Introduction
Head and neck cancer (HNC), the seventh most common
type of cancer worldwide, consists of numerous tumors affecting the
upper respiratory tract (1).
Although there are a number of different histological types,
squamous cell carcinoma (SCC) consists of ~95% of cases, covering
tumors of the lips, mouth, nasal cavity, sinuses, pharynx and
larynx (2). The major risk factors
include smoking, alcoholism and carcinogenic virus, as well as
human papilloma and Epstein-Barr viruses (3,4). HNC,
which is often diagnosed in patients who use tobacco and alcohol
heavily, presents a slowly declining incidence globally, in part
due to a decrease in tobacco use (5).
Over the past few decades, a comprehensive treatment
mode that integrates surgery, radiotherapy and chemotherapy has
been formed and developed, which has significantly improved the
local control rate of tumors but not the overall survival (OS),
with the 5-year OS rate remaining at only 40–50% (6–8).
Treatment of head and neck malignancies remains challenging and
requires a multidisciplinary approach. In this scenario, a
multi-specialty team evaluation is critical in determining the
treatment options for HNCs (9). Due
to the special anatomical structure of HNC, early diagnosis and
early detection of tumor recurrence are still difficult, various
forms of endoscopy and tumor tissue biopsy are the current gold
standard for diagnosis (10).
However, this invasive test has a number of drawbacks, including
the inability of patients to tolerate multiple biopsies, the high
cost, the inability to make a definitive diagnosis in
difficult-to-sample areas and the inability to consider the
heterogeneity of the tumor when a single sample is used to diagnose
the disease (11). Therefore, there
is an urgent need for convenient and accurate tests that can
identify patients at high risk of metastasis before the occurrence
of metastatic lesions, thereby improving the ability to
individualize treatment for patients.
Circulating tumor cells (CTCs) are shed from primary
tumors into the bloodstream, where they may seed metastases in
distant organs (12,13). Detecting CTCs serves as a valuable
biomarker for cancer progression and offers a target for early
detection, prognosis and monitoring treatment efficacy (14). Despite their infrequency, with
counts typically below 10 CTCs per 7.5 ml of blood in patients with
solid tumors, advancements in microfluidics and nanotechnology are
enhancing detection sensitivity and specificity (15–18).
The CELLSEARCH® (Menarini Silicon Biosystems, Inc.)
system, employing immunomagnetic enrichment and fluorescent
labeling, is a prevalent method for CTC identification. CTC
enumeration is a prognostic indicator in various types of cancer,
including metastatic breast, prostate and colorectal, and
fluctuations in CTC levels can signal treatment responses and
survival outcomes (19).
However, several challenges impede the clinical
application of CTCs. Their rarity complicates detection and
analysis. Additionally, the heterogeneity of CTCs necessitates
standardized protocols for isolation and analysis. The clinical
implications of single CTCs versus clusters also require further
clarification (20).
Looking ahead, the integration of CTC analysis with
other components of liquid biopsies, such as cell-free DNA, will
provide a more comprehensive assessment. The development of more
sensitive and specific technologies for CTC detection and analysis
is a priority. In essence, CTCs are a promising frontier in
oncology, with the potential to significantly enhance cancer
diagnosis, treatment and monitoring. The ongoing research and
development in this area are expected to yield discoveries that
will improve patient care and outcomes.
The present retrospective study aimed to investigate
the relationship between CTC count in the peripheral blood of
patients with HNC and patient outcomes, with the goal of offering
insights into the potential use of CTCs as a prognostic marker for
this patient population.
Materials and methods
Study subjects
The clinical data of 56 cases of patients
pathologically diagnosed with HNC that were admitted between
December 2013 to June 2018 to the Department of Otolaryngology,
Head and Neck Surgery (Beijing Tongren Hospital, Capital Medical
University, Beijing, China) were retrospectively reviewed (Table I). The diagnosis was confirmed by a
pathologist, who was independent from the study. The inclusion
criteria were as follows: i) All patients were pathologically
diagnosed with HNC; ii) no distant organ metastasis as confirmed by
routine imaging examination; and iii) complete clinical data and
follow-up information. The exclusion criteria were as follows: i)
Malignancies in other organs; ii) hematological disorders and/or
immune system diseases; iii) perioperative death or death caused by
non-tumor reasons; iv) patients requiring long-term oral
anticoagulation drugs; and v) incomplete clinical data and/or
follow-up information. The study was approved by the hospital
ethics committee of Beijing Tongren Hospital, Capital Medical
University.
 | Table I.Basic clinical information of 56
patients. |
Table I.
Basic clinical information of 56
patients.
| Pathological
features | Number of cases, n
(%) |
|---|
| Sex |
|
|
Male | 52 (92.86) |
|
Female | 4 (7.14) |
| Age |
|
|
<50 | 9 (16.07) |
|
50-60 | 14 (25.00) |
|
>60 | 33 (58.93) |
| Tumor type |
|
|
Oropharyngeal carcinoma | 5 (8.94) |
| Oral
carcinoma | 9 (16.07) |
|
Laryngeal carcinoma | 16 (28.57) |
|
Hypopharyngeal carcinoma | 13 (23.21) |
|
Nasopharyngeal carcinoma | 7 (12.50) |
|
Carcinoma of the nasal cavity
and sinuses | 6 (10.71) |
| Pathological
type |
|
|
Squamous cell carcinoma | 52 (92.86) |
|
Non-squamous cell
carcinoma | 4 (7.14) |
| Smoking
history |
|
|
Yes | 38 () |
| No | 18 () |
| Drinking
history |
|
|
Yes | 34 (67.86) |
| No | 22 (32.14) |
| TNM stage |
|
| I +
II | 16 (28.57) |
|
III | 13 (23.22) |
| IV | 27 (48.21) |
| Surgery |
|
|
With | 41 (73.21) |
|
Without | 15 (26.79) |
| Non-operative
management |
|
|
Radiotherapy | 21 (37.50) |
|
Chemoradiotherapy | 19 (33.93) |
|
None | 16 (28.57) |
CTC detection
CTC detection was performed at the time of sample
collection as part of routine clinical testing. The CellSearch
technique (21), utilized by Nuohai
Life Science (Shanghai) Co. Ltd, is a non-invasive diagnostic
method for detecting and quantifying CTCs in the peripheral blood
of cancer patients. The system comprises the Celltracks Autoprep,
Celltracks Analyzer II and CellSave Tube Preservative tubes and
kits, which include epithelial cell adhesion molecule (EpCAM)
antibody-coated immunomagnetic beads, fluorescent antibodies for
cytokeratin (CK) and CD45), cell fixatives and buffers. i) Specimen
Collection: Blood samples were collected from patients on an empty
stomach before surgery or treatment, with the first 1 ml discarded
to avoid contamination. A 10 ml sample was drawn into a CellSave
tube, mixed, stored at room temperature and tested within 72 h. ii)
Procedure: 7.5 ml of peripheral blood was transferred into a Cell
Search conical tube, followed by the addition of 6.5 ml of diluent.
After centrifugation at 800 g for 10 min, the sample was processed
in the CellTracks Autoprep System. The system automatically
enriched CTCs using antibodies and DAPI for co-incubation,
following the instrument's protocol. iii) Detection process: Blood
samples were collected (7.5 ml, and CTCs were enriched through
immunomagnetic separation based on EpCAM expression, a molecule
selectively present on epithelial cells including CTCs. iv)
Identification: Post-enrichment, CTCs were identified with
fluorescently labeled CK antibodies and specific epithelial cell
markers. DAPI staining visualized the nucleus, confirming intact
cells. v) Exclusion of white blood cells: CD45, specific for white
blood cells (22), ensured that
only CTCs were counted by excluding white blood cells from the
analysis. vi) Automation and sensitivity: The automated
CELLSEARCH® system minimized human error and enhanced
result reproducibility. It is sensitive and capable of detecting a
single CTC in 7.5 ml of blood.
Determination of experimental
results
The Cell Search system was used to perform CTC
detection. The scanning results obtained from the CellTracks
Analyzer II scanning system were then analyzed, and the cells with
immunofluorescent staining results showing EpCAM-positive,
CK-positive, DAPI-positive and CD45-negative with intact cell
membrane and nucleus staining were defined as CTCs. The count of
CTCs was recorded, and CTCs ≥1/7.5 ml was defined as CTC-positive.
CTC negative indicates the absence of circulating tumor cells in
blood samples.
Observational indicators
The relationship between CTCs and sex, age, smoking
history, alcohol consumption history, pathological type, tumor
site, TNM staging, non-operative management and whether the patient
underwent surgical treatment were analyzed. Furthermore, the impact
of CTCs on patient prognosis were examined. Age was categorized
into three levels: i) <50; ii) 50–60; and iii) >60 for
analysis.
Patients who had not undergone surgery, that is,
after comprehensive discussion by a multidisciplinary team, choose
to receive non-surgical treatment plans, mainly including
radiotherapy or combined chemoradiotherapy. Among these patients,
the majority refused surgical treatment due to personal preference,
hoping to preserve laryngeal function.
Statistical analysis
This study used SPSS 20.0 software (IBM Corp.) for
data analysis. Count data are expressed in the form of n (%). The
χ2 test was used to compare the distribution differences
in clinical and pathological characteristics between CTC-positive
and CTC-negative patients. Firstly, the patient's outcome of death
was defined: Overall survival (OS) was defined as the time from
diagnosis to death from any cause, and Kaplan-Meier estimation and
log-rank test were used to estimate and compare survival rates
between different variables. The impact factors on overall survival
were analyzed using univariate and multivariate Cox proportional
hazards regression models. P<0.05 was considered to indicate a
statistically significant difference. The experiments were
performed in triplicate.
Results
Basic pathological features of 56
patients
The total 56 patients included 52 males and 4
females, with a median age of 61.5 years (range, 42–75). Stage I +
II, III and IV HNC were found in 16, 13 and 27 cases, respectively.
All patients were confirmed by histopathology, including 52 cases
of SCC and 4 cases of non-squamous cell carcinoma, as presented in
Table I.
Detection of CTCs
Blood samples provided by all 56 patients reached a
volume of 10 ml without any apparent hemolysis or clotting
phenomena, meeting the quality requirements for the assay kit,
resulting in a sample qualification rate of 100%. The detection
outcomes from the CELLSEARCH® system revealed that out
of the 56 patients, 14 individuals exhibited the presence of CTCs
with a count of ≥1/7.5 ml, indicating a positivity rate of 25%. The
median CTC count stood at 2 (range, 1–32). CTCs, identified by the
presence of cytokeratin (CK+) and DAPI positivity (DAPI+), while
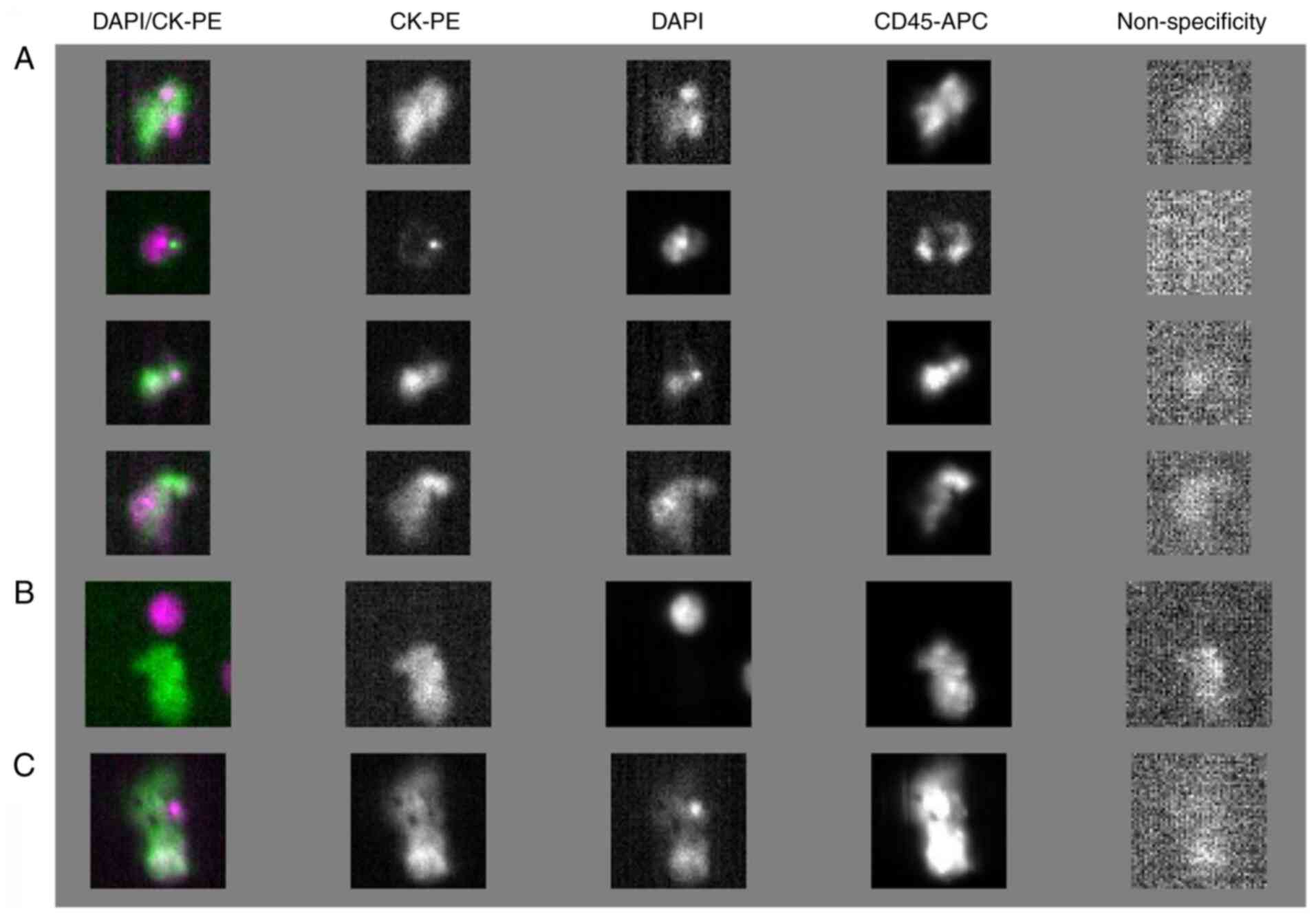
being negative for the hematopoietic marker CD45 (Fig. 1). Non-CTCs, which are negative for
CK (CK-), positive for DAPI (DAPI+), and positive for CD45 were
also identified.
Comparison of basic pathological
features between CTC-positive and CTC-negative patients
Table II provides a
comparative analysis of various basic pathological features between
patients who tested positive for CTCs and those who tested
negative. When comparing CTC-positive patients with CTC-negative
patients, no statistically significant differences were observed in
terms of age, sex, smoking/alcohol history (present or absent),
tumor location, pathological type, TNM staging and whether surgical
treatment was received (all P>0.05). The TNM staging was divided
into stages I + II, III and IV. The CTC-positive group had 3
(21.43%) in stages I + II, 5 (35.71%) in stage III and 6 (42.86%)
in stage IV. The CTC-negative group had 13 (30.95%) in stages I +
II, 8 (19.05%) in stage III and 21 (50.00%) in stage IV. The
difference in TNM stage distribution was not statistically
significant (χ2=1.701; P=0.427). In terms of
non-operative management, the CTC-positive group had fewer patients
undergoing radiotherapy [3 (21.43%)] and chemoradiotherapy [4
(28.57%)], with a larger proportion not receiving any treatment [7
(50.00%)]. The CTC-negative group had a higher number of patients
undergoing radiotherapy [20 (47.62%)] and chemoradiotherapy [15
(35.71%)], with fewer not receiving any treatment [7 (16.67%)]. The
difference in non-operative management was significant
(χ2=6.578; P=0.037).
 | Table II.Comparison of basic pathological
features between CTC-positive and CTC-negative patients. |
Table II.
Comparison of basic pathological
features between CTC-positive and CTC-negative patients.
|
| CTCs, n (%) |
|
|
|---|
|
|
|
|
|
|---|
| Pathological
features | Positive
(n=14) | Negative
(n=42) | χ2 | P-value |
|---|
| Sex |
|
| 1.436 | 0.231 |
|
Male | 12 (85.71) | 40 (95.24) |
|
|
|
Female | 2 (14.29) | 2 (5.00) |
|
|
| Age |
|
| 0.223 | 0.894 |
|
<50 | 2 (14.29) | 7 (16.67) |
|
|
|
50-60 | 3 (21.43) | 11 (26.19) |
|
|
|
>60 | 9 (64.29) | 24 (57.14) |
|
|
| Tumor type |
|
| 7.235 | 0.204 |
|
Oropharyngeal carcinoma | 3 (21.43) | 2 (4.76) |
|
|
| Oral
carcinoma | 2 (14.29) | 7 (16.67) |
|
|
|
Laryngeal carcinoma | 4 (28.57) | 12 (28.57) |
|
|
|
Hypopharyngeal carcinoma | 2 (14.29) | 11 (26.19) |
|
|
|
Nasopharyngeal carcinoma | 3 (21.43) | 4 (9.52) |
|
|
|
Carcinoma of the nasal cavity
and sinuses | 0 (0.00) | 6 (14.29) |
|
|
| Pathological
type |
|
| 1.436 | 0.281 |
|
Squamous cell carcinoma | 14 (100.00) | 38 (90.48) |
|
|
|
Non-squamous cell
carcinoma | 0 (0.00) | 4 (9.52) |
|
|
| Smoking
history |
|
| 0.109 | 0.741 |
|
Yes | 9 (64.29) | 29 (69.05) |
|
|
| No | 5 (35.71) | 13 (30.95) |
|
|
| Drinking
history |
|
| 0.898 | 0.343 |
|
Yes | 7 (50.00) | 27 (64.29) |
|
|
| No | 7 (50.00) | 15 (35.71) |
|
|
| TNM stage |
|
| 1.701 | 0.427 |
|
I–II | 3 (21.43) | 13 (30.95) |
|
|
|
III | 5 (35.71) | 8 (19.05) |
|
|
| IV | 6 (42.86) | 21 (50.00) |
|
|
| Surgical
treatment |
|
| 1.487 | 0.223 |
|
With | 12 (85.71) | 29 (69.05) |
|
|
|
Without | 2 (14.29) | 13 (30.95) |
|
|
| Non-operative
management |
|
| 6.578 | 0.037 |
|
Radiotherapy | 3 (21.43) | 20 (47.62) |
|
|
|
Chemoradiotherapy | 4 (28.57) | 15 (35.71) |
|
|
|
None | 7 (50.00) | 7 (16.67) |
|
|
Comparison of survival rates between
CTC-positive and CTC-negative patients
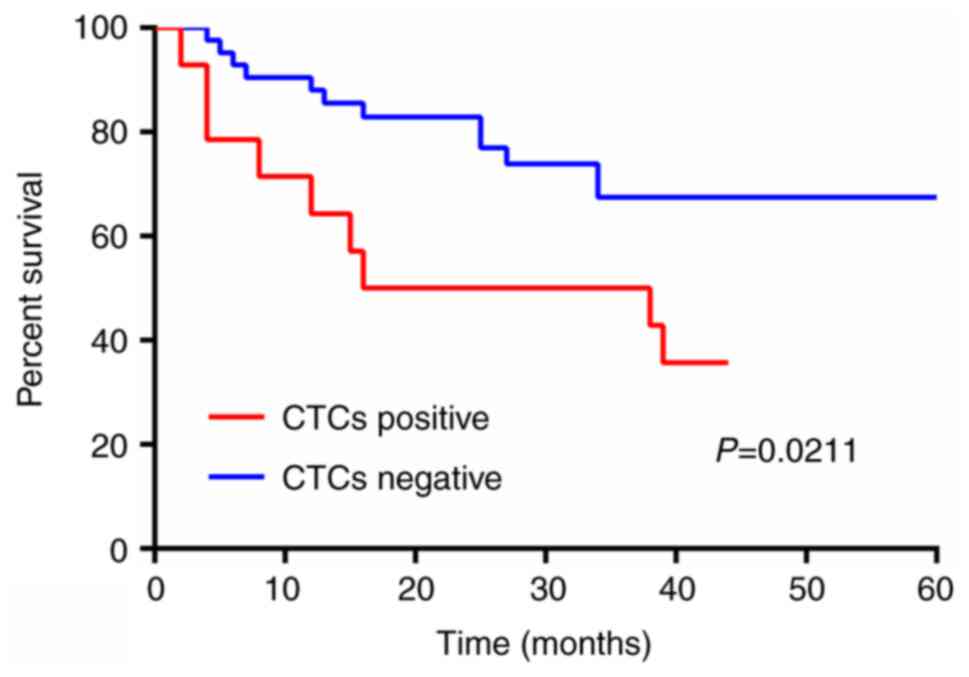
The Kaplan-Meier survival analysis results indicated
that the survival rate of CTC-negative patients is higher compared
with that of CTC-positive patients, with a statistically
significant difference (P=0.0211), as shown in Fig. 2.
Univariate and multivariate analyses
of prognosis in patients with HNC
The univariate Cox regression analysis indicated
that factors influencing the overall survival of patients include
CTCs (HR=1.373; 95% CI, 1.189–1.586; P<0.001), surgical
treatment (HR=0.294; 95% CI, 0.124–0.697; P=0.005) and
non-operative management (HR=0.404; 95% CI, 0.217–0.752; P=0.004),
demonstrating statistical significance for prognosis (P<0.05)
(Table III). Subsequently, the
multivariate Cox regression analysis revealed that CTCs (HR=1.274;
95% CI, 1.119–1.451; P<0.001) and non-operative management
(HR=0.268; 95% CI, 0.119–0.606; P=0.002) independently impact the
prognosis of patients with HNC (Table
IV).
 | Table III.Univariate survival analysis of
overall survival. |
Table III.
Univariate survival analysis of
overall survival.
| Pathological
features | β | SEM | P-value | HR | 95% CI |
|---|
| Sex | −0.359 | 0.744 | 0.630 | 0.699 | 0.162–3.005 |
| Age | 0.020 | 0.026 | 0.444 | 1.020 | 0.970–1.073 |
| CTCs | 0.317 | 0.074 | <0.001 | 1.373 | 1.189–1.586 |
| Tumor type | −0.243 | 0.157 | 0.122 | 0.784 | 0.576–1.067 |
| Pathological
type | −0.675 | 0.746 | 0.366 | 0.509 | 0.118–2.198 |
| Smoking
history | 0.163 | 0.464 | 0.725 | 1.177 | 0.474–2.924 |
| Drinking
history | 0.238 | 0.464 | 0.607 | 1.269 | 0.512–3.148 |
| TNM stage | 0.057 | 00.257 | 0.825 | 1.059 | 0.640–1.752 |
| Surgical
treatment | −1.224 | 0.440 | 0.005 | 0.294 | 0.124–0.697 |
| Non-operative
management | −0.907 | 0.317 | 0.004 | 0.404 | 0.217–0.752 |
 | Table IV.Multivariate survival analysis of
overall survival. |
Table IV.
Multivariate survival analysis of
overall survival.
| Pathological
features | β | SEM | P-value | HR | 95% CI |
|---|
| CTCs | 0.242 | 0.066 | <0.001 | 1.274 | 1.119–1.451 |
| Surgical
treatment | −0.519 | 0.484 | 0.284 | 0.595 | 0.230–1.538 |
| Non-operative
management | −1.316 | 0.416 | 0.002 | 0.268 | 0.119–0.606 |
Discussion
As an emerging ‘liquid biopsy’ technique, CTC
detection has the advantages of simple, real-time, non-invasive and
convenient sampling, which provides a novel and simpler tool for
real-time monitoring of tumor changes during treatment (14). CTCs, as one of the hot topics in the
field of oncology research, have sparked significant interest among
numerous researchers. Previous studies have demonstrated that based
on the physical characteristics of tumors and the expression or
functional features of biomarkers, different methods can be
employed to identify CTCs (23,24).
CTCs can serve as a biomarker for various stages of disease
development and progression. First, CTCs can potentially be
detected in the early stages of cancer, although they are generally
rarer in the early stages compared with advanced stages. Second,
the presence and quantity of CTCs may increase as the disease
progresses, reflecting the growth and potential spread of the
tumor. Third, CTC levels can be monitored during treatment to
assess response. A decrease in CTCs may indicate effective
treatment, while an increase might suggest treatment resistance or
disease progression (25,26). Fourth, elevated levels of CTCs can
be associated with a higher risk of recurrence or the presence of
metastatic disease, as these cells can colonize distant sites
(18). At present, the
CELLSEARCH® system is the first automated and
standardized CTC detection system approved by the U.S. Federal Drug
Administration to detect CTCs by identifying epithelial markers
EpCAM (positive selection) and leukocyte CD45 (negative selection)
using immunomagnetic separation technology (27). The quantity of CTCs can serve as an
indicator for predicting the prognosis of metastatic malignant
tumors, thus playing an increasingly crucial role in the course of
cancer treatment (28–30).
Research has demonstrated the detection of CTCs in
peripheral blood of patients with HNC (31,32).
Among the 56 patients included in the present study, 14 patients
tested positive for CTCs, resulting in a positivity rate of 25%.
This finding also indicated the presence of CTCs in the peripheral
blood of patients with HNC. Additionally, there are reports
indicating that CTCs are more easily detectable in the peripheral
blood of patients with advanced stage IV disease and a high tumor
burden (33,34). In addition, Buglione et al
(33) observed that CTCs are more
easily found in oropharynx, hypopharynx and paranasal sinuses
compared with the oral cavity and nasopharynx. Therefore, this
study analyzed the association of CTC level with age, sex, smoking,
drinking, pathological type, tumor type, surgical treatment (with
vs. without), TNM stage and other pathological features. It found
that these clinical characteristic parameters are not associated
with the level of CTCs, which is consistent with the previous
results (35,36). However, non-operative management
(radiotherapy or chemoradiotherapy) exerts a significant effect on
the levels of CTCs in patients with HNC in this study (33). Theoretically, the impact of
unresected primary tumors on the results of CTC detection is
multifaceted, encompassing aspects such as CTC count,
heterogeneity, epithelial-mesenchymal transition subtypes, PD-L1
expression, in vitro culture conditions, challenges in
detection technology and clinical application efficacy (37). The unresected primary tumor may
release a greater number of CTCs into the bloodstream, thereby
affecting the enumeration and detection outcomes of CTCs (37). However, the present study found that
patients who did not undergo surgery exhibited lower levels of
CTCs, which may suggest that these patients have tumors at a lower
stage. At present, only a few published studies have investigated
the effects of radiotherapy on CTCs (34,38–41).
In head and neck squamous cell carcinoma (HNSCC), Buglione et
al (33) studied the role of
CTCs in patients who only received radiotherapy, and found a lower
sensitivity (30%) in patients receiving radiotherapy compared with
those receiving chemotherapy. In addition, radiotherapy combined
with chemotherapy has been shown to reduce the CTC count in HNSCC
and prostate cancer patients (42–45).
Due to the association between the quantity and characteristics of
CTCs and the prognosis of cancer patients, it was hypothesized that
the detection results of circulating tumor cells can serve as
crucial indicators for evaluating patient prognosis and survival
outcomes.
In the present study, through Kaplan-Meier survival
analysis, it was revealed that the OS of patients testing positive
for CTCs exhibited a notably shorter duration in comparison to
their CTC-negative counterparts. Tinhofer et al (46) revealed the association of CTCs with
disease-free survival (DFS) and OS in patients with oropharyngeal
cancer, indicating that the presence of CTCs in these patients
indicates an adverse prognosis. The role of CTC detection in
evaluating the efficacy and prognosis of HNC is still under study,
with limited research indicating a certain role played by CTCs in
evaluating prognosis in HNC. For example, Jatana et al
(47) found that CTCs are related
to DFS and that patients with a high number of CTCs detected have a
poor prognosis. In addition to the prognostic information provided
by baseline CTCs, changes in the count of CTCs during treatment can
also provide additional information: The persistence of CTCs during
treatment can help identify tumors that do not respond to
treatment, allowing for timely adjustment of the treatment protocol
(48). Through the application of
multivariable Cox regression analysis, the present study
conclusively demonstrated that CTCs (HR=1.274; 95% CI, 1.119–1.451;
P<0.001) and non-operative management (HR=0.268; 95% CI,
0.119–0.606; P=0.002) stood out as independent factors exerting a
significant impact on the prognosis of patients with HNC.
Furthermore, this investigation not only underscored the critical
role of CTCs as a valuable clinical indicator for prognostic
evaluation but also provided compelling evidence to support their
incorporation in prognostic assessments.
Additionally, molecular therapies for patients with
HNC have been an area of significant advancement. These therapies
target specific molecular pathways or genetic alterations that
contribute to cancer growth and progression. The following are some
molecular therapy models and approaches that are being used or
explored for HNC: i) EGFR inhibitors (49); ii) PI3K/AKT/mTOR pathway inhibitors:
Drugs such as mTOR inhibitors everolimus and ridaforolimus have
been explored in clinical trials (50); iii) immune checkpoint inhibitors,
which include PD-1/PD-L1 inhibitors (such as pembrolizumab and
nivolumab) and CTLA-4 inhibitors (such as ipilimumab), have shown
promise in treating HNC, particularly in patients with advanced
disease (51); iv) fibroblast
growth factor receptor inhibitors (52); v) VEGF inhibitors: Bevacizumab, a
monoclonal antibody against VEGF, has been used to target
angiogenesis in HNC (53); and vi)
hedgehog pathway inhibitors: Smoothened inhibitors such as
vismodegib have been studied for HNC, as the hedgehog pathway is
implicated in tumor growth (54).
Use of molecular therapies in HNC is complex and
requires careful patient selection based on tumor molecular
profiling. A number of these therapies are part of ongoing research
and their integration into standard care depends on the results of
clinical trials and an understanding of their safety and efficacy.
Precision medicine, which involves tailoring treatments to the
molecular profile of a tumor, is a growing field in oncology
(55). The success of molecular
therapies in HNC will likely lead to more personalized treatment
strategies in the future (55). It
should be noted that although the CELLSEARCH® system is
the only CTC detection system approved by regulatory authorities in
the United States and China, it has standardized operating
procedures and good comparability of results, and
CELLSEARCH® technology has been used for the detection
of various types of cancer, such as breast cancer, renal cancer,
lung cancer, ovarian cancer and brain cancer (56). However, the diagnostic cutoff values
for CTC in different cancers are still unclear and not comparable
at present (57).
The present study has several limitations. While the
potential significance of CTC detection in the early diagnosis,
treatment monitoring, and prognosis assessment of HNC is under
scrutiny, and current research findings exhibit inconsistencies
with previous reports, significantly hindering the widespread
adoption of CTC detection in large-scale clinical settings. These
discrepancies may stem from the intricate and varied biological and
clinical characteristics displayed by the anatomical origins of HNC
(58,59). Owing to these divergent
characteristics, prior studies have often been confined to
relatively modest patient cohorts, further exacerbated by
substantial heterogeneity among these patient groups, inevitably
amplifying uncertainty and rendering comparisons between research
outcomes challenging. Moreover, the relatively limited sample sizes
utilized in studies have somewhat diminished the statistical
robustness of research findings, casting doubts on the reliability
of the conclusions drawn. Notably, research on the association
between CTC count and treatment response in HNC is still in its
nascent stages, with existing studies lacking the requisite depth
and breadth to underpin clinical decision-making (59). Due to these circumstances, there
exists a pressing need for larger-scale, prospective clinical
investigations to comprehensively explore the utility of CTC
detection in monitoring treatment efficacy and evaluating prognosis
across distinct clinical subtypes of patients with HNC (60). Through enlarging sample sizes,
refining research methodologies and fully acknowledging the
biological and clinical disparities inherent in HNC, we endeavor to
provide sturdier and dependable evidence to bolster the integration
of CTC detection in clinical practice for HNC. Due to the
relatively small sample size at present, this study can be
considered an initial research attempt. Looking forward, we hope to
increase the sample size in future research work to evaluate the
effectiveness of this technology. Additionally, as a retrospective
study, we do not have data on circulating tumor DNA and therefore,
regrettably, the present study lacked the relevant data to report.
Detection of circulating tumor DNA will be the future research
direction.
The present study revealed that patients with
elevated levels of CTCs had a poorer prognosis. Both univariate and
multivariate analyses demonstrated that CTC levels were one of the
independent factors influencing the prognosis of patients with HNC.
The detection of CTCs holds potential value in the diagnosis and
prognosis assessment of patients with HNC. By monitoring CTC
levels, physicians can gain a greater understanding of the disease
progression in patients, thereby providing a robust basis for
devising personalized treatment plans.
Acknowledgements
Not applicable.
Funding
This study was funded by the Beijing Natural Science Foundation
(grant no. 7122039).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HYL contributed to the conception and design of the
study. PDL, FL and TW collected and analyzed data. HYL wrote and
revised the manuscript. HYL, PDL, FL and TW confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study protocol was in accordance with the
Declaration of Helsinki of the World Medical Association. The study
was approved by the ethical committee of the Beijing Tongren
Hospital, Capital Medical University (approval no. 23-B4-03).
Informed consent was waived for this study because the research was
retrospective and conducted on anonymized data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CD45
|
cluster of differentiation 45
|
|
CK
|
cytokeratin
|
|
CTCs
|
circulating tumor cells
|
|
DFS
|
disease-free survival
|
|
EpCAM
|
epithelial cell adhesion molecule
|
|
HNC
|
head and neck cancer
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
OS
|
overall survival
|
|
SCC
|
squamous cell carcinoma
|
References
|
1
|
Mody MD, Rocco JW, Yom SS, Haddad RI and
Saba NF: Head and neck cancer. Lancet. 398:2289–2299. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Credico G, Polesel J, Dal Maso L, Pauli
F, Torelli N, Luce D, Radoï L, Matsuo K, Serraino D, Brennan P, et
al: Alcohol drinking and head and neck cancer risk: The joint
effect of intensity and duration. Br J Cancer. 123:1456–1463. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta I, Ghabreau L, Al-Thawadi H, Yasmeen
A, Vranic S, Al Moustafa AE and Malki MI: Co-incidence of human
papillomaviruses and Epstein-barr virus is associated with high to
intermediate tumor grade in human head and neck cancer in syria.
Front Oncol. 10:10162020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mourad M, Jetmore T, Jategaonkar AA,
Moubayed S, Moshier E and Urken ML: Epidemiological trends of head
and neck cancer in the united states: A SEER population study. J
Oral Maxillofac Surg. 75:2562–2572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Economopoulou P, Kotsantis I, Kyrodimos E,
Lianidou ES and Psyrri A: Liquid biopsy: An emerging prognostic and
predictive tool in Head and Neck Squamous Cell Carcinoma (HNSCC).
Focus on Circulating Tumor Cells (CTCs). Oral Oncol. 74:83–89.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takes RP, Rinaldo A, Silver CE, Haigentz M
Jr, Woolgar JA, Triantafyllou A, Mondin V, Paccagnella D, de Bree
R, Shaha AR, et al: Distant metastases from head and neck squamous
cell carcinoma. Part I. Basic aspects. Oral Oncol. 48:775–779.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kopczyński P and Flieger R: Tumor markers
used in the diagnosis, monitoring, treatment, and prognosis head
and neck cancer. Pol Merkur Lekarski. 35:37–38. 2013.(In Polish).
PubMed/NCBI
|
|
9
|
Eskander A, Irish J, Groome PA, Freeman J,
Gullane P, Gilbert R, Hall SF, Urbach DR and Goldstein DP:
Volume-outcome relationships for head and neck cancer surgery in a
universal health care system. Laryngoscope. 124:2081–2088. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leipzig B, Zellmer JE and Klug D: The role
of endoscopy in evaluating patients with head and neck cancer. A
multi-institutional prospective study. Arch Otolaryngol.
111:589–594. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chung CS, Lo WC, Wen MH, Hsieh CH, Lin YC
and Liao LJ: Long term outcome of routine Image-enhanced endoscopy
in newly diagnosed head and neck cancer: A prospective study of 145
patients. Sci Rep. 6:295732016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pantel K and Speicher MR: The biology of
circulating tumor cells. Oncogene. 35:1216–1224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Novel Biomarkers in the Continuum of
Breast Cancer. Anticancer Res. 36:32242016.
|
|
14
|
Shen Z, Wu A and Chen X: Current detection
technologies for circulating tumor cells. Chem Soci Rev.
46:2038–2056. 2017. View Article : Google Scholar
|
|
15
|
Bidard FC, Proudhon C and Pierga JY:
Circulating tumor cells in breast cancer. Mol Oncol. 10:418–430.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pantel K, Hille C and Scher HI:
Circulating tumor cells in prostate cancer: From discovery to
clinical utility. Clin Chem. 65:87–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia S, Zhang R, Li Z and Li J: Clinical
and biological significance of circulating tumor cells, circulating
tumor DNA, and exosomes as biomarkers in colorectal cancer.
Oncotarget. 8:55632–55645. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong M, Luo S, Gu L, Wang X, Zhang Z,
Liang C, Huang H, Lin Y and Huang J: SIMarker: Cellular similarity
detection and its application to diagnosis and prognosis of liver
cancer. Comput Biol Med. 171:1081132024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ramirez JM, Fehm T, Orsini M, Cayrefourcq
L, Maudelonde T, Pantel K and Alix-Panabières C: Prognostic
relevance of viable circulating tumor cells detected by EPISPOT in
metastatic breast cancer patients. Clin Chem. 60:214–221. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin D, Shen L, Luo M, Zhang K, Li J, Yang
Q, Zhu F, Zhou D, Zheng S, Chen Y and Zhou J: Circulating tumor
cells: Biology and clinical significance. Signal Transduct Target
Ther. 6:4042021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coumans F and Terstappen L: Detection and
characterization of circulating tumor cells by the cellsearch
approach. Methods Mol Biol. 1347:263–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rheinländer A, Schraven B and Bommhardt U:
CD45 in human physiology and clinical medicine. Immunol Lett.
196:22–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alix-Panabières C and Pantel K: Challenges
in circulating tumour cell research. Nat Rev Cancer. 14:623–631.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alix-Panabières C and Pantel K:
Circulating tumor cells: Liquid biopsy of cancer. Clin Chem.
59:110–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng Z, Wu S, Wang Y and Shi D:
Circulating tumor cell isolation for cancer diagnosis and
prognosis. EBioMedicine. 83:1042372022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pereira-Veiga T, Schneegans S, Pantel K
and Wikman H: Circulating tumor cell-blood cell crosstalk: Biology
and clinical relevance. Cell Rep. 40:1112982022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu M, Stott S, Toner M, Maheswaran S and
Haber DA: Circulating tumor cells: Approaches to isolation and
characterization. J Cell Biol. 192:373–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park CH, Lee JI, Sung J, Choi S and Ko KP:
A flow visualization model of duodenogastric reflux after
esophagectomy with gastric interposition. J Cardiothorac Surg.
8:1922013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Xiao Y, Zhao J, Chen M, Xu Y,
Zhong W, Xing J and Wang M: Relationship between circulating tumour
cell count and prognosis following chemotherapy in patients with
advanced non-small-cell lung cancer. Respirology. 21:519–525. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nieva J, Wendel M, Luttgen MS, Marrinucci
D, Bazhenova L, Kolatkar A, Santala R, Whittenberger B, Burke J,
Torrey M, et al: High-definition imaging of circulating tumor cells
and associated cellular events in non-small cell lung cancer
patients: A longitudinal analysis. Phys Biol. 9:0160042012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nichols AC, Lowes LE, Szeto CC, Basmaji J,
Dhaliwal S, Chapeskie C, Todorovic B, Read N, Venkatesan V, Hammond
A, et al: Detection of circulating tumor cells in advanced head and
neck cancer using the CellSearch system. Head Neck. 34:1440–1444.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bozec A, Ilie M, Dassonville O, Long E,
Poissonnet G, Santini J, Chamorey E, Ettaiche M, Chauvière D,
Peyrade F, et al: Significance of circulating tumor cell detection
using the CellSearch system in patients with locally advanced head
and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol.
270:2745–2749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buglione M, Grisanti S, Almici C, Mangoni
M, Polli C, Consoli F, Verardi R, Costa L, Paiar F, Pasinetti N, et
al: Circulating tumour cells in locally advanced head and neck
cancer: Preliminary report about their possible role in predicting
response to non-surgical treatment and survival. Eur J Cancer.
48:3019–3026. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guney K, Yoldas B, Ozbilim G, Derin AT,
Sarihan S and Balkan E: Detection of micrometastatic tumor cells in
head and neck squamous cell carcinoma. A possible predictor of
recurrences? Saudi Med J. 28:216–220. 2007.PubMed/NCBI
|
|
35
|
Li H, Li B, Pan Y, Zhang Y, Xiang J, Zhang
Y, Sun Y, Yu X, He W and Hu H: Preoperative folate
receptor-positive circulating tumor cell level is a prognostic
factor of long term outcome in non-small cell lung cancer patients.
Front Oncol. 10:6214352020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bertamini L and Oliva S: High levels of
circulating tumor plasma cells as a key hallmark of aggressive
disease in Transplant-Eligible patients with newly diagnosed
multiple myeloma. J Clin Oncol. 40:3120–3131. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ju L, Yang J, Zhai C, Chai S, Dong Z and
Li M: Survival, Chemotherapy and Chemosensitivity Predicted by CTC
Cultured In Vitro of SCLC Patients. Front Oncol. 11:6833182021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Martin OA, Anderson RL, Russell PA, Cox
RA, Ivashkevich A, Swierczak A, Doherty JP, Jacobs DH, Smith J,
Siva S, et al: Mobilization of viable tumor cells into the
circulation during radiation therapy. Int J Radiat Oncol Biol Phys.
88:395–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morosin T, Ashford B, Ranson M, Gupta R,
Clark J, Iyer NG and Spring K: Circulating tumour cells in
regionally metastatic cutaneous squamous cell carcinoma: A pilot
study. Oncotarget. 7:47111–47115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Balasubramanian P, Lang JC, Jatana KR,
Miller B, Ozer E, Old M, Schuller DE, Agrawal A, Teknos TN, Summers
TA Jr, et al: Multiparameter analysis, including EMT markers, on
negatively enriched blood samples from patients with squamous cell
carcinoma of the head and neck. PLoS One. 7:e420482012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dorsey JF, Kao GD, MacArthur KM, Ju M,
Steinmetz D, Wileyto EP, Simone CB II and Hahn SM: Tracking viable
circulating tumor cells (CTCs) in the peripheral blood of non-small
cell lung cancer (NSCLC) patients undergoing definitive radiation
therapy: Pilot study results. Cancer. 121:139–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jatana KR, Balasubramanian P, Lang JC,
Yang L, Jatana CA, White E, Agrawal A, Ozer E, Schuller DE, Teknos
TN and Chalmers JJ: Significance of circulating tumor cells in
patients with squamous cell carcinoma of the head and neck: Initial
results. Arch Otolaryngol Head Neck Surg. 136:1274–1279. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Winter SC, Stephenson SA, Subramaniam SK,
Paleri V, Ha K, Marnane C, Krishnan S and Rees G: Long term
survival following the detection of circulating tumour cells in
head and neck squamous cell carcinoma. BMC Cancer. 9:4242009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jatana KR, Balasubramanian P, McMullen KP,
Lang JC, Teknos TN and Chalmers JJ: Effect of surgical intervention
on circulating tumor cells in patients with squamous cell carcinoma
of the head and neck using a negative enrichment technology. Head
Neck. 38:1799–1803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao W, Li JZ, Ho WK, Chan JY and Wong TS:
Biomarkers for use in monitoring responses of nasopharyngeal
carcinoma cells to ionizing radiation. Sensors (Basel).
12:8832–8846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tinhofer I, Hristozova T, Stromberger C,
Keilhoiz U and Budach V: Monitoring of circulating tumor cells and
their expression of EGFR/phospho-EGFR during combined radiotherapy
regimens in locally advanced squamous cell carcinoma of the head
and neck. Int J Radiat Oncol Biol Phys. 83:e685–e690. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jatana KR, Lang JC and Chalmers JJ:
Identification of circulating tumor cells: A prognostic marker in
squamous cell carcinoma of the head and neck? Future Oncol.
7:481–484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schochter F, Friedl TWP, deGregorio A,
Krause S, Huober J, Rack B and Janni W: Are Circulating tumor cells
(CTCs) ready for clinical use in breast cancer? An overview of
completed and ongoing trials using CTCs for clinical treatment
decisions. Cells. 8:14122019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kyrodimos E, Chrysovergis A, Mastronikolis
N, Tsiambas E, Ragos V, Roukas D, Fotiades P and Papanikolaou V:
Targeting EGFR in nasopharyngeal carcinoma. J BUON. 26:759–761.
2021.PubMed/NCBI
|
|
50
|
Aguayo F, Perez-Dominguez F and Osorio JC:
PI3K/AKT/mTOR signaling pathway in HPV-Driven head and neck
carcinogenesis: Therapeutic implications. Biology (Basel).
12:6722023.PubMed/NCBI
|
|
51
|
O'Meara CH and Jafri Z: Immune checkpoint
inhibitors, Small-molecule immunotherapies and the emerging role of
neutrophil extracellular traps in therapeutic strategies for head
and neck cancer. Int J Mol Sci. 24:116952023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bao Y, Gabrielpillai J, Dietrich J, Zarbl
R, Strieth S, Schröck F and Dietrich D: Fibroblast growth factor
(FGF), FGF receptor (FGFR), and cyclin D1 (CCND1) DNA methylation
in head and neck squamous cell carcinomas is associated with
transcriptional activity, gene amplification, human papillomavirus
(HPV) status, and sensitivity to tyrosine kinase inhibitors. Clin
Epigenetics. 13:2282021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Khadela A, Shah Y, Mistry P, Mansuri M,
Sureja D and Bodiwala K: A review of efficacy and safety of
cetuximab and bevacizumab-based monoclonal antibodies in head and
neck cancer. Med Oncol. 40:662022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cierpikowski P, Leszczyszyn A and Bar J:
The role of hedgehog signaling pathway in head and neck squamous
cell carcinoma. Cells. 12:20832023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tsimberidou AM, Fountzilas E, Nikanjam M
and Kurzrock R: Review of precision cancer medicine: Evolution of
the treatment paradigm. Cancer Treat Rev. 86:1020192020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
de Wit S, van Dalum G and Terstappen LW:
Detection of circulating tumor cells. Scientifica (Cairo).
2014:8193622014.PubMed/NCBI
|
|
57
|
Hoeppener AE, Swennenhuis JF and
Terstappen LW: Immunomagnetic separation technologies. Recent
Results Cancer Res. 195:43–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hanna GJ, Patel N, Tedla SG, Baugnon KL,
Aiken A and Agrawal N: Personalizing surveillance in head and neck
cancer. Am Soc Clin Oncol Educ Book. 43:e3897182023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang X, Duijf PHG, Sriram S, Perera G,
Vasani S, Kenny L, Leo P and Punyadeera C: Circulating tumour DNA
alterations: Emerging biomarker in head and neck squamous cell
carcinoma. J Biomed Sci. 30:652023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tsutsuyama M and Nakanishi H: Detection of
circulating tumor cells in drainage venous blood from colorectal
cancer patients using a new filtration and cytology-based automated
platform. PLoS One. 14:e02122212019. View Article : Google Scholar : PubMed/NCBI
|