Introduction
Pancreatic ductal adenocarcinoma (PDAC) represents a
challenging type of cancer with increasing mortality counts,
projected to replace breast cancer as the third highest ranked
cause of cancer-related deaths in Europe by 2025 (1). Historically, the outcome for patients
with PDAC has been poor with almost no long-time survivors, yet
recent data indicate a positive trend with 10-year survival (all
stages combined) now climbing towards 10% (2).
One key factor for improved prognosis has been the
introduction of adjuvant chemotherapy following curative intent
resection in patients with disease limited to the locoregional area
and who are in reasonably good performance status. Initially,
5-fluorouracil (5-FU) and folinic acid was mainstay treatment
(3) however over the past 10–15
years protocols including gemcitabine single agent (Gem) (4), gemcitabine plus the 5-FU prodrug
capecitabine (GemCape) (5), and
5-FU, leucovorin, irinotecan and oxaliplatin (FOLFIRINOX) (6) have largely replaced this standard. In
addition, a combination of the 5-FU oral prodrug tegafur in
combination with enzyme inhibitors gimeracil and oteracil (S-1) has
evolved as a viable option mainly in Asian populations (7). Irrespective of the treatment regimen
administered, benefit from chemotherapy is highly variable between
patients and finding prognostic and treatment predictive markers to
better guide therapeutic strategy has proven to be a goal yet to be
accomplished.
Dihydropyrimidine dehydrogenase (DPD) is an enzyme
encoded by the DPYD gene that is involved in the catabolism
of thymine and uracil and is expressed in various tissues and cells
of the body, including the liver, bone marrow and mononuclear cells
of the blood, as well as in tumour tissue (8). In addition to its physiological
function, DPD is also a key enzyme in the conversion of 5-FU into
its pharmacologically inactive form dihydrofluorouracil (9). High expression of DPD mRNA and protein
has been reported in several types of adenocarcinoma including
those of the stomach, head and neck, and pancreas (10).
Germline variations of the DPYD gene are
closely linked to severe toxicity to 5-FU and other pyrimidine
analogues, and there is now a general recommendation to check all
patients for such variants prior to starting treatment (11). Beside this, additional reports
indicate that intratumoural expression of DPD (either due to
germline or somatic mutations, epigenetic alterations, or
post-transcriptional upregulation) is a marker for poor prognosis
in various types of cancer as well as poor response to
chemotherapeutic anti-metabolic drugs including 5-FU, capecitabine
and S-1 when given alone or in combination with gemcitabine
(12–17). In addition, in vitro and
in vivo studies in urinary bladder cancer have implied DPD
expression levels to interfere with sensitivity and resistance to
gemcitabine (18).
The current study aimed to evaluate the potential
prognostic impact of intratumoural DPD expression in a large
real-world multi-centre cohort of patients with resected PDAC
treated with adjuvant chemotherapy.
Materials and methods
TMA construction
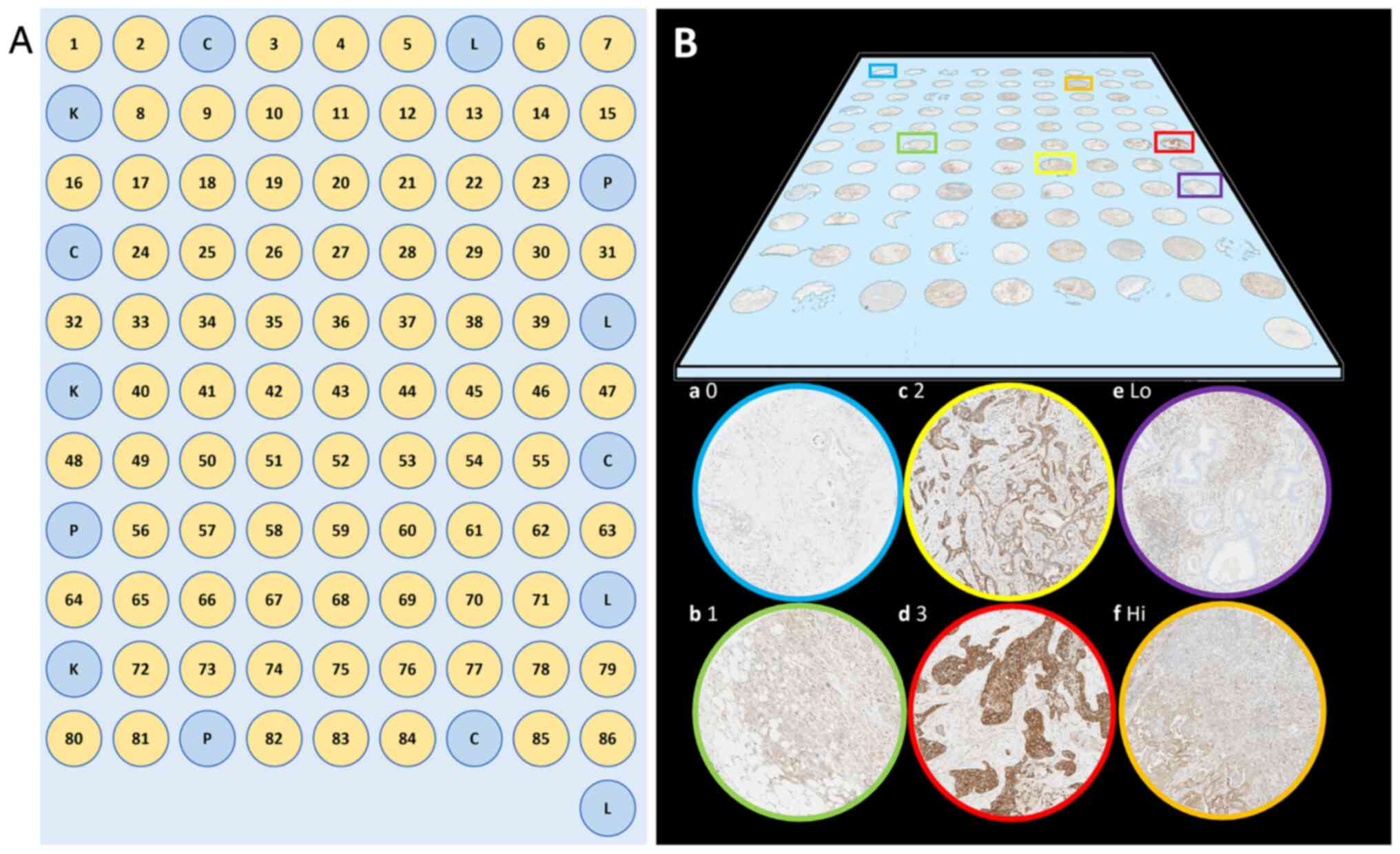
A tissue microarray (TMA) with multiple biopsies of
tumour tissue representing PDAC patients who underwent surgical
resection between 1993–2019 in any of the Northern, Western, and
Southeastern health care regions of Sweden, was constructed. The
cohort from the Southeast region included all resected cases
between 2009–2019 and has been described in detail in a previous
publication (19).
All available slides were reviewed for each case and
paraffin blocks corresponding to slides with the highest proportion
of tumour cells were selected for the TMA. The TMA was manufactured
with the previous ESPAC3 material-based TMA as a template (15). Two 1 mm in diameter micro core
biopsies were taken from tumour cell rich areas in two blocks from
the primary tumour and one 1 mm in diameter core biopsy from a
block with lymph node metastasis (if present), in total 4–5 cores
per case with an automated TMA Master or TMA Grandmaster (3DHistech
Kft., Budapest, Hungary). In a few cases where poor core quality
was readily detected during biopsy transferral (e.g., due to half
or broken biopsies), additional blocks were retrieved (if
available) to reach the total micro biopsy number of 4–5. In 86
other cases tumour tissue was embedded in new paraffin blocks as
tumour was only found in xl-blocks in the original case (not
compatible with the TMA machine). The biopsies were arranged in a
grid pattern in receiving paraffin blocks with micro biopsies
containing control tissue (alternating benign liver, pancreatic,
colonic, and renal tissue) arranged in a fence-like manner in the
perimeter of the grid (Fig. 1).
Receiving blocks were then mildly heated to melt the cores with
surrounding paraffin and subsequently 3.5 µm sections were taken
with a HM355S microtome (Thermo Fisher Scientific, Waltham MA, USA)
and mounted on Cut frosted microscope slides (Epredia, Kalamazoo
MI, USA). One section from each block was stained with
haematoxylin-eosin (HE) for reference and validation of tumour cell
content in the micro biopsies.
Immunohistochemical staining
One additional slide, sectioned at a thickness of
3.5 µm, was retrieved from all TMA blocks and baked for 1 h at
60°C. Deparaffinization and staining were performed in BOND III
stainers (Leica) using heat-induced epitope retrieval (HIER) with
Bond Epitope Retrieval Solution 2, effective heating time of 20 min
at 100°C, and Bond Polymer Refine Detection Kit (all reagents
supplied by Leica). The primary antibody (rabbit anti-DPD, Abcam ab
134922, Abcam, Cambridge, UK) was used at a dilution of 1:2,000,
with EnVision FLEX Antibody Diluent (Dako) and incubated for 15 min
at room temperature. The staining procedures were designed
according to a previously validated and optimised protocol for
immunohistochemical analysis of intratumoural DPD expression in
paraffin embedded TMA biopsies of PDAC (15).
DPD staining intensity assessment
Staining intensity was evaluated and scored by HB
and NE in an individual and blinded manner in four tiers 0–3,
replicating the methods used in the previous work utilizing the
ESPAC3 tissue material (15). If
heterogeneous staining intensity was present, the predominant
pattern was chosen. When the raters scored the same core
differently, the case was discussed, and a consensus score was
established.
Representative stained slides are displayed in
Fig. 1. Following completion of
scoring, the cores were deciphered and cases with less than two
evaluable tumour cores (e.g., no tumour in the core, section lost
during preparation, no tissue left in the TMA block etc.) were
excluded from further analysis. For included cases a mean score was
calculated based on all cores from the same case rounded to the
nearest integer. Cases were then dichotomized into low (0–1) or
high (2–3) expression.
Statistical analysis
All cases fulfilling the inclusion criteria were
included in the statistical analyses that were performed with SPSS
v29 (IBM, Armonk, USA) and R Statistical Software (v4.3.1; R
Foundation for Statistical Computing, Vienna, Austria). P<0.05
was considered to indicate a statistically significant difference.
If not else stated, descriptive statistics were reported as median
and interquartile range for continuous variables and as frequencies
and percentages for categorical values. Comparisons were made with
Mann Whitney U or χ2-test and unpaired Student's t-test
for categorical and continuous parameters, respectively. Cohen's κ
was used to assess interrater variability concerning IHC scoring.
Primary outcome was median overall survival (OS), defined as time
from the date of surgery until death or censoring, whatever
occurred first. Kaplan-Meier survival analysis was used to estimate
survival times and the log-rank test was utilized to detecting
significant differences between subgroups. Assuming proportional
hazards, univariate Cox regression analysis was used to identify
potentially prognostic factors. Spearman's rank correlation was
used to determine covariation between selected variables. A
subsequent multivariate Cox regression model, including factors
with P<0.10 in the univariate analysis, was used to determine
independent prognostic factors. To calculate median follow-up time,
the reverse Kaplan-Meier method was used (20).
Results
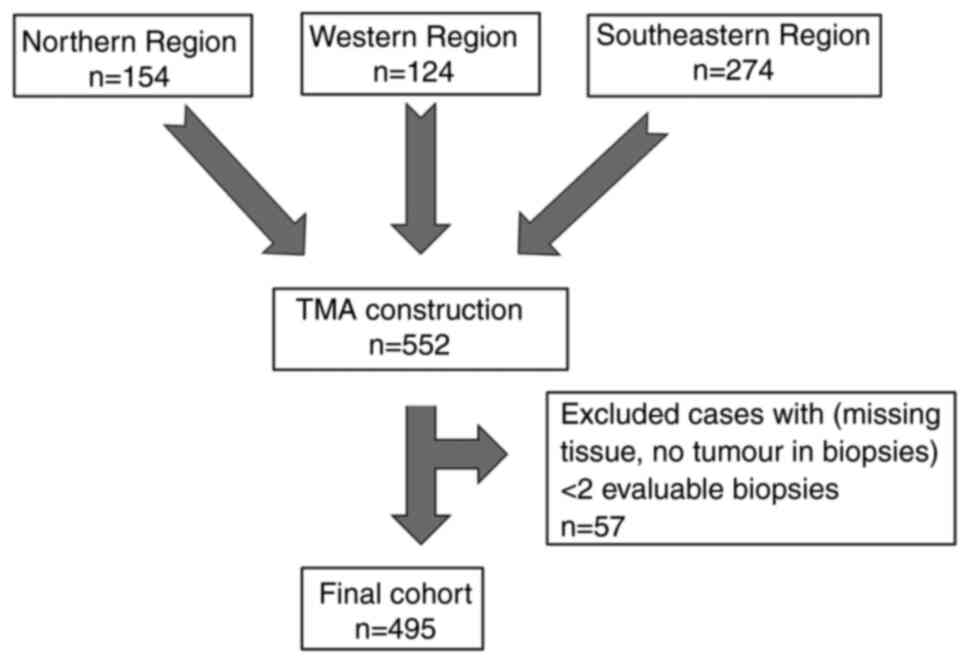
A total of 2,323 tumour cores were transferred to
the TMA blocks, representing the total cohort of 552 included cases
of resected PDAC. Fifty-seven cases were excluded due to less than
two evaluable tumour containing micro biopsies being available,
leaving a total of 495 cases in the final cohort available for
analysis (Fig. 2). DPD staining was
performed, and staining intensity was scored by two independent
assessors. The staining pattern was generally homogenous and clear,
with excellent inter-rater concordance (Cohen's κ=0.81).
Descriptives
Seventeen patients were diagnosed with PDAC of a
specified histological subtype, whilst 478 cases were diagnosed
with PDAC not otherwise specified (NOS). Descriptive patient
characteristics are shown for the full cohort and stratified as per
DPD intensity level in Table I. DPD
levels were significantly associated with tumour differentiation
grade and overall survival, with low levels generally being linked
with better prognostic features (high tumour differentiation grade
and longer overall survival).
 | Table I.Patient characteristics for the total
cohort and when divided into subgroups per DPD staining intensity
level. |
Table I.
Patient characteristics for the total
cohort and when divided into subgroups per DPD staining intensity
level.
| Characteristic | Total (n=495) | DPD low
(n=383) | DPD high
(n=112) |
P-valuea |
|---|
| Age,
yearsb | 69 (63–75) | 68 (9) | 69 (10) | 0.196 |
| Tumour size,
mmb | 30 (24–39) | 30 (23–39) | 30 (25–40) | 0.230c |
| Sex, female | 269 (54) | 201 (52.5) | 68 (60.7) | 0.152 |
| Year of
surgery |
|
|
| 0.181 |
|
1993-2005 | 23 (4.9) | 19 (6.3) | 4 (3.9) |
|
|
2006-2010 | 101 (21.7) | 79 (21.7) | 22 (21.5) |
|
|
2011-2015 | 207 (44.4) | 169 (46.4) | 38 (37.3) |
|
|
2016-2019 | 135 (29.0) | 97 (26.6) | 38 (37.3) |
|
| Differentiation
grade |
|
|
| <0.001 |
|
High | 48 (9.9) | 42 (11.2) | 6 (5.5) |
|
|
Medium | 237 (49.0) | 203 (54.1) | 34 (31.2) |
|
|
Low | 199 (41.1) | 130 (34.7) | 69 (63.3) |
|
| Margin status |
|
|
| 0.568 |
| R0 | 274 (56.1) | 217 (57.1) | 57 (52.8) |
|
| R1 | 205 (42.0) | 157 (41.3) | 48 (44.4) |
|
| R2 | 9 (1.8) | 6 (1.6) | 3 (2.8) |
|
| Sampled
nodesb | 15 (10–21) | 15 (9–20) | 17 (11–23) | 0.051d |
| Positive
nodesb | 2 (0–5) | 2 (0–5) | 2 (0.8–5.3) | 0.583d |
| TNM 7th ed
Stage |
|
|
| 0.151 |
| I | 50 (10.5) | 41 (11.1) | 9 (8.3) |
|
| II | 353 (78.2) | 292 (79.2) | 81 (75.0) |
|
|
III | 14 (2.9) | 8 (2.2) | 6 (5.6) |
|
| IV | 40 (8.4) | 28 (7.6) | 12 (11.1) |
|
| TNM 8th ed
Stage |
|
|
| 0.360 |
| I | 70 (19.5) | 56 (20.8) | 14 (15.7) |
|
| II | 144 (40.1) | 112 (41.5) | 32 (35.9) |
|
|
III | 105 (29.2) | 74 (27.4) | 31 (34.8) |
|
| IV | 40 (11.1) | 28 (10.4) | 12 (13.5) |
|
| Neoadjuvant
treatment | 25 (5.1) | 21 (5.5) | 4 (3.6) | 0.567 |
| Adjuvant
treatment |
|
|
| 0.971 |
|
None | 182 (37.1) | 140 (36.7) | 42 (38.2) |
|
|
5FU | 10 (2.0) | 9 (2.4) | 1 (0.9) |
|
|
Gem | 239 (48.7) | 184 (48.3) | 55 (50.0) |
|
|
GemCape | 39 (7.9) | 31 (8.1) | 8 (7.3) |
|
|
Gem/NabP | 4 (0.8) | 3 (0.8) | 1 (0.9) |
|
|
FOLFIRINOX | 6 (1.2) | 5 (1.3) | 1 (0.9) |
|
|
Other | 11 (2.2) | 9 (2.4) | 2 (1.8) |
|
| Relapse |
|
|
| 0.528 |
|
None | 68 (21.6) | 50 (20.9) | 18 (23.7) |
|
|
Local | 51 (16.2) | 35 (14.6) | 16 (21.1) |
|
|
Distant | 114 (36.2) | 90 (37.7) | 24 (31.6) |
|
| Local
and distant | 73 (23.2) | 58 (24.3) | 15 (19.7) |
|
| M1 at
surgery | 9 (2.9) | 6 (2.5) | 3 (3.9) |
|
| OSe | 19.6
(17.4–23.8) | 22.5
(18.6–26.1) | 16.2
(13.1–21.5) | 0.005f |
Overall survival
Median overall survival in the total cohort,
including all patients who had and who had not received any
adjuvant chemotherapy, was 19.6 months (95% CI 17.4–23.8, Table I).
Patients who did not receive adjuvant chemotherapy
had a worse outcome than those who did, with a median overall
survival of 11.6 months (95% CI 9.6–13.5) vs. 28.8 months (95% CI
25.0–32.6), P<0.001, log-rank test).
Further subgrouping of patients, according to the
type of chemotherapy received, revealed overall survival estimates
of 28.1 months (95% CI24.1–32.0) and 28.1 months (95% CI 15.0–41.2)
for the most commonly utilized protocols Gem (n=239) and GemCape
(n=39), respectively. The other subgroups, including FOLFIRINOX,
gemcitabine with nab-paclitaxel (Gem/nab-P), and 5-FU single agent,
were too small (n<11 for each individual regimen) to perform
separate subanalyses on.
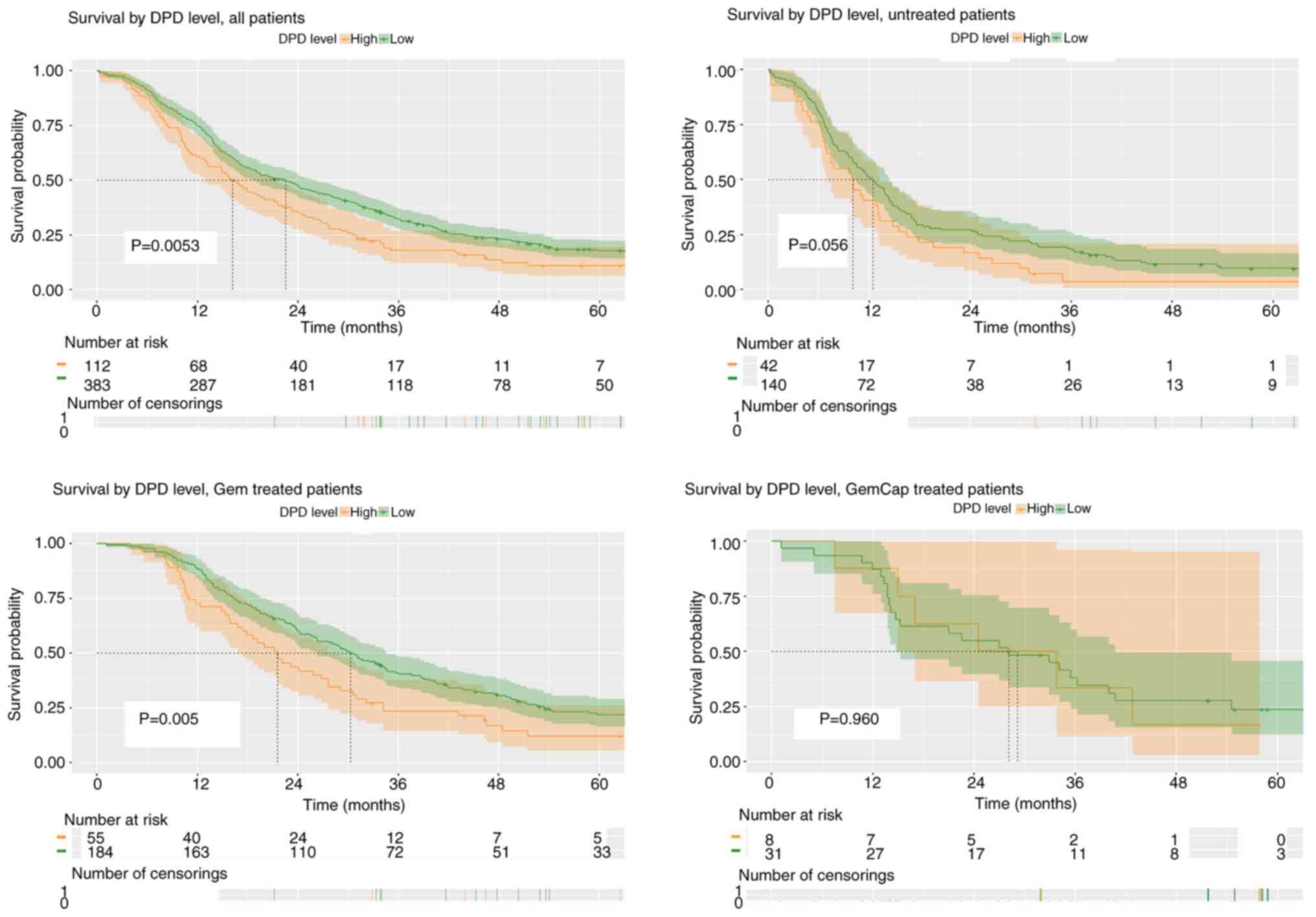
When categorising the patients according to the
intratumoural DPD expression levels, high expression was associated
with shorter survival in the total cohort of patients (log-rank
P=0.0053). This impact was most prominent in the Gem treated
population, with median OS of 30.3 months (95% CI 26.3–34.5) and
21.5 months (95% CI 17.4–27.7) in the DPD low and high expression
subgroups, respectively (P=0.005). A similar yet not statistically
significant trend was evident in the group of patients who did not
receive any postoperative chemotherapy (P=0.056), whereas no
difference was observed in the GemCape treated subgroup (P=0.960)
(Fig. 3).
Univariate analyses of overall
survival
Given the heterogenous nature of the main groups of
patients (no chemotherapy, Gem, and GemCape), these were separately
assessed with Cox regression analysis in terms of potential
prognostic factors and overall survival.
In the untreated group of patients, TNM stage,
R-status, year of surgery and tumour differentiation grade were all
statistically significant prognostic parameters (Table II).
 | Table II.Univariate and multivariate Cox
regression analyses on all patients combined, and in the subgroups
who received and who did not receive adjuvant chemotherapy. |
Table II.
Univariate and multivariate Cox
regression analyses on all patients combined, and in the subgroups
who received and who did not receive adjuvant chemotherapy.
|
| All patients | No adjuvant
chemotherapy (n=182) | Any adjuvant
chemotherapy (n=309) |
|---|
|
|
|
|
|
|---|
| Variable | HR Univariate | P-value | HR Univariate | P-value | HR
Multivariate | P-value | HR Univariate | P-value | HR
Multivariate | P-value |
|---|
| Adjuvant |
|
|
|
|
|
|
|
|
|
|
|
None | 1 | <0.001 | - | - | - | | - | | - |
|
|
5-FU | 0.18
(0.07–0.43) | <0.001 | - |
| - |
| - |
| - |
|
|
Gem | 0.47
(0.38–0.58) | <0.001 | - |
| - |
| - |
| - |
|
|
GemCape | 0.45
(0.31–0.68 | <0.001 | - |
| - |
| - |
| - |
|
|
Gem/NabP | 0.82
(0.26–2.57) | 0.734 | - |
| - |
| - |
| - |
|
|
FOLFIRINOX | 0.56
(0.21–1.50) | 0.248 | - |
| - |
| - |
| - |
|
| Age |
|
|
|
|
|
|
|
|
|
|
| <70
years | 1 |
| 1 |
| - |
| 1 |
| - |
|
| ≥70
years | 1.19
(0.98–1.44) | 0.078 | 0.79
(0.57–1.07) | 0.129 | - |
| 1.17
(0.91–1.51) | 0.211 | - |
|
| DPD-level |
|
|
|
|
|
|
|
|
|
|
|
Low | 1 |
| 1 |
| 1 |
| 1 |
| 1 |
|
|
High | 1.38
(1.10–1.73) | 0.005 | 1.19
(0.99–1.42) | 0.058 | 1.06
(0.70–1.59) | 0.787 | 1.17
(1.01–1.36) | 0.038 | 0.76
(0.56–1.02) | 0.070 |
| Sex |
|
|
|
|
|
|
|
|
|
|
|
Male | 1 |
| 1 |
| - | | 1 |
| 1 |
|
|
Female | 0.85
(0.71–1.03) | 0.104 | 1.12
(0.82–1.51) | 0.488 | - | | 0.85
(0.75–0.96) | 0.011 | 1.36
(1.06–1.74) | 0.016 |
| Grade |
|
|
|
|
|
|
|
|
|
|
|
Low | 1 |
| 1 | <0.001 | 1 | <0.001 | 1 | 0.333 | - |
|
|
Medium | 0.75
(0.61–0.91) | 0.005 | 0.54
(0.38–0.76) | <0.001 | 0.60
(0.42–0.87) | 0.006 | 0.85
(0.66–1.10) | 0.217 | - |
|
|
High | 0.54
(0.36–0.77) | <0.001 | 0.26
(0.15–0.45) | <0.001 | 0.33
(0.19–0.58) | <0.001 | 0.76
(0,48–1.20) | 0.242 | - |
|
| Neoadjuvant
treatment |
|
|
|
|
|
|
|
|
|
|
| No | 1 |
| 1 |
| - |
| 1 |
| - |
|
|
Yes | 0.83
(0.52–1.31) | 0.424 | 1.04
(0.53–2.04) | 0.912 | - |
| 0.70
(0.37–1.31) | 0.263 | - |
|
| Margin status |
|
|
|
|
|
|
|
|
|
|
| R0 | 1 |
| 1 |
| 1 |
| 1 |
| 1 |
|
|
R1-2 | 1.34
(1.10–1.63) | 0.004 | 1.41
(1.03–1.95) | 0.035 | 1.01
(0.72–1.41) | 0.981 | 1.43
(1.11–1.84) | 0.005 | 1.29
(1.00–1.68) | 0.054 |
| Year of
surgery |
|
|
|
|
|
|
|
|
|
|
|
1993-2006 | 1 |
| 1 | 0.008 | - | - | 1 | 0.604 | - |
|
|
2007-2010 | 0.64
(0.42–0.96) | 0.032 | 0.63
(0.36–1.08) | 0.090 | - | - | 0.93
(0.46–1.90) | 0.848 | - |
|
|
2011-2014 | 0.66
(0.45–0.97) | 0.033 | 0.91
(0.55–1.49) | 0.693 | - | - | 0.90
(0.46–1.79) | 0.765 | - |
|
|
2015-2019 | 0.47
(0.32–0.70) | <0.001 | 0.48
(0.28–0.83) | 0.008 | - | - | 0.77
(0.39–1.52) | 0.448 | - |
|
| TNM 7th |
|
|
|
|
|
|
|
|
|
|
| Stage
I | 1 |
| 1 | <0.001 | 1 | <0.001 | 1 | 0.024 | 1 | 0.097 |
| Stage
II | 2.07
(1.46–2.94) | <0.001 | 3.56
(1.89–6.69) | <0.001 | 3.55
(1.83–6.89) | 0.013 | 1.75
(1.14–2.70) | 0.011 | 1.63
(1.04–2.55) | 0.033 |
| Stage
III | 4.45
(2.34–8.45) | <0.001 | 6.10
(2.30–16.14) | <0.001 | 4.10
(1.35–12.43) | <0.001 | 2.91
(1.10–7.68) | 0.031 | 2.67
(1.00–7.16) | 0.050 |
| Stage
IV | 3.77
(2.38–5.99) | <0.001 | 5.92
(2.81–12.45) | <0.001 | 4.81
(2.21–10.46) | <0.001 | 2.33
(1.21–4.48) | 0.011 | 1.92
(0.97–3.79) | 0.062 |
| TNM 8th |
|
|
|
|
|
|
|
|
|
|
| Stage
I | 1 |
| 1 | <0.001 | - |
| 1 | <0.001 | - |
|
| Stage
II | 1.57
(1.12–2.22) | 0.010 | 0.42
(0.28–0.63) | <0.001 | - |
| 1.40
(0.90–2.16) | 0.135 | - |
|
| Stage
III | 2.66
(1.86–3.81) | <0.001 | 0.91
(0.68–1.22) | 0.535 | - |
| 2.69
(1.64–4.10) | <0.001 | - |
|
| Stage
IV | 3.66
(2.37–5.64) | <0.001 | 1.33
(0.98–1.80) | 0.069 | - |
| 2.45
(1.30–4.59) | 0.005 | - |
|
In the Gem treated group, DPD level, sex, R-status,
and TNM stage were prognostic whereas no factors showed significant
prognostic value in the GemCape group (Table III). When combining all patients
treated with any type of chemotherapy, DPD-level, sex, R-status,
and TNM stage were prognostic in terms of overall survival
(Table II).
 | Table III.Univariate and multivariate Cox
regression analyses on patients treated with adjuvant Gem and
GemCape. |
Table III.
Univariate and multivariate Cox
regression analyses on patients treated with adjuvant Gem and
GemCape.
|
| Gem (n=239) | GemCape (n=37) |
|---|
|
|
|
|
|---|
| Variable | HR Univariate | P-value | HR
Multivariate | P-value | HR Univariate | P-value |
|---|
| Age |
|
|
|
|
|
|
| <70
years | 1 | | - | | 1 |
|
| ≥70
years | 1.26
(0.95–1.67) | 0.105 | - |
| 1.43
(0.64–3.18) | 0.380 |
| DPD-level |
|
|
|
|
|
|
|
Low | 1 |
| 1 |
| 1 |
|
|
High | 1.25
(1.06–1.47) | 0.007 | 1.19
(1.01–1.41) | 0.036 | 1.02
(0.65–1.60) | 0.944 |
| Sex |
|
|
|
|
|
|
|
Male | 1 |
| 1 |
| 1 |
|
|
Female | 0.84
(0.73–0-96) | 0.010 | 0.87
(0.76–1.00) | 0.050 | 0.92
(0.64–1.34) | 0.664 |
| Grade |
|
|
|
|
|
|
|
Low | 1 | 0.47 | - |
| 1 |
|
|
Medium | 0.89
(0.67–1.21) | 0.48 | - |
| 0.75
(0.35–1.61) | 0.464 |
|
High | 0.74
(0.45–1.21) | 0.234 | - |
| - |
|
| Neoadjuvant
treatment |
|
|
|
|
|
|
| No | 1 |
| - |
| 1 |
|
|
Yes | 0.73
(0.33–1.36) | 0.457 | - |
| 1.02
(0.35–2.93) | 0.976 |
| Radicality |
|
|
|
|
|
|
| R0 | 1 |
| 1 |
| 1 |
|
|
R1-2 | 1.40
(1.05–1.86) | 0.021 | 1.23
(0.91–1.64) | 0.174 | 1.75
(0.74–4.13) | 0.203 |
| Surgery |
|
|
|
|
|
|
|
1993-2006 | 1 | 0.366 | - |
| - |
|
|
2007-2010 | 0.62
(0.30–1.28) | 0.200 | - |
| 1 | 0.735 |
|
2011-2014 | 0.67
(0.34–1.33) | 0.254 | - |
| 0.69
(0.10–4.96) | 0.713 |
|
2015-2019 | 0.56
(0.28–1.12) | 0.102 | - |
| 0.57
(0.13–2.45) | 0.448 |
| TNM 7th |
|
|
|
|
|
|
| Stage
I | 1 | 0.003 | 1 | 0.018 | 1 | 0.566 |
| Stage
II | 1.92
(1.20–3.06) | 0.006 | 0.48
(0.30–0.76) | 0.002 | 1.93
(0.26–14.30) | 0.520 |
| Stage
III | 5.41
(1.82–16.07) | 0.002 | 0.88
(0.63–1.22) | 0.429 | 1.24
(0.08–19.83) | 0.880 |
| Stage
IV | 2.77
(1.32–5.82) | 0.007 | 2.26
(1.05–4.87) | 0.037 | 6.64
(0.39–112.6) | 0.190 |
| TNM 8th |
|
|
|
|
|
|
| Stage
I | 1 | <0.001 | - |
| 1 | 0.261 |
| Stage
II | 1.40
(0.84–2.33) | 0.199 | - |
| 2.16
(0.70–6.72) | 0.183 |
| Stage
III | 3.01
(1.76–5.15) | <0.001 | - |
| 2.21
(0.69–7.05) | 0.181 |
| Stage
IV | 2.88
(1.38–6.04) | 0.005 | - |
| 8.77
(0.89–86.80) | 0.063 |
Multivariate analyses of overall
survival
All factors that returned P<0.10 in the
univariate regression analyses were included in the subsequent
multivariate Cox regression analyses (Kaplan-Meier curves for these
factors are seen in Fig. S1). As
Spearman's rank correlation analysis revealed no association
between the year of surgery variable and the dependent variable DPD
expression level (rho 0.109, negligible correlation; data not
shown), the year of surgery variable was excluded in the
multivariate analysis.
In the group of patients who had not received any
chemotherapy, differentiation grade and TNM stage were both
statistically significant independent prognostic factors with
regards to overall survival (Table
II).
In the Gem treated group of patients, TNM stage and
DPD expression levels were both independent parameters for survival
(with P=0.018 and P=0.036, respectively), whereas sex was
borderline significant (P=0.050, Table III). Amongst GemCape treated
patients, neither DPD-expression nor any other factors were found
statistically significant (Table
III). Upon grouping all types of chemotherapy together, only
sex was a statistically significant factor in terms of survival
(Table II).
Discussion
Over the past 20 years, adjuvant chemotherapy has
evolved as mainstay treatment in patients who have undergone
curative intent resection of pancreatic adenocarcinoma. Despite
significant therapeutic improvement, the prognosis remains poor,
and there are still patient groups that do not benefit from the
chemotherapy given. There are currently few, if any, treatment
predictive molecular biomarkers that tell us who will be at high
vs. low risk for recurrent disease and who will have good outcomes
following adjuvant treatment.
With newer and more intense multi-drug regimens at
hand, the need for prognostic profiling of the tumour, that may
indicate what type of patient that will need more intense
treatments and follow up, has become imminent.
Previous studies on DPD expression in various types
of cancer including colorectal and pancreatic adenocarcinomas have
indicated a potential prognostic and/or predictive value in
patients treated with 5-FU or other fluoropyrimidines alone or in
combination with Gem (12–17). In addition, DPD has been implied as
a molecular marker for response to Gem in urinary bladder cancer
cell lines and tumours (18).
The present study focused on the potential value of
intratumoural DPD expression levels in a large cohort of patients
with PDAC who underwent curative intent surgery over a period of 26
years (1993–2019) and covering three major catchment areas of
Sweden.
As expected, outcomes were very poor amongst
patients who did not receive any type of adjuvant chemotherapy.
This was not surprising and is at least partly likely to be
explained by selection bias in terms of patients with poor
performance status and/or severe complications following the
surgical resection being less likely to be fit for chemotherapy
and, notably, the survival in this group was closely mirroring the
observation arm of the ESPAC-1 trial (3). In addition, early relapses, preceding
the window of starting adjuvant chemotherapy, may have contributed
to the dismal outcome in this type of patients. Independent
prognostic factors (following multivariate regression analysis) in
patients who did not receive adjuvant chemotherapy were
differentiation grade and TNM stage.
Amongst patients who did receive adjuvant
chemotherapy, median overall survival was 28.8 months, which is in
line with outcomes reported in the literature (3–6). There
was no numerical difference between the two most common
chemotherapy regimens utilised (Gem and GemCape) as median overall
survival was 28.1 months in both groups. It should however be noted
that the Gem group made up the vast majority of the population
treated with chemotherapy (n=239, 75% of those who received any
type of chemotherapy) whereas just 39 patients (14%) received
GemCape. Therefore, selection bias cannot be excluded and any
inter-group comparisons should be made with greatest caution.
Independent prognostic factors remaining
statistically significant following multivariate Cox regression
analysis included TNM stage and DPD expression status in the Gem
group, whereas no factors appeared statistically significant in the
GemCape group of patients. Again, the relatively small number of
subjects in the latter (meaning a low power to detect any
significant findings) should prompt careful interpretation.
As other types of chemotherapy including 5-FU with
folinic acid, FOLFIRINOX, and gemcitabine plus nab-paclitaxel were
only sporadically given in this cohort (n<11 in each treatment
group), no meaningful analyses of prognostic factors including
DPD-high vs. low were possible to do within these subgroups.
The results of this study cannot be directly
compared with previous results on intratumoural DPD expression in
patients of the ESPAC3 randomised controlled trial (15), as this was a real world cohort where
no strict inclusion or exclusion criteria nor any randomisation to
various treatments were applied. In the ESPAC3 trial population,
DPD appeared as an independent prognostic marker in the 5-FU
treated arm of patients but not in the Gem arm (although a
non-significant numerical difference was still evident). On the
other hand, the Kondo study (16)
on patients receiving a combination of S-1 and gemcitabine revealed
that DPD was an independent prognostic marker, with high expression
being linked with worse prognosis.
Whereas the present results indicate that DPD is an
independent predictor of the outcome in PDAC patients treated with
postoperative Gem, the cohort studied here cannot be used to answer
whether guidance to any of the more intense multi-drug protocols
with GemCape (5), Gemcitabine and
Nab-paclitaxel (21), or FOLFIRINOX
(6) would have been beneficial for
patients with high expression of DPD. Although 39 patients in the
present population were treated with GemCape, and no statistically
significant factors were evident in the multivariate regression
model, statistical power would not be sufficient to rule out any
impact of DPD (or any of the other potentially prognostic markers)
in this or any of the even smaller subgroups. In addition, it would
be most relevant for a future prospective trial to explore whether
the addition of a DPD inhibitor such as gimeracil, one of three
active substances in the S-1 combination, might be able to override
the negative impact of high levels of DPD in the tumour. In theory,
such a Gem plus gimeracil combination might be particularly
valuable in patients with high expression of DPD in their
tumour.
The weaknesses of this study are mainly inherent to
the retrospective study design, and as there was no randomisation
to various treatment arms any inter-arm differences observed should
be interpreted with caution. Selection of treatment is likely to
have been affected by background patient factors and comorbidity
status as well as postoperative recovery and occurrence of
complications. During the studied period, the predominant adjuvant
protocol was Gem, with a smaller proportion of patients being
subjected to the more recent multi-drug regimens that are now
available and generally recommended.
The main strengths include the long term and
comprehensive multi-centre real-world approach, meaning that a
large number of patients with PDAC undergoing curative intent
resection in three major health care regions were included.
Detailed clinical information was available and follow up time was
sufficient to yield robust data on overall survival. To our
knowledge, this is the largest real-world cohort of patients with
resected PDAC treated with gemcitabine where DPD has been explored
as a prognostic marker.
Future studies should focus on exploring the value
of intratumoural DPD expression levels in patients undergoing
adjuvant chemotherapy with contemporary multi-drug regimens, as
well as exploring other potential enzymes and transport proteins
involved in the metabolism and turnover of nucleic acids hence
playing a potential role for sensitivity to-anti pyrimidine
chemotherapeutics. Such candidate biomarkers include (but would not
be limited to) thymidylate synthase, orotate phosphoribosyl
transferase, cytidine deaminase, human equilibrative nucleoside
transporter-1, and intratumoural human antigen all known to be
involved in the turnover of antimetabolic chemotherapeutics
(15,16,22–25).
In addition, preclinical research will be necessary to dissect the
mechanisms by which DPD and other metabolic enzymes affect the
sensitivity to 5-FU, gemcitabine and other compounds.
Ideally, individual or panels of predictive
biomarkers should be identified and explored as treatment guiding
tools in a prospective trial setting, to establish algorithms for
optimal adjuvant treatment strategies and drug(s) of choice in
patients with localised PDAC undergoing surgical resection.
In conclusion, intratumoural DPD expression is an
independent prognostic factor in patients with PDAC undergoing
surgical resection followed by adjuvant gemcitabine. Additional
studies of DPD as a potential predictive biomarker in cohorts of
PDAC patients treated with gemcitabine and non-gemcitabine based
multi-drug chemotherapy protocols are warranted.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Lisa Kölhed and
Ms. Liza Haglund (laboratory technicians, Department of Pathology,
Linköping University Hospital, Sweden) who remoulded the paraffin
blocks for this study, Dr Mats Fredrikson (Linköping University,
Sweden) for statistical advice, and the late Dr Alkwin Wanders
(Aalborg University Hospital, Denmark) who played a key role in the
early work-up of this study.
Funding
This work was supported by the non-profit funding bodies FORSS
(grant no. 941207), CKOC and Region Östergötland (grant nos.
RÖ-962449, RÖ-935580, RÖ-962548, RÖ-937640, RÖ-990631, RÖ-975579
and RÖ-978701). HB received funding from Lion's Research Fund,
Linköping. Oskar Franklin received funding from The Royal Swedish
Academy of Sciences (PE Lindahl Foundation; grant nos. LM2021-0010
and LM2023-0012), The Swedish Society of Medicine (grant no.
SLS-960379), Region Västerbotten in Umeå, Sweden (grant no. RV
967602), The Sjöberg Foundation, Cancerforskningsfonden i Norrland
(grant no. LP 23-2337), Bengt Ihre Foundation (grant nos.
SLS-960529 and SLS-986656) and Bengt Ihre Research Fellowship
Grant. Daniel Öhlund received funding from the Cancer Research
Foundation in Northern Sweden (grant nos. AMP23-1104 and LP
24-2377), the Swedish Cancer Society (grant no. 23 2707 Pj), The
Swedish Research Council (grant no. 2022-00855), Region
Västerbotten (grant no. RV-978812), the Knut and Alice Wallenberg
Foundation, and the Marianne and Marcus Wallenberg Foundation
(grant no. MMW 2020.0189). Malin Sund received funding from the
Swedish Research Council (grant no. 2019-01690), the Swedish Cancer
Society (grant no. 19 0273), Västerbotten Region (grant nos.
RV-583411, RV-549731, RV-583411 and RV-841551), the Sigrid Juselius
Foundation (grant no. 8166), Finska Läkaresällskapet, Medicinska
Understödsföreningen Liv och Hälsa, the Sjöberg Foundation and VTR
funding from Helsinki University Hospital (grant no. TYH2022329).
None of the funding bodies participated in the design,
conceptualization, data collection, analysis, interpretation of
data, or writing of the manuscript of this study.
Availability of data and materials
The data generated in this study are available at
reasonable request to the corresponding author.
Authors' contributions
Conceptualisation and design were performed by HB,
PN, MS, CV, HG, BB, DÖ, SL, OF and NOE. HB, MB, FG, PN, MS, CV, DÖ,
SL and OF constructed the TMA. HB, MB and FG were responsible for
tissue management and preparation. Staining was performed by MB and
FG, scoring by HB and NOE. Data analysis was conducted by HB, HG,
BB, DÖ, SL, OF and NE. HB and NOE drafted the manuscript. HB and
NOE confirm the authenticity of all the raw data. All authors
contributed to, and read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was performed according to The
Declaration of Helsinki and was approved by the Swedish Ethics
Review Board (approval number 2020-01511). Due to the retrospective
non-interventional nature of the study, the review board waived the
need for informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancerfonden, . The Swedish Cancer Society
Report. Cancerfonden; Stockholm: 2023
|
|
3
|
Neoptolemos JP, Stocken DD, Friess H,
Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C,
Lacaine F, et al: A randomized trial of chemoradiotherapy and
chemotherapy after resection of pancreatic cancer. N Engl J Med.
350:1200–1210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neoptolemos JP, Stocken DD, Bassi C,
Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger
S, Mariette C, et al: Adjuvant chemotherapy with fluorouracil plus
folinic acid vs. gemcitabine following pancreatic cancer resection:
A randomized controlled trial. JAMA. 304:1073–1081. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neoptolemos JP, Palmer DH, Ghaneh P,
Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA,
Cunningham D, Wadsley J, et al: Comparison of adjuvant gemcitabine
and capecitabine with gemcitabine monotherapy in patients with
resected pancreatic cancer (ESPAC-4): A multicentre, open-label,
randomised, phase 3 trial. Lancet. 389:1011–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conroy T, Hammel P, Hebbar M, Ben
Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi
JJ, et al: FOLFIRINOX or gemcitabine as adjuvant therapy for
pancreatic cancer. N Engl J Med. 379:2395–2406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uesaka K, Boku DN, Fukutomi A, Okamura Y,
Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto
H, et al: Adjuvant chemotherapy of S-1 versus gemcitabine for
resected pancreatic cancer: A phase 3, open-label, randomised,
non-inferiority trial (JASPAC 01). Lancet. 388:248–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Padmanabhan S: Handbook of
pharmacogenomics and stratified medicine. Academic Press;
Cambridge, MA, USA: pp. 341–364. 2014
|
|
9
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukui Y, Oka T, Nagayama S, Danenberg PV,
Danenberg KD and Fukushima M: Thymidylate synthase,
dihydropyrimidine dehydrogenase, orotate phosphoribosyltransferase
mRNA and protein expression levels in solid tumors in large scale
population analysis. Int J Mol Med. 22:709–716. 2008.PubMed/NCBI
|
|
11
|
European Medicines Agency, . EMA
recommendations on DPD testing prior to treatment with
fluorouracil, capecitabine, tegafur and flucytosine. EMA, Press
Release; Amsterdam: 2020
|
|
12
|
Falvella FS, Cheli S, Martinetti A,
Mazzali C, Iacovelli R, Maggi C, Gariboldi M, Pierotti MA, Di
Bartolomeo M, Sottotetti E, et al: DPD and UGT1A1 deficiency in
colorectal cancer patients receiving triplet chemotherapy with
fluoropyrimidines, oxaliplatin and irinotecan. Br J Clin Pharmacol.
80:581–588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang YH, Shi WN, Wu SH, Miao RR, Sun SY,
Luo DD, Wan SB, Guo ZK, Wang WY, Yu XF, et al: SphK2 confers
5-fluorouracil resistance to colorectal cancer via upregulating
H3K56ac-mediated DPD expression. Oncogene. 39:5214–5227. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vallböhmer D, Yang DY, Kuramochi H,
Shimizu D, Danenberg KD, Lindebjerg J, Nielsen JN, Jakobsen A and
Danenberg PV: DPD is a molecular determinant of capecitabine
efficacy in colorectal cancer. Int J Oncol. 31:413–418.
2007.PubMed/NCBI
|
|
15
|
Elander NO, Aughton K, Ghaneh P,
Neoptolemos JP, Palmer DH, Cox TF, Campbell F, Costello E, Halloran
CM, Mackey JR, et al: Expression of dihydropyrimidine dehydrogenase
(DPD) and hENT1 predicts survival in pancreatic cancer. Br J
Cancer. 118:947–954. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kondo N, Murakami Y, Uemura K, Sudo T,
Hashimoto Y, Nakashima A and Sueda T: Combined analysis of
dihydropyrimidine dehydrogenase and human equilibrative nucleoside
transporter 1 expression predicts survival of pancreatic carcinoma
patients treated with adjuvant gemcitabine plus S-1 chemotherapy
after surgical resection. Ann Surg Oncol. 19 (Suppl 3):S646–S655.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuramochi H, Hayashi K, Uchida K, Nakajima
G, Hatori T, Danenber KD, Danenberg PV and Yamamoto M: High
intratumoral dihydropyrimidine dehydrogenase mRNA levels in
pancreatic cancer associated with a high rate of response to S-1.
Cancer Chemother Pharmacol. 63:85–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsukahara S, Shiota M, Takamatsu D,
Nagakawa S, Matsumoto T, Kiyokoba R, Yagi M, Setoyama D, Noda N,
Matsumoto S, et al: Cancer genomic profiling identified
dihydropyrimidine dehydrogenase deficiency in bladder cancer
promotes sensitivity to gemcitabine. Sci Rep. 12:85352022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blomstrand H, Olsson H, Green H, Björnsson
B and Elander NO: Impact of resection margins and para-aortic lymph
node metastases on recurrence patterns and prognosis in resectable
pancreatic cancer-a long-term population-based cohort study. HPB
(Oxford). 25:1531–1544. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schemper M and Smith TL: A note on
quantifying follow-up in studies of failure time. Control Clin
Trials. 17:343–346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tempero M, Pelzer U, O'Reilly EM, Winter
J, Oh DY, Li CP, Tortora G, Chang HM, Lopez CD, Bekaii-Saab T, et
al: Adjuvant nab-paclitaxel + gemcitabine in resected pancreatic
ductal adenocarcinoma: Results from a randomized, open-label, phase
III trial. J Clin Oncol. 41:2007–2019. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyake K, Imura S, Yoshizumi T, Ikemoto T,
Morine Y and Shimada M: Role of thymidine phosphorylase and orotate
phosphoribosyltransferase mRNA expression and its ratio to
dihydropyrimidine dehydrogenase in the prognosis and
clinicopathological features of patients with pancreatic cancer.
Int J Clin Oncol. 12:111–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oba A, Ban D, Kirimura S, Akahoshi K,
Mitsunori Y, Matsumura S, Ochiai T, Kudo A, Tanaka S and Minoru T:
Clinical application of the biomarkers for the selection of
adjuvant chemotherapy in pancreatic ductal adenocarcinoma. J
Hepatobiliary Pancreat Sci. 23:480–488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aughton K, Elander NO, Evans A, Jackson R,
Campbell F, Costello E, Halloran CM, Mackey JR, Scarfe AG, Valle
JW, et al: hENT1 predicts benefit from gemcitabine in pancreatic
cancer but only with low CDA mRNA. Cancers (Basel). 13:57582021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei CH, Gorgan TR, Elashoff DA, Hines OJ,
Farrell JJ and Donahue TR: A meta-analysis of gemcitabine
biomarkers in patients with pancreaticobiliary cancers. Pancreas.
42:1303–1310. 2013. View Article : Google Scholar : PubMed/NCBI
|