Introduction
Breast cancer is one of the most prevalent malignant
tumors among women, with an estimated global incidence of ~2.3
million new cases each year (1).
Breast cancer has emerged as a major public health concern, and its
incidence is steadily increasing. According to the World Health
Organization, breast cancer is the leading cause of cancer-related
mortality among women globally; it severely impacts the physical
and mental health of patients as well as their quality of life
(2). Early detection is primarily
achieved through mammography (MG), resulting in a 5-year survival
rate of >90% in developed countries. However, in low-income
countries, breast cancer is frequently diagnosed at advanced
stages, leading to a marked reduction in the 5-year survival rate
to <40% (2). Thus, early and
accurate diagnosis is crucial for improving the prognosis of
patients with breast cancer.
Conventional MG, however, has limitations in early
diagnosis (3). The complex
anatomical structure of breast tissues and variations in tissue
density often hinder the accurate detection of early malignant
tumors, particularly those with small diameters or located deep
within the breast (4). To address
these challenges, new imaging techniques, including ultrasound
shear wave elastography (SWE) and magnetic resonance imaging (MRI),
have gained considerable attention (5,6).
SWE offers a distinct advantage in characterizing
the nature of breast lumps as it quantitatively evaluates the
stiffness of tissue and provides objective measurements of
elasticity parameters (7)
Conversely, MRI delivers extensive information on tissue perfusion
and facilitates a comprehensive assessment of lesion morphology,
boundaries and internal structure through the dynamic observation
of hemodynamic characteristics, which is particularly beneficial
for the diagnosis of lesions located deep within the tissue
(6).
The primary objective of the present study was to
investigate the diagnostic efficacy of a combination of MG, SWE and
MRI in the early of detection breast cancer. By comparing the
strengths of these imaging techniques in lesion detection,
characterization and localization, the study aims to provide an
accurate and comprehensive foundation for medical imaging in the
early diagnosis of breast cancer. The integration of MG, SWE and
MRI may leverage their respective advantages to result in a
complementary and comprehensive diagnostic strategy. Through the
comparative analysis of different techniques in breast cancer
diagnosis, the study aims to provide a reliable and comprehensive
diagnostic solution for clinicians, ultimately enhancing the early
screening and diagnosis of breast cancer.
Materials and methods
Study design
In the present study, prospective data collection
with retrospective analysis was conducted on patients with breast
tumors who underwent lumpectomy at Yancheng No. 1 People's Hospital
(Yancheng First Hospital, Affiliated Hospital of Nanjing University
Medical School; Yancheng, China) from December 2021 to January
2023. Patients were categorized into benign and malignant groups
based on histopathology results. Relevant data, including age,
medical history, imaging results and lesion characteristics, were
systematically collected. The study adhered to the Declaration of
Helsinki and was approved by the Ethics Committee of The First
People's Hospital of Yancheng (Yancheng, China; approval no.
2021-J-098).
Inclusion criteria
Participants were included based on the following
criteria: i) Meeting the diagnostic criteria for breast tumors as
outlined in the Chinese expert consensus on the clinical diagnosis
and treatment of advanced breast cancer (2021) (8); ii) undergoing lumpectomy at Yancheng
No. 1 People's Hospital, with pathological confirmation of breast
cancer; iii) being a female aged between 18 and 65 years; and iv)
having completed follow-up treatment at Yancheng No. 1 People's
Hospital, with comprehensive clinical data available. The exclusion
criteria were as follows: i) Prior receipt of surgery, chemotherapy
or medication before consultation; ii) pregnancy or lactation; iii)
presence of severe pulmonary or cardiac diseases, or abnormal liver
or kidney function; iv) coexistence of other diffuse breast
lesions; v) implantation of devices such as pacemakers that could
be affected by magnetic fields; vi) intolerance to ultrasound
contrast agents; vii) presence of breast implants; and viii)
lesions with a largest diameter >3 cm.
Study methods
MG
The patients were examined by MG using the
Selenia® Dimensions® Digital Mammography
System (Hologic, Inc.). Prior to the examination, patients were
assessed for contraindications and instructed to remove any
external objects that could interfere with the imaging process.
Patients were asked to maintain still during the procedure and to
position their arms at their sides to facilitate the upward and
forward positioning of the breast tissue. The examination utilized
an automatic compression system and automatic exposure control
system, with exposure settings ranging from 22 to 49 kV for voltage
and 4 to 500 mA for current. The image acquisition process complied
with the technical standards established by the Chinese Medical
Association in 2014 (9) The breasts
of the patients were imaged from the mediolateral oblique and
bilateral craniocaudal positions, with magnified views and
supplementary positions employed as necessary. It was crucial to
ensure that the axillary lymph nodes, parasternally located glands
and other relevant areas were thoroughly examined to avoid missed
diagnoses.
Ultrasound SWE
An AixPlorer Color Doppler Ultrasound machine
(SuperSonic Imagine), equipped with a probe operating at
frequencies of 4–15 MHz, was utilized for SWE. Patients were
positioned supine, with the upper limb on the affected side
elevated to fully expose the breast and axillary area. Initially, a
2-dimensional ultrasound examination was conducted to evaluate the
morphology and blood flow characteristics of the lesion. The SWE
mode was then activated, and the probe was gently placed over the
lesion. The size of the sampling frame in the elastic detection
area was adjusted until there was no red extrusion mark (a visual
indicator signaling that the elasticity measurement exceeds the
optimal range of the device) at the top of the frame. This
adjustment ensured that the sampling frame adequately covered the
lesion and the surrounding area of stiff tissue. For larger lesions
extending beyond the sampling frame, multiple sections were
measured separately. The part of the lesion exhibiting the highest
elasticity value was recorded. Subsequently, patients were
instructed to hold their breath for 3 sec. After acquiring a stable
image, the elastic parameters of the lesion were recorded,
including the maximum value of the elastic modulus (E-max), the
mean value of the elastic modulus (E-mean) and the elasticity ratio
of the lesion to fat (E-ratio). Each lesion was assessed three
times, and the average value was calculated. These parameters were
measured by using the Q-Box™ quantification tool
(SuperSonic Imagine).
MRI
Dynamic contrast-enhanced (DCE) MRI and
diffusion-weighted imaging (DWI) were performed using the MAGNETOM
Skyra 3.0T (Siemens AG). The scanning parameters were as follows:
For T2-weighted imaging in the axial position, the repetition time
(TR)/echo time (TE) values were set at 6,710/75 msec, with 2
excitations and a slice thickness of 4 mm; for T1-weighted imaging
in the axial position, the TR/TE values were 630/12 msec, with 2
excitations and a slice thickness of 5 mm; and for DWI, the TR/TE
values were set at 3,400/71 msec, with a slice thickness of 5 mm
and a b-value of 800 sec/mm2. For DCE MRI, gadopentetate
dimeglumine (Magnevist; Bayer AG) was used as the contrast agent at
a dosage of 0.2 ml/kg under high pressure, followed by axial
scanning to obtain five sequences. The parameters for this sequence
were TR/TE values of 4.66/1.68 msec, with 1 excitation, a slice
thickness of 1.6 mm, and an 80-sec interval between each
period.
Study indicators
The imaging parameters were compared between the
benign and malignant groups. This included MG-related parameters,
namely breast glandular density, margins, borders and axillary
lymph node involvement; SWE-related parameters, namely the E-ratio,
E-mean and E-max; and MRI-related parameters, namely the maximum
diameter measured by MRI, lesion morphology, mass margins, signal
enhancement and time-signal intensity curve (TIC).
Statistical analysis
Data analysis was conducted using SPSS version 20.0
(IBM Corp.). Basic characteristics of the samples were expressed as
the mean ± standard deviation. Independent samples t-tests were
employed to compare differences in continuous variables between the
two groups, while χ2 or Fisher's exact tests were used
for the analysis of categorical variables. The diagnostic efficacy
of each of the combinations of MG + SWE, MG + MRI, SWE + MRI and MG
+ SWE + MRI was assessed, and the sensitivity, specificity,
positive predictive value and negative predictive value were
calculated. Receiver operating characteristic (ROC) curves were
plotted to evaluate the diagnostic accuracy of each combination
method and DeLong's test was employed to compare the area under the
curve (AUC) values between different diagnostic combinations. To
adjust for potential type I errors arising from multiple
comparisons among the AUCs, a Bonferroni correction was applied. A
significance level of α=0.05 (two-tailed) was considered to
indicate statistical significance.
Results
Analysis of baseline data
A total of 93 patients with breast tumors were
included in the present study, of which 37 patients were in the
benign group (mean age, 43.75±9.22 years) and 56 patients were in
the malignant group (mean age, 46.42±9.27 years). No significant
differences were detected between the two groups regarding age and
tumor characteristics, including location, shape, growth direction
and posterior features. However, the malignant group exhibited
significantly larger tumor sizes (3.32±1.45 vs. 1.72±0.81 cm), as
well as a higher percentage of irregular mass margins (82.14 vs.
43.24%), weak echogenicity (89.29 vs. 35.14%) and
microcalcification (69.64 vs. 24.32%) compared with the benign
group (P<0.001; Table I).
 | Table I.Analysis of the baseline data of all
study subjects. |
Table I.
Analysis of the baseline data of all
study subjects.
| Parameters | Benign group
(n=37) | Malignant group
(n=56) |
t/χ2-value | P-value |
|---|
| Age, mean ± SD,
years | 43.75±9.22 | 46.42±9.27 | −1.362 | 0.176 |
| Tumor size, mean ±
SD, cm | 1.72±0.81 | 3.32±1.45 | −6.105 | <0.001 |
| Location, n (%) |
|
| 0.147 | 0.702 |
|
Right | 20 (54.05) | 28 (50.00) |
|
|
| Left | 17 (45.95) | 28 (50.00) |
|
|
| Tumor shape, n
(%) |
|
| 0.345 | 0.557 |
| Oval | 12 (32.43) | 15 (26.79) |
|
|
|
Irregular | 25 (67.57) | 41 (73.21) |
|
|
| Growing direction, n
(%) |
|
| 2.890 | 0.089 |
|
Parallel | 32 (86.49) | 40 (71.43) |
|
|
|
Vertical | 5 (13.51) | 16 (28.57) |
|
|
| Mass margin, n
(%) |
|
| 15.171 | <0.001 |
|
Smooth | 21 (56.76) | 10 (17.86) |
|
|
|
Irregular | 16 (43.24) | 46 (82.14) |
|
|
| Echogenicity, n
(%) |
|
| 29.896 | <0.001 |
|
Strong | 24 (64.86) | 6 (10.71) |
|
|
|
Weak | 13 (35.14) | 50 (89.29) |
|
|
| Calcification |
|
| 18.322 | <0.001 |
| No | 28 (75.68) | 17 (30.36) |
|
|
|
Microcalcification | 9 (24.32) | 39 (69.64) |
|
|
| Posterior features,
n (%) |
|
| - | 0.517 |
|
Enhanced | 34 (91.89) | 48 (85.71) |
|
|
|
Masked | 3 (8.11) | 8 (14.29) |
|
|
Imaging analysis of typical cases
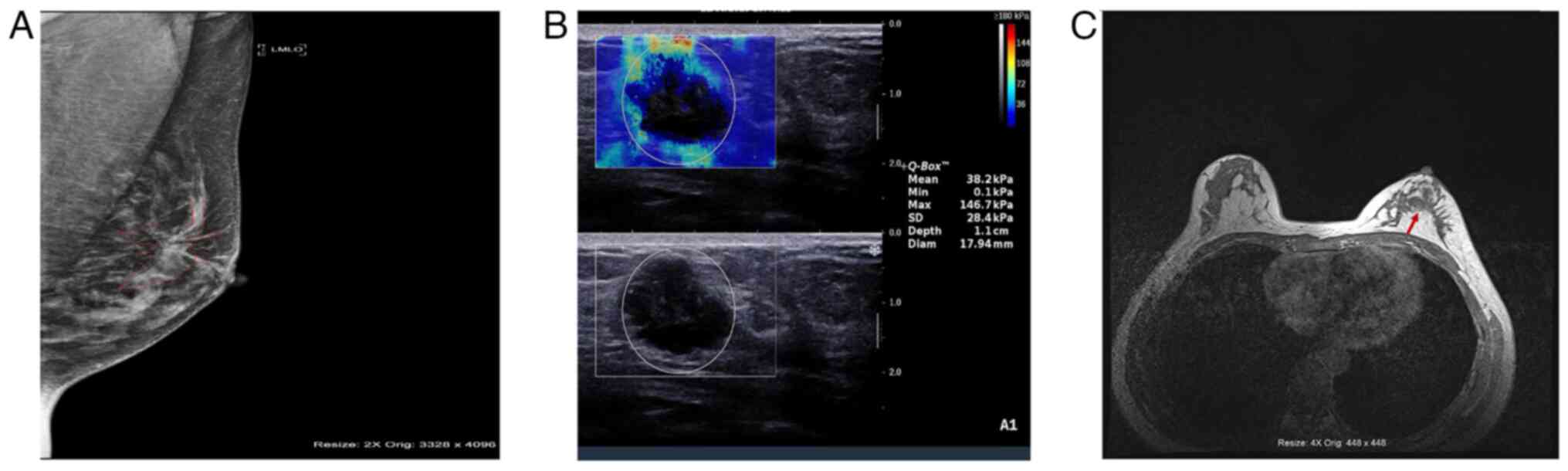
Typical imaging results from the three techniques in
different patients are presented in Fig. 1. The MG image reveals multiple
punctate and nodular calcified foci in the left breast, with larger
foci located in the central region (Fig. 1A). The SWE results include an E-mean
of 38.2 kPa and an E-max of 146.7 kPa, suggestive of malignant
lesions (Fig. 1B). An irregular
mass in the anterior left breast is revealed by MRI, characterized
by inhomogeneous mild-to-moderate enhancement and high-to-low mixed
signals, with a greater vascular presence on the anterior side
compared with the contralateral side (Fig. 1C).
Comparison of imaging parameters
MG examination results indicated that the malignant
group had a greater proportion of cases with high breast glandular
density (57.1 vs. 27.0%), irregular mass margins (66.1 vs. 35.1%),
unclear mass borders (69.6 vs. 43.2%) and axillary lymph node
involvement (75.0 vs. 48.6%) compared with the benign group
(P<0.05). SWE results showed that the benign group had
significantly lower E-ratio (3.25±0.92 vs. 15.30±2.75), E-mean
(21.14±8.70 vs. 44.42±14.26) and E-max values (99.88±37.07 vs.
287.63±89.97) compared with the malignant group (P<0.001). MRI
results demonstrated that the malignant group had a larger
MRI-measured maximum diameter (2.06±0.44 vs. 1.16±0.55 cm) and a
higher proportion of cases with irregular lesion morphology (67.9
vs. 35.1%), irregular mass margins (58.9 vs. 21.6%), signal
enhancement (78.6 vs. 54.1%) and Type III TICs (48.2 vs. 13.5%)
compared with the benign group (P<0.05; Table II).
 | Table II.Comparison of imaging parameters. |
Table II.
Comparison of imaging parameters.
| A, MG |
|---|
|
|---|
| Parameters | Benign group,
(n=37) | Malignant group
(n=56) |
χ2-value | P-value |
|---|
| Glandular density,
n (%) |
|
| 8.159 | 0.004 |
|
Low | 27 (73.0) | 24 (42.9) |
|
|
|
High | 10 (27.0) | 32 (57.1) |
|
|
| Mass margin, n
(%) |
|
| 8.578 | 0.003 |
|
Smooth | 24 (64.9) | 19 (33.9) |
|
|
|
Irregular | 13 (35.1) | 37 (66.1) |
|
|
| Mass border, n
(%) |
| | 6.426 | 0.011 |
|
Clear | 21 (56.8) | 17 (30.4) |
|
|
|
Unclear | 16 (43.2) | 39 (69.6) |
|
|
| Axillary lymph
nodes, n (%) |
|
| 6.758 | 0.009 |
| No | 19 (51.4) | 14 (25.0) |
|
|
|
Yes | 18 (48.6) | 42 (75.0) |
|
|
|
| B, SWE |
|
|
Parameters | Benign group,
(n=37) | Malignant group
(n=56) | t-value | P-value |
|
| E-ratio, mean ±
SD | 3.25±0.92 | 15.30±2.75 | −25.663 | <0.001 |
| E-mean, mean ±
SD | 21.14±8.70 | 44.42±14.26 | −8.888 | <0.001 |
| E-max, mean ±
SD | 99.88±37.07 | 287.63±89.97 | −12.020 | <0.001 |
|
| C, MRI |
|
|
Parameters | Benign group,
(n=37) | Malignant group
(n=56) |
χ2/t-value | P-value |
|
| MRI-measured
maximum diameter, mean ± SD, cm | 1.16±0.55 | 2.06±0.44 | −8.724 | <0.001 |
| Lesion morphology,
n (%) |
|
| 9.632 | 0.002 |
|
Regular | 24 (64.9) | 18 (32.1) |
|
|
|
Irregular | 13 (35.1) | 38 (67.9) |
|
|
| Mass margin, n
(%) |
|
| 12.580 | <0.001 |
|
Smooth | 29 (78.4) | 23 (41.1) |
|
|
|
Irregular | 8 (21.6) | 33 (58.9) |
|
|
| Signal enhancement,
n (%) |
|
| 6.241 | 0.012 |
|
Normal | 17 (45.9) | 12 (21.4) |
|
|
|
Enhanced | 20 (54.1) | 44 (78.6) |
|
|
| TIC, n (%) |
|
| 12.977 | 0.002 |
| Type
I | 14 (37.8) | 9 (16.1) |
|
|
| Type
II | 18 (48.6) | 20 (35.7) |
|
|
| Type
III | 5 (13.5) | 27 (48.2) |
|
|
Analysis of diagnostic efficacy
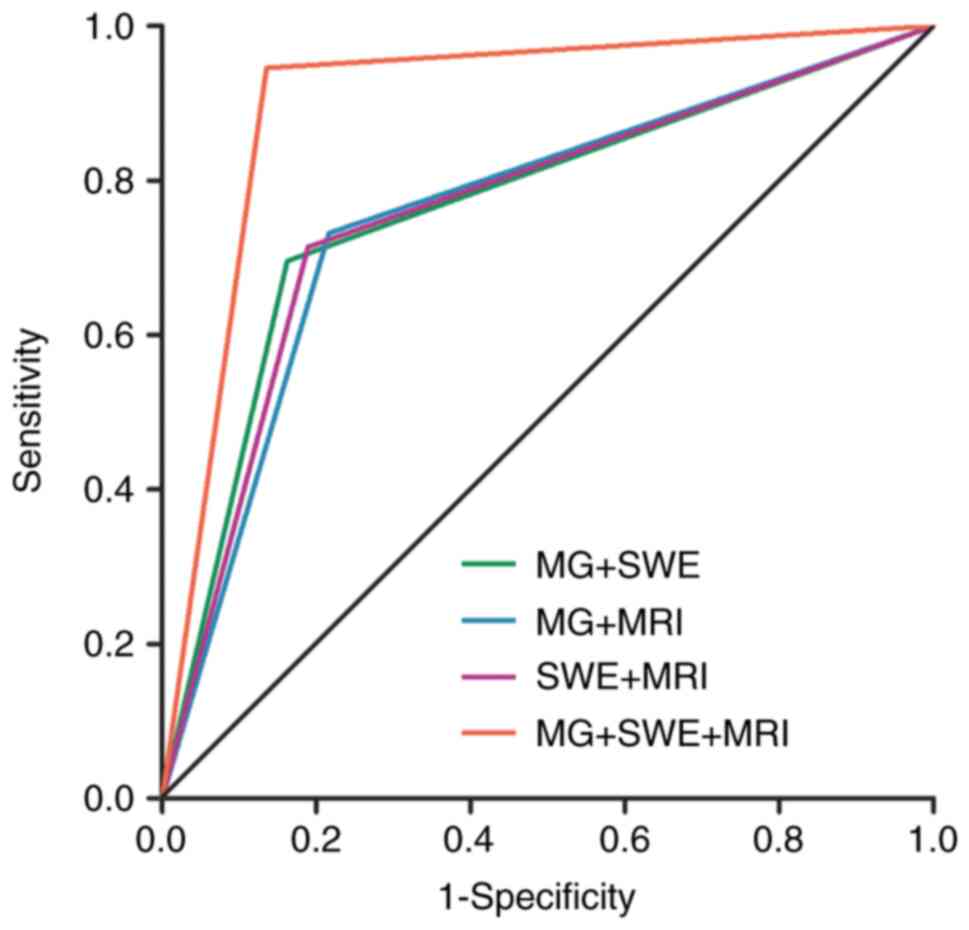
The diagnostic sensitivity, specificity and AUC for
MG + SWE were 69.6, 83.8 and 0.767% (95% CI, 0.667–0.867),
respectively. For MG + MRI, these values were 73.2, 78.4 and 0.758%
(95% CI, 0.655–0.860), respectively; for SWE + MRI, they were 71.4,
81.1 and 0.763% (95% CI, 0.661–0.864), respectively; and for MG +
SWE + MRI, they were 94.6, 86.5 and 0.906% (95% CI, 0.832–0.979),
respectively (Tables III and
IV). The diagnostic efficacy of MG
+ SWE + MRI was significantly superior to that of MG + SWE, MG +
MRI and SWE + MRI (P<0.001). However, no significant differences
were detected in diagnostic efficacy among the combinations of MG +
SWE, MG + MRI and SWE + MRI (Fig.
2, Table IV).
 | Table III.Results for different combinations of
imaging methods for benign and malignant breast tumors and their
diagnostic efficacy. |
Table III.
Results for different combinations of
imaging methods for benign and malignant breast tumors and their
diagnostic efficacy.
|
| Pathological
diagnosis, n |
|
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Parameters | Malignant | Benign | Sensitivity, % | Specificity, % | Positive predictive
value, % | Negative predictive
value, % |
|---|
| MG + SWE |
|
|
|
|
|
|
|
Malignant | 39 | 6 | 69.6 | 83.8 | 86.7 | 64.6 |
|
Benign | 17 | 31 |
|
|
|
|
| MG + MRI |
|
|
|
|
|
|
|
Malignant | 41 | 8 | 73.2 | 78.4 | 83.7 | 65.9 |
|
Benign | 15 | 29 |
|
|
|
|
| SWE + MRI |
|
|
|
|
|
|
|
Malignant | 40 | 7 | 71.4 | 81.1 | 85.1 | 65.2 |
|
Benign | 16 | 30 |
|
|
|
|
| MG + SWE + MRI |
|
|
|
|
|
|
|
Malignant | 53 | 5 | 94.6 | 86.5 | 91.4 | 91.4 |
|
Benign | 3 | 32 |
|
|
|
|
 | Table IV.Comparison of the AUCs for different
combinations of imaging methods. |
Table IV.
Comparison of the AUCs for different
combinations of imaging methods.
| Parameters | AUC | 95% CI |
P-valuea |
P-valueb |
P-valuec |
P-valued |
P-valuee |
P-valuef |
|---|
| MG + SWE | 0.767 | 0.667–0.867 | 0.8533 | 0.9303 | 0.0975 | <0.001 | <0.001 | <0.001 |
| MG + MRI | 0.758 | 0.655–0.860 |
|
|
|
|
|
|
| SWE + MRI | 0.763 | 0.661–0.864 |
|
|
|
|
|
|
| MG + SWE + MRI | 0.906 | 0.832–0.979 |
|
|
|
|
|
|
Discussion
The development of breast cancer is influenced by a
variety of factors, including heredity, hormone levels and
lifestyle choices, and its early diagnosis is challenging due to
the complex structure of breast tissue (10). Imaging is crucial in the early
screening and diagnosis of breast cancer, and imaging techniques
including MG, SWE and MRI are typically used for the detection of
breast cancer during the early stages. Each method has strengths
and limitations, particularly in the identification of dense or
early-stage lesions, which can impede diagnostic accuracy.
Therefore, the present study aimed to elucidate these limitations
by exploring the combination of MG, SWE and MRI. The findings
indicate that the diagnostic value of this combined approach was
superior to that of pairwise combinations, thereby providing a
strong foundation for medical imaging in the early screening and
diagnosis of breast cancer.
Beyond diagnostic efficacy, the cost-effectiveness
of these modalities is crucial for clinical decision-making. MG
remains widely accessible and inexpensive, making it a
cost-effective first-line screening tool (11). SWE is a newer technique that
provides affordable quantitative data on tissue stiffness, which
helps to differentiate benign from malignant lesions and
potentially reduces unnecessary biopsies for Breast
Imaging-Reporting and Data System (BI-RADS) IVa lesions (12). Although MRI is more expensive, it
delivers detailed information on vasculature and perfusion, which
is valuable for the imaging of complex or dense cases. A previous
study suggests that MRI is most cost-effective when used
selectively, to complement MG and SWE in challenging scenarios
(13). Integrating these methods
enhances diagnostic efficacy and helps to manage costs by tailoring
modality selection based on patient-specific factors.
Breast density influences the accuracy of diagnostic
imaging as dense breast tissue can obscure lesions, thereby
reducing the sensitivity of MG. Although MG is a traditional
imaging method favored for use in the diagnosis of breast disease
due to its simplicity, speed and non-invasive nature, it has
notable limitations in detection rates, particularly in cases with
high breast glandular density or early lesions (14). Conventional imaging techniques,
including MG and ultrasound, often exhibit low sensitivity and
specificity, particularly in young patients with dense breast
tissue, breast implants or post-surgical scarring (15). Thus, the density of breast tissue
continues to pose a challenge to the sensitivity of MG, emphasizing
that alternative imaging strategies are necessary (16).
By contrast, SWE is a valuable tool for the
quantitative assessment of breast tissue elasticity, allowing for
an objective evaluation of tissue stiffness. This technique has
shown considerable promise in distinguishing between benign and
malignant solid breast masses in previous studies (17–19).
MRI complements the aforementioned methods by monitoring the
dynamic perfusion processes within lesions, providing essential
physiological information about vascular structure, blood flow rate
and blood volume. This capability is crucial for determining the
blood supply in breast cancer and revealing the physiological
status of the lesion. Thus, the integration of MG, SWE and MRI
offers a comprehensive and complementary approach for the diagnosis
of breast cancer. By addressing the limitations of individual
imaging methods, this combined strategy enhances diagnostic
accuracy and reliability, ultimately improving the early detection
of breast cancer and contributing positively to patient
outcomes.
SWE provides quantitative measurements of tissue
stiffness in kilopascals by imaging the propagation of transverse
waves through tissue. This method enables non-invasive, real-time
assessments of tissue elasticity (20). Sravani et al (21) investigated the reproducibility of
SWE and its alignment with histological findings, which
demonstrated its potential for the classification of breast masses
as benign or malignant. In contrast to static elastography, which
uses grayscale ultrasound to indicate relative stiffness, SWE
offers the advantage of providing objective measurements of lesion
stiffness in kilopascals. SWE has shown diagnostic accuracy similar
to that of strain elastography (SE) in differentiating between
benign and malignant breast lesions. For example, Li et al
(22) conducted a screening study
involving 623 breast lesions, which revealed that SWE and
conventional ultrasound exhibited superior diagnostic performance
in the diagnosis of cystic solid lesions compared with non-cystic
solid lesions. Notably, the threshold for each SWE parameter varied
between the cystic and non-cystic lesion groups, being higher in
the former group than in the latter. The development of breast
cancer is associated with physiological changes, including cell
proliferation and neovascularization, which affect the mechanical
properties of the tissue. By directly measuring the elastic modulus
of tissue, SWE can indirectly provide information on the density
and arrangement of tissue cells, thereby reflecting the
physiological characteristics of breast cancer (23). Furthermore, Shahzad et al
(24) concluded that both SE and
SWE, when used as supplemental techniques to conventional B-mode
breast ultrasound, enhanced the characterization of solid breast
lesions and reduced unnecessary biopsies for BI-RADS IVa
lesions.
In addition to MG, SWE and MRI, it is important to
acknowledge other advanced imaging techniques such as positron
emission tomography-computed tomography (PET-CT) and 3-dimensional
(3D) MG. PET-CT is primarily used for the detection of metastatic
disease, and has limited use in the detection of early breast
cancer due to its low resolution for small lesions, as well as its
high cost (25). However, it is
highly sensitive at detecting metabolically active tumors, which
can complement the anatomical and functional data provided by SWE
and MRI (26) 3D MG, also known as
tomosynthesis, improves detection rates compared with traditional
2D mammography, particularly in women with dense breasts, by
providing a detailed, layered view of the breast tissue. However,
it does not offer the quantitative or functional insights provided
by SWE and MRI (27). Integrating
these other modalities may further enhance diagnostic accuracy,
particularly in complex cases, although cost-effectiveness and
accessibility remain considerations.
In the present study, when MG, SWE and MRI were
combined, specific indicators of diagnostic efficacy were
evaluated; specifically, the sensitivity, specificity, positive
predictive value and negative predictive values were 94.6, 86.5,
91.4 and 91.4%, respectively, with an AUC of 0.906 (95% CI,
0.832–0.979). This demonstrates the high sensitivity and
reliability of this combined approach in the accurate diagnosis of
breast cancer.
Additionally, the present study examined the
differences in diagnostic efficacy among various combinations of
imaging techniques. The results indicated that while there were
differences in diagnostic efficacy among MG + SWE, MG + MRI, and
SWE + MRI, these differences were not statistically significant.
Zhang et al (28) reported
that multiparametric MRI, incorporating DCE-MRI and DWI with
apparent diffusion coefficient mapping, enabled accurate breast
cancer diagnosis; models using both quantitative and qualitative
descriptors from DCE-MRI and DWI exhibited high diagnostic
accuracy. Similarly, Yadav et al (29) demonstrated that high-resolution DWI,
a contrast-free MRI technique, improved lesion detection compared
with DCE-MRI. Its diagnostic performance was found to be comparable
with that of MRI, suggesting a potentially adjunctive role for
high-resolution DWI in conjunction with MG.
The combination of MG, SWE and MRI may offer
considerable potential in the personalization of clinical breast
cancer management. Each of these imaging modalities provides
distinct information about tumor characteristics, which may be
beneficial when tailoring an individual treatment plan. For
example, MG can be used to detect calcifications and architectural
distortions, while SWE offers quantitative data on tissue stiffness
that indicates tumor aggressiveness, and MRI provides detailed
information on tumor vascularity and perfusion. By combining these
modalities, clinicians can obtain a comprehensive profile of the
tumor, enabling more precise risk stratification and treatment
planning. This integrated imaging approach may be used to inform
decisions regarding the necessity of neoadjuvant chemotherapy, the
extent of surgical intervention, or the use of targeted therapies,
in line with the principles of personalized medicine. Moreover,
advanced imaging parameters, such as the elasticity measurements
from SWE and the DCE patterns from MRI, could be used to monitor
the response to treatment, allowing therapy adjustments to be made
on the basis of real-time tumor changes. This approach should not
only enhance diagnostic accuracy but also support a more
individualized and adaptive treatment strategy, ultimately
improving patient outcomes.
The findings of the present study could have
translational impact, although further research is required to
clarify this. For instance, SWE assesses tissue stiffness, which is
associated with the composition of the extracellular matrix (ECM)
and the presence of fibrotic tissue. In breast cancer, increased
stiffness often arises from collagen deposition and other ECM
components, which contribute to tumor progression and metastasis
(30). This indicates that SWE can
quantitatively measure stiffness, thereby providing indirect
information on fibrosis and ECM remodeling within the tumor.
Moreover, MRI can capture hemodynamic changes associated with tumor
angiogenesis, which is a hallmark of cancer progression (31), and DCE-MRI evaluates vascular
permeability and blood flow, reflecting abnormal blood vessel
development in the tumor microenvironment. In additional,
alterations in perfusion and oxygenation detected by MRI may
indicate tumor hypoxia, which promotes aggressive phenotypes and
therapy resistance (32). By
associating these imaging features with factors within the tumor
microenvironment, the combined application of SWE and MRI enhances
diagnostic accuracy while offering valuable insights into tumor
biology.
The present study had certain limitations that
should be considered when interpreting the results. First, the
relatively small sample size of 93 cases may affect the
generalizability and statistical significance of the findings.
Future studies could address this by increasing the sample size
through multicenter collaborations to enhance the robustness and
external validity of the results. Second, the retrospective nature
of the study might have restricted data collection and recording,
leading to potential biases. Conducting prospective studies in the
future would allow for more controlled data collection and the
opportunity to explore additional clinical variables in real time.
Additionally, the present study lacks a separate validation group
to confirm the diagnostic performance of the combined imaging
modalities due to limitations in sample availability. Future
research should address this by including independent validation
datasets to confirm the reproducibility of the results and provide
stronger evidence for the clinical utility of the multimodal
imaging approach in breast cancer diagnosis. Furthermore, as the
present study primarily focused on diagnostic performance, the
applicability of this combination of imaging techniques for the
analysis of long-term follow-up outcomes, such as treatment
response and survival rates, requires further investigation. Future
research could include longitudinal follow-up studies to evaluate
the impact of these imaging modalities on patient outcomes,
providing a more comprehensive understanding of their clinical
utility in breast cancer management. Lastly, the present study did
not explore the biological or physical mechanisms underlying the
combined use of MG, SWE and MRI in breast cancer diagnosis. Future
research should focus on mechanistic and translational studies to
clarify these principles, providing insights into the potential
synergies and enhancing understanding of their diagnostic
roles.
In conclusion, the combination of MG with SWE and
MRI demonstrates a strong performance in the early diagnosis of
breast cancer, offering high diagnostic accuracy and reliability.
Overall, the present study provides a solid medical imaging
foundation for the early screening and diagnosis of breast
cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by Yancheng Medical Science and
Technology Development Plan Project (grant no. YK2021028).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
The study was conceived and designed by LXQ, XZ and
WYZ. Analysis and interpretation of data was performed by XZ and
YGF. The manuscript was drafted by LXQ and XZ, and critically
revised for important intellectual content by LXQ, XZ and WYZ. The
statistical analysis was performed by LXQ, XZ and YGF. Study
supervision was primarily conducted by WYZ, with contributions from
all authors. LXQ and XZ confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study complied with the Declaration of Helsinki
and was approved by the Ethics Committee of Yancheng No. 1 People's
Hospital (approval no. 2021-J-098). All subjects signed a consent
form before participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MG
|
mammography
|
|
SWE
|
shear wave elastography
|
|
MRI
|
magnetic resonance imaging
|
|
SE
|
strain elastography
|
References
|
1
|
Kashyap D, Pal D, Sharma R, Garg VK, Goel
N, Koundal D, Zaguia A, Koundal S and Belay A: Global increase in
breast cancer incidence: risk factors and preventive measures.
Biomed Res Int. 2022:96054392022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization, . Global breast
cancer initiative implementation framework: Assessing,
strengthening and scaling up of services for the early detection
and management of breast cancer: Executive summary. World Health
Organization; 2023
|
|
3
|
Zhu C, Chen M, Liu Y, Li P, Ye W, Ye H, Ye
Y, Liu Z, Liang C and Liu C: Value of mammographic
microcalcifications and MRI-enhanced lesions in the evaluation of
residual disease after neoadjuvant therapy for breast cancer. Quant
Imaging Med Surg. 13:5593–5604. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bodewes FTH, van Asselt AA, Dorrius MD,
Greuter MJW and de Bock GH: Mammographic breast density and the
risk of breast cancer: A systematic review and meta-analysis.
Breast. 66:62–68. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi J, Wang C, Ma Y, Wang J, Yang G, Wu Y,
Wang H and Mi C: The potential role of combined shear wave
elastography and superb microvascular imaging for early prediction
the pathological response to neoadjuvant chemotherapy in breast
cancer. Front Oncol. 13:11761412023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Zhou XX, Liu L, Liu AY, Zhao WJ,
Zhang HX, Zhu YM and Kuai ZX: Comparison of dynamic
contrast-enhanced MRI and non-mono-exponential model-based
diffusion-weighted imaging for the prediction of prognostic
biomarkers and molecular subtypes of breast cancer based on
radiomics. J Magn Reson Imaging. 58:1590–1602. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mao YJ, Lim HJ, Ni M, Yan WH, Wong DW and
Cheung JC: Breast tumour classification using ultrasound
elastography with machine learning: A systematic scoping review.
Cancers (Basel). 14:3672022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu B, Hu X, Feng J, Geng C, Jin F, Li H,
Li M, Li Q, Liao N, Liu D, et al: Chinese expert consensus on the
clinical diagnosis and treatment of advanced breast cancer (2018).
Cancer. 126:3867–3882. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chinese Medical Association, . Mammography
screening and diagnostic consensus. Chin J Radiol. 48:711–717.
2014.(In Chinese).
|
|
10
|
Arian A, Seyed-Kolbadi FZ, Yaghoobpoor S,
Ghorani H, Saghazadeh A and Ghadimi DJ: Diagnostic accuracy of
intravoxel incoherent motion (IVIM) and dynamic contrast-enhanced
(DCE) MRI to differentiate benign from malignant breast lesions: A
systematic review and meta-analysis. Eur J Radiol. 167:1110512023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim YX, Lim ZL, Ho PJ and Li J: Breast
cancer in asia: Incidence, mortality, early detection, mammography
programs, and risk-based screening initiatives. Cancers (Basel).
14:42182022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen Y, He J, Liu M, Hu J, Wan Y, Zhang T,
Ding J, Dong J and Fu X: Diagnostic value of contrast-enhanced
ultrasound and shear-wave elastography for small breast nodules.
PeerJ. 12:e176772024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdel Rahman RW, Refaie RMAE, Kamal RM,
Lasheen SF and Elmesidy DS: The diagnostic accuracy of
diffusion-weighted magnetic resonance imaging and shear wave
elastography in comparison to dynamic contrast-enhanced MRI for
diagnosing BIRADS 3 and 4 lesions. Egypt J Radiol Nucl Med.
52:1852021. View Article : Google Scholar
|
|
14
|
Asare B, White MJ and Rossi J: Metaplastic
carcinoma with osteosarcomatous differentiation in the breast: Case
report. Radiol Case Rep. 18:4272–4280. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alaref A, Hassan A, Sharma Kandel R,
Mishra R, Gautam J and Jahan N: Magnetic resonance imaging features
in different types of invasive breast cancer: A systematic review
of the literature. Cureus. 13:e138542021.PubMed/NCBI
|
|
16
|
Kubota K, Nakashima K, Nakashima K,
Kataoka M, Inoue K, Goto M, Kanbayashi C, Hirokaga K, Yamaguchi K
and Suzuki A: The Japanese breast cancer society clinical practice
guidelines for breast cancer screening and diagnosis, 2022 edition.
Breast Cancer. 31:157–164. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang H, Xu Y, Zhao Y, Yin J, Chen Z and
Huang P: The role of tissue elasticity in the differential
diagnosis of benign and malignant breast lesions using shear wave
elastography. BMC Cancer. 20:9302020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang H, Yu X, Zhang L, Song L and Gao X:
Diagnostic values of shear wave elastography and strain
elastography for breast lesions. Rev Med Chil. 148:1239–1245. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suvannarerg V, Chitchumnong P, Apiwat W,
Lertdamrongdej L, Tretipwanit N, Pisarnturakit P, Sitthinamsuwan P,
Thiravit S, Muangsomboon K and Korpraphong P: Diagnostic
performance of qualitative and quantitative shear wave elastography
in differentiating malignant from benign breast masses, and
association with the histological prognostic factors. Quant Imaging
Med Surg. 9:386–398. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bian J, Zhang J and Hou X: Diagnostic
accuracy of ultrasound shear wave elastography combined with superb
microvascular imaging for breast tumors: A protocol for systematic
review and meta-analysis. Medicine (Baltimore). 100:e262622021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sravani N, Ramesh A, Sureshkumar S,
Vijayakumar C, Abdulbasith KM, Balasubramanian G and Ch Toi P:
Diagnostic role of shear wave elastography for differentiating
benign and malignant breast masses. SA J Radiol.
24:19992020.PubMed/NCBI
|
|
22
|
Li J, Liu Y, Li Y, Li S, Wang J, Zhu Y and
Lu H: Comparison of diagnostic potential of shear wave elastography
between breast mass lesions and non-mass-like lesions. Eur J
Radiol. 158:1106092023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie L, Liu Z, Pei C, Liu X, Cui YY, He NA
and Hu L: Convolutional neural network based on automatic
segmentation of peritumoral shear-wave elastography images for
predicting breast cancer. Front Oncol. 13:10996502023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shahzad R, Fatima I, Anjum T and Shahid A:
Diagnostic value of strain elastography and shear wave elastography
in differentiating benign and malignant breast lesions. Ann Saudi
Med. 42:319–326. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hadebe B, Harry L, Ebrahim T, Pillay V and
Vorster M: The role of PET/CT in breast cancer. Diagnostics
(Basel). 13:5972023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paydary K, Seraj SM, Zadeh MZ,
Emamzadehfard S, Shamchi SP, Gholami S, Werner TJ and Alavi A: The
evolving role of FDG-PET/CT in the diagnosis, staging, and
treatment of breast cancer. Mol Imaging Biol. 21:1–10. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hodgson R, Heywang-Köbrunner SH, Harvey
SC, Edwards M, Shaikh J, Arber M and Glanville J: Systematic review
of 3D mammography for breast cancer screening. Breast. 27:52–61.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang M, Horvat JV, Bernard-Davila B,
Marino MA, Leithner D, Ochoa-Albiztegui RE, Helbich TH, Morris EA,
Thakur S and Pinker K: Multiparametric MRI model with dynamic
contrast-enhanced and diffusion-weighted imaging enables breast
cancer diagnosis with high accuracy. J Magn Reson Imaging.
49:864–874. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yadav P, Harit S and Kumar D: Efficacy of
high-resolution, 3-D diffusion-weighted imaging in the detection of
breast cancer compared to dynamic contrast-enhanced magnetic
resonance imaging. Pol J Radiol. 86:e277–e286. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Winkler J, Abisoye-Ogunniyan A, Metcalf KJ
and Werb Z: Concepts of extracellular matrix remodelling in tumour
progression and metastasis. Nat Commun. 11:51202020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Majidpoor J and Mortezaee K: Angiogenesis
as a hallmark of solid tumors-clinical perspectives. Cell Oncol
(Dordr). 44:715–737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roy S, Kumaravel S, Sharma A, Duran CL,
Bayless KJ and Chakraborty S: Hypoxic tumor microenvironment:
Implications for cancer therapy. Exp Biol Med (Maywood).
245:1073–1086. 2020. View Article : Google Scholar : PubMed/NCBI
|