Introduction
There has been an improvement in the outcomes of
chemotherapy for inoperable advanced non-small cell lung cancer
(NSCLC) in recent years owing to the use of targeted therapies for
driver mutations and immune checkpoint inhibitors (ICIs). Long-term
survival can be expected, especially when ICIs are used in cases
with high PD-L1 expression (1).
Additionally, the perioperative use of these drugs has increased
the number of curable cases (2).
However, the incidence of secondary primary cancers and multiple
primary malignancies is expected to increase with prolonged
treatment (3). Managing these
overlapping cancers can be challenging due to the difficulty in
determining treatment priorities and maintaining overall patient
condition. The administration of platinum-based agents such as
carboplatin, commonly used in NSCLC treatment, has been associated
with the development of therapy-related myeloid neoplasms (t-MNs)
due to their cytotoxic effects and propensity to accumulate in bone
tissue (4). This accumulation can
potentially impact bone marrow function and contribute to secondary
malignancies, including CML (5). As
the number of long-term survivors of NSCLC increases, recognizing
and managing the risk of t-MNs as a delayed complication is
essential. Chronic myeloid leukaemia (CML), a type of
myeloproliferative neoplasm (MPN), is characterised by the
translocation of t(9;22)(q34;q11), resulting in the formation of
the BCR-ABL1 fusion gene. Gene activation at the haematopoietic
stem cell level causes tumorigenesis. Introducing tyrosine kinase
inhibitors (TKIs) has improved treatment outcomes, and the
development of improved next-generation agents is ongoing (6,7).
However, few cases of CML development during NSCLC treatment have
been reported to date.
Case report
A 66-year-old woman, who was a former smoker,
presented with a neck mass at Asahikawa Medical University,
Asahikawa, Hokkaido, Japan. Computed tomography (CT) scans revealed
a tumour in the left upper lobe of the lung and bilateral cervical
lymphadenopathy. Right cervical lymph node biopsy revealed lung
adenocarcinoma (cT4N3M1b Stage IVA). The OncoMine Dx Target Test
Multi-CDx System (ODxTT) (Life Technologies Corporation, Frederick
Facility, USA) identified a KRAS G61L mutation. Programmed
cell death ligand 1 (PD-L1) tumour proportion score (TPS) was
80–90%, indicating high expression. The patient received
pembrolizumab as the first-line treatment, followed by carboplatin
and paclitaxel as second-line therapies. Following the reinitiation
of pembrolizumab as third-line therapy, the patient experienced
anorexia and was subsequently diagnosed with immune-related
gastritis. Corticosteroid treatment resulted in clinical
improvement. At this point, since the lesion was confined to the
left axillary lymph node, excisional surgery was performed.
However, local recurrence was observed three months
postoperatively. After delayed treatment for a urinary tract
infection, the patient was admitted for the initiation of
fourth-line treatment.
On admission to Asahikawa Medical University, her
Eastern Cooperative Oncology Group performance status (ECOG-PS) was
1. Physical examination revealed a painless, immobile,
hen-egg-sized lymph node in the left axilla. Blood tests showed an
increased white blood cell (WBC) count to 28,670/µl. Chest CT
revealed enlargement of the left axillary lymph node and
disappearance of the primary lesion in the left upper lobe. The
spleen was not enlarged. A tissue biopsy of the left axillary lymph
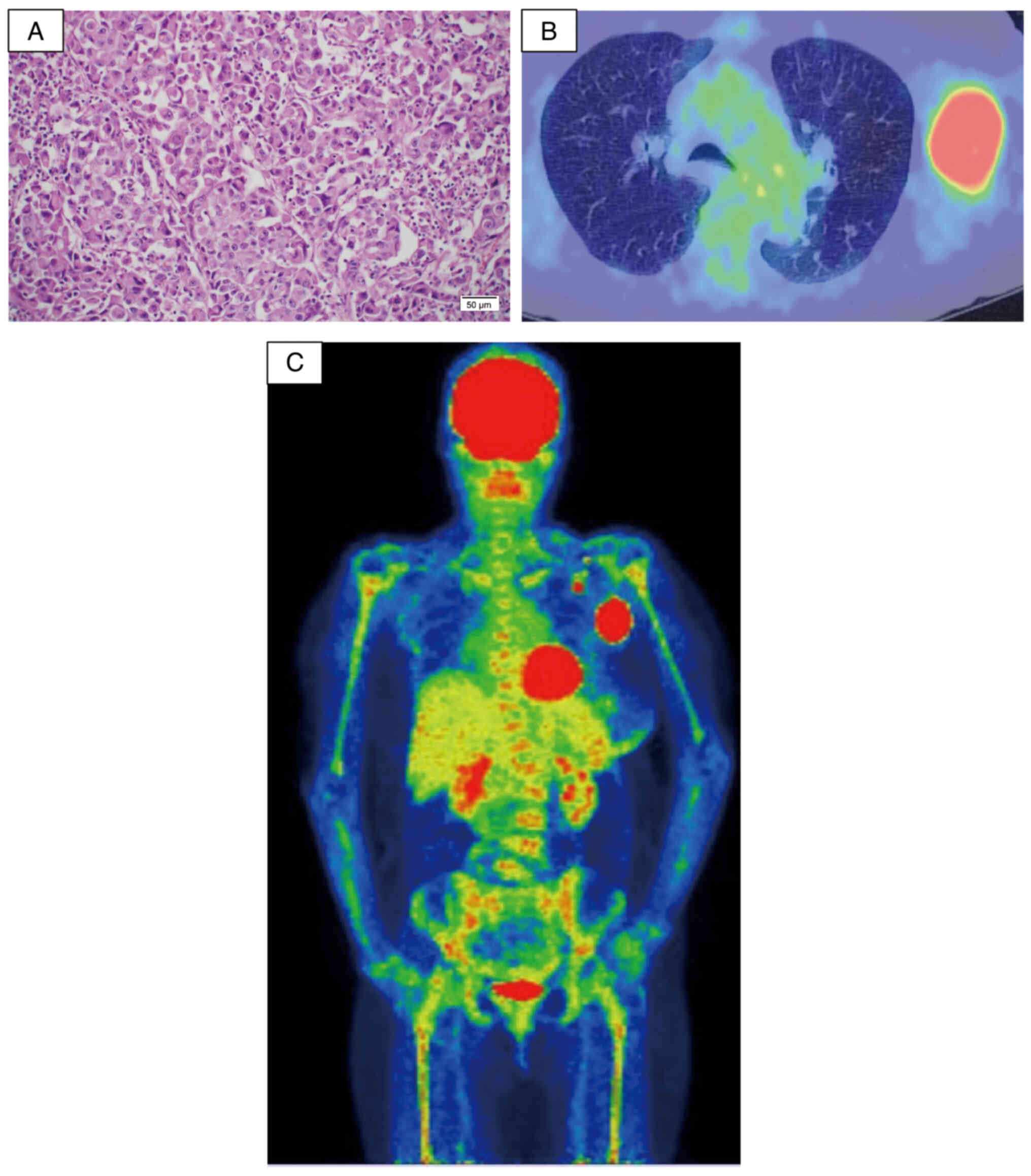
node indicated metastasis of poorly differentiated cancer cells
(Fig. 1A), consistent with
metastatic adenocarcinoma confirmed by immunohistochemistry. During
hospitalisation, despite no signs of infection or a history of
corticosteroid administration in the past month, there was a
gradual increase in the WBC count. 18F-fluoro-D-deoxyglucose
positron emission tomography (FDG-PET) revealed high uptake in the
left axillary lymph nodes and diffuse bone uptake (Fig. 1B and C). These imaging findings
suggested diffuse bone metastasis, haematological disease, and
local recurrence in the left axilla. Further examinations were
conducted, including serum granulocyte colony-stimulating factor
(G-CSF) levels and bone marrow biopsy. Serum G-CSF levels were
normal, ruling out G-CSF-producing tumours. The bone marrow smear
showed a nucleated cell count of 629,500/µl, a myeloid/erythroid
(M/E) ratio of 5.32, and megakaryocyte hyperplasia at 368/µl. No
dysplasia was observed in granulocytic, erythroid, or
megakaryocytic lineages. There was no increase in blast cells, and
the differentiation of the three haematopoietic lineages appeared
normal. No abnormal cells or cell clusters suggestive of bone
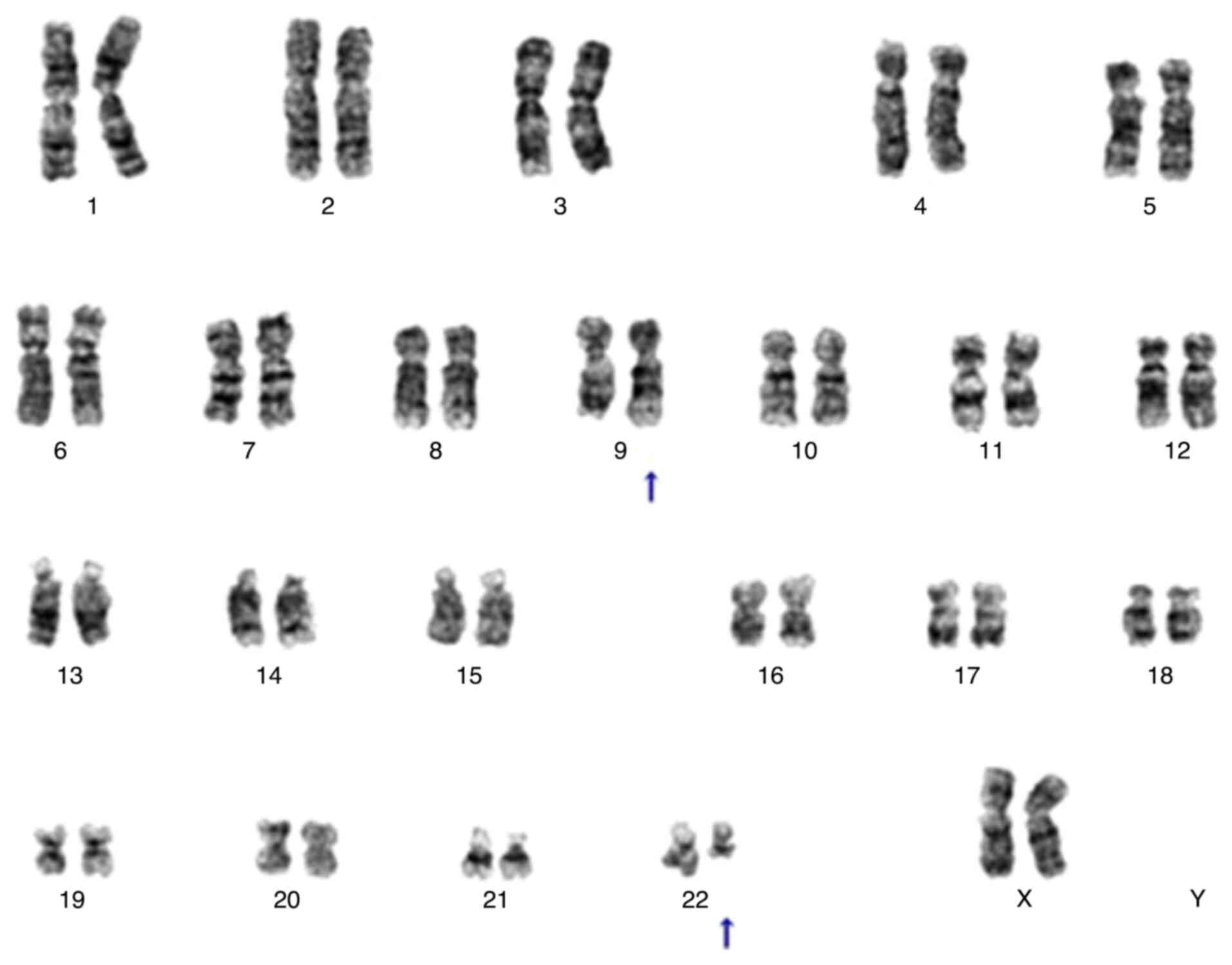
marrow metastasis from the cancer were detected. Chromosomal
analysis of bone marrow fluid using G-banding revealed a karyotype
of 46, XX, t(9;22) (q34.1;q11.2) [20/20], leading to a definitive
diagnosis of CML (Fig. 2). Based on
these findings, the patient was diagnosed with concurrent lung
adenocarcinoma and CML. Notably, the time from the initial
administration of carboplatin, an alkylating agent, to the
diagnosis of CML was approximately 22 months, a relatively short
period for disease onset.
Given the rapid postoperative regrowth of lung
cancer and the completion of third-line therapy, lung cancer is
considered a prognostic determinant. However, the treatment of CML
is conservative because of its chronic disease phase. Therefore,
when chemotherapy was administered for treating lung cancer, a
cautious observational approach was adopted for CML. Carboplatin +
pemetrexed + bevacizumab therapy was initiated as the fourth-line
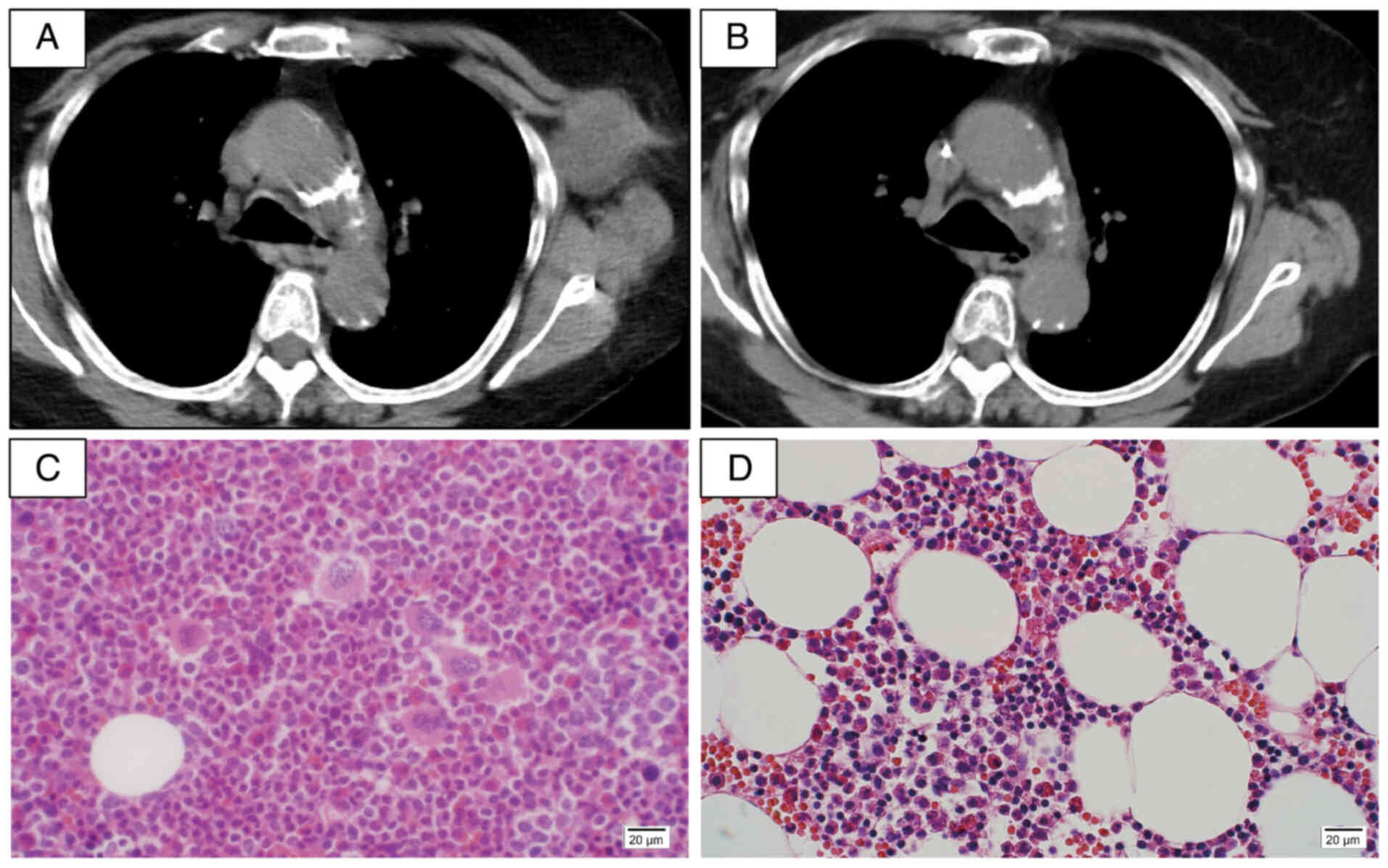
treatment. Six courses were completed without severe adverse
events, and CT showed a reduced size of the left axillary lymph
node (Fig. 3A and B). Upon
re-evaluation of the bone marrow aspirate, the smear showed a
nucleated cell count of 60,000/µl, an M/E ratio of 2.84, and
normocellular marrow with 16/µl megakaryocytes, indicating
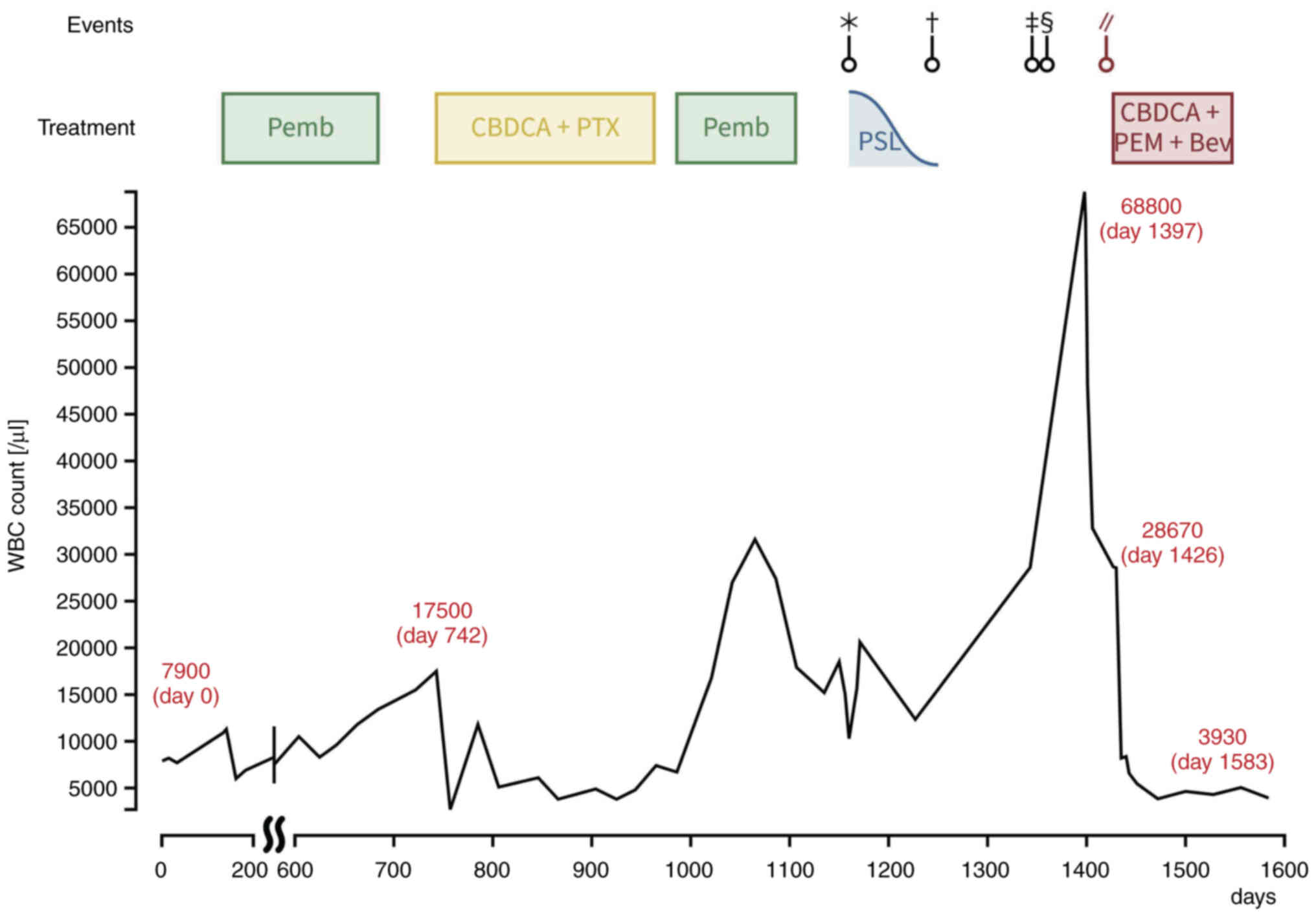
improvement compared to the time of CML diagnosis (Fig. 3C and D). Peripheral blood
BCR-ABL1IS, used to assess the therapeutic response in
CML, remained stable, shifting from 3.60 to 4.26% over three months
concurrent with chemotherapy for lung cancer, suggesting that the
disease activity of CML was under control (Fig. 4). Following the hospital stay, the
patient's follow-up care was transferred to a local hospital near
her residence, and further monitoring at our institution was
discontinued.
Discussion
The emergence of immune checkpoint inhibitors has
increased the number of long-term survivors of advanced NSCLC
(1). Consequently, the incidence of
secondary primary cancers and multiple primary malignancies is
expected to increase. Patients who undergo cytotoxic chemotherapy
or radiation therapy, which causes DNA damage, are at risk of
developing therapy-related myeloid neoplasms (t-MNs), including
acute myeloid leukaemia (t-AML), myelodysplastic syndrome (t-MDS),
and myelodysplastic syndrome/myeloproliferative neoplasm
(t-MDS/MPN) (8). The administration
of alkylating agents has been identified as a risk factor for the
development of t-MNs, and the time from administration to the onset
of t-MNs is 5–7 years (9).
Additionally, platinum-based agents such as carboplatin have a
known propensity to accumulate in bone tissue and impact bone
marrow function, potentially contributing to the development of
myeloid neoplasms (4). As the
number of long-term survivors of NSCLC increases, the potential of
t-MN as a delayed complication should be recognized. In the present
case, the time from the initial administration of carboplatin, an
alkylating agent, to the diagnosis of CML was approximately 22
months, a relatively short period for disease onset. Therefore, the
possibility of co-exposures or confounders other than alkylating
agents, such as radiation exposure or genetic mutations involved in
DNA repair, cannot be ruled out (9). The abbreviated onset period may be
related to using an immune checkpoint inhibitor. t-AML can develop
during pembrolizumab treatment (10). Immune checkpoint inhibitors enhance
the development of t-MNs after exposure to alkylating agents.
However, the patient's WBC count increased to approximately
20,000/µl before carboplatin administration (Fig. 3). De novo CML may have been present
before treatment with an alkylating agent; however, differentiation
between de novo carcinogenesis and t-MN is challenging because of
the lack of distinguishing molecular mechanisms.
The natural progression of CML typically commences
with a chronic phase lasting 3–5 years, characterised by minimal
symptoms, followed by an accelerated phase, during which there is
progressive impairment in the maturation and differentiation of
granulocytes, culminating in a blast phase. Disease staging was
performed according to the World Health Organization
classification, with the cases in question categorised under the
chronic phase (11). EUTOS and
Sokal scores were used for prognostic stratification (12). Based on the EUTOS score, a
prognostic model established from patient outcomes treated with
imatinib, this patient was categorised into the low-risk group and
exhibited a 90% progression-free survival five years post-treatment
initiation, markedly surpassing the outcomes associated with NSCLC.
In the present case, lung cancer was considered a prognostic factor
because of the rapid progression of postoperative re-growth and the
fact that the patient had completed tertiary treatment; therefore,
the patient was treated with chemotherapy for lung cancer while CML
was carefully monitored.
The patient's lung cancer had responded to treatment
by the end of six courses of carboplatin, pemetrexed, and
bevacizumab therapy. Simultaneously, bone marrow puncture showed
improvement in nucleated cell count, M/E ratio, and megakaryocyte
count, and BCR-ABL1IS in peripheral blood remained unchanged from
3.60 to 4.26% over 3 months following chemotherapy for lung cancer,
suggesting that the disease of CML was controlled. Carboplatin has
been used as multi-agent chemotherapy for CML in the blastic phase
and is effective in inducing remission (5). Pemetrexed is not generally used to
treat CML. However, it exerts an anticancer effect by inhibiting
DNA synthesis through the antagonism of folate metabolism, which
may suppress cell proliferation in leukaemia. Ponatinib, a TKI
indicated for CML, is expected to be used in NSCLC treatment but
evidence to support its use is insufficient (13). There are no reports on the safety of
TKIs in combination with chemotherapy for lung cancer. Therefore,
chemotherapy for lung cancer with the expectation of controlling
CML may be an effective treatment option. Naturally, observations
should be made with an eye toward the sequential use of TKI,
because deep remission cannot be expected without it.
In conclusion, this case illustrates that a lung
cancer chemotherapy regimen can mitigate CML progression. With the
evolving long-term survival rates of lung cancer, the incidence of
concurrent malignancies and secondary cancer development, such as
CML, along with lung cancer, as observed in this case, is
anticipated to increase. Therefore, it is crucial to identify such
cases and strategically prioritise their management for lung cancer
treatment to ensure optimal therapeutic outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The OncoMine Dx Target
Test Multi-CDx System data generated in the present study are not
publicly available due to ethical restrictions but may be requested
from the corresponding author.
Authors' contributions
KNa was involved in the material preparation, data
collection, data curation and visualization and wrote the original
draft. RK conceived the study and reviewed and edited the
manuscript. RS, KNi, AY, HY, YU, CM and YM were involved in the
material preparation and data collection. TF, MY and MT collected
data, were involved in revising the manuscript critically for
important intellectual content and supervised the study. RY
conceived the study, performed analysis, reviewed and edited the
manuscript, and supervised the study. TS conceived the study, was
involved in project administration and supervised the study. KNa,
RK, RY and TS confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
relative of the patient for publication of the details of the
medical case.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
CML
|
chronic myeloid leukaemia
|
|
MPN
|
myeloproliferative neoplasm
|
|
WBC
|
white blood cell
|
|
t-MN
|
therapy-related myeloid neoplasms
|
|
TKIs
|
tyrosine kinase inhibitors
|
|
PD-L1
|
programmed cell death ligand 1
|
|
TPS
|
tumour proportion score
|
|
ECOG-PS
|
Eastern Cooperative Oncology Group
performance status
|
|
FDG-PET
|
18F-fluoro-D-deoxyglucose
positron emission tomography
|
|
G-CSF
|
granulocyte colony-stimulating
factor
|
|
M/E
|
myeloid/erythroid
|
|
AML
|
acute myeloid leukaemia
|
|
MDS
|
myelodysplastic syndrome
|
|
MDS/MPN
|
myelodysplastic
syndrome/myeloproliferative neoplasm
|
References
|
1
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Five-year outcomes with pembrolizumab versus chemotherapy
for metastatic non-small-cell lung cancer with PD-L1 tumor
proportion score ≥ 50. J Clin Oncol. 39:2339–2349. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wakelee H, Liberman M, Kato T, Tsuboi M,
Lee SH, Gao S, Chen KN, Dooms C, Majem M, Eigendorff E, et al:
Perioperative pembrolizumab for early-stage non-small-cell lung
cancer. N Engl J Med. 389:491–503. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tabuchi T, Ito Y, Ioka A, Miyashiro I and
Tsukuma H: Incidence of metachronous second primary cancers in
Osaka, Japan: Update of analyses using population-based cancer
registry data. Cancer Sci. 103:1111–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis LB: The epidemiology of second
primary cancers. Cancer Epidemiol Biomarkers Prev. 15:2020–2026.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Montefusco E, Petti MC, Alimena G,
Latagliata R, Celesti F, Capria S, Amadori S, Avvisati G and
Mandelli F: Etoposide, intermediate-dose cytarabine and carboplatin
(VAC): A combination therapy for the blastic phase of chronic
myelogenous leukemia. Ann Oncol. 8:175–179. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kantarjian H, O'Brien S, Jabbour E,
Garcia-Manero G, Quintas-Cardama A, Shan J, Rios MB, Ravandi F,
Faderl S, Kadia T, et al: Improved survival in chronic myeloid
leukemia since the introduction of imatinib therapy: A
single-institution historical experience. Blood. 119:1981–1987.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wylie AA, Schoepfer J, Jahnke W,
Cowan-Jacob SW, Loo A, Furet P, Marzinzik AL, Pelle X, Donovan J,
Zhu W, et al: The allosteric inhibitor ABL001 enables dual
targeting of BCR-ABL1. Nature. 543:733–737. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koo SM, Kim KU, Kim YK and Uh ST:
Therapy-related myeloid leukemia during erlotinib treatment in a
non-small cell lung cancer patient: A case report. World J Clin
Cases. 9:7205–7211. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McNerney ME, Godley LA and Le Beau MM:
Therapy-related myeloid neoplasms: When genetics and environment
collide. Nat Rev Cancer. 17:513–527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HB, Park SG, Hong R, Kang SH and Na
YS: Acute myelomonocytic leukemia during pembrolizumab treatment
for non-small cell lung cancer: A case report. World J Clin Cases.
8:2833–2840. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hasford J, Baccarani M, Hoffmann V,
Guilhot J, Saussele S, Rosti G, Guilhot F, Porkka K, Ossenkoppele
G, Lindoerfer D, et al: Predicting complete cytogenetic response
and subsequent progression-free survival in 2060 patients with CML
on imatinib treatment: The EUTOS score. Blood. 118:686–692. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren M, Hong M, Liu G, Wang H, Patel V,
Biddinger P, Silva J, Cowell J and Hao Z: Novel FGFR inhibitor
ponatinib suppresses the growth of non-small cell lung cancer cells
overexpressing FGFR1. Oncol Rep. 29:2181–2190. 2013. View Article : Google Scholar : PubMed/NCBI
|