Introduction
Mucinous cystadenocarcinoma (MCA) is a rare
malignant breast tumour, first reported by Koenig and Tavasoli in
1998 (1). MCA was first classified
as a mucus-producing breast cancer, characterised by a cystic
structure with columnar cells and abundant intra- and extracellular
mucin, in 2003 (2). According to
the 2019 World Health Organisation classification of breast
tumours, it is recognised as an independent and specialised type of
breast cancer (3). Most MCA cases
present with a loss of oestrogen receptor (ER), progesterone
receptor (PR) and human epidermal growth factor 2 (HER2)
expression, and a better prognosis compared with other
triple-negative breast cancer of no specific type. To date, only a
limited number of MCA cases have been reported worldwide (1,4–38).
Most reports of MCA show that the tumour is not usually accompanied
by axillary lymph node metastasis, and the prognosis is good. MCA
is also confused with other mucus-secreting breast cancers and
metastases of ovarian or pancreatic cancers (9,16,39,40).
Therefore, MCA diagnosis demands precision through a comprehensive
evaluation of clinical, pathological, imaging and genetic
characteristics. It is also necessary to give an individualized
treatment plan for MCA.
The current study reports a case of primary MCA of
the breast with complete clinicopathological features and genomic
profiling using next-generation sequencing for a comprehensive
evaluation of this rare tumour.
Case report
A 51-year-old premenopausal woman presented to the
Peking Union Medical College Hospital (Beijing, China) in June
2022, due to a mass in the left breast that had been present for
nearly 1 year. The patient had no history of breast surgery,
hormonal treatment or malignant tumours; however, the patient's
mother had ben diagnosed with lung cancer. A clinical examination
confirmed a hard mass, with a diameter of 2 cm, which could be
palpated at the 2 o'clock position in the left breast. No nipple
discharge or enlarged lymph nodes in the axilla were observed.
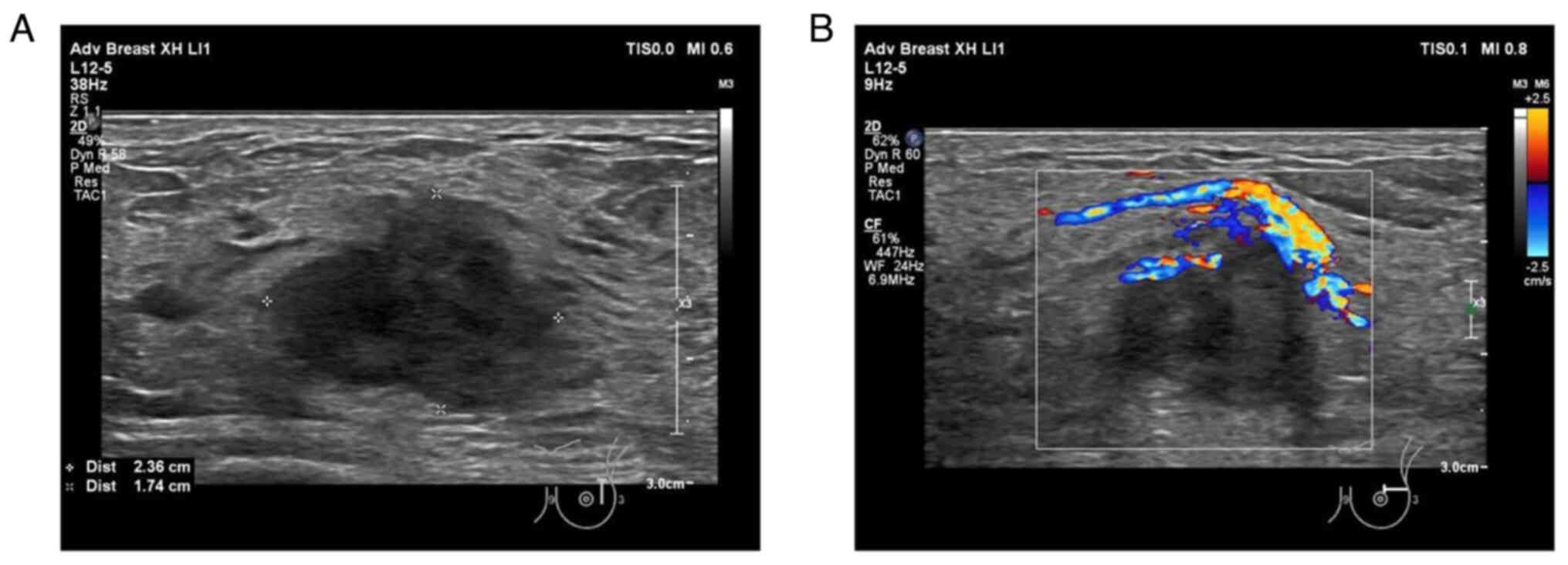
Ultrasound showed a 2.4-cm irregularly hypo-echoic mass with
abundant blood flow signals 2 cm away from the nipple in the
direction of the 2 o'clock position on the left breast (Fig. 1). The patient had undergone a
mammography examination in another hospital prior to attending the
Peking Union Medical College Hospital for treatment, and the
mammography had revealed an irregularly shaped high-density mass in
the upper left breast. Analysis of peripheral blood tumour
indicators included results for carcinoembryonic antigen (CEA),
cancer antigen (CA)153 and CA125. The serum level of CEA was
elevated to 60.1 ng/ml, which markedly exceeded the upper limit of
the normal range (5 ng/ml). While both the CA153 and CA125 were
within the normal levels.
A core needle biopsy (CNB) of the breast mass was
performed. The specimens were sent for routine pathological
examination. The tissue were fixed in 10% formalin neutral fixative
for at least 6 h at 25°C and then made into paraffin-embedded
tissue blocks. Sections (4-µm thick) were prepared for further
haematoxylin-eosin (H&E) staining and immunohistochemical
staining. After being deparaffinized with xylene and rehydrated
with a series of anhydrous ethanol, 95% ethanol, 70% ethanol and
PBS, some of the sections were stained with haematoxylin for 3 min
and eosin for 45 sec at room temperature. All immunohistochemical
staining (Table SI) was performed
using a Ventana Benchmark XT Autostainer (Ventana Medical Systems,
Inc.) according to the manufacturer's protocols. Finally,
visualization was performed using a DAB color development kit,
followed by counterstaining using haematoxylin for 3 min at 25°C.
All sections were sealed with neutral resin. Tumour morphology of
H&E staining and immunohistochemical results were observed
using Olympus light microscope BX53. Images were captured by a
microscope camera (BASLER, acA1920-150uc).
The pathological diagnosis was a high-grade
infiltrating adenocarcinoma with mucus secretion and papillary
formation. Immunohistochemical markers of biopsy included ER, PR,
HER2, androgen receptor (AR), cytokeratin (CK)7, CK14, CK20, CK5/6,
epidermal growth factor receptor (EGFR), tumour protein p53 (p53),
p63, GATA-binding protein 3 (GATA3), paired box 8 (PAX-8), special
AT-rich sequence-binding protein 2 (SATB2), homeobox protein CDX-2
(CDX-2) and Ki-67 (Fig. S1). No
in situ carcinoma was found on needle biopsy, and the
tumours were ER-, PR-, AR- and HER2-negative. The neoplastic cells
showed diffused strong expression of p53 and a high Ki-67 index of
70%, indicating their highly aggressive nature. The neoplastic
cells were CK7-positive, and both CK20- and SATB2-negative, which
excluded the possibility of a gastrointestinal origin. PAX-8
negativity excluded a gynaecological origin. The neoplastic cells
were GATA3-negative, which is common in triple-negative breast
cancer and is consistent with the lack of ER, PR and HER2
expression. The tumour was negative for CK5/6, CK14, p63 and CDX-2
expression, and positive for EGFR expression. Other tumours,
including mucinous adenocarcinoma of the lungs and pancreatic or
biliary tract cancers, should be excluded; however, the biopsy
tissues were limited. Further clinical examinations are also
required to distinguish metastatic adenocarcinomas from primary
breast lesions. Positron emission tomography (PET)/computed
tomography (CT) examination only showed a lesion with increased
radioactive uptake in the upper quadrant of the left breast,
measuring 1.5×1.2 cm, with a maximum standardised uptake value of
10.5 and no other lesions, confirming that it was a primary tumour
(Fig. S2).
The patient underwent a left mastectomy and sentinel
lymph node biopsy, and the absence of lymph node metastasis was
confirmed. The surgical specimens were fixed in 10% formalin
neutral fixative for at least 12 h at 25°C and then made into
paraffin-embedded tissue blocks. Sections (4-µm thick) were
prepared for further H&E staining and immunohistochemical
staining as aforementioned. The macroscopic appearance was a
greyish-white tumour with a maximum diameter of 2.4 cm, a tough
texture and a relatively well-circumscribed mass without obvious
cysts. A mucous-like lustre was observed on the cut surface of the
tumour (Fig. S3). Microscopically,
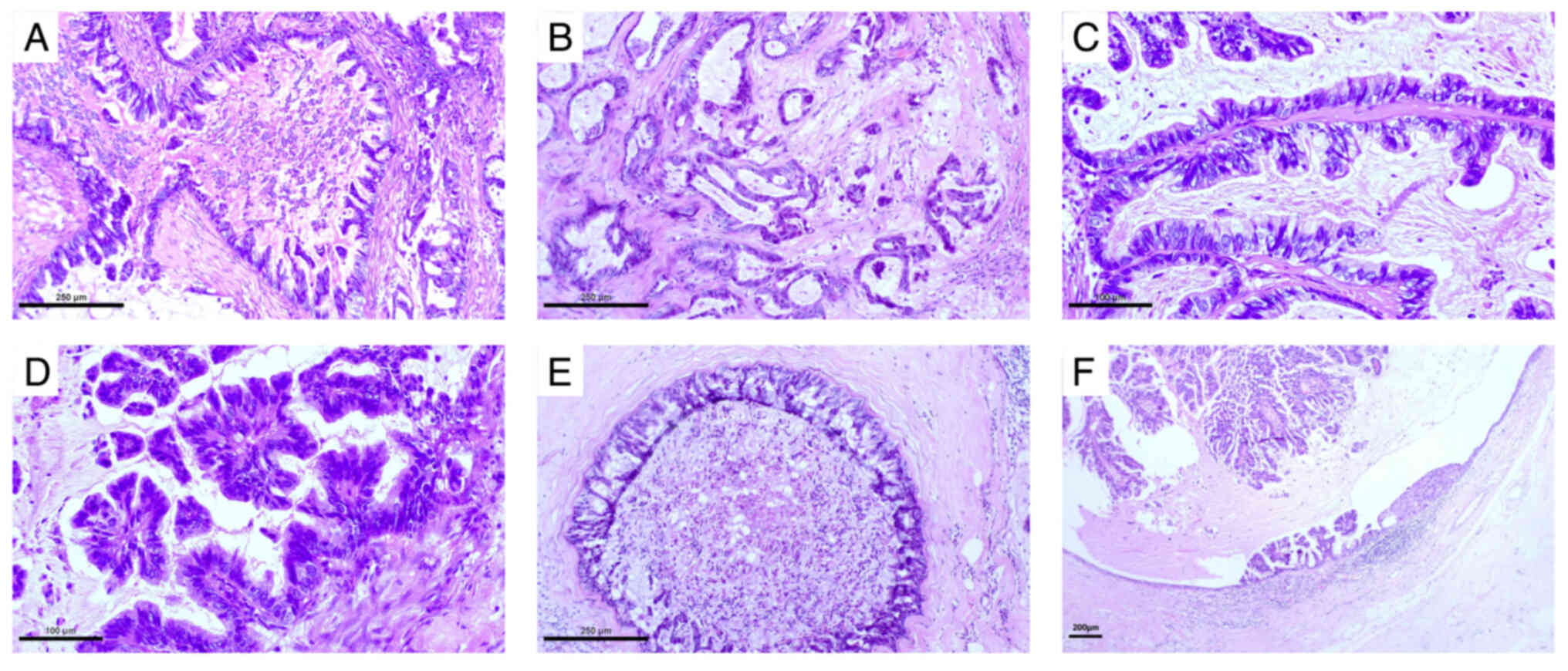
the tumour consisted of different sizes of irregular cysts and
ducts (Fig. 2A and B). Varying
degrees of branching papillary structures were observed in the
lumen and the cyst cavity (Fig. 2C and
D). The tumour cells were highly columnar in shape, with
high-grade nuclei arranged in a single or layered manner. There was
a large amount of mucus both inside and outside the cells. When the
tumour cells were arranged in a single layer, the intracellular
mucus was more prominent and the nuclei were often located at the
base (Fig. 2C). Extracellular mucus
filled the lumen and cyst, and overflowed into the tumour stroma.
Carcinoma in situ with similar morphology was observed
around the invasive tumour (Fig. 2E and
F).
The immunohistochemistry (IHC) staining, performed
as aforementioned, and the results of the surgical specimens were
similar to those of the needle biopsy. Quadruple-negative breast
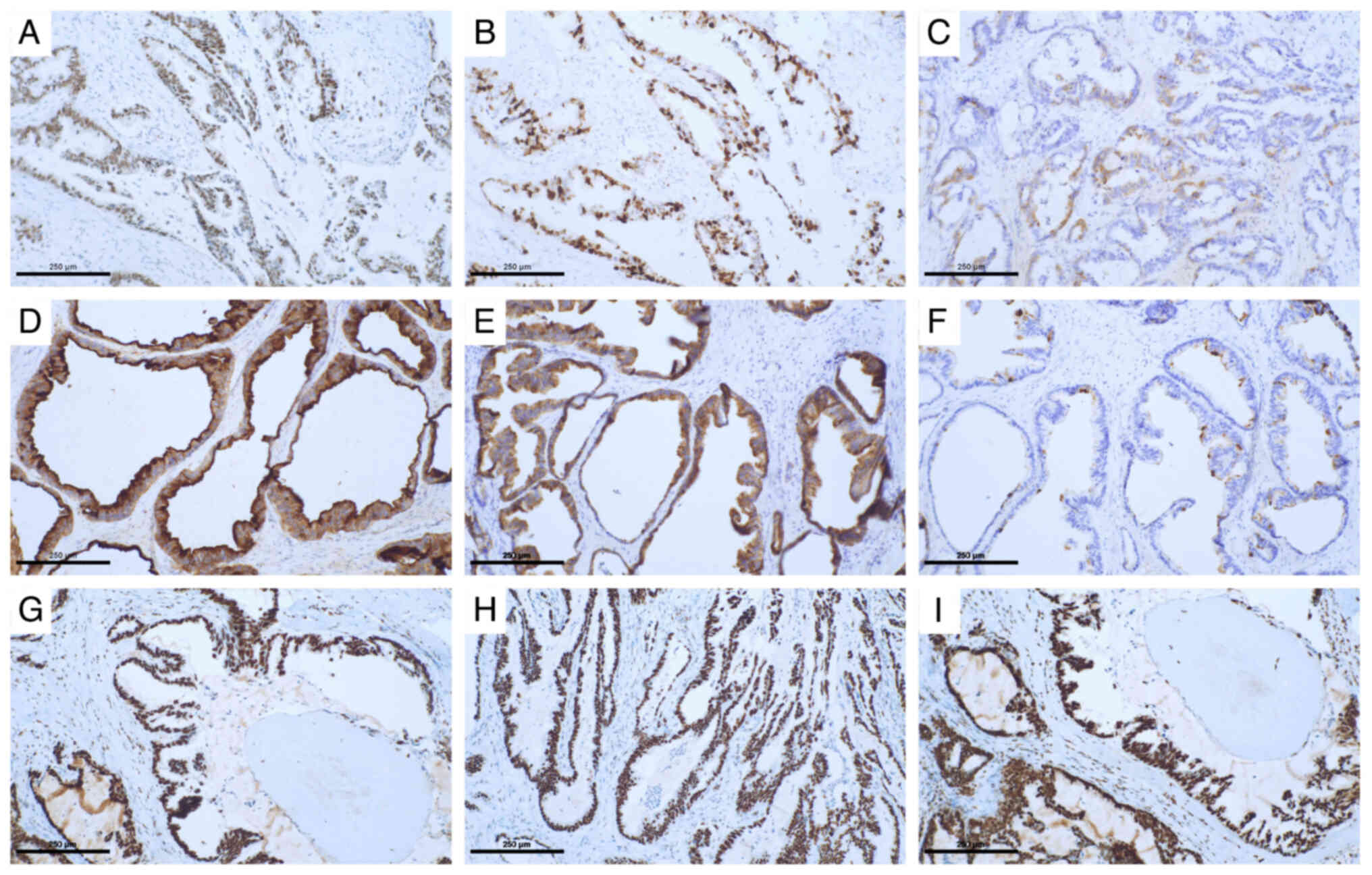
cancer (ER-, PR-, AR- and HER2-negative) (Fig. S1), with diffuse and strong positive
expression of p53 (Fig. 3A) and a
high Ki-67 index (Fig. 3B), was
diagnosed, which was different from mucinous carcinoma or
encapsulated papillary carcinoma that typically expresses hormone
receptors. Additional markers of breast cancer, such as mammaglobin
and gross cystic disease fluid protein 15 (GCDFP-15), were
identified. Mammaglobin was partially positive (Fig. 3C) and GCDFP15 was focal and weakly
positive, indicating that the tumour was a primary breast lesion.
The absence of the myoepithelium also excluded the possibility of
benign breast mucinous lesions. Papillary formation and abundant
intra/extracellular mucus excluded the possibility of invasive
papillary carcinomas. Finally, the patient was diagnosed with
primary MCA of the breast based on these morphological and
immunohistochemical features. Mucus subtype-related
immunohistochemistry showed that the tumour cells mainly expressed
mucin (MUC)1 and MUC6 (Fig. 3D and
E), with partial expression of MUC5AC (Fig. 3F) and no expression of MUC2. DNA
mismatch repair protein Msh2 (MSH-2) (Fig. 3G), MSH-6 (Fig. 3H), DNA mismatch repair protein Mlh1
(Fig. 3I) and PMS-2 were also
expressed, indicating microsatellite stability. A sentinel lymph
node biopsy did not reveal any metastatic tumours. For this
patient, the left breast tumour had a maximum diameter of 2.4 cm,
so the T stage was T2. An axillary sentinel lymph node biopsy
showed no metastatic cancer, so the N stage was N0. No
hypermetabolic lesions other than that in the left breast were
found on the whole-body PET/CT scan, so the M stage was M0. The TNM
stage (41) was therefore
determined to be T2N0M0.
DNA was extracted from formalin-fixed
paraffin-embedded (FFPE) tissues using FFPE tissue genomic DNA
one-step extraction kit (cat. no. RC1004; Kaishuo Biotech (Xiamen)
Co., Ltd.). Samples were quantified using the Qubit dsDNA BR Assay
Kit (cat. no. Q32853; Thermo Fisher Scientific, Inc.), and DNA
integrity was evaluated with 1% agarose gel electrophoresis.
Library Preparation was performed with the Twist Human Core Exome
EF Multiplex Complete Kit, 96 Samples (cat. no. PN100803; Twist
Bioscience) and library concentration was quantified using the
Qubit dsDNA BR Assay Kit (cat. no. Q32853; Thermo Fisher
Scientific, Inc.). Library length was evaluated on an Agilent 2100
Bioanalyzer (Agilent Technologies, Inc.). Concentration in moles
was calculated according to library length, and the concentration
of final library was 6.8 pM and sequenced using whole-exome
sequencing. The sequencing type was 150 bp for length and paired
end for direction of sequencing with the NovaSeq 6000 S4 Reagent
Kit v1.5 (300 cycles; cat. no. 20028312; Illumina Inc.). Two
variant callers, MuTect2 (v4.1.0.0) (42) for SNV and indels, and Strelka
(v2.9.10) (43) for indels, were
used to call somatic variants annotated by ANNOVAR (Version:
2023Jan05) (44). CNVkit (v 0.9.11)
(45) analysis was used to evaluate
copy number alterations. Mutations inbreast cancer 1-associated
RING domain 1 (BARD1), kinase domain-containing receptor (KDR),
mucin-6 (MUC6), tumour protein 53 (TP53) and breast cancer
1-interacting protein C-terminal helicase 1 (BRIP1) were
identified, and are summarized in Table
I.
 | Table I.Genetic profile identified in the
present case of primary mucinous cystadenocarcinoma of the
breast. |
Table I.
Genetic profile identified in the
present case of primary mucinous cystadenocarcinoma of the
breast.
| Gene | Chromosome | Exon | Type of
mutation | DNA sequence
change | Amino acid
change | Allele frequency,
% |
|---|
| BARD1 | 2 | 6 | Missense |
c.1518_1519delinsCA | p.V507M | 99.4 |
| KDR | 4 | 24 | Missense | c.G3207C | p.L1069F | 11 |
| MUC6 | 11 | 31 | Missense | c.C5146T | p.P1716S | 16.1 |
| TP53 | 17 | 10 | Missense | c.T1013G | p.F338C | 29.6 |
| BRIP1 | 17 | 6 | Missense | c.A587G | p.N196S | 32.3 |
After surgery, considering that the patient had no
distant metastasis and the TNM stage was T2N0M0, according to the
Chinese Society of Clinical Oncology Breast Cancer Guidelines 2022
(46), the patient received eight
cycles of chemotherapy (75 mg/m2 intravenous doxorubicin
on day 1 and 600 mg/m2 intravenous cyclophosphamide on
day 1, cycled every 21 days for 4 cycles; and sequential 85
mg/m2 intravenous docetaxel on day 1, cycled every 21
days for 4 cycles), followed by sequential capecitabine (650
mg/m2 orally twice daily for 6 months). The patient was
followed up every 6 months, including an assessment of any abnormal
signs, ultrasound examinations of breast and axillary lymph nodes,
neck and supraclavicular lymph nodes, abdomen and gynecological
regions, chest CT and contralateral breast mammogram once a year.
No recurrence was evident during 26 months of follow-up. The serum
CEA level markedly decreased to 3 ng/ml 16 months after
surgery.
Written informed consent was obtained from the
patient and all procedures followed the ethical standards of the
Declaration of Helsinki.
Discussion
Primary MCA of the breast is a rare invasive breast
cancer that is characterised by a cystic structure lined with tall
columnar cells and abundant intra-and extracellular mucus, and is
similar to pancreatic or ovarian mucinous cystadenocarcinoma.
According to the PubMed database (https://pubmed.ncbi.nlm.nih.gov/), a literature review
with key words including ‘primary’, ‘breast’ and ‘mucinous
cystadenocarcinoma’, and excluding any metastatic lesions of breast
cases, revealed that 40 cases were reported by December 2023, as
shown in Table II. Primary MCA of
the breast predominantly occurred in postmenopausal women, with a
median age of 59 years (range, 33–96 years) (1,4–38). The
tumour size ranged from 0.8 to 19 cm and 95% were single lesions.
Among the reported 35 cases with known lymph node status, 26 cases
had no lymph node metastasis, 1 case showed isolated tumour cells,
8 cases had lymph node metastasis and 5 cases had >3 lymph nodes
involved. In the present case, the patient was 51 years old with a
2.4-cm tumour and no lymph node metastasis. In the present case,
the CEA level was significantly elevated at diagnosis and decreased
to normal after surgery. In previous research, it has been reported
that both CA153 and CA125 are elevated at diagnosis and decreased
after surgery (26). However, there
is no previous report on the elevation of CEA in MCA.
 | Table II.Comparison of the clinicopathological
features of the present case with the other cases reported in the
literature. |
Table II.
Comparison of the clinicopathological
features of the present case with the other cases reported in the
literature.
| First author,
year | Age, years | Tumour size,
cm | pN stage | ER | PR | HER2 | Ki-67 % | CK7 | CK20 | Associated
findings | Surgery;
CT/RT/HT | Follow-up time,
status | (Refs.) |
|---|
| Koenig and
Tavasolli, | 54 | 19.0 | N2 | - | - | NA | 40 | + | - | None | M, LND | 24 months,
ANED | (1) |
| 1998 | 67 | 2.3 | N0 | - | - | NA | 30 | + | - | DCIS | M, LND | 22 months,
ANED |
|
|
| 49 | 8.5 | N0 | - | - | NA | 70 | + | - | DCIS | M, LND, CT +
RT | 11 months,
ANED |
|
|
| 61 | 0.8 | N0 | - | - | NA | 50 | + | - | None | L, LND | NA |
|
| Rosen and Scott,
1984 | 79 | 6.0 | NA | - | - | NA | NA | NA | NA | NA | M, LND | 108 months,
DOD | (4) |
| Domoto et
al, 2000 | 74 | 10.0 | N0 | - | - | - | 22 | + | - | NA | M, LND, | 24 months,
ANED | (5) |
| Honma et al,
2003 | 96 | 2.0 | N1 | - | - | - | 35 | NA | NA | None | L, LND | 46 months, DOD | (6) |
| Chen et al,
2004 | 65 | 3.0 | N0 | - | - | - | 20.5 | + | - | IDC, DCIS | M, LND, CT | 8 months, ANED | (7) |
| Coyne and Irion,
2006 | 51 | 4.0 | NA | - | - | NA | NA | + | - | None | L | NA | (8) |
| Lee and Chaung,
2008 | 55 | 2.5 | N0 | - | - | - | 10 | + | - | IDC, DCIS | M, LND | 6 months, ANED | (9) |
| Rakici et
al, 2009 | 52 | 10.0 | N0 | + | - | - | NA | - | - | ADH | M, LND, CT | 24 months,
ANED | (10) |
| Gulwani and Bhalla,
2010 | 61 | 3.0 | N0 | - | - | - | NA | NA | - | None | M, LND | 6 months, ANED | (11) |
| Petersson et
al, 2010 | 73 | 4.5 | N0 | - | - | 2+
(FISH+) | NA | + | - | DCIS | M, LND | NA | (12) |
| Sentani et
al, 2012 | 65 | 3.0 | N0 | - | - | - | NA | + | - | DCIS | L, LND | 6 months, ANED | (13) |
| Deng et al,
2012 | 41 | 7.0, 5.0, 2.5 | N3 | - | - | - | 50 | + | - | DCIS | M, LND | 24 months,
ANED | (14) |
| Li et al,
2012 | 52 | 6.5 | N0 | - | - | - | 10 | + | - | None | M, LND, CT | 12 months,
ANED | (15) |
| Kim et al,
2012 | 59 | 0.9 | N0 | - | - | 2+
(FISH−) | 5 | + | - | IDC, DCIS | L, SLNB, CT | 3 months, ANED | (16) |
| Witherspoon et
al, 2015 | 91 | 7.5 | N0 | - | - | - | 40 | + | - | IDC, DCIS | L, LND, RT | 14 months, DOD | (17) |
| Lin et al,
2013 | 62 | 3.2 | N0 | - | - | - | NA | + | - | None | M, LND | 5 months, ANED | (18) |
| Kucukzeybek et
al, 2014 | 55 | 2.0 | N0 | - | - | 2+
(FISH+) | 30 | + | - | DCIS | L, SLNB, CT, RT,
H | 10 months,
ANED | (19) |
| Seong et al,
2016 | 59 | 2.0 | NA | - | - | 3+ | NA | NA | NA | NA | NA | NA | (20) |
|
| 50 | 2.2 | NA | - | - | - | NA | NA | NA | NA | NA | NA |
|
| Koufopoulos et
al, 2017 | 63 | 1.6 | N1 | - | - | - | NA | + | - | None | L, LND, CT, RT | 48 months,
ANED | (21) |
| Nayak et al,
2018 | 68 | 6.2 | N0 | - | - | - | NA | + | - | DCIS | L, SLNB | 3 months, ANED | (22) |
|
| 51 | 2.0 | N0 | - | - | - | NA | + | - | DCIS | L, LND | 96 months,
recurrence |
|
| Kaur et al,
2019 | 45 | 12.0 | NA | - | - | - | NA | + | - | NA | M | NA | (23) |
| Sun et al,
2020 | 56 | 2.0 | N0 | + | + | - | 3-5 | - | - | Atypical lobular
lesion | M, LND, HT | 3 months, ANED | (24) |
| Wang et al,
2020 | 66 | 2.5 | N0 | - | - | - | 60 | + | - | DCIS | M, LND | 13months, ANED | (25) |
| Hu et al,
2020 | 50 | 5.8 | N0 | - | - | - | 70 | NA | NA | NA | L, SLNB | NA | (26) |
| Jain et al,
2021 | 45 | 4.3 | N0 | - | - | - | 45-50 | + | + | DCIS | M, LND, CT | 6 months, ANED | (27) |
| Lin et al,
2021 | 72 | 0.9 | N0 | - | - | - | 30 | + | - | None | L, SLNB, RT | 16 months,
ANED | (28) |
| Kamrani et
al, 2021 | 69 | 2.0 | N0 | + | + | - | NA | NA | NA | DCIS | M, LND | NA | (29) |
| Zuo et al,
2022 | 61 | 2.1 | N1 | - | - | - | 40 | + | - | DCIS | M, SLNB, LND,
CT | 10 months,
ANED | (30) |
| Kaur et al,
2022 | 65 | 18.0 | N0 | - | - | 3+ | 90 | + | Focal + | None | M | 6 months, ANED | (31) |
| Moatasim and
Mamoon, 2022 | 61 | 3.5 | N2 | + | + | - | <10 | NA | NA | DCIS | M, LND, | NA | (32) |
| Lei et al,
2023 | 59 | 3.0 | N0 | - | - | - | 40 | + | - | None | M, LND, CT | 108 months,
ANED | (33) |
| Gong et al,
2024 | 54 | 4.2 | N2 | - | - | - | 70 | + | - | None | Salvage CT | 6 months, PR | (34) |
| Vegni et al,
2023 | 41 | 3.0 | N0 (i+) | + | - | - | 35 | Focal + | Focal + | PILC | L, LND, CT, RT | 12 months,
ANED | (35) |
| Xiao et al,
2021 | 58 | 3.0 | N0 | - | - | - | 70 | + | - | IDC | L, SLNB, CT | 6 months, ANED | (36) |
| Yao et al,
2022 | 45 | 4.0 | N2 | - | - | - | 60 | + | - | IDC, DCIS | M, LND, CT, RT | 36 months,
recurrence | (37) |
| Luo et al,
2023 | 33 | 3.7 | N0 | + | + | - | 70 | - | - | None | M, LND, CT | 8 months, ANED | (38) |
| Present case | 51 | 2.4 | N0 | - | - | - | 70 | + | - | IDC | M, SLNB, CT | 26 months,
ANED |
|
Among the previously reported cases (1,4–38), 19
cases exhibited MCA with ductal carcinoma in situ (DCIS)
and/or invasive ductal carcinoma (IDC), 1 case exhibited
pleomorphic invasive lobular carcinoma, 1 case exhibited atypical
ductal hyperplasia and 13 cases exhibited pure MCA. A previous
report suggested that MCA accompanied with DCIS indicated that MCA
cells were derived from the mucinous metaplasia of epithelial cells
of DCIS, accompanied with loss of ER and PR expression (7). It is difficult to diagnose primary MCA
of the breast and exclude metastatic cancers when the breast
lesions only present with MCA, without other characteristic lesions
of the accompanying breast epithelial cells. Primary pancreatic and
ovarian MCA were positive for both CK7 and CK20, while
gastrointestinal carcinoma was CK7-negative and CK20- and
CDX-2-positive (25), and nearly
all the cases of primary MCA of the breast were CK7-positive and
CK20-negative, as summarized in Table
II. In the present case, CK7 positivity and CK20, CDX-2 and
PAX-8 negativity, and positive expression of breast origin-related
markers, such as GCDFP15 and mammaglobin, supported the diagnosis
of primary MCA of the breast. In the present study, four mucin
glycoprotein markers were analysed. The tumour mainly expressed
MUC1 and MUC6, and partially expressed MUC5AC, but not MUC2. In a
previous case of MCA (30), IHC
staining for mucin glycoprotein showed positive results for MUC1
and MUC5AC, but no staining for MUC2, which was similar to the
present case. The lack of expression of MUC2 in the present case
was different from the expression status in ovarian mucinous
carcinoma, which is mainly positive for MUC2 (39,40).
In the present case, MUC6 was mainly expressed and there was also a
mutation in the MUC6 gene in the molecular analysis. This
was different from previous reports (16,35,37),
which showed a negative expression status for MUC6 in breast MCA.
This may be related to the mutation of the MUC6 gene in the
present case and deserves further investigation. Therefore, when
considering the literature and the present case, it is necessary to
distinguish MCA from other breast diseases. Both MCA and mucinous
carcinoma of the breast have abundant extracellular mucus, but the
latter has no intracellular mucus (47,48).
Both MCA and encapsulated papillary carcinoma have a papillary
structure and lack myoepithelium, but the latter has no
intracellular mucus and strongly diffused expression of ER and PR
(47,48). Mucocele-like lesions are benign
mucinous cysts with uniformly arranged flat or cuboidal epithelium,
mostly accompanied by mucin exudation into the surrounding stroma,
and have a myoepithelium but no heterologous cells, unlike MCA
(4,49).
Most cases of primary MCA of the breast are negative
for ER, PR and HER2 expression; in the literature review, only 4
cases presented with HER2 amplification and 6 cases were hormone
receptor-positive. The median Ki-67 index was 40% (range, 5–90%).
Among the 41 reported cases (including the present case), of which
32 had follow-up information (median follow-up time, 12 months;
range, 3–108 months), 2 had recurrence (7,22). One
of these cases (22) was of a
51-year old female diagnosed with T1N0 triple-negative MCA
accompanied by DCIS, who underwent local surgical treatment and
experienced local recurrence after 96 months of follow-up. The
other case (37) was of a 45-year
old female diagnosed with T2N2 triple-negative MCA accompanied by
IDC and DCIS, with a high Ki-67 index of 60%. This patient received
chemotherapy and radiotherapy after modified radical surgery for
breast cancer and was followed up for 36 months with local
recurrence. There were 2 cases of recurrence among the 23
triple-negative MCA cases with follow-up information. In previous
case reports and systematic reviews of primary MCA of the breast,
researchers generally reported that MCA was a triple-negative
subtype with a high Ki-67 index and a good prognosis. However, with
an increasing number of case reports, it was found that the
recurrence risk of triple-negative MCA was not significantly lower
than that of triple-negative non-specific breast cancer. However,
the number of known cases of primary MCA of the breast remains
limited. In the present case, the patient underwent eight cycles of
chemotherapy followed by 6 months of oral capecitabine and showed
no evidence of recurrence at 26 months of follow-up; however, the
risk of MCA recurrence should not be underestimated.
Next-generation sequencing revealed TP53
missense mutations, similar to those in previous cases (28,33).
As a tumour suppressor gene, TP53 may cause abnormal protein
expression and function when mutated, resulting in tumour
development (50). A missense
mutation was also found in KDR in the present study, which
was similar to the result in a previous case (33). KDR mutations tend to occur
frequently in advanced gastric cancer (51) and renal/adrenal angiosarcomas
(52), suggesting that they might
be related to the occurrence and development of carcinoma, although
this requires further research. In the present case, MUC6-positive
expression was found, along with a missense mutation in
MUC6, which seemed to suggest an association between these
two results. Research on colon adenocarcinoma revealed that the
mutation of MUC6 was associated with a high tumour mutation
burden and microsatellite instability (53). Research on MUC6 mutations is
limited, but the findings of the present study warrant further
investigation. The BARD1 gene is structurally similar to
BRCA1; these two genes can form dimers and play important
roles in DNA repair and apoptosis (54). BARD1 is a moderate-risk gene
for hereditary breast cancer, particularly triple-negative breast
cancer (55). In the present case,
there was a high frequency of missense mutations in BARD1,
which are related to tumour development and the immunohistochemical
characteristics of triple-negative breast cancer. Further research
on this gene may be important to further distinguish between common
triple-negative breast cancer and breast MCA.
In summary, MCA is a rare breast cancer, with only
41 reported cases. The present study reports a case of MCA
accompanied by mutations in the TP53, KDR, MUC6 and
BARD1 genes, which mainly act as tumour suppressor genes and
affect DNA repair, with no recurrence after 26 months of follow-up.
Combining this case with a review of the literature helps us to
better understand the clinicopathological and genetic
characteristics of MCA, and guide treatment.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National High Level Hospital
Clinical Research Funding (grant no. 2022-PUMCH-A-166) and the
Chinese Academy of Medical Sciences Innovation Fund for Medical
Sciences (grant no. 2021-12M-1-053).
Availability of data and materials
The data generated in the present study may be found
in the SRA under accession number PRJNA1171987 or at the following
URL: https://www.ncbi.nlm.nih.gov/sra/PRJNA1171987.
Authors' contributions
XC and XYR designed the report of this case. XC, YCL
and SJS collected the clinical information and imaging examination
data of this case, and participated in the literature search. XYR
performed the pathological data. XYR and XC analyzed the datasets.
XC drafted the manuscript and all authors discussed the results and
commented on the manuscript. XC and XYR confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient, and all the procedures followed the ethical standards of
the Helsinki Declaration.
Patient consent for publication
The patient provided written informed consent for
the publication of this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Koenig C and Tavassoli FA: Mucinous
cystadenocarcinoma of the breast. Am J Surg Pathol. 22:698–703.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tavassoli FA and Devilee P: Pathology and
genetics of tumours of the breast and female genital organs. Lyon,
France: IARC Press, World Health Organization Classification of
Tumours; pp. pp30–31. 2003
|
|
3
|
Wen HY, Desmedt C, Reis-Filho JS and
Schmit F: Mucinous cystadenocarcinoma. In WHO Classifification of
Breast Tumours. 5th edition. Lokuhetty D: International Agency for
Research on Cancer; Lyon, France: pp. 126–127. 2019
|
|
4
|
Rosen PP and Scott M: Cystic
hypersecretory duct carcinoma of the breast. Am J Surg Pathol.
8:31–41. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Domoto H, Terahata S, Yamazaki T, Sato K,
Takeo H and Tamai S: Mucinous cystadenocarcinoma of the breast
showing sulfomucin production. Histopathology. 36:567–569. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Honma N, Sakamoto G, Ikenaga M, Kuroiwa K,
Younes M and Takubo K: Mucinous cystadenocarcinoma of the breast: A
case report and review of the literature. Arch Pathol Lab Med.
127:1031–1033. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen WY, Chen CS, Chen HC, Hung YJ and Chu
JS: Mucinous cystadenocarcinoma of the breast coexisting with
infiltrating ductal carcinoma. Pathol Int. 54:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coyne JD and Irion L: Mammary mucinous
cystadenocarcinoma. Histopathology. 49:659–660. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SH and Chaung CR: Mucinous metaplasia
of breast carcinoma with macrocystic transformation resembling
ovarian mucinous cystadenocarcinoma in a case of synchronous
bilateral infiltrating ductal carcinoma. Pathol Int. 58:601–605.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rakıcı S, Gönüllü G, Gürsel SB, Yıldız L,
Bayrak IK and Yücel I: Mucinous cystadenocarcinoma of the breast
with estrogen receptor expression: A case report and review of the
literature. Case Rep Oncol. 2:210–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gulwani H and Bhalla S: Mucinous
cystadenocarcinoma: A rare primary malignant tumor of the breast.
Indian J Pathol Microbiol. 53:200–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petersson F, Pang B, Thamboo TP and Putti
TC: Mucinous cystadenocarcinoma of the breast with amplification of
the HER2-gene confirmed by FISH: The first case reported. Hum
Pathol. 41:910–913. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sentani K, Tashiro T, Uraoka N, Aosaki Y,
Yano S, Takaeko F and Yasui W: Primary mammary mucinous
cystadenocarcinoma: Cytological and histological findings. Diagn
Cytopathol. 40:624–628. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng Y, Xue D, Wang X, Xu S, Ao Q, Hu Z
and Wang G: Mucinous cystadenocarcinoma of the breast with a
basal-like immunophenotype. Pathol Int. 62:429–432. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Peng J, Zhang Z and Zhang Y: Mammary
mucinous cystadenocarcinoma. Breast J. 18:282–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SE, Park JH, Hong S, Koo JS, Jeong J
and Jung WH: Primary mucinous cystadenocarcinoma of the breast:
Cytologic finding and expression of MUC5 Are different from
mucinous carcinoma. Korean J Pathol. 46:611–616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Witherspoon LE and Oxenhandler RW: A rare
tumor: Mucinous cystadenocarcinoma of the breast. Am Surg.
81:E106–E108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin DL, Hu JL, Shao SH, Sun DM and Wang
JG: Primary mucinous cystadenocarcinoma of the breast with
endocervical-like mucinous epithelium. Breast Care (Basel).
8:445–447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kucukzeybek BB, Yigit S, Sari AA, Rezanko
T, Durak E and Sadullahoglu C: Primary mucinous cystadenocarcinoma
of the breast with amplification of the HER2 gene confirmed by
FISH-case report and review of the literature. Pol J Pathol.
65:70–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seong M, Ko EY, Han BK, Cho SY, Cho EY,
Lee SK and Lee JE: Radiologic findings of primary mucinous
cystadenocarcinoma of the breast: A report of two cases and a
literature review. J Breast Cancer. 19:330–333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koufopoulos N, Goudeli C, Syrios J,
Filopoulos E and Khaldi L: Mucinous cystadenocarcinoma of the
breast: The challenge of diagnosing a rare entity. Rare Tumors.
9:70162017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nayak A, Bleiweiss IJ, Dumoff K and Bhuiya
TA: Mucinous cystadenocarcinoma of the Breast: Report of 2 cases
including one with Long-term local recurrence. Int J Surg Pathol.
26:749–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaur M, Tiwana KK and Singla N: Rare
breast malignancy subtypes: A cytological, histological, and
immunohistochemical correlation. Niger J Surg. 25:70–75. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun M, Su S, Liu Q, Li Q and Wang K:
Mammary synchronous mucinous cystadenocarcinoma and columnar cell
mucinous carcinoma: A case report. Int J Clin Exp Pathol.
13:2381–2386. 2020.PubMed/NCBI
|
|
25
|
Wang X, Li Y, Zhao P, Jia H, Dong X, Zhang
L and Wang C: Primary mucinous cystadenocarcinoma of the breast: A
clinicopathologic analysis of one case and review of the
literature. Int J Clin Exp Pathol. 13:2562–2568. 2020.PubMed/NCBI
|
|
26
|
Hu Y, Tian C, Zhang X, Wei Q and Bian Y:
18F-FDG PET/CT findings in a patient with primary mucinous
cystadenocarcinoma of the breast. Clin Nucl Med. 45:159–160. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jain E, Kumar A, Jain R and Sharma S:
Primary mucinous cystadenocarcinoma of the breast: A rare case
report with review of literature. Int J Surg Pathol. 29:740–746.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin LH, Hernandez O, Zhu K, Guth A, Cotzia
P and Darvishian F: Genetic profile of primary mucinous
cystadenocarcinoma of the breast-A case report. Breast J.
27:731–734. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kamrani G, Nikbakhsh N, Hosseini A,
Ghorbani H, Arefisigaroudi N and Davarian A: Mucinous
cystadenocarcinoma of breast in a 69-year-old woman with positive
hormone receptors, the first case reported. Caspian J Intern Med.
12 (Suppl 2):S444–S446. 2021.PubMed/NCBI
|
|
30
|
Zuo C and Xie J: Mixed primary mucinous
cystadenocarcinoma and invasive ductal carcinoma of the breast: A
case report and literature review. Transl Cancer Res. 11:4455–4464.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaur K, Shah A, Gandhi J and Trivedi P:
Mucinous cystadenocarcinoma of the breast: A new entity with broad
differentials-a case report. J Egypt Natl Canc Inst. 34:92022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moatasim A and Mamoon N: Primary breast
mucinous cystadenocarcinoma and review of literature. Cureus.
14:e230982022.PubMed/NCBI
|
|
33
|
Lei T, Shi YQ and Chen TB: Mammary
mucinous cystadenocarcinoma with long-term follow-up: Molecular
information and literature review. Diagn Pathol. 18:132023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong Y, Geng X, Liu Y, Zhang R, Liu Y and
Li H: Mucinous cystadenocarcinoma of the breast with bone
metastases: First case report and literature review. Oncol Res
Treat. 47:97–103. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vegni F, D'Alessandris N, Santoro A,
Angelico G, Scaglione G, Carlino A, Arciuolo D, Valente M, Sfregola
S, Natale M, et al: Primary mucinous cystadenocarcinoma of the
breast intermixed with pleomorphic invasive lobular carcinoma: The
first report of this rare association. J Pers Med. 13:9482023.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao N, Xiao SB, Chen CW and Gao YT:
Breast mucinous cystadenocarcinoma: Report of a case. Zhonghua Bing
Li Xue Za Zhi. 50:1302–1304. 2021.(In Chinese). PubMed/NCBI
|
|
37
|
Yao M, Cao LQ, Gao YH and Gao HW: Mixed
mucinous cystadenocarcinoma and columnar cell mucinous carcinoma of
the breast with axillary lymph node metastases: Report of a case.
Zhonghua Bing Li Xue Za Zhi. 51:567–569. 2022.(In Chinese).
PubMed/NCBI
|
|
38
|
Luo SY, Zhou ML, Jian L and Wang DZ:
Primary mucinous cystadenocarcinoma of the breast with hormone
receptor expression: Report of a case. Zhonghua Bing Li Xue Za Zhi.
52:1055–1057. 2023.(In Chinese). PubMed/NCBI
|
|
39
|
Hirabayashi K, Yasuda M, Kajiwara H, Itoh
J, Miyazawa M, Hirasawa T, Muramatsu T, Murakami M, Mikami M and
Osamura RY: Alterations in mucin expression in ovarian mucinous
tumors: Immunohistochemical analysis of MUC2, MUC5AC, MUC6, and
CD10 expression. Acta Histochem Cytochem. 41:15–21. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang J and El-Bahrawy M: Expression
profile of mucins (MUC1, MUC2, MUC5AC, and MUC6) in ovarian
mucinous tumours: Changes in expression from benign to malignant
tumours. Histopathology. 66:529–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cibulskis K, Lawrence MS, Carter SL,
Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES
and Getz G: Sensitive detection of somatic point mutations in
impure and heterogeneous cancer samples. Nat Biotechnol.
31:213–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saunders CT, Wong WS, Swamy S, Becq J,
Murray LJ and Cheetham RK: Strelka: Accurate somatic small-variant
calling from sequenced tumor-normal sample pairs. Bioinformatics.
28:1811–1817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang K, Li M and Hakonarson H: ANNOVAR:
Functional annotation of genetic variants from high-throughput
sequencing data. Nucleic Acids Res. 38:e1642010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Talevich E, Shain AH, Botton T and Bastian
BC: CNVkit: Genome-Wide copy number detection and visualization
from targeted DNA sequencing. PLoS Comput Biol. 12:e10048732016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jiang Z, Li J, Chen J, Liu Y, Wang K, Nie
J, Wang X, Hao C, Yin Y, Wang S, et al: Chinese society of clinical
oncology (CSCO) breast cancer guidelines 2022. Transl Breast Cancer
Res. 3:132022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tay TKY and Tan PH: Papillary neoplasms of
the breast-reviewing the spectrum. Mod Pathol. 34:1044–1061. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen S, Wang J, Yang L, Ji M and Chen S:
Comparative analysis of clinicopathologic characteristics and
molecular subtypes of invasive papillary carcinoma of the breast
and invasive ductal carcinoma: Results from SEER database. J BUON.
26:1991–2002. 2021.PubMed/NCBI
|
|
49
|
Ginter PS, Tang X and Shin SJ: A review of
mucinous lesions of the breast. Breast J. 26:1168–1178. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kato S, Han SY, Liu W, Otsuka K, Shibata
H, Kanamaru R and Ishioka C: Understanding the function-structure
and function-mutation relationships of p53 tumor suppressor protein
by high-resolution missense mutation analysis. Proc Natl Acad Sci
USA. 100:8424–8429. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Oh S, Nam SK, Lee KW, Lee HS, Park Y, Kwak
Y, Lee KS, Kim JW, Kim JW, Kang M, et al: Genomic and
Transcriptomic characterization of gastric cancer with bone
metastasis. Cancer Res Treat. 56:219–237. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Argani P, Saoud C and Antonescu CR:
Molecular analysis of Renal/adrenal angiosarcomas reveals high
frequency of recurrent genetic alterations. Genes Chromosomes
Cancer. 63:e232682024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen M, Zhang X, Ming Z, Lingy u, Feng X,
Han Z and An HX: Characterizing and forecasting
neoantigens-resulting from MUC mutations in COAD. J Transl Med.
22:3152024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu LC, Wang ZW, Tsan JT, Spillman MA,
Phung A, Xu XL, Yang MC, Hwang LY, Bowcock AM and Baer R:
Identification of a RING protein that can interact in vivo with the
BRCA1 gene product. Nat Genet. 4:430–440. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Breast Cancer Association Consortium, .
Dorling L, Carvalho S, Allen J, González-Neira A, Luccarini C,
Wahlström C, Pooley KA, Parsons MT, Fortuno C, et al: Breast cancer
risk genes-Association analysis in more than 113,000 women. N Engl
J Med. 384:428–439. 2021. View Article : Google Scholar : PubMed/NCBI
|