Introduction
The HER2 gene, located on the long arm of chromosome
17, encodes a transmembrane tyrosine kinase receptor protein
(1,2). Although normal breast epithelial cells
have approximately 20,000 receptors per cell, the number of
receptors is estimated to be about 2 million in HER2 overexpressing
breast cancer (1). The HER2
receptor has a tyrosine kinase within its intracellular domain.
Upon dimerization, HER2 stimulates tyrosine kinase, resulting in
the activation of signaling cascades. This cascade is involved in
the proliferation, invasion, and metastasis of HER2 positive breast
cancer (3). HER2 overexpression was
found in 15% of breast cancers and correlated with poor prognosis
(4). Identification of the
mechanism and aggressive feature of HER2 positive breast cancer has
led to the development of trastuzumab, which has improved the
prognosis of HER2 positive breast cancer.
NSABP-B31 showed an improvement in disease-free
survival (DFS) and overall survival (OS) with trastuzumab in
combination with adjuvant chemotherapy after surgery for HER2
positive early stage breast cancer (5). A reanalysis of HER2 expression in
patients in this trial showed that 174 of 1787 patients were HER2
negative, and further subgroup analysis showed an additive effect
of trastuzumab in HER2 negative patients [relative risk for DFS,
0.34; 95% confidence interval (CI), 0.14 to 0.80; P=0.014]
(6). NSABP B-47, a randomized trial
of adjuvant chemotherapy in combination with or without trastuzumab
for HER2-negative patients showed no additive effect of trastuzumab
[hazard ratio (HR), 0.98; 95% CI, 0.76 to 1.25; P=0.85] (7).
HER2 negative breast cancer account for
approximately 80% of all breast cancers. Moreover, HER2 expression
is observed in HER2 negative breast cancers, albeit at low levels
(HER2-low breast cancer) (8),
accounting for approximately half of all breast cancer cases
(9). Recently, trastuzumab
deruxtecan was shown to improve the progression-free survival (PFS)
and OS of patients with HER2-low breast cancer in DESTINY Breast04
trial. This new classification is very interesting and noteworthy.
In addition, the DESTINY Breast06 trial showed that trastuzumab
deruxtecan improve PFS in patients with HER2-low breast cancer as
well as HER2-ultralow breast cancer. Nevertheless, the
clinicopathological features and prognosis of HER2-low breast
cancer remain poorly defined. Many other studies (3,7,10–12)
regarding the clinical characteristics of HER2-low breast cancer
have used immunohistochemistry (IHC) to determine HER2 status.
In this study, we retrospectively analyzed the
clinicopathologic characteristics and prognosis of HER2-low breast
cancer using fluorescence in situ hybridization (FISH). This
is the first study to evaluate the HER2-low status by FISH
assay.
Materials and methods
Study design and population
A prospective database of patients with clinical
stage I to III breast cancer who underwent surgery between
September 2012 and October 2022 at Teikyo University Hospital was
analyzed. Patients with pathologically diagnosed invasive carcinoma
without HER2 amplification on presurgical core needle biopsy or
surgical specimens were included. HER2 gene amplification was
evaluated by SRL Inc., Japan using the PathVysion HER-2 DNA probe
kit (Abbott Molecular Inc., IL). The PathVysion HER-2 DNA probe kit
consists of Locus Specific Identifier (LSI) HER-2/neu spectrum
orange DNA probe and Chromosome Enumeration Probe (CEP) 17 spectrum
green DNA probe. The LSI HER-2/neu DNA probe is a 226 Kb
SpectrumOrange directly-labeled, fluorescent DNA probe specific for
the HER-2/neu gene locus (17q11.2-q12). The CEP17 DNA probe is a
5.4 Kb SpectrumGreen directly-labeled, fluorescent DNA probe
specific for the alpha satellite DNA sequence at the centromeric
region of chromosome 17 (17p11.1-q11.1). Tissues were fixed in 10%
neutral buffered formalin solution for 24–48 h, de-alcoholized, and
then paraffin-embedded. Section slides were prepared with a
thickness of 4 to 6 µm. Next, the samples were immersed in protease
solution (37±1°C) for 10–60 min for enzymatic treatment, and then
immersed in 10% neutral buffered formalin solution for 10 min at
room temperature for fixation. After that, hybridization was
performed. 10 µl of DNA probe was added to the sample and incubated
at 37±1°C for 14–18 h. The samples were washed by immersion in
posthybridization wash buffer at 72±1°C for 2 min, and 10 µl of 4,
6-diamidino-2-phenylindole counterstain was added for measurement.
The signal was measured using a fluorescent microscope. The number
of LSI HER-2/neu and CEP17 signals in 20 nuclei were counted using
a 63× or 100× objective lens. HER2-low and HER2-negative was
defined as HER2/CEP17 ratio ≧1.0, and <1.0, respectively.
Patients were considered hormone receptor (HR)-positive if more
than 1% of the infiltrating tumor cells showed immunostaining for
estrogen receptor (ER) or progesterone receptor (PgR). Lymph node
metastasis was assessed in presurgical core needle biopsy or
surgical specimens. Pathologic complete response (pCR) was defined
as ypT0/isN0.
Statistical analysis
To compare the patients' clinicopathological
characteristics, continuous variables are expressed as medians and
categorical variables are expressed as numbers and percentages.
Pearson χ2 test were used to compared categorical
variables. Two-sides P-values of <0.05 were considered
statistically significant. OS was defined as time from the date of
surgery to time of death or last follow-up. DFS was defined as time
from the date of surgery to the date of disease recurrence or death
or last follow-up. Prognostic analysis was performed using the
Kaplan-Meier curves and the log-rank test. Multivariate logistic
regression analyses were used to identify independent prognostic
factors. All statistical analyses were performed using the SPSS
Statistics 28.0.0.0 (IBM, Armonk, NY, USA).
Results
Clinicopathologic characteristics
The median age, tumor size, and ki67 of the entire
1024 patients was 56.0 years (range=20.0–93.0), 2.0 cm
(range=0.3–15.0 cm), and 15.0% (range=0.5–99.0%), respectively. 155
(15.1%) patients had lymph node metastasis. Additionally, 908
(88.7%) patients revealed HR positive (ER positive; 903 patients,
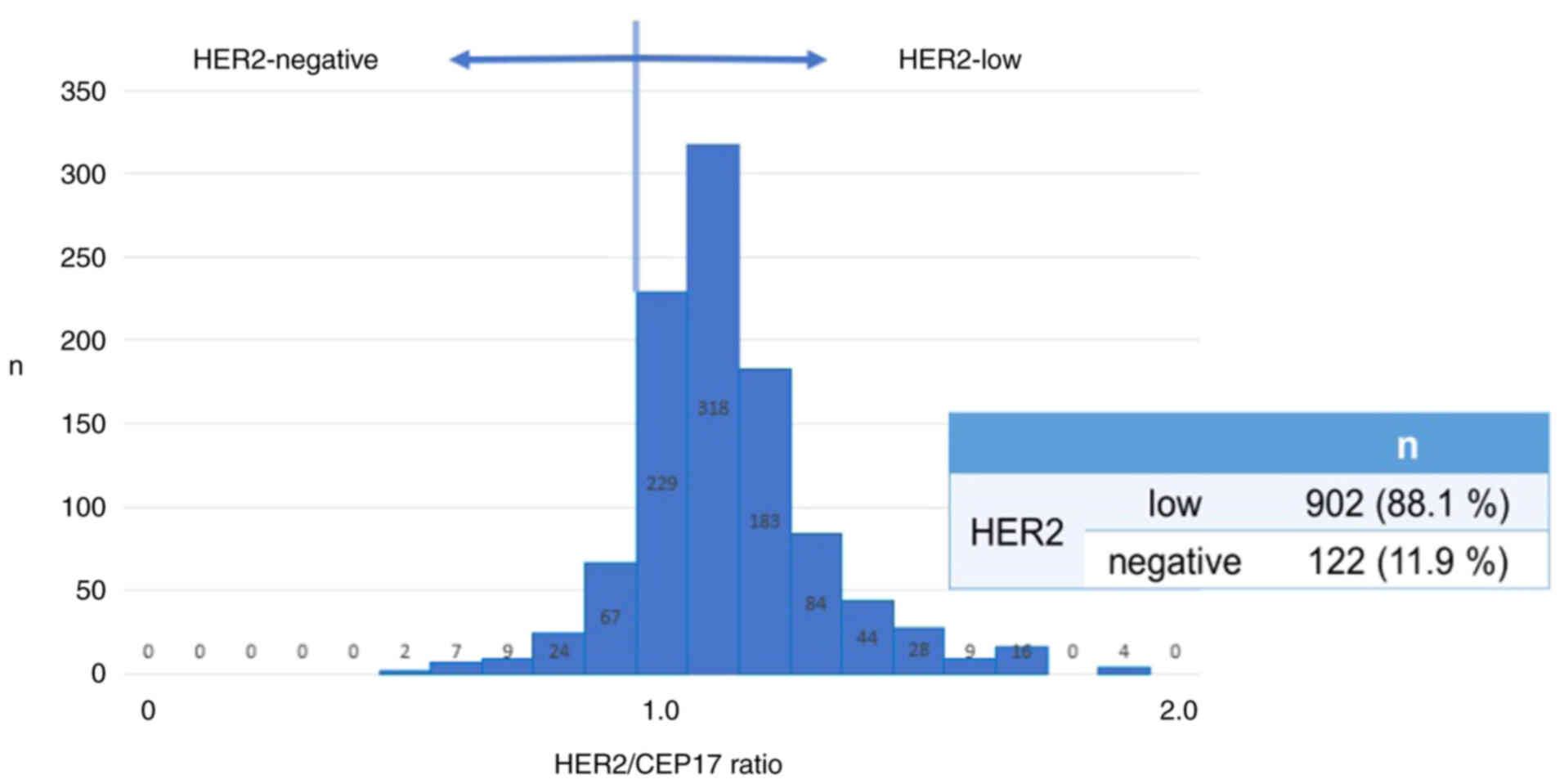
PgR positive; 810 patients). The number of HER2-low and
HER2-negative patients was 902 (88.1%) and 122 (11.9%),
respectively (Fig. 1).
No significant differences were observed between
HER2-low and HER2-negative patients in factors of age, tumor size,
clinical T stage, lymph node metastasis, nuclear grade, rate of
neoadjuvant chemotherapy (NAC), and adjuvant therapy (Table I). Positive rates for ER and PgR
were significantly higher in HER2-low patients compared to
HER2-negative patients [ER: 804 (89.0%) patients vs. 99 (82.0%)
patients; P=0.021, PgR: 723 (80.2%) patients vs. 86 (71.3%)
patients; P=0.023]. The median ki67 was significantly lower for
HER2-low compared to HER2-negative (14.5 vs. 18.5%, P=0.013)
patients.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Group | HER2-low (n=902) | HER2-negative
(n=122) | P-value |
|---|
| Median age, years
(range) |
| 56.0 (20–93) | 54.0 (23–90) | 0.286 |
| Median tumor size,
cm |
| 2.0 | 1.9 | 0.546 |
| cT stage, n (%) | 1 | 482 (53.4) | 66 (54.1) | 0.955 |
|
| 2 | 357 (39.6) | 46 (37.7) |
|
|
| 3 | 20 (2.2) | 5 (4.1) |
|
|
| 4 | 43 (4.8) | 5 (4.1) |
|
| Lymph node
metastasis, n (%) | Yes | 134 (14.9) | 21 (17.2) | 0.468 |
|
| No | 768 (85.1) | 101 (82.8) |
|
| Nuclear grade, n
(%) | 1 | 571 (63.3) | 71 (58.2) | 0.097 |
|
| 2 | 141 (15.6) | 20 (16.4) |
|
|
| 3 | 126 (14.0) | 25 (20.5) |
|
|
| Unknown | 64 (7.1) | 6 (4.9) |
|
| Estrogen receptor, n
(%) | Positive | 804 (89.0) | 99 (82.0) | 0.021 |
| Progesterone
receptor, n (%) | Positive | 723 (80.2) | 86 (71.3) | 0.023 |
| Median Ki67 (%) |
| 14.5 | 18.5 | 0.013 |
| Neoadjuvant
chemotherapy, n (%) | Yes | 168 (18.6) | 29 (23.8) | 0.160 |
|
| No | 735 (81.5) | 92 (75.4) |
|
| Adjuvant therapy, n
(%) | Chemotherapy | 141 (15.6) | 19 (15.6) | 0.894 |
|
| Endocrine | 815 (90.4) | 102 (83.6) | 0.056 |
|
| Radiation | 681 (75.5) | 100 (82.0) | 0.088 |
NAC was performed on 197 patients, including 168
HER2-low and 29 HER2-negative patients (Table II). Although the median ki67 was
significantly higher for HER2-negative compared to HER2-low in
patients treated with NAC (35.0 vs. 30.0%, P=0.036), the pCR rates
were not significantly different between the two groups [16.1%
(27/168 patients) vs. 17.2% (5/29 patients), P=0.528].
 | Table II.Patient characteristics of patients
treated with neoadjuvant chemotherapy. |
Table II.
Patient characteristics of patients
treated with neoadjuvant chemotherapy.
| Characteristics | Groups | HER2-low (n=168) | HER2-negative
(n=29) | P-value |
|---|
| Median age,
years |
| 55.5 | 53.0 | 0.474 |
| Median tumor size,
cm |
| 3.2 | 3.2 | 0.824 |
| Lymph node
metastasis, n (%) | Yes | 103 (61.3) | 19 (65.5) | 0.667 |
|
| No | 65 (38.7) | 10 (34.5) |
|
| HR, n (%) | Positive | 105 (62.5) | 14 (48.3) | 0.148 |
|
| Negative | 63 (37.5) | 15 (51.7) |
|
| Nuclear grade, n
(%) | 1.2 | 89 (53.0) | 14 (48.3) | 0.444 |
|
| 3 | 70 (41.7) | 15 (51.7) |
|
|
| Unknown | 9 (5.4) | 0 (0) |
|
| Median Ki67
(%) |
| 30.0 | 35.0 | 0.036 |
| pCR rate (%) |
| 16.1 | 17.2 | 0.528 |
Prognostic analysis
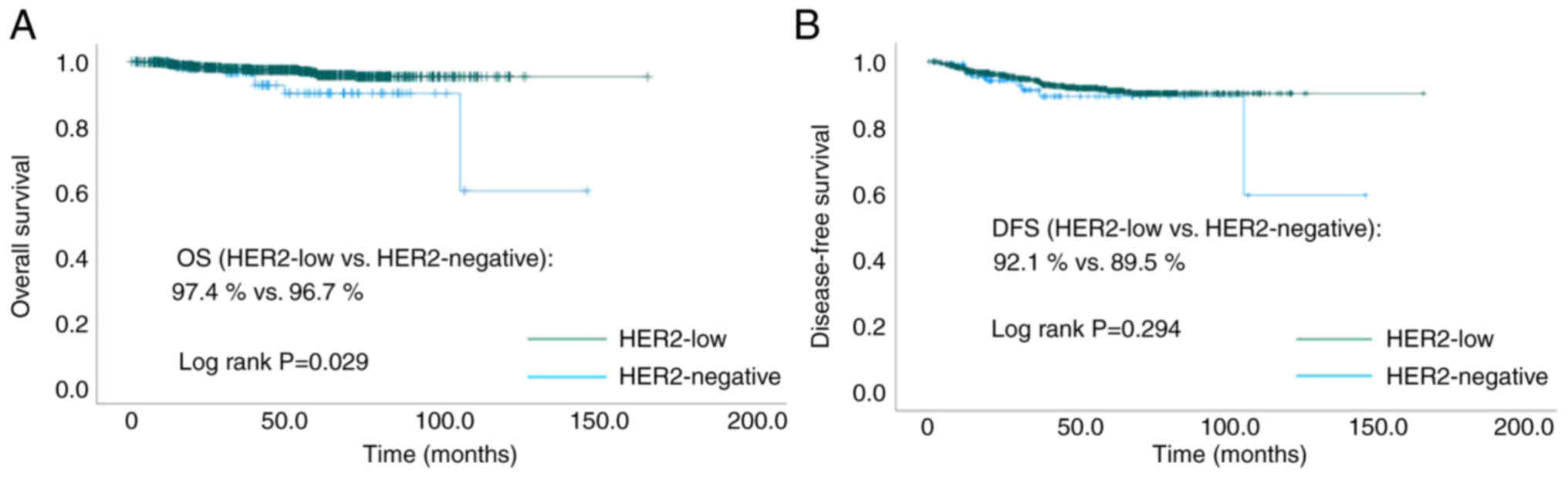
At a median follow-up time of 46.2 months, the OS
was significantly better in HER2-low compared to HER2-negative
(97.4 vs. 96.7%, P=0.029, Fig. 2A)
patient. DFS was not significantly different between the two groups
(92.1 vs. 89.5%, P=0.294, Fig. 2B).
In univariate analysis, lymph node metastasis, HR expression, HER2
status, nuclear grade, and ki67 were significantly associated with
OS. In the multivariate logistic analysis, lymph node metastasis
and HR expression were significantly associated with OS (Table III).
 | Table III.Analysis of prognostic factor for
OS. |
Table III.
Analysis of prognostic factor for
OS.
| Factor | Group | OS events, no. of
events/entire patients (%) | Univariate analysis
P-value | Multivariate
logistic regression analyses P-value |
|---|
| Age | ≥56.0 years | 21/529 (4.0) | 0.125 |
|
|
| <56.0 years | 12/496 (2.4) |
|
|
| Lymph node
metastasis | Yes | 16/155 (10.3) | <0.001 | <0.001 |
|
| No | 17/869 (2.0) |
|
|
| HR | Positive | 20/908 (2.2) | <0.001 | 0.005 |
|
| Negative | 13/116 (11.2) |
|
|
| HER2 | Low | 26/903 (2.9) | 0.029 | 0.340 |
|
| Negative | 7/121 (5.8) |
|
|
| NG | 1.2 | 17/803 (2.1) | <0.001 | 0.231 |
|
| 3 | 13/151 (8.6) |
|
|
| Ki67 | ≥15% | 21/463 (4.5) | 0.014 | 0.256 |
|
| <15% | 8/458 (1.7) |
|
|
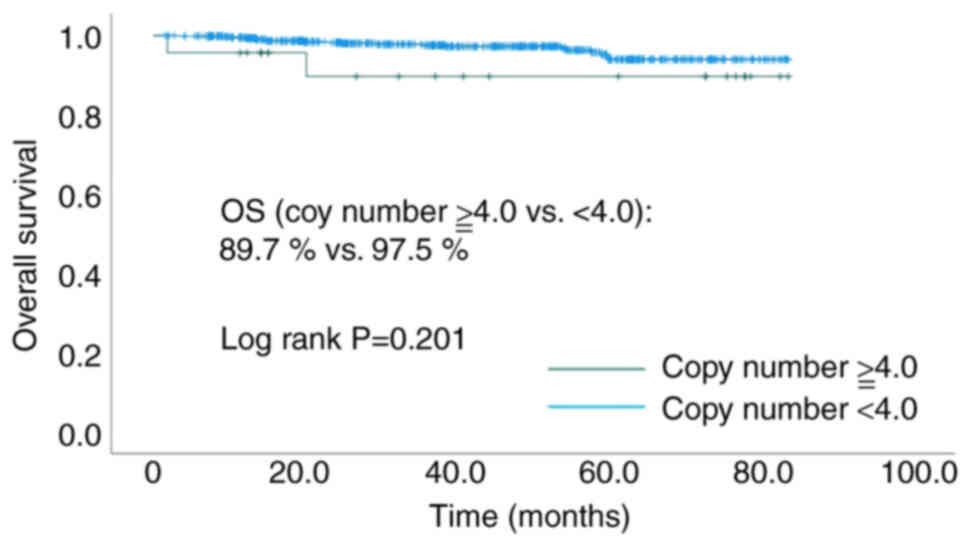
Among the HER2-low patients, 23 cases had a HER2
copy number of ≥4.0, and 662 cases had <4.0. Meanwhile, among
the HER2-negative patients, one case had a HER2 copy number of
≥4.0, and 99 cases had <4.0. No significant difference was
observed in the OS between the high copy number (≥4.0) and the low
copy number (<4.0) (89.7 vs. 97.5% P=0.201, Fig. 3) group among the HER2-low
patients.
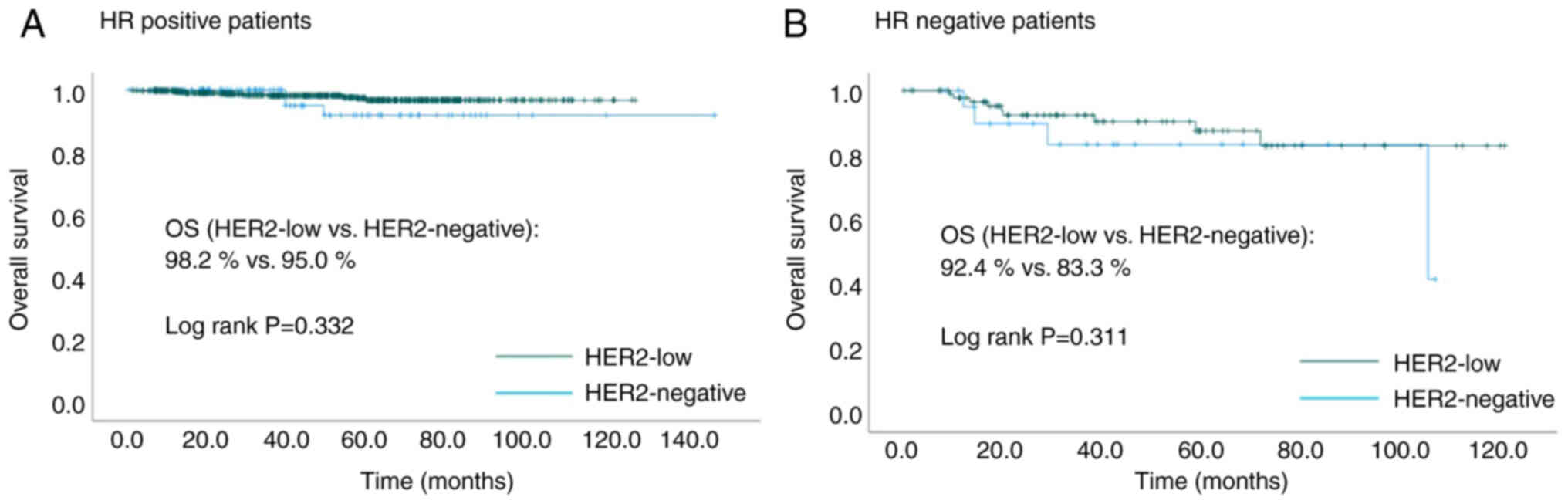
Among HR-positive patients, HER2-low and
HER2-negative patients were 808 (89.0%) and 100 (11.0%),
respectively. Among HR-negative patients, HER2-low and
HER2-negative patients were 94 (81.0%) and 22 (19.0%),
respectively. There was no significant difference in the OS between
HER2-low patients and HER2-negative patients in both HR positive
and HR negative cases (HR positive: 98.2 vs. 95.0%, P=0.332; HR
negative: 92.4 vs. 83.3%, P=0.311, Fig.
4).
Discussion
In this study, the positive rates of ER and PgR were
significantly higher, and the median ki67 was significantly lower
in HER2-low compared to HER2-negative patients. With a median
follow-up time of 46.2 months, the OS was significantly better in
HER2-low patients compared to HER2-negative patients, and HER2-low
expression was not independently related factors for OS.
Schettini et al reported that HER2-low tumors
were more frequently found within HR-positive disease compared to
triple-negative breast cancer (65.4 vs. 36.5%, P<0.001)
(13). Tarantino et al
reported that HER2-low breast cancer patients had a significantly
higher HR-positive rate than HER2-negative (90.6 vs. 81.8%,
P<0.001) which is consistent with our study (89.6 vs. 82.0%,
P=0.011) (10). Moreover, they
analyzed the rate of HER2-low breast cancer by ER expression levels
(negative, 0%; low, 1–9%; moderate, 10–49%; high, 50–95%; very
high, >95%) and found a positive correlation between ER
expression and the rate of HER2-low breast cancer (negative, 40.1%;
low, 46.3%; moderate, 55.2%; high, 57.8%; very high, 62.1%;
P<0.001) (10). A study
analyzing clinicopathologic characteristics of inflammatory breast
cancer with HER2-low also showed ER expressing tumors were more
common in patients with HER2-low vs. HER2-negative (65 vs. 38%,
P<0.01) (14). In addition,
considering that the median ki67 was significantly lower in
HER2-low than in HER2-negative (14.5 vs. 18.5%, P=0.013), HER2-low
was considered luminal-like and HER2-negative was considered triple
negative breast cancer-like. The PAM50 gene expression profile
showed a lower percentage of basal-like and higher percentage of
luminal A in HER2-low compared to HER2-negative breast cancer
patients (luminal A, 50.8 vs. 28.7%; basal-like, 13.3 vs. 43.7%,
P<0.001) (13). In other words,
most of these studies showed that HER2-low breast cancer is
luminal-like and HER2-negative breast cancer is
triple-negative-like. This finding is consistent with the
significantly higher positive rate of ER and PgR in HER2-low
patients compared to HER2-negative patients in our study.
Therefore, luminal-like feature might be one of the
clinicopathologic characteristics of HER2-low breast cancer.
There are various views on the prognostic impact of
HER2-low. Retrospective study of 3169 Japanese breast cancer
patients without HER2 overexpression found no statistically
significant differences between the prognosis of HER2-low and
HER2-0 patients, regardless of HR status, although patients in the
HER2-low group tended to have better prognosis than those in the
HER2-0 group (5 year OS: HR-positive, 96.7 vs. 94.9%, P=0.215;
HR-negative, 86.5 vs. 79.3%, P=0.152) (15). Another retrospective study of 804
primary breast cancer patients also confirmed no significant
differences in progression-free interval (PFI) between HER2-low and
HER2-subtype (5 year PFI rate: HER2-low/HR+ vs. HER2-/HR+, 82.6 vs.
78.0%, P=0.65; HER2-low/HR-vs. HER2-/HR-, 63 vs. 65%, P=0.81)
(16). Gampenrieder et al
analyzed the prognostic value of low HER2 expression in metastatic
breast cancer and reported that low HER2 expression was not
associated with OS regardless of HR expression (11). A study analyzing clinicopathologic
characteristics of inflammatory breast cancer (IBC) with HER2-low
reported that differences in OS were small, both among ER-positive
HER2-negative vs. HER2-low IBC (48 month OS: 80 vs. 81%, HR: 0.82,
95%CI:0.39–1.73) and ER-negative HER2-negative vs. HER2-low IBC (48
month OS: 34 vs. 47%, HR: 1.34, 95%CI: 0.74–2.41) (14). Moreover, Mouabbi et al
reported that progression-free survival and OS were not
statistically different between the patients with HER2-low and
HER2-negative treated with targeted therapy (cyclin-dependent
kinase 4/6 inhibitors, everolimus, or alpelisib) plus endocrine
therapy (17). Tarantino et
al reported that patients with HER2-low breast cancer had a
significantly better OS than patients with HER2-negative breast
cancer. However, this significance disappeared when HR-positive and
HR-negative were analyzed separately or when adjusted for
menopausal status, HR status, tumor grade, and histology
[HR-positive, 1.34 (P=0.26); HR-negative, 0.88 (P=0.65); overall,
1.14 (P=0.52)] (10). On the other
hand, Park et al retrospectively analyzed 2452
non-metastatic triple negative breast cancer and revealed that OS
was significantly better in patients with HER2-low compared to
HER2-0 (HR: 0.698, 95%CI: 0.517–0.943, P=0.019) (18). In our study, although OS was
significantly better in HER2-low, HER2-low was not significant
associated factor for OS in multivariate analysis. The high rate of
HR positivity in HER2-low patients and the positive correlation
between HER2 amplification and ER expression suggest that HR status
may be a confounding factor in prognosis.
Denkert et al performed a pooled analysis of
a cohort study of patients with HER2 non-amplified primary breast
cancer who underwent NAC, and found a significantly higher pCR rate
for HER2-negative compared to HER2-low across 2310 patients (39.0
vs. 29.2%, P=0.0002) (19).
Tarantino et al analyzed inflammatory breast cancer and
reported the higher pCR rate for HER2-negative breast cancer
compared to HER2-low breast cancer (11 vs. 6%, OR: 1.8,
95%CI:0.6–5.3) (14). In our study,
there was no significant difference in the pCR rate between the two
groups (17.2 vs. 16.1% vs., P=0.528). This could be attributed to a
small number of patients in our study.
This study had several limitations, the most
important limitation of this study is that the cutoff value for
HER2-low was defined as a HER2/CEP17 ratio ≧1.0. The definition of
HER2-low status using FISH assay requires careful consideration. In
general, HER2-low is defined as an IHC score of 1+ or 2+ with
negative FISH assay. Among 1024 cases in our study, HER2 status was
evaluated by both IHC and FISH assay in 280 patients, and there was
no significant correlation between IHC and FISH assay (HER2/CEP17
ratio; IHC 0, 0.8–1.7; 1+, 0.5–1.7; 2+, 0.0–1.7, P=0.118). In this
study, HER2-low was defined as HER2/CEP17 ratio ≧1.0 since copy
number of HER2 is more than the CEP. The validity of this
definition using FISH requires further investigation. The other
limitation is that the data for this study was collected at a
single site, so the results are exploratory. Moreover, our study
was underpowered to find statistical significance due to the small
sample size. Additionally, because of the short observation period,
caution is needed in interpreting the prognosis, especially
considering that most of the patients in our study were HR
positive. Therefore, this study is ongoing with additional patients
and an extended observation period. In the near future, more solid
data will be provided.
Many other studies (3,7,10–12)
regarding the clinical characteristics of HER2-low breast cancer
have used IHC to determine HER2 status. To the best of our
knowledge, this study is the only study evaluating HER2 status
using FISH assay, and this new definition of HER2-low is a strength
of this study. In the future, this new definition may help guide
treatment strategies for HER2-low breast cancer.
In this analysis, HER2-low patients had a
significantly higher HR-positive rate and lower median ki67
compared to HER2-negative patients. Multivariate logistic
regression analyses showed that HER2-low status was not an
independent factor for OS. These data suggested that HER2-low may
not be established as an independent subtype.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AS and HJ contributed to the concept and design of
the study and were major contributors to writing the manuscript. AM
contributed to the study design. YM, AM and TI contributed to data
acquisition. HJ contributed to review and editing. AS, YM, AM, TI
and HJ confirm the authenticity of all the data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by The Medical Ethics
Committee of Teikyo University School of Medicine (Tokyo, Japan;
approval no. 22-113). Patient informed consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ross JS, Fletcher JA, Linette GP, Stec J,
Clark E, Ayers M, Symmans WF, Pusztai L and Bloom KJ: The Her-2/neu
gene and protein in breast cancer 2003: Biomarker and target of
therapy. Oncologist. 8:307–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grant S, Qiao L and Dant P: Roles of ERBB
family receptor tyrosine kinases, and downstream signaling
pathways, in the control of cell growth and survival. Front Biosci.
7:d376–d389. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang H, Katerji H, Turner BM and Hicks
DG: HER2-low breast cancers: New opportunities and challenges. Am J
Clin Pathol. 157:328–336. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paik S, Kim C and Wolmark N: HER2 status
and benefit from adjuvant trastuzumab in breast cancer. N Engl J
Med. 358:1409–1411. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fehrenbacher L, Cecchini RS, Geyer CE Jr,
Rastogi P, Costantino JP, Atkins JN, Crown JP, Polikoff J, Boileau
JF, Provencher L, et al: NSABP B-47/NRG oncology phase III
randomized trial comparing adjuvant chemotherapy with or without
trastuzumab in high-risk invasive breast cancer negative for HER2
by FISH and with IHC 1+ or 2. J Clin Oncol. 38:444–453. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ergun Y, Ucar G and Akagunduz B:
Comparison of HER2-zero and HER2-low in terms of
clinicopathological factors and survival in early-stage breast
cancer: A systematic review and meta-analysis. Cancer Treat Rev.
115:1025382023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tarantino P, Hamilton E, Tolaney SM,
Cortes J, Morganti S, Ferraro E, Marra A, Viale G, Trapani D,
Cardoso F, et al: HER2-low breast cancer: Pathological and clinical
landscape. J Clin Oncol. 38:1951–1962. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tarantino P, Jin Q, Tayob N, Jeselsohn RM,
Schnitt SJ, Vincuilla J, Parker T, Tyekucheva S, Li T, Lin NU, et
al: Prognostic and biologic significance of ERBB2-low expression in
early-stage breast cancer. JAMA Oncol. 8:1177–1183. 2022.PubMed/NCBI
|
|
11
|
Gampenrieder SP, Rinnerthaler G, Tinchon
C, Petzer A, Balic M, Heibl S, Schmitt C, Zabernigg AF, Egle D,
Sandholzer M, et al: Landscape of HER2-low metastatic breast cancer
(MBC): Results from the Austrian AGMT_MBC-registry. Breast Cancer
Res. 23:1122021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miglietta F, Griguolo G, Bottosso M,
Giarratano T, Lo Mele M, Fassan M, Cacciatore M, Genovesi E, De
Bartolo D, Vernaci G, et al: HER2-low-positive breast cancer:
Evolution from primary tumor to residual disease after neoadjuvant
treatment. NPJ Breast Cancer. 8:662022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schettini F, Chic N, Brasó-Maristany F,
Paré L, Pascual T, Conte B, Martínez-Sáez O, Adamo B, Vidal M,
Barnadas E, et al: Clinical, pathological, and PAM50 gene
expression features of HER2-low breast cancer. NPJ Breast Cancer.
7:12021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tarantino P, Niman S, Erick T, Priedigkeit
N, Harrison BT, Giordano A, Nakhlis F, Bellon JR, Parker T, Strauss
S, et al: HER2-low inflammatory breast cancer: Clinicopathologic
features and prognostic implications. Eur J Cancer. 174:277–286.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Horisawa N, Adachi Y, Takatsuka D, Nozawa
K, Endo Y, Ozaki Y, Sugino K, Kataoka A, Kotani H, Yoshimura A, et
al: The frequency of low HER2 expression in breast cancer and a
comparison of prognosis between patients with HER2-low and
HER2-negative breast cancer by HR status. Breast Cancer.
29:234–241. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agostinetto E, Rediti M, Fimereli D,
Debien V, Piccart M, Aftimos P, Sotiriou C and de Azambuja E:
HER2-low breast cancer: Molecular characteristics and prognosis.
Cancers (Basel). 13:28242021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mouabbi J, Singareeka Raghavendra A,
Bassett R Jr, Hassan A, Tripathy D and Layman RM: Survival outcomes
in patients with hormone receptor-positive metastatic breast cancer
with low or no ERBB2 expression treated with targeted therapies
plus endocrine therapy. JAMA Netw Open. 6:e23130172023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park WK, Nam SJ, Kim SW, Lee JE, Yu J, Lee
SK, Ryu JM and Chae BJ: The prognostic impact of HER2-low and
menopausal status in triple-negative breast cancer. Cancers
(Basel). 16:25662024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Denkert C, Seither F, Schneeweiss A, Link
T, Blohmer JU, Just M, Wimberger P, Forberger A, Tesch H, Jackisch
C, et al: Clinical and molecular characteristics of
HER2-low-positive breast cancer: Pooled analysis of individual
patient data from four prospective, neoadjuvant clinical trials.
Lancet Oncol. 22:1151–1161. 2021. View Article : Google Scholar : PubMed/NCBI
|