Introduction
Small-cell lung cancer (SCLC) accounts for 13–15% of
all lung cancer cases and approximately one-third of cases are
limited-stage (LS)-SCLC (1).
LS-SCLC has a poor prognosis due to rapid growth and early distant
and loco-regional dissemination. The median survival time of
LS-SCLC is reported to be 16–20 months with a 5-year survival rate
of 10–20% (2). Brain metastasis
(BM) is a common complication of SCLC, occurring either at
diagnosis or throughout the course of the disease (3). Additionally, >10% of patients with
SCLC have BM at initial diagnosis and the cumulative incidence of
BM at 2 years is >50% (4).
Furthermore, ~65% of patients have detectable BM on autopsy
(5). The survival advantage of
prophylactic cranial irradiation (PCI) was first demonstrated in
the 1990s and a 5.4% increase in the rate of survival was acquired
at 3 years among patients with SCLC in complete remission (6). The National Comprehensive Cancer
Network guidelines recommend PCI as the standard treatment for
patients with LS-SCLC who have achieved remission after first-line
chemoradiation therapy (7).
However, these guidelines are primarily based on the results of a
meta-analysis of multiple clinical studies conducted in the
pre-magnetic resonance imaging (MRI) era (6,8).
During this phase, the enrollment criteria were based on the
absence of symptoms of BM or the use of computed tomography (CT)
imaging to confirm the absence of BM, rather than MRI examination.
In addition, the results of a previous study revealed that the
detection rate of BM was 10% in the CT era and 24% in the MRI era
(9), indicating that MRI may be
more sensitive than contrast-enhanced CT for the detection of BMs.
Thus, MRI may be more beneficial as an assessment tool for BM.
Numerous previous retrospective studies conducted in the MRI era
have re-evaluated the efficacy of PCI in patients with LS-SCLC
(10–30). Some of these studies (10,15,18,20,26)
suggest that PCI fails to significantly prolong the overall
survival (OS) time of patients with LS-SCLC in modern pretreatment
MRI staging. However, other studies (21–25,27–30)
suggest that PCI does significantly prolong the OS time of patients
with LS-SCLC in the era of MRI. As such, the results of these
studies remain controversial. Therefore, the present study aimed to
evaluate the efficacy of PCI in patients with LS-SCLC in the era of
MRI, to provide a reference for the clinical management of
LS-SCLC.
Materials and methods
Literature search strategy
The PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.cochranelibrary.com/) and Cochrane
Library (https://www.embase.com/) databases were
searched for clinical studies assessing the effectiveness of PCI in
patients with LS-SCLC during the MRI era, from the time of database
creation until May 24, 2023. The references of the obtained studies
were also examined for the identification of pertinent clinical
studies that were not discovered during the initial search. Search
terms included ‘small-cell lung carcinoma’, ‘small-cell lung
cancer’, ‘prophylactic brain irradiation’, ‘prophylactic cranial
irradiation’, ‘PCI’ and ‘whole-brain radiotherapy’. A combination
of subject terms and key words were used in the search.
Inclusion and exclusion criteria
Literature were included in the present study
according to the following criteria: i) Studies involving patients
with LS-SCLC; ii) studies involving patients without BM, as
confirmed using brain MRI at baseline or prior to PCI; iii)
clinical trials evaluating the effectiveness of PCI in the
treatment of LS-SCLC compared with non-PCI; and iv) clinical trials
reporting outcomes, such as overall survival (OS) and the rate of
BM. The following literature were excluded from the present
meta-analysis: i) Abstracts; ii) case reports; iii) reviews; iv)
study plans; v) studies that reported outcomes yet withheld raw
data; and vi) studies that were not available in English.
Literature screening and data
extraction
In total, two researchers independently evaluated
the literature and extracted data from the included studies. When a
consensus on inclusion could not be reached, discussions were held
within the research group. Primary information that were extracted
from the literature was as follows: i) General information,
including author names, year of publication, type of study, number
of patients, patient sex, patient age and patient history of
smoking; ii) clinical information, including tumor stage, initial
treatment, percentage of complete response (CR) and partial
response (PR) and PCI dose; and iii) outcome indices, including OS,
progression-free survival (PFS), BM rate and BM-free survival
(BMFS). The Newcastle-Ottawa scale (NOS) was used to assess the
quality of each study (31), and
those scored with seven stars or more were considered to be
high-quality studies.
Statistical analysis
Statistical analysis was performed using STATA 17.0
software (StataCorp LP). The included studies that provided
OS-related hazard ratios (HRs) and 95% confidence intervals (CIs)
were analyzed during the meta-analysis. For studies that only
provided Kaplan-Meier survival curves, the associated HR and 95% CI
were calculated using Engauge Digitizer v12.2.1 software
(http://markummitchell.github.io/engauge-digitizer/)
and methods as previously described (32). χ2 test and the
I2 index were used to investigate heterogeneity. The
Cochrane Guidance Manual for Systematic Evaluation (33) states that the significance level of
heterogeneity should be set at P=0.1 for the χ2 test and
I2 should be set at 50%. However, according to the
recommendations provided by the Cochrane Handbook for Systematic
Reviews of Interventions (33), the
choice between a fixed-effect and a random-effects meta-analysis
model should not be made on the basis of a statistical test for
heterogeneity. Additionally, heterogeneity is always expected when
examining the intervention effects among multiple studies from
different research groups and geographical locations. Therefore,
all data for the present meta-analysis were combined using the
random-effects model. Publication bias was evaluated using a funnel
plot.
Results
Literature screening and baseline
characteristics
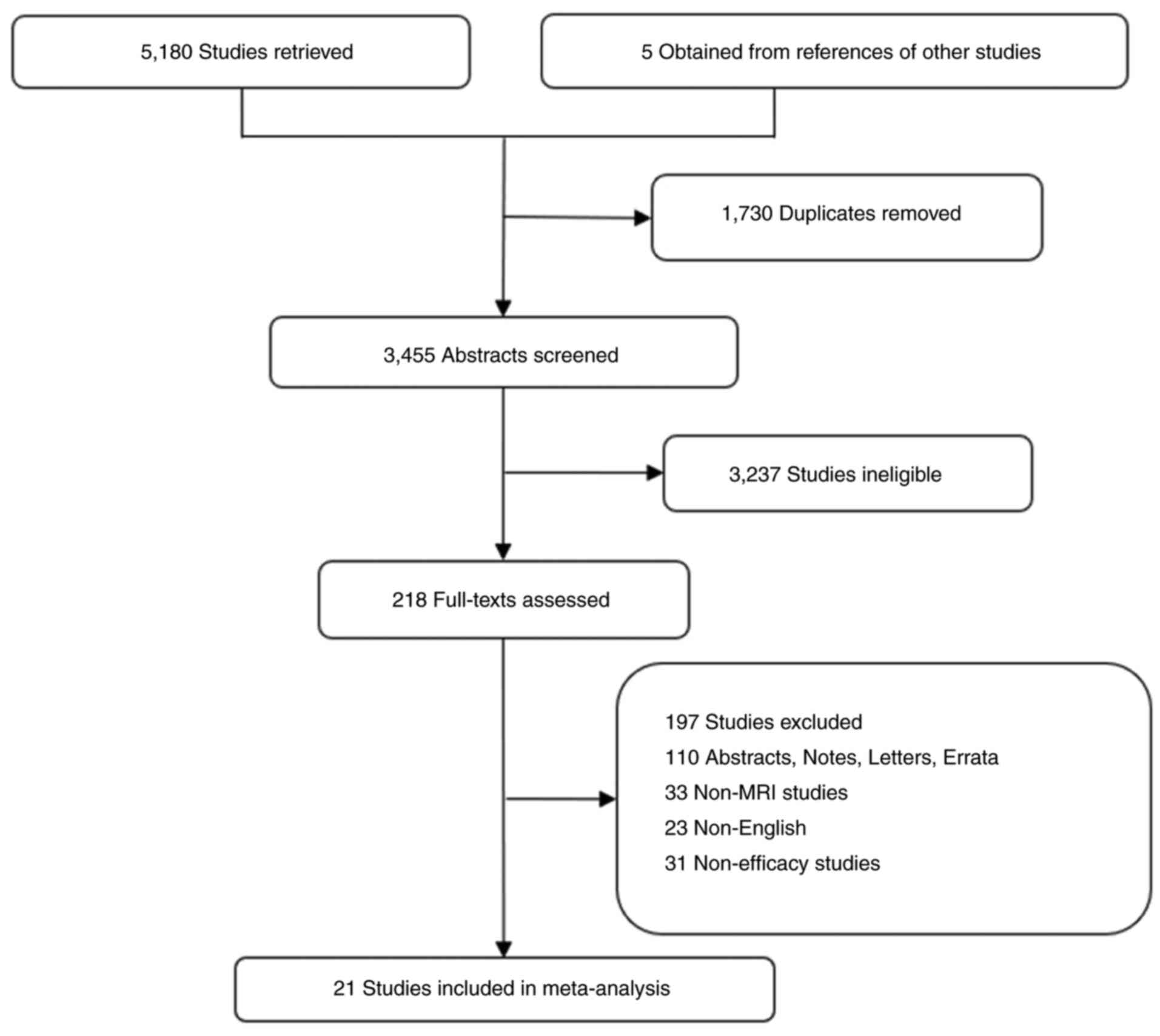
A total of 21 retrospective clinical studies
assessing the therapeutic efficacy of PCI in patients with LS-SCLC
during the MRI era were included in the present study. The
screening process is outlined in Fig.
1 and the baseline characteristics of the included studies are
displayed in Table I. All patients
with LS-SCLC included in the 21 retrospective studies underwent
baseline or pre-PCI brain MRI to exclude the presence of BM. In
total, 10 of the studies only used brain MRI at baseline to confirm
the absence of BM, 7 studies used brain MRI prior to PCI and at
baseline to confirm the absence of BM and only 4 studies used brain
MRI as active surveillance following PCI. In 1 study, the initial
treatment regimen consisted of surgery plus chemotherapy, 2 studies
used surgery plus chemotherapy and the remaining 18 studies used
chemoradiotherapy. In 10 of the included studies, the PCI dose was
25 Gy/10 fractions (F), compared with 30 Gy/15 F in 2 studies, and
between 25 and 40 Gy in 5 studies. Notably, the PCI dose was not
reported in the remaining 4 studies. The quality of all included
trials was evaluated using the NOS (8) and all were rated as high quality. The
results of the quality assessment are displayed in Table SI.
 | Table I.Baseline characteristics of the
included studies. |
Table I.
Baseline characteristics of the
included studies.
| First author,
year | Group | Time frame of the
study | Patients, n | Median age (range),
years | Male/female | I–II/III | CCRT/SCRT | EP/others | CR/PR | PCI dose, Gy/F | Brain MRI time
points | (Refs.) |
|---|
| Chen et al,
2022 | PCI | 2009.6–2019.6 | 324 | 58.0 (51–64) | 232/92 | 62/162 | 115/209 | 315/9 | 91/233 | 25-30/10 | Baseline, before
PCI, | (10) |
|
| Non-PCI |
| 324 | 59.0 (51–65) | 229/95 | 54/270 | 132/192 | 305/15 | 99/225 |
| MRI
surveillance |
|
| Eze et al,
2017 | PCI | 1998-2012 | 71 | - | 36/35 | - | 34/37 | 68/3 | - | 30/15 | Baseline, before
PCI | (11) |
|
| Non-PCI |
| 113 | - | 75/38 | - | 35/78 | 96/17 | - |
|
|
|
| Inoue et al,
2021 | PCI | 1998-2018 | 43 | - | 38/5 | 32/10 | 43/0 | - | 34/9 | 24-30/10-15 | Baseline, before
PCI | (12) |
|
| Non-PCI |
| 85 | - | 71/14 | 46/39 | 71/14 | - | 41/44 |
|
|
|
| Zhu et al,
2014 | PCI | 2003.1–2009.12 | 67 | 55.0 (34–82) | 49/18 | 27/40 | - | - | - | 25/10 | Baseline, before
PCI | (13) |
|
| Non-PCI |
| 126 | 55.0 (7–74) | 101/25 | 65/61 | - | - | - |
|
|
|
| Li et al,
2021 | PCI | 2013.7–2017.6 | 77 | - | 58/19 | - | 47/30 | - | - | 25/10 | Baseline, before
PCI, | (14) |
|
| Non-PCI |
| 113 | - | 74/39 | - | 43/70 | - | - |
| MRI
surveillance |
|
| Mamesaya et
al, 2018 | PCI | 2002.9–2015.8 | 60 | 64.0 (34–82) | 43/17 | 8/52 | 58/2 | - | 23/37 | 25/10 | Baseline, before
PCI | (15) |
|
| Non-PCI |
| 20 | 72.5 (56–83) | 11/9 | 6/14 | 11/9 | - | 4/16 |
|
|
|
| Ozawa et al,
2015 | PCI | 2006.1–2013.6 | 28 | - | - | - | - | - | - | NR | Before PCI | (16) |
|
| Non-PCI |
| 57 | - | - | - | - | - | - |
|
|
|
| Pan et al,
2023 | PCI | 2006.1–2017.12 | 57 | 57.0 | 44/19 | 6/77 | 69/15 | 77/6 | 38/45 | 25/10 | Baseline, before
PCI | (17) |
|
| Non-PCI |
| 61 | 61.0 | 28/5 | 2/31 | 14/19 | 29/4 | 15/18 |
|
|
|
| Qi et al,
2022 | PCI | 2012.1–2018.1 | 75 | - | 53/22 | 23/52 | 35/40 | - | 16/59 | - | Baseline, before
PCI, | (18) |
|
| Non-PCI |
| 75 | - | 59/16 | 25/50 | 29/46 | - | 8/67 |
| MRI
surveillance |
|
| Ghanta et
al, 2021 | PCI | 2009-2020 | 243 | 65.2 (60–71) | 91/152 | 58/185 | 221/13 | - | 69/174 | 25-36/10-18 | Before PCI, | (19) |
|
| Non-PCI |
| 106 | 68.9 (61–74) | 42/64 | 29/77 | 89/11 | - | 32/74 |
| MRI
surveillance |
|
| Pezzi et al,
2020 | PCI | 1992-2012 | 205 | 62.2 (27–85) | 110/95 | - | - | 67/14 | 70/23 | 25-30/10-15 | Baseline, before
PCI | (20) |
|
| Non-PCI |
| 92 | 68.6 (40–86) | 52/40 | - | - | 34/8 | 36/28 |
|
|
|
| Farooqi et
al, 2017 | PCI | 1985-2012 | 364 | 61.0 (34–85) | 187/177 | - | 301/63 | - | - | 25/10 | Baseline | (21) |
|
| Non-PCI |
| 294 | 64.0 (27–95) | 155/139 | - | 230/64 | - | - |
|
|
|
| Sas-Korczyńska
et al, 2017 | PCI | 2002-2015 | 167 | 59.0 (32–79) | 103/64 | - | 100/67 | - | - | 30/15 | Baseline | (22) |
|
| Non-PCI |
| 104 | 63.5 (35–79) | 71/33 | - | 22/82 | - | - |
|
|
|
| Chen et al,
2018 | PCI | 2003.1–2015.12 | 19 | (44–73) | 15/4 | 10/9 | - | - | - | 25/10 | Baseline | (23) |
|
| Non-PCI |
| 33 | (38–74) | 27/6 | 17/16 | - | - | - |
|
|
|
| Kim et al,
2019 | PCI | 1994.11–2010.6 | 139 | 60.0 (34–75) | 123/16 | 34/105 | - | - | - | - | Baseline | (24) |
|
| Non-PCI |
| 95 | 62.0 (40–77) | 81/14 | 20/75 | - | - | - |
|
|
|
| Lee et al,
2023 | PCI | 2004.1–2017.12 | 211 | 65.0 (39–79) | 175/36 | 41/170 | 191/20 | - | 32/158 | 25/10 | Baseline | (25) |
|
| Non-PCI |
| 60 | 70.0 (44–93) | 51/9 | 15/45 | 32/28 | - | 4/41 |
|
|
|
| Farris et
al, 2019 | PCI | 2007-2018 | 39 | 62.0 (38–80) | 20/19 | 12/27 | - | - | 12/27 | 25/10 | Baseline | (26) |
|
| Non-PCI |
| 53 | 66.0 (41–91) | 28/25 | 18/34 | - | - | 17/36 |
|
|
|
| Yin et al,
2018 | PCI | 2010.1–2015.12 | 160 | - |
| - | - | - | - | 30-40/10-20 | Baseline | (27) |
|
| Non-PCI |
| 109 | - |
| - | - | - | - |
|
|
|
| Held et al,
2022 | PCI | 2012.1–2019.12 | 28 | 62.2 (47–71) | 12/16 | - | - | 28/0 | - | 25/10 | Baseline | (28) |
|
| Non-PCI |
| 21 | 65.5 (57–71) | 11/10 | - | - | 21/0 | - |
|
|
|
| Lim et al,
2022 | PCI | - | 26 | 60.0 (43–76) | 22/4 | - | - | 25/1 | - | 25-37.5/10-25 | Baseline | (29) |
|
| Non-PCI |
| 81 | 69.0 (44–82) | 69/12 | - | - | 68/3 | - |
|
|
|
| Jeong et al,
2020 | PCI | 2005.8–2014.3 | 56 | - |
| - | - | - | - | 25/10 | Baseline | (30) |
|
| Non-PCI |
| 45 | - |
| - | - | - | - |
|
|
|
Overall outcomes
The results of the present study revealed that the
1-, 2-, 3- and 5-year OS rates of patients in the PCI groups were
89, 67, 50 and 40%, respectively (Fig.
S1), while in the non-PCI group the OS rates were 85, 48, 35
and 32% (Fig. S2). In addition,
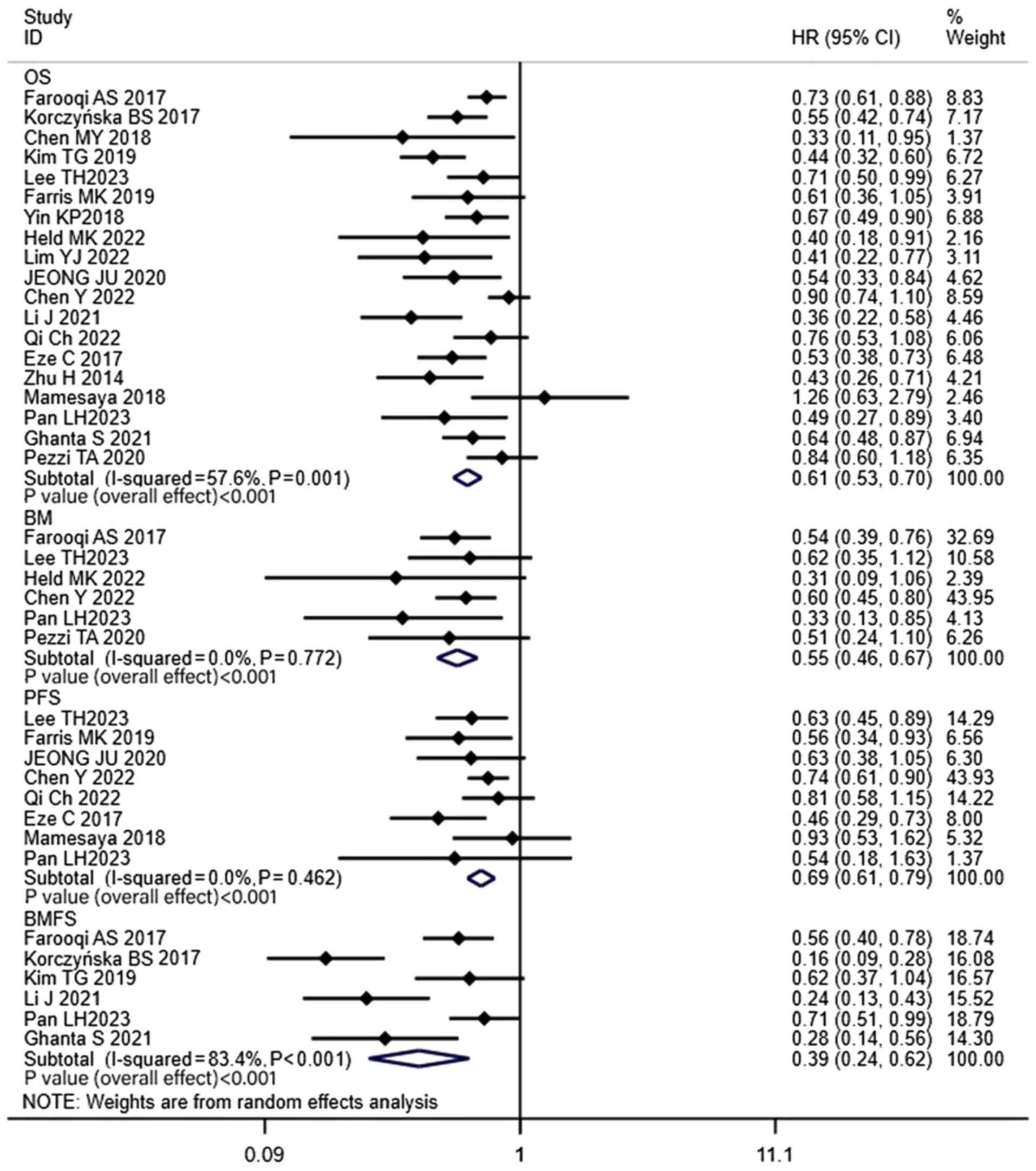
the combined HR was 0.61 (CI, 0.53–0.70; P<0.001; Fig. 2). Only 8 of the included studies
performed propensity-matched analysis, and the combined results
demonstrated that PCI significantly improved the OS of patients
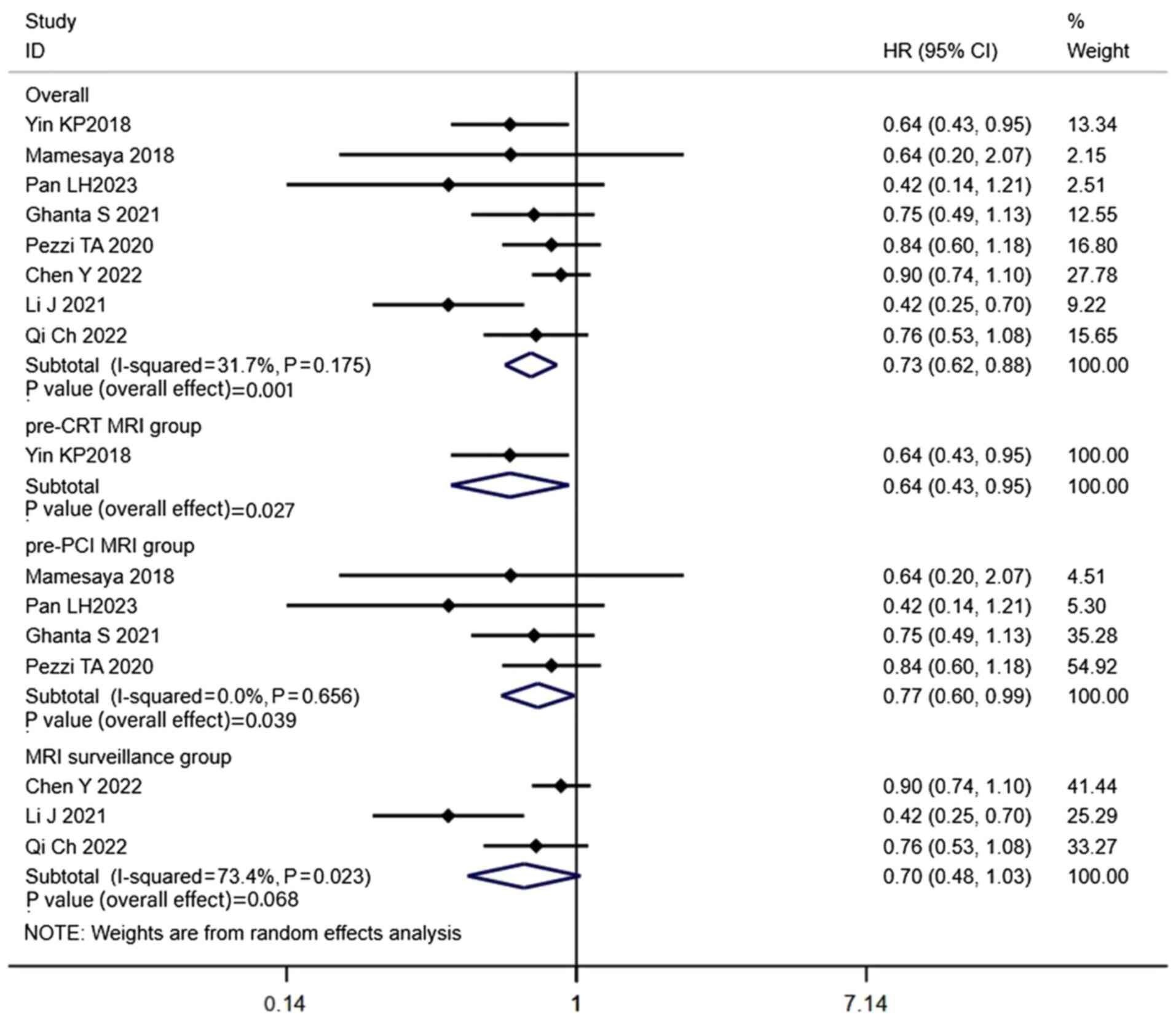
with LS-SCLC (HR, 0.73; CI, 0.62–0.88; P=0.001; Fig. 3). The 1-, 2-, 3- and 5-year BM rates
in the PCI groups were 6, 18, 20 and 25%, respectively (Fig. S1), while in the non-PCI group the
BM rates were 29, 38, 27 and 41% (Fig.
S2). Notably, PCI significantly reduced the incidence of BM in
patients with LS-SCLC (HR, 0.55; CI, 0.46–0.67; P<0.001;
Fig. 2). The combined results also
demonstrated that the PFS (HR, 0.69; CI, 0.61–0.79; P<0.001) and
BMFS (HR, 0.39; CI, 0.24–0.62; P<0.001) in the PCI group were
significantly improved compared with the non-PCI group (Fig. 2).
Results of the pre-chemoradiotherapy
(CRT) MRI group
In total, 10 studies included patients who had
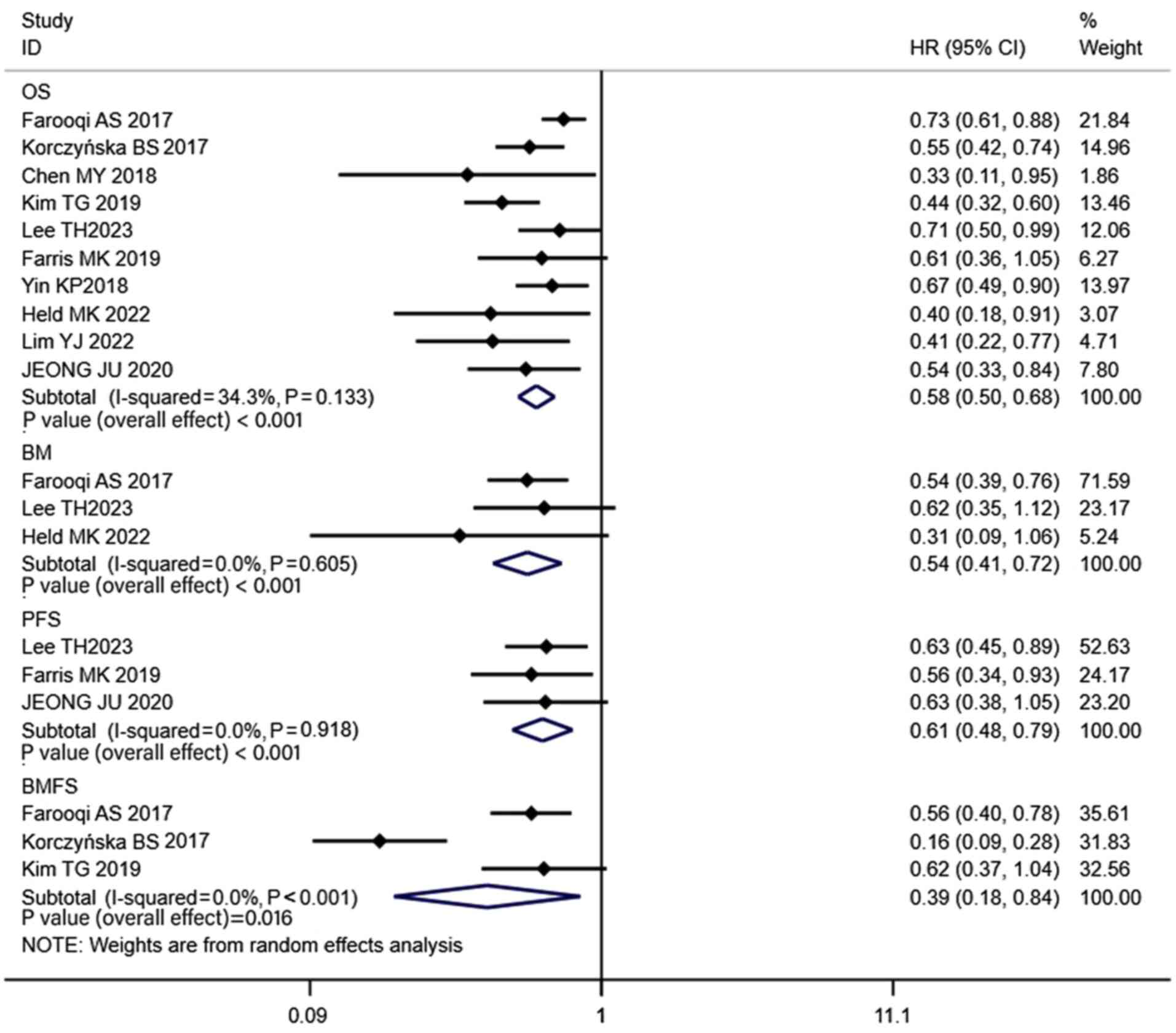
undergone brain MRI at baseline, which excluded the presence of BM.
The combined results of these studies revealed that the 1-, 2-, 3-
and 5-year OS rates of patients in the PCI groups were 84, 61, 50
and 36%, respectively (Fig. S3),
while in the non-PCI group the OS rates were 82, 42, 33 and 27%
(Fig. S4). In addition, the
combined HR was 0.58 (CI, 0.50–0.68; P<0.001; Fig. 4). Notably, the propensity-matched
analysis of 1 study revealed a significant increase in OS in
patients in the PCI group (HR, 0.64; CI, 0.43–0.95; P=0.027;
Fig. 3). The combined results
revealed that the PCI group exhibited a significantly lower BM rate
than the non-PCI group, with 1-, 2-, 3- and 5-year BM rates of 8,
14, 23 and 24%, respectively (Fig.
S3), while in the non-PCI group the BM rates were 23, 27, 27
and 40% (Fig. S4). In addition,
the combined HR was 0.54 (CI, 0.41–0.72; P<0.001; Fig. 4). Moreover, the PFS (HR, 0.61; CI,
0.48–0.79; P<0.001) and BMFS (HR, 0.39; CI, 0.18–0.84; P=0.016)
rates observed in the PCI group were significantly improved,
compared with the non-PCI group (Fig.
4).
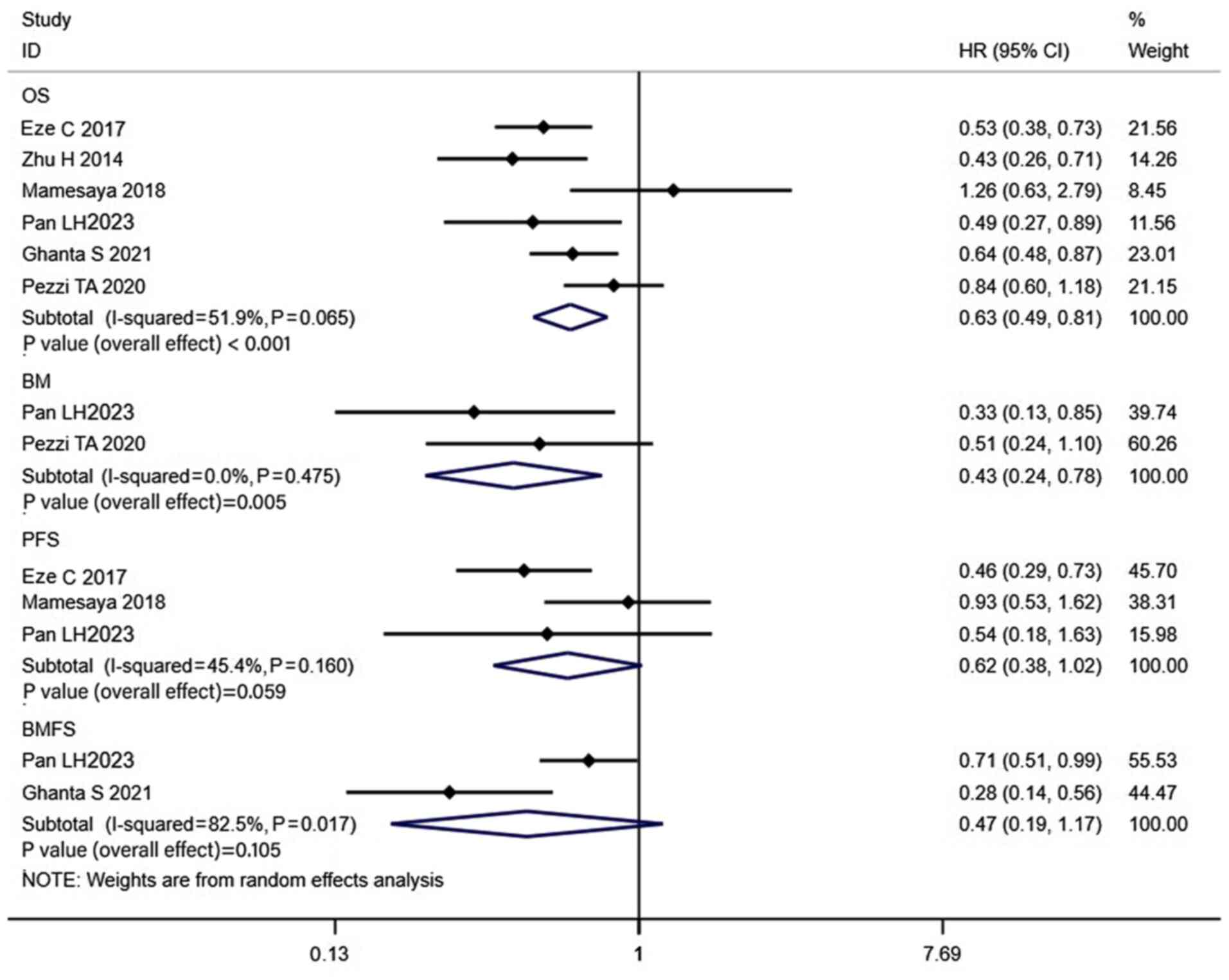
Results of the pre-PCI MRI group
The pooled analysis included 7 trials that comprised
of patients without BM, verified using brain MRI prior to PCI. The
results revealed that the 1-, 2-, 3- and 5-year OS rates of
patients in the PCI groups were 89, 78, 57 and 54%, respectively
(Fig. S5), while in the non-PCI
group the OS rates were 83, 58, 60 and 42% (Fig. S6). In addition, the combined HR was
0.63 (CI, 0.49–0.81; P<0.001; Fig.
5). Propensity-matched analysis was carried out in 4 of the 7
studies that used brain MRI prior to PCI to confirm the absence of
BMs, and the combined results also revealed that the OS was
significantly higher in the PCI group (HR, 0.77; CI, 0.60–0.99;
P=0.039; Fig. 3). The 1-, 2-, 3-
and 5-year BM rates of patients in the PCI groups were 4, 26, 16
and 18%, respectively (Fig. S5),
while in the non-PCI group the BM rates were 31, 40, 21 and 39%
(Fig. S6). The combined HR was
0.43 (CI, 0.24–0.78; P=0.005; Fig.
5), indicating a significant decrease in BM rate in the PCI
group. However, the combined results revealed that the PFS (HR,
0.62; CI, 0.38–1.02; P=0.059) and BMFS (HR, 0.47; CI, 0.19–1.17;
P=0.105) in the PCI group were not significantly different from
those in the non-PCI group (Fig.
5).
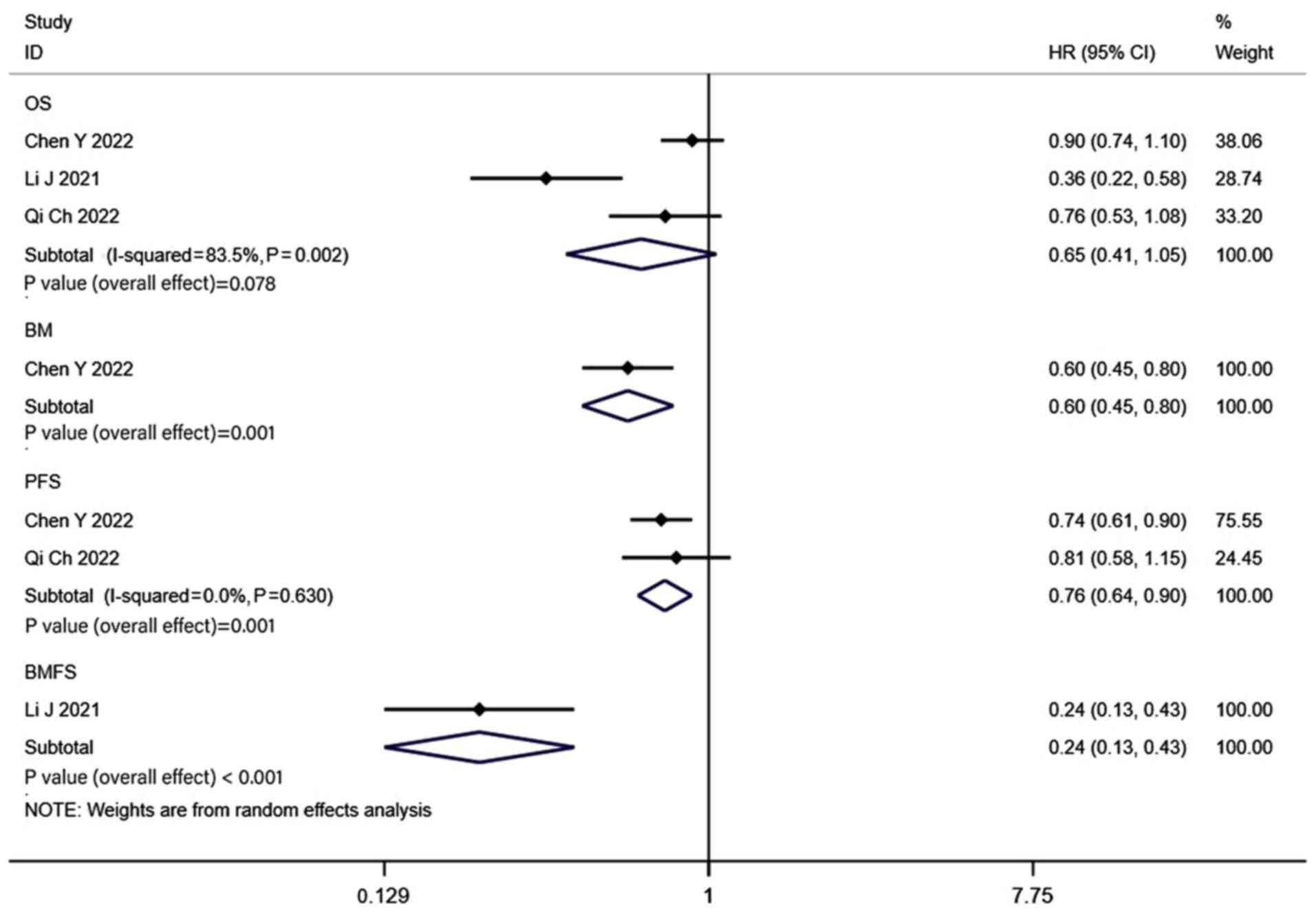
Results of the MRI surveillance
group
The results of the 4 studies that used brain MRI for
active surveillance following PCI revealed that the 1-, 2-, 3- and
5-year OS rates of patients in the PCI groups were 91, 74, 49 and
32%, respectively (Fig. S7), while
in the non-PCI group the OS rates were 87, 61, 33 and 27% (Fig. S8). Notably, the combined HR was
0.65 (CI, 0.41–1.05; P=0.078; Fig.
6). Propensity-matched analysis was performed in 3 of the 4
studies that used brain MRI for active surveillance following PCI,
and the pooled findings did not reveal a statistically significant
difference in OS between the two groups (HR, 0.70; CI, 0.48–1.03;
P=0.068; Fig. 3). The 1-, 2-, 3-
and 5-year BM rates of patients in the PCI groups were 6, 26, 22
and 34%, respectively (Fig. S7),
while in the non-PCI group the BM rates were 31, 57, 31 and 40%
(Fig. S8). The combined HR was 0.6
(CI, 0.45–0.80; P=0.001; Fig. 6),
indicating that the rate of BM in the PCI group was significantly
decreased. The combined results also demonstrated that the PCI
group exhibited significantly higher rates of PFS (HR, 0.76; CI,
0.64–0.90; P=0.001) and BMFS (HR, 0.24; CI, 0.13–0.43; P<0.001)
than the non-PCI group (Fig.
6).
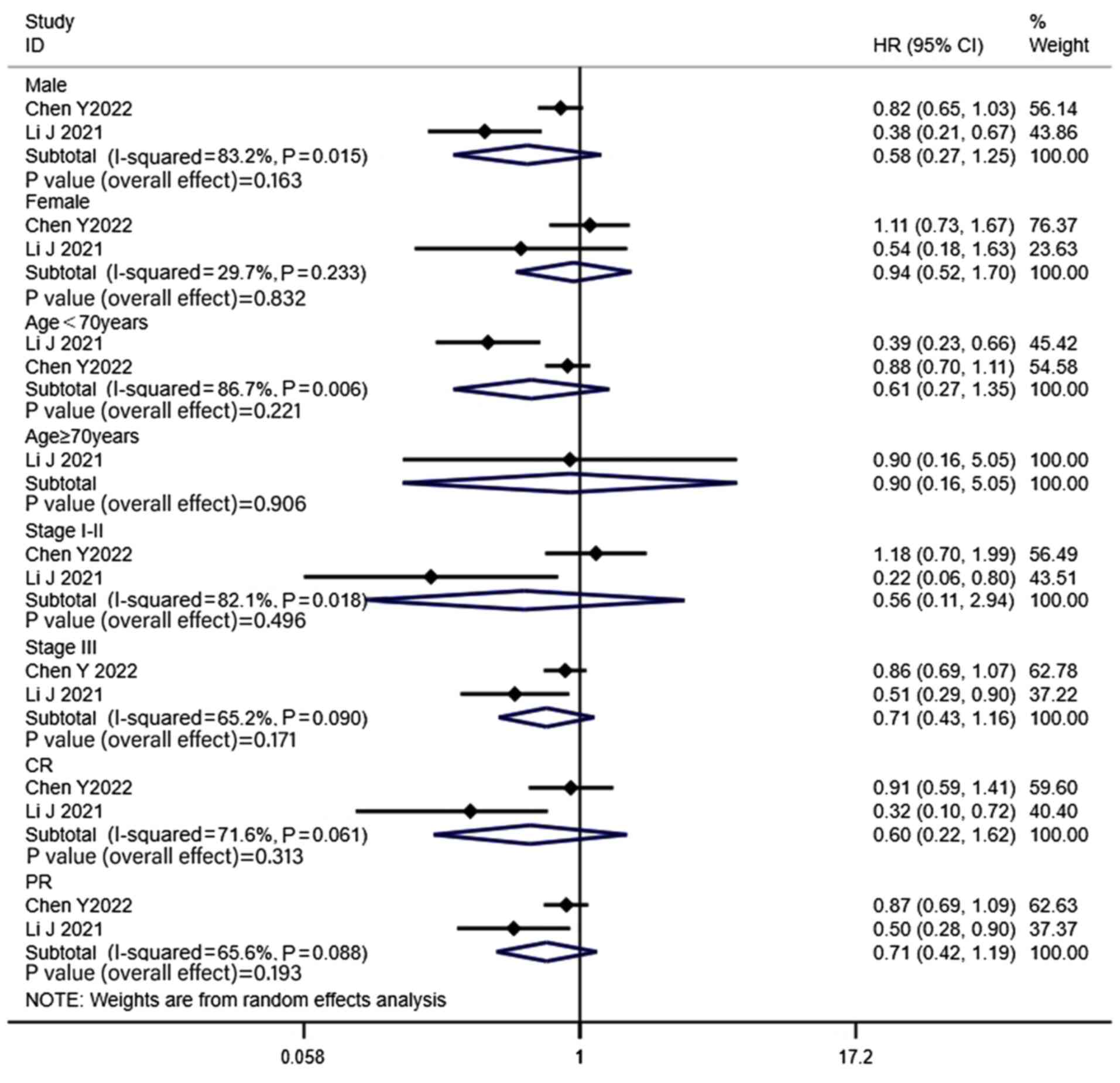
To investigate the heterogeneity of the OS analysis
in the MRI surveillance group, a subgroup analysis was performed
(Fig. 7). The variables used to
analyze the heterogeneity of OS were the male/female ratio, median
age (<70 vs. ≥70 years), proportion of stages (I–II/III) and
fraction of CR/PR. The results of the subgroup analysis revealed
that there was no significant difference in OS between the PCI and
non-PCI groups in any of the subgroups.
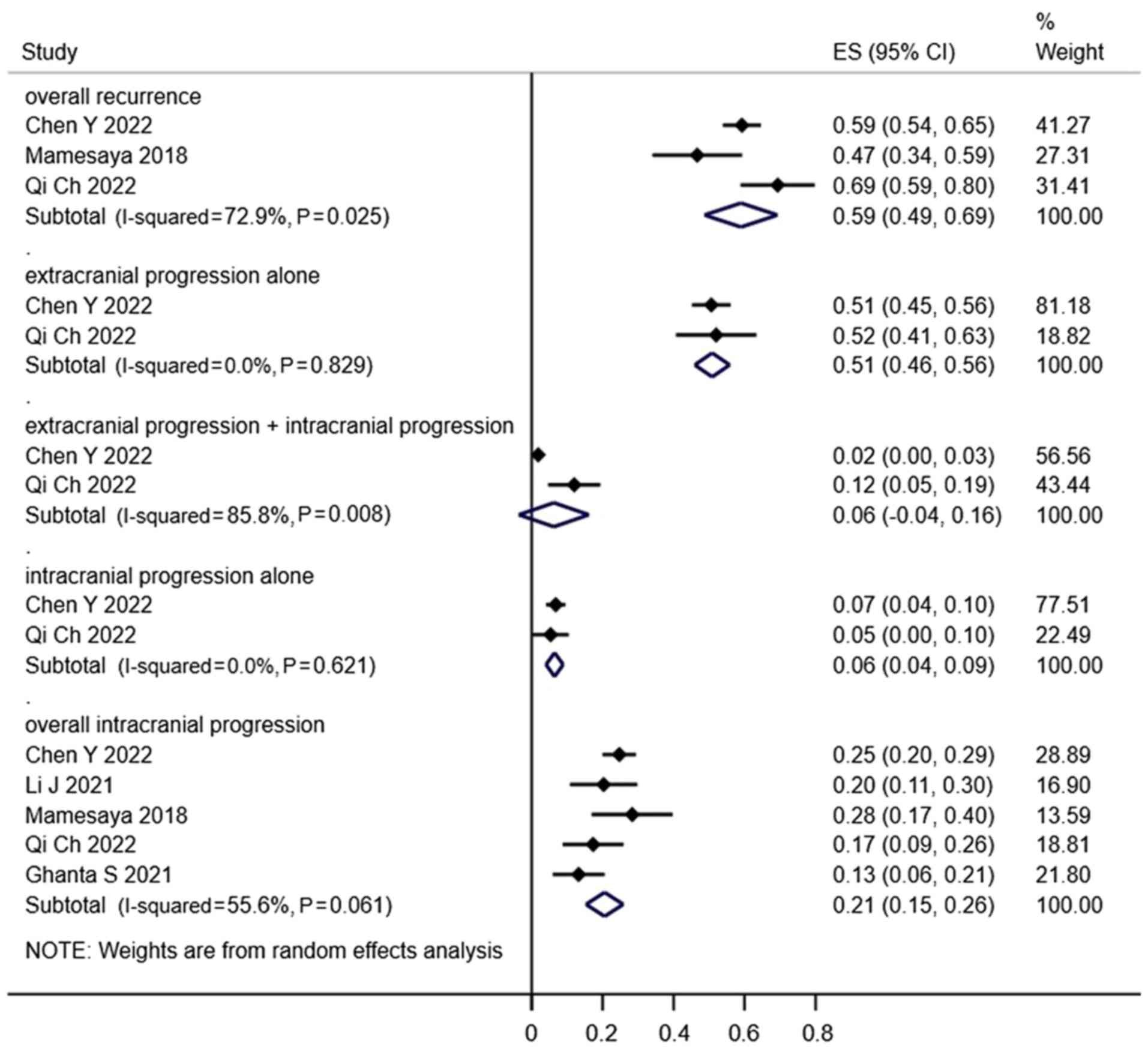
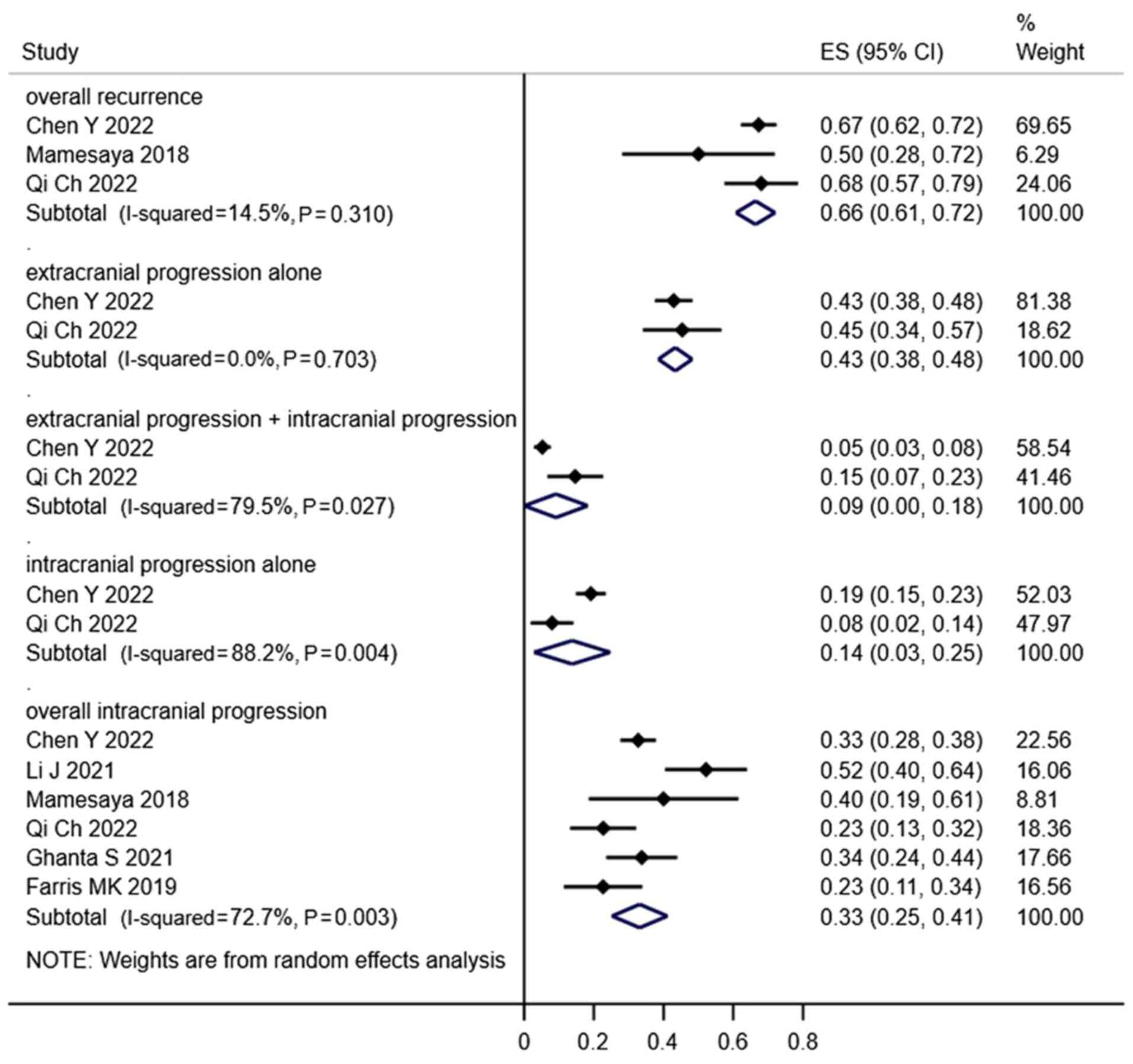
Failure patterns
In total, 5 studies examined the failure patterns of
LS-SCLC following treatment and demonstrated that the main cause of
failure was extracranial failure. Notably, overall recurrence,
extracranial progression alone, extracranial progression combined
with intracranial progression, intracranial progression alone and
overall intracranial progression rates in the PCI groups were 59,
51, 6, 6 and 21%, respectively (Fig.
8), while in the non-PCI groups the rates were 66, 43, 9, 14
and 33% (Fig. 9).
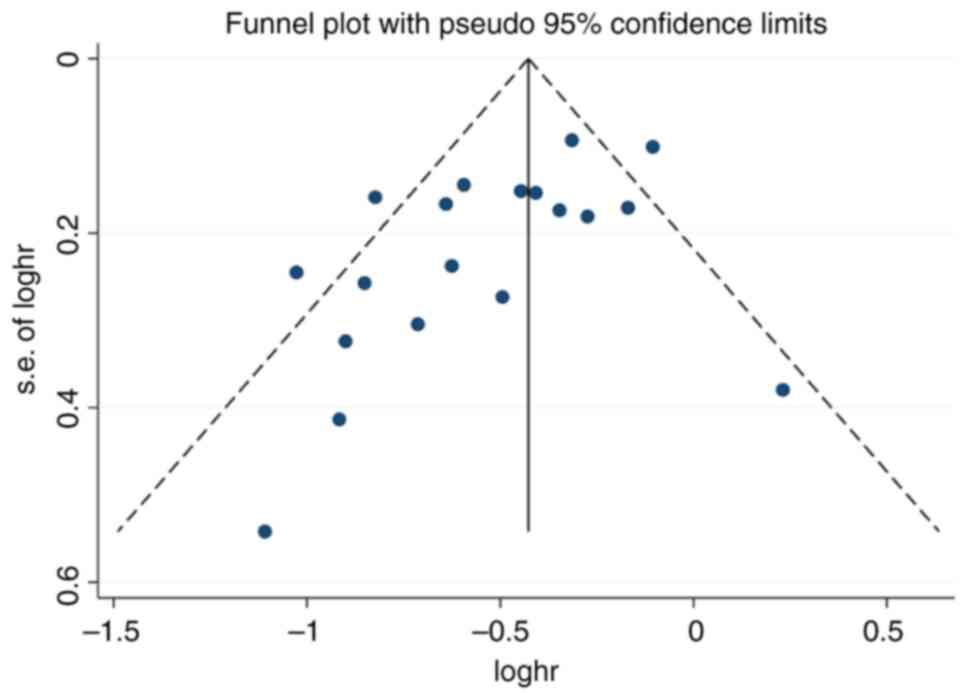
Bias and sensitivity analyses
The results of the funnel plot analysis revealed
that there was no indication of significant publication bias
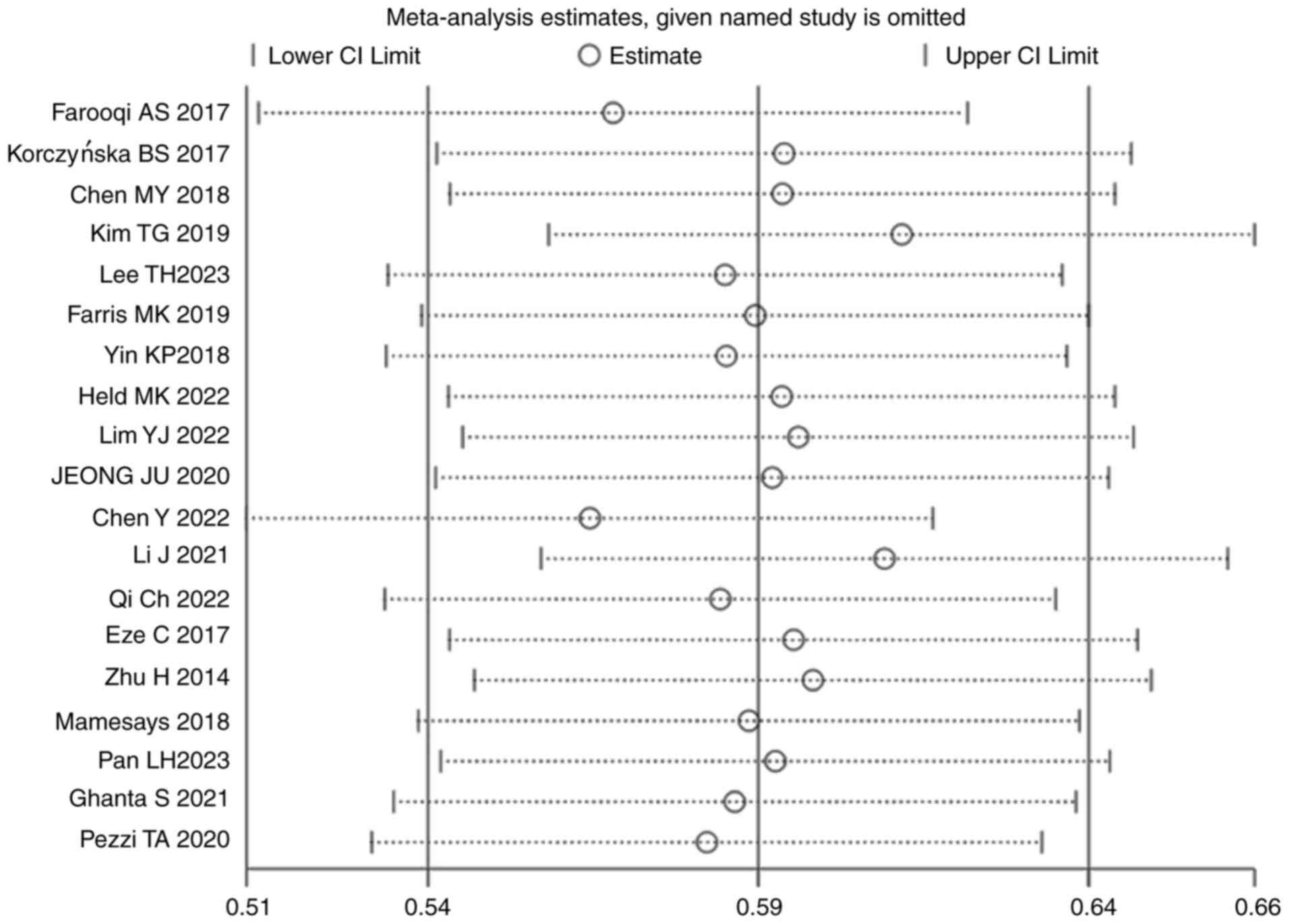
(Fig. 10). Sensitivity analyses
were performed to determine the effect of each study on the overall
meta-analysis estimate, through calculating the pooled HRs and
omitting one study at a time. The pooled results did not
significantly differ when single studies were removed, suggesting
that the results of the meta-analysis were stable (Fig. 11).
Discussion
The results of a previous meta-analysis revealed
that PCI significantly decreased the incidence of BM by 25.3% (33.3
vs. 58.6%; P<0.001) and increased the 3-year OS rate by 5.4%
(20.7 vs. 15.3%; P=0.01) in patients with LS-SCLC who achieved CR
after chemoradiotherapy (6). PCI is
the standard treatment recommendation for patients with LS-SCLC who
achieve CR or PR with first-line chemoradiotherapy (7). The results of a previous study
revealed a notable difference in the detection rates of BMs
originating from SCLC between the CT and MRI eras (9). Notably, the observed rates of BM were
10 and 24%, respectively, indicating that MRI may be more effective
for BM detection. However, the majority of the studies included in
the previous meta-analysis utilized CT rather than MRI to assess
the presence of BM in LS-SCLC. In a single-center study, 40
patients with LS-SCLC who achieved CR after chemoradiotherapy
underwent cranial MRI prior to PCI (34). The results demonstrated that BM was
detected in 13/40 patients (32.5%; 95% CI, 18–47%) and 11 cases
exhibited asymptomatic BM. These results suggested that patients
who did not undergo cranial MRI may have developed occult BM before
or during treatment; thus, patients who underwent PCI in the
pre-MRI era may have received treatment, rather than prevention.
Thus, these results may have overstated the benefits of PCI. Based
on the results of the aforementioned studies, numerous
retrospective clinical studies have re-evaluated the effectiveness
of PCI in patients with LS-SCLC during the MRI era (10–30).
The results of the present meta-analysis indicated that PCI
significantly decreased the incidence of BM (P<0.001) and
prolonged the OS time (P<0.001) in patients with LS-SCLC who had
the absence of BM confirmed via brain MRI at baseline or prior to
PCI. In addition, the results of the present meta-analysis revealed
that studies using propensity score matching also revealed that PCI
significantly prolonged OS time (P=0.039), which was consistent
with the findings of previous meta-analyses (35,36).
Thus, in the era of MRI, PCI is required for patients with LS-SCLC
who have undergone brain MRI at baseline or prior to PCI, to
exclude the presence of BM.
The results of a previous prospective study revealed
that PCI did not significantly increase the OS time of patients
with extensive-stage SCLC compared with active surveillance using
brain MRI. Notably, the median OS time in the PCI and non-PCI
groups was 11.6 and 13.7 months, respectively (HR, 1.27; 95% CI,
0.96–1.68; P=0.094) (37). Previous
retrospective clinical studies have also investigated whether
active surveillance using brain MRI is superior to PCI in patients
with LS-SCLC (10,14,18,19).
The results of the present meta-analysis revealed that PCI was able
to significantly reduce the incidence of BM in patients with
LS-SCLC compared with active surveillance using brain MRIs
(P=0.001); however, the observed benefit in OS was not significant
(P=0.078). The studies included in the present meta-analysis were
retrospective clinical studies. Thus, there may have been
differences in the baseline characteristics of the included
patients. In addition, propensity score matching analysis was
carried out in 3 of the studies to reduce the impact of potential
confounding, through reaching an equilibrium between the baseline
characteristics of the two patient groups. The combined results of
the studies that used propensity score matching also demonstrated
that PCI did not significantly prolong OS in patients with LS-SCLC,
compared with active surveillance using brain MRIs (P=0.068).
Notably, brain MRI follow-up may be for the early detection of BM,
leading to timely treatment with salvage radiation therapy and
improved patient survival. The results of the present meta-analysis
also revealed that the 1-, 2-, 3- and 5-year BM rates were higher
in the MRI surveillance group than in the pre-CRT and pre-PCI
groups, indicating that active surveillance using brain MRI may be
used for the early detection of asymptomatic BM. Moreover, studies
included in the present meta-analysis demonstrated that salvage
cerebrospinal radiotherapy was administered to 69.4–91.5% of
patients in the MRI surveillance group, compared with 23.1–58.5% of
patients in the PCI cohort. These results indicated that the
percentage of patients in the PCI group who received salvage
radiotherapy following BM was lower; however, the effectiveness of
systemic therapy for BM was suboptimal. This may have contributed
to the poor prognosis of these patients. In addition, the results
of previous studies demonstrated that patients with PR and stage
III disease were at a higher risk of developing BM and may have
been more likely to benefit from PCI (14,19).
However, the results of the subgroup analysis in the present study
revealed that PCI did not significantly improve the OS of patients
with PR and stage III LS-SCLC, compared with active surveillance
using brain MRI. Thus, even in patients at high risk of BM, brain
MRI active surveillance and early effective salvage therapy may be
not inferior to PCI in patients with LS-SCLC. Ongoing large-scale
randomized clinical trials are focused on the effects of active
surveillance using brain MRI and early effective salvage therapy in
patients with LS-SCLC [NCT04790253 (38), PRIMALung (39) and SWOG1827 (40)].
The results of the present meta-analysis revealed
that extracranial metastasis was the primary mode of recurrence
following initial treatment for LS-SCLC. In addition, the results
of a previous study revealed that intrathoracic recurrence alone
was the most common mode of recurrence following treatment for
LS-SCLC (28%), which was markedly higher than the incidence of BM
(9%) (41). Although PCI may
decrease the incidence of BM, the risk of extracranial metastasis
remains high, which may impact the therapeutic efficacy of PCI.
Thus, improved extracranial disease control may improve the
observed benefits of PCI in patients with LS-SCLC. Following the
introduction of immunotherapy, the ADRIATIC study (42) assessed the effectiveness of
immunoconsolidation following immunotherapy combined with
chemoradiotherapy for the treatment of LS-SCLC. The results
demonstrated that treatment was well-tolerated and that the PFS
(16.6 vs. 9.2 months; HR, 0.76; CI, 0.61–0.95; P=0.0161) and OS
(55.9 vs. 33.4 months; HR, 0.73; CI, 0.57–0.93; P=0.0104) times of
patients were improved. A trial including 40 patients with LS-SCLC
demonstrated that immunotherapy combined with chemoradiotherapy was
advantageous for patients with LS-SCLC, with median OS and median
PFS times of 39.5 (CI, 8.0–71.0) and 19.7 (CI, 8.8–30.5) months,
respectively (43). Notably,
immunotherapy significantly increased the rate of systemic disease
control in patients with LS-SCLC, which may have enhanced the
therapeutic benefits of PCI. Therefore, in the era of
immunotherapy, reassessing the curative value of PCI for patients
with LS-SCLC is crucial.
Notably, the present study exhibits several
limitations. The included studies were retrospective clinical
studies, which may lead to selection bias and selective reporting.
However, prospective studies focusing on the effects of PCI on
LS-SCLC in the MRI era are ongoing and the results are yet to be
reported. In addition, the included studies did not record or
analyze neurotoxicity or cognitive function following PCI. Thus,
post-PCI neurotoxicity response was not investigated in the present
study. Moreover, the present study did not determine the impact of
salvage therapy following BM on patient survival, as only a small
number of the included studies described the use of salvage therapy
following BM. In some cases, HR values were estimated based on
Kaplan-Meier survival curves, as numerous included studies did not
provide HR values. These factors may have limited the results of
the present meta-analysis.
In conclusion, the results of the present
meta-analysis revealed that PCI was effective in improving OS and
reducing BM in patients with LS-SCLC, when the absence of BM had
been confirmed using brain MRI at baseline or prior to PCI. In
patients with LS-SCLC who underwent active surveillance using brain
MRI following PCI, PCI reduced the rate of BM. However, PCI did not
significantly improve OS. Notably, studies included in the present
meta-analysis were retrospective; thus, additional randomized
controlled clinical trials are required to verify the results.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Gansu
Provincial Health Industry Scientific Research Project (grant no.
GSWSKY2021-057), the Lanzhou Science and Technology Program (grant
no. 2022-5-101) and the Lanzhou Talent Innovation and
Entrepreneurship Project (grant no. 2021-RC-130).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LS was involved in the conceptualization and design
the work and wrote the manuscript. YD, MJ, HS, LG and YQ were
responsible for acquiring, analyzing and interpreting the data. HS
and LG confirm the authenticity of all the raw data. JT and SW
interpreted the data and designed the study, and all of the authors
carefully reviewed the manuscript. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the United States over
the last 30 years: Analysis of the surveillance, epidemiologic, and
end results database. J Clin Oncol. 24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manapov F, Käsmann L, Roengvoraphoj O,
Dantes M, Schmidt-Hegemann NS, Belka C and Eze C: Prophylactic
cranial irradiation in small-cell lung cancer: Update on patient
selection, efficacy and outcomes. Lung Cancer (Auckl). 9:49–55.
2018.PubMed/NCBI
|
|
3
|
Alexopoulos CG, Vaslamatzis M, Patilla E
and Taranto L: Central nervous system involvement and the role of
prophylactic cranial irradiation in small cell lung cancer.
Oncologist. 2:153–159. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arriagada R, Le Chevalier T, Borie F,
Rivière A, Chomy P, Monnet I, Tardivon A, Viader F, Tarayre M and
Benhamou S: Prophylactic cranial irradiation for patients with
small-cell lung cancer in complete remission. J Natl Cancer Inst.
87:183–190. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Elliott JA, Osterlind K, Hirsch FR and
Hansen HH: Metastatic patterns in small-cell lung cancer:
Correlation of autopsy findings with clinical parameters in 537
patients. J Clin Oncol. 5:246–254. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aupérin A, Arriagada R, Pignon JP, Le
Péchoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka
H, Wagner H and Aisner J: Prophylactic cranial irradiation for
patients with small-cell lung cancer in complete remission.
Prophylactic cranial irradiation overview collaborative group. N
Engl J Med. 341:476–484. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network, .
Clinical practice guide-lines in oncology: small cell lung cancer
(version 1). 2022.https://www.nccn.org/professionals/physician_gls/default.aspx#sclc
|
|
8
|
Meert AP, Paesmans M, Berghmans T, Martin
B, Mascaux C, Vallot F, Verdebout JM, Lafitte JJ and Sculier JP:
Prophylactic cranial irradiation in small cell lung cancer: A
systematic review of the literature with meta-analysis. BMC Cancer.
1:52001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seute T, Leffers P, ten Velde GP and
Twijnstra A: Detection of brain metastases from small cell lung
cancer: Consequences of changing imaging techniques (CT versus
MRI). Cancer. 112:1827–1834. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Wang Y, Ren F, Huang Z, Tan B,
Zhao Z, Yu X, Dong P, Yu J and Meng X: Prophylactic cranial
irradiation (PCI) versus active surveillance in patients with
limited-stage small cell lung cancer: A retrospective, multicentre
study. Respir Res. 23:2742022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eze C, Roengvoraphoj O, Niyazi M,
Hildebrandt G, Fietkau R, Belka C and Manapov F: Treatment response
and prophylactic cranial irradiation are prognostic factors in a
real-life limited-disease small-cell lung cancer patient cohort
comprehensively staged with cranial magnetic resonance imaging.
Clin Lung Cancer. 18:e243–e249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inoue Y, Tsujino K, Sulaiman NS, Marudai
M, Kajihara A, Miyazaki S, Sekii S, Uezono H, Ota Y and Soejima T:
Re-evaluation of prophylactic cranial irradiation in limited-stage
small cell lung cancer: A propensity score matched analysis. J
Radiat Res. 62:877–883. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu H, Guo H, Shi F, Zhu K, Luo J, Liu X,
Kong L and Yu J: Prophylactic cranial irradiation improved the
overall survival of patients with surgically resected small cell
lung cancer, but not for stage I disease. Lung Cancer. 86:334–338.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Ding C, Yang C, Wang S and Qiao X:
Prophylactic cranial irradiation confers favourable prognosis for
patients with limited-stage small cell lung cancer in the era of
MRI: A propensity score-matched analysis. J Med Imaging Radiat
Oncol. 65:778–785. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mamesaya N, Wakuda K, Omae K, Miyawaki E,
Kotake M, Fujiwara T, Kawamura T, Kobayashi H, Nakashima K, Omori
S, et al: Efficacy of prophylactic cranial irradiation in patients
with limited-disease small-cell lung cancer who were confirmed to
have no brain metastasis via magnetic resonance imaging after
initial chemoradiotherapy. Oncotarget. 9:17664–17674. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozawa Y, Omae M, Fujii M, Matsui T, Kato
M, Sagisaka S, Asada K, Karayama M, Shirai T, Yasuda K, et al:
Management of brain metastasis with magnetic resonance imaging and
stereotactic irradiation attenuated benefits of prophylactic
cranial irradiation in patients with limited-stage small cell lung
cancer. BMC Cancer. 15:5892015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan L, Fan X, Wang L, Wang Y, Li Y, Cui Y,
Zheng H, Yi Q and Wu K: Prophylactic cranial irradiation for
limited-stage small-cell lung cancer in the magnetic resonance
imaging era. Cancer Med. 12:2484–2492. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi C, Li W, Li H, Wen F, Zhou L, Sun X and
Yu H: Benefits of prophylactic cranial irradiation in the MRI era
for patients with limited stage small cell lung cancer. Front
Oncol. 12:8334782022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghanta S, Keller A, Rodríguez-López JL,
Patel A and Beriwal S: Utility of prophylactic cranial irradiation
for limited stage small cell lung cancer in the modern era with
magnetic resonance imaging surveillance. Clin Oncol (R Coll
Radiol). 33:e323–e330. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pezzi TA, Fang P, Gjyshi O, Feng L, Liu S,
Komaki R and Lin SH: Rates of overall survival and intracranial
control in the magnetic resonance imaging era for patients with
limited-stage small cell lung cancer with and without prophylactic
cranial irradiation. JAMA Netw Open. 3:e2019292020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farooqi AS, Holliday EB, Allen PK, Wei X,
Cox JD and Komaki R: Prophylactic cranial irradiation after
definitive chemoradiotherapy for limited-stage small cell lung
cancer: Do all patients benefit? Radiother Oncol. 122:307–312.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sas-Korczyńska B, Łuczyńska E, Kamzol W
and Sokołowski A: Analysis of risk factors for pulmonary
complications in patients with limited-stage small cell lung
cancer: A single-centre retrospective study. Strahlenther Onkol.
193:141–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen MY, Hu X, Xu YJ and Chen M: The
impact of prophylactic cranial irradiation for post-operative
patients with limited stage small cell lung cancer. Medicine
(Baltimore). 97:e130292018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim TG, Pyo H, Ahn YC, Noh JM and Oh D:
Role of prophylactic cranial irradiation for elderly patients with
limited-disease small-cell lung cancer: Inverse probability of
treatment weighting using propensity score. J Radiat Res.
60:630–638. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee TH, Chung JH, Wu HG, Kim S, Lee JH,
Keam B, Kim JS, Kim KH, Kim BH and Kim HJ: Efficacy of prophylactic
cranial irradiation according to the risk of extracranial
recurrence in limited-stage small cell lung cancer. Cancer Res
Treat. 55:875–884. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Farris MK, Wheless WH, Hughes RT, Soike
MH, Masters AH, Helis CA, Chan MD, Cramer CK, Ruiz J, Lycan T, et
al: Limited-stage small cell lung cancer: Is prophylactic cranial
irradiation necessary? Pract Radiat Oncol. 9:e599–e607. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin K, Song D, Zhang H, Cai F, Chen J and
Dang J: Efficacy of surgery and prophylactic cranial irradiation in
stage II and III small cell lung cancer. J Cancer. 9:3500–3506.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Held MK, Hansen O, Schytte T, Hansen KH,
Bahij R, Nielsen M, Nielsen TB and Jeppesen SS: Outcomes of
prophylactic cranial irradiation in patients with small cell lung
cancer in the modern era of baseline magnetic resonance imaging of
the brain. Acta Oncol. 61:185–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim YJ, Song C and Kim HJ; Korean
Association for Lung Cancer and Korea Central Cancer Registry, :
survival impact of prophylactic cranial irradiation in small-cell
lung cancer in the modern era of magnetic resonance imaging
staging. Radiat Oncol. 17:262022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeong JU, Jeon W, Ahn SJ, Kim YC, Oh IJ,
Park CK, Yoon MS, Song JY, Nam TK and Chung WK: Treatment time to
the end of thoracic radiotherapy has more predictive power for
survival than radiation dose intensity in patients with
limited-stage small-cell lung cancer receiving concurrent
chemoradiation of more than 45 Gy. Oncol Lett. 19:239–246.
2020.PubMed/NCBI
|
|
31
|
Cook DA and Reed DA: Appraising the
quality of medical education research methods: The medical
education research study quality instrument and the
newcastle-ottawa scale-education. Acad Med. 90:1067–1076. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Higgins JPT, Thomas J, Chandler J,
Cumpston M, Li T, Page MJ and Welch VA: Cochrane handbook for
systematic reviews of interventions version 6.5 (updated August
2024). Cochrane; 2024, Available from. www.training.cochrane.org/handbook
|
|
34
|
Manapov F, Klautke G and Fietkau R:
Prevalence of brain metastases immediately before prophylactic
cranial irradiation in limited disease small cell lung cancer
patients with complete remission to chemoradiotherapy: A single
institution experience. J Thorac Oncol. 3:652–655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chi C, Wang C, Dong L, Li X and Xu L:
Prophylactic cranial irradiation in limited-stage small cell lung
cancer: A meta-analysis. J Coll Physicians Surg Pak. 34:461–467.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Shen B, Yang Y, Guo J, Ren W, Zhou
Q, Mao J, Ye W and Wu D: Survival benefit of prophylactic cranial
irradiation in limited-stage small-cell lung cancer in modern
magnetic resonance imaging staging: A systematic review and
meta-analysis. Acta Oncol. 62:305–314. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takahashi T, Yamanaka T, Seto T, Harada H,
Nokihara H, Saka H, Nishio M, Kaneda H, Takayama K, Ishimoto O, et
al: Prophylactic cranial irradiation versus observation in patients
with extensive-disease small-cell lung cancer: A multicentre,
randomised, open-label, phase 3 trial. Lancet Oncol. 18:663–671.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Levy A, Rusthoven CG, Brown PD, Le Péchoux
C and Faivre-Finn C: Prophylactic cranial irradiation for patients
with SCLC-A new perspective in the immunotherapy era. J Thorac
Oncol. 15:S1556-0864-02446-8. 2024.
|
|
39
|
Levy A, Berghmans T, Koller M, Fournier B,
Mauer M, Andratschke N and Faivre-Finn C: PRIMALung (EORTC-1901):
PRophylactic cerebral irradiation (PCI) or active brain MAgnetic
resonance imaging (MRI) surveillance in small-cell lung cancer
(SCLC) patients. Lung Cancer. 198:1079932024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
S1827 (MAVERICK) Testing whether the use
of brain scans alone instead of brain scans plus preventive brain
radiation affects lifespan in patients with small cell lung cancer.
https://clinicaltrials.gov/study/NCT04155034
|
|
41
|
Ellis PM, Swaminath A and Pond GR:
Patterns of relapse in small cell lung cancer: Competing risks of
thoracic versus CNS relapse. Curr Oncol. 28:2778–2788. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
David RS, Ying C, Byoung CC, Laktionov KK,
Fang J, Chen Y, Zenke Y, Lee KH, Wang Q, Navarro A, et al:
ADRIATIC: Durvalumab (D) as consolidation treatment (tx) for
patients (pts) with limited-stage small-cell lung cancer (LS-SCLC).
ASCO; LBA5: 2024
|
|
43
|
Welsh JW, Heymach JV, Guo C, Menon H,
Klein K, Cushman TR, Verma V, Hess KR, Shroff G, Tang C, et al:
Phase 1/2 Trial of Pembrolizumab and Concurrent Chemoradiation
Therapy for Limited-Stage SCLC. J Thorac Oncol. 15:1919–1927. 2020.
View Article : Google Scholar : PubMed/NCBI
|