Introduction
Renal cell carcinoma (RCC) is a common malignant
tumor of the urinary system, accounting for >85% of renal
malignant tumors (1). The most
common pathological type of RCC is clear cell (cc)RCC, which is a
malignant tumor that arises from the renal parenchymal urinary
tubular epithelial system. The clinical symptoms of early ccRCC are
minimal and the minority of symptomatic patients exhibit different
manifestations, such as abdominal pain, hematuria, weight loss or
abdominal mass. In total, ~30% of patients present with metastases
of varying severity and 25% of patients develop metastases
following radical nephrectomy (2).
The prognosis of patients with early ccRCC is
positive; however, in the advanced disease stage, ccRCC often
invades surrounding nearby organs and metastasizes to the
bloodstream. ccRCC commonly metastasizes to organs such as the
lungs, bones, liver and brain (3–5). The
prognosis of patients with metastatic ccRCC is poor, and the 5-year
survival rate is ~9% (6,7). Metastasis of ccRCC to the thyroid
gland, specifically within a primary thyroid tumor (PTT), is
rare.
The results of a previous study have indicated that
the average duration from radical nephrectomy to the diagnosis of
ccRCC metastasis to the thyroid is 8.7 years (8). To the best of our knowledge, only 11
cases of ccRCC metastasizing to a PTT have been reported (5,9–17).
These reports demonstrate that the immunohistochemical results of
cells from metastatic ccRCC to PTT are typically negative for
thyroglobulin, calcitonin and thyroid transcription factor 1
(TTF-1), and positive for common acute lymphoblastic leukemia
antigen (CD10) and vimentin. Thyroid metastasis may be facilitated
by hematogenous dissemination through the paravertebral venous
network. However, the etiology underlying the phenomenon of
metastasis from one tumor to another remains elusive (18).
Thyroid tumors are classified into primary and
metastatic tumors. Metastatic tumors are less common compared with
primary tumors. Primary tumors include thyroid papillary carcinoma,
follicular carcinoma, medullary carcinoma and hyalinizing
trabecular tumor (HTT). Notably, HTT of the thyroid gland is a rare
primary tumor with a female predominance (19), which accounts for ~0.2% of PTTs
(19). HTT was initially reported
by Carney et al in 1987 (20), and this tumor originates from the
thyroid follicular epithelium. It is named for its stromal
hyalinization and the trabecular and cord-like arrangement of the
tumor. HTT often presents as a single, solid, well-defined nodule
with a gray-yellow cut surface, medium texture and a fibrous
capsule of varying thickness. HTT also exhibits unique histological
features and protein expression level; for examples, the tumor
cells of HTT are arranged in a trabecular pattern, with hyaline
material visible between the trabecular cell nests, and the tumor
cells are positive for TTF-1 and CD56 (21). As the observed nuclear
characteristics and hyalinization are comparable with papillary
thyroid carcinoma, HTT is often misdiagnosed via cytological
examination using fine needle aspiration biopsy, leading to poor
patient outcomes and further complications in tumor classification
(22). In the early stages of the
disease, no specific indicators are observed during clinical
examination, only presentation with a thyroid nodule.
The present study presents a case of ccRCC
metastasizing to a HTT of the thyroid.
Case report
In April 2023, a 60-year-old female was referred to
Ningbo Beilun District People's Hospital (Ningbo, China) with
radiographic findings indicating thyroid nodules. The previous
medical history of the patient included a left radical nephrectomy
in February 2021, performed for the treatment of ccRCC.
Histological examination revealed grade 1 ccRCC (23), and no other distant metastases were
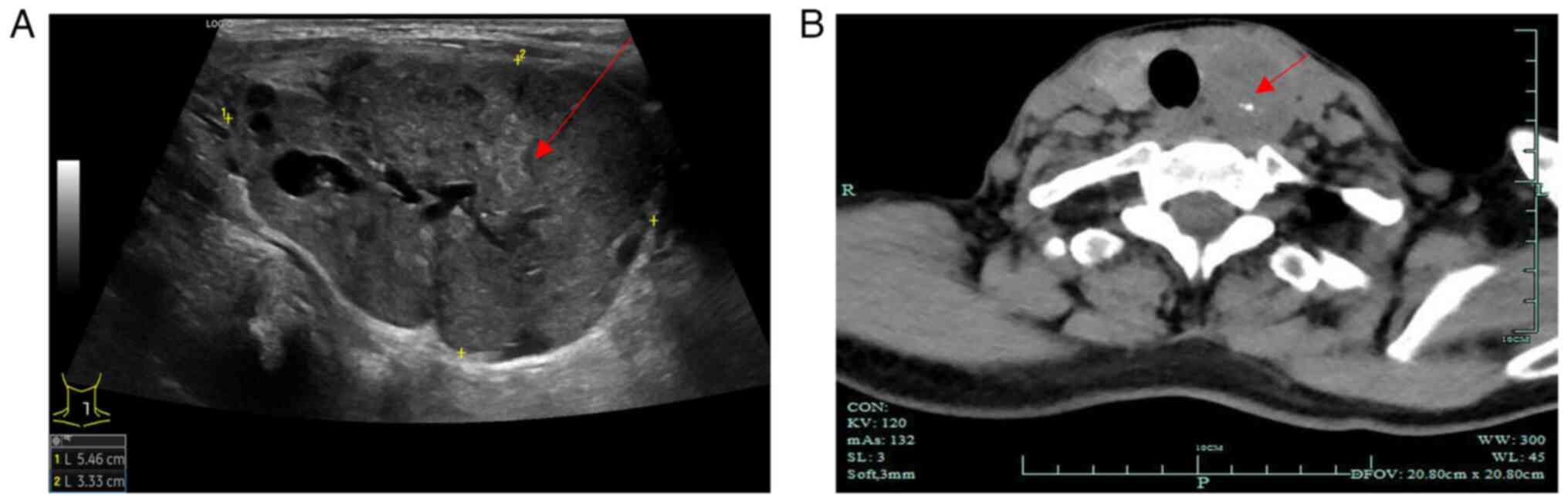
observed during follow-up. As shown in Fig. 1A, preoperative thyroid ultrasound
examination results indicated a hypoechoic nodule in the left lobe
of the thyroid [Thyroid Imaging Reporting and Data System (TI-RADS)
4a] and a nodule in the right lobe of the thyroid (TI-RADS 3).
TI-RADS is a classification level for thyroid nodules, where
TI-RADS 4a indicates the presence of one of the following
ultrasonographic malignant features: Extremely hypoechoic,
microcalcifications or irregular microlobulated margins; the
probability of malignancy in this category ranges from 5 to 10%
(24). The results of computed
tomography revealed enlargement of the left lobe of the thyroid
with a mass-like low-density shadow inside, measuring ~40×32 mm
(Fig. 1B). In addition, the left
lobe exhibited poorly defined borders and an uneven density with
visible calcifications inside the mass (Fig. 1B). Multiple small nodules were
observed in the right lobe of the thyroid, with no abnormal density
shadows observed in the isthmus of the thyroid. Notably, the
patient did not present with enlarged lymph nodes on either side of
the neck. The results of urine and blood tests were normal, and
normal functioning of the thyroid and parathyroid was observed.
Given the large size of the mass, there was a risk of tracheal
deviation and compression. The patient's history of ccRCC and the
potential for malignancy in the thyroid nodules was considered, and
after the patient was informed, they refused fine needle aspiration
cytology and opted for total thyroidectomy.
Specimens were surgically obtained via total
thyroidectomy. Intraoperatively, diffuse enlargement of the left
lobe of the thyroid was observed, along with multiple nodules with
solid, clear borders. Notably, the largest nodule measured
~55×41×33 mm. Multiple nodules were also observed in the right lobe
of the thyroid, with solid, clear borders, measuring ~24×14×11 mm.
No nodules were observed in the isthmus.
Thyroidectomy specimens were fixed in 10% formalin
for 48 h at 37°C, routinely dehydrated, embedded in paraffin and
cut into 4-µm sections. Subsequently, H&E staining was
performed for 40 min at 37°C, then the sections were examined under
a light microscope (DM2000; Leica Microsystems, Inc.).
Immunohistochemical analyses were performed
automatically using the Bench Mark ULTRA immunohistochemistry
system (Ventana Medical Systems, Inc.), for which the
paraffin-embedded tissues were sectioned (4 µm) using a paraffin
slicing machine, mounted onto poly-L-lysine-coated glass slides and
allowed to dry at 65°C for 60 min. The sections were subsequently
put into the immunohistochemistry instrument for automated antigen
retrieval (pH 9.0 EDTA, 100°C, 36 min) and blocking (3% hydrogen
peroxide, 37°C, 4 min), followed by incubation with primary
antibodies and the secondary antibody, and chromogenic development.
The sections were incubated with the following primary antibodies:
Neural cell adhesion molecule (CD56; mouse; cat. no. MAB-0743),
TTF-1 (mouse; cat. no. MAB-0599), cytokeratin 19 (CK19; mouse; cat.
no. MAB-0829), chromogranin-A (CgA; rabbit; cat. no. RMA-0548),
paired box 8 (PAX-8; mouse; cat. no. MAB-0837), carbonic anhydrase
IX (CAIX; rabbit; cat. no. RAB-0615), CD10 (mouse; cat. no.
MAB-0668), mucin-1 (MUC-1; mouse; cat. no. Kit-0011), cytokeratin 7
(CK7; mouse; cat. no. MAB-0828), Ki-67 (mouse; cat. no. MAB-0672)
(all from Fuzhou Maixin Biotechnology Development Co., Ltd.) and
BRAF-VE600E (mouse; cat. no. 790-5095; Roche Diagnostics) for 1 h
at 37°C. The bound primary antibody was detected using a
ready-to-use secondary antibody (UltraView Universal DAB detection
kit; cat. no. 760-500; Roche Diagnostics) for 36 min at 37°C, and
DAB (from the UltraView Universal DAB detection kit) was utilized
as a chromogen for 8 min at 37°C. After removing the sections from
the instrument, they were immersed in distilled water containing
detergent for washing and then rinsed with distilled water.
Subsequently, the sections were dehydrated through a series of
ethanol solutions and cleared with xylene before mounting the
sections in an automated slide stainer. The sections were then
ready to be observed under a microscope (Leica Microsystems, Inc.
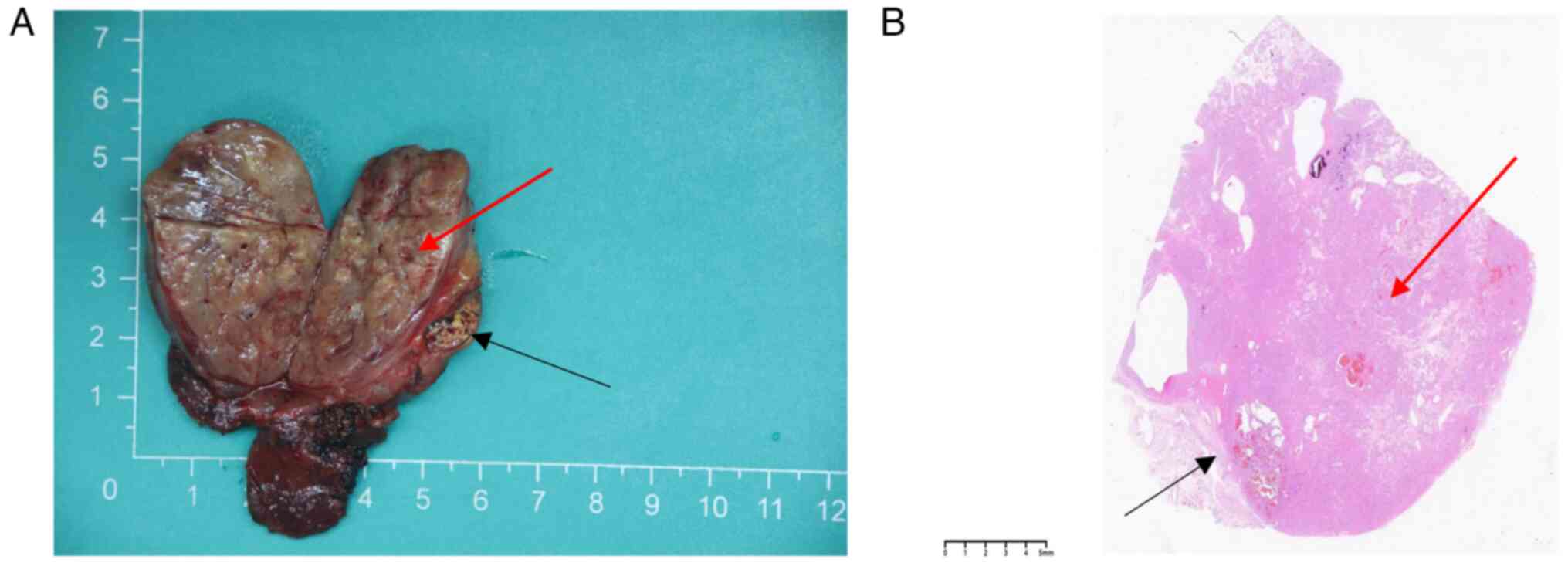
DM2000). A tumor measuring 55×41×33 mm was observed in the left
thyroid tissue section. In addition, the tumor appeared gray-yellow
in color, with areas of hemorrhage and a fibrous capsule on the
periphery. A small area of ~8×5 mm was visible near the periphery
of the tumor, with a multicolored appearance (Fig. 2A).
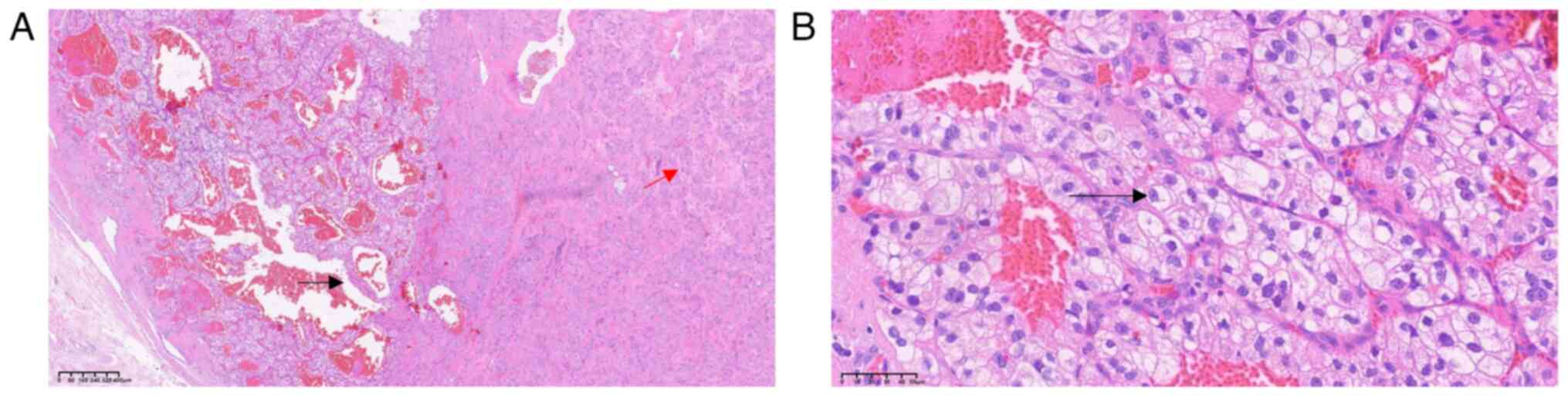
At a low magnification, the tumor boundary was
clearly visible with a fibrous capsule (Fig. 2B). Tumor cells were composed of two
histologically distinct cell types. In addition, the tumors
exhibited solid growth with small beam- and nest-like structures,
with transparent degeneration between the beams. Tumor cells were
polygonal, fusiform, oval or elliptical in shape, with nuclear
grooves, intranuclear pseudo-inclusions and small nucleoli. Rare
nuclear division figures were observed (Fig. 3A). Microscopic examination also
revealed that a number of tumor cells were located within the inner
side of the capsule and arranged in a nest-like or glandular
pattern. Regular reticular patterns were observed in the tumor and
these were composed of small thin-walled blood vessels. The tumor
cell cytoplasm was transparent, with clear capsules and circular
nuclei, and the size was relatively uniform throughout. Results of
the microscopic examination revealed nucleoli of several sizes, and
acidophilic nucleoli (black arrow) were observed at ×400
magnification (Fig. 3B).
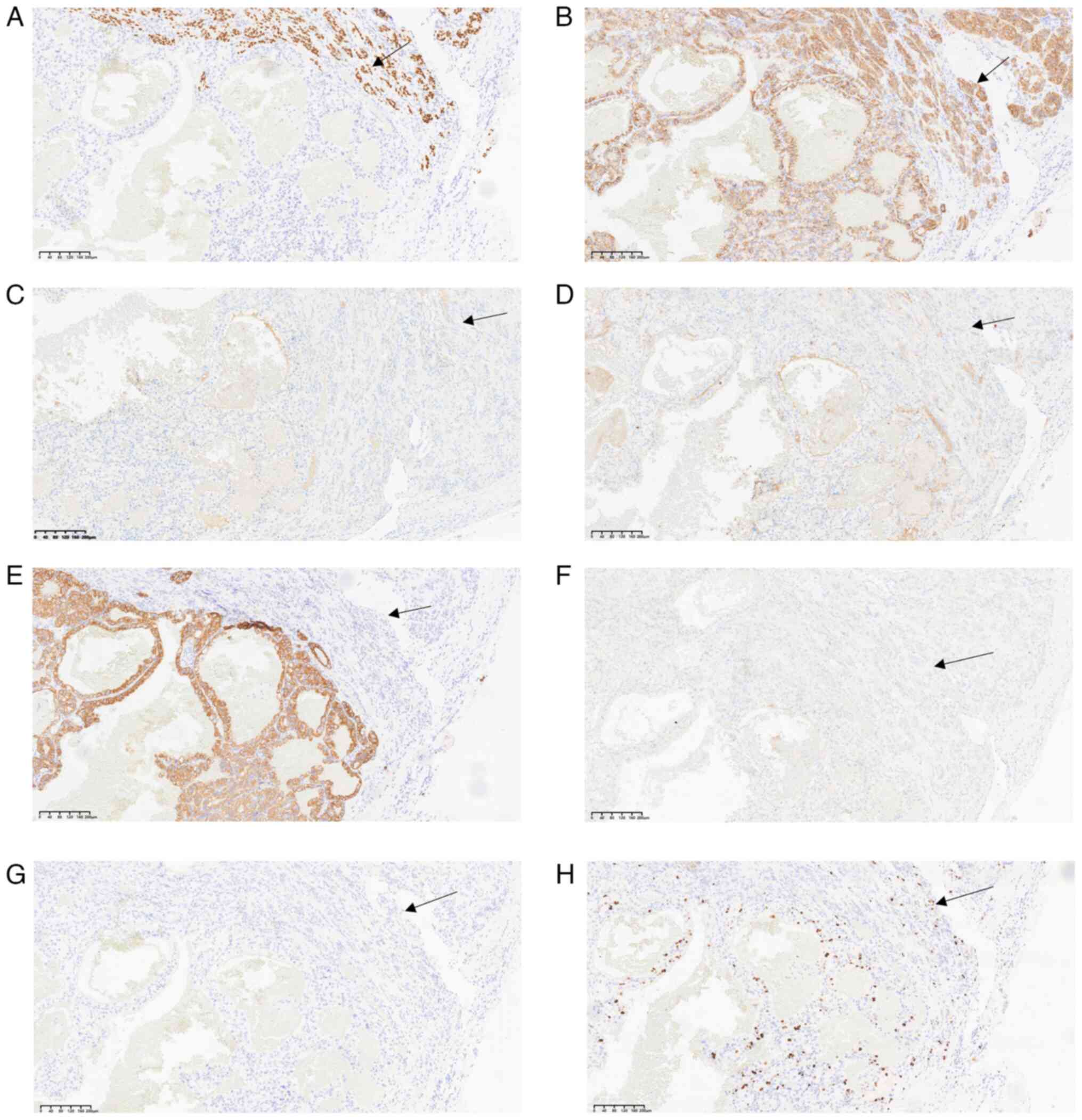
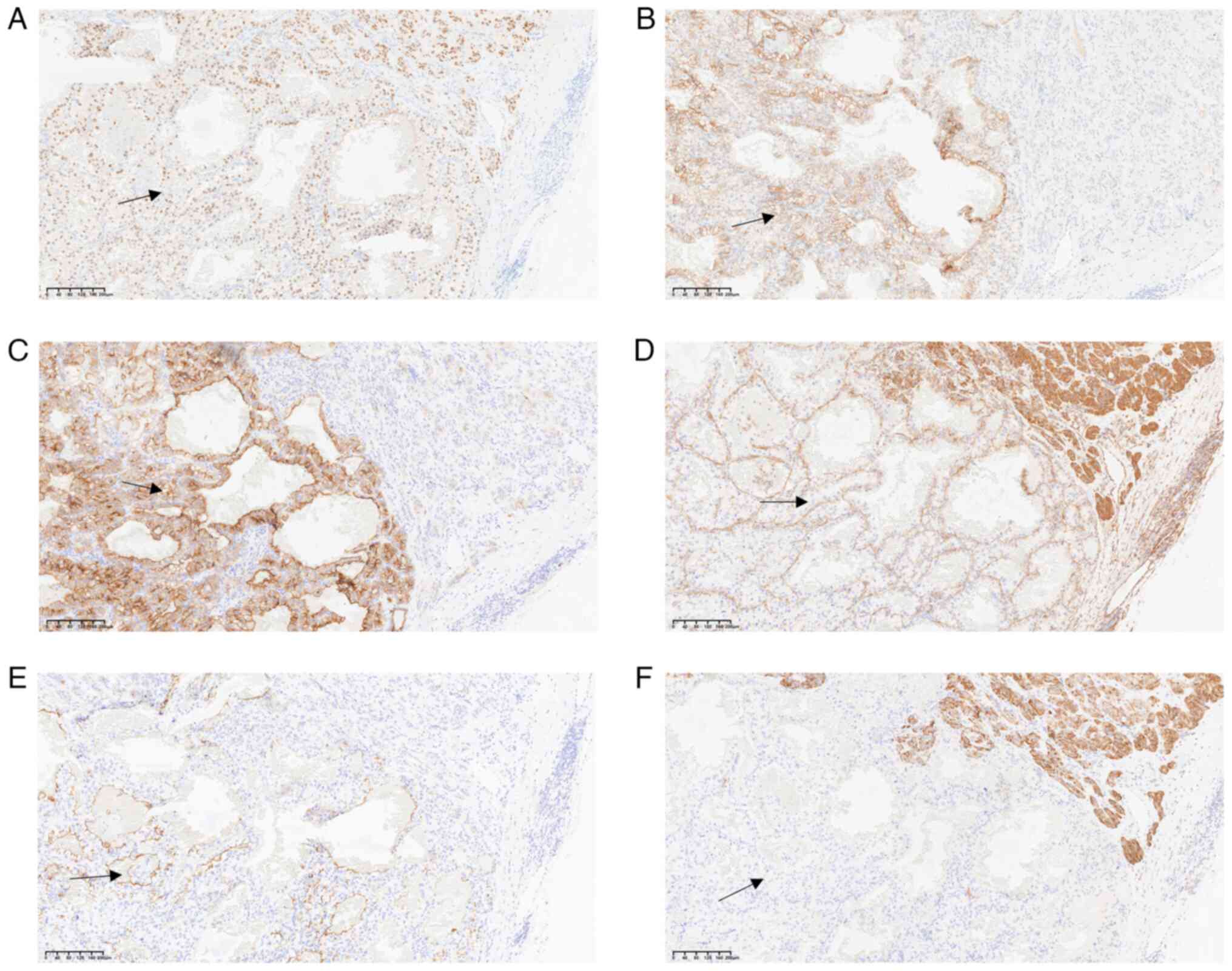
Immunohistochemical analysis revealed that clear
trabecular areas of the thyroid exhibited positive TTF-1 (Fig. 4A) and CD56 staining (Fig. 4B), whilst calcitonin (Fig. 4C), mesothelin (Fig. 4D), CK19 (Fig. 4E), BRAF-V600E (Fig. 4F) and CgA (Fig. 4G) expression was negative.
Positivity for Ki-67 was <5% of tumor cells (Fig. 4H). By contrast, the clear cell areas
exhibited positive PAX-8 (Fig. 5A),
CAIX (Fig. 5B), CD10 (Fig. 5C), vimentin (Fig. 5D) and MUC-1 (Fig. 5E) expression, while CK7 (Fig. 5F) expression was negative. The
observed histological features and immune profile were consistent
with metastatic ccRCC to the thyroid, with clear cell changes in
the lamellar tumor.
In the present case, the patient was diagnosed with
metastatic ccRCC to a HTT of the thyroid. Following surgery, the
patient received levothyroxine therapy, with a regimen of 75 µg
levothyroxine sodium tablets taken orally once daily, 1 h before
breakfast. Thyroid function was followed up every 3 months to serve
as the basis for dosage adjustment. she also underwent an enhanced
computed tomography scan, which indicated that no metastasis to
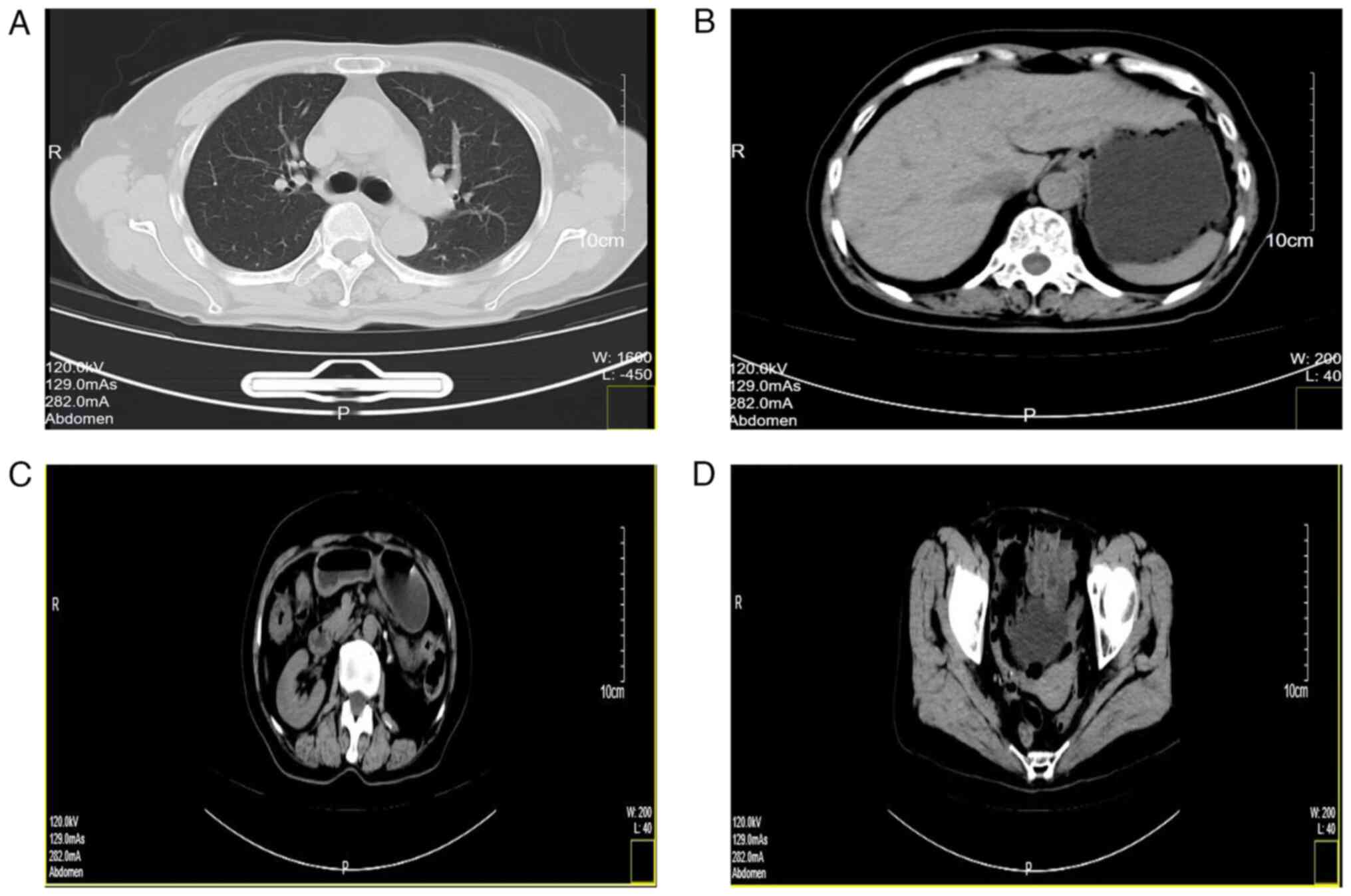
other organs had occurred (Fig. 6).
The lungs and mediastinum (Fig.
6A), liver, gallbladder and stomach (Fig. 6B), intestinal tract and right kidney
(Fig. 6C), as well as the uterus,
bilateral adnexa and bladder (Fig.
6D), all showed no evidence of tumorous lesions. From May 18,
2023 to the present date, the patient has undergone programmed
death-1 monoclonal antibody immunotherapy and small molecule
tyrosine kinase inhibitor targeted therapy. As of the latest visit
(October 2024), the patient was in a good condition with no sign of
metastasis or recurrence, and regular follow-ups every 3 months
have been arranged.
Discussion
Most patients with metastatic ccRCC to the thyroid
present with painless masses, which are often misdiagnosed as a
nodular goiter. Clinical symptoms associated with nodular goiters
include dysphagia, respiratory distress, hoarseness, neck pain and
a cough (25). In the present case,
the patient presented with a painless cervical mass and no
additional clinical symptoms. Notably, diagnosis of metastatic
ccRCC to the thyroid remains challenging, due to the reliance on
H&E staining. Using imaging, metastatic ccRCC to the thyroid
typically appears as a single nodule, which may be misdiagnosed as
a primary tumor. During diagnosis of PTTs, HTTs of the thyroid may
be misdiagnosed as medullary thyroid carcinoma, follicular adenoma
or paraganglioma. Notably, immunohistochemistry and molecular
testing are commonly used to distinguish between them (26). Specific tumor markers aid in
determining the primary site of the metastatic tumor, for example,
calcitonin is a specific marker for medullary thyroid carcinoma
(27) and CAIX is a specific marker
for ccRCC (28).
Immunohistochemical analysis of the tumor revealed positive TTF-1
expression, which verified the presence of a PTT. Moreover,
negative CK19, CgA and BRAF-V600E expression and positive CD56
expression, in combination with positive periodic acid-Schiff
staining in the stroma, was indicative of a HTT of the thyroid
gland. Subsequently, the primary objective was to determine the
specific type of clear cell area within the tumor. The following
markers were utilized: PAX-8, CAIX, CD10, CK7, MUC-1 and vimentin.
Notably, CK7 exhibited negative expression, whilst CAIX, CD10,
PAX-8, MUC-1 and vimentin all demonstrated positive expression.
These findings had led to the diagnosis of the clear cell area as
ccRCC.
In the diagnosis of RCC, to differentiate and
distinguish between types, transcription factor 3 (TFE3) is
commonly used for diagnosing TFE3 rearranged RCC (29,30);
fumarate hydratase (FH) is used for diagnosing FH-deficient RCC
(29,30); and cytokeratin (CK) is used as an
auxiliary diagnostic tool for ccRCC (29). When CAIX, CD10, PAX-8 and vimentin
all exhibit positive expression, the CK marker can be omitted.
Consequently, it seemed that it was unnecessary to employ
additional markers such as CK, TFE3 and FH for further
characterization in the present study.
Collectively, immunohistochemical expression levels,
the patient's medical history and H&E staining were used to
verify ccRCC metastasis to a HTT of the thyroid. In clinical
pathology, when tumor tissues outside the kidney exhibit
morphological features similar to those of ccRCC, the
aforementioned immunohistochemical markers can be used to assist in
diagnosis.
In the 11 previously reported cases of ccRCC
metastases to PTTs, there were four instances of the follicular
variant of papillary thyroid carcinoma (9–12),
four cases of follicular adenoma (5,13–15),
one case of papillary thyroid carcinoma (16), one case of Hurthle cell (oncocytic)
carcinoma (15) and one case of
Hurthle cell adenoma (17).
Although all cases were metastatic to thyroid tumors following
ccRCC-mediated radical nephrectomy, the histological features and
biological behaviors of the tumor included in the present study
differed from those previously reported. Among the 11 cases
reported, there were 4 cases of follicular adenoma and 1 case of
Hurthle cell adenoma, both of which were benign tumors. The
morphology of their tumor tissues was characterized by complete
encapsulation. However, they also exhibited certain distinct
features. Specifically, Hurthle cell adenoma presented with
abundant granules in the eosinophilic cytoplasm, whilst follicular
adenoma displayed cells arranged in follicles of varying sizes.
None of these five cases showed metastasis to other organs. The
remaining six cases were malignant tumors. The follicular variant
of papillary thyroid carcinoma displayed follicles of varying sizes
and shared characteristics with papillary thyroid carcinoma cells.
Both Hurthle cell (oncocytic) carcinoma and papillary thyroid
carcinoma demonstrated invasive growth. Among these malignant
cases, four cases exhibited metastasis to other organs, including
the liver, pancreas, contralateral kidney, spine and subcutaneous
tissue. In the present case, the tumor cell exhibited unique clear
cell alterations with hyaline stroma, which was different from
previously reported cases. The biological behavior of the tumor was
intermediate between that of benign and malignant tumors, showing
differences from both.
Few studies have focused on the mechanisms
underlying metastatic ccRCC to the thyroid. The rarity of this
tumor may be due to high levels of oxygen and iodine as a result of
abundant blood flow in the thyroid (31). In diseases such as chronic
thyroiditis or nodular goiter, the oxygen and iodine content in the
thyroid decreases, leading to higher levels of metastasis to the
thyroid (32–34). In a previous study, a metabolic
hypothesis was proposed, also known as the ‘seed and soil’
hypothesis. This hypothesis emphasizes the interaction between
tumor cells (seeds) and the microenvironment of the target organ
for metastasis (soil). This theory denotes that the metastasis and
growth of tumor cells are not only determined by the biological
characteristics of the tumor cells themselves but are also
influenced by the microenvironment of the target organ (5). In addition, a mechanical hypothesis
was proposed, which suggested that tumor cells metastasize to other
tumor tissues through the circulatory system, where they colonize
and grow. During metastasis, tumor cells may easily invade the
recipient tumor tissue due to an abundant vascular supply (35). The interstitium of a HTT contains an
abundant microvascular network (36), and this rich vascular network not
only provides nutrients and oxygen for metastatic tumor cells, but
also alters the microenvironment of the recipient tumor cells
(9). Moreover, the abundant
vascular network may also alter the micromechanical environment of
the recipient tumor cell matrix, and such changes may promote the
metastasis of tumor cells (37).
The mechanism underlying ccRCC metastasis to an HTT is complex, and
both metabolic and mechanical hypotheses are valid. However,
further investigations are required to determine the specific
mechanisms involved.
Treatment of metastatic thyroid tumors may include
surgery, radiotherapy or chemotherapy. Surgical intervention is the
primary integrative therapeutic approach, involving total or
partial thyroidectomy, along with cervical lymph node dissection.
Following surgery, patients are treated with long-term oral thyroid
hormone replacement therapy (38).
Thyroidectomy is considered a more effective treatment option than
radiotherapy and chemotherapy (39). However, there is ongoing debate
regarding the surgical management of metastatic thyroid tumors. In
cases involving unilateral nodules, unilateral thyroidectomy is
favored to minimize damage to the recurrent laryngeal nerve and
parathyroid glands (40). However,
patients that undergo total thyroidectomy exhibit a lower rate of
recurrence (41). For patients with
multiple and small bilateral lesions, total thyroidectomy may be
recommended to prevent relapse (42). Determining the appropriate surgical
approach for patients requires a comprehensive assessment by the
clinician, for the development of an individualized treatment
plan.
In conclusion, metastasis of ccRCC to a HTT is rare,
with clinical manifestations that are not specific to the tumor
type, leading to challenges in clinical diagnosis. Accurate
pathological diagnosis combined with clinical information and
immunohistochemical analysis are crucial for the development of an
individualized treatment plan.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CL contributed to the study design, writing and
revisions of the present study. MJ and JL were responsible for
drafting the manuscript and analysing disease mechanisms. HM and WX
were responsible for the case analysis and pathological diagnosis
assistance. JS was responsible for the sampling of surgical
specimens and immunohistochemical analysis, and made key revisions
to the content of the manuscript and. CL and MJ confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent for
participation
The present study was approved by the Beilun
District People's Hospital Medical Ethics Committee (Ningbo, China;
April 23, 2024; approval no. 2024LP023). Written informed consent
was obtained from the patient, prior to investigations using tumor
samples.
Patient consent for publication
Written informed consent for the publication of this
article was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deng FM and Melamed J: Histologic variants
of renal cell carcinoma: Does tumor type influence outcome? Urol
Clin North Am. 39:119–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leibovich BC, Blute ML, Cheville JC, Lohse
CM, Frank I, Kwon ED, Weaver AL, Parker AS and Zincke H: Prediction
of progression after radical nephrectomy for patients with clear
cell renal cell carcinoma: A stratification tool for prospective
clinical trials. Cancer. 97:1663–1671. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bianchi M, Sun M, Jeldres C, Shariat SF,
Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P,
et al: Distribution of metastatic sites in renal cell carcinoma: A
population-based analysis. Ann Oncol. 23:973–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xue J, Chen W, Xu W, Xu Z, Li X, Qi F and
Wang Z: Patterns of distant metastases in patients with clear cell
renal cell carcinoma-A population-based analysis. Cancer Med.
10:173–187. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koo HL, Jang J, Hong SJ, Shong Y and Gong
G: Renal cell carcinoma metastatic to follicular adenoma of the
thyroid gland. A case report. Acta Cytol. 48:64–68. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsimafeyeu I, Zolotareva T, Varlamov S,
Zukov R, Petkau V, Mazhbich M, Statsenko G, Safina S, Zaitsev I,
Sakaeva D, et al: Five-year survival of patients with metastatic
renal cell carcinoma in the Russian federation: Results from the
RENSUR5 registry. Clin Genitourin Cancer. 15:e1069–e1072. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walton J, Ng ASN, Arevalo K, Apostoli A,
Meens J, Karamboulas C, St-Germain J, Prinos P, Dmytryshyn J, Chen
E, et al: PRMT1 inhibition perturbs RNA metabolism and induces DNA
damage in clear cell renal cell carcinoma. Nat Commun. 15:82322024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khaddour K, Marernych N, Ward WL, Liu J
and Pappa T: Characteristics of clear cell renal cell carcinoma
metastases to the thyroid gland: A systematic review. World J Clin
Cases. 7:3474–3485. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Badawi F and Meliti A: Tumor-to-tumor
metastasis of renal cell carcinoma to a follicular variant of
papillary thyroid carcinoma: A case report and literature review.
Cureus. 14:e237422022.PubMed/NCBI
|
|
10
|
Baloch ZW and LiVolsi VA: Tumor-to-tumor
metastasis to follicular variant of papillary carcinoma of thyroid.
Arch Pathol Lab Med. 123:703–706. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kefeli M and Mete O: An unusual solitary
thyroid nodule with bloody follicles: Metastatic renal cell
carcinoma within an infiltrative follicular variant papillary
carcinoma. Endocr Pathol. 27:171–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu J, Nikiforova MN, Hodak SP, Yim JH, Cai
G, Walls A, Nikiforov YE and Seethala RR: Tumor-to-tumor metastases
to follicular variant of papillary thyroid carcinoma: histologic,
immunohistochemical, and molecular studies of two unusual cases.
Endocr Pathol. 20:235–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wolf G, Aigner RM, Humer-Fuchs U, Schwarz
T, Krippl P and Wehrschuetz M: Renal cell carcinoma metastasis in a
microfollicular adenoma of the thyroid gland. Acta Med Austriaca.
29:141–142. 2002.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Medas F, Calò PG, Lai ML, Tuveri M, Pisano
G and Nicolosi A: Renal cell carcinoma metastasis to thyroid tumor:
A case report and review of the literature. J Med Case Rep.
7:2652013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryska A and Cáp J: Tumor-to-tumor
metastasis of renal cell carcinoma into oncocytic carcinoma of the
thyroid. Report of a case and review of the literature. Pathol Res
Pract. 199:101–106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bohn OL, De las Casas LE and Leon ME:
Tumor-to-tumor metastasis: Renal cell carcinoma metastatic to
papillary carcinoma of thyroid-report of a case and review of the
literature. Head Neck Pathol. 3:327–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qian L, Pucci R, Castro CY and Eltorky MA:
Renal cell carcinoma metastatic to Hurthle cell adenoma of thyroid.
Ann Diagn Pathol. 8:305–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tjahjono R, Phung D, Gurney H, Gupta R,
Riffat F and Palme CE: Thyroid gland metastasis from renal cell
carcinoma: A case series and literature review. ANZ J Surg.
91:708–715. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee S, Han BK, Ko EY, Oh YL, Choe JH and
Shin JH: The ultrasonography features of hyalinizing trabecular
tumor of the thyroid are more consistent with its benign behavior
than cytology or frozen section readings. Thyroid. 21:253–259.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carney JA, Ryan J and Goellner JR:
Hyalinizing trabecular adenoma of the thyroid gland. Am J Surg
Pathol. 11:583–591. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Huang X, Hu Y, Wang F, Du T, He W,
Chen L, Lang B, Pu Q and Chen H: Hyalinizing trabecular tumor of
the thyroid: a clinicopathological analysis of four cases and
review of the literature. Int J Clin Exp Pathol. 10:7616–7626.
2017.PubMed/NCBI
|
|
22
|
Howard BE, Gnagi SH, Ocal IT and Hinni ML:
Hyalinizing trabecular tumor masquerading as papillary thyroid
carcinoma on fine-needle aspiration. ORL J Otorhinolaryngol Relat
Spec. 75:309–313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Warren AY and Harrison D: WHO/ISUP
classification, grading and pathological staging of renal cell
carcinoma: Standards and controversies. World J Urol. 36:1913–1926.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malhi HS and Grant EG: Ultrasound of
thyroid nodules and the thyroid imaging reporting and data system.
Neuroimaging Clin N Am. 31:285–300. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang Q and Wang Z: Metastases to the
thyroid gland: What can we do? Cancers (Basel). 14:30172022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chu S: Hyalinizing trabecular tumor of the
thyroid: A case report. Asian J Surg. 46:5559–5560. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng J and Wang J: Expression and clinical
significance of Ki67 and calcitonin in medullary thyroid carcinoma.
Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 28:1921–1924.
2014.(In Chinese). PubMed/NCBI
|
|
28
|
Luong-Player A, Liu H, Wang HL and Lin F:
Immunohistochemical reevaluation of carbonic anhydrase IX (CA IX)
expression in tumors and normal tissues. Am J Clin Pathol.
141:219–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mahmut A and Sean RW: Immunohistochemistry
for the diagnosis of renal epithelial neoplasms. Semin Diagn
Pathol. 39:1–16. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanakaraj J, Chang J, Hampton LJ and Smith
SC: The New WHO category of ‘molecularly defined renal carcinomas’:
clinical and diagnostic features and management implications. Urol
Oncol. 42:211–219. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cilengir AH, Kalayci TO, Duygulu G,
Rezanko TA and İnci MF: Metastasis of renal clear cell carcinoma to
thyroid gland mimicking adenomatous goiter. Pol J Radiol.
81:618–621. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zivaljevic V, Jovanovic M, Perunicic V and
Paunovic I: Surgical treatment of metastasis to the thyroid gland:
A single center experience and literature review. Hippokratia.
22:137–140. 2018.PubMed/NCBI
|
|
33
|
Mohammadi A, Toomatari SB and Ghasemi-Rad
M: Metastasis from renal cell carcinoma to thyroid presenting as
rapidly growing neck mass. Int J Surg Case Rep. 5:1110–1112. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cordes M and Kuwert T: Metastases of
non-thyroidal tumors to the thyroid gland: A regional survey in
middle franconia. Exp Clin Endocrinol Diabetes. 122:273–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carney JA, Hirokawa M, Lloyd RV, Papotti M
and Sebo TJ: Hyalinizing trabecular tumors of the thyroid gland are
almost all benign. Am J Surg Pathol. 32:1877–1889. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Neophytou CM, Panagi M, Stylianopoulos T
and Papageorgis P: The role of tumor microenvironment in cancer
metastasis: Molecular mechanisms and therapeutic opportunities.
Cancers (Basel). 13:20532021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cao J, Yu YE, Li NN, Wu YX, Shi JN and
Fang MY: Thyroid metastasis from non-small cell lung cancer. Int J
Clin Exp Pathol. 12:3013–3021. 2019.PubMed/NCBI
|
|
39
|
Straccia P, Mosseri C, Brunelli C, Rossi
ED, Lombardi CP, Pontecorvi A and Fadda G: Diagnosis and treatment
of metastases to the thyroid gland: A meta-analysis. Endocr Pathol.
28:112–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nixon IJ, Coca-Pelaz A, Kaleva AI,
Triantafyllou A, Angelos P, Owen RP, Rinaldo A, Shaha AR, Silver CE
and Ferlito A: Metastasis to the Thyroid Gland: A Critical Review.
Ann Surg Oncol. 24:1533–1539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Russell JO, Yan K, Burkey B and Scharpf J:
Nonthyroid metastasis to the thyroid gland: Case series and review
with observations by primary pathology. Otolaryngol Head Neck Surg.
155:961–968. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iesalnieks I, Winter H, Bareck E,
Sotiropoulos GC, Goretzki PE, Klinkhammer-Schalke M, Bröckner S,
Trupka A, Anthuber M, Rupprecht H, et al: Thyroid metastases of
renal cell carcinoma: Clinical course in 45 patients undergoing
surgery. Assessment of factors affecting patients' survival.
Thyroid. 18:615–624. 2008. View Article : Google Scholar : PubMed/NCBI
|