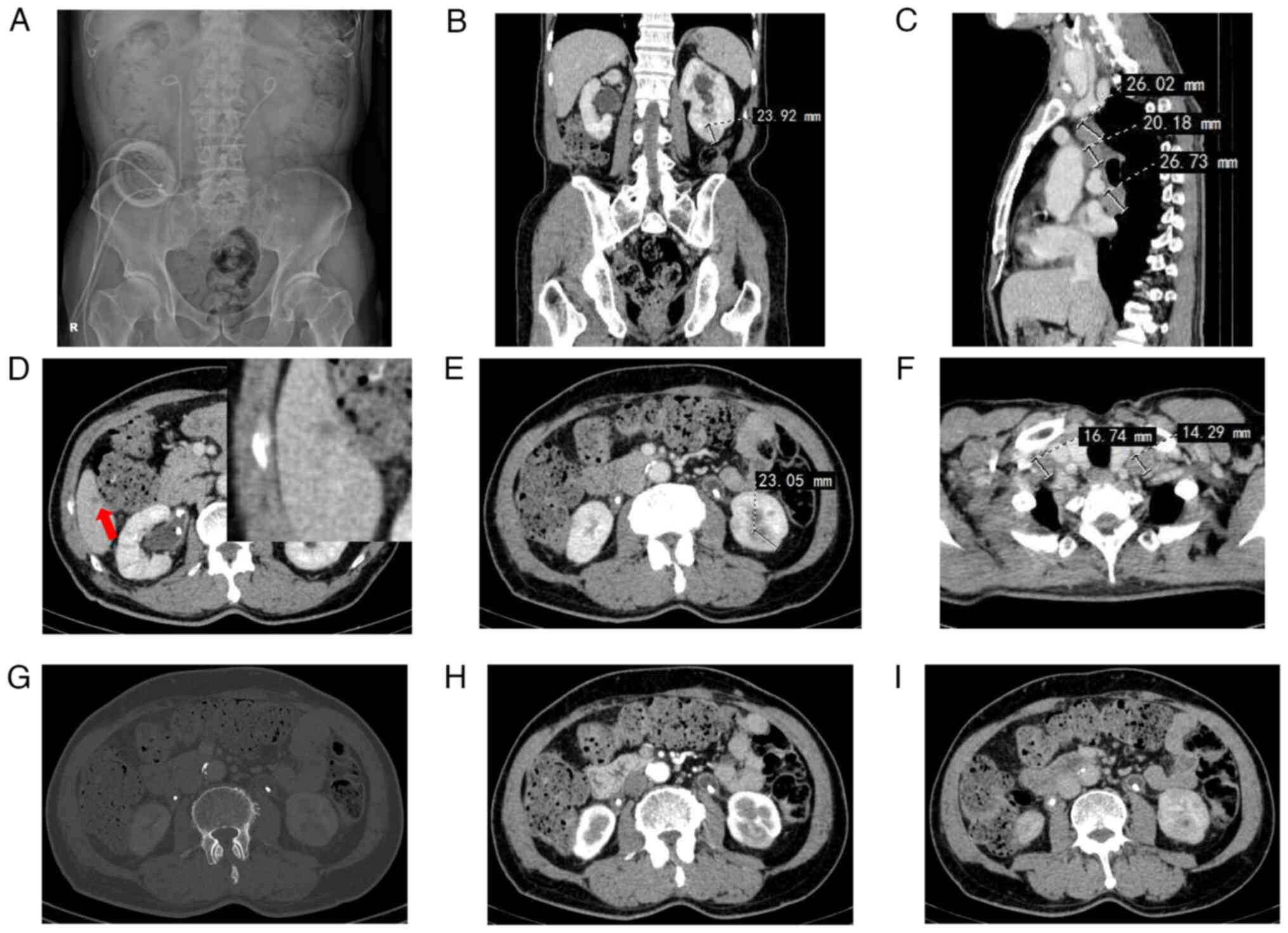
thickening of the bladder wall (Fig.
1A) and hyperechoic right kidney (Fig. 1B). Blood flow signals were observed
in a strongly echogenic area of the right kidney (Fig. 1C). The patient underwent further
urinary system computed tomography urography (CTU) examination to
assist in the assessment of their condition. A total of 1 h before
the examination, the patient drank 500–1,000 ml of water and held
their urine. During the examination, the patient was in a supine
position with both arms raised. After routine scanning using a
dual-source 64-row spiral CT machine (spiral mode; scanning layer
thickness, 5 mm; rotation time, 0.8 sec; pitch, 1.375:1; tube
voltage, 120 kV; Siemens Healthineers), iohexol (300 mg/ml; GE
Healthcare) was injected at a flow rate of 3.0–3.5 ml/sec
(injection volume of ~90 ml), and then arterial phase, venous phase
and excretion phase scanning were performed (tube current 250–300
mA in the arterial phase and venous phase, and 80–150 mA in the
excretion phase). Urinary system CTU showed an oval mass measuring
~20 mm in the upper pole of the right kidney (Fig. 1D and E). The renal mass was
significantly enhanced in the arterial phase, showing a ‘fast-in
and fast-out’ pattern (‘fast-in’ refers to the contrast agent
entering the renal tumor, causing it to appear earlier than the
renal cortex; ‘fast-out’ means that the contrast agent leaves the
renal tumor earlier than it leaves the renal cortex) (Fig. 1D and E). Bladder CTU examination
revealed no obvious abnormal tumors (Fig. 1F).
After 9 days, the patient underwent cystoscopy,
which revealed multiple carpet-like tumor protrusions in the
trigone area, posterior wall, and the left and right walls of the
bladder. The largest mass was ~5×5 mm, located at the ureteral
orifice on the left side of the bladder (data not shown).
Pathological examination after transurethral resection of bladder
tumor (TURBT) revealed high-grade invasive bUC. Firstly, the
resected specimens were stained with hematoxylin and eosin
(H&E). The resected specimens were fixed with 10% formalin,
embedded in paraffin, and cut into 4-µm tissue sections. After the
paraffin sections were dewaxed in xylene I and II for 5 min, they
were incubated in 100, 100, 95 and 85% ethanol solutions for 1 min,
1 min, 30 and 20 sec, respectively, and then rinsed with distilled
water for 30 sec. After staining with hematoxylin (Shandong Chiwell
Biotechnology Co., Ltd.) for 6–10 min, the sections were rinsed
with running water for ~15 min to obtain blue color. Subsequently,
the sections were placed in a 1% hydrochloric acid-ethanol solution
for ~5 sec, and were rinsed with running water for 30 sec,
incubated in 95% ethanol for 1 min for dehydration, counterstained
with eosin stain (Shandong Chiwell Biotechnology Co., Ltd.) for
1.0–1.5 min, and then incubated in 85, 95, 100 and 100% ethanol
solutions for 10, 20, 30 sec and 1 min, respectively, for
dehydration. After excess alcohol was removed, the sections were
soaked in xylene I (20 sec) and II (40 sec) until they became
transparent, and sealed with neutral gum. H&E staining was
performed at room temperature (~25°C). Secondly,
immunohistochemistry was performed on the resected specimens. The
resected specimens were fixed with 10% formaldehyde, embedded in
paraffin, sliced (~4 µm thick), dewaxed and rehydrated as
aforementioned. The sections were rinsed with purified water three
times (5 min each) to remove the ethanol, placed in preheated 0.50
M EDTA buffer (pH 8.0) in a boiling water bath for 20 min, and
naturally cooled down to room temperature. The slides were rinsed
with purified water three times (5 min each) to remove the EDTA
buffer, and subsequently placed in 3% hydrogen peroxide at room
temperature (5 min) to block the endogenous peroxidases. After
washing three times with PBS (pH 7.2–7.4; 5 min each), primary
antibodies from Shanghai Jiehao Biotechnology Co., Ltd., were added
to the sections (diluted in PBS) and incubated at 37°C for 60 min.
The detailed information of the primary antibodies used in the
present study is as follows: Cytokeratin (CK)20 (1:100; cat. no.
CRM-0632); CK5/6 (1:100; cat. no. CM-0531); CK7 (1:100; cat. no.
CM-0541); GATA-3 (1:100; cat. no. GM-0091); p40 (1:100; cat. no.
PRM-0271); p53 (1:100; cat. no. PM-0051); Ki-67 (1:100; cat. no.
KM-0021); α-smooth muscle actin (α-SMA; 1:100; cat. no. AM-0051);
CD117 (1:100; cat. no. CRM-0421); transcription factor E3 (TFE-3;
1:100; cat. no. TRM-0141); CK(Pan) (1:100; cat. no. CM-0641); CD34
(1:100; cat. no. CM-0271); PAX-8 (1:100; cat. no. PRM-0291);
vimentin (1:100; cat. no. VM-0031); epithelial membrane antigen
(EMA; 1:100; cat. no. EM-0041); and carbonic anhydrase 9 (CA-9;
1:100; cat. no. CR-0811). After washing three times with PBS (pH
7.2–7.4; 5 min each), the secondary antibody (anti-mouse/rabbit IgG
peroxidase polymer; 1:20, cat. no. KY-202; Shanghai Jiehao
Biotechnology Co., Ltd.) was added and incubated at room
temperature for 30 min. 3,3′-Diaminobenzidine was used for color
development and hematoxylin was used for counterstaining for 8 min
at room temperature. After ethanol dehydration, the slides were
sealed with resin. The pathological sections were observed using a
light microscope (DM1000; Leica Microsystems, Inc.). The
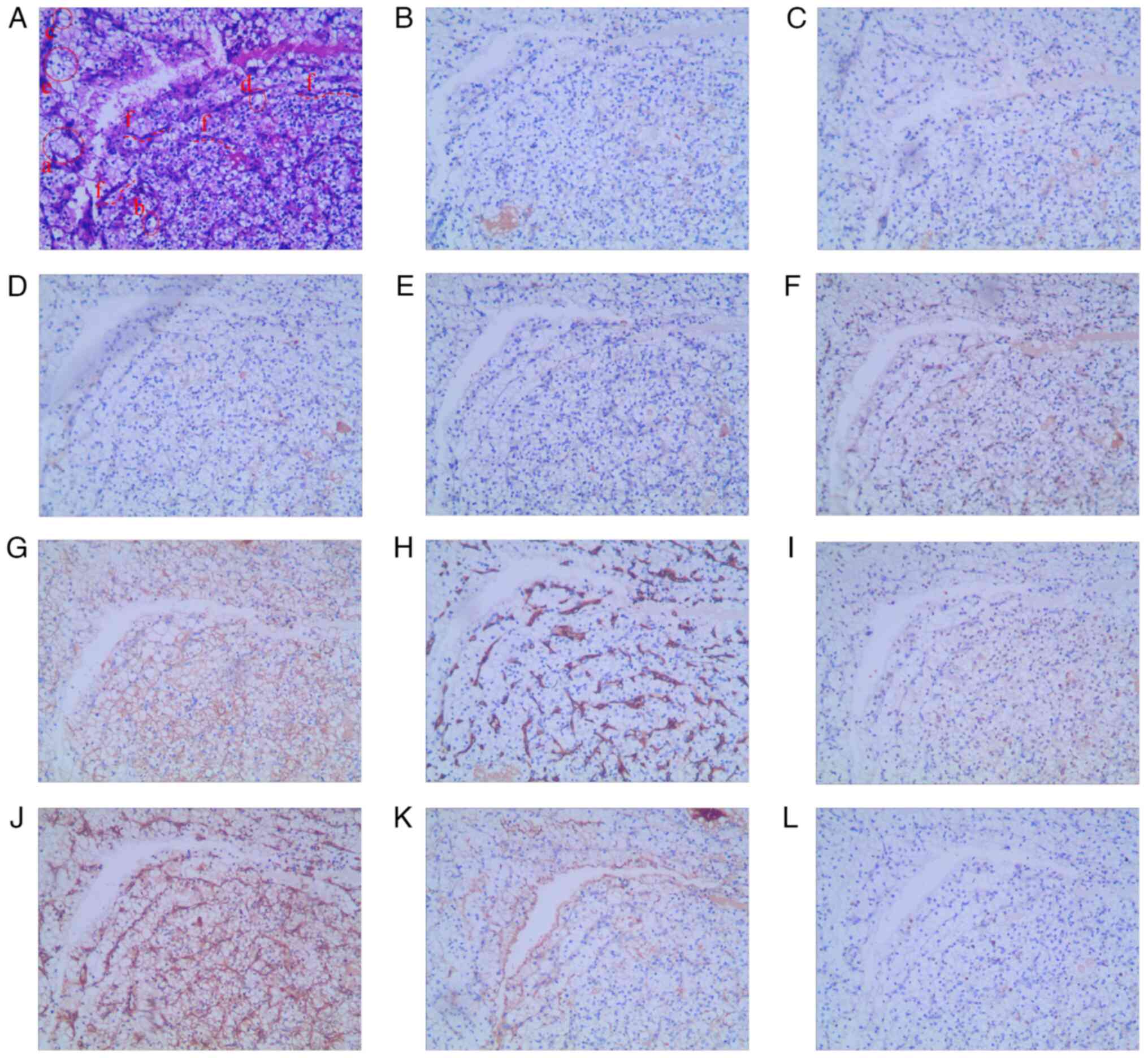
immunohistochemistry results were as follows: CK20(+) (Fig. 2B), CK5/6(−) (Fig. 2C), CK7(+) (Fig. 2D), GATA-3(+) (Fig. 2E), p40(−) (Fig. 2F), p53(+, mutant) (Fig. 2G), Ki-67(+, 30%) (Fig. 2H) and α-SMA(+) (Fig. 2I). H&E results showed high-grade
cancer with cells exhibiting disordered polarity (the long axis of
the nucleus is not perpendicular to the fibrovascular axis; a),
enlarged nuclei (>3 times larger than the normal size; b),
chromatin condensation (c), nipple fusion (d) and low intercellular
connectivity (e) (Fig. 2A). SMA is
part of the microfilament system of cytoskeletal proteins and is
present in the smooth muscle, muscularis and muscularis propria of
the vascular wall (10). The
present immunohistochemical results showed a α-SMA(+) phenotype
(Fig. 2I); therefore, this was
considered to be a muscle-invasive carcinoma. CK20, CK7 and GATA-3
are markers for the joint diagnosis of UC. The pathological results
were all positive (Fig. 2B, D and
E); therefore, a diagnosis of UC was made. TP53 is a tumor
suppressor gene that is mutated in half of all human cancers
(11). p53 expression is
upregulated in urothelial carcinoma tissue compared with normal
urothelial tissue, and high p53 expression predicts shorter
survival in patients with non-muscle-invasive bladder cancer, but
is not associated with prognosis in the muscle-invasive group
(12). The present result was
p53(+, mutant), which further confirmed the diagnosis of UC. Since
the specimen was bladder tissue, a diagnosis of bUC was considered.
Other possible diagnoses were excluded as follows: i) Squamous cell
carcinoma was excluded, as there was no urothelial component,
predominantly basal cell or clear cell features (if a urothelial
component is present, UC with squamous differentiation can be
considered); and ii) adenocarcinoma was excluded, as there was no
urothelial differentiation with true glandular elements
(mucus-secreting ductules or intestinal glands) in the tumor (if
adenocarcinoma is mixed with UC, UC with glandular differentiation
is considered) (13). If there was
no metastasis, combined with the patient's good condition at the
time and the presence of a right kidney tumor, radical cystectomy
and partial right nephrectomy could be performed simultaneously.
The patient was recommended to undergo a whole-body single-photon
emission computed tomography (SPECT) examination to determine
whether there was metastasis. However, the patient refused to
undergo a total cystectomy and therefore requested a partial right
nephrectomy only.
 | Figure 2.(A) Hematoxylin and eosin staining
indicating that the tumor is bladder urothelial carcinoma. Tumor
cells are (B) CK20(+), (C) CK5/6(−), (D) CK7(+), (E) GATA-3(+), (F)
p40(−), (G) p53(+, mutant), (H) Ki-67(+, 30%) and (I) α-smooth
muscle actin (+). Magnification, ×100. CK, cytokeratin. |
A total of 9 days later, a laparoscopic right
partial nephrectomy was performed to remove the right renal mass
completely. Pathological examination of the specimen revealed a
ccRCC in the right kidney with clean resection margins. The
immunohistochemistry results were as follows: CK20(−) (Fig. 3B), CK7(−) (Fig. 3C), GATA-3(−) (Fig. 3D), CD117(−) (Fig. 3E), TFE-3(−) (Fig. 3F), CK(+) (Fig. 3G), CD34(+) (Fig. 3H), PAX-8(+) (Fig. 3I), vimentin(+) (Fig. 3J), EMA(focal +) (Fig. 3K) and Ki-67(+, <1%) (Fig. 3L). H&E results revealed ccRCC
with tumor cells arranged in nests and alveoli (a; Fig. 3A). The tumor cells were round (b) or
polygonal (c) and relatively large, with transparent cytoplasm (d).
The nuclei were centrally located (e), round and uniform in size,
and the interstitium contained a network of small, thin-walled
sinusoidal vessels (f; Fig. 3A).
CD34 immunohistochemical images showed typical ‘chicken cage-like’
changes (grid-like changes in blood vessels; Fig. 3H). The immunohistochemical results
of CK20 and GATA-3 were negative thus excluding UC (14), the results for CD117 and CK7 were
negative thus excluding chromophobe cell carcinoma (15), and the PAX-8 result was positive, as
found in both renal tumors (16)
and UC (17). EMA is expressed in
several tumors, therefore its diagnostic value is not high and it
needs to be used in combination with specific epithelial
differentiation markers (18);
however, the aforementioned immunohistochemical results showed that
CK and vimentin were strongly expressed in renal tubular epithelial
cells, suggesting renal carcinoma. No tumor cells were found on the
pathological section resection margins; therefore, the resection
margins were considered clean. Other possible diagnoses were
excluded as follows: i) In chromophobe RCC, the tumor cell membrane
is thick and clear, the cytoplasm is rich, the nuclei are shrunken,
the interstitial blood vessels are mostly thick-walled, and CD117
and CK7 are diffusely positive (15); and ii) in clear cell papillary RCC,
the tumor cell cytoplasm is clear, the cell nuclei are arranged
linearly away from the basement membrane, there are various growth
patterns, such as cystic, tubular, papillary and solid, and CK7 is
diffusely positive (19). According
to the TNM system of the American Joint Committee on Cancer (AJCC)
(20) and the clinical staging of
renal cancer (21), the
pathological stage was T1a. However, confirmation of the presence
or absence of lymph node metastasis and distant metastasis requires
further whole-body SPECT examination. The patient refused SPECT and
requested to discuss with their family whether to undergo radical
cystectomy.
 | Figure 3.(A) Hematoxylin and eosin staining
indicating that the tumor is renal cell carcinoma. Tumor cells are
(B) CK20(−), (C) CK7(−), (D) GATA-3(−), (E) CD117(−), (F)
transcription factor E3(−), (G) CK(+), (H) CD34(+), (I) PAX-8(+),
(J) vimentin(+), (K) epithelial membrane antigen (focal +) and (L)
Ki-67(+, <1%). Magnification, ×100. CK, cytokeratin. |
After surgery (laparoscopic right partial
nephrectomy), the patient received regular intravesical
instillations of epirubicin (50 mg per week for 2 months). At 2
months after the surgery, the patient developed an intermittent
fever (maximum temperature, 39.1°C) and urinary tract infections
caused by Klebsiella pneumoniae and Pantoea
agglomerans (bacterial detection was performed using the MA-120
microbial identification and drug sensitivity analysis system;
Zhuhai Meihua Medical Technology Co., Ltd.). The patient was then
treated with sulbactam [0.5 g; every 12 h (q12 h)], cefoperazone (1
g; q12 h) and nitrofurantoin (50 mg; three times a day) for 5 days.
The patient finally agreed to undergo a radical cystectomy. A SPECT
scan was performed on the patient in September 2021. The patient
was intravenously injected with 25 mCi of technetium (99mTc)
methylenediphosphonate injection contrast agent 2–4 h before the
examination. After emptying their bladder, the patient laid flat on
the SPECT scanning bed (Symbia T16 SPECT/CT; Siemens Healthineers)
and underwent whole-body bone imaging, cervical and pelvic SPECT,
and CT enhanced scanning. The following SPECT parameters were used:
Acquisition angle, 6°/frame; acquisition time, 15 sec/frame;
matrix, 128×128; and rotation, 360°. The CT scanning parameters
were as follows: Tube voltage, 110 kV; tube current, 160 mA; and
slice thickness, 1.5 mm. Image fusion was performed using the
dedicated software Syngo MI VA70A (Syngo MI Applications VA70A;
Siemens AG) for the image post-processing workstation. SPECT showed
postoperative changes without distant or lymph node metastasis
(Fig. 1G-K). According to the AJCC
TNM system and clinical staging (21), the pathological stage of ccRCC was
pT1aN0M0 and the clinical stage was I, with the right renal tumor
being ~2 cm in size.
Combined with the results of the SPECT examination
and previous bladder pathological examination, according to the TNM
system of the AJCC and the clinical staging of bladder cancer
(22), the pathological stage of
bladder cancer was T2 and the clinical stage was II. However, the
specific pathological staging needs to be determined by
pathological examination after radical cystectomy. According to the
guidelines for the diagnosis and treatment of bladder cancer
(22), radical cystectomy is
recommended for T2 MIBC without lymph node metastasis.
In October 2021, a laparoscopic radical resection of
the bladder, prostate, seminal vesicle and distal ureter, a pelvic
lymph node dissection and bilateral cutaneous ureterostomies were
performed. The pathological results of H&E staining performed
according to the aforementioned method revealed high-grade invasive
bUC that had infiltrated the entire bladder (Fig. S1A). The bilateral seminal vesicles
(Fig. S1B and C), upper bladder
resection margin (Fig. S1D), lymph
nodes (Fig. S1E and F), junction
of the prostate and bladder (Fig.
S1G), vas deferens (Fig. S1H and
I), prostate (Fig. S1J) and
ureters (Fig. S1K and L) were not
invaded. Bladder cancer was evaluated according to the AJCC TNM and
aforementioned clinical staging (22), and it was confirmed that the
pathological stage of bladder cancer was pT2N0M0 and the clinical
stage was II. The pathological results of this case showed that the
tumor invaded the entire bladder layer (Fig. S1A), but did not invade the
perivesical fat tissue (Fig. S1D),
and no distant metastasis was found.
After the diagnosis of dual cancer, it was
recommended that the patient and their family undergo genetic
testing to exclude, or prevent in advance, any gene-related
diseases. However, the patient and their family refused for
financial reasons, and as some patients do not believe in the
concept of preventing diseases before their occurrence.
Postoperative chemotherapy and regular follow-up
were recommended according to the postoperative pathology findings
suggestive of MIBC. However, the patient refused chemotherapy and
regular review, and only underwent bilateral ureteral single J tube
replacement in the outpatient clinic every 3 months. Abdominal
X-ray showed bilateral ureteral single J tubes exiting from the
right abdominal wall (Fig. 4A). In
September 2022, the patient visited the Outpatient Department of
the Huanghe Sanmenxia Hospital Affiliated to Henan University of
Science and Technology for the treatment of hoarseness, cough while
drinking water and dyspnea. Physical examination revealed multiple
swollen lymph nodes in the neck and armpits on both sides, the
largest of which was ~20 mm and was located at level IV of the
right side of the neck. SPECT examination results in September 2022
showed no tumor recurrence in the right kidney and pelvis, and a
19×24×23-mm mass was observed in the lower pole of the left kidney
(Fig. 4B and E); multiple swollen
lymph nodes were observed in the bilateral neck (Fig. 4F), mediastinum (Fig. 4C), axilla and portal vein (data not
shown), considering the possibility of metastasis; and nodules with
a diameter of ~5 mm were observed in the S6 segment of the liver
(Fig. 4D), indicating a potential
metastasis.
A right neck lymph node biopsy was performed in
October 2022. Lymph node pathology results suggested metastatic UC.
The immunohistochemistry results were as follows: CK5/6(+)
(Fig. 5B), CK7(+) (Fig. 5C), GATA-3(+) (Fig. 5D), PAX-8(+) (Fig. 5E), Ki-67(+, 80%) (Fig. 5F), p40(focal +) (Fig. 5G), CA-9(−) (Fig. 5H) and CK20(−) (Fig. 5I). H&E staining images showed
tumor cell infiltration (Fig. 5A).
Positive expression of PAX-8 indicates that the tumor originates
from the kidney or urothelial tissue (Fig. 5E). CA-9 is a specific tumor marker
for metastatic renal cancer, and negative expression excludes renal
cancer metastasis (Fig. 5H)
(23). p40 positivity indicates
squamous cell cancer, but in this case the expression was focal and
had no practical significance (Fig.
5G) (24). CK5/6, CK7, GATA-3
and CK20 are all epithelial tissue tumor markers, which are used
together for the diagnosis of UC, among which the relative
specificity of GATA-3 is 86% (14,25).
In this case, the pathological results showed that CK5/6 (Fig. 5B), CK7 (Fig. 5C) and GATA-3 (Fig. 5D) were all positive, and CK20
(Fig. 5I) was negative; therefore,
the lymph node tissue was invaded with metastatic bUC. Bladder
cancer was evaluated based on the AJCC TNM and clinical staging.
The patient had distant lymph node metastasis, the pathological
stage was M1, and the clinical stage was IV.
It was recommended that the patient undergo a liver
and left kidney biopsy to clarify the pathological type and assist
in further diagnosis and treatment. The patient rejected these
suggestions as they already had multiple tumors and had been
confirmed with UC with lymph node metastasis. Regardless of the
results of the pathological examination of the liver and left
kidney, the patient was already in the late stage of cancer;
therefore, they did not wish to experience further pain from biopsy
punctures, surgery, radiotherapy and chemotherapy. The only
treatment they would accept was oral medication to relieve the
current pain.
Combined with the patient's clinical data, empirical
analysis suggested that the left renal mass may be a recurrence of
ccRCC. Firstly, Fig. 4G shows
bilateral renal SPECT scans. SPECT examination results showed that
the tumor was unevenly enhanced during the cortical phase (Fig. 4H); the tumor disappeared rapidly
during the excretory phase, with a density lower than that of
normal renal parenchyma, and the tumor was observed to compress the
renal pelvis (Fig. 4I). Secondly,
the ‘Guidelines for the Diagnosis and Treatment of Urological and
Men's Diseases in China (2022 Edition)’ (26) mentioned that metastatic renal cancer
caused by other cancers is relatively rare, and its clinical
characteristics are bilateral renal metastasis and multiple
metastatic lesions. The patient only had a single tumor in the
lower pole of the left kidney, which did not meet the clinical
characteristics of renal metastatic carcinoma, and they had a
history of right-sided ccRCC; therefore, it was more likely that
the left kidney may be a recurrence of ccRCC.
For liver tumors, since both bUC and ccRCC can
metastasize to the liver, it is impossible to clearly determine
their pathological type. According to the AJCC bladder cancer
staging (22), the following
assumptions can be made: If the liver metastasis comes from UC, the
pathological stage is M1b and the clinical stage is IVB; if the
metastasis comes from ccRCC, the pathological stage is M1a and the
clinical stage is IVA. Therefore, the patient was diagnosed with
metastatic UC, with pathological stage M1 and clinical stage IV.
The patient and their family refused further radiotherapy and
chemotherapy, as they believed that the side effects of
chemotherapy and radiotherapy would outweigh the therapeutic
effects to the disease itself.
Sunitinib is a multi-targeted receptor tyrosine
kinase inhibitor (TKI) that inhibits tumor cell proliferation and
antitumor angiogenesis by acting on targets such as vascular
endothelial growth factor receptors 1–3 (VEGFR1-3), c-KIT,
platelet-derived growth factor receptor-α and -β and FMS-like
tyrosine kinase 3 (27). Both the
United States Food and Drug Administration (FDA) (21) and the Chinese FDA (28) have approved sunitinib for the
treatment of metastatic RCC. Tislelizumab, a programmed cell death
protein 1 (PD-1) inhibitor originally developed in China, is
specifically designed to minimize Fcγ receptor binding of anti-PD-1
antibodies, thereby limiting antibody-dependent phagocytosis.
Tislelizumab has higher affinity than pembrolizumab or nivolizumab,
all of which provide a potential mechanism of resistance to
anti-PD-1 therapies (29).
Tislelizumab was approved by the China National Medical Products
Administration for the treatment of locally advanced or metastatic
UC (30). The antitumor mechanisms
of pembrolizumab, nivolizumab and tislelizumab are similar. The
present patient had both ccRCC and bUC, and there is currently no
unified guideline for this type of patient. The use of tislelizumab
+ sunitinib is empirically recommended to treat patients, aiming to
maximize the benefits to patients through dual antitumor
mechanisms. The reason for choosing tislelizumab instead of
pembrolizumab or nivolizumab is that the target affinity of
tislelizumab is 35–60 times higher than that of pembrolizumab or
nivolizumab (31).
After communicating with the patient and their
family, a regimen of tislelizumab (200 mg once every 3 weeks;
BeiGene, Ltd.) + sunitinib (50 mg/day; administered for 4 weeks
with a 2-week interval) was adopted to control disease progression
for 2 months. In January 2023, the patient died of tumor
metastasis, and no autopsy was performed.
Discussion
From the establishment of the databases to May 19,
2024, a comprehensive literature search was conducted in the China
National Knowledge Infrastructure database (https://www.cnki.net/) and Wanfang Data (https://wanfangdata.com.cn/) using ‘clear cell renal
cell carcinoma’ and ‘bladder urothelial carcinoma’ as Chinese
search terms. In addition, relevant English studies were searched
in Web of Science (webofscience.com) and PubMed (https://pubmed.ncbi.nlm.nih.gov) using the search
terms ‘clear cell renal cell carcinoma’ and ‘bladder urothelial
carcinoma’ from the inception of the databases to May 19, 2024. The
initial search identified 3,656 studies. The inclusion criteria
were as follows: i) Case reports or case series of ccRCC combined
with bUC or TCC; ii) all included cases had clear pathological
types; iii) the included studies had available full texts; and iv)
the included studies presented patient information, such as sex,
age, smoking, previous history, diagnosis time of renal cancer and
bUC, and follow-up status. The exclusion criteria were as follows:
i) Irrelevant studies; ii) unable to download full text; and iii)
incomplete patient information. Two researchers independently
screened the studies according to the inclusion criteria and a
third researcher was involved in the decision-making process for
study inclusion. When reading the references of the 31 included
studies, three further studies met the inclusion criteria, and
ultimately 34 studies were included in the literature review. There
were 7 articles in Chinese (to ensure high quality of evidence,
only core journals were included, because core journals usually
have stricter review processes, more authentic information and
greater academic influence), 11 articles in Japanese (indexed by
PubMed or Web of Science) and 16 articles in English. Japanese
articles could be read using Google Translate (https://translate.google.com). The literature
screening process is illustrated in Fig. 6.
 | Figure 6.Steps in the literature search. From
the establishment of the database to May 19, 2024, relevant studies
were searched in CNKI, WANFANG, WOS and PubMed databases using
‘clear cell renal cell carcinoma’ and ‘bladder urothelial
carcinoma’ as search terms, and 3,656 studies were initially
retrieved (CNKI, 1,180; WANFANG, 42; PubMed, 2,284; WOS, 150).
After screening the inclusion criteria, 34 studies were obtained,
and after excluding three duplicate studies, 31 studies were
obtained. When reading the 31 studies, three studies that met the
inclusion criteria were found in the references. Finally, 34
studies were included for review, of which one study contained 2
patients, and a total of 35 patients were included. CNKI, China
National Knowledge Infrastructure; WOS, Web of Science. |
The relevant survival analyses were performed using
the data extracted in Table SI.
For patients with multiple primary cancers associated with ccRCC
and bUC, the Kaplan-Meier method in GraphPad Prism 5 software
(Dotmatics) was used to evaluate the effects of the tumor onset
time interval and ccRCC and bUC pathological stage on survival, and
the log-rank test was used to test the differences between the
survival curves. P<0.05 was considered to indicate a
statistically significant difference.
A total of 35 patients were included in 34 articles,
and together with the present case, a cohort of 36 patients
diagnosed with ccRCC complicated by bUC was included in the
literature review. The clinical characteristics of all the cases
reviewed in the literature are presented in Tables SI and SII. The median age at first cancer
diagnosis was 56.5 years (range, 31–82 years). Most cases occurred
in males, with a male-to-female ratio of 6:1. A total of 16 (44.4%)
patients had a history of smoking. However, only 11.1% (4 cases) of
the patients had a family history of cancer. Regardless of whether
renal or bladder cancer is the first type of cancer, the first
symptom in most patients is hematuria, which may be intermittent,
continuous, macroscopic or microscopic. A small number of patients
(11.1%) have symptoms of prostatitis, such as difficulty in
urination, frequent urination, urgency and incomplete urination.
Few patients with renal cancer (5.6%) as the primary cancer
experience lower back or hip pain. A total of 9 patients had dual
primary cancers of the urinary system (including ccRCC and bUC)
(32–39). A total of 11 patients were diagnosed
with triple primary cancers of the urinary tract, including
prostate cancer, ccRCC and bUC (40–50).
The remaining 16 patients also had other types of cancer, such as
von Hippel-Lindau disease (51),
uterine cancer (52), renal UC
(53), renal pelvic cancer
(54), ureteral UC (55), renal pelvic and ureteral TCC
(32,56,57),
hepatic carcinoma (58), metastatic
clear cell carcinoma (59), tubular
cystic RCC (60), ureteral inverted
papilloma cancer (61), esophageal
cancer (62), ureteral
fibroepithelial polyps (63),
neuroendocrine tumor (64), rectal
adenocarcinoma (65), and
metastatic UC (present case).
In published reports on multiple primary urinary
tract cancers, ccRCC was mainly in stages T1 (32,34–38,40,45–49,
54,56,60,62)
(present case) and T2 (33,39,41,44,51–55,57,64,65),
accounting for 47.2 and 33.3% of the cases, respectively. A total
of 20 patients (55.6%) underwent total (33,36,37,39,40,43,45,51,53,58,59) or
partial (44,48,49,52,54,60,62,63)
(present case) nephrectomy as the primary treatment, and 8 patients
underwent nephroureterectomy (41,54,57) or
nephroureter and partial cystectomy (32,34,47,56,61)
according to their clinical stage and condition. A total of 3
patients with concurrent bladder cancer underwent
nephroureterectomy and cystectomy simultaneously (43,46,53).
To prevent recurrence or progression of renal cancer after surgery,
certain patients receive chemotherapy with fluorouracil (54), and others use immunosuppressants,
such as interferon (41,42,44),
interleukin-2 (44), nivolumab
(49), ipilimumab (49), tislelizumab and sunitinib (present
case). A patient who had previously been receiving hemodialysis for
renal failure was found to have bladder, kidney and ureteral tumors
and continued to receive hemodialysis treatment after surgery
(57).
Among the multiple primary urinary tract cancers in
the present review, the pathological stages of bUC were mainly T1
(32–35,38–42,44–46,48,49,51,53,54,57,59,63,64)
and T2 (37,38,43,47,60,65)
(present case), accounting for 58.3 and 19.4% of cases,
respectively, including 1 case (52) in the T3 stage and 1 case (55) in the T4 stage. The main surgical
methods for bUC were TURBT (63.9%) (32–35,38–44,48–51,54,56–59,61,62)
and radical cystectomy (30.6%) (36,45,46,52,53,60),
among which 13.9% of the patients underwent radical cystectomy
after being diagnosed with bladder cancer by TURBT (37,47,64,65)
(present case). Intravesical chemotherapy (35,37,38,41,50,55,61,62,65)
and Bacillus Calmette-Guérin (39)
therapy were the main treatments after TURBT.
Based on the different onset times of ccRCC and
bUC-related multiple cancers, they can be divided into synchronous
multiple primary malignant neoplasms (SMPMN; ≤6 months) and
metachronous MPMN (MMPMN; >6 months). In the present review, 20
patients had SMPMN (34,35,37–39,42,43,45–47,49–51,53,54,57,59,60,64)
(present case) and 16 patients had MMPMN (32,33,36,38,40,41,44,48,52,55,56,58,61–63,65).
The median survival or follow-up time since the last cancer
diagnosis was 20 and 48 months in the SMPMN and MMPMN groups,
respectively.
For patients with multiple primary cancers
associated with ccRCC and bUC, the tumor onset time interval
(P=0.533; Fig. 7A) and ccRCC
pathological stage (P=0.455; Fig.
7B) had no significant effect on the survival rate; however,
the bUC pathological stage had a significant effect on the survival
rate (P=0.021; Fig. 7C).
Bladder cancer is currently the 9th most common
malignant tumor worldwide (1).
According to statistics in 2022, there were ~614,000 new cases of
bladder cancer and 220,000 deaths worldwide (1). The incidence and mortality rates in
men were higher than those in women (1). Bladder cancer is the sixth most common
cancer in men and the ninth leading cause of cancer death (1). bUC accounts for >90% of all bladder
cancer cases (5). Smoking is the
most common risk factor, while other risk factors include
occupational factors, such as exposure to paint, rubber, petroleum,
dyes and coal, and iatrogenic factors, such as pelvic radiotherapy,
chemotherapy, long-term indwelling catheterization, chronic
inflammatory stimulation and schistosomiasis infection, associated
with geographical or socioeconomic reasons (6,66). The
main clinical manifestation in most patients is painless gross
hematuria; however, if lower urinary tract symptoms are present,
such as frequency, urgency and difficulty in urination, bladder
cancer should be suspected (6,22,67).
Since the advent of cystoscopy, TURBT has been the
main method for the initial diagnosis and staging of bladder
cancer, and is also the main method for the treatment of NMIBC
(6,22).
The current standard surgical approach for MIBC is
radical cystectomy combined with urinary diversion (68). With advancements in technology, the
surgical methods of radical cystectomy have evolved from
traditional open surgery to laparoscopic or robotic surgery. Khan
et al (69) compared the
prognosis of patients with bladder cancer who underwent three
surgical procedures (open radical cystectomy, laparoscopic radical
cystectomy and robotic-assisted radical cystectomy) and found no
difference in 90-day complication rates among the three surgeries
according to the Clavien-Dindo complication classification system
(70).
Griffiths et al (71) confirmed that neoadjuvant chemo-
therapy with cisplatin, methotrexate and vinblastine is beneficial
for the treatment of MIBC. This view is also supported by the
results of a systematic review and meta-analysis of neoadjuvant
chemotherapy for invasive bladder cancer (72). Currently, the main treatments for
metastatic UC include chemotherapy, such as gemcitabine combined
with cisplatin (73,74), immune checkpoint inhibitors, such as
avelumab (75) and pembrolizumab
(76), and radiotherapy; however,
radiotherapy alone has no obvious benefit on overall survival
(22).
For patients who are unable or unwilling to undergo
radical cystectomy and who clearly understand the risks and need
for careful follow-up, the American Urological Association
guidelines recommend ‘trimodal therapy’, which is maximal TURBT
combined with chemoradiotherapy (77).
New guidelines from the FDA and the European
Society for Medical Oncology recommend enfortumab vedotin plus
pembrolizumab as the first-line standard treatment for advanced UC,
with an advantage shown in terms of patient survival compared with
chemotherapy (78,79). Nivolumab plus gemcitabine-cisplatin
or platinum plus avelumab are alternative treatments (78).
The prognosis and treatment of bUC mainly depends
on histopathology and TNM staging. The degree of muscle layer
invasion determines whether a patient is staged as pT1 (NMIBC) or
pT2 (MIBC). The 5-year overall survival rates of pT1, pT2 and pT3
bUC are 75, 50 and 20%, respectively (22,67).
According to the recommendations of domestic
(26) and international (22) guidelines, the patient in the present
report was first diagnosed with bladder cancer through TURBT, and
then underwent radical cystectomy combined with urinary diversion.
Regular follow-up and treatment were not performed after the
operation, which eventually led to the occurrence of metastatic UC.
Tislelizumab was administered to treat metastatic bladder cancer,
but the patient ultimately had a poor prognosis.
Renal cancer is a growing disease with an estimated
400,000 new cases per year worldwide and an annual mortality rate
of nearly 175,000 individuals (80); it accounts for ~3% of adult
malignancies (81). ccRCC is the
predominant and aggressive histological subtype of adult renal
cancer. The NCCN guidelines state that ~85% of renal tumors are
RCC, of which ~70% are ccRCC (3).
However, Wilms' tumors account for 90% of childhood renal cancer
cases (80).
High blood pressure (80,82),
smoking (80,83,84),
high body mass index, obesity (80), alcohol consumption, lack of exercise
and multiple births in women (84)
are risk factors for renal cancer.
Most cases of renal cancer are discovered
incidentally during abdominal ultrasonography or CT scans. Only a
minority (<10%) of patients present with the classic triad of
kidney cancer, which includes hematuria, flank pain and a palpable
abdominal mass (85). The main
treatments for localized renal cancer include partial nephrectomy,
total nephrectomy and tumor ablation (85,86).
Patients with metastatic renal cancer, which is usually not
treatable with surgery, may benefit from immune checkpoint and
targeted protease inhibitors (85).
Since 2005, protein kinase inhibitors have been used to treat
metastatic RCC by targeting growth factor receptors, such as
fibroblast growth factor receptor, MET oncogene and VEGFR. Since
2015, PD-1 inhibitors (nivolumab and ipilimumab) have been used to
inhibit renal cancer cells. Currently, the standard treatment for
most patients with metastatic renal cancer is a combination of
immune checkpoint inhibitors and protein TKIs. For patients who
cannot tolerate immunosuppressants, single-drug therapy can be used
(85,86).
A total of three studies (41,42,44)
included in the present review reported the use of interferon and
IL-2 alone or in combination for postoperative adjuvant therapy of
RCC, all of which were published before 2010. However, it was
subsequently confirmed that there was no clinical benefit in the
use of interferon-α and/or IL-2 for RCC above stage T2 (87).
Routine preoperative renal mass biopsy of small
renal masses is not recommended by the current guidelines (26). The main reasons include inaccurate
biopsy results, complications of renal mass biopsies and needle
tract implantation (88), which
ultimately lead to a high rate of unnecessary nephrectomy/partial
nephrectomy for benign renal tumors (18–26%) (89). Gao et al (88) reported that a preoperative renal
mass biopsy could reduce the rate of unnecessary partial/radical
nephrectomy to 3%.
A partial nephrectomy was performed on the right
renal tumor of the present patient in accordance with the
guidelines (26). During follow-up,
the right renal tumor did not recur, but a mass appeared in the
left kidney. After evaluation, it was suspected that the mass in
the left kidney could be a recurrence of renal cancer. Sunitinib
was administered for treatment, but the patient eventually died of
multiple advanced tumors.
The presence of two or more histologically
different malignancies in the same individual is known as MPMN. In
1932, Warren (90) proposed
diagnostic criteria for MPMN, namely that each tumor must occur in
a different site or organ, have its own pathological morphology and
be histologically malignant, and that metastasis or recurrence is
excluded. According to the literature, the incidence of multiple
primary neoplasms in patients with cancer is 2.4–8.0% and can be as
high as 17.0% at 20 years of follow-up. The main factors causing
their occurrence include the host (genetics, hormones and previous
cancer history) and the environment (tobacco and alcohol
consumption, geography, pathogens and occupation) (91).
Gul et al (92) showed that the incidence of RCC and
bladder cancer is higher in men compared with that in women, and
that the higher smoking rate in men is partly responsible for the
difference in incidence between men and women; however, hormones,
genetics and differences in gene mutation patterns between men and
women also play a role. Based on the SEER database, Wu et al
(8) studied the clinical and
pathological characteristics of 704 patients with RCC and UC, 566
of whom had bUC, with a male-to-female ratio of 4.66:1. The results
showed that the risk of co-occurrence of RCC and UC increased in
older (>65 years old), male and Caucasian populations. The
results of the present review showed that the median age at onset
of ccRCC- and bUC-related multiple primary cancers was 56.5 years,
the incidence rate in males was six times (30:5) higher than that
in females, the incidence rate in smoker was 2.67 times (16:6)
higher than that in non-smokers and only 1 female patient was
diagnosed with this disease among all smokers. Therefore, we
hypothesized that male sex and smoking may be risk factors for the
co-occurrence of ccRCC and bUC. This is consistent with previously
published results (92). However,
only 11.1% of the patients with cancer had a family history of
cancer. Therefore, whether a family history of cancer is a risk
factor remains to be verified. The patient in the present report
was male with a history of smoking and was diagnosed with two types
of cancer, which is consistent with previous reports (32,37–39).
The patient had no family history of cancer; therefore, whether the
patient had a genetic mutation needs to be considered. However, the
patient and their family refused genetic testing.
The clinical manifestations of ccRCC- and
bUC-related multiple malignancies are similar to those of single
ccRCC and bUC. Most patients present with hematuria, which may be
accompanied by lower urinary tract irritation or lower back pain.
The main clinical manifestation of the current case was hematuria,
accompanied by urinary tract irritation symptoms, and no lower back
pain.
Wu et al (8)
showed that the proportions of pathological stage I/II for RCC and
UC were 81.1 and 93.3%, respectively. Most patients with RCC
underwent a total or partial nephrectomy, whereas 87.1% of patients
with bUC underwent a partial resection and only 1.4% underwent a
local tumor resection. In the present review, 80.5% of patients
with ccRCC and 77.7% of patients with bUC were in stages T1 and T2;
therefore, the current results show that the proportion of renal
cancer in the T1/2 stage was consistent with the results reported
by Wu et al (8), while the
proportion of bladder cancer in the T1/2 stage was different.
However, this result may have a certain publication bias, as most
patients in the early stage can undergo surgery to obtain clear
pathological data, whereas most patients in the late stage do not
have the opportunity for surgery (since patients with advanced
cancer often have metastasis to distant or surrounding tissues,
which usually indicates a poor prognosis) and cannot obtain clear
pathological data; therefore, patients in the late stage may be
omitted from publications. In the present review, 27.8% of patients
underwent nephrectomy, 27.8% underwent partial nephrectomy, 27.8%
underwent nephrectomy and/or ureterectomy and/or cystectomy, and
only 1 patient underwent renal biopsy for RCC, there was no
difference in the proportion of surgical approach for RCC, but for
bUC, 63.9% of patients underwent TURBT, 16.7% underwent radical
cystectomy and 13.9% underwent radical cystectomy after TURBT. The
SEER database only includes cancer data from the United States. The
data collected in the present review were from studies performed in
China, the United States, Japan, France and other countries, which
were mainly case reports or small studies. Differences in the
economic, regional and medical levels may explain the differences
in the surgical methods used. The patient in the present report
underwent partial nephrectomy and radical cystectomy in stages.
Initially, the patient was recommended to undergo a whole-body
SPECT examination to evaluate their systemic condition. If there
was no metastasis to other organs or lymph nodes, a partial
nephrectomy and a radical cystectomy would be performed at the same
time. However, partial nephrectomy combined with radical cystectomy
is more difficult. Because it involves multiple organs of the human
body (kidneys, ureters, bladder and prostate) at the same time, it
requires superb surgical skills. The surgery time involving
multiple organs at the same time is longer than the surgery time
for a single organ, and it is also a great challenge for the
postoperative recovery of the patient.
Qi et al (9)
followed up 27 patients with combined RCC and UC, 17 of whom had
both renal cancer and bladder tumors. The study showed no
difference in survival between patients who underwent partial
nephrectomy and total nephrectomy (9). However, detailed information on the
patients of the study by Qi et al (9) could not be obtained; therefore, these
patients were not included in the present review. Wu et al
(8) demonstrated that the
co-occurrence of bladder cancer and RCC was not a risk factor for
survival outcomes in RCC. Previous studies have suggested that
individuals with papillary RCC have an increased risk of
subsequently developing bladder (93,94) or
prostate (94) cancer, while
another study did not confirm this association (95). The present review demonstrated that
the survival rate of ccRCC- and bUC-related multiple primary tumors
was not associated with the tumor onset time interval (P=0.533) or
the pathological stage of ccRCC (P=0.455), but may be related to
the pathological stage of bUC (P=0.021), which is inconsistent with
the conclusion by Wu et al (8). The present literature review results
showed that the median survival time for ccRCC- and bUC-related
multiple primary tumors was 47.5 months, with a median survival of
20 months for SMPMN and 48 months for MMPMN. The follow-up of the
present case lasted only 19 months from onset to death, which is
consistent with the median survival time of 20 months drawn from
the literature review. Due to the low incidence of the disease,
more studies are needed in the future to clarify whether the
occurrence of bladder cancer or RCC affects the prognosis of
patients with ccRCC- and bUC-related multiple primary tumors.
Some cases of multiple primary malignancies
associated with gene deletions or mutations are called gene-related
syndromes, such as Lynch syndrome. Lynch syndrome, also known as
hereditary non-polyposis colorectal cancer, is an autosomal
dominant disorder caused by germline mutations in one of the four
mismatch repair genes or the EpCAM gene. The clinical
manifestations of the syndrome include UTUC (2–20%), colorectal
cancer (30–73%), endometrial cancer (30–51%) and other
extraintestinal tumors (96).
According to Lynch syndrome diagnosis and treatment guidelines,
renal and bladder cancers do not belong to Lynch syndrome (97). Results of a systematic review and
meta-analysis showed that Lynch syndrome was associated with a
significantly increased relative risk of renal and bladder cancer,
although the quality of the evidence was assessed as ‘low’
(98). Among the reports included
in the present literature review, six involved UTUC or rectal
adenocarcinoma in addition to bUC and ccRCC (53–57,65).
Therefore, it is important to perform genetic testing in patients
with both bUC- and ccRCC-related primary multiple cancers to
determine whether they have Lynch syndrome. Although the patient in
the present report rejected genetic testing, it is believed that
genetic testing is important for further studying this disease.
Currently, there are no uniform clinical guidelines
or pieces of prospective experimental evidence for the treatment of
patients with multiple primary neoplasms, and most cases are
managed based on previous case reports (91). The treatment of ccRCC- and
bUC-related multiple primary malignant tumors requires the
formulation of a surgical plan that maximizes the patient's own
interests based on the actual condition of the patient and the
determination of further diagnostic and treatment measures, such as
radiotherapy, chemotherapy, targeted therapy or combined therapy,
according to changes in the patient's condition.
The present study has certain limitations. First,
due to insufficient information from the included literature, it
was not possible to analyze whether alcohol consumption,
hypertension, diabetes and aristolochic acid exposure history were
risk factors for the comorbidity of ccRCC and bUC. Second, certain
patients included in the literature review had other cancers
besides ccRCC and bUC, such as prostate cancer, UTUC, esophageal
cancer and rectal cancer, which may be factors leading to the
occurrence of other primary cancers. Third, the present study
focused on ccRCC and bUC, and did not include UTUC. According to
the study by Wu et al (99),
the most common secondary tumors of UTUC in Taiwan are RCC and
hepatocellular carcinoma. Patients with UTUC and a history of
cancer have a higher risk of developing other primary malignancies
(99). Fourth, due to the low
incidence of the co-occurrence of bUC and ccRCC, only 36 patients
were included for analysis. In the future, a multicenter joint
study on this type of patients with multiple primary cancers needs
to be conducted to gain a deeper understanding of the disease.
Fifth, dual and multiple cancers may be related to gene mutations.
Although none of the included cases underwent genetic testing, the
importance of genetic testing in identifying multiple cancers
cannot be excluded. Last, coexistence of RCC and bUC may be related
to Lynch syndrome, but genetic testing has not been performed to
verify this, and more cases are needed in the future to supplement
the present study.
In summary, ccRCC and bUC comorbidity is a rare
phenomenon, and there are no clear epidemiological, therapeutic and
prognostic characteristics. Previous studies have reported the
comorbidity of ccRCC and bUC; however, these studies were limited
by the lack of detailed case data or small sample sizes. The
present study comprehensively summarized the typical
characteristics of ccRCC- and bUC-related multiple primary
malignancies. In conclusion, the risk factors for ccRCC- and
bUC-related multiple primary malignancies are male sex and smoking,
and whether a family history of tumors is a risk factor remains to
be confirmed. The median age at the onset of ccRCC- and bUC-related
multiple primary malignancies was 56 years, and the median survival
was 47.5 months (SMPMN, 20 months; MMPMN, 48 months). The
cumulative survival rate is not related to the interval between
tumor onset and the pathological stage of ccRCC, but may be related
to the pathological stage of bUC. In the present case, the patient
had both ccRCC and bUC, namely SMPMN. After surgery, the patient
recovered well but eventually developed cervical lymph node
metastasis with a UC origin, suggesting that the pathological stage
of bladder cancer is crucial for prognosis. The overall survival
rate from disease onset to death was 19 months.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are
included in the figures and/or tables of this article.
Authors' contributions
SW, YZ and KW conceived and designed the present
study, and collected the data for the case report. SW, XW and XY
analyzed and interpreted the data. MY obtained pathological data.
SW wrote the manuscript, and all authors discussed the results and
commented on the manuscript. CW made substantial contributions to
the conception and design of the study. SW and CW confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Huanghe Sanmenxia Hospital Affiliated to Henan
University of Science and Technology (Sanmenxia, China; approval
no. 202402190018). The patient's wife provided initial written
informed consent to participate in the treatment.
Patient consent for publication
Written informed consent for publication of the
clinical details and images was obtained from the patient's
wife.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar
|
|
2
|
Patard JJ, Leray E, Rioux-Leclercq N,
Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani
W, Abbou CC, et al: Prognostic value of histologic subtypes in
renal cell carcinoma: A multicenter experience. J Clin Oncol.
23:2763–2771. 2005. View Article : Google Scholar
|
|
3
|
Motzer RJ, Jonasch E, Agarwal N, Alva A,
Bagshaw H, Baine M, Beckermann K, Carlo MI, Choueiri TK, Costello
BA, et al: NCCN guidelines® insights: Kidney cancer,
version 2.2024. J Natl Compr Canc Netw. 22:4–16. 2024. View Article : Google Scholar
|
|
4
|
Mohanty SK, Lobo A and Cheng L: The 2022
revision of the World Health Organization classification of tumors
of the urinary system and male genital organs: Advances and
challenges. Hum Pathol. 136:123–143. 2023. View Article : Google Scholar
|
|
5
|
Jones RJ, Crabb SJ, Linch M, Birtle AJ,
Mcgrane J, Enting D, Stevenson R, Liu K, Kularatne B and Hussain
SA: Systemic anticancer therapy for urothelial carcinoma: UK
oncologists' perspective. Br J Cancer. 130:897–907. 2024.
View Article : Google Scholar
|
|
6
|
Lopez-Beltran A, Cookson MS, Guercio BJ
and Cheng L: Advances in diagnosis and treatment of bladder cancer.
BMJ. 384:e0767432024. View Article : Google Scholar
|
|
7
|
Rouprêt M, Seisen T, Birtle AJ, Capoun O,
Compérat EM, Dominguez-Escrig JL, Andersson IG, Liedberg F,
Mariappan P, Mostafid AH, et al: European association of urology
guidelines on upper urinary tract urothelial carcinoma: 2023
Update. Eur Urol. 84:49–64. 2023. View Article : Google Scholar
|
|
8
|
Wu K, Liu X, Wang Y, Wang X and Li X:
Clinicopathological characteristics and outcomes of synchronous
renal cell carcinoma and urothelial carcinoma: A population-based
analysis. Front Public Health. 10:9943512022. View Article : Google Scholar
|
|
9
|
Qi N, Chen Y, Gong K and Li H: Concurrent
renal cell carcinoma and urothelial carcinoma: Long-term follow-up
study of 27 cases. World J Surg Oncol. 16:162018. View Article : Google Scholar
|
|
10
|
Council L and Hameed O: Differential
expression of immunohistochemical markers in bladder smooth muscle
and myofibroblasts, and the potential utility of desmin,
smoothelin, and vimentin in staging of bladder carcinoma. Mod
Pathol. 22:639–650. 2009. View Article : Google Scholar
|
|
11
|
Olivier M, Hollstein M and Hainaut P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2:a0010082010. View Article : Google Scholar
|
|
12
|
Borowczak J, Szczerbowski K, Maniewski M,
Zdrenka M, Słupski P, Andrusewicz H, Bysik-Miśkurka J, Rutkiewicz
P, Bodnar M and Szylberg A: The prognostic role of p53 and its
correlation with CDK9 in urothelial carcinoma. Clin Transl Oncol.
25:830–840. 2023. View Article : Google Scholar
|
|
13
|
Vlachou E, Johnson BR, Baraban E, Nadal R
and Hoffman-Censits J: Current advances in the management of
nonurothelial subtypes of bladder cancer. Am Soc Clin Oncol Educ
Book. 44:e4386402024. View Article : Google Scholar
|
|
14
|
Naik M, Rao BV, Challa S, Fonseca D, Sudha
SM, Giridhar A, Sharma R, Raju KVVN and Rao TS: Utility of GATA-3
and associated immunohistochemical markers in the differential
diagnosis of poorly differentiated urothelial carcinoma. J Cancer
Res Ther. 19 (Suppl):S02023. View Article : Google Scholar
|
|
15
|
Zhou J, Yang X, Zhou L, Zhang P and Wang
C: Combined immunohistochemistry for the ‘three 7’ markers (CK7,
CD117, and claudin-7) is useful in the diagnosis of chromophobe
renal cell carcinoma and for the exclusion of mimics: Diagnostic
experience from a single institution. Dis Markers.
2019:47081542019. View Article : Google Scholar
|
|
16
|
Barr ML, Jilaveanu LB, Camp RL, Adeniran
AJ, Kluger HM and Shuch B: PAX-8 expression in renal tumours and
distant sites: A useful marker of primary and metastatic renal cell
carcinoma? J Clin Pathol. 68:12–17. 2014. View Article : Google Scholar
|
|
17
|
Gorbokon N, Baltruschat S, Lennartz M,
Luebke AM, Höflmayer D, Kluth M, Hube-Magg C, Hinsch A, Fraune C,
Lebok P, et al: PAX8 expression in cancerous and non-neoplastic
tissue: A tissue microarray study on more than 17,000 tumors from
149 different tumor entities. Virchows Arch. 485:491–507. 2024.
View Article : Google Scholar
|
|
18
|
Leroy X, Copin MC, Devisme L, Buisine MP,
Aubert JP, Gosselin B and Porchet N: Expression of human mucin
genes in normal kidney and renal cell carcinoma. Histopathology.
40:450–457. 2002. View Article : Google Scholar
|
|
19
|
Yan WX, Cao WR, Zhao J, Zhang W, Wang XL,
Yuan Q and Dang SQ: Clear cell papillary renal cell carcinoma: A
clinicopathologic analysis of 6 cases. Int J Clin Exp Pathol.
8:4595–4599. 2015.
|
|
20
|
Amin MB, Edge SB, Greene FL and Brierley
JD: AJCC cancer staging manual. 8th edition. New York: Springer;
2017
|
|
21
|
Pandey J and Syed W: Renal cancer.
StatPearls [Internet] Treasure Island (FL): StatPearls Publishing;
2024
|
|
22
|
Kaseb H, Leslie SW, Soon-Sutton TL and
Aeddula NR: Bladder cancer. StatPearls Treasure Island: StatPearls
Publishing; 2023
|
|
23
|
Kim HL, Seligson D, Liu X, Janzen N, Bui
MHT, Yu H, Shi T, Belldegrun AS, Horvath S and Figlin RA: Using
tumor markers to predict the survival of patients with metastatic
renal cell carcinoma. J Urol. 173:1496–1501. 2005. View Article : Google Scholar
|
|
24
|
Gailey MP and Bellizzi AM:
Immunohistochemistry for the novel markers glypican 3, PAX8, and
p40 (ΔNp63) in squamous cell and urothelial carcinoma. Am J Clin
Pathol. 140:872–880. 2013. View Article : Google Scholar
|
|
25
|
Queipo FJ, Unamunzaga GM, Negro BF,
Fuertes SG, Cortés MÁ, Tejedor EC, Mañas CMB, Ariño AB, Sjödahl G
and Beorlegui C: Immunohistochemistry subtyping of urothelial
carcinoma is feasible in the daily practice. Virchows Arch.
481:191–200. 2022. View Article : Google Scholar
|
|
26
|
Huang J and Zhang X: Chinese guidelines
for diagnosis and treatment of urology and andrology diseases (2022
edition). 2022.
|
|
27
|
Motzer RJ, Michaelson MD, Redman BG, Hudes
GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE,
et al: Activity of SU11248, a multitargeted inhibitor of vascular
endothelial growth factor receptor and platelet-derived growth
factor receptor, in patients with metastatic renal cell carcinoma.
J Clin Oncol. 24:16–24. 2006. View Article : Google Scholar
|
|
28
|
Guo J, Ma J, Sun Y, Qin S, Ye D, Zhou F,
He Z, Sheng X, Bi F, Cao D, et al: Chinese guidelines on the
management of renal cell carcinoma (2015 edition). Chin Clin Oncol.
5:122016. View Article : Google Scholar
|
|
29
|
Ye D, Liu J, Zhou A, Zou Q, Li H, Fu C, Hu
H, Huang J, Zhu S, Jin J, et al: Tislelizumab in Asian patients
with previously treated locally advanced or metastatic urothelial
carcinoma. Cancer Sci. 112:305–313. 2021. View Article : Google Scholar
|
|
30
|
Liu Y, Liu Q, Huang L, Ren X, Huang J and
Feng YF: A programmed death receptor-1 inhibitor-Tislelizumab. Clin
Med J. 20:37–42. 2022.(In Chinese).
|
|
31
|
Hong Y, Feng Y, Sun H, Zhang B, Wu H, Zhu
Q, Li Y, Zhang T, Zhang Y, Cui X, et al: Tislelizumab uniquely
binds to the CC' loop of PD-1 with slow-dissociated rate and
complete PD-L1 blockage. FEBS Open Bio. 11:782–792. 2021.
View Article : Google Scholar
|
|
32
|
Yokoyama I, Berman E, Rickert RR and
Bastidas J: Simultaneous occurrence of renal cell adenocarcinoma
and urothelial carcinoma of the renal pelvis in the same kidney
diagnosed by preoperative angiography. Cancer. 48:2762–2766. 1981.
View Article : Google Scholar
|
|
33
|
Oka H, Kobayashi S, Kobayashi T, Shugino
Y, Matsui Y, Fujikawa K, Iwamura H, Hukuzawa S, Soeda A and
Takeuchi H: Multiple primary cancers limited to the urological
field. Hinyokika Kiyo. 47:405–409. 2001.(In Japanese).
|
|
34
|
Srinath N, Dinesh US and Sharma GP: A case
of synchronous renal cell carcinoma (Rt) and transitional cell
carcinoma urinary bladder. Med J Armed Forces India. 62:190–191.
2011. View Article : Google Scholar
|
|
35
|
Zhang H, Ye D, Yao X, Zhang S, Dai B, Shen
Y, Zhu Y, Zhu Y, Shi G, et al: Clinical analysis of multiple
primary malignant tumors and concurrent renal cell carcinoma. J
Army Med Univ. 31:1261–1263. 2009.(In Chinese).
|
|
36
|
Rotellini M, Fondi C, Paglierani M,
Stomaci N and Raspollini MR: Clear cell carcinoma of the bladder in
a patient with a earlier clear cell renal cell carcinoma: A case
report with morphologic, immunohistochemical, and cytogenetical
analysis. Appl Immunohistochem Mol Morphol. 18:396–399. 2010.
View Article : Google Scholar
|
|
37
|
Smith MT, Taylor FD, Gianakopoulus WP and
Brown RR: Two separate synchronous primary genitourinary tumors.
Rev Urol. 14:104–107. 2012.
|
|
38
|
Zhu Q, Gu J, Zhang Y, Shi J, Sun F, Shen
J, Yang Y and Wang N: Clinical analysis of 21 cases of multiple
primary cancers associated with bladder cancer in elderly patients.
Chin J Clin Oncol. 39:993–996. 2012.(In Chinese).
|
|
39
|
Djafari AA, Moradi A, Rahnama H and
Rahavian A: The first case report of synchronous primary papillary
type 2 renal cell carcinoma of kidney and transitional cell
carcinoma of bladder. Urol Case Rep. 37:1016012021. View Article : Google Scholar
|
|
40
|
Dieckmann KP and Nekarda H: Triple
malignancy of the genitourinary tract. Int Urol Nephrol.
20:485–488. 1988. View Article : Google Scholar
|
|
41
|
Harima M, Narita K, Kobayakawa H, Tsujino
T, Yamamoto S, Fukushima S and Kishimoto T: A case of synchronous
triple primary cancers of prostate, kidney and bladder. Hinyokika
Kiyo. 44:675–678. 1998.(In Japanese).
|
|
42
|
Takada T, Honda M, Momohara C, Komori K
and Fujioka H: Synchronous triple urogenital cancer (renal cancer,
bladder cancer, prostatic cancer): A case report. Hinyokika Kiyo.
48:239–242. 2002.(In Japanese).
|
|
43
|
Satoh H, Momma T, Saito S and Hirose S: A
case of synchronous triple primary carcinomas of the kidney,
bladder and prostate. Hinyokika Kiyo. 49:261–264. 2003.(In
Japanese).
|
|
44
|
Arikan-Sengul C, Pehlivan Y, Sevinc A,
Karakok M, Kalender ME and Camci C: A case of metachronous triple
primary urogenital cancer: Urinary bladder, prostate, and renal
cancer. Onkologie. 32:122–124. 2009.
|
|
45
|
Nishikawa K, Soga N, Kato M, Masui S,
Hasegawa Y, Yamada Y, Kise H, Arima K and Sugimura Y: Case of
bladder cancer following adjuvant external beam radiation for
prostate cancer. Hinyokika Kiyo. 55:39–41. 2009.(In Japanese).
|
|
46
|
Pérez MPM, Bazán AA, Dorrego JMA, Herrero,
Ledo JC and de la Peña Barthel J: Simultaneous cystectomy and
nephroureterectomy due to synchronous upper urinary tract tumors
and invasive bladder cancer: Open and laparoscopic approaches. Curr
Urol. 6:76–81. 2012. View Article : Google Scholar
|
|
47
|
Tiwari P, Tripathi A, Bansal P, Vijay M,
Gupta A and Kundu AK: Synchronous primary cancers of urinary
bladder and kidney and prostate. Saudi J Kidney Dis Transpl.
23:786–789. 2012. View Article : Google Scholar
|
|
48
|
Okumura A, Tsuritani S, Takagawa K and
Fuse H: Case of heterochronous triple urogenital cancer (renal cell
carcinoma, bladder cancer, prostatic cancer). Nihon Hinyokika
Gakkai Zasshi. 104:702–705. 2013.(In Japanese).
|
|
49
|
Kurose H, Ueda K, Nakiri M, Matsuo M,
Suekane S and Igawa T: Synchronous primary triple urogenital
malignant tumors of kidney, prostate and bladder. Urol Case Rep.
33:1012772020. View Article : Google Scholar
|
|
50
|
Zhang G, Liao H, Liu L, Tang D and Zhang
F: A case report of synchronous triple cancers in the urinary and
male reproductive system. Chin J Urol. 44:861–862. 2023.(In
Chinese).
|
|
51
|
Kinbara H, Suzuki S, Nakano S, Yamakawa K,
Hioki T, Okabe S, Sugimura Y, Tajima K, Tochigi H and Kawamura J: A
case of renal cell carcinoma and bladder carcinoma associated with
von Hippel-Lindau disease. Hinyokika Kiyo. 36:823–826. 1990.(In
Japanese).
|
|
52
|
Nakano S, Suzuki S, Kinbara H, Maeda Y,
Yanagawa M, Tajima K, Tochigi H, Kawamura J, Hounoki S and Yamamoto
I: A case of double cancer: Renal cell carcinoma and transitional
cell carcinoma of urinary bladder. Hinyokika Kiyo. 36:831–835.
1990.(In Japanese).
|
|
53
|
Soda T, Nishimura K, Kobayashi Y, Kato T,
Tokugawa S, Kishikawa H, Ihara H and Ichikawa Y: A case of
synchronous contralateral renal cell carcinoma and urothelial
carcinoma. Hinyokika Kiyo. 55:491–494. 2009.(In Japanese).
|
|
54
|
Ando M, Arisawa C and Okano T: A case of
renal cell carcinoma associated with synchronous contralateral
renal pelvic cancer and bladder tumor. Int J Urol. 3:310–312. 1996.
View Article : Google Scholar
|
|
55
|
Lian Y, Wang D, Chen Q, Zhang Y, Shangguan
Z, Jiang M, Hu K and Hao W: A clinical analysis for 9 cases of
multiple primary malignant neoplasms associated with renal cell
carcinoma. Oncol Prog. 18:815–818. 2020.(In Chinese).
|
|
56
|
Tsujimura A, Takahara S and Koide T: A
case of synchronous ipsilateral renal cell carcinoma and
transitional cell carcinoma. Hinyokika Kiyo. 37:1303–1306. 1991.(In
Japanese).
|
|
57
|
Chuang HC, Chuang CK and Ng KF:
Simultaneous development of renal cell carcinoma and multifocal
urothelial carcinoma. Chang Gung Med J. 31:515–519. 2008.
|
|
58
|
Morikawa Y, Shiomi K, Ishihara Y and
Matsuura N: Triple primary cancers involving kidney, urinary
bladder, and liver in a dye worker. Am J Ind Med. 31:44–49. 1997.
View Article : Google Scholar
|
|
59
|
Kamota S, Harabayashi T, Suzuki S,
Takeyama Y, Mitsui T, Mouri G, Hashimoto A, Nakamura M, Shinohara
N, Nonomura K and Koyanagi T: Ureteral and bladder metastases of
renal cell carcinoma following synchronous renal cell carcinoma and
bladder cancer; a case report. Nihon Hinyokika Gakkai Zasshi.
94:705–708. 2003.(In Japanese).
|
|
60
|
Gönül II, Cakr A, Sözen S, Ataoglu O and
Alkibay T: A case of tubulocystic carcinoma simultaneously
occurring with clear cell type renal cell carcinoma and
micropapillary urothelial carcinoma of bladder. South Med J.
102:754–757. 2009. View Article : Google Scholar
|
|
61
|
Wang Y, Yu Z, He H, Wang X, Ge H and Xie
X: Cancerization of ureteral inverted papilloma complicated with
ipsilateral renal cell cancer and bladder transitional cell
carcinoma: One case report. J Chin Oncol. 22:691–692. 2016.(In
Chinese).
|
|
62
|
Xu G, Bu S and Wang X: A case report of
four primary malignant tumors. Cancer Res Prev Treat. 46:1141–1142.
2019.(In Chinese).
|
|
63
|
Akan S and Ediz C: A rare association in a
patient with non-muscle invasive bladder cancer: Ureteral
fibroepithelial polyp and ipsilateral renal cell carcinoma: A case
report. J Med Case Rep. 15:4752021. View Article : Google Scholar
|
|
64
|
Mitchell K, El Naili R, Pillai L, Lopez
EM, Riordan J, Marsh W, Luchey A and Hajiran A: Triple threat:
Three primary malignancies simultaneously involving three
genitourinary organs. Case Rep Urol. 2023:32429862023.
|
|
65
|
Huang J, Deng S, Li Q, Li J, Li W, Zhang X
and Xue S: Multiple primary malignancies of the bladder, rectum and
kidney: A case report. Chin J Urol. 44:383–384. 2023.(In
Chinese).
|
|
66
|
Lobo N, Afferi L, Moschini M, Mostafid H,
Porten S, Psutka SP, Gupta S, Smith AB, Williams SB and Lotan Y:
Epidemiology, screening, and prevention of bladder cancer. Eur Urol
Oncol. 5:628–639. 2022. View Article : Google Scholar
|
|
67
|
Dyrskjøt L, Hansel DE, Efstathiou JA,
Knowles MA, Galsky MD, Teoh J and Theodorescu D: Bladder cancer.
Nat Rev Dis Primers. 9:582023. View Article : Google Scholar
|
|
68
|
Aminoltejari K and Black PC: Radical
cystectomy: A review of techniques, developments and controversies.
Transl Androl Urol. 9:3073–3081. 2020. View Article : Google Scholar
|
|
69
|
Khan MS, Gan C, Ahmed K, Ismail AF,
Watkins J, Summers JA, Peacock JL, Rimington P and Dasgupta P: A
single-centre early phase randomised controlled three-arm trial of
open, robotic, and laparoscopic radical cystectomy (CORAL). Eur
Urol. 69:613–621. 2016. View Article : Google Scholar
|
|
70
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004. View Article : Google Scholar
|
|
71
|
Griffiths G, Hall R, Sylvester R, Raghavan
D and Parmar MK: International phase III trial assessing
neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy
for muscle-invasive bladder cancer: long-term results of the BA06
30894 trial. J Clin Oncol. 29:2171–2177. 2011. View Article : Google Scholar
|
|
72
|
Advanced Bladder Cancer (ABC)
Meta-analysis Collaboration, . Neoadjuvant chemotherapy in invasive
bladder cancer: update of a systematic review and meta-analysis of
individual patient data advanced bladder cancer (ABC) meta-analysis
collaboration. Eur Urol. 48:202–206. 2005. View Article : Google Scholar
|
|
73
|
van der Heijden MS, Sonpavde G, Powles T,
Necchi A, Burotto M, Schenker M, Sade JP, Bamias A, Beuzeboc P,
Bedke J, et al: Nivolumab plus gemcitabine-cisplatin in advanced
urothelial carcinoma. N Engl J Med. 389:1778–1789. 2023. View Article : Google Scholar
|
|
74
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 41:3881–3890. 2023.
View Article : Google Scholar
|
|
75
|
Powles T, Park SH, Voog E, Caserta C,
Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén
A, et al: Avelumab maintenance therapy for advanced or metastatic
urothelial carcinoma. N Engl J Med. 383:1218–1230. 2020. View Article : Google Scholar
|
|
76
|
Massari F, Santoni M, Takeshita H, Okada
Y, Tapia JC, Basso U, Maruzzo M, Scagliarini S, Büttner T,
Fornarini G, et al: Global real-world experiences with
pembrolizumab in advanced urothelial carcinoma after platinum-based
chemotherapy: The ARON-2 study. Cancer Immunol Immunother.
73:1062024. View Article : Google Scholar
|
|
77
|
Chang SS, Bochner BH, Chou R, Dreicer R,
Kamat AM, Lerner SP, Lotan Y, Meeks JJ, Michalski JM, Morgan TM, et
al: Treatment of non-metastatic muscle-invasive bladder cancer:
AUA/ASCO/ASTRO/SUO guideline. J Urol. 198:552–559. 2017. View Article : Google Scholar
|
|
78
|
Powles T, Bellmunt J, Comperat E, De
Santis M, Huddart R, Loriot Y, Necchi A, Valderrama BP, Ravaud A,
Shariat SF, et al: ESMO clinical practice guideline interim update
on first-line therapy in advanced urothelial carcinoma. Ann Oncol.
35:485–490. 2024. View Article : Google Scholar
|
|
79
|
Benjamin DJ, Rezazadeh KA and Prasad V:
The overall survival benefit in EV-302: Is enfortumab vedotin plus
pembrolizumab the new frontline standard of care for metastatic
urothelial carcinoma? Eur Urol Oncol. 7:313–315. 2024. View Article : Google Scholar
|
|
80
|
Cirillo L, Innocenti S and Becherucci F:
Global epidemiology of kidney cancer. Nephrol Dial Transplant.
39:920–928. 2024. View Article : Google Scholar
|
|
81
|
Deng J, Tu S, Li L, Li G and Zhang Y:
Diagnostic, predictive and prognostic molecular biomarkers in clear
cell renal cell carcinoma: A retrospective study. Cancer Rep
(Hoboken). 7:e21162024. View Article : Google Scholar
|
|
82
|
Safiri S, Hassanzadeh K, Jolfayi AG,
Mousavi SE, Asghari KM, Nejadghaderi SA, Naghdi-Sedeh N, Noori M,
Sullman MJM, Collins GS and Kolahi AA: Kidney cancer in the Middle
East and North Africa region: A 30-year analysis (1990–2019). Sci
Rep. 14:137102024. View Article : Google Scholar
|
|
83
|
Hunt JD, van der Hel OL, Mcmillan GP,
Boffetta P and Brennan P: Renal cell carcinoma in relation to
cigarette smoking: Meta-analysis of 24 studies. Int J Cancer.
114:101–108. 2005. View Article : Google Scholar
|
|
84
|
Chow WH, Dong LM and Devesa SS:
Epidemiology and risk factors for kidney cancer. Nat Rev Urol.
7:245–257. 2010. View Article : Google Scholar
|
|
85
|
Roskoski R Jr: Combination immune
checkpoint and targeted protein kinase inhibitors for the treatment
of renal cell carcinomas. Pharmacol Res. 203:1071812024. View Article : Google Scholar
|
|
86
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar
|
|
87
|
Goswamy R, Kalemoglu E, Master V and Bilen
MA: Perioperative systemic treatments in renal cell carcinoma.
Front Oncol. 14:13621722024. View Article : Google Scholar
|
|
88
|
Gao B, Gorgen ARH, Bhatt R, Tano ZE,
Morgan KL, Vo K, Zarandi SS, Ali SN, Jiang P, Patel RM, et al:
Avoiding ‘Needless’ nephrectomy: What is the role of small renal
mass biopsy in 2024? Urol Oncol. 42:236–244. 2024. View Article : Google Scholar
|
|
89
|
Johnson DC, Vukina J, Smith AB, Meyer AM,
Wheeler SB, Kuo TM, Tan HJ, Woods ME, Raynor MC, Wallen EM, et al:
Preoperatively misclassified, surgically removed benign renal
masses: A systematic review of surgical series and United States
population level burden estimate. J Urol. 193:30–35. 2015.
View Article : Google Scholar
|
|
90
|
Warren S: Multiple primary malignant
tumors, a survey of the literature and a statistical study. Am J
Cancer. 16:1358–1414. 1932.
|
|
91
|
Vogt A, Schmid S, Heinimann K, Frick H,
Herrmann C, Cerny T and Omlin A: Multiple primary tumours:
Challenges and approaches, a review. ESMO Open. 2:e0001722017.
View Article : Google Scholar
|
|
92
|
Gul ZG, Liaw CW and Mehrazin R: Gender
differences in incidence, diagnosis, treatments, and outcomes in
clinically localized bladder and renal cancer. Urology.
151:176–181. 2021. View Article : Google Scholar
|
|
93
|
Czene K and Hemminki K: Familial papillary
renal cell tumors and subsequent cancers: A nationwide
epidemiological study from Sweden. J Urol. 169:1271–1275. 2003.
View Article : Google Scholar
|
|
94
|
Rabbani F, Reuter VE, Katz J and Russo P:
Second primary malignancies associated with renal cell carcinoma:
Influence of histologic type. Urology. 56:399–403. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Antonelli A, Calza S, Arrighi N, Zani D,
Corti S, Cozzoli A, Zanotelli T, Cunico SC and Simeone C: Clinical
features and prognosis of patients with renal cancer and a second
malignancy. Urol Oncol. 30:294–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lonati C, Simeone C, Suardi N, Spiess PE,
Necchi A and Moschini M: Genitourinary manifestations of Lynch
syndrome in the urological practice. Asian J Urol. 9:443–450. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Umar A, Boland CR, Terdiman JP, Syngal S,
de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ,
Hamelin R, et al: Revised Bethesda guidelines for hereditary
nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite
instability. J Natl Cancer Inst. 96:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nassour AJ, Jain A, Hui N, Siopis G,
Symons J and Woo H: Relative risk of bladder and kidney cancer in
lynch syndrome: Systematic review and meta-analysis. Cancers
(Basel). 15:5062023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wu KY, Cheong IS, Lai JN, Hu CY, Hung KC,
Chen YT, Chiu LT, Tsai HT, Jou YC, Tzai TS and Tsai YS: Risk of
secondary primary malignancies in survivors of upper tract
urothelial carcinoma: A nationwide population-based analysis.
Cancer Epidemiol. 89:1025362024. View Article : Google Scholar : PubMed/NCBI
|