Introduction
Pancreatic cancer is a common malignancy of the
digestive system with increasing global incidence and an overall
5-year survival rate of 5% (1).
Pancreatic cancer is a highly malignant tumour, which is currently
treated through surgery, chemotherapy and radiotherapy; however,
there are no effective therapeutic methods (2). In recent years, immune surveillance,
immune escape, immune tolerance, T-lymphocyte signalling,
inflammatory mediators, cytokines and the downregulation of
costimulatory molecules have been recognized as potential targets
for therapy (3,4).
The analysis of tumour-infiltrating lymphocyte (TIL)
subsets, which include CD3-, CD4- and CD8-positive cells, natural
killer cells and myeloid cells, is a key indicator of cellular
immune function, which is important for determining the recurrence,
metastasis and prognosis of malignancies (5,6). Our
previous research demonstrated that the infiltration of T
lymphocytes is associated with the prognosis of patients with
colorectal cancer (6).
Cytokines are messengers that lead to immune and
inflammatory responses, and they are important for cell growth and
differentiation (7). IL-2, IFN-α
and IFN-γ are secreted mainly by T helper (Th)1 cells, which can
enhance the cytotoxic effects of killer effector cells and mediate
antitumour cellular immune responses (8). IFN-γ is an important cytokine that can
change the surface composition of tumour cells, enhance the
antigenicity of MHC-I and tumour-related antigens, induce the
differentiation of tumour cells into normal cells, and activate
cytotoxic cells [such as macrophages, natural killer (NK) cells and
lymphokine-activated killer cells] to exert antitumour immune
effects (9). Cytokines such as
IL-4, IL-5, IL-6 and IL-10 are secreted mainly by Th2 cells, which
stimulate B-lymphocyte proliferation and produce specific
antibodies associated with humoral immunity (10), while also inhibiting cytokines
secreted by Th1 cells. Under normal conditions in the body, Th1 and
Th2 cells are in equilibrium, restricting the function of the other
through their respective secreted cytokines; however, when the
level of IL-4 in the immune microenvironment increases, it can bind
to the IL-4R on the surface of undifferentiated Th cells. Once the
IL-4R is activated, it will initiate intracellular signal
transduction pathways to promote the transition of Th1 cells to Th2
cells, and immune suppression and tumour escape can occur. In
addition, cytokines such as IL-17, IL-21, IL-22 and IL-6 are mainly
secreted by Th17 cells (11). IL-17
enhances the expression of inflammatory cytokines, such as IL-1,
IL-6 and IL-23, thus exacerbating the inflammatory response. In
addition, IL-17 can stimulate cancer cells to secrete VEGF and
indirectly promote blood vessel formation, promote tumour
development by inhibiting CD8+ cells and enhance the
entry of myeloid-derived suppressor cells into tumour tissue
(12). Increased IL-17 expression
in patients with colon cancer and hepatocellular carcinoma has been
reported to predict a poor prognosis (13,14).
4-1 BBL is a member of the tumour necrosis factor
(TNF) family and is a type II membrane protein, which is mostly
expressed on activated antigen-presenting cells. 4-1 BBL acts
primarily in the late stage of the immune response and mainly
regulates the proliferation of T cells (especially CD8-positive
cells) after activation (15,16).
It has been reported that the transfection of the 4-1BBL gene into
antigen-presenting cells can significantly enhance its
co-stimulatory effect on T cells (17). Previous studies have shown that the
expression of the costimulatory molecule 4-lBBL is closely related
to the occurrence and metastasis of prostate cancer, gastric
cancer, glioma and laryngeal cancer (18–21),
and its low expression confirms the existence of immune escape in
tumours, indicating a poor prognosis.
The present study used immunohistochemistry and
reverse transcription-quantitative PCR (RT-qPCR) to determine the
expression of TILs, cytokines and costimulatory molecules in
chronic pancreatitis and pancreatic cancer tissues. Subsequently,
the study analysed the relationships of TILs, cytokines and
costimulatory molecules with clinicopathological characteristics
and prognosis, explored the significance of immune function in the
occurrence and development of pancreatic cancer, and provided a
theoretical basis for the immunotherapeutic treatment of pancreatic
cancer.
Materials and methods
Patient characteristics
A total of 60 paraffin-embedded tissue samples
(including 20 samples from patients with chronic pancreatitis and
40 samples from patients with pancreatic cancer) were obtained from
patients who had been treated at The First Affiliated Hospital of
Soochow University (Suzhou, China) between November 2006 and
December 2016, and their data were accessed. Prior to the
collection of these tissue samples, the patients had provided their
informed consent for their tissues to be used in scientific
research. None of the patients had previously undergone
radiotherapy, chemotherapy or immunotherapy. Postoperative
pathology confirmed chronic pancreatitis or pancreatic cancer, and
Tumour-Node-Metastasis classification and differentiation grading
for pancreatic cancer were performed according to the criteria
described by the Union for International Cancer Control (22,23).
The present study was a retrospective analysis approved by the
research Ethics Committee of The First Affiliated Hospital of
Soochow University (approval no. 2023-410).
Immunohistochemical staining for CD3,
CD4, CD8, CD56, IFN-γ, IL-17 and 4-1BBL
Tissue sections (4 µm) were prepared from
paraffin-embedded specimens. Following deparaffinization with
xylene (three times; 5 min each) and rehydration with anhydrous
ethanol, and 95, 75 and 50% ethanol (5 min each), the slides were
heated to 100°C in 10 mmol/l sodium citrate buffer (pH 6) for 15
min for antigen retrieval. Endogenous peroxidase activity was
blocked by incubating the sections at 25°C with 3% H2O2 in methanol
for 10 min. The sections were subsequently blocked with 10% normal
horse serum (Wuhan Boster Biological Technology, Ltd.) for 10 min
at 25°C, and were then incubated with the following anti-human
antibodies at room temperature for 2 h in moisture chambers in the
dark: Monoclonal mouse IgG against CD3 (cat. no. sc-20047),
polyclonal rabbit IgG against CD4 (cat. no. sc-7219) and CD8 (cat.
no. sc-7188), monoclonal mouse IgG against IFN-γ (cat. no.
sc-373727) and 4-1BBL (cat. no. sc-398933) (all from Santa Cruz
Biotechnology, Inc.; dilution, 1:100), monoclonal mouse IgG against
CD56 (cat. no. ab9272; Abcam; dilution, 1:500) and polyclonal
rabbit IgG against IL-17 (cat. no. ab79056; Abcam; dilution,
1:100). The sections were subsequently washed with PBS and
incubated for 1 h in moisture chambers in the dark at room
temperature with polyclonal goat anti-mouse/rabbit IgG biotinylated
secondary antibodies (cat. no. K5007; Dako; Agilent Technologies,
Inc.; dilution, 1:2,000). Finally, the sections were developed with
3,3′-diaminobenzidine tetrahydrochloride hydrate and counterstained
with haematoxylin for 5 min at room temperature. A total of five
randomly selected fields were assessed using a BX53 light
microscope (Olympus Corporation), and areas of necrosis were
avoided. A paraffin-embedded section of human tonsillar tissue,
provided by the Pathology Department of the First Affiliated
Hospital of Soochow University was used as a positive control, and
the volunteer who provided this tissue provided written informed
consent for it to be used in subsequent scientific research. PBS
was used instead of primary antibody as a negative control.
Scoring system for
immunohistochemistry
The expression levels of CD3, CD4, CD8, CD56, IFN-γ,
IL-17 and 4-1BBL were scored using a semi-quantitative system
(24). PBS was used instead of
primary antibody as a negative control, and tonsillar tissue was
used as a positive control, with a double-blind reading by two
pathologists with generally consistent results. The staining
intensity was scored as 0 (achromatic), 1 (light yellow), 2
(brownish yellow) or 3 (brown). In addition, the percentage of
positive cells was scored as 0 (<5%), 1 (5–24%), 2 (25–49%), 3
(50–74%) or 4 (>75%). The two scores were added together and the
samples were assigned to one of four levels as follows: (−), score
0–1; (+), score 2; (++), score 3–4; or (+++), score ≥5. (−) and (+)
were defined as negative expression, (++) as weak expression and
(+++) as strong expression.
RT-qPCR
Total RNA from formalin-fixed paraffin-embedded
(FFPE) tissue sections was purified using an RNeasy FFPE Kit (cat.
no. 73504; Qiagen, Inc.); all of the reagents used for RNA
extraction were obtained from this kit. Firstly, the paraffin was
removed from freshly cut FFPE tissue sections by treatment with a
deparaffinization solution (Qiagen, Inc.). The samples were then
incubated in optimized lysis buffer to release RNA from the
sections. A short incubation at 80°C partially reversed the
formalin cross-linking of the released nucleic acids; this was
followed by deoxyribonuclease treatment, which was optimized to
eliminate all genomic DNA. The lysate was then mixed with Buffer
RBC. Ethanol was added to provide appropriate binding conditions
for RNA and the samples were applied to the provided RNeasy
MinElute spin columns. The RNA was then eluted in a minimum of 14
µl RNase-free water. cDNA was subsequently synthesized from total
RNA using RevertAid™ First Strand cDNA Synthesis kit
(cat. no. K1622; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. mRNA levels were quantified with qPCR
using a FastStart Universal SYBR Green Master (Rox) kit (cat. no.
4913914001; Sigma-Aldrich KGaA). The qPCR cycling conditions were
as follows: One cycle of initial template degeneration at 95°C for
1 min; followed by 45 cycles of template degeneration at 95°C for
20 sec, annealing at 58°C for 30 sec and extension at 68°C for 45
sec. β-actin was used as an internal reference. Three independent
experiments were performed to analyse relative target gene
expression. The expression levels of RNA were quantified using Cq
values and were normalized to β-actin using the 2-ΔΔCq
method (25). All primers were
supplied by Sangon Biotech Co., Ltd., as shown in Table I. The success rate of mRNA
extraction was determined by calculating the ratio of the number of
samples from which mRNA was successfully extracted to the total
number of samples.
 | Table I.Primer pairs used for reverse
transcription-quantitative PCR. |
Table I.
Primer pairs used for reverse
transcription-quantitative PCR.
| Gene name (GenBank
no.) | Sequence | Product size,
bp |
|---|
| CD3
(NM_000732.4) | F:
5′-GGGAGTCTTCTGCTTTGCTG-3′ | 153 |
|
| R:
5′-TTGTTCCGAGCCCAGTTTC-3′ |
|
| CD4
(NM_000616.4) | F:
5′-GTGAACCTGGTGGTGATG-3′ | 122 |
|
| R:
5′-GAGACCTTTGCCTCCTTG-3′ |
|
| CD8
(NM_001768.7) | F:
5′-ATGGCCTTACCAGTGACCG-3′ | 104 |
|
| R:
5′-AGGTTCCAGGTCCGATCCAG-3′ |
|
| CD56
(NM_000615.6) | F:
5′-CCAACCATCATCTGGAAACA-3′ | 137 |
|
| R:
5′-CTGCCCTCACAGCGATAAGT-3′ |
|
| IFN-γ
(NM_000619) | F:
5′-GAGTGTGGAGACCATCAAGGA-3′ | 128 |
|
| R:
5′-GTATTGCTTTGCGTTGGACA-3′ |
|
| IL-17
(U32659.1) | F:
5′-AACGATGACTCCTGGGAAGA-3′ | 115 |
|
| R:
5′-CTCAGAATTTGGGCATCCTG-3′ |
|
| 4-1BBL
(NM_003811.3) | F:
5′-GCCTGGGCGTCCATCTTC-3′ | 112 |
|
| R:
5′-AGTCCGGCTGGGATTTCG-3′ |
|
| β-actin
(NM_001101.3) | F:
5′-CACTGTGCCCATCTACGAGG-3′ | 154 |
|
| R:
5′-AATGTCACGCACGATTTCC-3′ |
|
Statistical analysis
All statistical analyses were carried out using SPSS
(v25; IBM Corporation). The data are presented as the means ±
standard deviation. The χ2 test or Fisher's exact test
were employed to assess the association between expression and
patient characteristics. For comparing the immunohistochemical
staining scores among different groups, the Kruskal-Wallis test
followed by Dunn's test was utilized. The mRNA expression levels in
various groups were assessed by one-way ANOVA followed by the
Tukey's honest significant difference post hoc test. The survival
of each group was depicted as a Kapan-Meier curve, and differences
were compared using the log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of CD3, CD4, CD8, CD56,
IFN-γ, IL-17, and 4-1BBL in chronic pancreatitis, and stages I–II
and III–IV pancreatic cancer
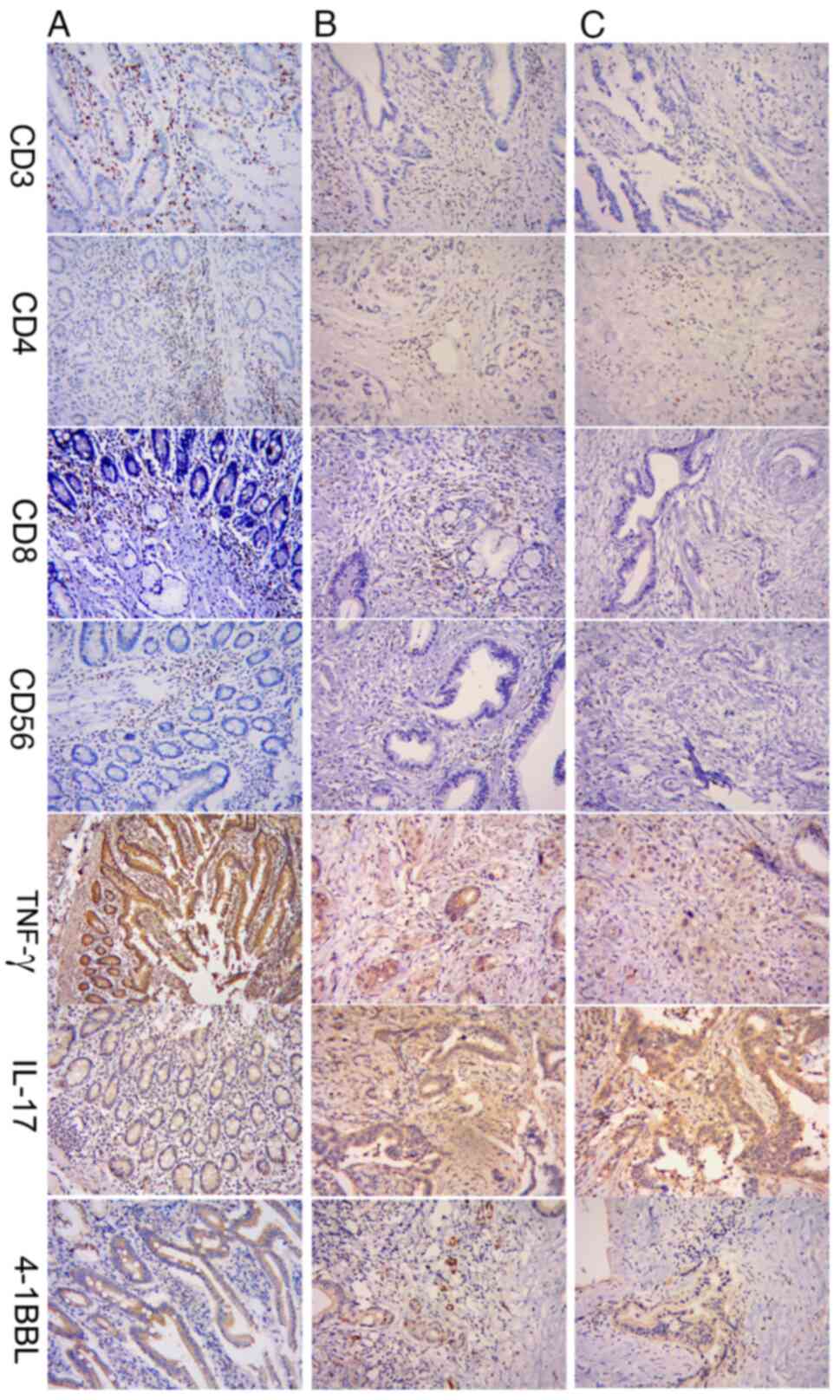
Immunohistochemical staining revealed that CD3, CD4,
CD8 and CD56 were located mainly in the cell membrane and appeared
yellow or brown, and IFN-γ, IL-17 and 4-1BBL were located in the
cell membrane or cytoplasm (Fig.
1). The expression of CD3, CD4, CD8, CD56, IFN-γ and 4-1BBL
were gradually decreased in samples from patients with stages I–II
and III–IV pancreatic cancer compared with those from patients with
chronic pancreatitis. By contrast, the expression of IL-17 was
gradually increased in samples from patients with stages I–II and
III–IV pancreatic cancer compared with those from patients with
chronic pancreatitis (Fig. 1).
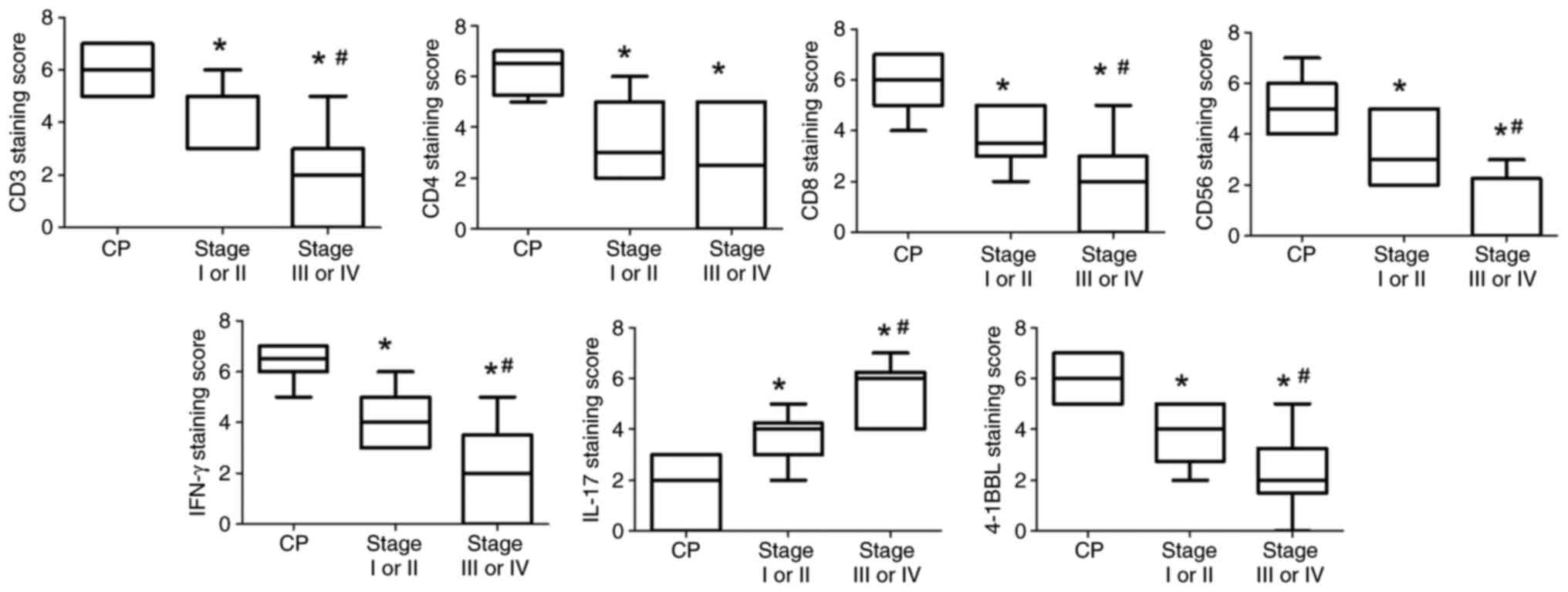
The expression levels of CD3, CD4, CD8, CD56, IFN-γ
and 4-1BBL were significantly lower in pancreatic cancer tissues
than those in chronic pancreatitis tissues (P<0.05; Fig. 2). By contrast, the expression levels
of IL-17 were significantly increased in patients with pancreatic
cancer than in those with chronic pancreatitis (P<0.05).
Furthermore, the expression levels of CD3, CD8, CD56, IFN-γ and
4-1BBL was significantly lower in stage III–IV than in stage I–II
(P<0.05), and the expression of IL-17 was significantly greater
in stage III–IV than in stage I–II (P<0.05). However, no
significant difference was observed between stages I–II and III–IV
with respect to CD4 staining (Fig.
2).
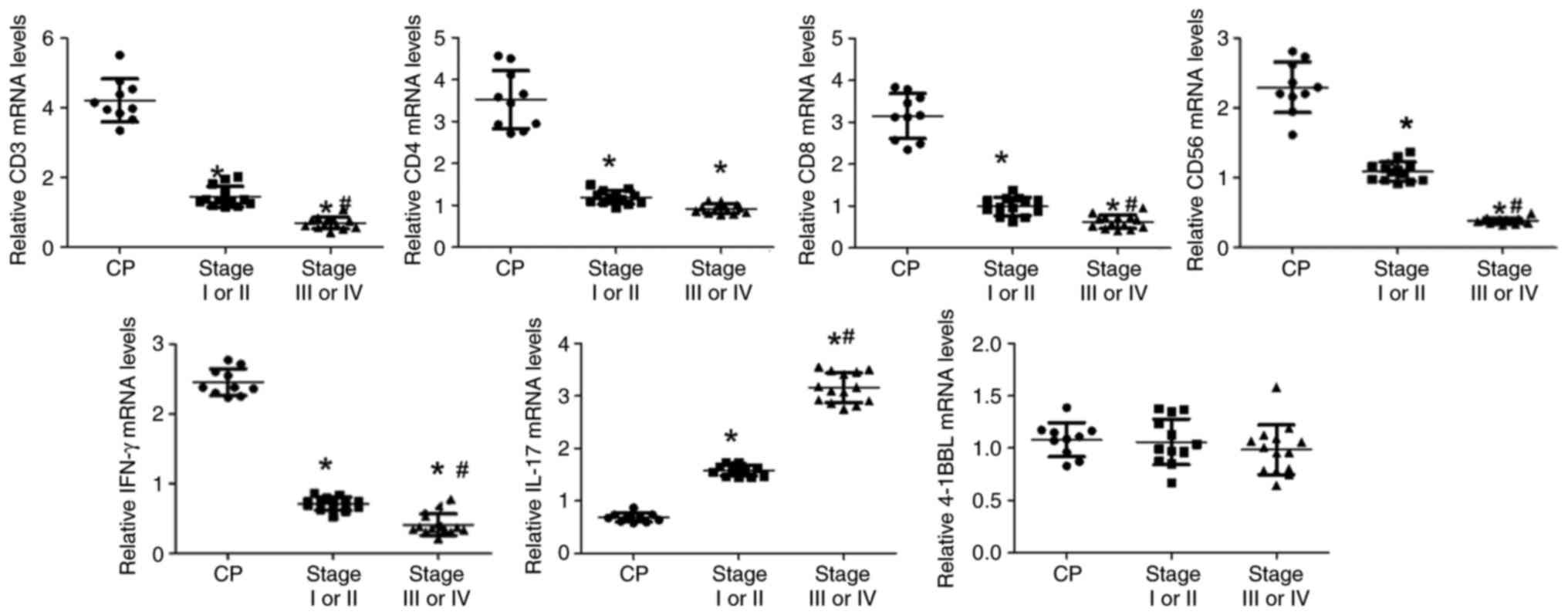
CD3, CD4, CD8, CD56, IFN-γ, IL-17, and
4-1BBL mRNA expression levels in chronic pancreatitis, and stages
I–II and III–IV pancreatic cancer
A total of 50 blocks were randomly selected from the
60 paraffin-embedded samples and were used for mRNA extraction,
including 16 chronic pancreatitis samples, 17 stage I–II samples
and 17 stage III–IV samples. The total extraction rate of mRNA from
the wax blocks of pancreatic tissue was ~74% (37/50), and the
extraction rates of the chronic pancreatitis, stage I–II and stage
III–IV tissue samples were 10/16 (62.5%), 13/17 (76.5%), and 14/17
(82.4%), respectively. Similar to the immunohistochemistry results,
CD3, CD4, CD8, CD56 and IFN-γ mRNA expression levels were
significantly lower in pancreatic cancer tissues than those in
chronic pancreatitis tissues (P<0.05; Fig. 3). By contrast, the mRNA expression
levels of IL-17 were significantly increased in tissues from
patients with pancreatic cancer than in those from patients with
chronic pancreatitis (P<0.05; Fig.
3). The mRNA expression levels of CD3, CD8, CD56 and IFN-γ were
significantly lower in samples from patients with stage III–IV
pancreatic cancer than in those from patients with stage I–II
pancreatic cancer (P<0.05), whereas the opposite was shown
regarding IL-17. The mRNA expression levels of CD4 were not
significantly different between stages I–II and III–IV. In contrast
to the immunohistochemistry results, the mRNA expression levels of
4-1BBL were not significant different among the chronic
pancreatitis, stage I–II and stage III–IV groups (P>0.05;
Fig. 3).
Association between CD3, CD4, CD8,
CD56, IFN-γ, IL-17 and 4-1BBL expression and clinicopathological
data
The infiltration of CD3-, CD8- and CD56-positive
cells was related to the differentiation and stage of pancreatic
cancer, with greater infiltration detected in patients with highly
differentiated cancer (P<0.05) and in patients with stage I–II
cancer (P<0.05), independent of the patient age, sex and tumour
site (P>0.05) (Table II). The
infiltration of CD4-positive cells was not related to age, sex,
tumour site differentiation or stage (P>0.05) (Table II). Furthermore, the expression
levels of IFN-γ and 4-1BBL were greater in the patients with highly
differentiated cancer (P<0.05) and in patients with stage I–II
cancer (P<0.05); however, the expression levels of IL-17 were
lower in patients with highly differentiated cancer (P<0.05) and
in patients with stage I–II cancer (P<0.05) (Table III).
 | Table II.Relationship between
clinicopathological parameters and CD3+, CD4+, CD8+ and CD56+
T-cell infiltration in patients with pancreatic cancer. |
Table II.
Relationship between
clinicopathological parameters and CD3+, CD4+, CD8+ and CD56+
T-cell infiltration in patients with pancreatic cancer.
|
|
| CD3+ T cells | CD4+ T cells | CD8+ T cells | CD56+ T cells |
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | n | Strong | Negative/weak | P-value | Strong | Negative/weak | P-value | Strong | Negative/weak | P-value | Strong | Negative/weak | P-value |
|---|
| Age, years |
|
|
| 0.481a |
|
| 0.822a |
|
| 0.730b |
|
| 0.505b |
|
≤60 | 17 | 10 | 7 |
| 8 | 9 |
| 4 | 13 |
| 4 | 13 |
|
|
>60 | 23 | 16 | 7 |
| 10 | 13 |
| 7 | 16 |
| 8 | 15 |
|
| Sex |
|
|
| 0.445a |
|
| 0.641a |
|
| 0.715b |
|
| 0.484b |
|
Male | 26 | 18 | 8 |
| 11 | 15 |
| 8 | 18 |
| 9 | 17 |
|
|
Female | 14 | 8 | 6 |
| 7 | 7 |
| 3 | 11 |
| 3 | 11 |
|
| Tumour site |
|
|
| 0.343a |
|
| 0.897a |
|
| 0.148b |
|
| 0.398a |
|
Pancreatic head | 24 | 17 | 7 |
| 11 | 13 |
| 9 | 15 |
| 6 | 18 |
|
|
Nonpancreatic head | 16 | 9 | 7 |
| 7 | 9 |
| 2 | 14 |
| 6 | 10 |
|
|
Differentiation |
|
|
| 0.040b |
|
| 0.140a |
|
|
<0.001b |
|
| 0.003b |
|
High | 15 | 13 | 2 |
| 9 | 6 |
| 10 | 5 |
| 9 | 6 |
|
|
Moderate/Low | 25 | 13 | 12 |
| 9 | 16 |
| 1 | 24 |
| 3 | 22 |
|
| Stage |
|
|
| 0.014a |
|
| 0.949a |
|
| 0.011b |
|
| 0.035b |
| I and
II | 22 | 18 | 4 |
| 10 | 12 |
| 10 | 12 |
| 10 | 12 |
|
| III and
IV | 18 | 8 | 10 |
| 8 | 10 |
| 1 | 17 |
| 2 | 16 |
|
 | Table III.Relationship between
clinicopathological parameters and IL-17, IFN-γ and 4-1BBL
expression in patients with pancreatic cancer. |
Table III.
Relationship between
clinicopathological parameters and IL-17, IFN-γ and 4-1BBL
expression in patients with pancreatic cancer.
|
|
| IFN-γ | IL-17 | 4-1BBL |
|---|
|
|
|
|
|
|
|---|
| Characteristic | n | Strong | Negative/weak | P-value | Strong | Negative/weak | P-value | Strong | Negative/weak | P-value |
|---|
| Age, years |
|
|
| 0.151a |
|
| 0.973a |
|
| 0.131a |
|
≤60 | 17 | 9 | 8 |
| 6 | 11 |
| 10 | 7 |
|
|
>60 | 23 | 7 | 16 |
| 8 | 15 |
| 8 | 15 |
|
| Sex |
|
|
| 0.104a |
|
| 0.730b |
|
| 0.842a |
|
Male | 26 | 8 | 18 |
| 10 | 16 |
| 12 | 14 |
|
|
Female | 14 | 8 | 6 |
| 4 | 10 |
| 6 | 8 |
|
| Tumour site |
|
|
| 0.792a |
|
| 0.787a |
|
| 0.243a |
|
Pancreatic head | 24 | 10 | 14 |
| 8 | 16 |
| 9 | 15 |
|
|
Nonpancreatic head | 16 | 6 | 10 |
| 6 | 10 |
| 9 | 7 |
|
|
Differentiation |
|
|
| 0.001a |
|
| 0.005b |
|
| 0.033a |
|
High | 15 | 11 | 4 |
| 1 | 14 |
| 10 | 5 |
|
|
Moderate/Low | 25 | 5 | 20 |
| 13 | 12 |
| 8 | 17 |
|
| Stage |
|
|
| 0.038a |
|
| 0.014a |
|
| 0.009a |
| I and
II | 22 | 12 | 10 |
| 4 | 18 |
| 14 | 8 |
|
| III and
IV | 18 | 4 | 14 |
| 10 | 8 |
| 4 | 14 |
|
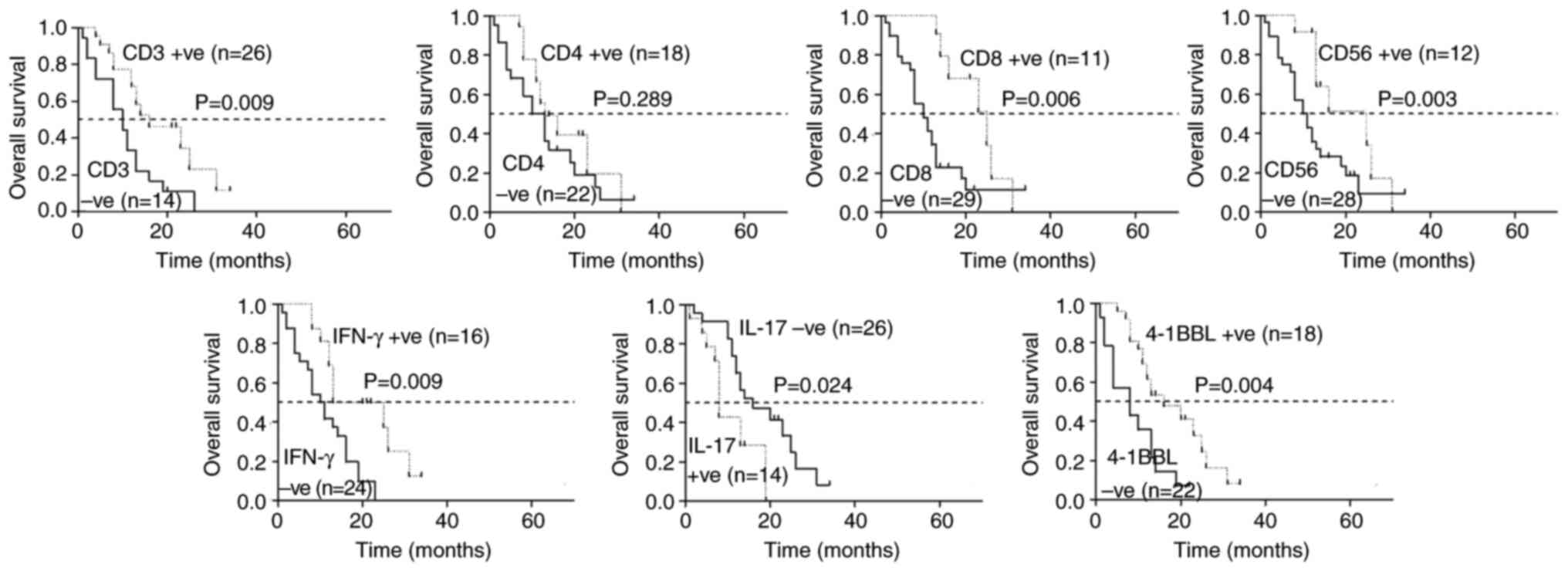
Association between CD3, CD4, CD8,
CD56, IFN-γ and 4-1BBL expression and the prognosis of patients
with pancreatic cancer
The median survival times of patients with strongly
positive CD3, CD8, CD56, IFN-γ and 4-1BBL expression were 18.7,
22.5, 20.3, 20.1 and 18.3 months, respectively, longer than those
with weakly positive and negative expression (P<0.05; Fig. 4). The median survival time of those
with strong positive CD4 expression was 17.3 months, which was
longer than those with weak positive and negative CD4 expression,
but the difference was not statistically significant (P>0.05;
Fig. 4). The median survival time
of those with strong positive IL-17 expression was 9.8 months,
which was shorter than that of those with weak positive and
negative expression (P<0.05; Fig.
4).
Discussion
The immune system has contradictory and complex
functions in the development of pancreatic cancer, and the role of
immune cells in the pancreatic cancer microenvironment varies. The
occurrence and development of chronic pancreatitis are related to
immunity. An immunohistochemical study revealed that CD4- and
CD8-positive T lymphocytes, macrophages and mast cells were the
main infiltrates in chronic pancreatitis; however, CD56-positive NK
lymphocytes and B lymphocytes were less abundant (5). The present study analysed the changes
in CD3-, CD4-, CD8- and CD56-positive cells in chronic pancreatitis
and pancreatic cancer tissues at different stages via
immunohistochemistry and RT-qPCR, and revealed that when
inflammation developed in the pancreas, the number of CD3-, CD4-
and CD8-positive cells increased through autoimmune regulation,
thus killing target cells and carrying out immune functions.
However, the decrease in the number of these cells in pancreatic
cancer tissues indicated that the cellular immune function of
patients with malignant tumours may be inhibited, and that the
ability of these patients to identify and kill mutant cells
decreases, resulting in an imbalance in the immune state of the
body, and the growth of tumour cells and even metastasis. The
present study also revealed that CD3-, CD4-, CD8-, CD56-positive
cells and IFN-γ expression were present in highly differentiated
pancreatic cancer. In addition to CD4-positive cells, CD3-,
CD8-positive cells, NK cells, and IFN-γ were also associated with
clinical stage; the higher the stage, the lower the expression.
Moreover, it was revealed that high expression was associated with
a good prognosis during the follow-up process, similar to the
findings of previous studies (26,27).
It is possible that T lymphocytes prevent the infiltration of
tumour cells into deep tissue, preventing their invasion into the
lymphatic tract and metastasis, thus delaying the development of
the tumour and prolonging the life of the patient.
Some studies have shown that IFN-γ can directly
inhibit fibroblast activation and proliferation, and inhibit or
block the occurrence and development of pancreatic fibrosis
(28,29). The present study revealed that IFN-γ
expression was increased in chronic pancreatitis compared with in
pancreatic cancer, suggesting that IFN-γ may have an antifibrotic
effect on chronic pancreatitis. However, in pancreatic cancer,
IFN-γ levels may decrease due to the depletion of body substances
in the antitumour process, suggesting that IFN-γ is
immunosuppressive in patients with tumours (30).
Previous studies have shown that IL-17 is expressed
in various tumour tissues (12,13);
however, its role in tumours is controversial. On the one hand,
IL-17 can selectively enhance the production of proangiogenic
chemokines, such as CXCL1, CXCL5, CXCL6 and CXCL8, in tumour cells
and endothelial cells (31). By
contrast, IL-17 has been confirmed to inhibit certain hematopoietic
tumours, such as mast cell tumours and plasma cytomas (32). Most studies have confirmed that
IL-17 mainly affects tumour growth by stimulating cancer cells to
secrete VEGF and indirectly promoting blood vessel formation
(11,33). The results of the present study
showed that the expression levels of IL-17 were greater in
pancreatic cancer tissues than those in chronic pancreatitis
tissues. The present study also revealed that IL-17 was related to
tumour differentiation and Tumour-Node-Metastasis (TNM) stage
(22); lower differentiation and
higher TNM stage indicated higher expression levels. In the initial
stage of pancreatic cancer, Th17 cells that produce IL-17 may serve
a proinflammatory role and inhibit tumour growth. With the
development of pancreatic cancer, the immune system is disrupted
and inflammation cannot be alleviated; at this time, Thl7 cells may
participate in the protumour effects or block the antitumour
effects (34), eventually leading
to the occurrence of pancreatic cancer. During clinical follow-up,
the strong positive expression of IL-17 was associated with a poor
prognosis in patients with pancreatic cancer and the median
survival time of patients with strong positive IL-17 expression was
9.8 months, which was significantly shorter than that of patients
with weak positive or negative expression. These findings suggested
that IL-17 may have an important role in predicting the prognosis
of patients with pancreatic cancer.
4-1BB/4-1BBL-mediated costimulatory signals are
involved in immunomodulatory processes in autoimmune diseases,
tumours, viral infections and other diseases (16–20).
Chronic pancreatitis is an immunological process characterized by
increased expression of 4-1BBL. However, studies on tumours have
shown that there is an antitumour immune response during tumour
growth and tumour cell surface expression of 4-1 BBL may
participate in antitumour immunity (16,35).
Our previous animal experiments revealed that 4-1BBL can promote
bone marrow-derived dendritic cell (antigen-presenting cell)
maturation leading to the secretion of IL-6 and IL-12 cytokines,
which may enhance immunity (36,37).
Although antitumour immunity and tumour growth coexist, the low
expression levels of costimulatory molecules such as 4-1BBL may not
be enough to fully activate the immune system, preventing tumour
removal. In the present study, 4-1BBL expression was significantly
lower in patients with pancreatic cancer compared with in patients
with chronic pancreatitis, confirming the low degree of local
immunity in patients with pancreatic cancer. The
immunohistochemical results revealed differences between stages
I–II and III–IV, indicating that a further decline in immune
function occurred with the progression of the tumour. RT-qPCR
revealed little difference among the three groups, possibly due to
the detection of 4-1BBL expression in the tumour cell stroma by
immunohistochemistry, which mainly involved various activated
immune cells such as T cells, B cells and antigen-presenting cells
(dendritic cells, macrophages). However, 4-1BBL is also expressed
in tumour cells, and qPCR can detect mRNA expression levels in all
tissues; thus, the results of qPCR may be inconsistent with those
of immunohistochemistry (38).
The present study used qPCR of paraffin-embedded
pancreatic tissue to detect TILs, cytokines and costimulatory
molecule gene expression. The results revealed that the mRNA
extraction rate of chronic pancreatitis (62.5%) was slightly lower
than that of pancreatic cancer (76.5% and 82.4%), which may be due
to two aspects: i) Chronic pancreatitis mainly involves fibrosis of
the pancreatic parenchyma, leading to a reduction in the glands and
the interstitium. ii) Some chronic pancreatitis tissues have high
fat content, resulting in reduced expression of the corresponding
target genes. Therefore, attention should be paid to addressing the
issue concerning the accuracy, uniformity, quantity and appropriate
method of obtaining pancreatic tissues for RNA extraction.
Chronic pancreatitis remains one of the highest risk
factors for pancreatic cancer. Initially, the immune response is
strong in chronic pancreatitis and the immune system can actively
recognize tumour-specific antigens, leading to the activation of
the innate and adaptive immune responses that eliminate transformed
cells. However, with increasing age, environmental factors or
certain genetic syndromes, such as mutations in the K-RAS gene,
evade immune surveillance, leading to the occurrence of pancreatic
cancer (39). Multiple mechanisms
are immediately involved in pancreatic cancer progression.
Extracellular vesicles derived from the pancreas can inhibit the
immune response and are associated with the immune escape via the
suppression of NK cells, antigen-presenting cells and cytotoxic T
cells, the induction and activation of immunosuppressive cells, the
secretion of cytokines, such as TNF-β, or the induction of
fibroblasts, which secrete large amounts of fibrin and
proteoglycans to form the extracellular matrix and a protective
physical barrier for tumour cells (40). As cancer progresses, the immune
response is further suppressed in stages III–IV, as shown in the
present study.
The lack of a normal control without pancreatic
disease is a limitation of the present study. The study focused on
the changes in these indicators during the process from benign
lesions to cancerous transformation and metastasis, examining the
trends of these indicators in chronic pancreatitis, stage I–II
pancreatic cancer and stage III–IV pancreatic cancer. The present
study reviewed the relevant literature and revealed that a number
of studies, including animal experiments, clinical trials using
tissues adjacent to pancreatic cancer as negative controls or
studies comparing pancreatic benign diseases, such as pancreatic
cysts, with pancreatic cancer, arrived at conclusions consistent
with those of the present study (41–43).
This provides additional support for the validity of the current
research findings. In addition, as the present study assessed only
40 pancreatic cancer specimens, a multivariate analysis was not
performed, since a requirement of multivariate analyses is that the
sample size should be 10-15 times the number of independent
variables. Therefore, only a univariate survival analysis was
applied in the present study, which is also a limitation.
In conclusion, the changes in CD3-, CD8- and
CD56-positive cells, the cytokines IL-17 and IFN-γ, and the
costimulatory molecule 4-1BBL in patients with pancreatic cancer
were closely related to the degree of differentiation, TNM staging
and prognosis. Notably, only the high expression of IL-17 was
revealed to be an adverse prognostic factor of pancreatic cancer,
all of the others indicated a good prognosis. These findings
suggested that the suppression of antitumour immunity, the decrease
in the infiltration density of TILs and the downregulation of
costimulatory molecules may lead to the transformation from chronic
pancreatitis to pancreatic cancer; however, the underlying
mechanism requires further study.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81172166).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YYL, KZ, JXY and TTX conceived and designed the
study, and contributed to data collection, data analysis, article
editing and interpretation of the results. YYL drafted the initial
manuscript. XDC and JKZ participated in the design of the research
methodology and supervised the study. YXC and YJG contributed to
data collection and carried out a part of the data analysis. YYL,
JXY, TTX and KZ confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study is a retrospective analysis and
the data are anonymous; it was approved by the research ethics
committee of the First Affiliated Hospital of Soochow University
(approval no. 2023-410).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torphy RJ, Fujiwara Y and Schulick RD:
Pancreatic cancer treatment: Better, but a long way to go. Surg
Today. 50:1117–1125. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kolbeinsson HM, Chandana S, Wright GP and
Chung M: Pancreatic cancer: A review of current treatment and novel
therapies. J Invest Surg. 36:21298842023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leidner R, Sanjuan Silva N, Huang H,
Sprott D, Zheng C, Shih YP, Leung A, Payne R, Sutcliffe K, Cramer
J, et al: Neoantigen T-cell receptor gene therapy in pancreatic
cancer. N Engl J Med. 386:2112–2119. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhatia R, Bhyravbhatla N, Kisling A, Li X,
Batra SK and Kumar S: Cytokines chattering in pancreatic ductal
adenocarcinoma tumor microenvironment. Semin Cancer Biol.
86:499–510. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zimnoch L, Szynaka B and Puchalski Z: Mast
cells and pancreatic stellate cells in chronic pancreatitis with
differently intensified fibrosis. Hepatogastroenterology.
49:1135–1138. 2002.PubMed/NCBI
|
|
6
|
Jin C, Duan X, Liu Y, Zhu J, Zhang K,
Zhang Y, Xia T, Fei Y and Ye J: T cell immunity induced by a
bivalent Salmonella-based CEACAM6 and 4-1BBL vaccines in a rat
colorectal cancer model. Oncol Lett. 13:3753–3759. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong C: Cytokine regulation and function
in T cells. Annu Rev Immunol. 39:51–76. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu X and Zhu J: CD4 T helper cell subsets
and related human immunological disorders. Int J Mol Sci.
21:80112020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar S, Jeong Y, Ashraf MU and Bae YS:
Dendritic cell-mediated Th2 Immunity and immune disorders. Int J
Mol Sci. 20:21592019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang W, Sung N, Gilman-Sachs A and
Kwak-Kim J: T Helper (Th) Cell profiles in pregnancy and recurrent
pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Front Immunol.
11:20252020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alspach E, Lussier DM and Schreiber RD:
Interferon γ and its important roles in promoting and inhibiting
spontaneous and therapeutic cancer immunity. Cold Spring Harb
Perspect Biol. 11:a0284802019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McGeachy MJ, Cua DJ and Gaffen SL: The
IL-17 family of cytokines in health and disease. Immunity.
50:892–906. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Razi S, Baradaran Noveiry B,
Keshavarz-Fathi M and Rezaei N: IL-17 and colorectal cancer: From
carcinogenesis to treatment. Cytokine. 116:7–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee YH, Chuah S, Nguyen PHD, Lim CJ, Lai
HLH, Wasser M, Chua C, Lim TKH, Leow WQ, Loh TJ, et al: IFNγ-IL-17+
CD8 T cells contribute to immunosuppression and tumor progression
in human hepatocellular carcinoma. Cancer Lett. 552:2159772023.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Claus C, Ferrara C, Xu W, Sam J, Lang S,
Uhlenbrock F, Albrecht R, Herter S, Schlenker R, Hüsser T, et al:
Tumor-targeted 4-1BB agonists for combination with T cell
bispecific antibodies as off-the-shelf therapy. Sci Transl Med.
11:eaav59892019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho E, Singh R, Han C, Kim SH, Kim KH,
Park BM, Shin DH, Han S, Kim YH, Kwon BS, et al: 4-1BB-4-1BBL
cis-interaction contributes to the survival of self-reactive
CD8+ T cell. Cell Mol Immunol. 20:1077–1080. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martinez-Perez AG, Perez-Trujillo JJ,
Garza-Morales R, Loera-Arias MJ, Saucedo-Cardenas O, Garcia-Garcia
A, Rodriguez-Rocha H and Montes-de-Oca-Luna R: 4-1BBL as a mediator
of cross-talk between innate, adaptive, and regulatory immunity
against cancer. Int J Mol Sci. 22:621012021. View Article : Google Scholar
|
|
18
|
Zhu H, Wang M, Du Y, Liu X, Weng X and Li
C: 4-1BBL has a possible role in mediating castration-resistant
conversion of prostate cancer via up-regulation of androgen
receptor. J Cancer. 10:2464–2471. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
An HW, Seok SH, Kwon JW, Choudhury AD, Oh
JS, Voon DC, Kim DY and Park JW: The loss of epithelial Smad4
drives immune evasion via CXCL1 while displaying vulnerability to
combinatorial immunotherapy in gastric cancer. Cell Rep.
41:1118782022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Puigdelloses M, Garcia-Moure M, Labiano S,
Laspidea V, Gonzalez-Huarriz M, Zalacain M, Marrodan L,
Martinez-Velez N, De la Nava D, Ausejo I, et al: CD137 and PD-L1
targeting with immunovirotherapy induces a potent and durable
antitumor immune response in glioblastoma models. J Immunother
Cancer. 9:e0026442021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Hu H, Liu Z, Xu J, Gao Y, Zhan X,
Zhou S, Zhong W, Wu D, Wang P, et al: Macrophage STING signaling
promotes NK cell to suppress colorectal cancer liver metastasis via
4-1BBL/4-1BB co-stimulation. J Immunother Cancer. 11:e0064812023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging manual. 7th edition.
Springer; New York, NY: 2010
|
|
23
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board, : The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma Y, Ma L, Guo Q and Zhang S: Expression
of bone morphogenetic protein-2 and its receptors in epithelial
ovarian cancer and their influence on the prognosis of ovarian
cancer patients. J Exp Clin Cancer Res. 29:852010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roxburgh CSD and McMillan DC: The role of
the in situ local inflammatory response in predicting recurrence
and survival in patients with primary operable colorectal cancer.
Cancer Treat Rev. 38:451–466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li F, Chen B, Li L, Zha M, Zhou S, Wu T,
Bachem MG and Sun Z: INS-1 cells inhibit the production of
extracellular matrix from pancreatic stellate cells. J Mol Histol.
45:321–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Q, Yang Q, Wu Z, Chen Y, Xu M, Zhang
H, Zhao J, Liu Z, Guan Z, Luo J, et al: IL-1β-activated mTORC2
promotes accumulation of IFN-γ+ γδ T cells by
upregulating CXCR3 to restrict hepatic fibrosis. Cell Death Dis.
13:2892022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ren J, Li N, Pei S, Lian Y, Li L, Peng Y,
Liu Q, Guo J, Wang X, Han Y, et al: Histone methyltransferase WHSC1
loss dampens MHC-I antigen presentation pathway to impair
IFN-γ-stimulated antitumor immunity. J Clin Invest.
132:e1531672022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JW, Wang P, Kattah MG, Youssef S,
Steinman L, DeFea K and Straus DS: Differential regulation of
chemokines by IL-17 in colonic epithelial cells. J Immunol.
181:6536–6545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benchetrit F, Ciree A, Vives V, Warnier G,
Gey A, Sautès-Fridman C, Fossiez F, Haicheur N, Fridman WH and
Tartour E: Interleukin-17 inhibits tumor cell growth by means of a
T-cell-dependent mechanism. Blood. 99:2114–2121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu N, Wang Y, Wang K, Zhong B, Liao Y,
Liang J and Jiang N: Cathepsin K regulates the tumor growth and
metastasis by IL-17/CTSK/EMT axis and mediates M2 macrophage
polarization in castration-resistant prostate cancer. Cell Death
Dis. 13:8132022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gorczynski RM: IL-17 signaling in the
tumor microenvironment. Birbrair A: Tumor Microenvironment.
Advances in Experimental Medicine and Biology. Vol 1240. Springer;
Cham: pp. pp47–58. 2020, View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen X, Zhang R, Nie X, Yang Y, Hua Y and
Lü P: 4-1BB targeting immunotherapy: Mechanism, antibodies, and
chimeric antigen receptor T. Cancer Biother Radiopharm. 38:431–444.
2023.PubMed/NCBI
|
|
36
|
Ye JX, Zhang YT, Zhang XG, Ren DM and Chen
WC: Recombinant attenuated Salmonella harboring 4-1BB ligand gene
enhances cellular immunity. Vaccine. 27:1717–1723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ye J, Li L, Zhang Y, Zhang X, Ren D and
Chen W: Recombinant Salmonella-based 4-1BBL vaccine enhances T cell
immunity and inhibits the development of colorectal cancer in rats:
In vivo effects of vaccine containing 4-1BBL. J Biomed Sci.
20:82013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leech MD, Barr TA, Turner DG, Brown S,
O'Connor RA, Gray D, Mellanby RJ and Anderton SM: Cutting edge:
IL-6-dependent autoimmune disease: Dendritic cells as a sufficient,
but transient, source. J Immunol. 190:881–885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hingorani SR: Epithelial and stromal
co-evolution and complicity in pancreatic cancer. Nat Rev Cancer.
23:57–77. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu C, He D, Li L, Zhang S, Wang L, Fan Z
and Wang Y: Extracellular vesicles in pancreatic cancer immune
escape: Emerging roles and mechanisms. Pharmacol Res.
183:1063642022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Picard FSR, Lutz V, Brichkina A, Neuhaus
F, Ruckenbrod T, Hupfer A, Raifer H, Klein M, Bopp T, Pfefferle PI,
et al: IL-17A-producing CD8+ T cells promote PDAC via
induction of inflammatory cancer-associated fibroblasts. Gut.
72:1510–1522. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Teramatsu K, Oono T, Oyama K, Fujimori N,
Murakami M, Yasumori S, Ohno A, Matsumoto K, Takeno A, Nakata K, et
al: Circulating CD8+CD122+ T cells as a
prognostic indicator of pancreatic cancer. BMC Cancer. 22:11342022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song Y, Ji B, Jiang CX, Chen ZM, Yao NH,
Mukaida N and Huang H: IL17RB expression might predict prognosis
and benefit from gemcitabine in patients with resectable pancreatic
cancer. Pathol Res Pract. 215:1526502019. View Article : Google Scholar : PubMed/NCBI
|